1. Introduction
Liver fibrosis is a chronic condition characterized by the excessive accumulation of extracellular matrix proteins, primarily due to the differentiation of hepatic stellate cells (HSCs) into myofibroblasts during persistent liver injury. This pathological process disrupts the normal hepatic architecture and function, ultimately leading to cirrhosis and liver failure if untreated [
1]. Managing liver fibrosis remains a major clinical challenge because effective anti-fibrotic therapies are scarce. Current treatments mainly address the underlying cause of injury—for example, antiviral therapy for hepatitis or alcohol abstinence in alcoholic liver disease—but they achieve only limited reversal of established fibrosis [
2]. To date, no universally approved anti-fibrotic drug exists for broad clinical use. For instance, Rezdiffra (resmetirom) has demonstrated therapeutic benefit in patients with noncirrhotic NASH (nonalcoholic steatohepatitis) and fibrosis [
3]; however, its efficacy across other etiologies of liver fibrosis remains unproven. These limitations underscore the urgent need for new therapeutic strategies that directly target established fibrosis, irrespective of etiology.
The gut–liver axis has been increasingly recognized as a critical determinant of liver health and disease. The gut microbiota, through its metabolic activities and cellular components, can influence the progression of liver fibrosis [
4]. Dysbiosis—an imbalance in the gut microbial community—can increase intestinal permeability, allowing bacterial products such as endotoxins to translocate into the portal circulation. These products trigger hepatic inflammation and exacerbate fibrogenesis. Consequently, modulating the gut microbiota has emerged as a promising therapeutic strategy for liver disease. Previous studies have shown that scutellarin (SCU), a natural flavonoid derived from
Erigeron breviscapus (Compositae), regulates intestinal microbiota composition [
5,
6]. This finding suggests that SCU’s anti-fibrotic activity may partly depend on its ability to improve gut microbial balance. In line with the evolving paradigm of multi-target therapies for complex diseases [
7], we hypothesized that SCU might also influence the liver’s local microbiome. Emerging evidence, including our recent work, supports the existence of a resident “hepatic microbiota” that may contribute to liver fibrosis progression [
8]. Thus, SCU’s therapeutic effects may extend to modulating hepatic microbiota and, in turn, fibrogenesis. Nonetheless, research on the liver microbiome remains at an early stage, and further studies are needed to identify microbial changes that are beneficial or harmful in liver fibrosis.
SCU, a well-studied bioactive ingredient in traditional Chinese medicine, exhibits a broad spectrum of pharmacological activities. These include cardioprotective effects, such as the attenuation of cardiac hypertrophy [
9] and the amelioration of ischemia–reperfusion injury [
10,
11], as well as antimicrobial [
12] and antiviral properties [
13]. SCU also exhibits neuroprotective effects, for example, in glaucoma [
14], and antitumor activities through modulation of immune responses [
15]. Notably, SCU has demonstrated anti-fibrotic efficacy in other organ systems, including the amelioration of cardiac interstitial fibrosis post-infarction [
16] and the attenuation of pulmonary fibrosis [
17]. Collectively, these findings suggest that SCU has potential as an anti-fibrotic agent in hepatic disease. Given its diverse pharmacological profile and anti-fibrotic activity in the heart and lung, it is reasonable to propose that SCU could also play a beneficial role in ameliorating liver fibrosis, especially if delivered effectively to hepatic targets.
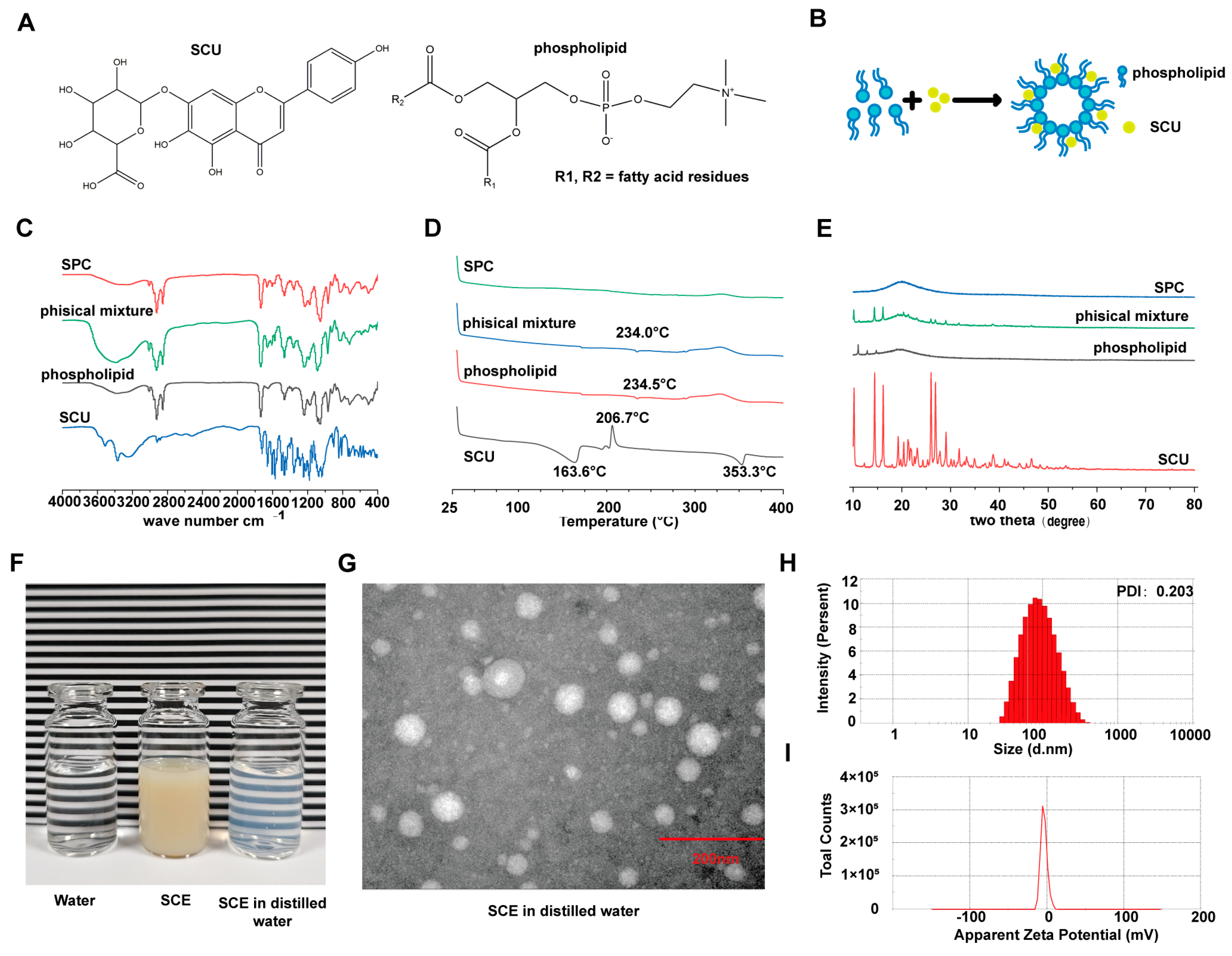
Despite its therapeutic potential, the clinical use of SCU is limited by its unfavorable biopharmaceutical properties. Specifically, SCU’s poor water solubility markedly limits its oral absorption and bioavailability, thereby reducing its efficacy. Overcoming these challenges requires advanced drug delivery strategies that improve solubility, protect SCU from degradation during gastrointestinal transit, and enable targeted delivery to the liver. Recent innovations, including phospholipid complexation and nanoformulations, have enhanced the solubility and bioavailability of poorly water-soluble drugs and, in some cases, conferred organ-targeting capabilities [
18].
