Metabolic Regulation of Ferroptosis in Breast Cancer
Abstract
1. Introduction
2. Iron Metabolism: The Catalytic Foundation
2.1. Iron Uptake and Regulation
2.2. Mitochondrial Iron Dynamics
2.3. Iron Storage and Release
3. Glutathione Metabolism and Amino Acid Regulation
3.1. The Central GPX4-GSH Axis
3.2. Cysteine Transport and Synthesis
3.3. Alternative Cysteine Actions
3.4. Nucleotide Metabolism Connections
4. Lipid Metabolism: The Primary Battleground
4.1. Polyunsaturated Fatty Acid (PUFA) Synthesis and Incorporation
4.2. ACSL4: The Critical Enzyme
4.3. Lipid Peroxidation Mechanisms
4.4. Monounsaturated vs. Polyunsaturated Fatty Acids
4.5. Lipid Availability and Trafficking
5. Breast Cancer-Specific Metabolic Reprogramming
5.1. Breast Tumor Microenvironment Factors
5.2. Breast Cancer Subtype Variations
5.3. Oncogene and Tumor Suppressor Influences
6. Additional Metabolic Pathways
6.1. Glucose Metabolism Connections
6.2. Selenium and Antioxidant Systems
7. Future Directions and Challenges
7.1. Multi-Omics Integration and Tumor Organoids
7.2. Immunotherapy Synergy
7.3. Photodynamic-Ferroptosis Synergy Treatment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Arachidonic Acid |
| ACSL3 | Acyl-CoA Synthetase Long-Chain Family Member 3 |
| ACSL4 | Acyl-CoA Synthetase Long-Chain Family Member 4 |
| AdA | Adrenic Acid |
| AGPS | Alkylglycerone Phosphate Synthase |
| ALOX15 | Arachidonate 15-Lipoxygenase |
| ATGL | Adipose Triglyceride Lipase |
| BH4 | Tetrahydrobiopterin |
| CAF | Cancer-Associated Fibroblast |
| CBS | Cystathionine Beta-Synthase |
| CISD1 | CDGSH Iron Sulfur Domain 1 |
| CTH | Cystathionine Gamma-Lyase |
| CTNS | Cystinosin |
| DGLA | Dihomo-Gamma-Linolenic Acid |
| DLD | Dihydrolipoamide Dehydrogenase |
| DMT1 | Divalent Metal Transporter 1 |
| ER | Estrogen Receptor |
| FADS1 | Fatty Acid Desaturase 1 |
| FADS2 | Fatty Acid Desaturase 2 |
| FASN | Fatty Acid Synthase |
| FERscore | Ferroptosis Score |
| FSP1 | Ferroptosis Suppressor Protein 1 |
| FTH1 | Ferritin Heavy Chain 1 |
| G6PD | Glucose-6-Phosphate Dehydrogenase |
| GCH1 | GTP Cyclohydrolase 1 |
| GGT1 | Gamma-Glutamyltransferase 1 |
| GLA | Gamma-Linolenic Acid |
| GLS2 | Glutaminase 2 |
| GPX4 | Glutathione Peroxidase 4 |
| GSH | Glutathione |
| HERC2 | HECT Domain and RCC1-Like Domain-Containing Protein 2 |
| HIF1α | Hypoxia-Inducible Factor 1-Alpha |
| HK2 | Hexokinase 2 |
| HSL | Hormone-Sensitive Lipase |
| ICD | Immunogenic Cell Death |
| IFNγ | Interferon-Gamma |
| IKE | Imidazole Ketone Erastin |
| IL-6 | Interleukin-6 |
| KGDH | Alpha-Ketoglutarate Dehydrogenase |
| LA | Linoleic Acid |
| LAR | Luminal Androgen Receptor |
| LIP | Labile Iron Pool |
| LPCAT | Lysophosphatidylcholine Acyltransferase |
| MBOAT | Membrane-Bound O-Acyltransferase |
| MGL | Monoglyceride Lipase |
| MFSD12 | Major Facilitator Superfamily Domain-Containing Protein 12 |
| MUFA | Monounsaturated Fatty Acid |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NCOA4 | Nuclear Receptor Coactivator 4 |
| NRF2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| OxPE | Oxidized Phosphatidylethanolamine |
| PC | Phosphatidylcholine |
| PDK4 | Pyruvate Dehydrogenase Kinase 4 |
| PD-L1 | Programmed Death-Ligand 1 |
| PE | Phosphatidylethanolamine |
| PKA | Protein Kinase A |
| PKM2 | Pyruvate Kinase M2 |
| PLA2 | Phospholipase A2 |
| PPP | Pentose Phosphate Pathway |
| PRMT5 | Protein Arginine Methyltransferase 5 |
| PUFA | Polyunsaturated Fatty Acid |
| RNR | Ribonucleotide Reductase |
| ROS | Reactive Oxygen Species |
| SAT1 | Spermidine/Spermine N1-Acetyltransferase 1 |
| SCD | Stearoyl-CoA Desaturase |
| SREBP1 | Sterol Regulatory Element-Binding Protein 1 |
| STEAP3 | Six-Transmembrane Epithelial Antigen of Prostate 3 |
| TAM | Tumor-Associated Macrophage |
| TCA | Tricarboxylic Acid |
| TF | Transferrin |
| TFR1 | Transferrin Receptor 1 |
| TNBC | Triple-Negative Breast Cancer |
| TOS | D-α-Tocopherol Succinate |
| TrxR1 | Thioredoxin Reductase 1 |
| UBIAD1 | UbiA Prenyltransferase Domain-Containing Protein 1 |
| VDAC3 | Voltage-Dependent Anion Channel 3 |
| xCT | Cystine/Glutamate Antiporter (SLC7A11) |
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Singh, M.; Mugler, K.; Hailoo, D.W.; Burke, S.; Nemesure, B.; Torkko, K.; Shroyer, K.R. Differential expression of transferrin receptor (TfR) in a spectrum of normal to malignant breast tissues: Implications for in situ and invasive carcinoma. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 417–423. [Google Scholar] [CrossRef]
- Habashy, H.O.; Powe, D.G.; Staka, C.M.; Rakha, E.A.; Ball, G.; Green, A.R.; Aleskandarany, M.; Paish, E.C.; Douglas Macmillan, R.; Nicholson, R.I.; et al. Transferrin Receptor (CD71) is a Marker of Poor Prognosis in Breast Cancer and can Predict Response to Tamoxifen. Breast Cancer Res. Treat. 2010, 119, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, P.; Duan, X.; Cheng, M.; Xu, L.X. Deferoxamine-Induced High Expression of TfR1 and DMT1 Enhanced Iron Uptake in Triple-Negative Breast Cancer Cells by Activating IL-6/PI3K/AKT Pathway. Onco Targets Ther. 2019, 12, 4359–4377. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.P.; Elliott, R.L.; Head, J.F. Manipulation of Iron Transporter Genes Results in the Suppression of Human and Mouse Mammary Adenocarcinomas. Anticancer Res. 2010, 30, 759–765. [Google Scholar]
- Li, J.; He, K.; Liu, P.; Xu, L.X. Iron Participated in Breast Cancer Chemoresistance by Reinforcing IL-6 Paracrine Loop. Biochem. Biophys. Res. Commun. 2016, 475, 154–160. [Google Scholar] [CrossRef]
- Tubbesing, K.; Khoo, T.C.; Bahreini Jangjoo, S.; Sharikova, A.; Barroso, M.; Khmaladze, A. Iron-Binding Cellular Profile of Transferrin using Label-Free Raman Hyperspectral Imaging and Singular Value Decomposition (SVD). Free Radic. Biol. Med. 2021, 169, 416–424. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Sheng, X.; Zhou, W.; Sun, A.; Dai, H. Mitochondria-Related Signaling Pathways Involved in Breast Cancer Regulate Ferroptosis. Genes Dis. 2023, 11, 358–366. [Google Scholar] [CrossRef]
- Battaglia, A.M.; Chirillo, R.; Aversa, I.; Sacco, A.; Costanzo, F.; Biamonte, F. Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells 2020, 9, 1505. [Google Scholar] [CrossRef]
