Modulation of Paraoxonase 1 Activity and Asymmetric Dimethylarginine by Immunomodulatory Therapies in Multiple Sclerosis
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics
2.2. Serum PON1 and ARE Activity in MS
2.3. PON1 Genetic Phenotype
2.4. Treatment Effects on PON1 and ARE Activity
- Dimethyl fumarate (DMF) showed the lowest PON activity (60.41 U/mL).
- For ARE activity, natalizumab-treated patients had the lowest values (112.60 U/mL), whereas DMF-treated patients had the highest (135.65 U/mL).
| No Treatment | INF | GA | Natalizumab | Fingolimod | DMF | Immunosuppressive | p | |
|---|---|---|---|---|---|---|---|---|
| Number of patients: 261 | 110 | 59 | 40 | 11 | 19 | 17 | 5 | |
| Age (year) median | 38 | 40 | 38.5 | 33 | 36 | 32 | 38 | 0.2795 |
| EDSS median | 2.5 | 2 | 2.25 | 4 | 3 | 1.5 | 3 | 0.2722 |
| Paraoxonase (IU/L) median | 122.28 | 63.30 | 136.63 | 109.00 | 76.78 | 60.41 | 123.04 | 0.0382 |
| Arylesterase (IU/L) median | 130.18 | 118.67 | 128.48 | 112.60 | 132.73 | 135.65 | 125.13 | 0.034 |
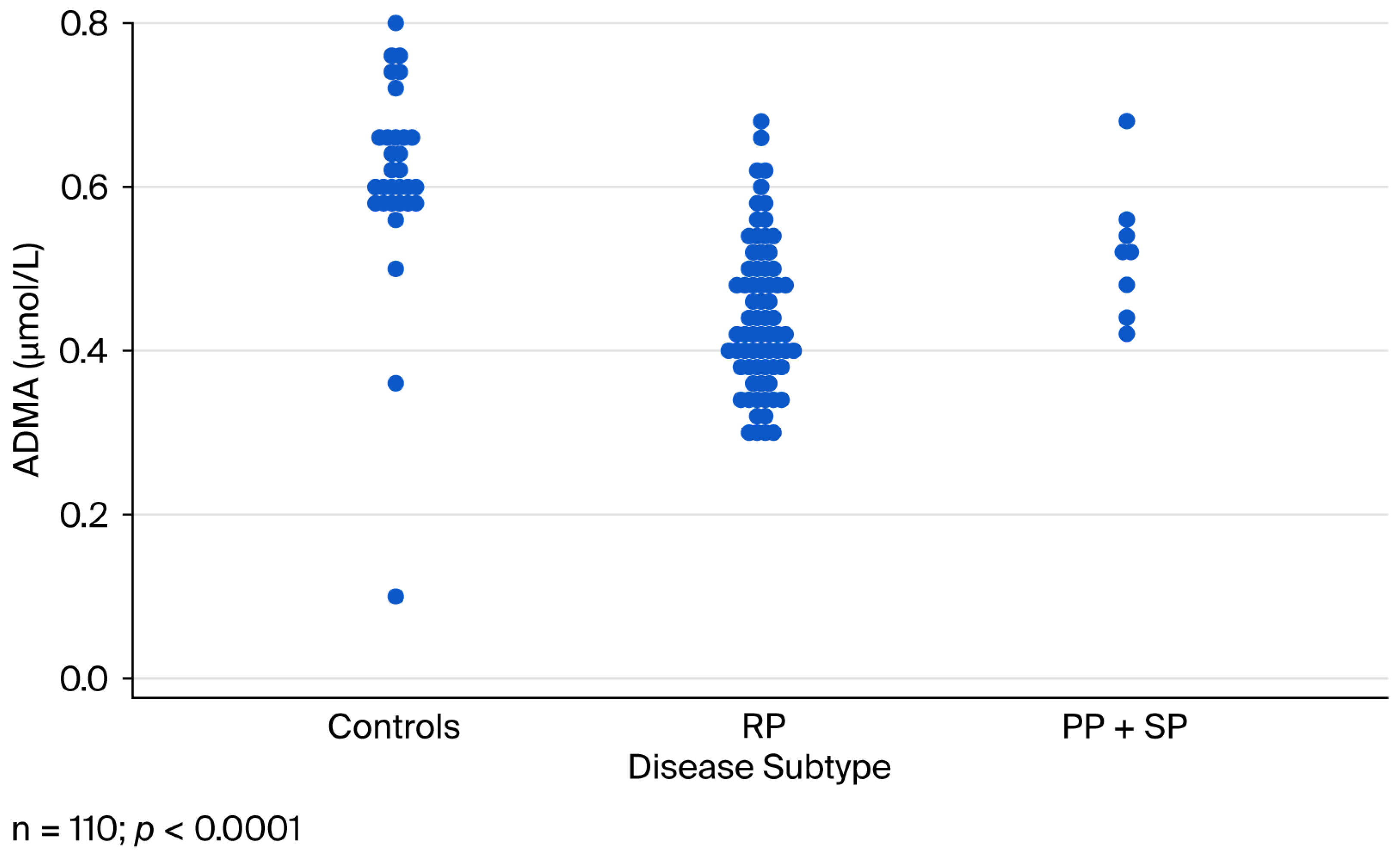
2.5. Serum ADMA Concentrations
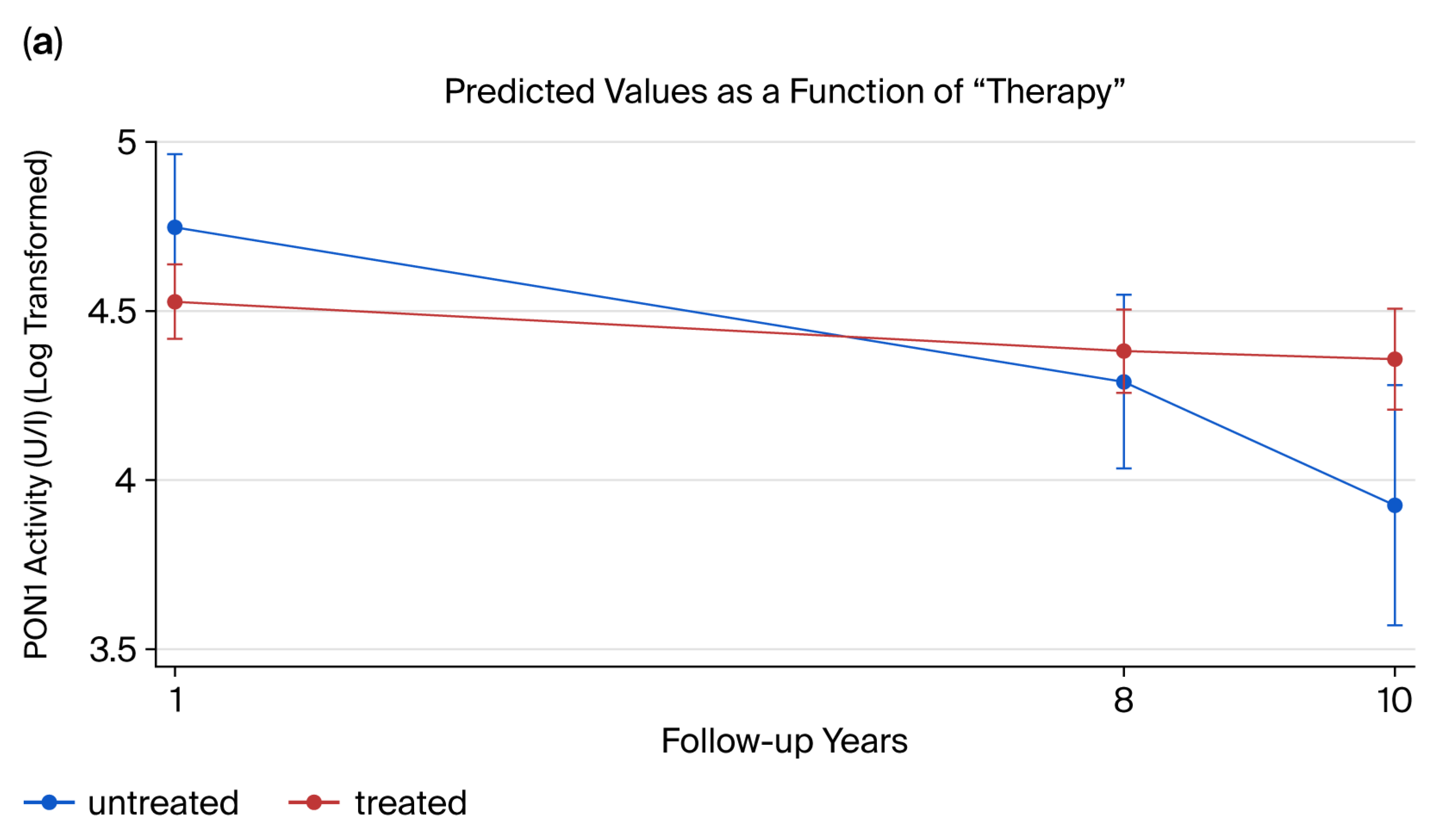
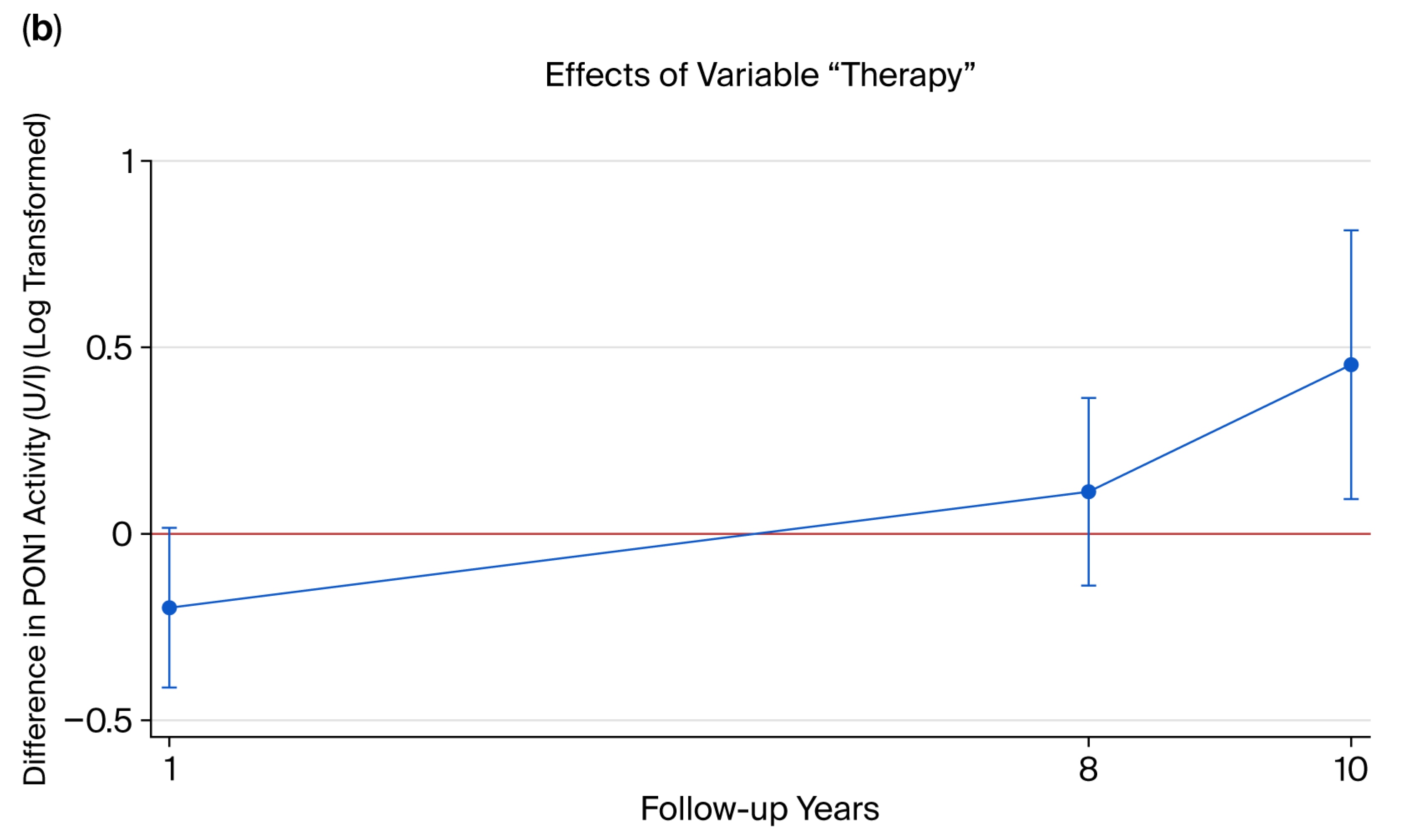
2.6. Longitudinal Analysis of PON1 Activity
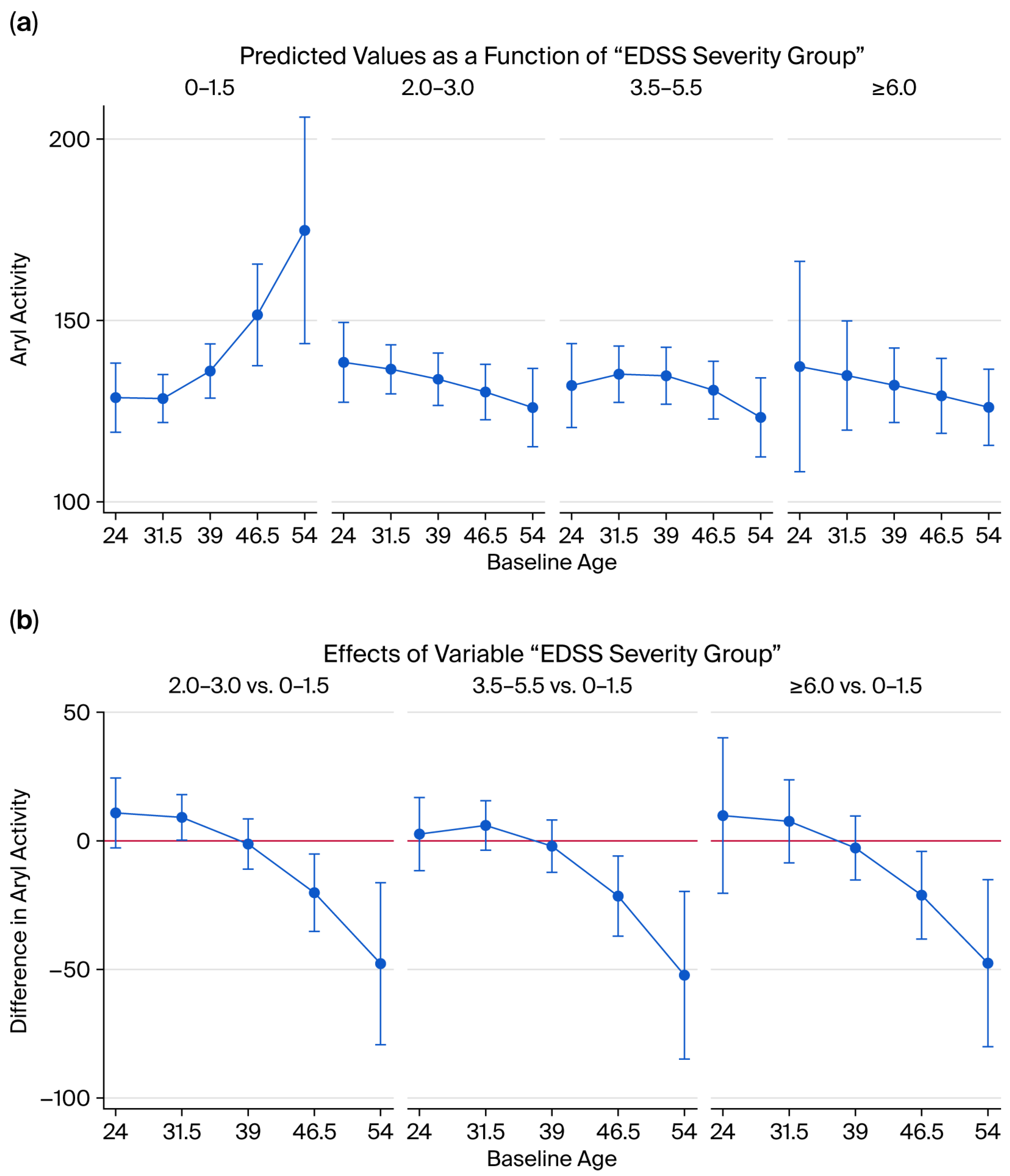
2.7. Disability and Enzyme Activity
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Blood Collection and Lipid Measurements
4.3. Measurement of Serum Paraoxonase (PON1) and Arylesterase (ARE) Activity
4.4. PON1 Phenotyping
4.5. Measurement of ADMA
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | Multiple sclerosis |