In this study, we developed a novel nanoemulsion-based delivery system for SCU to improve its solubility and liver-targeting capability. Initially, we prepared a scutellarin–phospholipid complex (SPC) to increase the lipophilicity of SCU and facilitate its incorporation into the nanoemulsion. Using this complex, we formulated a scutellarin-loaded nanoemulsion (SCE) stabilized with chitosan oligosaccharide. We then conducted a comprehensive physicochemical characterization of both SPC and SCE and evaluated their performance in vitro and in vivo. Specifically, we examined the ability of SCE to enhance cellular uptake and hepatic accumulation of SCU, along with its pharmacokinetic and pharmacodynamic profiles in a bile duct ligation (BDL)-induced liver fibrosis mouse model. We further assessed the therapeutic efficacy of SCE against liver fibrosis in vivo and in vitro, using a TGF-β1-activated HSC (LX-2 cell) model. In addition, we investigated whether free and nanoformulated SCU could modulate gut and liver microbiota in fibrotic mice, exploring the gut–liver axis as a potential mechanism of action. Finally, we confirmed the safety and biocompatibility of SCE through both in vitro assays (normal liver cells and LX-2 cells) and in vivo assessments (histopathology and blood chemistry in mice). Overall, our findings demonstrate that SCE significantly alleviates liver fibrosis by enhancing hepatic delivery and multi-target mechanisms, offering a promising therapeutic approach that combines microbiome modulation with direct anti-fibrotic activity.
3. Discussion
Liver fibrosis is characterized by excessive deposition of extracellular matrix components, particularly collagen, leading to distortion of liver architecture and function. If unresolved, fibrosis progresses to cirrhosis and eventually hepatocellular carcinoma [
38]. Although researchers have extensively elucidated the mechanisms of fibrogenesis, clinically approved anti-fibrotic therapies remain scarce. Current treatment strategies primarily target underlying etiologies (e.g., antiviral therapy for viral hepatitis) and provide supportive care, but they show limited efficacy in reversing established fibrosis.
SCU has emerged as a potential multi-target agent due to its broad pharmacological activities. Prior studies have documented its anti-fibrotic properties in non-hepatic tissues. In this context, multi-target approaches that simultaneously address different aspects of fibrogenesis are highly desirable. Our study focused on scutellarin (SCU) because of its well-documented pharmacological profile and its critical limitation of poor solubility and bioavailability, which made it an ideal candidate to demonstrate the utility of our nanoemulsion platform. Other flavonoids such as silybin and quercetin are also promising anti-fibrotic candidates, and the nanoemulsion strategy described here could, in principle, be extended to these compounds in future work.
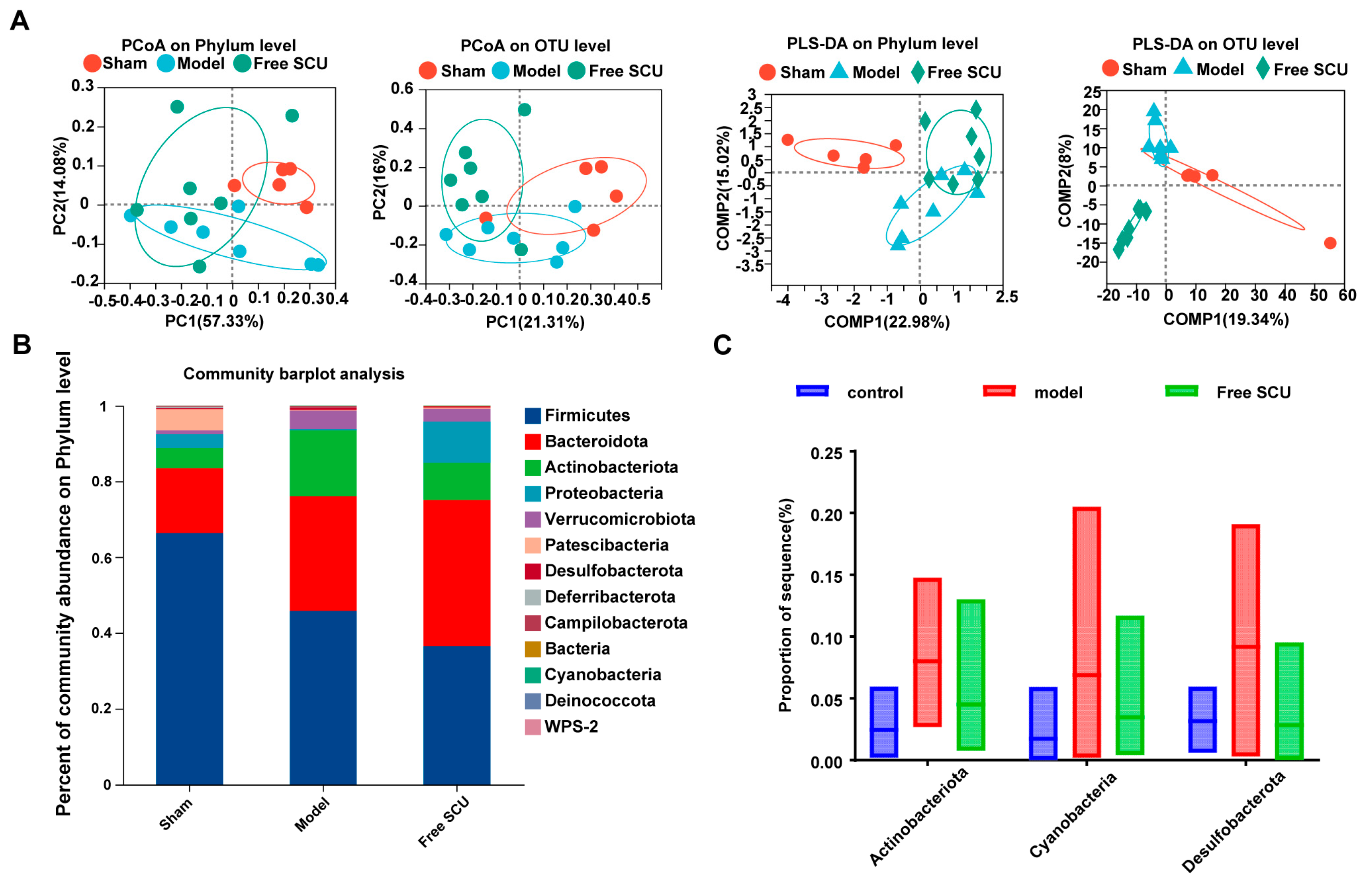
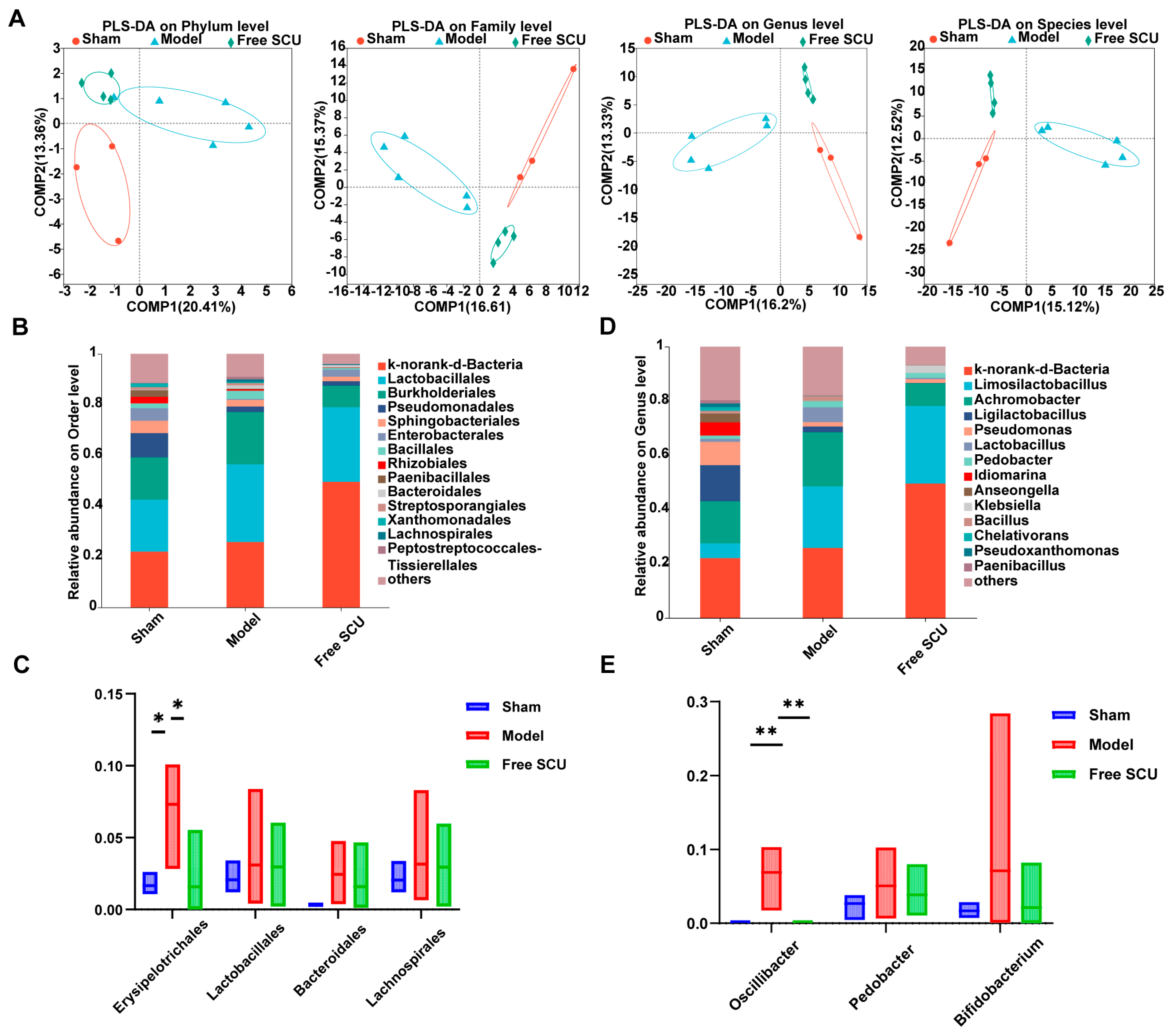
Scutellarin (SCU) has emerged as a promising candidate due to its multitarget pharmacological profile. Previous studies have shown that SCU reduces fibrosis in non-hepatic organs [
16,
17] and protects the liver [
9,
10]. Other researchers demonstrated that SCU reshapes gut microbiota in liver disease models [
5], matters because gut-derived factors drive liver inflammation. In this study, we showed that SCU treatment altered both intestinal and hepatic microbiota under fibrotic conditions to some extent. SCU reduced the abundance of several potentially pathogenic taxa (such as