- Gan, H.; Huang, X.; Luo, X.; Li, J.; Mo, B.; Cheng, L.; Shu, Q.; Du, Z.; Tang, H.; Sun, W.; et al. A Mitochondria-Targeted Ferroptosis Inducer Activated by Glutathione-Responsive Imaging and Depletion for Triple Negative Breast Cancer Theranostics. Adv. Healthc. Mater. 2023, 12, 2300220. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. CISD1 Inhibits Ferroptosis by Protection Against Mitochondrial Lipid Peroxidation. Biochem. Biophys. Res. Commun. 2016, 478, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an Autophagic Cell Death Process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Mancias, J.D.; Pontano Vaites, L.; Nissim, S.; Biancur, D.E.; Kim, A.J.; Wang, X.; Liu, Y.; Goessling, W.; Kimmelman, A.C.; Harper, J.W. Ferritinophagy Via NCOA4 is Required for Erythropoiesis and is Regulated by Iron Dependent HERC2-Mediated Proteolysis. eLife 2015, 4, e10308. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, J.; Deng, H.; Yang, J.; Xu, R.; Wang, Z.; Shen, Q.; Fang, T. pH-Sensitive Molecular-Switch-Containing Polymer Nanoparticle for Breast Cancer Therapy with Ferritinophagy-Cascade Ferroptosis and Tumor Immune Activation. Adv. Healthc. Mater. 2021, 10, 2100683. [Google Scholar] [CrossRef]
- Cañeque, T.; Baron, L.; Müller, S.; Carmona, A.; Colombeau, L.; Versini, A.; Solier, S.; Gaillet, C.; Sindikubwabo, F.; Sampaio, J.L.; et al. Activation of Lysosomal Iron Triggers Ferroptosis in Cancer. Nature 2025, 642, 492–500. [Google Scholar] [CrossRef]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, 1800311. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Liu, Z.-Y.; Wang, M.-S.; Guo, Y.-X.; Wang, X.-K.; Luo, K.; Huang, S.; Li, R.-F. Mechanisms and Regulations of Ferroptosis. Front. Immunol. 2023, 14, 1269451. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Lee, N.; Carlisle, A.E.; Peppers, A.; Park, S.J.; Doshi, M.B.; Spears, M.E.; Kim, D. xCT-Driven Expression of GPX4 Determines Sensitivity of Breast Cancer Cells to Ferroptosis Inducers. Antioxidants 2021, 10, 317. [Google Scholar] [CrossRef]
- Hasegawa, M.; Takahashi, H.; Rajabi, H.; Alam, M.; Suzuki, Y.; Yin, L.; Tagde, A.; Maeda, T.; Hiraki, M.; Sukhatme, V.P.; et al. Functional Interactions of the Cystine/Glutamate Antiporter, CD44v and MUC1-C Oncoprotein in Triple-Negative Breast Cancer Cells. Oncotarget 2016, 7, 11756–11769. [Google Scholar] [CrossRef]
- Li, F.; Long, H.; Zhou, Z.; Luo, H.; Xu, S.; Gao, L. System Xc −/GSH/GPX4 Axis: An Important Antioxidant System for the Ferroptosis in Drug-Resistant Solid Tumor Therapy. Front. Pharmacol. 2022, 13, 910292. [Google Scholar] [CrossRef] [PubMed]
- Jyotsana, N.; Ta, K.T.; DelGiorno, K.E. The role of cystine/glutamate antiporter SLC7A11/xCT in the pathophysiology of cancer. Front. Oncol. 2022, 12, 858462. [Google Scholar] [CrossRef] [PubMed]
- Azizi, R.; Ahmed, H.H.; Abdul Kareem, R.; Taher Waam, W.M.; Alwan, M.; Jawad, M.J.; Hamad, A.K.; Darzi, S. SLC7A11 Inhibitors Represent a Promising Therapeutic Target by Facilitating the Induction of Ferroptosis in Breast Cancer. Int. J. Mol. Cell. Med. 2025, 14, 496–516. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, P.; Wang, J.; Zhang, J.; Ma, Q.; Jiang, Y.; Wu, Y.; Han, T.; Xiang, D. HLF Regulates Ferroptosis, Development and Chemoresistance of Triple-Negative Breast Cancer by Activating Tumor Cell-Macrophage Crosstalk. J. Hematol. Oncol. 2022, 15, 2. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Y.; Xu, Y.; Liu, X.; Kang, X.; Zhu, J.; Long, S.; Han, Y.; Xue, C.; Sun, Z.; et al. SLC7A11 Protects Luminal A Breast Cancer Cells Against Ferroptosis Induced by CDK4/6 Inhibitors. Redox Biol. 2024, 76, 103304. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine Transporter SLC7A11/xCT in Cancer: Ferroptosis, Nutrient Dependency, and Cancer Therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.; Hsu, C.; Yin, P.; Yeh, T.; Lee, H.; Tseng, L. CHAC1 Degradation of Glutathione Enhances Cystine-Starvation-Induced Necroptosis and Ferroptosis in Human Triple Negative Breast Cancer Cells Via the GCN2-eIF2α-ATF4 Pathway. Oncotarget 2017, 8, 114588–114602. [Google Scholar] [CrossRef]
- Erdélyi, K.; Ditrói, T.; Johansson, H.J.; Czikora, Á.; Balog, N.; Silwal-Pandit, L.; Ida, T.; Olasz, J.; Hajdú, D.; Mátrai, Z.; et al. Reprogrammed Transsulfuration Promotes Basal-Like Breast Tumor Progression Via Realigning Cellular Cysteine Persulfidation. Proc. Natl. Acad. Sci. USA 2021, 118, e2100050118. [Google Scholar] [CrossRef]
- Xue, W.; Yu, Y.; Yao, Y.; Zhou, L.; Huang, Y.; Wang, Y.; Chen, Z.; Wang, L.; Li, X.; Wang, X.; et al. Breast Cancer Cells have an Increased Ferroptosis Risk Induced by System X(C)(-) Blockade After Deliberately Downregulating CYTL1 to Mediate Malignancy. Redox Biol. 2024, 70, 103034. [Google Scholar] [CrossRef]
- Swanda, R.V.; Ji, Q.; Wu, X.; Yan, J.; Dong, L.; Mao, Y.; Uematsu, S.; Dong, Y.; Qian, S. Lysosomal Cystine Governs Ferroptosis Sensitivity in Cancer Via Cysteine Stress Response. Mol. Cell 2023, 83, 3347–3359.e9. [Google Scholar] [CrossRef]
- He, L.; Chen, J.; Deng, P.; Huang, S.; Liu, P.; Wang, C.; Huang, X.; Li, Y.; Chen, B.; Shi, D.; et al. Lysosomal Cyst(E)Ine Storage Potentiates Tolerance to Oxidative Stress in Cancer Cells. Mol. Cell 2023, 83, 3502–3519.e11. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Xing, X.; Zhang, W.; Wang, Y.; Jin, X.; Tian, M.; Ba, X.; Hao, F. Cysteine and Homocysteine can be Exploited by GPX4 in Ferroptosis Inhibition Independent of GSH Synthesis. Redox Biol. 2023, 69, 102999. [Google Scholar] [CrossRef] [PubMed]
- Daher, B.; Vučetić, M.; Pouysségur, J. Cysteine Depletion, a Key Action to Challenge Cancer Cells to Ferroptotic Cell Death. Front. Oncol. 2020, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a Glutathione-Independent Ferroptosis Suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ Oxidoreductase FSP1 Acts Parallel to GPX4 to Inhibit Ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C.; DelGiorno, K.E.; Lee, H.; Purohit, V.; Sagalovskiy, I.R.; Ma, A.; Kapilian, J.; Firl, C.E.M.; et al. Cysteine Depletion Induces Pancreatic Tumor Ferroptosis in Mice. Science 2020, 368, 85–89. [Google Scholar] [CrossRef]
- Tarangelo, A.; Rodencal, J.; Kim, J.T.; Magtanong, L.; Long, J.Z.; Dixon, S.J. Nucleotide Biosynthesis Links Glutathione Metabolism to Ferroptosis Sensitivity. Life. Sci. Alliance 2022, 5, e202101157. [Google Scholar] [CrossRef]
- Tarangelo, A.; Magtanong, L.; Bieging-Rolett, K.T.; Li, Y.; Ye, J.; Attardi, L.D.; Dixon, S.J. p53 Suppresses Metabolic Stress-Induced Ferroptosis in Cancer Cells. Cell Rep. 2018, 22, 569–575. [Google Scholar] [CrossRef]
- Allen, A.E.; Sun, Y.; Wei, F.; Reid, M.A.; Locasale, J.W. Nucleotide Metabolism is Linked to Cysteine Availability. J. Biol. Chem. 2023, 299, 103039. [Google Scholar] [CrossRef]
- Zhang, Y.; Swanda, R.V.; Nie, L.; Liu, X.; Wang, C.; Lee, H.; Lei, G.; Mao, C.; Koppula, P.; Cheng, W.; et al. mTORC1 Couples Cyst(E)Ine Availability with GPX4 Protein Synthesis and Ferroptosis Regulation. Nat. Commun. 2021, 12, 1589. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, Y.; Jiang, Y.; Zhang, L.; Cheng, W. GPX4: The Hub of Lipid Oxidation, Ferroptosis, Disease and Treatment. Biochim. Biophys. Acta-Rev. Cancer 2023, 1878, 188890. [Google Scholar] [CrossRef] [PubMed]