| PON1 | Paraoxonase 1 |
| ADMA | Asymmetric dimethylarginine |
| CIS | Clinically isolated syndrome |
| RRMS | Relapsing-remitting multiple sclerosis |
| PPMS | Primary-progressive multiple sclerosis |
| SPMS | Secondary-progressive multiple sclerosis |
| RPMS | Relapsing-progressive multiple sclerosis |
| ARE | Arylesterase |
| ROS | Reactive oxygen species |
| EDSS | Expanded Disability Status Scale |
| BMI | Body mass index |
| IFN | Interferon |
| GA | Glatiramer acetate |
| DMF | Dimethyl fumarate |
References
- Biernacki, T.; Sandi, D.; Fricska-Nagy, Z.; Kincses, Z.T.; Füvesi, J.; Laczkó, R.; Kokas, Z.; Klivényi, P.; Vécsei, L.; Bencsik, K. Epidemiology of multiple sclerosis in Central Europe, update from Hungary. Brain Behav. 2020, 10, e01598. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Leray, E.; Yaouanq, J.; Le Page, E.; Coustans, M.; Laplaud, D.; Oger, J.; Edan, G. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010, 133, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- University of California, San Francisco MS-EPIC Team; Cree, B.A.C.; Hollenbach, J.A.; Bove, R.; Kirkish, G.; Sacco, S.; Caverzasi, E.; Bischof, A.; Gundel, T.; Msc, A.H.Z.; et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann. Neurol. 2019, 85, 653–666. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Moccia, M.; Coetzee, T.; Cohen, J.A.; Correale, J.; Graves, J.; Marrie, R.A.; Montalban, X.; Yong, V.W.; Thompson, A.J.; et al. Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol. 2023, 22, 78–88. [Google Scholar] [CrossRef]
