Chemical Constituents from the Vietnamese Mangrove Avicennia marina: Two New Iridoid Glycosides and Their Cytotoxicity Against Cancer Cell Lines
Abstract
1. Introduction
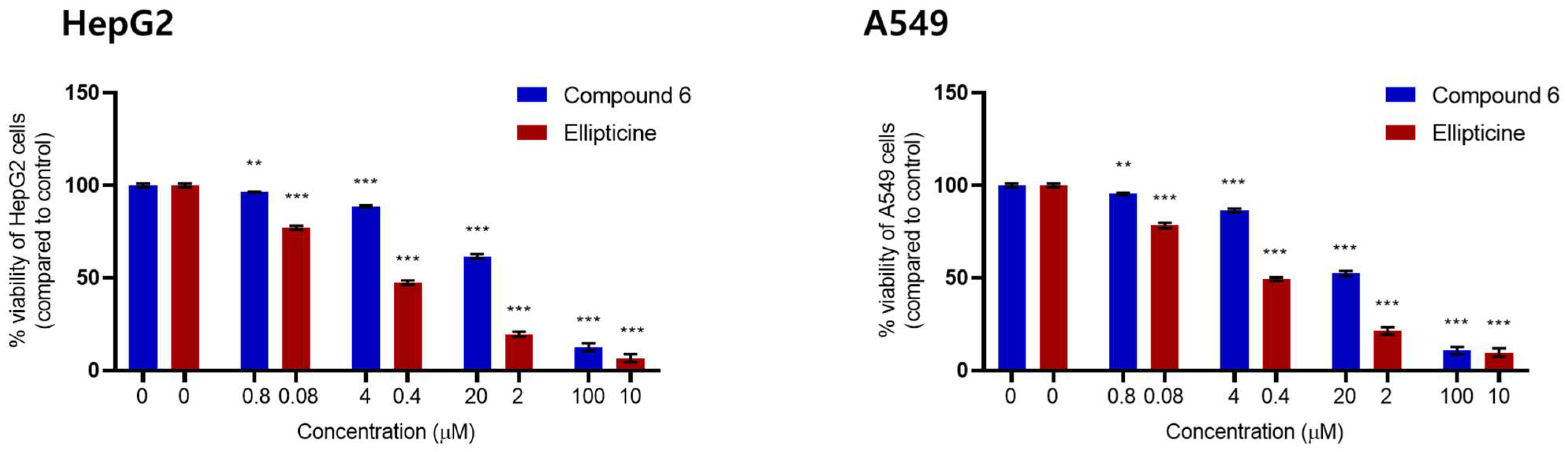
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Identification
3.3. Extraction and Isolation
3.4. Acid Hydrolysis and Identification of Absolute Configuration of Sugars
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friess, D.A.; Rogers, K.; Lovelock, C.E.; Krauss, K.W.; Hamilton, S.E.; Lee, S.Y.; Lucas, R.; Primavera, J.; Rajkaran, A.; Shi, S. The state of the world’s mangrove forests: Past, present, and future. Annu. Rev. Environ. Resour. 2019, 44, 89–115. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Simlai, A.; Roy, A. Biological activities and chemical constituents of some mangrove species from Sundarban estuary: An overview. Pharmacogn. Rev. 2013, 7, 170. [Google Scholar] [CrossRef]
- Zhou, P.; Hu, H.; Wu, X.; Feng, Z.; Li, X.; Tavakoli, S.; Wu, K.; Deng, L.; Luo, H. Botany, traditional uses, phytochemistry, pharmacological activities, and toxicity of the mangrove plant Avicennia marina: A comprehensive review. Phytochem. Rev. 2025, 1–36. [Google Scholar] [CrossRef]
- Warner, R.; Kaidonis, M.; Dun, O.; Rogers, K.; Shi, Y.; Nguyen, T.T.; Woodroffe, C.D. Opportunities and challenges for mangrove carbon sequestration in the Mekong River Delta in Vietnam. Sustain. Sci. 2016, 11, 661–677. [Google Scholar] [CrossRef]
- Khan, W.R.; Nazre, M.; Akram, S.; Anees, S.A.; Mehmood, K.; Ibrahim, F.H.; Edrus, S.S.O.A.; Latiff, A.; Fitri, Z.A.; Yaseen, M. Assessing the productivity of the matang mangrove forest reserve: Review of one of the best-managed mangrove forests. Forests 2024, 15, 747. [Google Scholar] [CrossRef]
- Le, L.B.; Hoang, T.T.; Nguyen, M.T.A.; Truong, A.T.L.; Tran, N.T.; Nguyen, T.K.; Tran, V.T.; Hoang, K.V.B.; Nguyen, B.V.; Phan, D.H. Plant composition and economic potential on the foredunes of the nearshore islands from Vietnam. Biodiversity 2025, 26, 1–11. [Google Scholar] [CrossRef]
- Vinh, L.B.; Nguyet, N.T.M.; Thanh, C.D.; Huong, T.T.; Tram, L.H.; Van Thong, N.; Minh, N.H.; Thao, N.P.; Hwang, I.; Yang, S.Y. Chemical constituents of Vietnamese mangrove Hibiscus tiliaceus with antioxidant and alpha-glucosidase inhibitory activity. Nat. Prod. Res. 2021, 35, 2899–2904. [Google Scholar] [CrossRef]