- Beatty, A.; Fink, L.; Singh, T.; Strigun, A.; Peter, E.; Ferrer, C.; Nicolas, E.; Cai, K.; Moran, T.; Reginato, M.; et al. Metabolite Profiling Reveals the Glutathione Biosynthetic Pathway as a Therapeutic Target in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2017, 17, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Combs, J.A.; DeNicola, G.M. The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival. Cancers 2019, 11, 678. [Google Scholar] [CrossRef]
- Chen, R.; Wen, L.; Guo, F.; He, J.; Wong, K.H.; Chen, M. Glutathione-Scavenging Natural-Derived Ferroptotic Nano-Amplifiers Strengthen Tumor Therapy through Aggravating Iron Overload and Lipid Peroxidation. J. Control. Release 2025, 379, 866–878. [Google Scholar] [CrossRef]
- Ryu, C.S.; Kwak, H.C.; Lee, K.S.; Kang, K.W.; Oh, S.J.; Lee, K.H.; Kim, H.M.; Ma, J.Y.; Kim, S.K. Sulfur Amino Acid Metabolism in Doxorubicin-Resistant Breast Cancer Cells. Toxicol. Appl. Pharmacol. 2011, 255, 94–102. [Google Scholar] [CrossRef]
- Grammatikos, S.I.; Subbaiah, P.V.; Victor, T.A.; Miller, W.M. N-3 and N-6 Fatty Acid Processing and Growth Effects in Neoplastic and Non-Cancerous Human Mammary Epithelial Cell Lines. Br. J. Cancer 1994, 70, 219–227. [Google Scholar] [CrossRef]
- González-Bengtsson, A.; Asadi, A.; Gao, H.; Dahlman-Wright, K.; Jacobsson, A. Estrogen Enhances the Expression of the Polyunsaturated Fatty Acid Elongase Elovl2 Via ERα in Breast Cancer Cells. PLoS ONE 2016, 11, e0164241. [Google Scholar] [CrossRef]
- Hilvo, M.; Denkert, C.; Lehtinen, L.; Müller, B.; Brockmöller, S.; Seppänen-Laakso, T.; Budczies, J.; Bucher, E.; Yetukuri, L.; Castillo, S.; et al. Novel Theranostic Opportunities Offered by Characterization of Altered Membrane Lipid Metabolism in Breast Cancer Progression. Cancer Res. 2011, 71, 3236–3245. [Google Scholar] [CrossRef]
- Mukerjee, S.; Saeedan, A.S.; Ansari, M.N.; Singh, M. Polyunsaturated Fatty Acids Mediated Regulation of Membrane Biochemistry and Tumor Cell Membrane Integrity. Membranes 2021, 11, 479. [Google Scholar] [CrossRef]
- Azrad, M.; Turgeon, C.; Demark-Wahnefried, W. Current Evidence Linking Polyunsaturated Fatty Acids with Cancer Risk and Progression. Front. Oncol. 2013, 3, 224. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.F.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized Arachidonic and Adrenic PEs Navigate Cells to Ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, C.; Wen, X.; Li, C.; Xiong, S.; Yue, T.; Long, P.; Shi, J.; Zhang, Z. ACSL4 as a Potential Target and Biomarker for Anticancer: From Molecular Mechanisms to Clinical Therapeutics. Front. Pharmacol. 2022, 13, 949863. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Bode, A.M.; Luo, X. ACSL Family: The Regulatory Mechanisms and Therapeutic Implications in Cancer. Eur. J. Pharmacol. 2021, 909, 174397. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. Identification of ACSL4 as a Biomarker and Contributor of Ferroptosis. Biochem. Biophys. Res. Commun. 2016, 478, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, Y.; Li, Y.; Chen, X.; Du, P.; Wang, Z.; Tian, A.; Zhao, Y. Reversing Ferroptosis Resistance in Breast Cancer Via Tailored Lipid and Iron Presentation. ACS Nano 2023, 17, 25257–25268. [Google Scholar] [CrossRef]
- Shintoku, R.; Takigawa, Y.; Yamada, K.; Kubota, C.; Yoshimoto, Y.; Takeuchi, T.; Koshiishi, I.; Torii, S. Lipoxygenase-Mediated Generation of Lipid Peroxides Enhances Ferroptosis Induced by Erastin and RSL3. Cancer Sci. 2017, 108, 2187–2194. [Google Scholar] [CrossRef]
- Imam, M.; Ji, J.; Zhang, Z.; Yan, S. Targeting the Initiator to Activate both Ferroptosis and Cuproptosis for Breast Cancer Treatment: Progress and Possibility for Clinical Application. Front. Pharmacol. 2025, 15, 1493188. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Chen, C.; Zhou, Y.; Hu, D.; Yang, J.; Chen, Y.; Zhuo, W.; Mao, M.; Zhang, X.; et al. Targeting Ferroptosis in Breast Cancer. Biomark. Res. 2020, 8, 58. [Google Scholar] [CrossRef]
- Li, J.; He, D.; Li, S.; Xiao, J.; Zhu, Z. Ferroptosis: The Emerging Player in Remodeling Triple-Negative Breast Cancer. Front. Immunol. 2023, 14, 1284057. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Pittalà, V.; Greish, K.; D’Amico, A.G.; Romeo, G.; Intagliata, S.; Salerno, L.; Vanella, L. Heme Oxygenase Modulation Drives Ferroptosis in TNBC Cells. Int. J. Mol. Sci. 2022, 23, 5709. [Google Scholar] [CrossRef]
- Sen, U.; Coleman, C.; Sen, T. Stearoyl Coenzyme A Desaturase-1: Multitasker in Cancer, Metabolism, and Ferroptosis. Trends Cancer 2023, 9, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, T.; Shokry, E.; Deshmukh, R.; Anand, J.; Galbraith, L.C.A.; Mitchell, L.; Rodriguez-Blanco, G.; Villar, V.H.; Sterken, B.A.; Nixon, C.; et al. Breast Cancer Secretes Anti-Ferroptotic MUFAs and Depends on Selenoprotein Synthesis for Metastasis. EMBO Mol. Med. 2024, 16, 2749–2774. [Google Scholar] [CrossRef] [PubMed]
- Magtanong, L.; Ko, P.; To, M.; Cao, J.Y.; Forcina, G.C.; Tarangelo, A.; Ward, C.C.; Cho, K.; Patti, G.J.; Nomura, D.K.; et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem. Biol. 2019, 26, 420–432.e9. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Feng, Y.; Zandkarimi, F.; Wang, H.; Zhang, Z.; Kim, J.; Cai, Y.; Gu, W.; Stockwell, B.R.; Jiang, X. Ferroptosis Surveillance Independent of GPX4 and Differentially Regulated by Sex Hormones. Cell 2023, 186, 2748–2764.e22. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, T.; Wang, X.; Xiong, F.; Hu, Z.; Qiao, X.; Yuan, X.; Wang, D. ACSL3 and ACSL4, Distinct Roles in Ferroptosis and Cancers. Cancers 2022, 14, 5896. [Google Scholar] [CrossRef]
- Lorito, N.; Subbiani, A.; Smiriglia, A.; Bacci, M.; Bonechi, F.; Tronci, L.; Romano, E.; Corrado, A.; Longo, D.L.; Iozzo, M.; et al. FADS1/2 Control Lipid Metabolism and Ferroptosis Susceptibility in Triple-Negative Breast Cancer. EMBO Mol. Med. 2024, 16, 1533–1559. [Google Scholar] [CrossRef]
- Danielli, M.; Perne, L.; Jarc Jovičić, E.; Petan, T. Lipid Droplets and Polyunsaturated Fatty Acid Trafficking: Balancing Life and Death. Front. Cell Dev. Biol. 2023, 11, 1104725, Erratum in Front. Cell Dev. Biol. 2023, 22, 1175493. [Google Scholar] [CrossRef]
- Xie, H.; Heier, C.; Kien, B.; Vesely, P.W.; Tang, Z.; Sexl, V.; Schoiswohl, G.; Strießnig-Bina, I.; Hoefler, G.; Zechner, R.; et al. Adipose Triglyceride Lipase Activity Regulates Cancer Cell Proliferation Via AMP-Kinase and mTOR Signaling. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158737. [Google Scholar] [CrossRef]
