Neurofilament Biomarkers in Neurology: From Neuroinflammation to Neurodegeneration, Bridging Established and Novel Analytical Advances with Clinical Practice
Abstract
1. Introduction
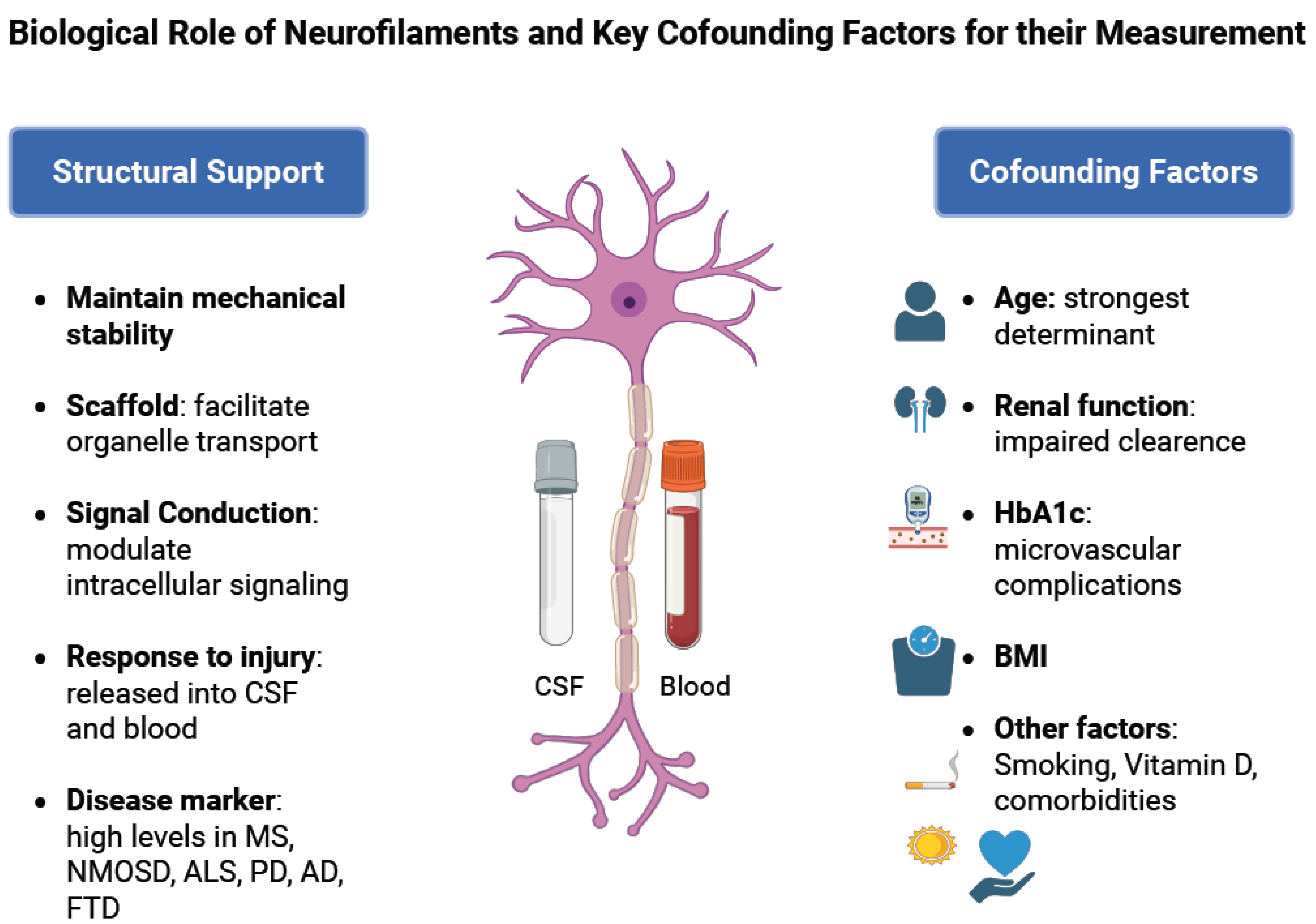
1.1. Structure of Neurofilaments and Their Role in Healthy Neurons
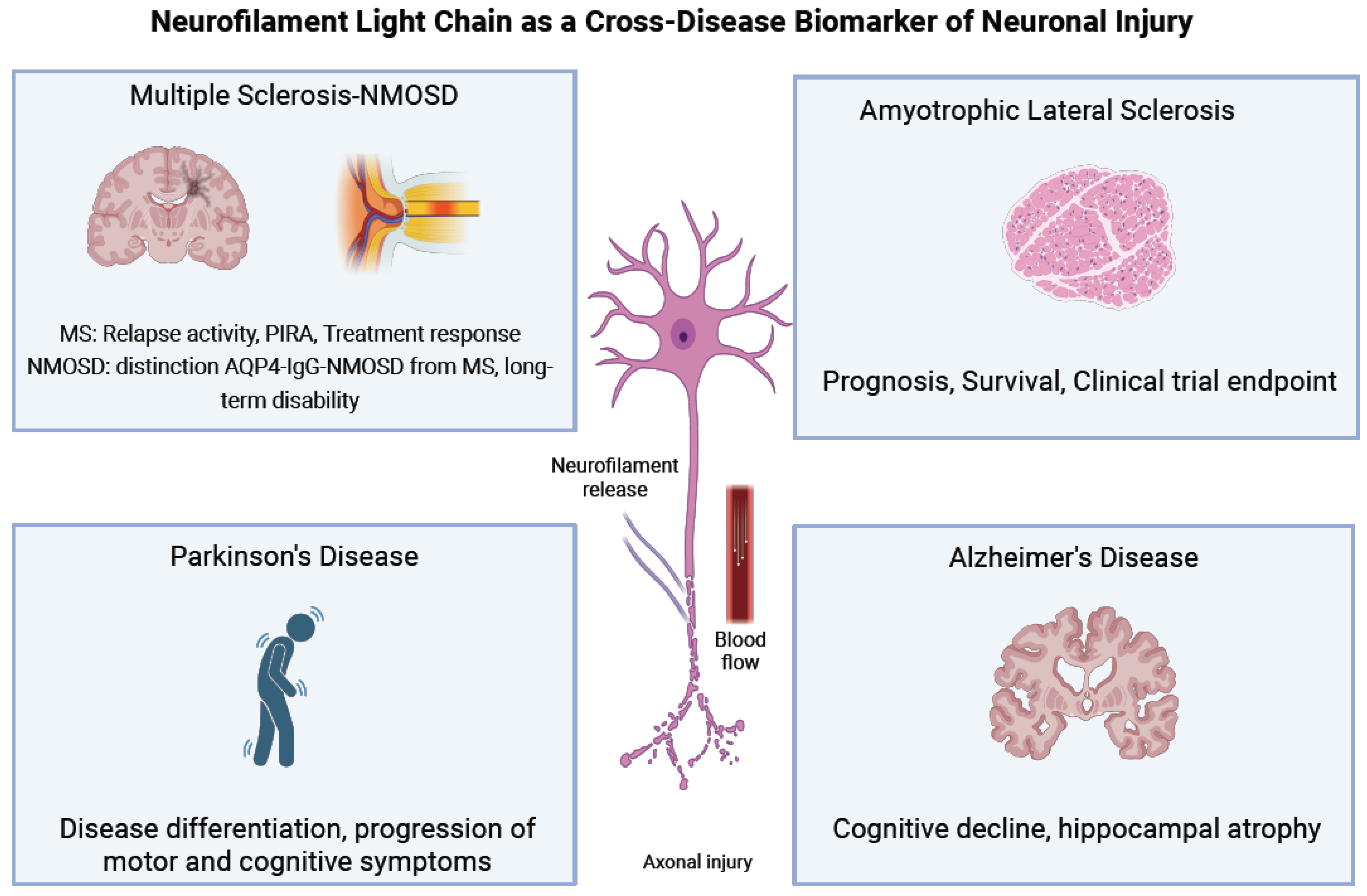
1.2. Role of Neurofilaments in Diseases with Axonal Injury
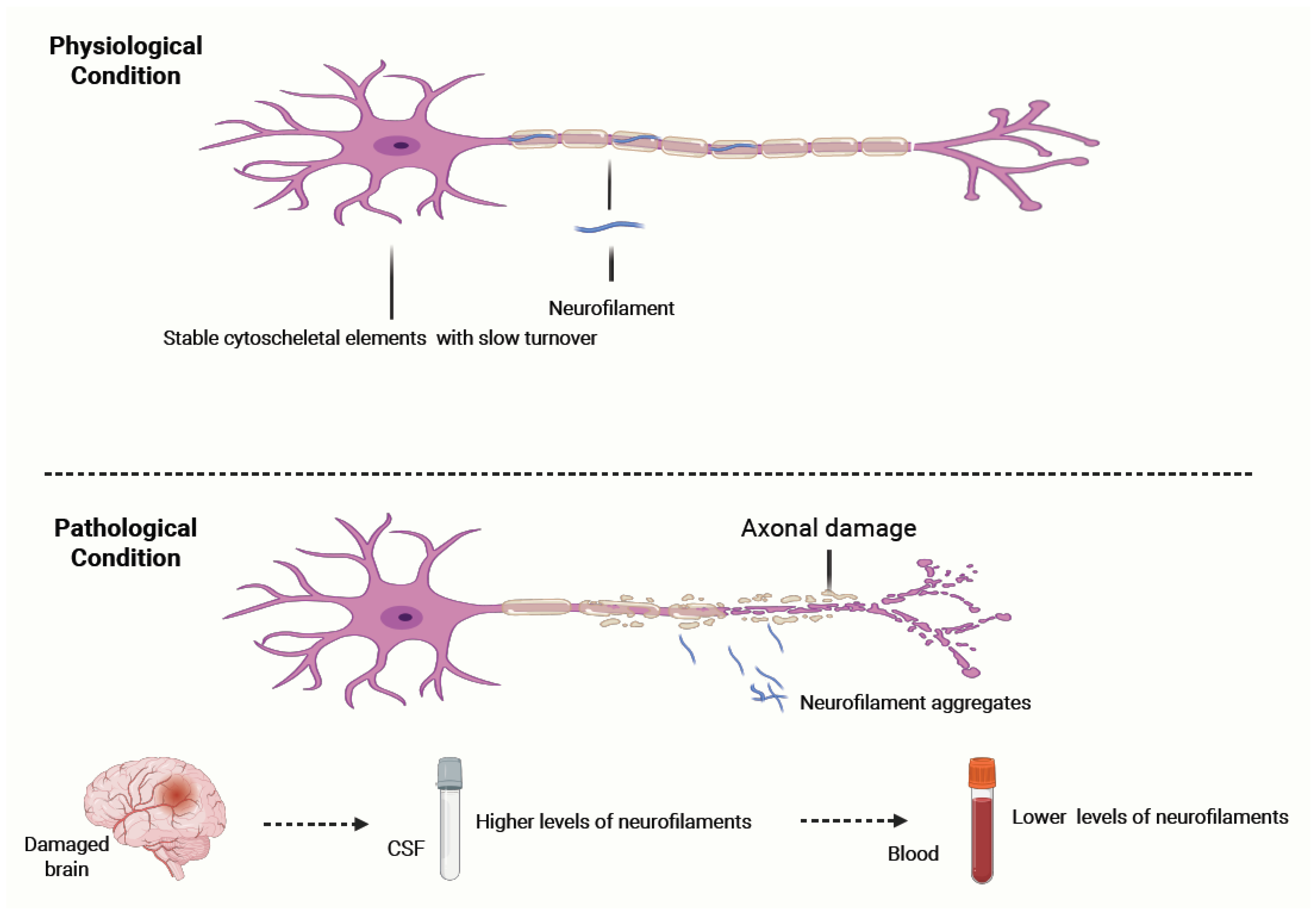
- Under physiological conditions, neurofilaments (NFs) are stable cytoskeletal components with slow turnover, maintaining axonal structure and integrity.
- In response to axonal injury caused by inflammatory, traumatic, ischemic, or degenerative mechanisms, NFs aggregate and are released into the cerebrospinal fluid (CSF) and subsequently into the blood. Their levels correlate with the extent of axonal damage and can remain elevated for weeks to months before clearance. NF concentrations are significantly higher in CSF than in blood, with a typical gradient of ~1:40. Clinically, NF measurement serves as a sensitive, though non-specific, biomarker of neuroaxonal injury and can help differentiate conditions with higher versus lower rates of neuronal degeneration.
1.3. Confounding Factors Influencing Nf Levels
2. Methods of NfL Measurement
2.1. Biological Sources of NfL
2.2. Analytical Platforms for NfL Quantification
2.3. Pre-Analytical Considerations
- Sample handling: Studies have shown that appropriate storage, typically at −80 °C, is essential to maintain protein stability, particularly by avoiding repeated freeze–thaw cycles. The type of collection tube does not significantly influence NfL levels [32].
- Physiological confounders: NfL levels increase with age and are influenced by renal function, metabolic status (e.g., HbA1c), and other comorbidities. Age-adjusted reference values and awareness of comorbidities are essential when interpreting NfL results in both healthy individuals and patients with neurological disease [19]. Even though most laboratories only use age-adjusted reference values, prediction models have been developed and published so that they can be used for patients with such confounders [19,33].
- Comparison of different assays: Different assays, like SIMOA and chemiluminescent immunoassays, correlate well with each other. However, it should be taken into account that the absolute NfL levels are not identical, and are thus not comparable [34]. Specialized conversion models have been developed and can be utilized for comparing NfL levels by different assays, for example, in patients with longitudinal NfL measurements [19,34].
3. Neurofilaments in Multiple Sclerosis
3.1. Introduction to Multiple Sclerosis
3.2. Clinical Utility of NfL in Multiple Sclerosis
3.2.1. The Use of sNfL in MS Diagnosis
3.2.2. The Use of NFL in Disease Monitoring
3.2.3. NFL as a Biomarker of Treatment Response
3.2.4. NfL as Biomarker of Subclinical Disease Activity
3.3. Prognostic and Therapeutic Implications in MS Subtypes
3.3.1. Secondary Progressive Multiple Sclerosis (SPMS)
3.3.2. Primary Progressive Multiple Sclerosis (PPMS)
3.3.3. Pediatric-Onset MS (POMS)
3.4. Integration with Other Biomarkers
3.5. Biomarkers for Neuromyelitis Optica Spectrum Disorders (NMOSD) and Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD)
4. Neurofilaments as Biomarkers in Amyotrophic Lateral Sclerosis
4.1. Introduction to Amyotrophic Lateral Sclerosis
4.2. Biomarkers in ALS
4.3. Neurofilaments as Diagnostic Biomarkers
4.4. Neurofilaments as Prognostic Biomarkers
4.5. Neurofilaments in Clinical Trials
4.5.1. NFs in Clinical Trials as Predictive Biomarkers
4.5.2. NFs in Clinical Trials as Susceptibility and Risk Biomarkers
4.5.3. NFs in Clinical Trials as Pharmacodynamic Biomarkers
4.6. Combination of NFs with Other Biomarkers
5. Neurofilaments in Parkinson’s Disease
5.1. Parkinson’s Disease
5.2. Nf Dysfunction in PD
5.3. NFs as a Biomarker in Parkinson’s Disease
5.4. Potential Applications of NFs Early in Pd Diagnosis
5.5. Combination of NFs with Other Biomarkers in Pd
6. Neurofilaments in Alzheimer’s Disease
6.1. Alzheimer’s Disease
6.2. NFs as Biomarker in Alzheimer’s Disease
7. Neurofilaments in Frontotemporal Dementia and Atypical Parkinsonism
8. Limitations in the Use of Neurofilaments and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zucchi, E.; Bonetto, V.; Sorarù, G.; Martinelli, I.; Parchi, P.; Liguori, R.; Mandrioli, J. Neurofilaments in Motor Neuron Disorders: Towards Promising Diagnostic and Prognostic Biomarkers. Mol. Neurodegener. 2020, 15, 58. [Google Scholar] [CrossRef]
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef]
- Verde, F.; Otto, M.; Silani, V. Neurofilament Light Chain as Biomarker for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front. Neurosci. 2021, 15, 679199. [Google Scholar] [CrossRef]
- Gafson, A.R.; Barthélemy, N.R.; Bomont, P.; Carare, R.O.; Durham, H.D.; Julien, J.-P.; Kuhle, J.; Leppert, D.; Nixon, R.A.; Weller, R.O.; et al. Neurofilaments: Neurobiological Foundations for Biomarker Applications. Brain 2020, 143, 1975–1998. [Google Scholar] [CrossRef]
- van Asperen, J.V.; Kotaich, F.; Caillol, D.; Bomont, P. Neurofilaments: Novel Findings and Future Challenges. Curr. Opin. Cell Biol. 2024, 87, 102326. [Google Scholar] [CrossRef]
- Barry, D.M.; Stevenson, W.; Bober, B.G.; Wiese, P.J.; Dale, J.M.; Barry, G.S.; Byers, N.S.; Strope, J.D.; Chang, R.; Schulz, D.J.; et al. Expansion of Neurofilament Medium C Terminus Increases Axonal Diameter Independent of Increases in Conduction Velocity or Myelin Thickness. J. Neurosci. 2012, 32, 6209–6219. [Google Scholar] [CrossRef]
- Menke, R.A.L.; Gray, E.; Lu, C.; Kuhle, J.; Talbot, K.; Malaspina, A.; Turner, M.R. CSF Neurofilament Light Chain Reflects Corticospinal Tract Degeneration in ALS. Ann. Clin. Transl. Neurol. 2015, 2, 748–755. [Google Scholar] [CrossRef]
- Shepherd, C.E.; McCann, H.; Thiel, E.; Halliday, G.M. Neurofilament-Immunoreactive Neurons in Alzheimer’s Disease and Dementia with Lewy Bodies. Neurobiol. Dis. 2002, 9, 249–257. [Google Scholar] [CrossRef]
