Active Inclusion Bodies in the Multienzymatic Synthesis of UDP-N-acetylglucosamine
Abstract
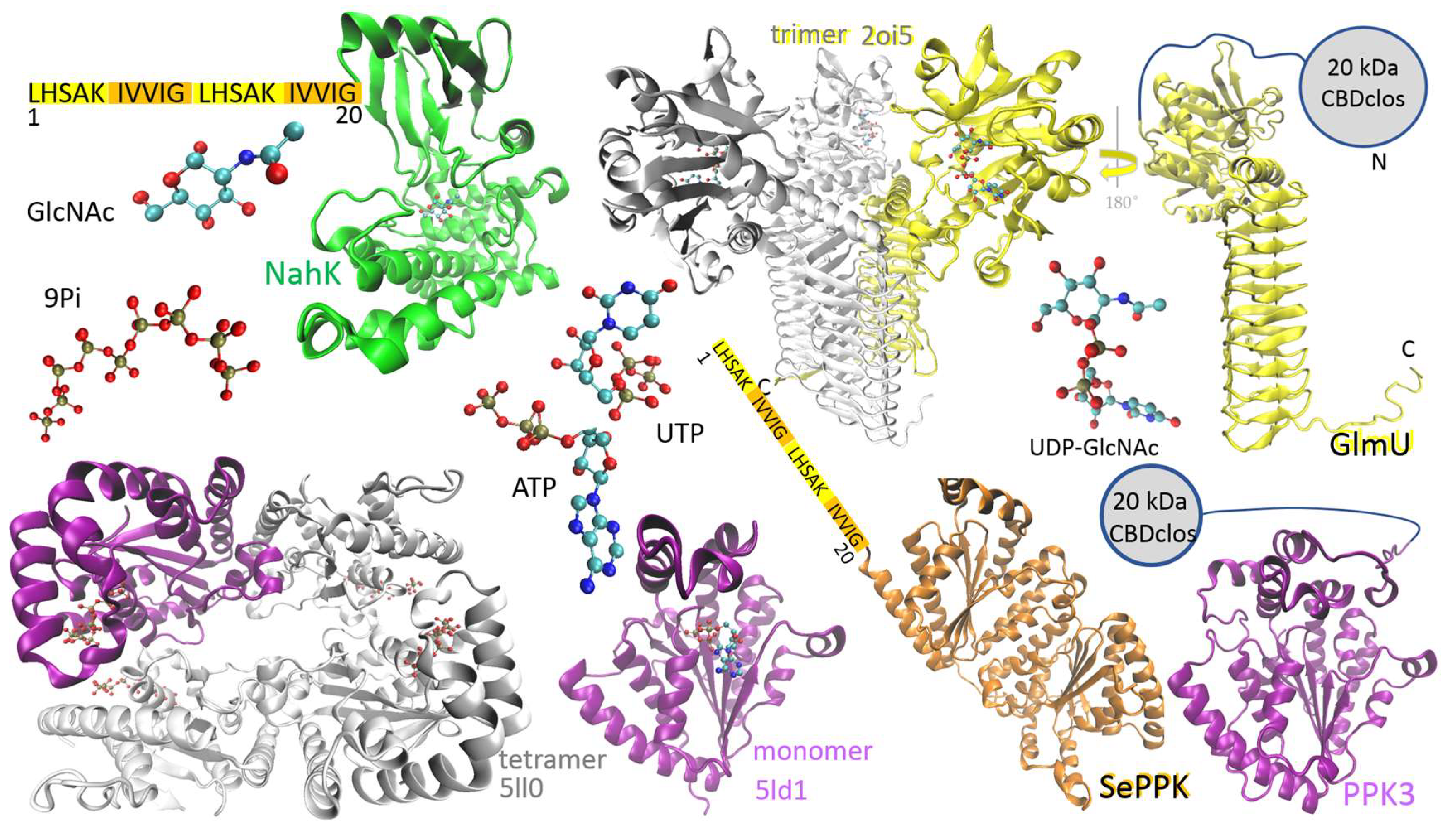
1. Introduction
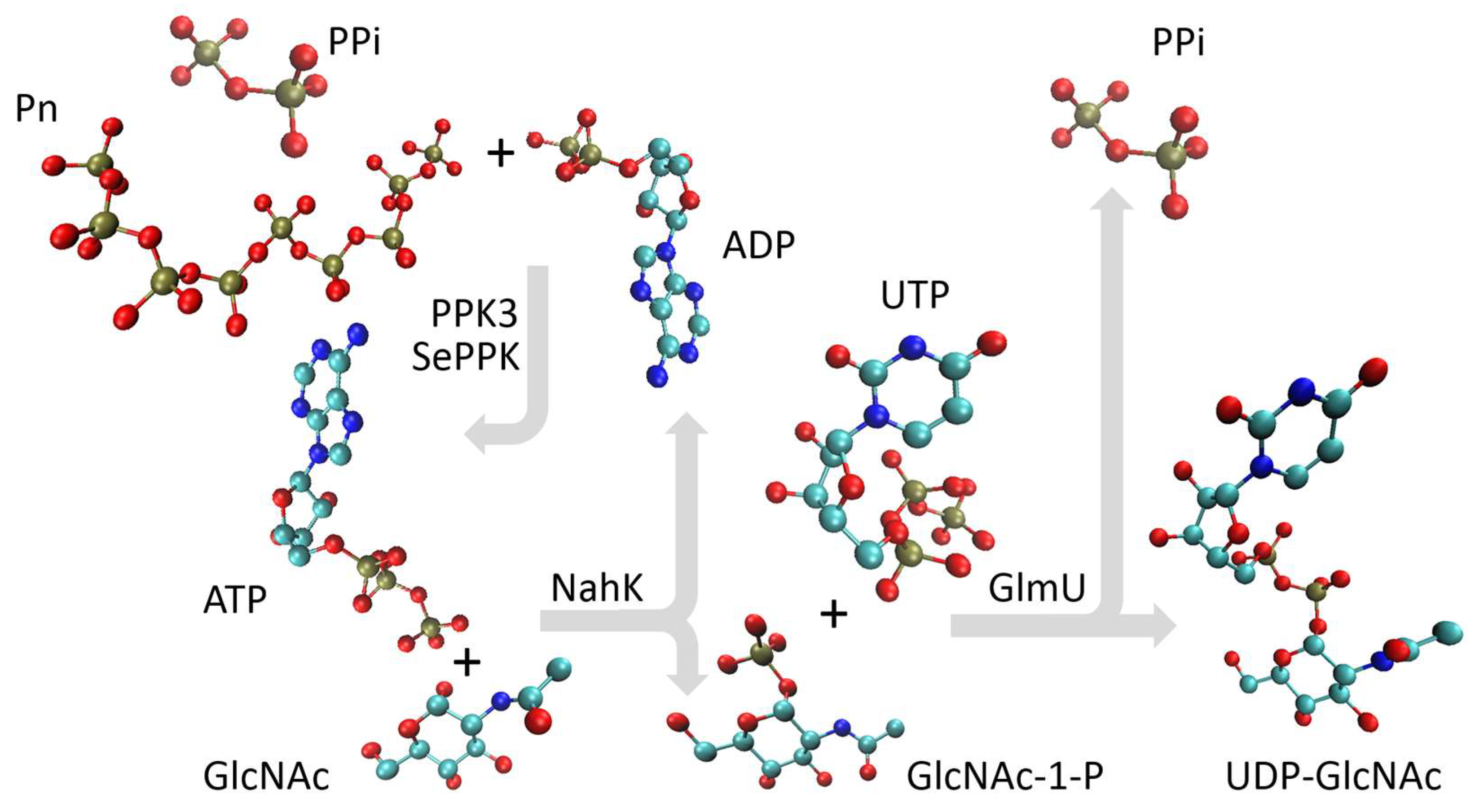
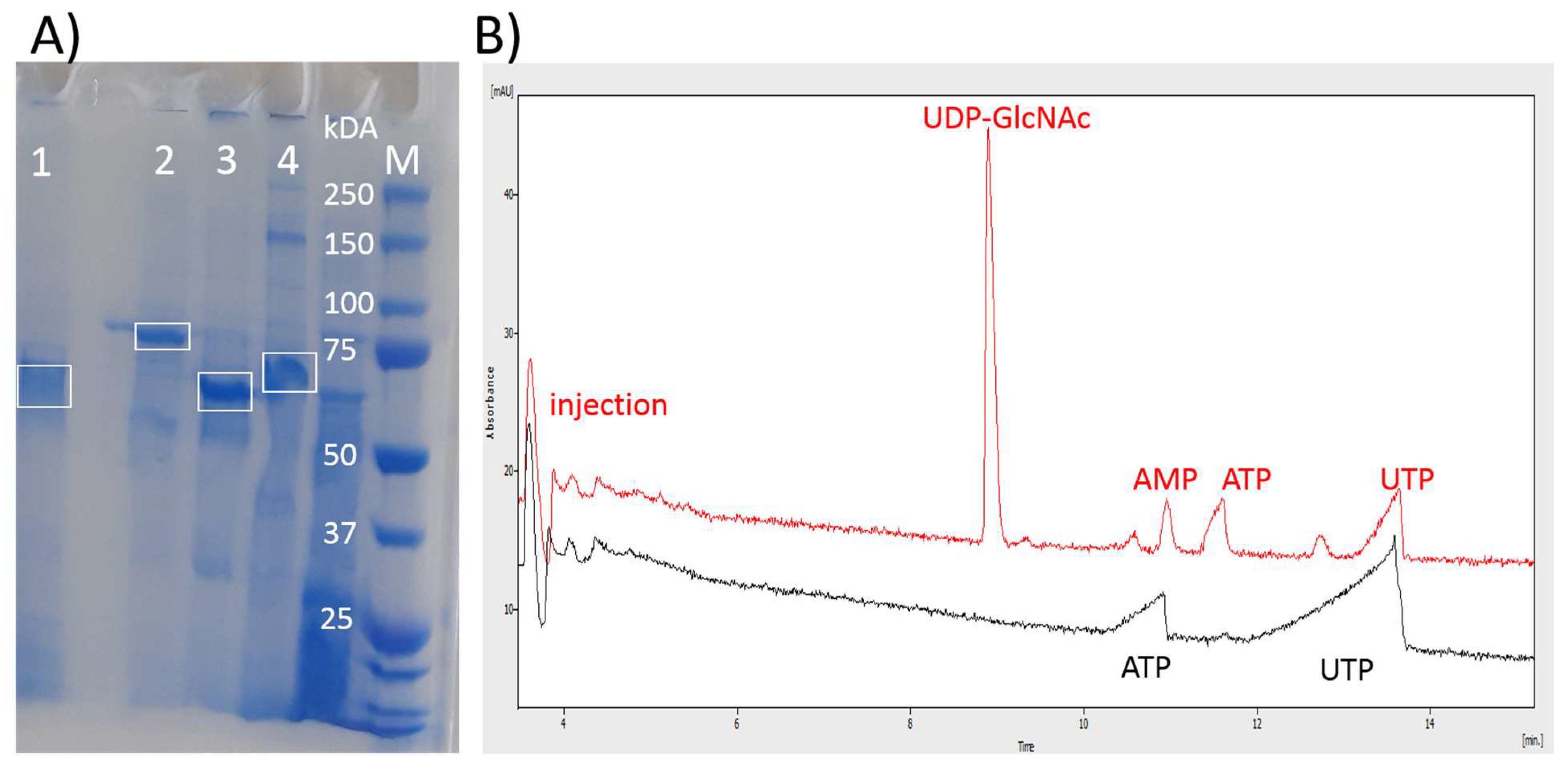
2. Results
3. Discussion
4. Materials and Methods
4.1. Cloning, Expression, and Isolation of aIBs
4.2. Multienzymatic Synthesis of UDP-GlcNAc by the aIBs
4.3. Capillary Electrophoresis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shively, J.M. Inclusion bodies of prokaryotes. Annu. Rev. Microbiol. 1974, 28, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Slouka, C.; Kopp, J.; Spadiut, O.; Herwig, C. Perspectives of inclusion bodies for bio-based products: Curse or blessing? Appl. Microbiol. Biotechnol. 2019, 103, 1143–1153. [Google Scholar] [CrossRef]
- De Marco, A.; Ferrer-Miralles, N.; Garcia-Fruitós, E.; Mitraki, A.; Peternel, S.; Rinas, U.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Vázquez, E.; Villaverde, A. Bacterial inclusion bodies are industrially exploitable amyloids. FEMS Microbiol. Rev. 2019, 43, 53–72. [Google Scholar] [CrossRef]
- Köszagová, R.; Nahálka, J. Inclusion bodies in biotechnology. J. Microbiol. Biotech. Food Sci. 2020, 9, 1191–1196. [Google Scholar] [CrossRef]
- Belková, M.; Köszagová, R.; Nahálka, J. Active Inclusion Bodies: The Unexpected Journey. J. Microbiol. Biotech. Food Sci. 2022, 12, 1–7. [Google Scholar] [CrossRef]
- Jäger, V.D.; Lamm, R.; Küsters, K.; Ölçücü, G.; Oldiges, M.; Jaeger, K.E.; Büchs, J.; Krauss, U. Catalytically-active inclusion bodies for biotechnology—General concepts, optimization, and application. Appl. Microbiol. Biotechnol. 2020, 104, 7313–7329. [Google Scholar] [CrossRef]