- Lee, J.H.; Kong, J.; Jang, J.Y.; Han, J.S.; Ji, Y.; Lee, J.; Kim, J.B. Lipid Droplet Protein LID-1 Mediates ATGL-1-Dependent Lipolysis during Fasting in Caenorhabditis Elegans. Mol. Cell. Biol. 2014, 34, 4165–4176. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Olzmann, J.A. The Cell Biology of Ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, J.; Oh, M.; Lee, E. An Integrated View of Lipid Metabolism in Ferroptosis Revisited Via Lipidomic Analysis. Exp. Mol. Med. 2023, 55, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, Y.; Zheng, H.; Li, M.; Liu, Y.; Feng, R.; Li, X.; Zhang, S.; Tang, M.; Yang, M.; et al. LPCAT1-Mediated Membrane Phospholipid Remodelling Promotes Ferroptosis Evasion and Tumour Growth. Nat. Cell Biol. 2024, 26, 811–824. [Google Scholar] [CrossRef]
- Wan, M.; Pan, S.; Shan, B.; Diao, H.; Jin, H.; Wang, Z.; Wang, W.; Han, S.; Liu, W.; He, J.; et al. Lipid Metabolic Reprograming: The Unsung Hero in Breast Cancer Progression and Tumor Microenvironment. Mol. Cancer 2025, 24, 61. [Google Scholar] [CrossRef]
- Li, D.; Li, Y. The Interaction between Ferroptosis and Lipid Metabolism in Cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar] [CrossRef]
- Lange, M.; Olzmann, J.A. Ending on a Sour Note: Lipids Orchestrate Ferroptosis in Cancer. Cell Metab. 2021, 33, 1507–1509. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and Functions of Lipid Droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, C.; Dai, R.; Zeng, Y. Ferroptosis-Mediated Crosstalk in the Tumor Microenvironment Implicated in Cancer Progression and Therapy. Front. Cell Dev. Biol. 2021, 9, 739392. [Google Scholar] [CrossRef]
- Blanchette-Farra, N.; Kita, D.; Konstorum, A.; Tesfay, L.; Lemler, D.; Hegde, P.; Claffey, K.P.; Torti, F.M.; Torti, S.V. Contribution of Three-Dimensional Architecture and Tumor-Associated Fibroblasts to Hepcidin Regulation in Breast Cancer. Oncogene 2018, 37, 4013–4032. [Google Scholar] [CrossRef]
- Zhu, Y.; Fang, S.; Fan, B.; Xu, K.; Xu, L.; Wang, L.; Zhu, L.; Chen, C.; Wu, R.; Ni, J.; et al. Cancer-Associated Fibroblasts Reprogram Cysteine Metabolism to Increase Tumor Resistance to Ferroptosis in Pancreatic Cancer. Theranostics 2024, 14, 1683–1700. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, L.; Li, X.; Hu, Y.; Luo, N. Cancer-Associated Fibroblasts Promote Doxorubicin Resistance in Triple-Negative Breast Cancer through Enhancing ZFP64 Histone Lactylation to Regulate Ferroptosis. J. Transl. Med. 2025, 23, 247. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, B.; Zhao, Y.; Tao, Z.; Wang, Y.; Chen, G.; Hu, X. Mammary Adipocytes Protect Triple-Negative Breast Cancer Cells from Ferroptosis. J. Hematol. Oncol. 2022, 15, 72. [Google Scholar] [CrossRef]
- Balaban, S.; Shearer, R.F.; Lee, L.S.; van Geldermalsen, M.; Schreuder, M.; Shtein, H.C.; Cairns, R.; Thomas, K.C.; Fazakerley, D.J.; Grewal, T.; et al. Adipocyte Lipolysis Links Obesity to Breast Cancer Growth: Adipocyte-Derived Fatty Acids Drive Breast Cancer Cell Proliferation and Migration. Cancer Metab. 2017, 5, 1. [Google Scholar] [CrossRef]
- Urbanelli, L.; Buratta, S.; Logozzi, M.; Mitro, N.; Sagini, K.; Raimo, R.D.; Caruso, D.; Fais, S.; Emiliani, C. Lipidomic Analysis of Cancer Cells Cultivated at Acidic pH Reveals Phospholipid Fatty Acids Remodelling Associated with Transcriptional Reprogramming. J. Enzyme Inhib. Med. Chem. 2020, 35, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Deng, T.; Zhang, H.; Zuo, D.; Zhu, Q.; Bai, M.; Liu, R.; Ning, T.; Zhang, L.; Yu, Z.; et al. Adipocyte-Derived Exosomal MTTP Suppresses Ferroptosis and Promotes Chemoresistance in Colorectal Cancer. Adv. Sci. 2022, 9, e2203357. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Bai, X.; Guo, L.; Tuoheti, K.; Zhan, S.; Liu, T. M1 Macrophage Inhibits Ferroptosis in Pseudomonas Aeruginosa-Induced Kidney Epithelial Cell Injury through the iNOS/ NO Pathway without Thiol. Front. Cell Dev. Biol. 2025, 13, 1597160. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Liao, C.; Zhou, H.; Wu, Y.; Zhang, W. Impact of Ferroptosis-Related Risk Genes on Macrophage M1/M2 Polarization and Prognosis in Glioblastoma. Front. Cell. Neurosci. 2024, 17, 1294029. [Google Scholar] [CrossRef]
- Gan, B. ACSL4, PUFA, and Ferroptosis: New Arsenal in Anti-Tumor Immunity. Signal Transduct. Target. Ther. 2022, 7, 128. [Google Scholar] [CrossRef]
- Liao, P.; Wang, W.; Wang, W.; Kryczek, I.; Li, X.; Bian, Y.; Sell, A.; Wei, S.; Grove, S.; Johnson, J.K.; et al. CD8(+) T Cells and Fatty Acids Orchestrate Tumor Ferroptosis and Immunity Via ACSL4. Cancer Cell 2022, 40, 365–378.e6. [Google Scholar] [CrossRef]
- Brown, C.W.; Amante, J.J.; Goel, H.L.; Mercurio, A.M. The α6β4 Integrin Promotes Resistance to Ferroptosis. J. Cell Biol. 2017, 216, 4287–4297. [Google Scholar] [CrossRef]
- Brown, C.W.; Amante, J.J.; Mercurio, A.M. Cell Clustering Mediated by the Adhesion Protein PVRL4 is Necessary for α6β4 Integrin-Promoted Ferroptosis Resistance in Matrix-Detached Cells. J. Biol. Chem. 2018, 293, 12741–12748. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, F.; Xiao, Y.; Jiang, Y.; Shao, Z. Conceptualizing the Complexity of Ferroptosis to Treat Triple-Negative Breast Cancer: Theory-to-Practice. Cancer Biol. Med. 2023, 20, 98–103. [Google Scholar] [CrossRef]
- Tan, L.; Liu, J.; Ma, C.; Huang, S.; He, F.; Long, Y.; Zheng, Z.; Liang, J.; Xu, N.; Wang, G.; et al. Iron-Dependent Cell Death: Exploring Ferroptosis as a Unique Target in Triple-Negative Breast Cancer Management. Cancer Manag. Res. 2025, 17, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, X.; Liu, Z.; Yu, Z. Role of GPX4-Mediated Ferroptosis in the Sensitivity of Triple Negative Breast Cancer Cells to Gefitinib. Front. Oncol. 2020, 10, 597434. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, Y.; Ding, J.; Jin, X.; Ma, D.; Li, D.; Shi, J.; Huang, W.; Wang, Y.; Jiang, Y.; et al. Ferroptosis Heterogeneity in Triple-Negative Breast Cancer Reveals an Innovative Immunotherapy Combination Strategy. Cell Metab. 2023, 35, 84–100.e8. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Qiu, J.; Hu, Y.; Wang, Y.; Yu, C.; Wu, Y. Efficacy of FERscore in Predicting Sensitivity to Ferroptosis Inducers in Breast Cancer. NPJ Breast Cancer 2024, 10, 74. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, T.; Wu, T.; Lin, R.; Huang, J.; Shi, D.; Yu, J.; Ren, Y.; Qian, C.; He, L.; et al. Targeting Estrogen-Regulated System X(C)(-) Promotes Ferroptosis and Endocrine Sensitivity of ER+ Breast Cancer. Cell Death Dis. 2025, 16, 30. [Google Scholar] [CrossRef]