- Shi, J.; Qin, X.; Chang, X.; Wang, H.; Guo, J.; Zhang, W. Neurofilament Markers in Serum and Cerebrospinal Fluid of Patients with Amyotrophic Lateral Sclerosis. J. Cell. Mol. Med. 2022, 26, 583–587. [Google Scholar] [CrossRef]
- Zetterberg, H.; Jacobsson, J.; Rosengren, L.; Blennow, K.; Andersen, P.M. Cerebrospinal Fluid Neurofilament Light Levels in Amyotrophic Lateral Sclerosis: Impact of SOD1 Genotype. Eur. J. Neurol. 2007, 14, 1329–1333. [Google Scholar] [CrossRef]
- Alshehri, R.S.; Abuzinadah, A.R.; Alrawaili, M.S.; Alotaibi, M.K.; Alsufyani, H.A.; Alshanketi, R.M.; AlShareef, A.A. A Review of Biomarkers of Amyotrophic Lateral Sclerosis: A Pathophysiologic Approach. Int. J. Mol. Sci. 2024, 25, 10900. [Google Scholar] [CrossRef]
- Zou, K.; Abdullah, M.; Michikawa, M. Current Biomarkers for Alzheimer’s Disease: From CSF to Blood. J. Pers. Med. 2020, 10, 85. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Miller, C.C.J. Neurofilaments and Neurological Disease. BioEssays 2003, 25, 346–355. [Google Scholar] [CrossRef]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Di Filippo, M.; Parnetti, L.; Zetterberg, H. Neurofilament Light Chain as a Biomarker in Neurological Disorders. J. Neurol. Neurosurg. Psychiatry 2019, 90, 870–881. [Google Scholar] [CrossRef]
- Koros, C.; Kitraki, E. Neurofilament Isoform Alterations in the Rat Cerebellum Following Cytosine Arabinoside Administration. Toxicol. Lett. 2009, 189, 215–218. [Google Scholar] [CrossRef]
- Eriksson, J.E.; Dechat, T.; Grin, B.; Helfand, B.; Mendez, M.; Pallari, H.-M.; Goldman, R.D. Introducing Intermediate Filaments: From Discovery to Disease. J. Clin. Investig. 2009, 119, 1763–1771. [Google Scholar] [CrossRef]
- Omary, M.B. “IF-Pathies”: A Broad Spectrum of Intermediate Filament–Associated Diseases. J. Clin. Investig. 2009, 119, 1756–1762. [Google Scholar] [CrossRef]
- Mousele, C.; Holden, D.; Gnanapavan, S. Neurofilaments in Neurologic Disease. Adv. Clin. Chem. 2024, 123, 65–128. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Sotirchos, E.S.; Smith, M.D.; Lord, H.; DuVal, A.; Mowry, E.M.; Calabresi, P.A. Contributors to Serum NfL Levels in People without Neurologic Disease. Ann. Neurol. 2022, 92, 688–698. [Google Scholar] [CrossRef]
- Yilmaz, A.; Blennow, K.; Hagberg, L.; Nilsson, S.; Price, R.W.; Schouten, J.; Spudich, S.; Underwood, J.; Zetterberg, H.; Gisslén, M. Neurofilament Light Chain Protein as a Marker of Neuronal Injury: Review of Its Use in HIV-1 Infection and Reference Values for HIV-Negative Controls. Expert Rev. Mol. Diagn. 2017, 17, 761–770. [Google Scholar] [CrossRef]
- Itorralba, J.; Schneider, R. The Prognostic Utility of Neurofilament Light Chain in Multiple Sclerosis: A Narrative Review. Pract. Neurol. 2024, 23, 18–21. [Google Scholar]
- Freedman, M.S.; Abdelhak, A.; Bhutani, M.K.; Freeman, J.; Gnanapavan, S.; Hussain, S.; Madiraju, S.; Paul, F. The Role of Serum Neurofilament Light (sNfL) as a Biomarker in Multiple Sclerosis: Insights from a Systematic Review. J. Neurol. 2025, 272, 400. [Google Scholar] [CrossRef]
- Freedman, M.S.; Gnanapavan, S.; Booth, R.A.; Calabresi, P.A.; Khalil, M.; Kuhle, J.; Lycke, J.; Olsson, T. Guidance for Use of Neurofilament Light Chain as a Cerebrospinal Fluid and Blood Biomarker in Multiple Sclerosis Management. eBioMedicine 2024, 101, 104970. [Google Scholar] [CrossRef]
- Varhaug, K.N.; Torkildsen, Ø.; Myhr, K.-M.; Vedeler, C.A. Neurofilament Light Chain as a Biomarker in Multiple Sclerosis. Front. Neurol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Eva, L.; Pleș, H.; Covache-Busuioc, R.-A.; Glavan, L.A.; Bratu, B.-G.; Bordeianu, A.; Dumitrascu, D.-I.; Corlatescu, A.D.; Ciurea, A.V. A Comprehensive Review on Neuroimmunology: Insights from Multiple Sclerosis to Future Therapeutic Developments. Biomedicines 2023, 11, 2489. [Google Scholar] [CrossRef]
- Shaw, G. The use and potential of pNF-H as a general blood biomarker of axonal loss: An immediate application for CNS injury. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015; ISBN 978-1-4665-6598-2. [Google Scholar]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as Biomarkers in Neurological Disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Katsavos, S.; Anagnostouli, M. Biomarkers in Multiple Sclerosis: An Up-to-Date Overview. Mult. Scler. Int. 2013, 2013, 340508. [Google Scholar] [CrossRef]
- Gisslén, M.; Price, R.W.; Andreasson, U.; Norgren, N.; Nilsson, S.; Hagberg, L.; Fuchs, D.; Spudich, S.; Blennow, K.; Zetterberg, H. Plasma Concentration of the Neurofilament Light Protein (NFL) Is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine 2016, 3, 135–140, Erratum in EBioMedicine 2016, 7, 287–288. [Google Scholar] [CrossRef]
- Ashrafzadeh-Kian, S.; Figdore, D.; Larson, B.; Deters, R.; Abou-Diwan, C.; Bornhorst, J.; Algeciras-Schimnich, A. Head-to-Head Comparison of Four Plasma Neurofilament Light Chain (NfL) Immunoassays. Clin. Chim. Acta Int. J. Clin. Chem. 2024, 561, 119817. [Google Scholar] [CrossRef]
- Budelier, M.M.; He, Y.; Barthelemy, N.R.; Jiang, H.; Li, Y.; Park, E.; Henson, R.L.; Schindler, S.E.; Holtzman, D.M.; Bateman, R.J. A Map of Neurofilament Light Chain Species in Brain and Cerebrospinal Fluid and Alterations in Alzheimer’s Disease. Brain Commun. 2022, 4, fcac045. [Google Scholar] [CrossRef]
- Sotirchos, E.S.; Fitzgerald, K.C.; Smith, M.D.; Vasileiou, E.S.; Resto, Y.; Lord, H.-N.; Mowry, E.M.; Calabresi, P.A. Type of Serum Collection Tube Does Not Impact Neurofilament Light Chain Levels. Mult. Scler. Relat. Disord. 2022, 59, 103676. [Google Scholar] [CrossRef]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, Ö.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; et al. Serum Neurofilament Light Chain for Individual Prognostication of Disease Activity in People with Multiple Sclerosis: A Retrospective Modelling and Validation Study. Lancet Neurol. 2022, 21, 246–257. [Google Scholar] [CrossRef]
