Antidepressants Target the ST3GAL5–GM3 Lipid Pathway to Suppress Microglial Inflammation
Abstract
1. Introduction
2. Results
2.1. Selection of Clinically Relevant Concentrations for Transcriptomic Analyses
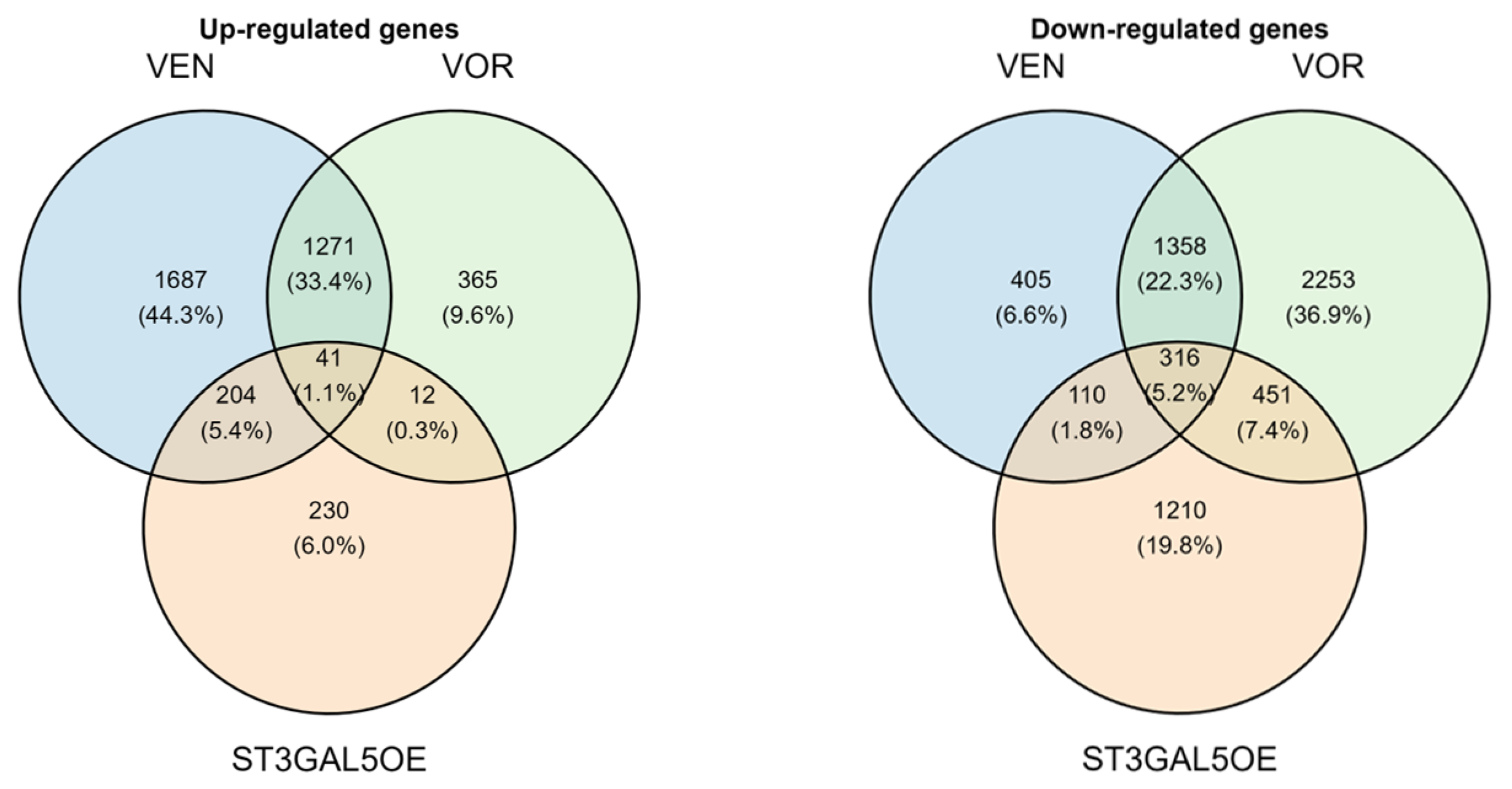
2.2. Transcriptomic Profiling Identifies a Convergent Gene Expression Signature Across VEN, VOR, and ST3GAL5 Overexpression
2.3. Functional Validation of Cytokine-Induced Signaling Responses
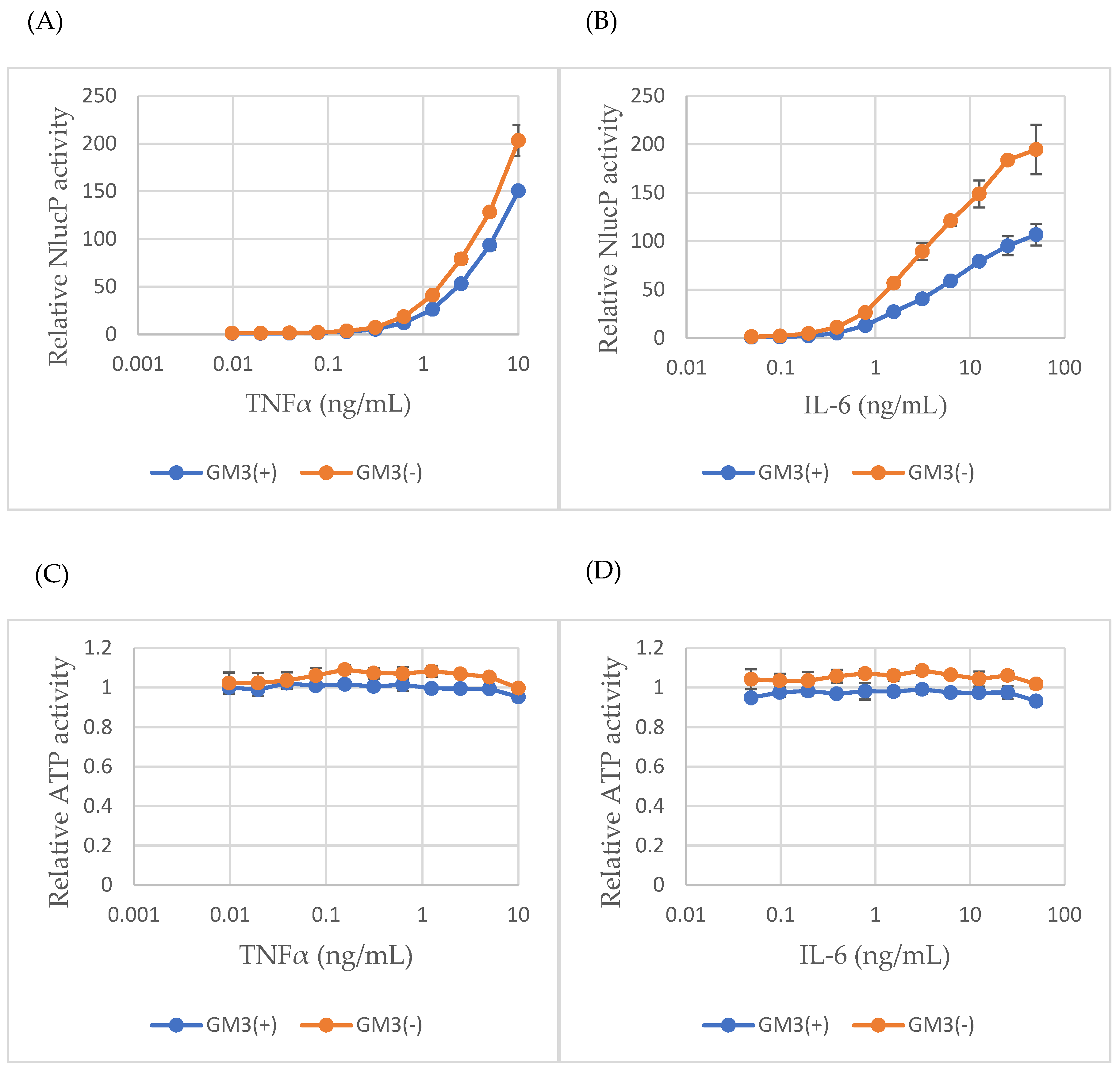
2.4. GM3 Suppresses Cytokine-Induced NF-κB and STAT3 Activity
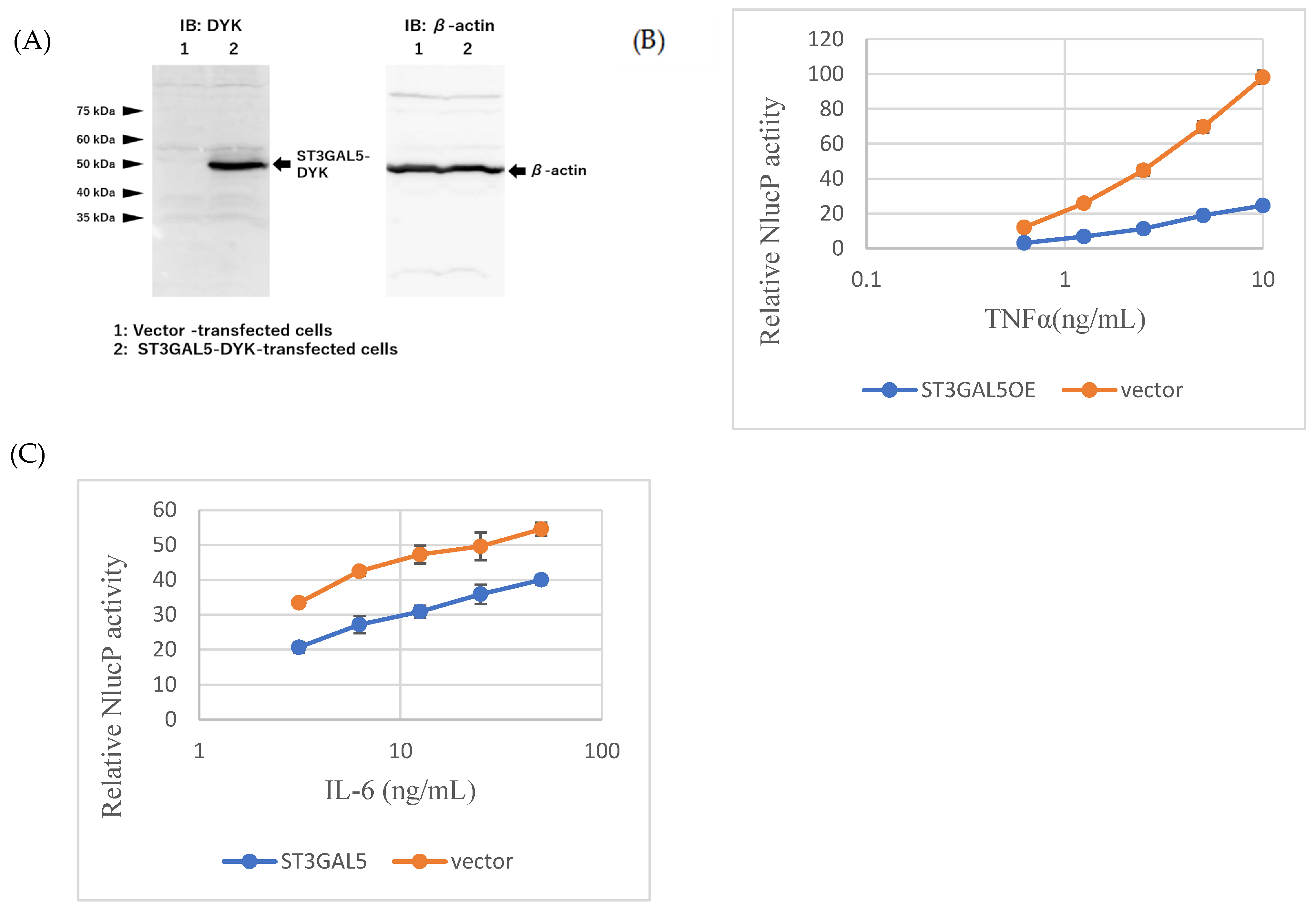
2.5. ST3GAL5 Overexpression Recapitulates GM3-Mediated Suppression
2.6. Chronic Antidepressant Exposure Phenocopies ST3GAL5OE Effects
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cytotoxicity Assay
4.4. NanoLuc Reporter Assays
4.5. RNA Extraction and Microarray
4.6. Differential Expression and Enrichment Analysis
4.7. Data Visualization and Statistics
4.8. Western Blotting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| BDNF | Brain-derived neurotrophic factor |
| DMSO | dimethyl sulfoxide |
| FDR | False discovery rate |
| GO | Gene Ontology |
| GM3 | monosialodihexosylganglioside |
| GSEA | Gene set enrichment analysis |
| IL | Interleukin |
| MD | Major depression |
| MG | Microglia |
| NES | Normalized enrichment score |
| NF-κB | Nuclear factor kappa B |
| NlucP | NanoLuc luciferase-PEST |
| ROS | Reactive oxygen species |
| SIE | Sis-inducible element |
| SSRI | Selective serotonin reuptake inhibitors |
| ST3GAL5 | ST3 beta-galactoside alpha-2,3-sialyltransferase 5 |
| TNF | Tumor necrosis factor |
| VEN | venlafaxine |
| VOR | vortioxetine |