- Herrera-Abreu, M.; Guan, J.; Khalid, U.; Ning, J.; Costa, M.R.; Chan, J.; Li, Q.; Fortin, J.; Wong, W.R.; Perampalam, P.; et al. Inhibition of GPX4 Enhances CDK4/6 Inhibitor and Endocrine Therapy Activity in Breast Cancer. Nat. Commun. 2024, 15, 9550. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, H.; Zhang, Y.; Qu, F.; Tang, Z.; Qu, C.; Tian, J.; Zong, B.; Wang, Y.; Ren, H.; et al. A Ferroptosis-Associated Gene Signature for the Prediction of Prognosis and Therapeutic Response in Luminal-Type Breast Carcinoma. Sci. Rep. 2021, 11, 17610. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-Tolerant Persister Cancer Cells are Vulnerable to GPX4 Inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef]
- Park, S.Y.; Jeong, K.J.; Poire, A.; Zhang, D.; Tsang, Y.H.; Blucher, A.S.; Mills, G.B. Irreversible HER2 Inhibitors Overcome Resistance to the RSL3 Ferroptosis Inducer in Non-HER2 Amplified Luminal Breast Cancer. Cell Death Dis. 2023, 14, 532. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Liu, Y.; Wang, L.; Gu, W. Regulation of SLC7A11 as an Unconventional Checkpoint in Tumorigenesis through Ferroptosis. Genes Dis. 2024, 12, 101254. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Carter, B.Z.; Andreeff, M.; Ishizawa, J. Molecular Mechanisms of Ferroptosis and Updates of Ferroptosis Studies in Cancers and Leukemia. Cells 2023, 12, 1128. [Google Scholar] [CrossRef]
- Yan, R.; Lin, B.; Jin, W.; Tang, L.; Hu, S.; Cai, R. NRF2, a Superstar of Ferroptosis. Antioxidants 2023, 12, 1739. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 Pathway Protects Against Ferroptosis in Hepatocellular Carcinoma Cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W.; Zheng, Y.; Dai, W.; Ji, J.; Wu, L.; Cheng, Z.; Zhang, J.; Li, J.; Xu, X.; et al. Targeting Fatty Acid Synthase Modulates Sensitivity of Hepatocellular Carcinoma to Sorafenib Via Ferroptosis. J. Exp. Clin. Cancer Res. 2023, 42, 6. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, R.; Wang, C.; Pan, M.; Geng, F.; Zhong, Y.; Su, H.; Kou, Y.; Mo, X.; Lefai, E.; et al. STAT3 Activation of SCAP-SREBP-1 Signaling Upregulates Fatty Acid Synthesis to Promote Tumor Growth. J. Biol. Chem. 2024, 300, 107351. [Google Scholar] [CrossRef]
- Chen, X.; Tsvetkov, A.S.; Shen, H.; Isidoro, C.; Ktistakis, N.T.; Linkermann, A.; Koopman, W.J.H.; Simon, H.; Galluzzi, L.; Luo, S.; et al. International Consensus Guidelines for the Definition, Detection, and Interpretation of Autophagy-Dependent Ferroptosis. Autophagy 2024, 20, 1213–1246. [Google Scholar] [CrossRef]
- Hadian, K. Ferroptosis Suppressor Protein 1 (FSP1) and Coenzyme Q10 Cooperatively Suppress Ferroptosis. Biochemistry 2020, 59, 637–638. [Google Scholar] [CrossRef]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.; Schulze, A. SREBP Activity is Regulated by mTORC1 and Contributes to Akt-Dependent Cell Growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef]
- Yi, J.; Zhu, J.; Wu, J.; Thompson, C.B.; Jiang, X. Oncogenic Activation of PI3K-AKT-mTOR Signaling Suppresses Ferroptosis Via SREBP-Mediated Lipogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 31189–31197. [Google Scholar] [CrossRef]
- Su, H.; Peng, C.; Liu, Y. Regulation of Ferroptosis by PI3K/Akt Signaling Pathway: A Promising Therapeutic Axis in Cancer. Front. Cell Dev. Biol. 2024, 12, 1372330. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Motohashi, H.; Yamamoto, M. The Keap1-Nrf2 System in Cancers: Stress Response and Anabolic Metabolism. Front. Oncol. 2012, 2, 200. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, Y.; Waguri, S.; Sou, Y.; Kageyama, S.; Hasegawa, J.; Ishimura, R.; Saito, T.; Yang, Y.; Kouno, T.; Fukutomi, T.; et al. Phosphorylation of p62 Activates the Keap1-Nrf2 Pathway during Selective Autophagy. Mol. Cell 2013, 51, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Cosialls, E.; Pacreau, E.; Duruel, C.; Ceccacci, S.; Elhage, R.; Desterke, C.; Roger, K.; Guerrera, C.; Ducloux, R.; Souquere, S.; et al. mTOR Inhibition Suppresses Salinomycin-Induced Ferroptosis in Breast Cancer Stem Cells by Ironing Out Mitochondrial Dysfunctions. Cell Death Dis. 2023, 14, 744. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-Mediated Activity during Tumour Suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, S.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 Engages Polyamine Metabolism with p53-Mediated Ferroptotic Responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, C.; Wu, R.; Sun, Y.; Levine, A.; Feng, Z. Glutaminase 2, a Novel p53 Target Gene Regulating Energy Metabolism and Antioxidant Function. Proc. Natl. Acad. Sci. USA 2010, 107, 7455–7460. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in Cancer: From Molecular Mechanisms to Therapeutic Strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef]
- Lei, G.; Mao, C.; Horbath, A.D.; Yan, Y.; Cai, S.; Yao, J.; Jiang, Y.; Sun, M.; Liu, X.; Cheng, J.; et al. BRCA1-Mediated Dual Regulation of Ferroptosis Exposes a Vulnerability to GPX4 and PARP Co-Inhibition in BRCA1-Deficient Cancers. Cancer Discov. 2024, 14, 1476–1495. [Google Scholar] [CrossRef]
- Cahuzac, K.M.; Lubin, A.; Bosch, K.; Stokes, N.; Shoenfeld, S.M.; Zhou, R.; Lemon, H.; Asara, J.; Parsons, R.E. AKT Activation because of PTEN Loss Upregulates xCT Via GSK3β/NRF2, Leading to Inhibition of Ferroptosis in PTEN-Mutant Tumor Cells. Cell Rep. 2023, 42, 112536. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Dong, Y.; Liu, W.; Fan, X.; Sun, Y. Pan-Cancer Analyses Confirmed the Ferroptosis-Related Gene SLC7A11 as a Prognostic Biomarker for Cancer. Int. J. Gen. Med. 2022, 15, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, J.; Lu, Y.; Bawcom, A.R.; Wu, J.; Ou, J.; Asara, J.M.; Armstrong, A.J.; Wang, Q.; Li, L.; et al. RB1-Deficient Prostate Tumor Growth and Metastasis are Vulnerable to Ferroptosis Induction Via the E2F/ACSL4 Axis. J. Clin. Investig. 2023, 133, e166647. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.M.; Leprivier, G. The Impact of Oncogenic RAS on Redox Balance and Implications for Cancer Development. Cell Death Dis. 2019, 10, 955. [Google Scholar] [CrossRef]
- Fan, Z.; Wirth, A.; Chen, D.; Wruck, C.J.; Rauh, M.; Buchfelder, M.; Savaskan, N. Nrf2-Keap1 Pathway Promotes Cell Proliferation and Diminishes Ferroptosis. Oncogenesis 2017, 6, e371. [Google Scholar] [CrossRef]
- Xie, Y.; Lei, X.; Zhao, G.; Guo, R.; Cui, N. mTOR in Programmed Cell Death and its Therapeutic Implications. Cytokine Growth Factor Rev. 2023, 71–72, 66–81. [Google Scholar] [CrossRef]
- Drake, L.E.; Springer, M.Z.; Poole, L.P.; Kim, C.J.; Macleod, K.F. Expanding Perspectives on the Significance of Mitophagy in Cancer. Semin. Cancer Biol. 2017, 47, 110–124. [Google Scholar] [CrossRef]
- Woo, Y.; Lee, H.; Jung, Y.M.; Jung, Y. mTOR-Mediated Antioxidant Activation in Solid Tumor Radioresistance. J. Oncol. 2019, 2019, 5956867. [Google Scholar] [CrossRef]