- Nahalka, J.; Nidetzky, B. Fusion to a pull-down domain: A novel approach of producing Trigonopsis variabilis D-amino acid oxidase as insoluble enzyme aggregates. Biotechnol. Bioeng. 2007, 97, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Hrabarova, E.; Belkova, M.; Koszagova, R.; Nahalka, J. Pull-Down Into Active Inclusion Bodies and Their Application in the Detection of (Poly)-Phosphates and Metal-Ions. Front. Bioeng. Biotechnol. 2022, 10, 1–10. [Google Scholar] [CrossRef]
- Komolov, A.S.; Sannikova, E.P.; Gorbunov, A.A.; Gubaidullin, I.I.; Plokhikh, K.S.; Konstantinova, G.E.; Bulushova, N.V.; Kuchin, S.V.; Kozlov, D.G. Synthesis of biologically active proteins as L6KD-SUMO fusions forming inclusion bodies in Escherichia coli. Biotechnol. Bioeng. 2024, 121, 535–550. [Google Scholar] [CrossRef]
- Mestrom, L.; Przypis, M.; Kowalczykiewicz, D.; Pollender, A.; Kumpf, A.; Marsden, S.R.; Bento, I.; Jarzębski, A.B.; Szymańska, K.; Chruściel, A.; et al. Leloir glycosyltransferases in applied biocatalysis: A multidisciplinary approach. Int. J. Mol. Sci. 2019, 20, 5263. [Google Scholar] [CrossRef] [PubMed]
- Palenčárová, K.; Köszagová, R.; Nahálka, J. Hyaluronic Acid and Its Synthases—Current Knowledge. Int. J. Mol. Sci. 2025, 26, 7028. [Google Scholar] [CrossRef]
- Li, S.; Lu, A.J.; Nagueh, E.S.; Li, Y.; Graber, M.; Carter, K.N.; Morales, E.; Kriss, C.L.; Chen, K.; Liu, J.; et al. O-GlcNAcylation promotes angiogenic transdifferentiation to reverse vascular ischemia. Nat. Cardiovasc. Res. 2025, 4, 904–920. [Google Scholar] [CrossRef]
- Li, S.; Ren, W.; Zheng, J.; Li, S.; Zhi, K.; Gao, L. Role of O-linked N-acetylglucosamine protein modification in oxidative stress-induced autophagy: A novel target for bone remodeling. Cell Commun. Signal. 