- Sotirchos, E.; Fitzgerald, K.; Smith, M.; Williams, J.; De Moor, C.; Szak, S.; Singh, C.; Fisher, E.; Bermel, R.; Hersh, C.; et al. Associations of Serum Neurofilament Light Chain with Clinico-Radiological Characteristics in the MSPATHS Network: A Cross-Sectional Evaluation (1722). Neurology 2021, 96, 1722. [Google Scholar] [CrossRef]
- Kantarci, O.H. Genetics and Natural History of Multiple Sclerosis. Semin. Neurol. 2008, 28, 7–16. [Google Scholar] [CrossRef]
- Skarlis, C.; Anagnostouli, M. The Role of Melatonin in Multiple Sclerosis. Neurol. Sci. 2020, 41, 769–781. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Skarlis, C.; Angelopoulou, E.; Rentzos, M.; Papageorgiou, S.G.; Anagnostouli, M. Monoclonal Antibodies as Therapeutic Agents in Autoimmune and Neurodegenerative Diseases of the Central Nervous System: Current Evidence on Molecular Mechanisms and Future Directions. Int. J. Mol. Sci. 2025, 26, 9398. [Google Scholar] [CrossRef]
- Yong, H.Y.F.; Camara-Lemarroy, C. Progression Independent of Relapsing Biology in Multiple Sclerosis: A Real-Word Study. Front. Neurol. 2025, 16, 1595929. [Google Scholar] [CrossRef]
- Damasceno, A.; Dias-Carneiro, R.P.C.; Moraes, A.S.; Boldrini, V.O.; Quintiliano, R.P.S.; Da Silva, V.A.d.P.G.; Farias, A.S.; Brandão, C.O.; Damasceno, B.P.; Dos Santos, L.M.B.; et al. Clinical and MRI Correlates of CSF Neurofilament Light Chain Levels in Relapsing and Progressive MS. Mult. Scler. Relat. Disord. 2019, 30, 149–153. [Google Scholar] [CrossRef]
- Novakova, L.; Zetterberg, H.; Sundström, P.; Axelsson, M.; Khademi, M.; Gunnarsson, M.; Malmeström, C.; Svenningsson, A.; Olsson, T.; Piehl, F.; et al. Monitoring Disease Activity in Multiple Sclerosis Using Serum Neurofilament Light Protein. Neurology 2017, 89, 2230–2237. [Google Scholar] [CrossRef]
- Barro, C.; Benkert, P.; Disanto, G.; Tsagkas, C.; Amann, M.; Naegelin, Y.; Leppert, D.; Gobbi, C.; Granziera, C.; Yaldizli, Ö.; et al. Serum Neurofilament as a Predictor of Disease Worsening and Brain and Spinal Cord Atrophy in Multiple Sclerosis. Brain J. Neurol. 2018, 141, 2382–2391. [Google Scholar] [CrossRef]
- Wong, Y.Y.M.; Bruijstens, A.L.; Barro, C.; Michalak, Z.; Melief, M.-J.; Wierenga, A.F.; van Pelt, E.D.; Neuteboom, R.F.; Kuhle, J.; Hintzen, R.Q. Serum Neurofilament Light Chain in Pediatric MS and Other Acquired Demyelinating Syndromes. Neurology 2019, 93, e968–e974. [Google Scholar] [CrossRef]
- Wattjes, M.P.; Ciccarelli, O.; Reich, D.S.; Banwell, B.; de Stefano, N.; Enzinger, C.; Fazekas, F.; Filippi, M.; Frederiksen, J.; Gasperini, C.; et al. 2021 MAGNIMS-CMSC-NAIMS Consensus Recommendations on the Use of MRI in Patients with Multiple Sclerosis. Lancet Neurol. 2021, 20, 653–670. [Google Scholar] [CrossRef]
- Delaby, C.; Bousiges, O.; Bouvier, D.; Fillée, C.; Fourier, A.; Mondésert, E.; Nezry, N.; Omar, S.; Quadrio, I.; Rucheton, B.; et al. Neurofilaments Contribution in Clinic: State of the Art. Front. Aging Neurosci. 2022, 14, 1034684. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Steffen, F.; Uphaus, T.; Muthuraman, M.; Fleischer, V.; Salmen, A.; Luessi, F.; Berthele, A.; Klotz, L.; Meuth, S.G.; et al. Clinical Implications of Serum Neurofilament in Newly Diagnosed MS Patients: A Longitudinal Multicentre Cohort Study. EBioMedicine 2020, 56, 102807, Correction in EBioMedicine 2021, 66, 103295. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Munger, K.L.; Cortese, M.; Barro, C.; Healy, B.C.; Niebuhr, D.W.; Scher, A.I.; Kuhle, J.; Ascherio, A. Serum Neurofilament Light Chain Levels in Patients with Presymptomatic Multiple Sclerosis. JAMA Neurol. 2020, 77, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Matute-Blanch, C.; Villar, L.M.; Álvarez-Cermeño, J.C.; Rejdak, K.; Evdoshenko, E.; Makshakov, G.; Nazarov, V.; Lapin, S.; Midaglia, L.; Vidal-Jordana, A.; et al. Neurofilament Light Chain and Oligoclonal Bands Are Prognostic Biomarkers in Radiologically Isolated Syndrome. Brain J. Neurol. 2018, 141, 1085–1093. [Google Scholar] [CrossRef]
- van der Vuurst de Vries, R.M.; Wong, Y.Y.M.; Mescheriakova, J.Y.; van Pelt, E.D.; Runia, T.F.; Jafari, N.; Siepman, T.A.; Melief, M.-J.; Wierenga-Wolf, A.F.; van Luijn, M.M.; et al. High Neurofilament Levels Are Associated with Clinically Definite Multiple Sclerosis in Children and Adults with Clinically Isolated Syndrome. Mult. Scler. Houndmills Basingstoke Engl. 2019, 25, 958–967. [Google Scholar] [CrossRef]
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schädelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum Neurofilament Light: A Biomarker of Neuronal Damage in Multiple Sclerosis. Ann. Neurol. 2017, 81, 857–870. [Google Scholar] [CrossRef]
- Ning, L.; Wang, B. Neurofilament Light Chain in Blood as a Diagnostic and Predictive Biomarker for Multiple Sclerosis: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0274565. [Google Scholar] [CrossRef]
- Manouchehrinia, A.; Piehl, F.; Hillert, J.; Kuhle, J.; Alfredsson, L.; Olsson, T.; Kockum, I. Confounding Effect of Blood Volume and Body Mass Index on Blood Neurofilament Light Chain Levels. Ann. Clin. Transl. Neurol. 2020, 7, 139–143. [Google Scholar] [CrossRef]
- Ramo-Tello, C.; Blanco, Y.; Brieva, L.; Casanova, B.; Martínez-Cáceres, E.; Ontaneda, D.; Ramió-Torrentá, L.; Rovira, À. Recommendations for the Diagnosis and Treatment of Multiple Sclerosis Relapses. J. Pers. Med. 2021, 12, 6. [Google Scholar] [CrossRef]
- Aktas, O.; Renner, A.; Huss, A.; Filser, M.; Baetge, S.; Stute, N.; Gasis, M.; Lepka, K.; Goebels, N.; Senel, M.; et al. Serum Neurofilament Light Chain: No Clear Relation to Cognition and Neuropsychiatric Symptoms in Stable MS. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e885. [Google Scholar] [CrossRef]
- Barro, C.; Healy, B.C.; Saxena, S.; Glanz, B.I.; Paul, A.; Polgar-Turcsanyi, M.; Guttmann, C.R.; Bakshi, R.; Weiner, H.L.; Chitnis, T. Serum NfL but Not GFAP Predicts Cognitive Decline in Active Progressive Multiple Sclerosis Patients. Mult. Scler. J. 2023, 29, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Tur, C.; Eshaghi, A.; Doshi, A.; Chan, D.; Binks, S.; Wellington, H.; Heslegrave, A.; Zetterberg, H.; Chataway, J. Serum Neurofilament Light and MRI Predictors of Cognitive Decline in Patients with Secondary Progressive Multiple Sclerosis: Analysis from the MS-STAT Randomised Controlled Trial. Mult. Scler. J. 2022, 28, 1913–1926. [Google Scholar] [CrossRef]