- Su, F.; Koeberle, A. Regulation and Targeting of SREBP-1 in Hepatocellular Carcinoma. Cancer Metastasis Rev. 2024, 43, 673–708. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, W. P53 in Ferroptosis Regulation: The New Weapon for the Old Guardian. Cell Death Differ. 2022, 29, 895–910. [Google Scholar] [CrossRef]
- Wang, S.; Li, D.; Ou, Y.; Jiang, L.; Chen, Y.; Zhao, Y.; Gu, W. Acetylation is Crucial for p53-Mediated Ferroptosis and Tumor Suppression. Cell Rep. 2016, 17, 366–373. [Google Scholar] [CrossRef]
- Murphy, M.E. Ironing Out how p53 Regulates Ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, 12350–12352. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Tanaka, T.; Poyurovsky, M.V.; Nagano, H.; Mayama, T.; Ohkubo, S.; Lokshin, M.; Hosokawa, H.; Nakayama, T.; Suzuki, Y.; et al. Phosphate-Activated Glutaminase (GLS2), a p53-Inducible Regulator of Glutamine Metabolism and Reactive Oxygen Species. Proc. Natl. Acad. Sci. USA 2010, 107, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Jiang, P. The Crisscross between p53 and Metabolism in Cancer. Acta Biochim. Biophys. Sin. 2023, 55, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, W.; Zhang, W. Ferroptosis and the Bidirectional Regulatory Factor p53. Cell Death Discov. 2023, 9, 197. [Google Scholar] [CrossRef]
- Sen, U.; Coleman, C.; Gandhi, N.; Jethalia, V.; Demircioglu, D.; Elliott, A.; Vanderwalde, A.M.; Hayatt, O.; de Stanchina, E.; Halmos, B.; et al. SCD1 Inhibition Blocks the AKT-NRF2-SLC7A11 Pathway to Induce Lipid Metabolism Remodeling and Ferroptosis Priming in Lung Adenocarcinoma. Cancer Res. 2025, 85, 2485–2503. [Google Scholar] [CrossRef]
- Patra, K.C.; Hay, N. The Pentose Phosphate Pathway and Cancer. Trends Biochem. Sci. 2014, 39, 347–354. [Google Scholar] [CrossRef]
- Choi, J.; Kim, E.; Koo, J.S. Expression of Pentose Phosphate Pathway-Related Proteins in Breast Cancer. Dis. Markers 2018, 2018, 9369358. [Google Scholar] [CrossRef]
- Mi, T.; Kong, X.; Chen, M.; Guo, P.; He, D. Inducing Disulfidptosis in Tumors:Potential Pathways and Significance. MedComm 2024, 5, e791. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, Y.; Yu, Z.; Wang, H. SLC7A11-Mediated Cell Death Mechanism in Cancer: A Comparative Study of Disulfidptosis and Ferroptosis. Front. Cell Dev. Biol. 2025, 13, 1559423. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, D. Role of Disulfide Death in Cancer (Review). Oncol. Lett. 2024, 29, 55. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, J.; Kuang, F.; Chen, X.; Zeh, H.J.; Kang, R.; Kroemer, G.; Xie, Y.; Tang, D. PDK4 Dictates Metabolic Resistance to Ferroptosis by Suppressing Pyruvate Oxidation and Fatty Acid Synthesis. Cell Rep. 2021, 34, 108767. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, X.; Wang, X.; Wei, X.; Wang, D.; Liu, X.; Xu, L.; Batu, W.; Li, Y.; Guo, B.; et al. RSL3 Enhances the Antitumor Effect of Cisplatin on Prostate Cancer Cells Via Causing Glycolysis Dysfunction. Biochem. Pharmacol. 2021, 192, 114741. [Google Scholar] [CrossRef]
- Maldonado, R.; Talana, C.A.; Song, C.; Dixon, A.; Uehara, K.; Weichhaus, M. β-hydroxybutyrate does Not Alter the Effects of Glucose Deprivation on Breast Cancer Cells. Oncol. Lett. 2020, 21, 65. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Yao, X.; Fei, Y.; Lin, Z.; Li, Z.; Cai, K.; Zhao, Y.; Luo, Z. HCAR1/MCT1 Regulates Tumor Ferroptosis through the Lactate-Mediated AMPK-SCD1 Activity and its Therapeutic Implications. Cell Rep. 2020, 33, 108487. [Google Scholar] [CrossRef]
- Subburayan, K.; Thayyullathil, F.; Pallichankandy, S.; Cheratta, A.R.; Galadari, S. Superoxide-Mediated Ferroptosis in Human Cancer Cells Induced by Sodium Selenite. Transl. Oncol. 2020, 13, 100843. [Google Scholar] [CrossRef]
- Xu, M.; Gao, X.; Yue, L.; Li, J.; Feng, X.; Huang, D.; Cai, H.; Qi, Y. Sensitivity of Triple Negative Breast Cancer Cells to ATM-Dependent Ferroptosis Induced by Sodium Selenite. Exp. Cell Res. 2024, 442, 114222. [Google Scholar] [CrossRef]
- Gao, J.; Ma, N.; Ni, S.; Han, X. Intersection of Ferroptosis and Nanomaterials Brings Benefits to Breast Cancer. Cell Biol. Toxicol. 2025, 41, 119. [Google Scholar] [CrossRef]
- Tosi, G.; Paoli, A.; Zuccolotto, G.; Turco, E.; Simonato, M.; Tosoni, D.; Tucci, F.; Lugato, P.; Giomo, M.; Elvassore, N.; et al. Cancer Cell Stiffening Via CoQ(10) and UBIAD1 Regulates ECM Signaling and Ferroptosis in Breast Cancer. Nat. Commun. 2024, 15, 8214. [Google Scholar] [CrossRef]
- Woldeselassie, M.; Tamene, A. Therapeutic Controversies Over use of Antioxidant Supplements during Cancer Treatment: A Scoping Review. Front. Nutr. 2024, 11, 1480780. [Google Scholar] [CrossRef] [PubMed]
- Kraft, V.A.N.; Bezjian, C.T.; Pfeiffer, S.; Ringelstetter, L.; Müller, C.; Zandkarimi, F.; Merl-Pham, J.; Bao, X.; Anastasov, N.; Kössl, J.; et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent. Sci. 2020, 6, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, T.; Sun, L.; Yao, Y.; Li, F.; Mao, L. Triggered Ferroptotic Albumin-Tocopherol Nanocarriers for Treating Drug-Resistant Breast Cancer. Front. Oncol. 2024, 14, 1464909. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wang, J.; Zhao, S.; Wei, Y.; Qi, Y.; Liu, S.; Wang, Y.; Ge, H.; Yang, X.; Tan, Y.; et al. Multi-Cohort Validation of a Lipid Metabolism and Ferroptosis-Associated Index for Prognosis and Immunotherapy Response Prediction in Hormone Receptor-Positive Breast Cancer. Int. J. Biol. Sci. 2025, 21, 3968–3992. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Zhai, D.; Xie, C.; Kuang, X.; Lin, Y.; Shao, N. Quantification of Ferroptosis Pathway Status Revealed Heterogeneity in Breast Cancer Patients with Distinct Immune Microenvironment. Front. Oncol. 2022, 12, 956999. [Google Scholar] [CrossRef]
- Glibetic, N.; Bowman, S.; Skaggs, T.; Weichhaus, M. The use of Patient-Derived Organoids in the Study of Molecular Metabolic Adaptation in Breast Cancer. Int. J. Mol. Sci. 2024, 25, 10503. [Google Scholar] [CrossRef]
- Lu, D.; Xia, B.; Feng, T.; Qi, G.; Ma, Z. The Role of Cancer Organoids in Ferroptosis, Pyroptosis, and Necroptosis: Functions and Clinical Implications. Biomolecules 2025, 15, 659. [Google Scholar] [CrossRef]
- Ye, L.; Zhong, F.; Sun, S.; Ou, X.; Yuan, J.; Zhu, J.; Zeng, Z. Tamoxifen Induces Ferroptosis in MCF-7 Organoid. J. Cancer Res. Ther. 2023, 19, 1627–1635. [Google Scholar] [CrossRef]
- Havas, K.M.; Milchevskaya, V.; Radic, K.; Alladin, A.; Kafkia, E.; Garcia, M.; Stolte, J.; Klaus, B.; Rotmensz, N.; Gibson, T.J.; et al. Metabolic Shifts in Residual Breast Cancer Drive Tumor Recurrence. J. Clin. Investig. 2017, 127, 2091–2105. [Google Scholar] [CrossRef]
- Desterke, C.; Xiang, Y.; Elhage, R.; Duruel, C.; Chang, Y.; Hamaï, A. Ferroptosis Inducers Upregulate PD-L1 in Recurrent Triple-Negative Breast Cancer. Cancers 2024, 16, 155. [Google Scholar] [CrossRef]