References
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Hirschfeld, R.M. History and evolution of the monoamine hypothesis of depression. J. Clin. Psychiatry 2000, 61 (Suppl. S6), 4–6. [Google Scholar] [PubMed]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2018, 72, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, J.; Cooper, R.E. The serotonin theory of depression: A systematic umbrella review of evidence. Mol. Psychiatry 2023, 28, 3243–3256. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. Role of inflammation in depression: From an evolutionary imperative to modern treatment targets. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Dobrek, L.; Głowacka, K. Depression and its phytopharmacotherapy: A narrative review. Int. J. Mol. Sci. 2023, 24, 4772. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia biology: One century of evolving concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Li, B.; Yang, W.; Ge, T.; Wang, Y.; Cui, R. Stress-induced microglial activation contributes to depression. Pharmacol. Res. 2022, 179, 106145. [Google Scholar] [CrossRef] [PubMed]
- Yirmiya, R. Inflammatory underpinning of depression: A historical perspective. Brain Behav. Immun. 2024, 122, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Maziz, M.N.H.; Chakravarthi, S.; Aung, T.; Htoo, P.M.; Shwe, W.H.; Gupalo, S.; Udayah, M.W.; Singh, H.; Kabir, M.S.; Thangarajan, R.; et al. Microglia-mediated neuroinflammation through phosphatidylinositol 3-kinase signaling causes cognitive dysfunction. Int. J. Mol. Sci. 2025, 26, 7212. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, E.; Wilson, A.A.; Mizrahi, R.; Rusjan, P.M.; Miler, L.; Rajkowska, G.; Suridjan, I.; Kennedy, J.L.; Rekkas, P.V.; Houle, S.; et al. Role of translocator protein density, a marker of neuroinflammation in the brain during major depressive episodes. JAMA Psychiatry 2015, 72, 268–275. [Google Scholar] [CrossRef]
- Setiawan, E.; Attwells, S.; Wilson, A.A.; Rusjan, P.M.; Mizrahi, R.; Miler, L.; Xu, C.; Sharma, S.; Kish, S.; Houle, S.; et al. Association of total translocator protein distribution volume with duration of untreated major depressive disorder. JAMA Psychiatry 2018, 5, 339–347. [Google Scholar] [CrossRef]
- Tynan, R.J.; Weidenhofer, J.; Hinwood, M.; Cairns, M.J.; Day, T.A.; Walker, F.R. Comparative examination of the anti-inflammatory effects of SSRI and SNRI on LPS-stimulated microglia. Brain Behav. Immun. 2012, 26, 469–479. [Google Scholar] [CrossRef]
- Manikowska, K.; Mikołajczyk, M.; Mikołajczak, P.Ł.; Bobkiewicz-Kozłowska, T. The influence of mianserin on TNF-α, IL-6 and IL-10 serum levels in rats under chronic mild stress. Pharmacol. Rep. 2014, 66, 22–27. [Google Scholar] [CrossRef]
- Alboni, S.; Benatti, C.; Montanari, C.; Tascedda, F.; Brunello, N. Chronic antidepressant treatments resulted in altered expression of genes involved in inflammation in the rat hypothalamus. Eur. J. Pharmacol. 2013, 721, 158–167. [Google Scholar] [CrossRef]
- Hung, Y.Y.; Huang, K.W.; Kang, H.Y.; Huang, G.Y.-L.; Huang, T.-L. Antidepressants normalize elevated Toll-like receptor profile in major depressive disorder. Psychopharmacology 2016, 233, 1707–1714. [Google Scholar] [CrossRef]