2024, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pederick, J.L.; Kumar, A.; Pukala, T.L.; Bruning, J.B. Functional and structural characterization of Staphylococcus aureus N-acetylglucosamine 1-phosphate uridyltransferase (GlmU) reveals a redox-sensitive acetyltransferase activity. Protein Sci. 2025, 34, e70111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Guan, W.; Cai, L.; Wang, P.G. Enzymatic route to preparative-scale synthesis of UDP-GlcNAc/GalNAc, their analogues and GDP-fucose. Nat. Protoc. 2010, 5, 636–646. [Google Scholar] [CrossRef]
- Zhai, Y.; Liang, M.; Fang, J.; Wang, X.; Guan, W.; Liu, X.W.; Wang, P.; Wang, F. NahK/GlmU fusion enzyme: Characterization and one-step enzymatic synthesis of UDP-N-acetylglucosamine. Biotechnol. Lett. 2012, 34, 1321–1326. [Google Scholar] [CrossRef]
- Li, X.; Qi, C.; Wei, P.; Huang, L.; Cai, J.; Xu, Z. Efficient chemoenzymatic synthesis of uridine 5′-diphosphate N-acetylglucosamine and uridine 5′-diphosphate N-trifluoacetyl glucosamine with three recombinant enzymes. Prep. Biochem. Biotechnol. 2017, 47, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Mahour, R.; Klapproth, J.; Rexer, T.F.T.; Schildbach, A.; Klamt, S.; Pietzsch, M.; Rapp, E.; Reichl, U. Establishment of a five-enzyme cell-free cascade for the synthesis of uridine diphosphate N-acetylglucosamine. J. Biotechnol. 2018, 283, 120–129. [Google Scholar] [CrossRef]
- Nahálka, J.; Pätoprstý, V. Enzymatic synthesis of sialylation substrates powered by a novel polyphosphate kinase (PPK3). Org. Biomol. Chem. 2009, 7, 1778–1780. [Google Scholar] [CrossRef]
- Achbergerová, L.; Nahálka, J. Degradation of polyphosphates by polyphosphate kinases from Ruegeria pomeroyi. Biotechnol. Lett. 2014, 36, 2029–2035. [Google Scholar] [CrossRef]
- Hoang, T.S.; Wormstall, S.T.; Hoang, N.H.; Reichl, U.; Rexer, T.F.T. Establishment of a cell-free multi-enzyme cascade for the synthesis of UDP-GalNAc. N. Biotechnol. 2025, 89, 20–28. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Hu, L.; Xu, R.; Zhang, W.; Hu, S.; Wang, Y.; Du, G.; Kang, Z. Construction of immobilized enzyme cascades for the biosynthesis of nucleotide sugars UDP-N-acetylglucosamine and UDP-glucuronic acid. Syst. Microbiol. Biomanufacturing 2024, 4, 895–905. [Google Scholar] [CrossRef]