Actinobacteriota and
Desulfobacterota in the gut), which BDL had elevated. Elevated
Actinobacteriota abundance worsens liver pathology in steatosis and cancer models [
19,
20], whereas elevated
Desulfobacterota abundance is linked to hepatic inflammation [
21]. Although the nanoemulsion primarily facilitated efficient hepatic targeting, its oral route of administration also allowed for transient interaction with the gut microbiota. This was associated with partial normalization of dysbiotic taxa, which—while not the principal mechanism of action—may have contributed additively to the observed therapeutic benefits. By attenuating these dysbiotic shifts, SCU likely blocked the translocation of pro-fibrogenic microbial products such as endotoxins from the gut to the liver. SCU also restored normal levels of
Erysipelotrichales and
Oscillibacter in the hepatic microbiota, which indicates that SCU directly or indirectly regulates bacteria that colonize or translocate to the liver during fibrogenesis. The liver microbiome is a relatively new research frontier. We observed that SCU shifted microbial abundance, but we have not yet determined how these changes drive anti-fibrotic outcomes. The liver microbiota results were obtained from low-biomass samples and should therefore be regarded as highly exploratory and inherently prone to contamination—a well-recognized limitation in this field. Although we implemented stringent precautions, including processing all samples under a biosafety cabinet with sterile, DNA-free reagents, the absence of dedicated negative controls and formal bioinformatic decontamination necessitates cautious interpretation. Accordingly, we do not present these findings as definitive evidence of a resident liver microbiota, but rather as preliminary, hypothesis-generating observations that are consistent with the emerging concept of a gut–liver axis in fibrosis. We classify our hepatic microbiota findings as exploratory. Future studies using germ-free or microbiota-depleted models containing adequate mice must establish whether microbiome modulation directly improves fibrosis. Despite this limitation, our results strengthen the concept of a gut–liver axis in fibrosis and show that SCU restores microbial balance to improve this axis. Furthermore, we acknowledge that microbiota abundance data may not follow a normal distribution, which limits the robustness of parametric comparisons.
SCU shows poor water solubility and low oral bioavailability, which likely hinders its therapeutic effectiveness in vivo [
12,
18]. In this study, we overcame this barrier by developing the scutellarin–phospholipid complex (SPC). Characterization techniques, including FTIR, DSC, and XRD, confirmed that SCU was present in an amorphous state within the lipid matrix, likely at least partially molecularly dispersed. Amorphization is known to enhance the dissolution of hydrophobic drugs [
39]. By incorporating SPC into a nanoemulsion (SCE), we formulated SCU at therapeutically relevant concentrations with greater solubility and absorption. Nanoemulsions commonly increase the oral bioavailability of lipophilic compounds by promoting lymphatic transport and preventing precipitation or metabolism [
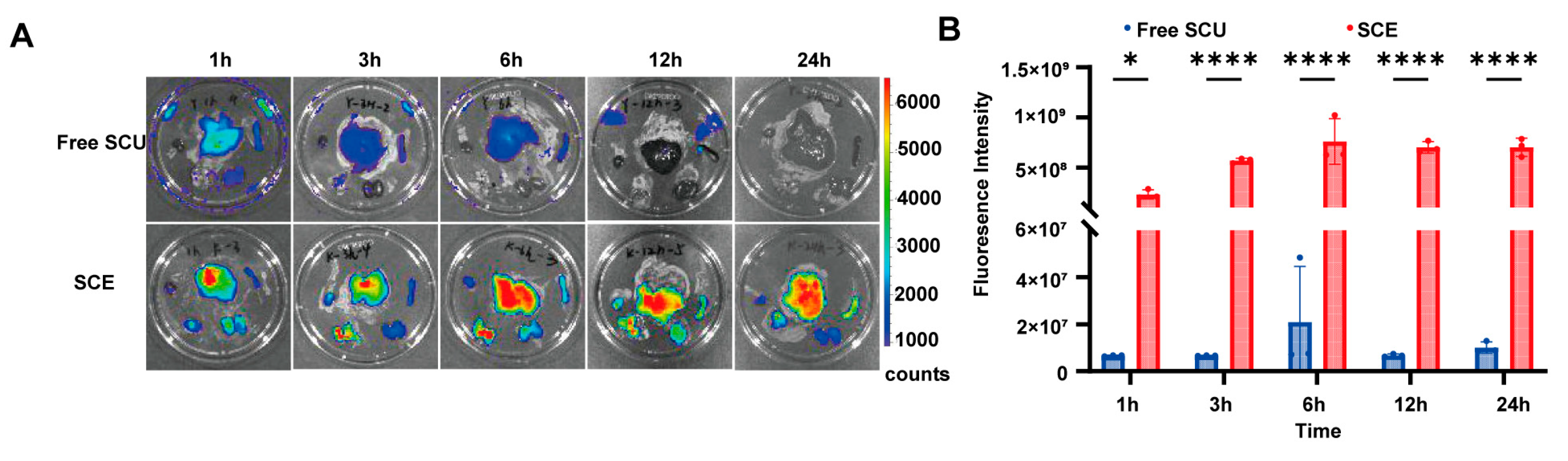
40]. Our findings support this mechanism: the SCE nanoemulsion likely promoted intestinal absorption via lymphatic transport pathways, thereby enhancing hepatic delivery of SCU, as corroborated by fluorescence imaging.