- Yu, L.; Huang, K.; Liao, Y.; Wang, L.; Sethi, G.; Ma, Z. Targeting Novel Regulated Cell Death: Ferroptosis, Pyroptosis and Necroptosis in Anti-PD-1/PD-L1 Cancer Immunotherapy. Cell Prolif. 2024, 57, e13644. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R.; Hou, N.; Zhang, J.; Wang, T.; Fan, P.; Ji, C.; Zhang, B.; Liu, L.; Wang, Y.; et al. PRMT5 Reduces Immunotherapy Efficacy in Triple-Negative Breast Cancer by Methylating KEAP1 and Inhibiting Ferroptosis. J. Immunother. Cancer. 2023, 11, e006890. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Huang, F.; Wu, R.; Xie, W.; Chen, M.; Dai, S.; Xu, W.; Zheng, W.; Tan, G. Toxicarioside H Induces Ferroptosis in Triple-Negative Breast Cancer Cells through Nrf2/HO-1 Pathway. Discov. Oncol. 2025, 16, 772. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Xie, R.; Cao, Y.; Tang, J.; Men, Y.; Peng, H.; Yang, W. Simvastatin Induced Ferroptosis for Triple-Negative Breast Cancer Therapy. J. Nanobiotechnol. 2021, 19, 311. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Fan, Y.; He, L.; Hu, N.; Xue, H.; Guan, X.; Zheng, Z. Enhanced Cancer Immunotherapy through Synergistic Ferroptosis and Immune Checkpoint Blockade using Cell Membrane-Coated Nanoparticles. Cancer Nanotechnol. 2023, 14, 83. [Google Scholar] [CrossRef]
- Han, N.; Li, L.; Peng, X.; Ma, Q.; Yang, Z.; Wang, X.; Li, J.; Li, Q.; Yu, T.; Xu, H.; et al. Ferroptosis Triggered by Dihydroartemisinin Facilitates Chlorin E6 Induced Photodynamic Therapy Against Lung Cancer through Inhibiting GPX4 and Enhancing ROS. Eur. J. Pharmacol. 2022, 919, 174797. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, C.; Guan, Q.; Wei, M.; Li, Z. Nanoparticle-Mediated Synergistic Anticancer Effect of Ferroptosis and Photodynamic Therapy: Novel Insights and Perspectives. Asian J. Pharm. Sci. 2023, 18, 100829. [Google Scholar] [CrossRef]
- Kojima, Y.; Tanaka, M.; Sasaki, M.; Ozeki, K.; Shimura, T.; Kubota, E.; Kataoka, H. Induction of Ferroptosis by Photodynamic Therapy and Enhancement of Antitumor Effect with Ferroptosis Inducers. J. Gastroenterol. 2024, 59, 81–94. [Google Scholar] [CrossRef]
- Luo, J.; Xu, L.; Feng, J.; Xu, K.; Tian, P.; Bai, X.; Xu, S.; Wen, L.; Lu, C.; Song, J. Tumor Microenvironment-Activated and ROS-Augmented Nanoplatform Amplified PDT Against Colorectal Cancer through Impairing GPX4 to Induce Ferroptosis. ACS Appl. Mater. Interfaces 2025, 17, 41586–41596. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Li, X.; Shen, X.; Yang, G.; Deng, Y.; Hu, Z.; Zhang, J.; Lu, Y. 5-ALA-PDT Induced Ferroptosis in Keloid Fibroblasts Via ROS, Accompanied by Downregulation of xCT, GPX4. Photodiagn. Photodyn. Ther. 2023, 42, 103612. [Google Scholar] [CrossRef]
- Le, Y.; Yunjie, X.; Kim, J.S. Photo-Activated Ferroptosis for Cancer Therapy: Advances, Challenges, and Prospects. CCS Chem. 2023, 5, 1718–1736. [Google Scholar] [CrossRef]
- Mishchenko, T.A.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Ferroptosis and Photodynamic Therapy Synergism: Enhancing Anticancer Treatment. Trends Cancer. 2021, 7, 484–487. [Google Scholar] [CrossRef]
- Luo, J.; Deng, Y.; Lu, S.; Chen, S.; He, R.; Qin, D.; Chi, B.; Chen, G.; Yang, X.; Peng, W. Current Status and Future Directions of Ferroptosis Research in Breast Cancer: Bibliometric Analysis. Interact. J. Med. Res. 2025, 14, e66286. [Google Scholar] [CrossRef]
- Yang, X.; Wu, L.; Xu, S. An Overview of GPX4-Targeting TPDs for Cancer Therapy. Bioorg. Med. Chem. 2025, 118, 118046. [Google Scholar] [CrossRef]
- Guo, J.; Yan, Y.; Zhang, L.; Chen, H.; Zhang, W.; Yuan, H.; Lin, J.; Sun, Q.; Yan, L.; Wang, B.; et al. Dual Ferroptosis Induction in N2-TANs and TNBC Cells Via FTH1 Targeting: A Therapeutic Strategy for Triple-Negative Breast Cancer. Cell Rep. Med. 2025, 6, 101915. [Google Scholar] [CrossRef]
- Santana-Codina, N.; Mancias, J. The Role of NCOA4-Mediated Ferritinophagy in Health and Disease. Pharmaceuticals 2018, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Zhuang, L.; Gan, B. The Roles of Ferroptosis in Cancer: Tumor Suppression, Tumor Microenvironment, and Therapeutic Interventions. Cancer Cell 2024, 42, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, J.; Hooi, S.C.; Jiang, Y.; Lu, G. Fatty Acid Activation in Carcinogenesis and Cancer Development: Essential Roles of Long-Chain Acyl-CoA Synthetases. Oncol. Lett. 2018, 16, 1390–1396. [Google Scholar] [CrossRef]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium Utilization by GPX4 is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422.e21. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Nakamura, T.; Toyama, T.; Chen, D.; Berndt, C.; Poschmann, G.; Mourão, A.S.D.; Doll, S.; Suzuki, M.; Zhang, W.; et al. PRDX6 Dictates Ferroptosis Sensitivity by Directing Cellular Selenium Utilization. Mol. Cell 2024, 84, 4629–4644.e9. [Google Scholar] [CrossRef]
- Conrad, M.; Proneth, B. Selenium: Tracing another Essential Element of Ferroptotic Cell Death. Cell Chem. Biol. 2020, 27, 409–419. [Google Scholar] [CrossRef]
- Dai, Q.; Wei, X.; Zhao, J.; Zhang, D.; Luo, Y.; Yang, Y.; Xiang, Y.; Liu, X. Inhibition of FSP1: A New Strategy for the Treatment of Tumors (Review). Oncol. Rep. 2024, 52, 105. [Google Scholar] [CrossRef]
- Koppula, P.; Lei, G.; Zhang, Y.; Yan, Y.; Mao, C.; Kondiparthi, L.; Shi, J.; Liu, X.; Horbath, A.; Das, M.; et al. A Targetable CoQ-FSP1 Axis Drives Ferroptosis- and Radiation-Resistance in KEAP1 Inactive Lung Cancers. Nat. Commun. 2022, 13, 2206. [Google Scholar] [CrossRef]
- Li, W.; Liang, L.; Liu, S.; Yi, H.; Zhou, Y. FSP1: A Key Regulator of Ferroptosis. Trends Mol. Med. 2023, 29, 753–764. [Google Scholar] [CrossRef]
- Martinez, S.; Sentis, S.; Poulard, C.; Trédan, O.; Le Romancer, M. Role of PRMT1 and PRMT5 in Breast Cancer. Int. J. Mol. Sci. 2024, 25, 8854. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liang, C.; Qu, X.; Zhang, T.; Mou, X.; Cai, Y.; Wang, W.; Shao, J.; Dong, X. Metal-Free Polymer Nano-Photosensitizer Actuates Ferroptosis in Starved Cancer. Biomaterials 2023, 292, 121944. [Google Scholar] [CrossRef] [PubMed]
- Szurko, A.; Rams-Baron, M.; Montforts, F.; Bauer, D.; Kozub, P.; Gubernator, J.; Altmann, S.; Stanek, A.; Sieroń, A.; Ratuszna, A. Photodynamic Performance of Amphiphilic Chlorin E6 Derivatives with Appropriate Properties: A Comparison between Different-Type Liposomes as Delivery Systems. Photodiagn. Photodyn. Ther. 2020, 30, 101799. [Google Scholar] [CrossRef] [PubMed]