- Trofin, D.M.; Sardaru, D.P.; Trofin, D.; Onu, I.; Tutu, A.; Onu, A.; Onită, C.; Galaction, A.I.; Matei, D.V. Oxidative Stress in Brain Function. Antioxidants 2025, 14, 297. [Google Scholar] [CrossRef]
- Khalaf, F.K.; Connolly, J.; Khatib-Shahidi, B.; Albehadili, A.; Tassavvor, I.; Ranabothu, M.; Eid, N.; Dube, P.; Khouri, S.J.; Malhotra, D.; et al. Paraoxonases at the Heart of Neurological Disorders. Int. J. Mol. Sci. 2023, 24, 6881. [Google Scholar] [CrossRef]
- Reichert, C.O.; Levy, D.; Bydlowski, S.P. Paraoxonase Role in Human Neurodegenerative Diseases. Antioxidants 2021, 10, 11. [Google Scholar] [CrossRef]
- Paragh, G.; Seres, I.; Balogh, Z.; Varga, Z.; Kárpáti, I.; Mátyus, J.; Ujhelyi, L.; Kakuk, G. The serum paraoxonase activity in patients with chronic renal failure and hyperlipidemia. Nephron 1998, 80, 166–170. [Google Scholar] [CrossRef]
- Kiss, E.; Seres, I.; Tarr, T.; Kocsis, Z.; Szegedi, G.; Paragh, G. Reduced paraoxonase1 activity is a risk for atherosclerosis in patients with systemic lupus erythematosus. Ann. N. Y. Acad. Sci. 2007, 1108, 83–91. [Google Scholar] [CrossRef]
- Jamroz-Wisniewska, A.; Beltowski, J.; Stelmasiak, Z.; Bartosik-Psujek, H. Paraoxonase 1 activity in different types of multiple sclerosis. Mult. Scler. 2009, 15, 399–402. [Google Scholar] [CrossRef]
- Szántó, A.; Harangi, M.; Seres, I.; Paragh, G.; Zeher, M. Decreased human paraoxonase-1 activity in patients with Sjögren’s syndrome. Int. Immunol. 2010, 22, 605–609. [Google Scholar] [CrossRef]
- Eckerson, H.W.; Wyte, C.M.; La Du, B.N. The human serum paraoxonase/arylesterase polymorphism. Am. J. Hum. Genet. 1983, 35, 1126–1138. [Google Scholar]