- Olsen, L.R.; Vetting, M.W.; Roderick, S.L. Structure of the E. coli bifunctional GlmU acetyltransferase active site with substrates and products. Protein Sci. 2007, 16, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Lyu, S.Y.; Liu, Y.C.; Chang, C.Y.; Wu, C.J.; Li, T.L. Insights into the binding specificity and catalytic mechanism of N-acetylhexosamine 1-phosphate kinases through multiple reaction complexes. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Kuge, M.; Keppler, M.; Friedrich, F.; Saleem-Batcha, R.; Winter, J.; Prucker, I.; Germer, P.; Gerhardt, S.; Einsle, O.; Jung, M.; et al. Structural Insights into Broad-Range Polyphosphate Kinase 2-II Enzymes Applicable for Pyrimidine Nucleoside Diphosphate Synthesis. ChemBioChem 2025, 26, e202400970. [Google Scholar] [CrossRef] [PubMed]
- Alissandratos, A. In vitro multi-enzymatic cascades using recombinant lysates of E. coli: An emerging biocatalysis platform. Biophys. Rev. 2020, 12, 175–182. [Google Scholar] [CrossRef]
- Achbergerová, L.; Nahálka, J. Polyphosphate—an ancient energy source and active metabolic regulator. Microb. Cell Fact. 2011, 10, 63. [Google Scholar] [CrossRef]
- Motomura, K.; Hirota, R.; Okada, M.; Ikeda, T.; Ishida, T.; Kuroda, A. A new subfamily of polyphosphate kinase 2 (Class III PPK2) catalyzes both nucleoside monophosphate phosphorylation and nucleoside diphosphate phosphorylation. Appl. Environ. Microbiol. 2014, 80, 2602–2608. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, W.; Xi, X.; Chen, J.; Wang, Y.; Du, G.; Li, J.; Chen, J.; Kang, Z. Engineering sulfonate group donor regeneration systems to boost biosynthesis of sulfated compounds. Nat. Commun. 