SCE significantly enhanced the pharmacokinetic and tissue distribution profiles of SCU. In vivo imaging results showed that SCE achieved higher and more sustained liver concentrations than free SCU. This liver-targeting effect benefits anti-fibrotic therapy by concentrating the drug at the site of action and reducing systemic exposure, thereby lowering the risk of off-target effects. SCE also prolonged hepatic retention, maintaining significant levels even 24 h after treatment. This sustained presence may extend drug action and permit less frequent dosing in clinical applications.
At the cellular level, the nanoemulsion also proved advantageous. Our results showed that SCE is readily taken up by liver cells (stellate cells, hepatocytes, and possibly liver macrophages) once it reaches the liver. The mechanism involved caveolae-mediated endocytosis, a pathway that nanoparticles often exploit to enter cells efficiently. Through this process, the nanoemulsion allowed SCU to cross the cellular barriers of fibrotic tissue more effectively. In fibrotic livers, the dense extracellular matrix typically impedes drug diffusion. However, nanoemulsions of suitable size can exploit disrupted sinusoidal endothelium and increased vascular permeability to access activated HSCs, which overexpress endocytic receptors. This mechanism likely explains why SCE suppressed HSC activation more effectively than free SCU in vivo—greater intracellular delivery enabled the drug to exert its pharmacological effect.
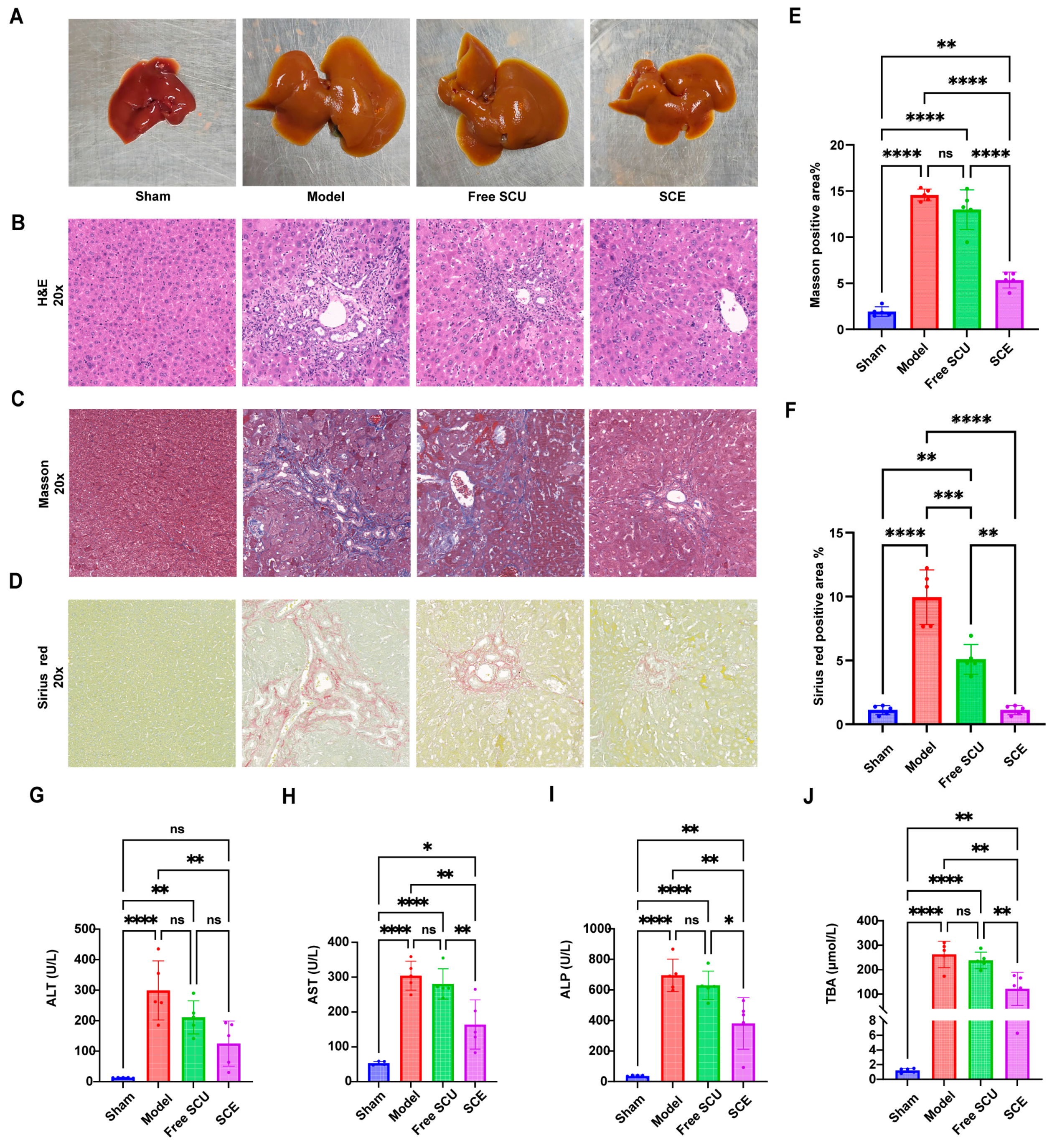
Therapeutically, SCE treatment produced certain outcomes in the BDL-induced fibrosis model. SCE improved the progression of fibrosis, as indicated by the lower collagen content and improved histology compared to untreated fibrotic mice. In contrast, free SCU exhibited only minimal effects, underscoring the critical role of the nanoemulsion delivery system in unlocking the therapeutic potential of SCU. The limited efficacy of free SCU at a dose of 10 mg/kg is consistent with previous reports that its poor bioavailability hinders in vivo activity [
12,
41,
42]. Using SCE, we effectively increased the bioavailability and hepatic concentration of SCU, thereby achieving the desired anti-fibrotic action.