- Kado, T.; Fujimoto, A.; Giang, L.H.; Tuan, M.; Hong, P.N.; Harada, K.; Tachida, H. Genetic structures of natural populations of three mangrove species, Avicennia marina, Kandelia candel and Lumnitzera racemosa, in Vietnam revealed by maturase sequences of plastid DNA. Plant Species Biol. 2004, 19, 91–99. [Google Scholar] [CrossRef]
- ElDohaji, L.M.; Hamoda, A.M.; Hamdy, R.; Soliman, S.S. Avicennia marina a natural reservoir of phytopharmaceuticals: Curative power and platform of medicines. J. Ethnopharmacol. 2020, 263, 113179. [Google Scholar] [CrossRef] [PubMed]
- Cerri, F.; Giustra, M.; Anadol, Y.; Tomaino, G.; Galli, P.; Labra, M.; Campone, L.; Colombo, M. Natural products from mangroves: An overview of the anticancer potential of Avicennia marina. Pharmaceutics 2022, 14, 2793. [Google Scholar] [CrossRef]
- Ba Vinh, L.; Thi Minh Nguyet, N.; Young Yang, S.; Hoon Kim, J.; Thi Vien, L.; Thi Thanh Huong, P.; Van Thanh, N.; Xuan Cuong, N.; Hoai Nam, N.; Van Minh, C. A new rearranged abietane diterpene from Clerodendrum inerme with antioxidant and cytotoxic activities. Nat. Prod. Res. 2018, 32, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Vinh, L.B.; Nguyet, N.T.M.; Yang, S.Y.; Kim, J.H.; Thanh, N.V.; Cuong, N.X.; Nam, N.H.; Minh, C.V.; Hwang, I.; Kim, Y.H. Cytotoxic triterpene saponins from the mangrove Aegiceras corniculatum. Nat. Prod. Res. 2019, 33, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Van Thanh, N.; Jang, H.-J.; Vinh, L.B.; Linh, K.T.P.; Huong, P.T.T.; Cuong, N.X.; Nam, N.H.; Van Minh, C.; Kim, Y.H.; Yang, S.Y. Chemical constituents from Vietnamese mangrove Calophyllum inophyllum and their anti-inflammatory effects. Bioorganic Chem. 2019, 88, 102921. [Google Scholar] [CrossRef]
- Vinh, L.B.; Phong, N.V.; Ali, I.; Dan, G.; Koh, Y.S.; Anh, H.L.T.; Van Anh, D.T.; Yang, S.Y.; Kim, Y.H. Identification of potential anti-inflammatory and melanoma cytotoxic compounds from Aegiceras corniculatum. Med. Chem. Res. 2020, 29, 2020–2027. [Google Scholar] [CrossRef]
- Dinda, B.; Debnath, S.; Harigaya, Y.J.C. Naturally occurring iridoids. A review, part 1. Chem. Pharm. Bull. 2007, 55, 159–222. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.; Le, V.D.; Do, T.H.; Nguyen, T.L.; Nguyen, P.T.; Nguyen, T.T.; Nguyen, T.D. Anti-inflammatory constituents from Psychotria prainii H. Lév. Nat. Prod. Res. 2019, 33, 695–700. [Google Scholar] [CrossRef]