2023, 14, 7297. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Y.; Huang, H.; Jin, X.; Li, J.; Du, G.; Kang, Z. Closed-Loop System Driven by ADP Phosphorylation from Pyrophosphate Affords Equimolar Transformation of ATP to 3′-Phosphoadenosine-5′-phosphosulfate. ACS Catal. 2021, 11, 10405–10415. [Google Scholar] [CrossRef]
- Okuyama, K.; Hamamoto, Y.; Ishige, K.; Takenouchi, K.; Noguchi, T. An efficient method for production of uridine 5′-diphospho-N-Acetzlglucosamine. Biosci. Biotechnol. Biochem. 2000, 64, 386–392. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, J.; Nahálka, J.; Wang, P.G. Biocatalytic synthesis of uridine 5′-diphosphate N-acetylglucosamine by multiple enzymes co-immobilized on agarose beads. Chem. Commun. 2002, 21, 2586–2587. [Google Scholar] [CrossRef]
- Caschera, F.; Noireaux, V. A cost-effective polyphosphate-based metabolism Fuels an all E. coli cell-free expression system. Metab. Eng. 2015, 27, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Fujishima, K.; Berhanu, S.; Kuruma, Y.; Jia, T.Z.; Khusnutdinova, A.N.; Yakunin, A.F.; McGlynn, S.E. A Bifunctional Polyphosphate Kinase Driving the Regeneration of Nucleoside Triphosphate and Reconstituted Cell-Free Protein Synthesis. ACS Synth. Biol. 2020, 9, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Tavanti, M.; Hosford, J.; Lloyd, R.C.; Brown, M.J.B. Recent Developments and Challenges for the Industrial Implementation of Polyphosphate Kinases. ChemCatChem 2021, 13, 3565–3580. [Google Scholar] [CrossRef]
- Siedentop, R.; Claaßen, C.; Rother, D.; Lütz, S.; Rosenthal, K. Getting the most out of enzyme cascades: Strategies to optimize in vitro multi-enzymatic reactions. Catalysts 2021, 11, 1183. [Google Scholar] [CrossRef]