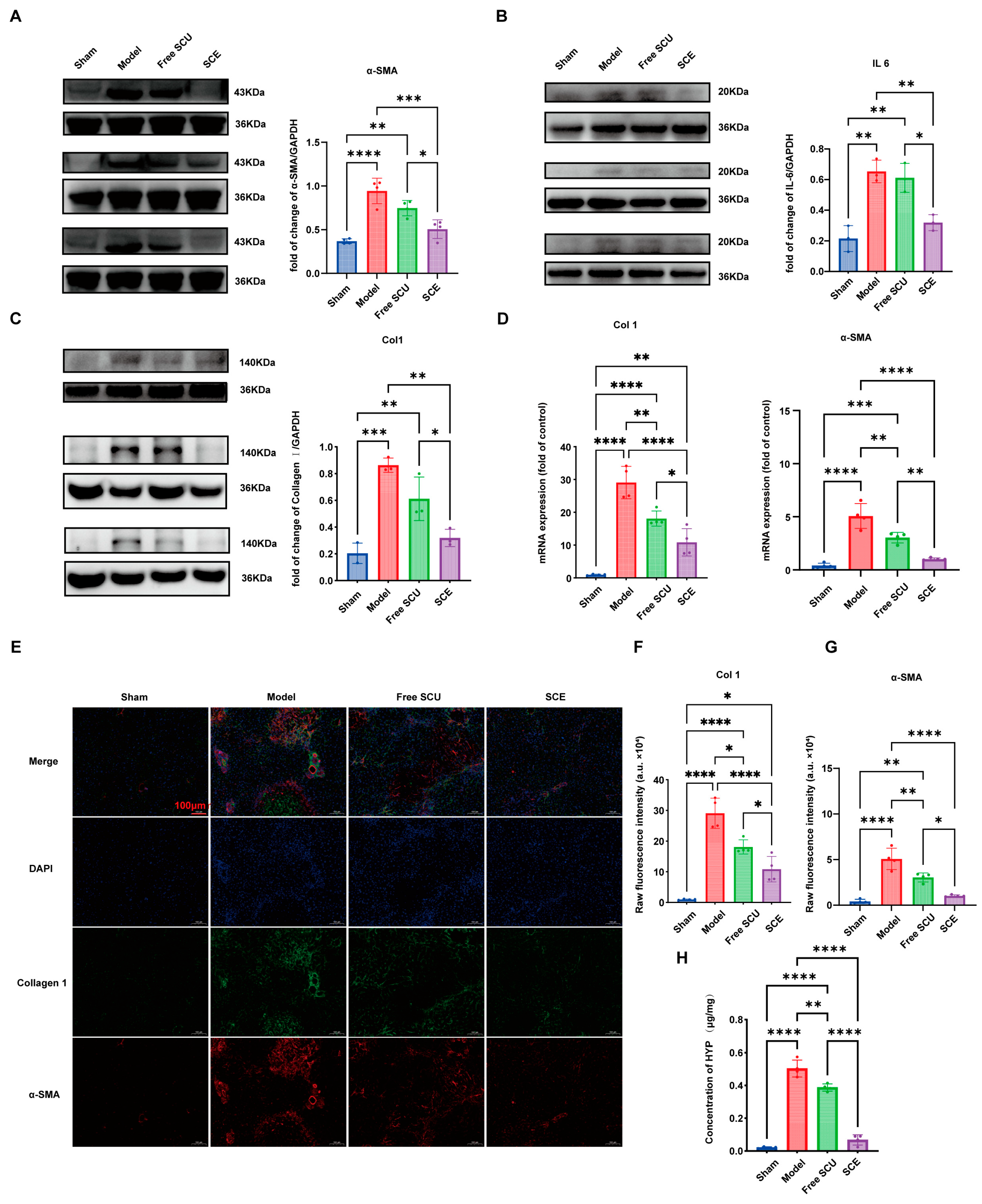
Mechanistically, SCU exerts anti-fibrotic effects through several interconnected pathways. Known for its antioxidant and anti-inflammatory properties [
10], SCU disrupts TGF-β/SMAD signaling, the central fibrogenic pathway, and downregulates key fibrotic markers such as α-SMA and collagen I. SCU also modulates inflammatory cascades, likely through NF-κB signaling, as evidenced by the pronounced reduction in hepatic IL-6 expression in SCE-treated mice. The downregulation is particularly significant, since IL-6 not only signals inflammation but also drives fibrosis and carcinogenesis in chronic liver disease. By suppressing IL-6, SCU helps dampen the inflammatory milieu that fuels fibrogenesis. We also found that SCU reduced MMP2 expression in HSCs in vitro. This modulation of MMP2 suggests that SCU influences matrix remodeling dynamics, promoting a more balanced environment where collagen deposition and degradation can proceed toward the resolution of fibrosis.
Our study also highlights the favorable safety of the SCE system. We were careful to demonstrate that the formulation components (lipid, surfactant, and COS polymer) did not introduce toxicity. COS is generally regarded as biocompatible and has been reported to confer additional biological benefits, such as promoting intestinal health and enhancing mucosal permeability. The surfactant (Cremophor EL), while sometimes causing hypersensitivity at high doses in intravenous formulations, is in a relatively low dose orally and was well-tolerated. Furthermore, the negative surface charge and nanoscale size of SCE contributed to its stability and low immune recognition, preventing unwanted immune responses.
From a translational perspective, SCE shows promise as a therapy for liver fibrosis. Nevertheless, several considerations and future directions must be addressed. First, although our results in the BDL model were compelling, liver fibrosis is a heterogeneous condition with diverse etiologies. Future work should evaluate the efficacy of SCE in additional models, such as those induced by carbon tetrachloride or NASH, to confirm its broader applicability. Second, the observed microbiome-modulating effects of SCU raise the possibility of combining SCU with specific probiotics or prebiotics to enhance therapeutic outcomes via synergistic modulation of the gut–liver axis. Third, while nanoemulsions are relatively straightforward to scale up, we must confirm long-term stability beyond seven days. Transforming the nanoemulsion into a solid dosage form, such as a freeze-dried powder for reconstitution, could improve practicality for clinical use. Fourthly, another limitation of this study is the use of a single dose of SCE. While this dose was effective and informed by previous studies [
41,
42], future work will include a comprehensive dose–response evaluation to determine the optimal therapeutic window and maximize the potential of this formulation. Finally, another limitation of this study is the absence of a blank nanoemulsion control in the in vitro experiments. Although our primary objective was to evaluate the therapeutic potential of SCU when formulated into a nanoemulsion, we acknowledge that vehicle-only controls would have further strengthened the interpretation of these findings. Without this control, we cannot fully exclude the possibility that some of the observed effects may be partially attributable to the components of the vehicle rather than SCU itself. Future studies will incorporate such vehicle controls to rigorously confirm the specificity of the nanoemulsion-mediated effects.
In addition, we acknowledge that effect sizes and confidence intervals were not provided, which limits the interpretability of some results.
The strategy of targeting both fibrotic processes and the microbiome is still relatively novel. Our work provides proof-of-concept that a single agent, when properly formulated, may simultaneously modulate HSC activity and the gut–liver axis. SCU exerts a dual mechanism of action by directly suppressing fibrotic processes and simultaneously ancillary modulating the microbiota, thereby exemplifying a polypharmacological strategy [
7]. In complex diseases such as liver fibrosis, where inflammation, cell activation, and gut–liver signaling interact through tightly linked pathways, multi-pronged agents like SCU achieve greater therapeutic effectiveness.
In conclusion, this work demonstrates that formulating SCU into a nanoemulsion markedly enhances its therapeutic efficacy against liver fibrosis. SCE improves pharmacokinetics and liver targeting, which increases anti-fibrotic effects by reducing collagen deposition and HSC activation. Additionally, SCE preserves the ability of SCU to modulate the gut–liver axis through microbiota changes. These results highlight the critical role of drug delivery systems in unlocking the clinical potential of poorly soluble natural products like SCU. The nanoemulsion approach described herein may apply to other phytochemicals or therapeutic agents facing similar solubility and bioavailability challenges. Overall, our findings contribute to the development of an effective, multi-target therapy for liver fibrosis and highlight the innovative angle of targeting the microbiome as part of the therapeutic mechanism.