- Hou, T.Y.; Huang, T.-L.; Lin, C.-C.; Wu, M.-K.; Hung, Y.-Y. Effects of selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors on toll-like-receptors expression profiles. Neuropsychiatry 2018, 8, 243–248. [Google Scholar]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef] [PubMed]
- Viljetić, B.; Blažetić, S.; Labak, I.; Ivić, V.; Zjalić, M.; Heffer, M.; Balog, M. Lipid Rafts: The Maestros of Normal Brain Development. Biomolecules 2024, 14, 362. [Google Scholar] [CrossRef] [PubMed]
- Itokazu, Y.; Wang, J.; Yu, R.K. Gangliosides in Nerve Cell Specification. Prog. Mol. Biol. Transl. Sci. 2018, 156, 241–263. [Google Scholar] [CrossRef]
- Itokazu, Y.; Yu, R.K. Ganglioside Microdomains on Cellular and Intracellular Membranes Regulate Neuronal Cell Fate Determination. Adv. Neurobiol. 2023, 29, 281–304. [Google Scholar] [CrossRef]
- Vasques, J.F.; de Jesus Gonçalves, R.G.; da Silva-Junior, A.J.; Martins, R.S.; Gubert, F.; Mendez-Otero, R. Gangliosides in nervous system development, regeneration, and pathologies. Neural Regen. Res. 2023, 18, 81–86. [Google Scholar] [CrossRef]
- Inokuchi, J.I.; Kanoh, H.; Inamori, K.I.; Nagafuku, M.; Nitta, T.; Fukase, K. Homeostatic and pathogenic roles of the GM3 ganglioside. FEBS J. 2022, 289, 5152–5165. [Google Scholar] [CrossRef]
- Gordon-Lipkin, E.; Cohen, J.S.; Srivastava, S.; Soares, B.P.; Levey, E.; Fatemi, A. ST3GAL5-Related Disorders: A Deficiency in Ganglioside Metabolism and a Genetic Cause of Intellectual Disability and Choreoathetosis. J. Child Neurol. 2018, 33, 825–831. [Google Scholar] [CrossRef]
- Inamori, K.-I.; Inokuchi, J.-I. Roles of Gangliosides in Hypothalamic Control of Energy Balance: New Insights. Int. J. Mol. Sci. 2020, 21, 5349. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [CrossRef]