- Sejbaek, T.; Nielsen, H.H.; Penner, N.; Plavina, T.; Mendoza, J.P.; Martin, N.A.; Elkjaer, M.L.; Ravnborg, M.H.; Illes, Z. Dimethyl Fumarate Decreases Neurofilament Light Chain in CSF and Blood of Treatment Naïve Relapsing MS Patients. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Bar-Or, A.; Cohen, J.A.; Comi, G.; Correale, J.; Coyle, P.K.; Cross, A.H.; de Seze, J.; Leppert, D.; Montalban, X.; et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N. Engl. J. Med. 2020, 383, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Preziosa, P.; Rocca, M.A.; Filippi, M. Current State-of-Art of the Application of Serum Neurofilaments in Multiple Sclerosis Diagnosis and Monitoring. Expert Rev. Neurother. 2020, 20, 747–769. [Google Scholar] [CrossRef]
- Delcoigne, B.; Manouchehrinia, A.; Barro, C.; Benkert, P.; Michalak, Z.; Kappos, L.; Leppert, D.; Tsai, J.A.; Plavina, T.; Kieseier, B.C.; et al. Blood Neurofilament Light Levels Segregate Treatment Effects in Multiple Sclerosis. Neurology 2020, 94, e1201–e1212. [Google Scholar] [CrossRef]
- Barrero Hernández, F.J.; Romero Villarrubia, A.; Muñoz Fernández, C.; Guillén Martinez, V.; Aguilera Del Moral, A.; Barrios-López, J.M.; Ramírez Rivas, M.A.; Gálvez Muñoz, A.J.; Piñar Morales, R. Real-World Study of Serum Neurofilament Light Chain Levels in Ocrelizumab-Treated People with Relapsing Multiple Sclerosis. J. Pers. Med. 2024, 14, 692. [Google Scholar] [CrossRef]
- Gontika, M.; Skarlis, C.; Markoglou, N.; Tzanetakos, D.; Vakrakou, A.; Toulas, P.; Koutsis, G.; Evangelopoulos, M.-E.; Pons, R.; Dardiotis, E.; et al. Natalizumab Therapy in Patients with Pediatric-Onset Multiple Sclerosis in Greece: Clinical and Immunological Insights of Time-Long Administration and Future Directions—A Single-Center Retrospective Observational Study. Naunyn. Schmiedebergs Arch. Pharmacol. 2022, 395, 933–943. [Google Scholar] [CrossRef]
- Fyfe, I. Trials Take MS Treatment Forward. Nat. Rev. Neurol. 2019, 15, 620. [Google Scholar] [CrossRef]
- Sormani, M.P.; Haering, D.A.; Kropshofer, H.; Leppert, D.; Kundu, U.; Barro, C.; Kappos, L.; Tomic, D.; Kuhle, J. Blood Neurofilament Light as a Potential Endpoint in Phase 2 Studies in MS. Ann. Clin. Transl. Neurol. 2019, 6, 1081–1089. [Google Scholar] [CrossRef]
- Bar-Or, A.; Montalban, X.; Hu, X.; Kropshofer, H.; Kukkaro, P.; Coello, N.; Ludwig, I.; Willi, R.; Zalesak, M.; Ramanathan, K.; et al. Serum Neurofilament Light Trajectories and Their Relation to Subclinical Radiological Disease Activity in Relapsing Multiple Sclerosis Patients in the APLIOS Trial. Neurol. Ther. 2023, 12, 303–317. [Google Scholar] [CrossRef]
- Bridel, C.; van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; NFL Group; Alvarez-Cermeño, J.C.; Andreasson, U.; Axelsson, M.; Bäckström, D.C.; et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-Analysis. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef]
- Meier, S.; Willemse, E.A.J.; Schaedelin, S.; Oechtering, J.; Lorscheider, J.; Melie-Garcia, L.; Cagol, A.; Barakovic, M.; Galbusera, R.; Subramaniam, S.; et al. Serum Glial Fibrillary Acidic Protein Compared with Neurofilament Light Chain as a Biomarker for Disease Progression in Multiple Sclerosis. JAMA Neurol. 2023, 80, 287. [Google Scholar] [CrossRef]
- Gibiansky, E.; Petry, C.; Mercier, F.; Günther, A.; Herman, A.; Kappos, L.; Hauser, S.; Yamamoto, Y.; Wang, Q.; Model, F.; et al. Ocrelizumab in Relapsing and Primary Progressive Multiple Sclerosis: Pharmacokinetic and Pharmacodynamic Analyses of OPERA I, OPERA II and ORATORIO. Br. J. Clin. Pharmacol. 2021, 87, 2511–2520. [Google Scholar] [CrossRef]
- Saucier, L.; Healy, B.C.; Saxena, S.; Sanon, E.; Chitnis, T. Glial Fibrillary Acidic Protein and Neurofilament Light Chain as Biomarkers in Pediatric Multiple Sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2024, 10, 20552173241274567. [Google Scholar] [CrossRef] [PubMed]
- Kodosaki, E.; Watkins, W.J.; Loveless, S.; Kreft, K.L.; Richards, A.; Anderson, V.; Hurler, L.; Robertson, N.P.; Zelek, W.M.; Tallantyre, E.C. Combination Protein Biomarkers Predict Multiple Sclerosis Diagnosis and Outcomes. J. Neuroinflamm. 2024, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Zeydan, B.; Azevedo, C.J.; Makhani, N.; Cohen, M.; Tutuncu, M.; Thouvenot, E.; Siva, A.; Okuda, D.T.; Kantarci, O.H.; Lebrun-Frenay, C. Early Disease-Modifying Treatments for Presymptomatic Multiple Sclerosis. CNS Drugs 2024, 38, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Huss, A.; Otto, M.; Senel, M.; Ludolph, A.C.; Abdelhak, A.; Tumani, H. A Score Based on NfL and Glial Markers May Differentiate Between Relapsing-Remitting and Progressive MS Course. Front. Neurol. 2020, 11, 608. [Google Scholar] [CrossRef]
- Di Filippo, M.; Gaetani, L.; Centonze, D.; Hegen, H.; Kuhle, J.; Teunissen, C.E.; Tintoré, M.; Villar, L.M.; Willemse, E.A.J.; Zetterberg, H.; et al. Fluid Biomarkers in Multiple Sclerosis: From Current to Future Applications. Lancet Reg. Health Eur. 2024, 44, 101009. [Google Scholar] [CrossRef]
- Engel, S.; Friedrich, M.; Muthuraman, M.; Steffen, F.; Poplawski, A.; Groppa, S.; Bittner, S.; Zipp, F.; Luessi, F. Intrathecal B-Cell Accumulation and Axonal Damage Distinguish MRI-Based Benign from Aggressive Onset in MS. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e595. [Google Scholar] [CrossRef]
- Magliozzi, R.; Pitteri, M.; Ziccardi, S.; Pisani, A.I.; Montibeller, L.; Marastoni, D.; Rossi, S.; Mazziotti, V.; Guandalini, M.; Dapor, C.; et al. CSF Parvalbumin Levels Reflect Interneuron Loss Linked with Cortical Pathology in Multiple Sclerosis. Ann. Clin. Transl. Neurol. 2021, 8, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Rodin, R.E.; Chitnis, T. Soluble Biomarkers for Neuromyelitis Optica Spectrum Disorders: A Mini Review. Front. Neurol. 2024, 15, 1415535. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakamura, Y.; Michalak, Z.; Isobe, N.; Barro, C.; Leppert, D.; Matsushita, T.; Hayashi, F.; Yamasaki, R.; Kuhle, J.; et al. Serum GFAP and Neurofilament Light as Biomarkers of Disease Activity and Disability in NMOSD. Neurology 2019, 93, e1299–e1311. [Google Scholar] [CrossRef]