4. Materials and Methods
4.1. Materials
Scutellarin (SCU, >98% purity, CAS No. 27740-01-8) was purchased from J&K Scientific (Wuhan, China). Soybean phospholipid (lecithin) served as the excipient for phospholipid complex preparation. Caprylic/capric triglycerides (medium-chain triglycerides) and Cremophor EL (polyoxyethylated castor oil) were used as the oil phase and surfactant, respectively, in the nanoemulsion. Chitosan oligosaccharide (COS) was used as a stabilizer. Recombinant human TGF-β1 (transforming growth factor beta 1) was purchased from R&D Systems (Minneapolis, MN, USA) for HSC activation in vitro. The human HSC line LX-2 was purchased from Shanghai Mingjin Biotechnology Co., Ltd. (Shanghai, China) (RRID:CVCL_5792) on 16 May 2023. The human normal liver cell line LO2 was kindly provided by Professor He (Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences & Peking Union Medical College). Antibodies against α-SMA (α-smooth muscle actin, Cat# 14395-1-AP), MMP2 (matrix metalloproteinase-2, Cat# 66366-1-Ig), and collagen type I (COL1A1, Cat# 14695-1-AP) were purchased from Proteintech (Wuhan, China). GAPDH antibody (Cat# ABL-1021) was purchased from Abbkine (Wuhan, China). All other reagents and chemicals were of analytical reagent grade and were used as received without further purification.
4.2. Preparation of Scutellarin–Phospholipid Complex (SPC)
A scutellarin–phospholipid complex (SPC) was prepared to enhance the lipophilicity of SCU, following a previously reported method with slight modifications [
39]. Briefly, SCU and soybean phospholipid (mass ratio 1:5) were co-dissolved in absolute ethanol to obtain a solution containing 0.5 mg/mL of SCU. The solvent was subsequently removed by rotary evaporation under reduced pressure at approximately 40 °C. During evaporation, the mixture was sonicated to facilitate molecular interaction between SCU and the phospholipid. The resulting solid residue was dried to constant weight to yield the SPC. For comparison, a physical mixture of SCU and phospholipid was prepared by manually blending the two components at the same 1:5 mass ratio using a mortar and pestle, without the use of any solvent.
4.3. Preparation of SCU-Loaded Nanoemulsion (SCE)
The SCU-loaded nanoemulsion (SCE) was prepared using the previously prepared SPC. First, a primary coarse emulsion was prepared using the phase inversion method. Caprylic/capric triglyceride (1 mL) was mixed with an equal mass of Cremophor EL to form the oil-surfactant phase. Subsequently, 48 mg of SPC (containing SCU) was added to the oil-surfactant phase and dissolved with gentle heating and sonication until a clear solution was obtained. Separately, chitosan oligosaccharide (15 mg) was dissolved in 3 mL of deionized water to prepare the aqueous phase. The oil phase was added dropwise into the aqueous phase under magnetic stirring at approximately 1500 rpm at room temperature, resulting in a crude oil-in-water emulsion. This primary emulsion was then subjected to high-energy ultrasonication to reduce droplet size, using an ultrasonic cell disruptor (Xinzhi, Ningbo, China) (200 Hz) in an ice-water bath with pulse cycles of 10 s on and 5 s off for a total of 15 min. The resulting nanoemulsion was equilibrated to room temperature and stored at 4 °C until use.
4.4. Characterization of SPC and SCE
To confirm SPC formation, differential scanning calorimetry (DSC), Fourier-transform infrared spectroscopy (FTIR), and X-ray powder diffraction (XRD) analyses were conducted on SCU, phospholipid, their physical mixture, and the SPC product. DSC thermograms were acquired using a DSC analyzer (Mettler-Toledo, Switzerland) by heating samples from 30 °C to 400 °C at a programmed rate to detect alterations in melting or crystallization behavior indicative of complex formation. FTIR spectra were recorded using a Nicolet 5700 FTIR spectrometer (Thermo, Waltham, MA, USA) over the range 4000–400 cm−1 to identify chemical interactions, such as hydrogen bonding, between SCU and the phospholipid. XRD patterns were recorded using a Bruker D8 Advance diffractometer (Billerica, MA, USA) (Cu Kα radiation, λ = 1.5406 Å, 40 kV, 40 mA) to assess the crystallinity of SCU in each sample; the disappearance of SCU’s characteristic crystalline peaks in the SPC would suggest an amorphous or molecularly dispersed state.
SCE was characterized in terms of particle size, size distribution, zeta potential, morphology, and drug content. The hydrodynamic particle size (mean diameter) and polydispersity index (PDI) were measured by dynamic light scattering (DLS) using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) at 25 °C. Zeta potential, indicating the surface charge of nanoemulsion droplets, was determined by electrophoretic light scattering with the same instrument. The morphology of SCE was observed using transmission electron microscopy (TEM). A drop of diluted SCE was placed on a carbon-coated copper grid, allowed to sit for 1 min, and excess fluid was removed by blotting. The grid was then air-dried and examined under a TEM (JEM-2100, JEOL, Akishima, Japan) operated at an accelerating voltage of 200 kV. The TEM images provided visual confirmation of particle size and morphology. The SCU concentration in the nanoemulsion was determined using a validated high-performance liquid chromatography (HPLC) method (Shimadzu, Kyoto, Japan) with UV detection at 335 nm. Briefly, SCE samples were diluted in methanol and analyzed on a C18 column. The mobile phase consisted of methanol and 0.5% acetic acid (4:6, v/v) and was delivered at a flow rate of 1 mL/min. The column temperature was maintained at 30 °C. Quantification was achieved using a standard calibration curve.
4.5. Storage Stability Study
The short-term physical and chemical stability of SCE was assessed over 7 days at 4 °C. Aliquots were stored in sealed vials and sampled on days 0 (initial), 1, 3, 5, and 7. At each time point, mean particle size, PDI, and zeta potential were measured by DLS as described in
Section 4.4. Additionally, the SCU concentration within the formulation was analyzed by HPLC to monitor any potential drug degradation or precipitation. All measurements were performed in triplicate. Stability was considered acceptable if no significant particle growth or aggregation occurred and if the SCU content remained above 90% of the initial drug content over the storage period.
4.6. Cell Culture and Cytotoxicity Assay
LX-2 and LO2 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Waltham, MA, USA) and Roswell Park Memorial Institute (RPMI) 1640 (Gibco, USA), respectively. Both media were supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2. The culture medium was replaced every 2–3 days, and cells were subcultured using trypsin-EDTA upon reaching 80–90% confluence.
The cytotoxicity of SCE was evaluated in vitro using the Cell Counting Kit-8 (CCK-8, Meilunbio, Dalian, China) assay in both LX-2 and LO2 cell lines. Cells were seeded into 96-well plates at a density of 5 × 103 cells per well and allowed to adhere for 12 h. Subsequently, the medium was replaced with 100 µL of fresh medium containing either free SCU or SCE at various concentrations (ranging from 5 µM to 30 µM SCU equivalent). Control wells received only drug-free medium. After 24 h of treatment, 10 µL of CCK-8 reagent was added to each well, followed by an additional 4 h incubation period. Absorbance was measured at 450 nm using a microplate reader (Synergy H1, BioTek, Winooski, VT, USA). Cell viability was expressed as a percentage of the untreated control. Each concentration was tested in quadruplicate, and data are presented as mean ± SD. For all cell-based assays (cytotoxicity, cellular uptake, gene/protein expression, and migration), at least three independent experiments were performed on different days, and each experiment contained multiple technical replicates.