- Sokol, K.H.; Lee, C.J.; Rogers, T.J.; Waldhart, A.; Ellis, A.E.; Madireddy, S.; Daniels, S.R.; House, R.J.; Ye, X.; Olesnavich, M.; et al. Lipid Availability Influences Ferroptosis Sensitivity in Cancer Cells by Regulating Polyunsaturated Fatty Acid Trafficking. Cell Chem. Biol. 2025, 32, 408–422.e6. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Chen, H.; Gao, R.; Ning, P.; Wang, Y.; Huang, W.; Chen, E.; Fang, L.; Guo, X.; et al. A Lysosome-Targeted Magnetic Nanotorquer Mechanically Triggers Ferroptosis for Breast Cancer Treatment. Adv. Sci. 2024, 11, e2302093. [Google Scholar] [CrossRef]
- Lorito, N.; Bacci, M.; Smiriglia, A.; Mannelli, M.; Parri, M.; Comito, G.; Ippolito, L.; Giannoni, E.; Bonechi, M.; Benelli, M.; et al. Glucose Metabolic Reprogramming of ER Breast Cancer in Acquired Resistance to the CDK4/6 Inhibitor Palbociclib. Cells 2020, 9, 668. [Google Scholar] [CrossRef]
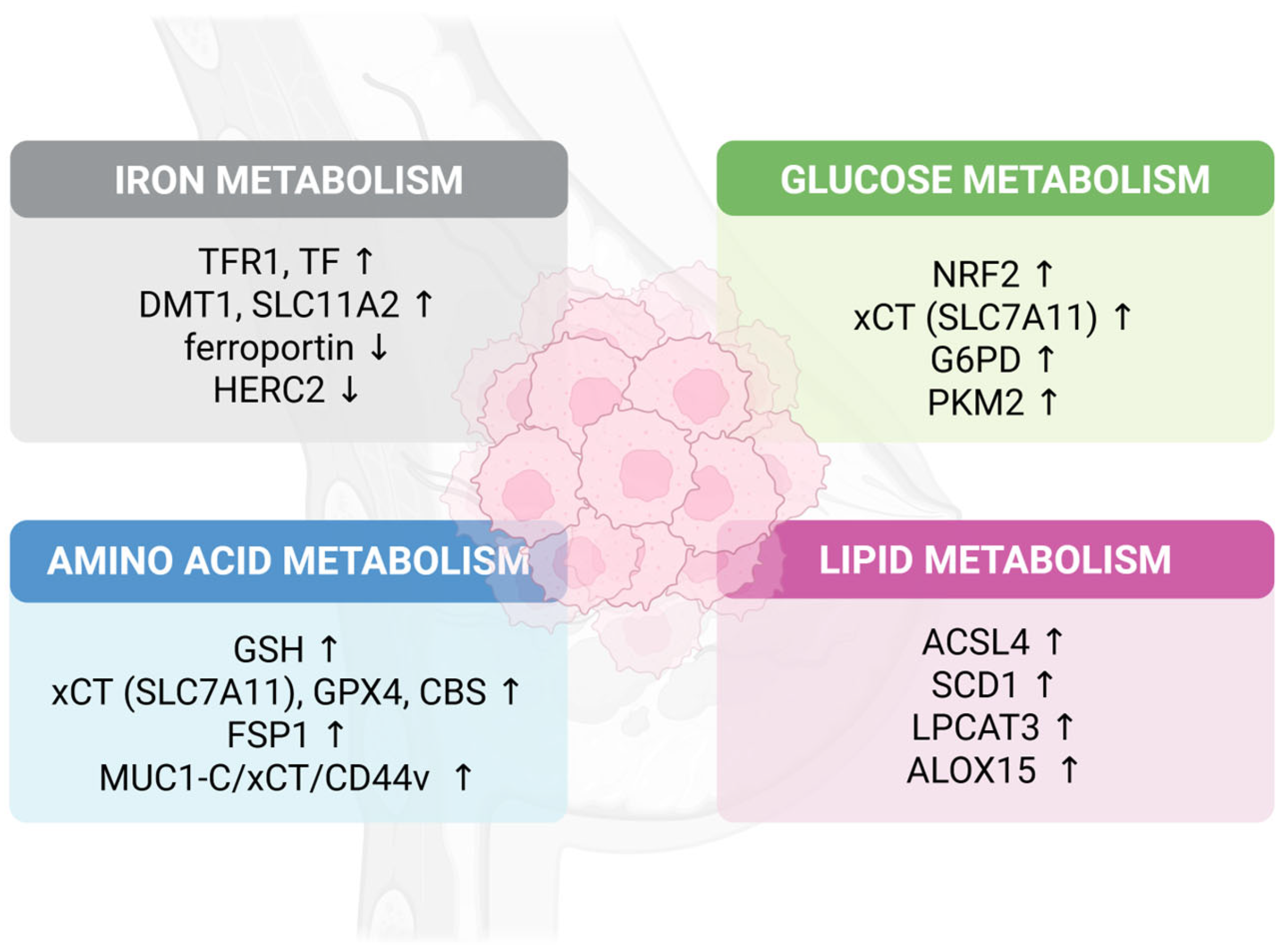
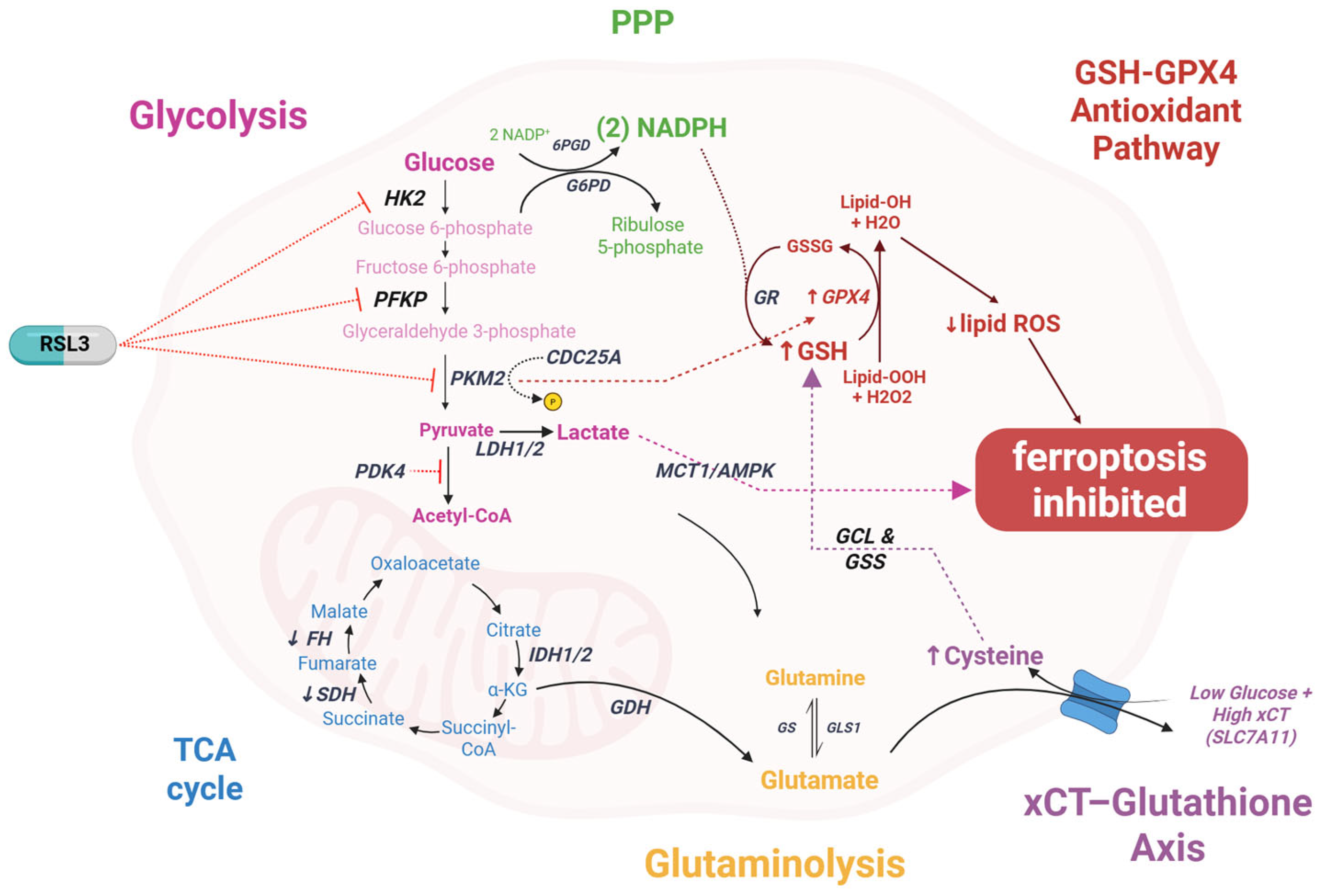
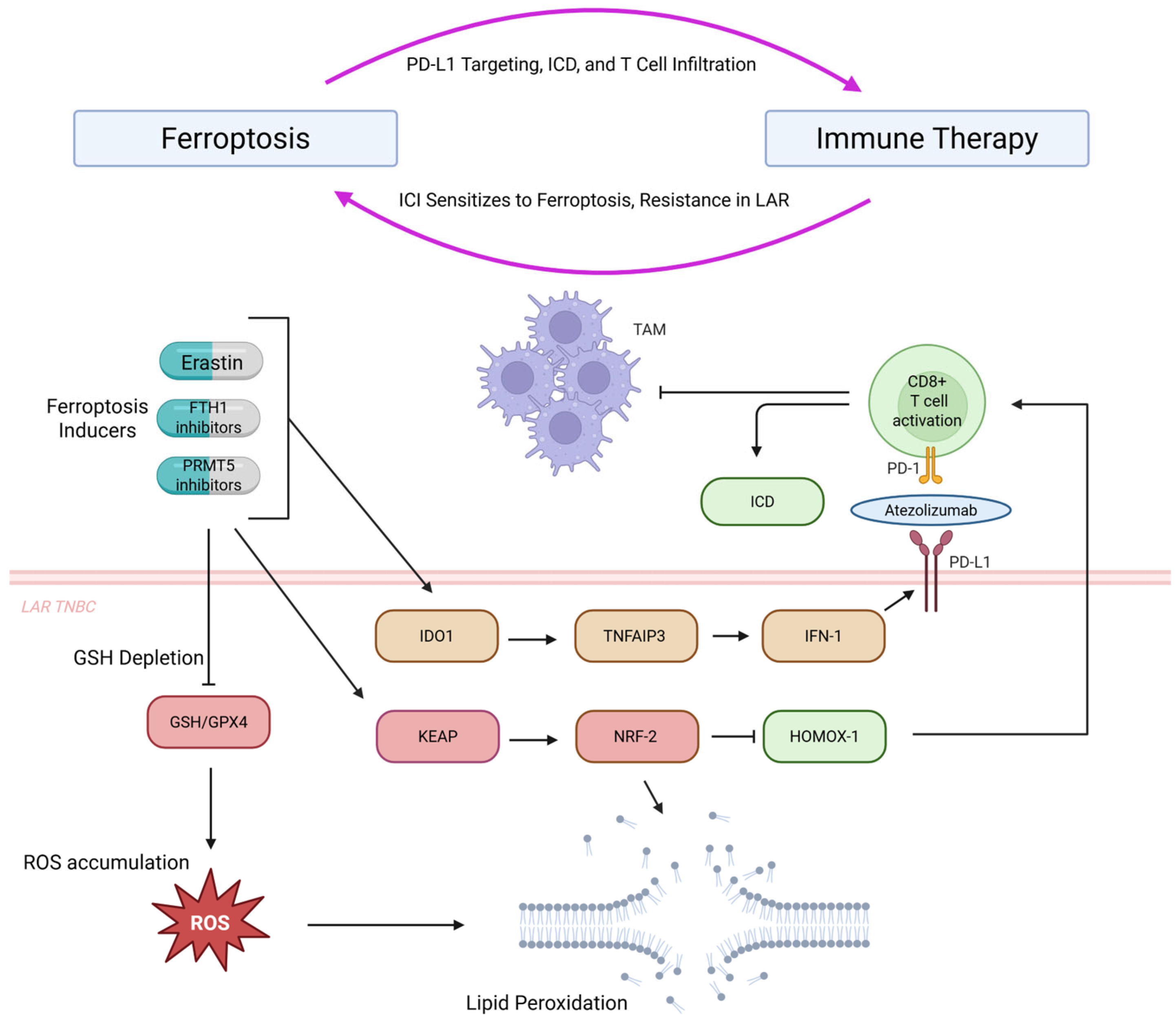
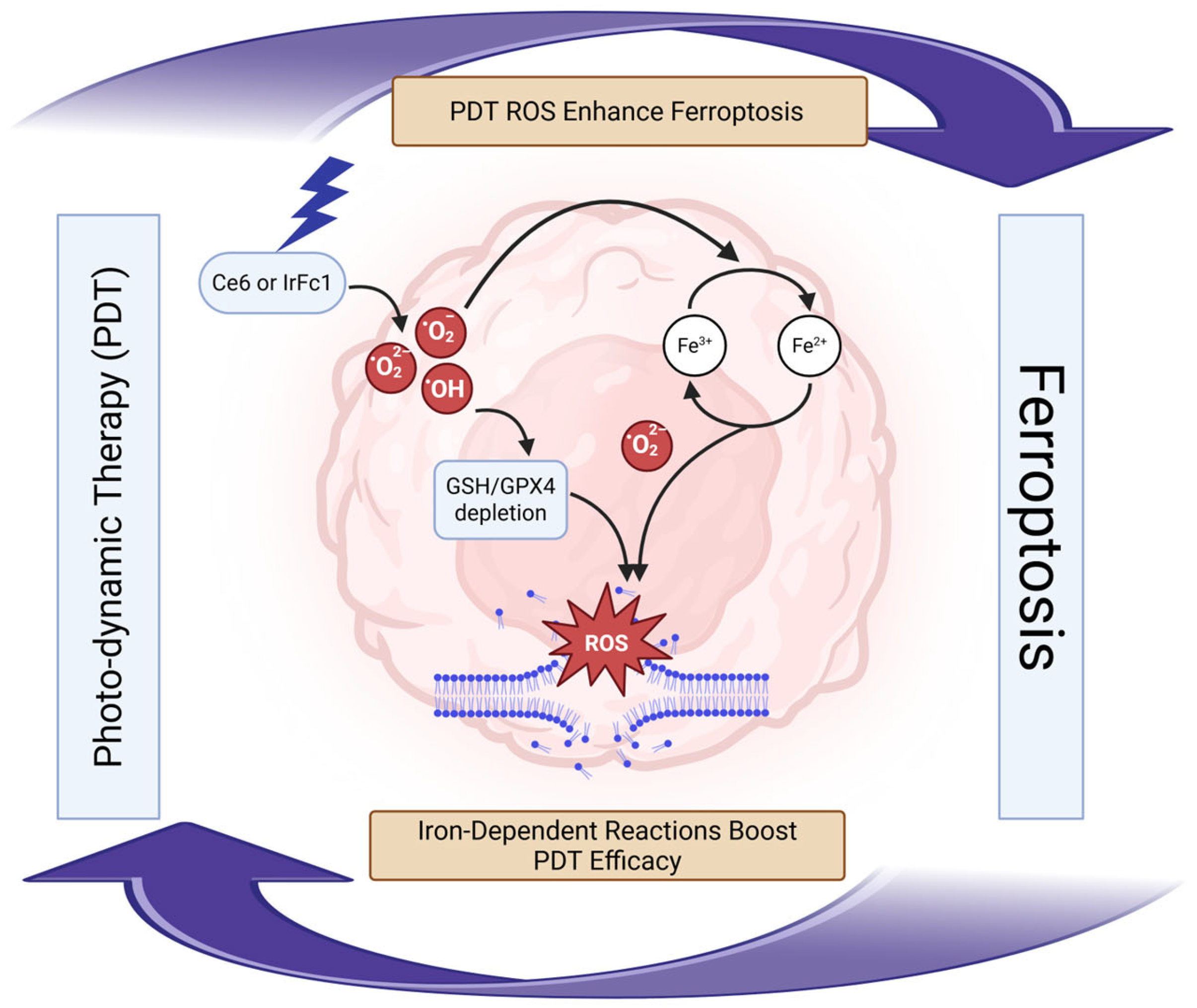
| Pathway/Mechanism | Clinical Status | Critical Gaps/Paradoxes | Key References |
|---|---|---|---|
| GPX4-GSH Axis | RSL3: preclinical only; hepatotoxicity at therapeutic doses | TNBC shows both xCT/GPX4 addiction AND high ferroptosis sensitivity; mechanism unclear | [11,17,18,19,20,21,42] |
| Cystine Import | Erastin: preclinical; poor bioavailability | TAM-supplied cysteine may override cell-autonomous targeting | [22,23,24,25,26,43,44,45] |
| Transsulfuration | No CBS inhibitors in development | Both import and synthesis can be simultaneously upregulated | [27,28,29,30,46] |
| Lysosomal Storage | CysRx: proof-of-concept only | Enhancing lysosomal cystine paradoxically increases ferroptosis | [31,32] |
| Alternative GPX4-Independent | No FSP1 inhibitors in trials | Relative contribution vs. GPX4 pathway undefined in breast cancer | [33,34,35,36,37] |
| Nucleotide Competition | No selective RNR modulators | Cannot target without affecting normal cell proliferation | [38,39,40,41] |
| TNBC Subtype | Ferroptosis Sensitivity | Key Regulatory Features |
|---|---|---|
| Luminal Androgen Receptor (LAR) | High | Upregulated GPX4, OxPE, glutathione metabolism |
| Mesenchymal (MES) | Moderate | Enriched iron metabolism, low FA/ROS activity |
| Immunomodulatory (IM) and Basal-Like Immune-Suppressed (BLIS) | Low | Minimal ferroptosis features, other cell death pathways dominant |
| Gene/ Pathway | Category | Effect on Ferroptosis | Primary Mechanisms | Clinical Relevance | Key References |
|---|---|---|---|---|---|
| RAS | Oncogene | Strong Resistance | ETS1→xCT upregulation NRF2 activation; FASN-HIF1α→MUFA FSP1 induction | Common in aggressive BC | [103,104,105,106,107,108,109,110,125,126] |
| mTORC1 | Oncogene | Context-dependent | SREBP1-SCD1→MUFA Suppresses ferritinophagy p62-KEAP1-NRF2 Glutaminolysis paradox | Activated in most BC | [111,112,113,114,115,116,127,128,129,130] |
| p53 | Tumor Suppressor | Promotes | Direct xCT repression SAT1→ALOX15 GLS2→glutamine depletion Acetylation-dependent | Lost in 30% BC | [39,117,118,119,131,132,133,134,135,136] |
| BRCA1 | Tumor Suppressor | Differential | Erastin resistance GPX4 inhibitor sensitive VDAC3-dependent | 5–10% hereditary BC | [121] |
| PTEN | Tumor Suppressor | Loss→Resistance | AKT→GSK3β→NRF2 xCT upregulation Pan-cancer mechanism | Lost in 30–40% BC | [122,123,137] |
| RB | Tumor Suppressor | Loss→Sensitivity | E2F→ACSL4 upregulation Increased PUFA incorporation | Lost in TNBC | [124] |
| HER2 | Oncogene | Context-dependent | Baseline resistance Sensitivity when inhibited | Amplified 15–20% BC | [101,102] |
| ER | Nuclear Receptor | Resistance | MBOAT1 upregulation ELOVL2→AdA synthesis | Positive in 70% BC | [48,66,98] |
| Strategy | Target/ Mechanism | Representative Agents | Development Status | Efficacy | Major Limitations | Key References |
|---|---|---|---|---|---|---|
| GPX4 Direct Inhibition | GPX4 enzymatic activity | RSL3 ML162 FIN56 | Preclinical only | 60–90% growth inhibition in vitro | Poor bioavailability (t½ < 2 h), hepatotoxicity | [19,42,95,101,174] |
| Cystine Import Blockade | System xc− (SLC7A11) | Erastin IKE Sulfasalazine | Preclinical; Sulfasalazine FDA-approved | Variable (30–70% inhibition) | Poor solubility, compensatory CBS upregulation | [22,24,26,28,43] |
| Iron Overload/Ferritinophagy | NCOA4 FTH1 iron import | Sorafenib-NPs CT-1 Salinomycin | Preclinical | 70–80% tumor reduction | Systemic iron toxicity | [15,16,175,176] |
| ACSL4 Modulation | PUFA incorporation | No specific inhibitors | Target identified | Context-dependent | Dual role, no selective agents | [52,54,56,67,177,178] |
| SCD1 Inhibition | MUFA synthesis blockade | A939572 CAY10566 | Preclinical | Sensitizes to ferroptosis | Normal tissue toxicity | [63,64,137] |
| Selenium Manipulation | GPX4 cofactor | Sodium selenite | Preclinical | TNBC-specific toxicity | Narrow therapeutic window | [147,148,179,180,181] |
| FSP1 Inhibition | CoQ10-ubiquinol system | iFSP1 | Early preclinical | Limited single-agent | Redundancy with GPX4 | [35,36,149,182,183,184] |
| Immunotherapy Combination | PD-1/PD-L1 + ferroptosis | RSL3/Erastin + ICIs | Preclinical | 60–90% tumor inhibition | T-cell toxicity | [160,161,162,165] |
| PRMT5 Inhibition | KEAP1 methylation | GSK3326595 | Phase I (not BC) | 60–80% with anti-PD-1 | Not ferroptosis-specific | [162,185] |
| PDT Combination | ROS + ferroptosis | Ce6/PpIX + light | Preclinical | >95% local control | Limited penetration | [166,167,168,171,186,187] |
| Lipid/Metabolic Intervention | Lipid availability, glucose | Dietary GLUT inhibitors | Observational/Preclinical | Context-dependent | Patient compliance | [69,141,145,188] |
| Nanoparticle Delivery | Targeted delivery | Fe3+-NPs glutathione-scavenging | Preclinical | 90% tumor inhibition | Manufacturing complexity | [45,149,165,189] |
| Hormone Therapy Combination | ER/HER2 + ferroptosis | Tamoxifen/Lapatinib + GPX4i | Proof of concept | Enhanced in resistant | Requires resistance | [98,99,101,102,190] |
| UBIAD1/CoQ10 Exploitation | Antioxidant paradox | No specific agents | Concept only | Unknown | Mechanism unclear | [150,151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glibetic, N.; Weichhaus, M. Metabolic Regulation of Ferroptosis in Breast Cancer. Int. J. Mol. Sci. 2025, 26, 9686. https://doi.org/10.3390/ijms26199686
Glibetic N, Weichhaus M. Metabolic Regulation of Ferroptosis in Breast Cancer. International Journal of Molecular Sciences. 2025; 26(19):9686. https://doi.org/10.3390/ijms26199686
Chicago/Turabian StyleGlibetic, Natalija, and Michael Weichhaus. 2025. "Metabolic Regulation of Ferroptosis in Breast Cancer" International Journal of Molecular Sciences 26, no. 19: 9686. https://doi.org/10.3390/ijms26199686
APA StyleGlibetic, N., & Weichhaus, M. (2025). Metabolic Regulation of Ferroptosis in Breast Cancer. International Journal of Molecular Sciences, 26(19), 9686. https://doi.org/10.3390/ijms26199686