- Chang, X.; Huang, W.; Wang, L.; ZhangBao, J.; Zhou, L.; Lu, C.; Wang, M.; Yu, J.; Li, H.; Li, Y.; et al. Serum Neurofilament Light and GFAP Are Associated with Disease Severity in Inflammatory Disorders with Aquaporin-4 or Myelin Oligodendrocyte Glycoprotein Antibodies. Front. Immunol. 2021, 12, 647618. [Google Scholar] [CrossRef] [PubMed]
- Carta, S.; Dinoto, A.; Capobianco, M.; Valentino, P.; Montarolo, F.; Sala, A.; Reindl, M.; Lo Re, M.; Chiodega, V.; Branger, P.; et al. Serum Biomarker Profiles Discriminate AQP4 Seropositive and Double Seronegative Neuromyelitis Optica Spectrum Disorder. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200188. [Google Scholar] [CrossRef]
- Aktas, O.; Hartung, H.-P.; Smith, M.A.; Rees, W.A.; Fujihara, K.; Paul, F.; Marignier, R.; Bennett, J.L.; Kim, H.J.; Weinshenker, B.G.; et al. Serum Neurofilament Light Chain Levels at Attack Predict Post-Attack Disability Worsening and Are Mitigated by Inebilizumab: Analysis of Four Potential Biomarkers in Neuromyelitis Optica Spectrum Disorder. J. Neurol. Neurosurg. Psychiatry 2023, 94, 757–768. [Google Scholar] [CrossRef]
- Luo, W.; Chen, Y.; Mao, S.; Jin, J.; Liu, C.; Zhong, X.; Sun, X.; Kermode, A.G.; Qiu, W. Serum Neurofilament Light Chain in Adult and Pediatric Patients with Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease: Correlation with Relapses and Seizures. J. Neurochem. 2022, 160, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef]
- Bradford, D.; Rodgers, K.E. Advancements and Challenges in Amyotrophic Lateral Sclerosis. Front. Neurosci. 2024, 18, 1401706. [Google Scholar] [CrossRef] [PubMed]
- Devarakonda, S.S.; Basha, S.; Pithakumar, A.; Thoshna, L.B.; Mukunda, D.C.; Rodrigues, J.; Ameers, K.; Biswas, S.; Pai, A.R.; Belurkar, S.; et al. Molecular Mechanisms of Neurofilament Alterations and Its Application in Assessing Neurodegenerative Disorders. Ageing Res. Rev. 2024, 102, 102566. [Google Scholar] [CrossRef]
- Corcia, P.; Lunetta, C.; Vourc’h, P.; Pradat, P.-F.; Blasco, H. Time for Optimism in Amyotrophic Lateral Sclerosis. Eur. J. Neurol. 2023, 30, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. Blueprint of Collapse: Precision Biomarkers, Molecular Cascades, and the Engineered Decline of Fast-Progressing ALS. Int. J. Mol. Sci. 2025, 26, 8072. [Google Scholar] [CrossRef]
- Shahim, P.; Norato, G.; Sinaii, N.; Zetterberg, H.; Blennow, K.; Chan, L.; Grunseich, C. Neurofilaments in Sporadic and Familial Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Genes 2024, 15, 496. [Google Scholar] [CrossRef]
- Falzone, Y.M.; Domi, T.; Mandelli, A.; Pozzi, L.; Schito, P.; Russo, T.; Barbieri, A.; Fazio, R.; Volontè, M.A.; Magnani, G.; et al. Integrated Evaluation of a Panel of Neurochemical Biomarkers to Optimize Diagnosis and Prognosis in Amyotrophic Lateral Sclerosis. Eur. J. Neurol. 2022, 29, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.; Swash, M. Diagnosis and differential diagnosis of MND/ALS: IFCN handbook chapter. Clin. Neurophysiol. Pract. 2024, 9, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Verde, F.; Steinacker, P.; Weishaupt, J.H.; Kassubek, J.; Oeckl, P.; Halbgebauer, S.; Tumani, H.; von Arnim, C.A.F.; Dorst, J.; Feneberg, E.; et al. Neurofilament Light Chain in Serum for the Diagnosis of Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 157–164. [Google Scholar] [CrossRef]
- Feneberg, E.; Oeckl, P.; Steinacker, P.; Verde, F.; Barro, C.; Van Damme, P.; Gray, E.; Grosskreutz, J.; Jardel, C.; Kuhle, J.; et al. Multicenter Evaluation of Neurofilaments in Early Symptom Onset Amyotrophic Lateral Sclerosis. Neurology 2018, 90, e22–e30. [Google Scholar] [CrossRef]
- Behzadi, A.; Pujol-Calderón, F.; Tjust, A.E.; Wuolikainen, A.; Höglund, K.; Forsberg, K.; Portelius, E.; Blennow, K.; Zetterberg, H.; Andersen, P.M. Neurofilaments Can Differentiate ALS Subgroups and ALS from Common Diagnostic Mimics. Sci. Rep. 2021, 11, 22128. [Google Scholar] [CrossRef]
- Davies, J.C.; Dharmadasa, T.; Thompson, A.G.; Edmond, E.C.; Yoganathan, K.; Gao, J.; Talbot, K.; Turner, M.R. Limited Value of Serum Neurofilament Light Chain in Diagnosing Amyotrophic Lateral Sclerosis. Brain Commun. 2023, 5, fcad163. [Google Scholar] [CrossRef]
- García-Casanova, P.H.; Vázquez-Costa, J.F. Advances in the Early Diagnosis of Amyotrophic Lateral Sclerosis. Expert Rev. Neurother. 2025, 25, 415–425. [Google Scholar] [CrossRef]
- Wohnrade, C.; Seeliger, T.; Gingele, S.; Bjelica, B.; Skripuletz, T.; Petri, S. Diagnostic Value of Neurofilaments in Differentiating Motor Neuron Disease from Multifocal Motor Neuropathy. J. Neurol. 2024, 271, 4441–4452. [Google Scholar] [CrossRef] [PubMed]
- De Schaepdryver, M.; Jeromin, A.; Gille, B.; Claeys, K.G.; Herbst, V.; Brix, B.; Van Damme, P.; Poesen, K. Comparison of Elevated Phosphorylated Neurofilament Heavy Chains in Serum and Cerebrospinal Fluid of Patients with Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, P.; Feneberg, E.; Weishaupt, J.; Brettschneider, J.; Tumani, H.; Andersen, P.M.; von Arnim, C.A.F.; Böhm, S.; Kassubek, J.; Kubisch, C.; et al. Neurofilaments in the Diagnosis of Motoneuron Diseases: A Prospective Study on 455 Patients. J. Neurol. Neurosurg. Psychiatry 2016, 87, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, E.; Bedin, R.; Fasano, A.; Fini, N.; Gessani, A.; Vinceti, M.; Mandrioli, J. Cerebrospinal Fluid Neurofilaments May Discriminate Upper Motor Neuron Syndromes: A Pilot Study. Neurodegener. Dis. 2018, 18, 255–261. [Google Scholar] [CrossRef]
- Lombardi, V.; Querin, G.; Ziff, O.J.; Zampedri, L.; Martinelli, I.; Heller, C.; Foiani, M.; Bertolin, C.; Lu, C.-H.; Malik, B.; et al. Muscle and Not Neuronal Biomarkers Correlate with Severity in Spinal and Bulbar Muscular Atrophy. Neurology 2019, 92, e1205–e1211, Erratum in Neurology 2020, 94, 852. [Google Scholar] [CrossRef]