- Richter, R.J.; Furlong, C.E. Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics 1999, 9, 745–753. [Google Scholar] [CrossRef]
- Rejdak, K.; Petzold, A.; Stelmasiak, Z.; Giovannoni, G. Cerebrospinal fluid brain specific proteins in relation to nitric oxide metabolites during relapse of multiple sclerosis. Mult. Scler. 2008, 14, 59–66. [Google Scholar] [CrossRef]
- Sandoo, A.; Dimitroulas, T.; Hodson, J.; Smith, J.P.; Douglas, K.M.; Kitas, G.D. Cumulative inflammation associates with asymmetric dimethylarginine in rheumatoid arthritis: A 6 year follow-up study. Rheumatology 2015, 54, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yue, W.; Quan, X.; Wang, Y.; Zhao, B.; Lu, Z. Asymmetric dimethylarginine exacerbates Aβ-induced toxicity and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free Radic. Biol. Med. 2015, 79, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Principi, F.; Di Ludovico, F.; Viti, B.; Angeleri, V.A.; Danni, M.; Provinciali, L. Increased levels of lipid hydroperoxides in plasma of patients with multiple sclerosis: A relationship with paraoxonase activity. Mult. Scler. 2005, 11, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Kayacelebi, A.A.; Beckmann, B.; Hanff, E.; Gold, R.; Haghikia, A.; Tsikas, D. Serum and cerebrospinal fluid concentrations of homoarginine, arginine, asymmetric and symmetric dimethylarginine, nitrite and nitrate in patients with multiple sclerosis and neuromyelitis optica. Amino Acids 2015, 47, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Racz, L.; Padra, J.; Mezei, Z.; Paragh, G.; Csiba, L.; Seres, I.; Csepany, T. An anti-oxidant, serum paraoxonase1 activity in multiple sclerosis. Eur. J. Neurol. 2014, 21, 679. [Google Scholar]