4.7. Cellular Uptake Study in LX-2 Cells
The cellular uptake of SCU delivered via SCE versus free drug was examined in LX-2 cells using a fluorescent probe. Nile Red (NR), a hydrophobic fluorescent dye, was used as a surrogate to visualize and quantify uptake. NR-loaded SCE was prepared by adding a small amount of NR to the SPC (at 10%
w/
w of SCU) during the nanoemulsion preparation process (
Section 4.3). A solution of free NR in PBS (containing a small amount of DMSO to aid solubilization) was used as a control corresponding to free SCU.
For qualitative uptake visualization, LX-2 cells were seeded in glass-bottomed confocal dishes at 2 × 105 cells per dish and cultured for 24 h. Cells were then treated with either free NR (in PBS) or NR-loaded SCE (NR/SCE) at an equivalent NR concentration. After incubation for predetermined periods (5 min, 15 min, 30 min, 1 h, 2 h, and 4 h) at 37 °C, the cells were washed three times with PBS to remove extracellular NR, fixed with 4% paraformaldehyde for 15 min, and stained with DAPI (4′,6-diamidino-2-phenylindole) to label nuclei. Fluorescent images were acquired using a laser scanning confocal microscope (Zeiss LSM 710, Oberkochen, Germany). NR (red) and DAPI (blue) signals were visualized to evaluate intracellular localization and uptake intensity.
For quantitative analysis, cellular uptake was evaluated by flow cytometry. LX-2 cells were seeded in 6-well plates (3 × 105 cells/well) and treated with free NR or NR-loaded SCE as described above. At selected time points (up to 4 h), cells were washed with ice-cold PBS, detached with trypsin, and resuspended in PBS. Intracellular fluorescence was measured using a flow cytometer (BD FACSCalibur, Franklin Lakes, NJ, USA) in the FL2 channel. At least 10,000 events were recorded per sample. The mean fluorescence intensity (MFI) was calculated to compare the cellular uptake efficiency of NR/SCE versus free NR.
To elucidate the cellular internalization pathways of SCE, LX-2 cells were pre-treated for 15 min with one of several pharmacological endocytosis inhibitors before exposure to NR-loaded SCE (NR/SCE). The inhibitors included: chlorpromazine (10 µg/mL, inhibitor of clathrin-mediated endocytosis), nystatin (25 µg/mL, inhibits caveolae-mediated endocytosis by cholesterol sequestration), methyl-β-cyclodextrin (MβCD, 5 mM, depletes membrane cholesterol and disrupts caveolae), genistein (100 µM, tyrosine kinase inhibitor that also disrupts caveolae-mediated endocytosis), imipramine (10 µg/mL, reported to inhibit caveolae pathway), and 5-(N-ethyl-N-isopropyl) amiloride (EIPA, 50 µM, inhibitor of macropinocytosis). Following inhibitor pretreatment, cells were washed with PBS and incubated with NR/SCE for 2 h at a concentration previously determined to produce intense fluorescence. Cells were then processed for flow cytometry as described in the cellular uptake section. A significant reduction in MFI in the presence of a specific inhibitor, relative to the untreated control, was interpreted as indicative of that pathway’s involvement in SCE internalization.
4.8. In Vitro Anti-Fibrotic Activity in LX-2 Cells
LX-2 cells were employed as an in vitro model to assess HSC activation and evaluate the anti-fibrotic potential of SCU formulations. Cells were seeded in 6-well plates at a density of approximately 1 × 105 cells per well and cultured to 80–90% confluence. To induce a fibrogenic phenotype, the cells were serum-starved in DMEM containing 2% FBS for 24 h, followed by stimulation with TGF-β1 (2 ng/mL) for an additional 24 h. This treatment activates LX-2 cells, resulting in upregulation of fibrotic markers. After activation, the medium was replaced, and SCE was added. The cells were then incubated for 12 h. Subsequently, the cells were harvested for analysis of fibrogenic gene and protein expression by quantitative real-time PCR (qPCR) and Western blot.
Total RNA was isolated using the RaPure Total RNA Kit (Magen, Guangzhou, China) according to the manufacturer’s protocol. RNA concentration and purity were verified by spectrophotometry. cDNA was synthesized and amplified using a one-step RT-qPCR SYBR Green kit (Vazyme, Nanjing, China) on a 7500 Fast Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). The primer sequences for target genes (collagen I [COL1A1], α-SMA [ACTA2], and β-actin [ACTB] as a housekeeping gene) were as follows: COL1A1: forward 5′-TGACCTTCCTGCGCCTAATG-3′, reverse 5′-GCTACGCTGTTCTTGCAGTG-3′; ACTA2 (α-SMA): forward 5′-CTCTGTCTGGATCGGTGGC-3′, reverse 5′-TTCGTCGTATTCCTGTTTGCT-3′; ACTB: forward 5′-CCTGGACTTCGAGCAAGAGATGG-3′, reverse 5′-GTGGTTTCGCTCGGCACATT-3′. The thermal cycling conditions were set according to kit protocols. The relative gene expression was calculated using the 2(−ΔΔCt) method, with normalization to ACTB and comparison to the untreated control group.
For Western blot analysis, we lysed LX-2 cells using radioimmunoprecipitation assay (RIPA) buffer supplemented with protease and phosphatase inhibitors to extract total protein. We measured protein concentrations and loaded equal amounts (20 µg per sample) onto SDS-PAGE gels, then transferred the separated proteins onto polyvinylidene difluoride (PVDF) membranes. We blocked the membranes with 5% non-fat milk for 1 h, followed by overnight incubation at 4 °C with primary antibodies against key fibrogenic proteins: collagen I (1:2000 dilution) and MMP2 (1:2000). GAPDH (1:5000) was used as an internal loading control. After washing, we incubated the membranes with horseradish peroxidase-conjugated secondary antibodies (1:10,000) for 1 h at room temperature. We visualized protein bands using enhanced chemiluminescence (ECL) substrate and captured images with a ChemiDoc imaging system (Bio-Rad, Hercules, CA, USA). We quantified band intensities using ImageJ software (1.54) and normalized target protein levels to GAPDH.
4.9. Cell Migration Assay
We evaluated the effect of SCU on HSC migration, a characteristic of activated HSCs that contributes to fibrotic tissue remodeling, using a wound healing (scratch) assay. LX-2 cells were seeded in 6-well plates and cultured until they formed a nearly confluent monolayer. We created a linear scratch approximately 1 mm wide through the cell monolayer using a sterile pipette tip. After gently washing the wells with PBS to remove detached cells and debris, we treated the remaining cells in serum-reduced medium containing TGF-β1 and SCU. We captured images of the wound area at 0 h (immediately after scratching), 12 h, and 24 h post-treatment using an inverted phase-contrast microscope. We quantified cell migration by measuring the remaining wound width or calculating the wound area at each time point relative to the initial wound area at 0 h.
4.10. In Vivo Tissue Distribution and Liver Targeting
We assessed the liver-targeting efficiency of SCE using a near-infrared fluorescent probe, DIR (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide), which is lipophilic and suitable for tracking nanoemulsion distribution via fluorescence imaging. We prepared DIR-loaded SCE similarly to NR/SCE by dissolving DIR in the oil phase before emulsification. As a control, we prepared free DIR by dissolving the dye in a Cremophor/ethanol mixture and subsequently diluting it with saline to mimic the free drug formulation.