- Benatar, M.; Wuu, J.; Andersen, P.M.; Lombardi, V.; Malaspina, A. Neurofilament Light: A Candidate Biomarker of Presymptomatic Amyotrophic Lateral Sclerosis and Phenoconversion. Ann. Neurol. 2018, 84, 130–139. [Google Scholar] [CrossRef]
- Benatar, M.; Wuu, J. Presymptomatic Studies in ALS: Rationale, Challenges, and Approach. Neurology 2012, 79, 1732–1739. [Google Scholar] [CrossRef]
- Benatar, M.; Wuu, J.; Lombardi, V.; Jeromin, A.; Bowser, R.; Andersen, P.M.; Malaspina, A. Neurofilaments in Pre-Symptomatic ALS and the Impact of Genotype. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 538–548. [Google Scholar] [CrossRef]
- Lu, C.-H.; Macdonald-Wallis, C.; Gray, E.; Pearce, N.; Petzold, A.; Norgren, N.; Giovannoni, G.; Fratta, P.; Sidle, K.; Fish, M.; et al. Neurofilament Light Chain: A Prognostic Biomarker in Amyotrophic Lateral Sclerosis. Neurology 2015, 84, 2247–2257, Erratum in Neurology 2015, 85, 921. [Google Scholar] [CrossRef]
- Sanchez-Tejerina, D.; Llaurado, A.; Sotoca, J.; Lopez-Diego, V.; Vidal Taboada, J.M.; Salvado, M.; Juntas-Morales, R. Biofluid Biomarkers in the Prognosis of Amyotrophic Lateral Sclerosis: Recent Developments and Therapeutic Applications. Cells 2023, 12, 1180. [Google Scholar] [CrossRef]
- Benatar, M.; Wuu, J.; Andersen, P.M.; Bucelli, R.C.; Andrews, J.A.; Otto, M.; Farahany, N.A.; Harrington, E.A.; Chen, W.; Mitchell, A.A.; et al. Design of a Randomized, Placebo-Controlled, Phase 3 Trial of Tofersen Initiated in Clinically Presymptomatic SOD1 Variant Carriers: The ATLAS Study. Neurother. J. Am. Soc. Exp. Neurother. 2022, 19, 1248–1258. [Google Scholar] [CrossRef]
- Irwin, K.E.; Sheth, U.; Wong, P.C.; Gendron, T.F. Fluid Biomarkers for Amyotrophic Lateral Sclerosis: A Review. Mol. Neurodegener. 2024, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- De Schaepdryver, M.; Goossens, J.; De Meyer, S.; Jeromin, A.; Masrori, P.; Brix, B.; Claeys, K.G.; Schaeverbeke, J.; Adamczuk, K.; Vandenberghe, R.; et al. Serum Neurofilament Heavy Chains as Early Marker of Motor Neuron Degeneration. Ann. Clin. Transl. Neurol. 2019, 6, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Vacchiano, V.; Mastrangelo, A.; Zenesini, C.; Masullo, M.; Quadalti, C.; Avoni, P.; Polischi, B.; Cherici, A.; Capellari, S.; Salvi, F.; et al. Plasma and CSF Neurofilament Light Chain in Amyotrophic Lateral Sclerosis: A Cross-Sectional and Longitudinal Study. Front. Aging Neurosci. 2021, 13, 753242. [Google Scholar] [CrossRef] [PubMed]
- Gaiani, A.; Martinelli, I.; Bello, L.; Querin, G.; Puthenparampil, M.; Ruggero, S.; Toffanin, E.; Cagnin, A.; Briani, C.; Pegoraro, E.; et al. Diagnostic and Prognostic Biomarkers in Amyotrophic Lateral Sclerosis: Neurofilament Light Chain Levels in Definite Subtypes of Disease. JAMA Neurol. 2017, 74, 525–532. [Google Scholar] [CrossRef]
- Gong, Z.-Y.; Lv, G.-P.; Gao, L.-N.; Lu, Y.; Guo, J.; Zang, D.-W. Neurofilament Subunit L Levels in the Cerebrospinal Fluid and Serum of Patients with Amyotrophic Lateral Sclerosis. Neurodegener. Dis. 2018, 18, 165–172. [Google Scholar] [CrossRef]
- Zhou, Y.-N.; Chen, Y.-H.; Dong, S.-Q.; Yang, W.-B.; Qian, T.; Liu, X.-N.; Cheng, Q.; Wang, J.-C.; Chen, X.-J. Role of Blood Neurofilaments in the Prognosis of Amyotrophic Lateral Sclerosis: A Meta-Analysis. Front. Neurol. 2021, 12, 712245. [Google Scholar] [CrossRef]
- Miller, T.; Cudkowicz, M.; Shaw, P.J.; Andersen, P.M.; Atassi, N.; Bucelli, R.C.; Genge, A.; Glass, J.; Ladha, S.; Ludolph, A.L.; et al. Phase 1-2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2020, 383, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; O’Reilly, E.J.; Molsberry, S.; Kolonel, L.N.; Le Marchand, L.; Paganoni, S.; Schwarzschild, M.A.; Benkert, P.; Kuhle, J.; Ascherio, A. Prediagnostic Neurofilament Light Chain Levels in Amyotrophic Lateral Sclerosis. Neurology 2021, 97, e1466–e1474. [Google Scholar] [CrossRef]
- Benatar, M.; Zhang, L.; Wang, L.; Granit, V.; Statland, J.; Barohn, R.; Swenson, A.; Ravits, J.; Jackson, C.; Burns, T.M.; et al. Validation of Serum Neurofilaments as Prognostic and Potential Pharmacodynamic Biomarkers for ALS. Neurology 2020, 95, e59–e69. [Google Scholar] [CrossRef] [PubMed]
- Brodovitch, A.; Boucraut, J.; Delmont, E.; Parlanti, A.; Grapperon, A.-M.; Attarian, S.; Verschueren, A. Combination of Serum and CSF Neurofilament-Light and Neuroinflammatory Biomarkers to Evaluate ALS. Sci. Rep. 2021, 11, 703. [Google Scholar] [CrossRef]
- Masrori, P.; De Schaepdryver, M.; Floeter, M.K.; De Vocht, J.; Lamaire, N.; D’Hondt, A.; Traynor, B.; Poesen, K.; Van Damme, P. Prognostic Relationship of Neurofilaments, CHIT1, YKL-40 and MCP-1 in Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 681–682. [Google Scholar] [CrossRef]
- Basha, S.; Mukunda, D.C.; Rodrigues, J.; Gail D’Souza, M.; Gangadharan, G.; Pai, A.R.; Mahato, K.K. A Comprehensive Review of Protein Misfolding Disorders, Underlying Mechanism, Clinical Diagnosis, and Therapeutic Strategies. Ageing Res. Rev. 2023, 90, 102017. [Google Scholar] [CrossRef]
- Basso, M.; Giraudo, S.; Corpillo, D.; Bergamasco, B.; Lopiano, L.; Fasano, M. Proteome Analysis of Human Substantia Nigra in Parkinson’s Disease. Proteomics 2004, 4, 3943–3952. [Google Scholar] [CrossRef]
- Hill, W.D.; Arai, M.; Cohen, J.A.; Trojanowski, J.Q. Neurofilament mRNA Is Reduced in Parkinson’s Disease Substantia Nigra Pars Compacta Neurons. J. Comp. Neurol. 1993, 329, 328–336. [Google Scholar] [CrossRef]
- Krüger, R.; Fischer, C.; Schulte, T.; Strauss, K.M.; Müller, T.; Woitalla, D.; Berg, D.; Hungs, M.; Gobbele, R.; Berger, K.; et al. Mutation Analysis of the Neurofilament M Gene in Parkinson’s Disease. Neurosci. Lett. 2003, 351, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ollé, R.; López-Toledano, M.A.; Goryunov, D.; Cabrera-Poch, N.; Stefanis, L.; Brown, K.; Liem, R.K.H. Mutations in the Neurofilament Light Gene Linked to Charcot-Marie-Tooth Disease Cause Defects in Transport. J. Neurochem. 2005, 93, 861–874. [Google Scholar] [CrossRef]