- Roberts, T.L.; Dolan, J.P.; Miller, G.J.; Lima, M.A.D.; Cosgrove, S.C. A modular, reusable biocatalytic flow system for UDP-GlcNAc production. React. Chem. Eng. 2025, 10, 1221–1226. [Google Scholar] [CrossRef]
- Fischöder, T.; Wahl, C.; Zerhusen, C.; Elling, L. Repetitive Batch Mode Facilitates Enzymatic Synthesis of the Nucleotide Sugars UDP-Gal, UDP-GlcNAc, and UDP-GalNAc on a Multi-Gram Scale. Biotechnol. J. 2019, 14, 1800386. [Google Scholar] [CrossRef]
- Koszagova, R.; Krajcovic, T.; Palencarova-Talafova, K.; Patoprsty, V.; Vikartovska, A.; Pospiskova, K.; Safarik, I.; Nahalka, J. Magnetization of active inclusion bodies: Comparison with centrifugation in repetitive biotransformations. Microb. Cell Fact. 2018, 17, 139. [Google Scholar] [CrossRef]
- Belkova, M.; Janegova, T.; Hrabarova, E.; Nahalka, J. Physiologically Aggregated LacZ Applied in Trehalose Galactosylation in a Recycled Batch Mode. Life 2023, 13, 1619. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köszagová, R.; Palenčárová, K.; Nahálka, J. Active Inclusion Bodies in the Multienzymatic Synthesis of UDP-N-acetylglucosamine. Int. J. Mol. Sci. 2025, 26, 9679. https://doi.org/10.3390/ijms26199679
Köszagová R, Palenčárová K, Nahálka J. Active Inclusion Bodies in the Multienzymatic Synthesis of UDP-N-acetylglucosamine. International Journal of Molecular Sciences. 2025; 26(19):9679. https://doi.org/10.3390/ijms26199679
Chicago/Turabian StyleKöszagová, Romana, Klaudia Palenčárová, and Jozef Nahálka. 2025. "Active Inclusion Bodies in the Multienzymatic Synthesis of UDP-N-acetylglucosamine" International Journal of Molecular Sciences 26, no. 19: 9679. https://doi.org/10.3390/ijms26199679
APA StyleKöszagová, R., Palenčárová, K., & Nahálka, J. (2025). Active Inclusion Bodies in the Multienzymatic Synthesis of UDP-N-acetylglucosamine. International Journal of Molecular Sciences, 26(19), 9679. https://doi.org/10.3390/ijms26199679