- Kirbas, A.; Kirbas, S.; Anlar, O.; Efe, H.; Yilmaz, A. Serum paraoxonase and arylesterase activity and oxidative status in patients with multiple sclerosis. J. Clin. Neurosci. 2013, 20, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Moghtaderi, A.; Hashemi, M.; Sharafaddinzadeh, N.; Dabiri, S.; Moazeni-Roodi, A.; Ramroodi, N.; Zolfaghari, M. Lack of association between paraoxonase 1 Q192R polymorphism and multiple sclerosis in relapse phase: A case-control study. Clin. Biochem. 2011, 44, 795–798. [Google Scholar] [CrossRef]
- Kurtuluş, F.; Yaman, A.; Ellidağ, H.Y.; Eren, E.; Gömceli, Y.B.; Yılmaz, N. Paraoxonase1 activity and phenotype distribution in multiple sclerosis. Ideggyogy. Szle. 2016, 69, E047–E054. [Google Scholar] [CrossRef]
- Jamroz-Wisniewska, A.; Beltowski, J.; Wójcicka, G.; Bartosik-Psujek, H.; Rejdak, K. Cladribine Treatment Improved Homocysteine Metabolism and Increased Total Serum Antioxidant Activity in Secondary Progressive Multiple Sclerosis Patients. Oxid. Med. Cell. Longev. 2020, 14, 1654754. [Google Scholar] [CrossRef]
- Seres, I.; Paragh, G.; Deschene, E.; Fulop, T., Jr.; Khalil, A. Study of factors influencing the decreased HDL associated PON1 activity with aging. Exp. Gerontol. 2004, 39, 59–66. [Google Scholar] [CrossRef]
- Cherki, M.; Berrougui, H.; Isabelle, M.; Cloutier, M.; Koumbadinga, G.A.; Khalil, A. Effect of PON1 polymorphism on HDL antioxidant potential is blunted with aging. Exp. Gerontol. 2007, 42, 815–824. [Google Scholar] [CrossRef]