- Chen, G.; Lee, R.; Højer, A.M.; Buchbjerg, J.K.; Serenko, M.; Zhao, Z. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin. Drug Investig. 2013, 33, 727–736. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; de Andrade, N.Q.; Morris, G.; Fernandes, B.S.; Brunoni, A.R.; et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: A systematic review and meta-analysis. Mol. Neurobiol. 2018, 55, 4195–4206. [Google Scholar] [CrossRef]
- Hannestad, J.; DellaGioia, N.; Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of Depression with C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayasaki, G.; Izumi, H.; Morimoto, Y.; Yoshimura, R. Antidepressants Target the ST3GAL5–GM3 Lipid Pathway to Suppress Microglial Inflammation. Int. J. Mol. Sci. 2025, 26, 9733. https://doi.org/10.3390/ijms26199733
Hayasaki G, Izumi H, Morimoto Y, Yoshimura R. Antidepressants Target the ST3GAL5–GM3 Lipid Pathway to Suppress Microglial Inflammation. International Journal of Molecular Sciences. 2025; 26(19):9733. https://doi.org/10.3390/ijms26199733
Chicago/Turabian StyleHayasaki, Gaku, Hiroto Izumi, Yasuo Morimoto, and Reiji Yoshimura. 2025. "Antidepressants Target the ST3GAL5–GM3 Lipid Pathway to Suppress Microglial Inflammation" International Journal of Molecular Sciences 26, no. 19: 9733. https://doi.org/10.3390/ijms26199733
APA StyleHayasaki, G., Izumi, H., Morimoto, Y., & Yoshimura, R. (2025). Antidepressants Target the ST3GAL5–GM3 Lipid Pathway to Suppress Microglial Inflammation. International Journal of Molecular Sciences, 26(19), 9733. https://doi.org/10.3390/ijms26199733