Male ICR mice (6–8 weeks old, approximately 25 g) were obtained from Beijing Vital River Labs (Beijing, China) and acclimatized for one week under standard laboratory conditions. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the Institute of Medicinal Biotechnology, CAMS & PUMC (Approval No. IMB-20231109D102), and conducted per national ethical guidelines. The mice were fasted for 12 h before the experiment and randomly divided into two treatment groups (n = 3 per group per time point): free DIR and DIR-loaded SCE groups. Each mouse received a single oral gavage of DIR (0.5 mg/kg). At 1, 3, 6, 12, and 24 h post-administration, we anesthetized and euthanized three mice from each group and excised their major organs (heart, liver, spleen, lung, and kidneys). We immediately performed ex vivo fluorescence imaging using the IVIS system (PerkinElmer, Waltham, MA, USA) with DIR-appropriate filter settings. We drew regions of interest (ROI) over each organ to quantify fluorescence intensity (radiant efficiency), with particular focus on the liver to assess targeting efficiency. The mean fluorescence intensity of the liver at each time point was calculated and compared between the two groups to evaluate the extent and duration of hepatic accumulation under the appropriate excitation/emission filter settings for DIR.
4.11. Bile Duct Ligation-Induced Liver Fibrosis Model and Treatment Protocol
We employed a bile duct ligation (BDL) mouse model to induce liver fibrosis and evaluate the therapeutic effects of SCU formulations. Male ICR mice (6–8 weeks old) were randomly divided into four groups (n = 5–6 per group): sham (sham operation + vehicle treatment), BDL model (BDL + vehicle), free SCU (BDL + free SCU treatment), and SCE (BDL + SCU nanoemulsion treatment). The BDL procedure was performed under anesthesia (isoflurane, gas anesthesia) by double ligation and transection of the common bile duct using sterile technique. In the sham-operated group, we exposed the bile duct but left it intact. Following surgery, we administered buprenorphine for postoperative analgesia and monitored all animals closely until full recovery. Each experimental group included 5 animals (biological replicates). For each endpoint, the exact number of samples analyzed is indicated in the figure legends. All data are presented as mean ± SD, and statistical analyses were performed using the full dataset (n = 5), unless otherwise specified.
We began treatment on postoperative day 2. Free SCU was freshly prepared by dissolving scutellarin in saline containing a minimal amount of NaOH to enhance solubility. This solution was then administered at a dose of 10 mg/kg. We formulated SCE to deliver an equivalent SCU dose. The Sham and BDL control groups received an equivalent volume of normal saline. We administered all treatments once daily by oral gavage for 14 consecutive days, recorded body weights regularly, and noted any signs of distress.
At the end of the treatment period, we anesthetized the mice and collected blood samples via the orbital sinus. Serum was separated by centrifugation at 3500 rpm for 10 min at 4 °C and stored at −80 °C for subsequent biochemical analyses. We then euthanized the mice and harvested liver tissues. Portions of the liver were fixed in 10% neutral-buffered formalin for histological and immunofluorescence analysis, whereas other portions were snap-frozen in liquid nitrogen and stored at −80 °C for biochemical assays, including Western blotting and hydroxyproline quantification. Additionally, we aseptically collected 50 mg of fresh liver tissue and fecal pellets from the colon and immediately frozen for microbiome analysis via 16S rRNA gene sequencing.
We performed quantitative real-time PCR (qPCR) and Western blot analyses to assess the expression of fibrogenic genes and proteins in liver tissues, following the procedures described in
Section 4.8. Notably, the primer sequences used for liver tissue qPCR differed from those used for cellular analysis. The primer sequences were as follows: COL1A1: forward 5′-CATGTTCAGCTTTGTGGACCT-3′, reverse 5′-GCAGCTGACTTCAGGGATGT-3; ACTA2: 5′-TTCCTTCGTGACTACTGCCG-3′, reverse 5′-TATAGGTGGTTTCGTGGATGCC-3′; ACTB: forward 5′-CGTTCAATACCCCAGCCATG-3′, reverse 5′-GACCCCGTCACCAGAGTCC-3′.
4.12. Serum Biochemistry Analysis
To evaluate liver injury and systemic responses to treatment, we quantified serum levels of key liver function markers. We measured alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) activities using an automated biochemical analyzer or colorimetric assay kits (Nanjing Jiancheng Bioengineering, Nanjing, China) following the manufacturer’s protocols. We also assessed serum total bile acids (TBA) as an indicator of BDL-induced cholestasis. We performed all assays in duplicate, and results were reported as mean ± SD for each group.
4.13. Histological Analysis
For histopathological examination, liver and other major organs—including the heart, spleen, lungs, and kidneys—were fixed in formalin, embedded in paraffin, and sectioned at a thickness of 4 µm. We stained liver sections with hematoxylin and eosin (H&E) to evaluate general liver architecture and injury. To visualize collagen deposition and fibrosis, we performed Masson’s trichrome and Sirius Red staining. Masson’s stain renders collagen fibers blue, whereas Sirius Red binds specifically to collagen, appearing red under light microscopy and exhibiting birefringence under polarized light.
To evaluate potential off-target toxicity, we stained heart, lung, spleen, and kidney sections from mice treated with saline, free SCU, or SCE (without BDL surgery) with H&E. A pathologist blinded to the treatment groups examined all tissues for signs of inflammation, necrosis, or other histological abnormalities.
4.14. Hydroxyproline Assay
We quantified hepatic hydroxyproline content as a surrogate marker for collagen accumulation and liver fibrosis. For each mouse, we assayed ~50 mg of liver tissue using a commercial hydroxyproline assay kit (Nanjing Jiancheng Bioengineering Institute, China), following the manufacturer’s instructions. Briefly, liver samples were hydrolyzed in concentrated hydrochloric acid at 110 °C for several hours to release free hydroxyproline from collagen. After hydrolysis, the samples were neutralized and treated with, followed by reaction with Ehrlich’s reagent (p-dimethylaminobenzaldehyde) to generate a chromogenic complex. The absorbance of the resulting solution was then measured at 550 nm and compared to a standard hydroxyproline calibration curve. Hydroxyproline content was expressed as micrograms per gram of liver tissue. All measurements were performed in duplicate.
4.16. Immunofluorescence Staining of Liver Sections
Immunofluorescence was performed to visualize key fibrosis markers (collagen I and α-SMA) in liver tissue sections. Paraffin-embedded liver sections (4 µm) were deparaffinized, rehydrated, and subjected to antigen retrieval (heating in citrate buffer, pH 6.0). After blocking with 5% bovine serum albumin and 0.3% Triton X-100 in PBS for 1 h, sections were incubated overnight at 4 °C with primary antibodies against collagen I (rabbit polyclonal, 1:200 dilution) and α-SMA (mouse monoclonal, 1:200). The next day, sections were washed and incubated for 1 h at room temperature in the dark with species-specific secondary antibodies (Alexa Fluor 594–conjugated goat anti-rabbit for collagen I, Alexa Fluor 488–conjugated goat anti-mouse for α-SMA). Nuclei were counterstained with DAPI, and Slides were mounted with antifade medium before fluorescence microscopy.
4.17. Statistical Analysis
For normally distributed data, results are expressed as mean ± SD and analyzed using one-way ANOVA with Tukey’s post hoc test. For data not following a normal distribution (e.g., microbiota abundances), values are presented as median with interquartile range (IQR), and non-parametric tests were applied as appropriate. p < 0.05 (corrected with q value if necessary) was considered statistically significant.