- Onmaz, D.E.; Isık, S.M.T.; Abusoglu, S.; Ekmekci, A.H.; Sivrikaya, A.; Abusoglu, G.; Ozturk, S.; Aydemir, H.Y.; Unlu, A. Serum ADMA levels were positively correlated with EDSS scores in patients with multiple sclerosis. J. Neuroimmunol. 2021, 353, 577497. [Google Scholar] [CrossRef]
- Monti, L.; Morbidelli, L.; Bazzani, L.; Rossi, A. Influence of Circulating Endothelin-1 and Asymmetric Dimethylarginine on Whole Brain Circulation Time in Multiple Sclerosis. Biomark. Insights 2017, 12, 1177271917712514. [Google Scholar] [CrossRef]
- Carlsson, H.; Abujrais, S.; Herman, S.; Khoonsari, P.E.; Åkerfeldt, T.; Svenningsson, A.; Burman, J.; Kultima, K. Targeted metabolomics of CSF in healthy individuals and patients with secondary progressive multiple sclerosis using high-resolution mass spectrometry. Metabolomics 2020, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- van Pesch, V.; Sindic, C.J.; Fernández, O. Effectiveness and safety of natalizumab in real-world clinical practice: Review of observational studies. Clin. Neurol. Neurosurg. 2016, 149, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Diouf, I.; Taylor, B.V.; Kalincik, T.; van der Mei, I. Superior effects of natalizumab versus other DMTs on patient-reported outcomes in people with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Spelman, T.; Herring, W.L.; Acosta, C.; Hyde, R.; Jokubaitis, V.G.; Pucci, E.; Lugaresi, A.; Laureys, G.; Havrdova, E.K.; Horakova, D.; et al. Comparative effectiveness and cost-effectiveness of natalizumab and fingolimod in rapidly evolving severe relapsing-remitting multiple sclerosis in the United Kingdom. J. Med. Econ. 2024, 27, 109–125. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Mezei, Z.; Bereczki, D.; Racz, L.; Csiba, L.; Csepany, T. Can a physician predict the clinical response to first-line immunomodulatory treatment in relapsing-remitting multiple sclerosis? Neuropsychiatr. Dis. Treat. 2012, 8, 465–473. [Google Scholar] [CrossRef]
- Schiavon, R.; Battaglia, P.; De, F.E.; Fasolin, A.; Biasioli, S.; Targa, L.; Guidi, G. HDL3-related decreased serum paraoxonase (PON) activity in uremic patients: Comparison with the PON1 allele polymorphism. Clin. Chim. Acta 2002, 324, 39–44. [Google Scholar] [CrossRef]
- La Du, B.N.; Eckerson, H.W. The polymorphic paraoxonase/arylesterase isozymes of human serum. Fed. Proc. 1984, 43, 2338–2341. [Google Scholar]
- Schulze, F.; Wesemann, R.; Schwedhelm, E.; Sydow, K.; Albsmeier, J.; Cooke, J.P.; Böger, R.H. Determination of asymmetric dimethylarginine (ADMA) using a novel ELISA assay. Clin. Chem. Lab. Med. 2004, 42, 1377–1383. [Google Scholar] [CrossRef]


| Disease Subtype | ||||||
|---|---|---|---|---|---|---|
| CIS | RR | PP+RP | SP | Total | p | |
| Total [N] | 10 | 208 | 19 | 25 | 262 | |
| Age (year) mean ± SD | 36.2 ± 9.3 | 36.7 ± 9.9 | 46 ± 9.6 | 50.96 ± 9.0 | 38.6 ± 10.7 | <0.0001 |
| Duration of disease (year) median | 0 | 4.5 | 4 | 14 | 5 | <0.0001 |
| EDSS (median) | 2.0 | 2.0 | 4.5 | 7.0 | 2.0 | <0.0001 |
| HDL-C (mM/L) median | 1.37 | 1.4 | 1.4 | 1.3 | 1.4 | 0.4015 |
| LDL-C (mM/L) | 2.59 | 2.88 | 2.9 | 2.92 | 2.9 | 0.9190 |
| ApoA1 (g/L) | 1.47 | 1.51 | 1.5 | 1.33 | 1.48 | 0.0775 |