- Lavedan, C.; Buchholtz, S.; Nussbaum, R.L.; Albin, R.L.; Polymeropoulos, M.H. A Mutation in the Human Neurofilament M Gene in Parkinson’s Disease That Suggests a Role for the Cytoskeleton in Neuronal Degeneration. Neurosci. Lett. 2002, 322, 57–61. [Google Scholar] [CrossRef]
- Holmberg, B.; Rosengren, L.; Karlsson, J.; Johnels, B. Increased Cerebrospinal Fluid Levels of Neurofilament Protein in Progressive Supranuclear Palsy and Multiple-system Atrophy Compared with Parkinson’s Disease. Mov. Disord. 1998, 13, 70–77. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Bougea, A.; Papadopoulos, A.; Papagiannakis, N.; Simitsi, A.-M.; Koros, C.; Georgakis, M.K.; Stefanis, L. CSF and Circulating NfL as Biomarkers for the Discrimination of Parkinson Disease from Atypical Parkinsonian Syndromes: Meta-Analysis. Neurol. Clin. Pract. 2021, 11, e867–e875. [Google Scholar] [CrossRef]
- Bäckström, D.C.; Eriksson Domellöf, M.; Linder, J.; Olsson, B.; Öhrfelt, A.; Trupp, M.; Zetterberg, H.; Blennow, K.; Forsgren, L. Cerebrospinal Fluid Patterns and the Risk of Future Dementia in Early, Incident Parkinson Disease. JAMA Neurol. 2015, 72, 1175. [Google Scholar] [CrossRef]
- Gordon, B.A. Neurofilaments in Disease: What Do We Know? Curr. Opin. Neurobiol. 2020, 61, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Dakna, M.; Kruse, N.; Galasko, D.; Foroud, T.; Zetterberg, H.; Schade, S.; Gera, R.G.; Wang, W.; Gao, F.; et al. Validation of Serum Neurofilament Light Chain as a Biomarker of Parkinson’s Disease Progression. Mov. Disord. 2020, 35, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Ashton, N.J.; Lupini, A.; Battaglio, B.; Zatti, C.; Trasciatti, C.; Gipponi, S.; Cottini, E.; Grossi, I.; Salvi, A.; et al. Plasma NfL, GFAP, Amyloid, and p-Tau Species as Prognostic Biomarkers in Parkinson’s Disease. J. Neurol. 2024, 271, 7537–7546. [Google Scholar] [CrossRef]
- Liu, Q. Neurofilamentopathy in Neurodegenerative Diseases. Open Neurol. J. 2011, 5, 58–62. [Google Scholar] [CrossRef]
- Zetterberg, H.; Skillbäck, T.; Mattsson, N.; Trojanowski, J.Q.; Portelius, E.; Shaw, L.M.; Weiner, M.W.; Blennow, K.; for the Alzheimer’s Disease Neuroimaging Initiative. Association of Cerebrospinal Fluid Neurofilament Light Concentration with Alzheimer Disease Progression. JAMA Neurol. 2016, 73, 60. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Cheng, D.; Wang, J.; Duong, D.M.; Losik, T.G.; Gearing, M.; Rees, H.D.; Lah, J.J.; Levey, A.I.; Peng, J. Proteomic Characterization of Postmortem Amyloid Plaques Isolated by Laser Capture Microdissection. J. Biol. Chem. 2004, 279, 37061–37068. [Google Scholar] [CrossRef]
- Sonoda, Y.; Mukai, H.; Matsuo, K.; Takahashi, M.; Ono, Y.; Maeda, K.; Akiyama, H.; Kawamata, T. Accumulation of Tumor-Suppressor PTEN in Alzheimer Neurofibrillary Tangles. Neurosci. Lett. 2010, 471, 20–24. [Google Scholar] [CrossRef]
- Wheelock, M.D.; Strain, J.F.; Mansfield, P.; Tu, J.C.; Tanenbaum, A.; Preische, O.; Chhatwal, J.P.; Cash, D.M.; Cruchaga, C.; Fagan, A.M.; et al. Brain Network Decoupling with Increased Serum Neurofilament and Reduced Cognitive Function in Alzheimer’s Disease. Brain J. Neurol. 2023, 146, 2928–2943. [Google Scholar] [CrossRef] [PubMed]
- Bacioglu, M.; Maia, L.F.; Preische, O.; Schelle, J.; Apel, A.; Kaeser, S.A.; Schweighauser, M.; Eninger, T.; Lambert, M.; Pilotto, A.; et al. Neurofilament Light Chain in Blood and CSF as Marker of Disease Progression in Mouse Models and in Neurodegenerative Diseases. Neuron 2016, 91, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Syrjanen, J.A.; Blennow, K.; Zetterberg, H.; Skoog, I.; Waern, M.; Hagen, C.E.; Van Harten, A.C.; Knopman, D.S.; Jack, C.R.; et al. Association of Cerebrospinal Fluid Neurofilament Light Protein with Risk of Mild Cognitive Impairment Among Individuals Without Cognitive Impairment. JAMA Neurol. 2019, 76, 187. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Andreasson, U.; Zetterberg, H.; Blennow, K.; for the Alzheimer’s Disease Neuroimaging Initiative. Association of Plasma Neurofilament Light with Neurodegeneration in Patients with Alzheimer Disease. JAMA Neurol. 2017, 74, 557. [Google Scholar] [CrossRef]
- Schultz, S.A.; Strain, J.F.; Adedokun, A.; Wang, Q.; Preische, O.; Kuhle, J.; Flores, S.; Keefe, S.; Dincer, A.; Ances, B.M.; et al. Serum Neurofilament Light Chain Levels Are Associated with White Matter Integrity in Autosomal Dominant Alzheimer’s Disease. Neurobiol. Dis. 2020, 142, 104960. [Google Scholar] [CrossRef]
- Sanchez, E.; Wilkinson, T.; Coughlan, G.; Mirza, S.; Baril, A.; Ramirez, J.; Binns, M.A.; Black, S.E.; Borrie, M.; Dilliott, A.A.; et al. Association of Plasma Biomarkers with Cognition, Cognitive Decline, and Daily Function across and within Neurodegenerative Diseases: Results from the Ontario Neurodegenerative Disease Research Initiative. Alzheimers Dement. 2024, 20, 1753–1770. [Google Scholar] [CrossRef]
- Moscoso, A.; Grothe, M.J.; Ashton, N.J.; Karikari, T.K.; Lantero Rodríguez, J.; Snellman, A.; Suárez-Calvet, M.; Blennow, K.; Zetterberg, H.; Schöll, M.; et al. Longitudinal Associations of Blood Phosphorylated Tau181 and Neurofilament Light Chain with Neurodegeneration in Alzheimer Disease. JAMA Neurol. 2021, 78, 396. [Google Scholar] [CrossRef]
- Freudenberg-Hua, Y.; Giliberto, L.; d’Abramo, C.; Li, W.; Ma, Y.; Goate, A.; Koppel, J. Differential Associations of APOE and TREM2 Variants with Glial Fibrillary Acidic Protein and Neurofilament Light in Plasma of UK Biobank Participants Support Distinct Disease Mechanisms. Mol. Psychiatry 2025, 30, 4985–4991. [Google Scholar] [CrossRef]
- Karantali, E.; Kazis, D.; Chatzikonstantinou, S.; Petridis, F.; Mavroudis, I. The Role of Neurofilament Light Chain in Frontotemporal Dementia: A Meta-Analysis. Aging Clin. Exp. Res. 2021, 33, 869–881. [Google Scholar] [CrossRef]
- Rojas, J.C.; Wang, P.; Staffaroni, A.M.; Heller, C.; Cobigo, Y.; Wolf, A.; Goh, S.-Y.M.; Ljubenkov, P.A.; Heuer, H.W.; Fong, J.C.; et al. Plasma Neurofilament Light for Prediction of Disease Progression in Familial Frontotemporal Lobar Degeneration. Neurology 2021, 96, e2296–e2312. [Google Scholar] [CrossRef] [PubMed]