- Sun, Y.; Ouyang, J.; Deng, Z.; Li, Q.; Lin, W. Structure elucidation of five new iridoid glucosides from the leaves of Avicennia marina. Magn. Reson. Chem. 2008, 46, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Duyen, N.T.; Vinh, L.B.; Phong, N.V.; Khoi, N.M.; Ha, D.T.; Long, P.Q.; Dung, L.V.; Hien, T.T.; Dat, N.T.; Lee, K.Y. Steroid glycosides isolated from Paris polyphylla var. chinensis aerial parts and paris saponin II induces G1/S-phase MCF-7 cell cycle arrest. Carbohydr. Res. 2022, 519, 108613. [Google Scholar] [CrossRef]
- Han, Y.K.; Vinh, L.B.; Nam, M.-h.; Lee, K.Y. Identification of compounds using HPLC-QTOF-MS online antioxidant activity mapping from aerial parts of Ligularia stenocephala. Appl. Biol. Chem. 2023, 66, 53. [Google Scholar] [CrossRef]
- Giang, V.H.; Thuy, L.T.; Cham, P.T.; Vinh, L.B.; Ban, N.K.; Linh, T.M.; Mai, N.C.; Hoe, P.T.; Huong, T.T.; Dang, N.H. Chemical constituents from Lycopodiella cernua and their anti-inflammatory and cytotoxic activities. Nat. Prod. Res. 2022, 36, 4045–4051. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Vinh, L.B.; Tuan, N.Q.; Lee, H.; Kim, E.; Kim, Y.-C.; Sohn, J.H.; Yim, J.H.; Lee, H.-J.; Lee, D.-S. Macrosphelides from Antarctic fungus Pseudogymnoascus sp. (strain SF-7351) and their neuroprotective effects on BV2 and HT22 cells. Chem.-Biol. Interact. 2023, 385, 110718. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.K.; Vinh, L.B.; Lee, K.S.; Lee, M.K.; Lee, K.Y. Aurantiosides A–D: New furofuran lignan glucosides with neuroprotective activity from Osmanthus fragrans var. aurantiacus enabled via a MS/MS-based molecular networking strategy. ACS Omega 2025, 10, 41233–41246. [Google Scholar] [CrossRef] [PubMed]



| Position | δC a | δH b (mult., J in Hz) | δC a | δH b (mult., J in Hz) |
|---|---|---|---|---|
| 1 | 96.1 | 5.08 (1H, d, J = 7.2 Hz, H-1) | 94.0 | 5.14 (1H, d, J = 5.4 Hz, H-1) |
| 3 | 150.9 | 7.43 (1H, s, H-3) | 149.8 | 7.30 (1H, overlapped, H-3) |
| 4 | 111.7 | - | 112.0 | - |
| 5 | 34.9 | 3.08 (1H, m, H-5) | 31.1 | 3.00 (1H, overlapped, H-5) |
| 6 | 38.6 | 2.10 (1H, m, H-6a) 2.77 (1H, m, H-6b) | 29.5 | 1.22 (1H, m, H-6a) 2.09 (1H, m, H-6b) |
| 7 | 129.7 | 5.89 (1H, brs, H-7) | 38.8 | 1.52 (2H, m, H-7) |
| 8 | 138.2 | - | 78.4 | - |
| 9 | 45.8 | 2.71 (1H, m, H-9) | 50.4 | 1.97 (1H, m, H-9) |
| 10 | 61.8 | 4.80 (2H, s, H-10) | 24.5 | 1.16 (3H, s, H-10) |
| 11 | 168.1 | - | 167.8 | - |
| 1′ | 98.8 | 4.56 (1H, d, J = 7.8 Hz, H-1′) | 98.3 | 4.57 (1H, d, J = 8.4 Hz, H-1′) |
| 2′ | 73.3 | 3.00 (1H, m, H-2′) | 73.0 | 3.00 (1H, overlapped, H-2′) |
| 3′ | 76.6 | 3.17 (1H, m, H-3′) | 73.8 | 3.45 (1H, m, H-3′) |