| ApoB (g/L) median | 0.78 | 0.8 | 0.86 | 0.83 | 0.81 | 0.4057 |
| Lp(a) (mg/L) median | 228 | 110 | 156 | 130 | 116 | 0.3534 |
| BMI (kg/m2) median | 23 | 24 | 25 | 24 | 24 | 0.669 |
| Paraoxonase (IU/L) median | 196.3 | 74.9 | 176.5 | 99.3 | 86.6 | 0.0231 |
| Arylesterase (IU/L) median | 124.6 | 126.8 | 122.4 | 131.5 | 127.2 | 0.7728 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racz, L.; Lorincz, H.; Seres, I.; Kardos, L.; Paragh, G.; Csepany, T. Modulation of Paraoxonase 1 Activity and Asymmetric Dimethylarginine by Immunomodulatory Therapies in Multiple Sclerosis. Int. J. Mol. Sci. 2025, 26, 9728. https://doi.org/10.3390/ijms26199728
Racz L, Lorincz H, Seres I, Kardos L, Paragh G, Csepany T. Modulation of Paraoxonase 1 Activity and Asymmetric Dimethylarginine by Immunomodulatory Therapies in Multiple Sclerosis. International Journal of Molecular Sciences. 2025; 26(19):9728. https://doi.org/10.3390/ijms26199728
Chicago/Turabian StyleRacz, Lilla, Hajnalka Lorincz, Ildiko Seres, Laszlo Kardos, Gyorgy Paragh, and Tunde Csepany. 2025. "Modulation of Paraoxonase 1 Activity and Asymmetric Dimethylarginine by Immunomodulatory Therapies in Multiple Sclerosis" International Journal of Molecular Sciences 26, no. 19: 9728. https://doi.org/10.3390/ijms26199728
APA StyleRacz, L., Lorincz, H., Seres, I., Kardos, L., Paragh, G., & Csepany, T. (2025). Modulation of Paraoxonase 1 Activity and Asymmetric Dimethylarginine by Immunomodulatory Therapies in Multiple Sclerosis. International Journal of Molecular Sciences, 26(19), 9728. https://doi.org/10.3390/ijms26199728