- Pijnenburg, Y.A.L.; Janssen, J.C.; Schoonenboom, N.S.M.; Petzold, A.; Mulder, C.; Stigbrand, T.; Norgren, N.; Heijst, H.; Hack, C.E.; Scheltens, P.; et al. CSF Neurofilaments in Frontotemporal Dementia Compared with Early Onset Alzheimer’s Disease and Controls. Dement. Geriatr. Cogn. Disord. 2007, 23, 225–230. [Google Scholar] [CrossRef]
- Sheth, U.; Öijerstedt, L.; Heckman, M.G.; White, L.J.; Heuer, H.W.; Lario Lago, A.; Forsberg, L.K.; Faber, K.M.; Foroud, T.M.; Rademakers, R.; et al. Comprehensive Cross-Sectional and Longitudinal Comparisons of Plasma Glial Fibrillary Acidic Protein and Neurofilament Light across FTD Spectrum Disorders. Mol. Neurodegener. 2025, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Pirpamer, L.; Hofer, E.; Voortman, M.M.; Barro, C.; Leppert, D.; Benkert, P.; Ropele, S.; Enzinger, C.; Fazekas, F.; et al. Serum Neurofilament Light Levels in Normal Aging and Their Association with Morphologic Brain Changes. Nat. Commun. 2020, 11, 812. [Google Scholar] [CrossRef]
- Shahim, P.; Gren, M.; Liman, V.; Andreasson, U.; Norgren, N.; Tegner, Y.; Mattsson, N.; Andreasen, N.; Öst, M.; Zetterberg, H.; et al. Serum Neurofilament Light Protein Predicts Clinical Outcome in Traumatic Brain Injury. Sci. Rep. 2016, 6, 36791. [Google Scholar] [CrossRef]
- De Marchis, G.M.; Katan, M.; Barro, C.; Fladt, J.; Traenka, C.; Seiffge, D.J.; Hert, L.; Gensicke, H.; Disanto, G.; Sutter, R.; et al. Serum Neurofilament Light Chain in Patients with Acute Cerebrovascular Events. Eur. J. Neurol. 2018, 25, 562–568. [Google Scholar] [CrossRef]
- Sotirchos, E.S.; Hu, C.; Smith, M.D.; Lord, H.-N.; DuVal, A.L.; Arrambide, G.; Montalban, X.; Akgün, K.; Ziemssen, T.; Naismith, R.T.; et al. Agreement Between Published Reference Resources for Neurofilament Light Chain Levels in People With Multiple Sclerosis. Neurology 2023, 101, e2448–e2453. [Google Scholar] [CrossRef]
- Hviid, C.V.B.; Madsen, A.T.; Winther-Larsen, A. Biological Variation of Serum Neurofilament Light Chain. Clin. Chem. Lab. Med. CCLM 2022, 60, 569–575. [Google Scholar] [CrossRef]
- Brum, W.S.; Ashton, N.J.; Simrén, J.; Di Molfetta, G.; Karikari, T.K.; Benedet, A.L.; Zimmer, E.R.; Lantero-Rodriguez, J.; Montoliu-Gaya, L.; Jeromin, A.; et al. Biological Variation Estimates of Alzheimer’s Disease Plasma Biomarkers in Healthy Individuals. Alzheimers Dement. 2024, 20, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Carobene, A.; Maiese, K.; Abou-Diwan, C.; Locatelli, M.; Serteser, M.; Coskun, A.; Unsal, I. Biological Variation Estimates for Serum Neurofilament Light Chain in Healthy Subjects. Clin. Chim. Acta 2023, 551, 117608. [Google Scholar] [CrossRef]
- Bar-Or, A.; Nicholas, J.; Feng, J.; Sorrell, F.; Cascione, M. Exploring the Clinical Utility of Neurofilament Light Chain Assays in Multiple Sclerosis Management. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, e200427. [Google Scholar] [CrossRef] [PubMed]
- Thebault, S.; Booth, R.A.; Rush, C.A.; MacLean, H.; Freedman, M.S. Serum Neurofilament Light Chain Measurement in MS: Hurdles to Clinical Translation. Front. Neurosci. 2021, 15, 654942. [Google Scholar] [CrossRef] [PubMed]

| Platform | Principle | Sample Type | Advantages | Limitations | Clinical Applicability |
|---|---|---|---|---|---|
| Enzyme-linked immunosorbent Assays (ELISA) | Antibody-based detection | Mainly CSF | Established method; low cost; significant accuracy | Low sensitivity for blood; labor-intensive; research use only | Research: limited clinical use |
| Electrochemiluminescence (ECLIA) | Luminescence generated by the electrochemical reactions of antibodies | CSF and serum | Semi-sensitive; partially automated | Less sensitive than SIMOA; moderate throughput | Emerging clinical utility |
| Single Molecule Array (SIMOA) | Ultra-sensitive bead-based digital immunoassay | CSF and serum | Ultrasensitive; Detects sub-pg/mL levels; strong CSF–serum correlation; high reproducibility | Higher cost; requires specialized platform | Widely used; FDA Breakthrough Device designation |
| High-Throughput Chemiluminescent Immunoassays | Automated chemiluminescent detection | Serum (routine) | Full automation; scalable; robust reproducibility | Limited availability; requires large clinical analyzers | Clinical integration (e.g., Siemens Atellica®); the first CE-marked blood test in Europe |
| Emerging Technologies | Immunoprecipitation with mass spectrometry | CSF and tissue | Identifies NfL proteoforms; potential CNS vs. PNS distinction | Currently research only; technically complex | Future personalized biomarker assays |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daponte, A.; Koros, C.; Skarlis, C.; Siozios, D.; Rentzos, M.; Papageorgiou, S.G.; Anagnostouli, M. Neurofilament Biomarkers in Neurology: From Neuroinflammation to Neurodegeneration, Bridging Established and Novel Analytical Advances with Clinical Practice. Int. J. Mol. Sci. 2025, 26, 9739. https://doi.org/10.3390/ijms26199739
Daponte A, Koros C, Skarlis C, Siozios D, Rentzos M, Papageorgiou SG, Anagnostouli M. Neurofilament Biomarkers in Neurology: From Neuroinflammation to Neurodegeneration, Bridging Established and Novel Analytical Advances with Clinical Practice. International Journal of Molecular Sciences. 2025; 26(19):9739. https://doi.org/10.3390/ijms26199739
Chicago/Turabian StyleDaponte, Ariadne, Christos Koros, Charalampos Skarlis, Daphne Siozios, Michail Rentzos, Sokratis G. Papageorgiou, and Maria Anagnostouli. 2025. "Neurofilament Biomarkers in Neurology: From Neuroinflammation to Neurodegeneration, Bridging Established and Novel Analytical Advances with Clinical Practice" International Journal of Molecular Sciences 26, no. 19: 9739. https://doi.org/10.3390/ijms26199739
APA StyleDaponte, A., Koros, C., Skarlis, C., Siozios, D., Rentzos, M., Papageorgiou, S. G., & Anagnostouli, M. (2025). Neurofilament Biomarkers in Neurology: From Neuroinflammation to Neurodegeneration, Bridging Established and Novel Analytical Advances with Clinical Practice. International Journal of Molecular Sciences, 26(19), 9739. https://doi.org/10.3390/ijms26199739