| 4′ | 70.0 | 3.05 (1H, m, H-4′) | 70.1 | 3.18 (1H, m, H-4′) |
| 5′ | 77.3 | 3.14 (1H, m, H-5′) | 76.5 | 3.20 (1H, m, H-5′) |
| 6′ | 61.2 | 3.40 (1H, m, H-6′a) 3.65 (1H, m, H-6′b) | 63.1 | 4.22 (1H, m, H-6′a) 4.40 (1H, m, H-6′b) |
| 1″ | 125.6 | - | 125.5 | - |
| 2″ | 111.2 | 7.32 (1H, d, J = 1.8 Hz, H-2″) | 111.0 | 7.30 (1H, d, J = 1.8 Hz, H-2″) |
| 3″ | 149.4 | - | 149.3 | - |
| 4″ | 147.9 | - | 147.9 | - |
| 5″ | 115.5 | 6.79 (1H, d, J = 7.8 Hz, H-5″) | 115.4 | 6.78 (1H, d, J = 7.8 Hz, H-5″) |
| 6″ | 123.2 | 7.12 (1H, dd, J = 1.8, 7.8 Hz, H-2″) | 123.2 | 7.11 (1H, dd, J = 1.8, 7.8 Hz, H-6″) |
| 7″ | 145.2 | 7.56 (1H, d, J = 16.2 Hz, H-7″) | 145.1 | 7.56 (1H, d, J = 16.2 Hz, H-7″) |
| 8″ | 114.3 | 6.48 (1H, d, J = 16.2 Hz, H-8″) | 114.3 | 6.47 (1H, d, J = 16.2 Hz, H-8″) |
| 9″ | 166.3 | - | 166.5 | - |
| 4″- OCH3 | 55.7 | 3.82 (3H, s, 4″-OCH3) | 55.7 | 3.81 (3H, s, 4″-OCH3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hieu, N.V.; Vinh, L.B.; Mai, P.T.; Hung, L.N.; Dat, N.T.; Phuong, L.H.; Anh, T.P.; Tuan, D.T.; Phong, N.V.; Hien, T.T.T.; et al. Chemical Constituents from the Vietnamese Mangrove Avicennia marina: Two New Iridoid Glycosides and Their Cytotoxicity Against Cancer Cell Lines. Int. J. Mol. Sci. 2025, 26, 9694. https://doi.org/10.3390/ijms26199694
Hieu NV, Vinh LB, Mai PT, Hung LN, Dat NT, Phuong LH, Anh TP, Tuan DT, Phong NV, Hien TTT, et al. Chemical Constituents from the Vietnamese Mangrove Avicennia marina: Two New Iridoid Glycosides and Their Cytotoxicity Against Cancer Cell Lines. International Journal of Molecular Sciences. 2025; 26(19):9694. https://doi.org/10.3390/ijms26199694
Chicago/Turabian StyleHieu, Ngo Van, Le Ba Vinh, Pham Thi Mai, Le Ngoc Hung, Nguyen Tien Dat, Lai Ha Phuong, Tran Phương Anh, Do Thanh Tuan, Nguyen Viet Phong, Truong Thi Thu Hien, and et al. 2025. "Chemical Constituents from the Vietnamese Mangrove Avicennia marina: Two New Iridoid Glycosides and Their Cytotoxicity Against Cancer Cell Lines" International Journal of Molecular Sciences 26, no. 19: 9694. https://doi.org/10.3390/ijms26199694
APA StyleHieu, N. V., Vinh, L. B., Mai, P. T., Hung, L. N., Dat, N. T., Phuong, L. H., Anh, T. P., Tuan, D. T., Phong, N. V., Hien, T. T. T., & Tuan Anh, H. L. (2025). Chemical Constituents from the Vietnamese Mangrove Avicennia marina: Two New Iridoid Glycosides and Their Cytotoxicity Against Cancer Cell Lines. International Journal of Molecular Sciences, 26(19), 9694. https://doi.org/10.3390/ijms26199694






