1. Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by impairments in social communication and the presence of restricted, repetitive behaviors. According to the World Health Organization, approximately 1 in 100 children worldwide is affected by ASD [
1]. In the United States, recent surveillance data indicate a prevalence of 1 in 36 among 8-year-old children, with a male-to-female ratio of approximately 4:1 [
2]. European estimates vary between 0.6% and 1.5%, reflecting differences in diagnostic practices and healthcare accessibility [
3]. While prevalence estimates in Poland remain limited, available data suggest rates comparable to those observed in other European countries [
4]. The observed increase in prevalence over recent decades is attributed to changes in diagnostic criteria, improved awareness, and broader access to diagnostic services, rather than a true rise in incidence [
5]. The disorder exhibits high heritability, with genetic factors contributing substantially to its etiology [
6]. Autism risk is influenced by the cumulative effect of numerous common genetic variants, each contributing a small effect. Genome-wide association studies (GWAS) suggest that these common variants may account for a significant portion of ASD heritability [
7]. In some cases, autism may result from the combined effect of a few rare genetic variants with moderate to large effects. This model suggests that multiple rare variants can interact to increase ASD risk [
8]. Dedicated databases focusing on the genetic underpinnings of autism, such as SFARI Gene, catalog a wide spectrum of candidate genes. Genes are stratified into three evidence-based tiers: Category 1 (High Confidence), Category 2 (Strong Candidate), and Category 3 (Suggestive Evidence). In addition, SFARI designates a distinct syndromic category, which encompasses genes that confer a substantial increase in risk and are consistently associated with additional clinical features beyond those required for an ASD diagnosis [
https://gene.sfari.org, accessed on 12 December 2024].
Careful examination of inheritance is indispensable, since it provides critical insights into the prediction of intricate epigenetic mechanisms underlying ASD. Some families exhibit a pattern where autism is transmitted in a dominant fashion, particularly affecting male offspring. This may occur when a parent, often an unaffected female carrier, passes a variant to her children, with sons being more likely to express the phenotype. [
9] In certain populations or consanguineous families, autosomal recessive inheritance has been observed. This involves both parents carrying one copy of a changed nucleotide, with a 25% chance of passing two copies to their child, leading to ASD. Recent studies have significantly expanded our understanding of autosomal recessive inheritance in autism: A 2023 study from University of California, Los Angeles, identified new candidate genes such as
PLEKHA8,
PRR25,
FBXL13,
VPS54,
SLFN5,
SNCAIP, and
TGM1 in families with multiple autistic children [
10]. Whole-exome sequencing in Qatari families identified
TRPC4 and
SCFD2 as novel autism-related genes under this inheritance pattern [
11,
12]. The “Building a Resource for the Advancement of Knowledge of Autism in Qatar” genome project found 13 new candidate genes with autosomal recessive transmission in consanguineous families [
13].
Over 150 genes on the X chromosome have now been associated with ASD. Many of these genes are involved in chromatin remodeling, synaptic function, and neuronal signaling, underscoring the genetic complexity of autism and the critical role of the X chromosome [
12,
14]. Recent studies underscore the contribution of inherited genetic variants transmitted from parents to the overall risk architecture of autism spectrum disorder. Although both rare de novo variants and common polygenic risk factors have been implicated [
15,
16], the genetic architecture of ASD remains complex and heterogeneous. Recent advances in next-generation sequencing (NGS) have enabled detailed investigation of familial genetic contributions, providing new insights into inherited molecular burdens. In this study, we applied NGS to 42 families with at least one affected individual to explore patterns of inherited and potentially pathogenic variants contributing to ASD susceptibility.
2. Results
From 42 families comprising 138 individuals, high-quality genetic material and sequencing data were obtained for 83 participants, including 33 with autism. Only a subset of these variants was classified as rare in the general population and deemed potentially relevant to ASD pathogenesis. Variants annotated as either benign or pathogenic based on ACMG SF v3.1 criteria were highlighted, and their segregation patterns were analyzed within families. We analyzed the localization of each gene on chromosomes to predict the potential mechanism of inheritance, classified as autosomal (46 genes) or X-linked (HUWE1).
2.1. Candidate Genes in Autism—Insights from DOMINO and SFARI
A total of multiple variants were identified across 47 genes in affected families, with inheritance patterns including maternal, paternal, biparental, and de novo origins. The probability of the inheritance was classified as very likely recessive or likely recessive (30.2%), either dominant or recessive (9.3%), likely dominant or very likely dominant (51.2%) according to DOMINO predictor. Inheritance classification using DOMINO was unavailable for four genes. The selected genes were further classified according to the SFARI framework. Within our cohort, we identified one de novo exonic variant in
HUWE1. For variants with undetermined parental origin due to insufficient data, we detected one exonic variant in
PCDHA1, three exonic variants in
PCDHA7, and one intronic variant in
SNHG14. The list of genes with DOMINO and SFARI classification was shown in
Table 1,
Table 2 and
Table 3, variants identified exclusively in unaffected siblings were shown in
Table 4.
A total of 55 variants were classified by DOMINO analysis as following a dominant mode of inheritance. Among these, eight were maternally inherited, located in CHD8 (chromatin remodeling, autism), EN2 (cerebellar development), SHANK3 (synaptic scaffolding, Phelan–McDermid syndrome), SCN1A (sodium channel, epilepsy), SHANK2 (synaptic scaffolding), NRXN1 (synapse formation, autism/schizophrenia), and RAI1 (transcriptional regulation, Smith–Magenis syndrome).
Eight were paternally inherited, including variants in ARID1A (chromatin remodeling, neurodevelopment), CACNA1C (calcium channel, Timothy syndrome), EN2, SHANK3, and RAI1.
Fifteen were transmitted from both parents, mapping to TBL1XR1 (transcriptional co-regulator, intellectual disability), CACNA1C, EN2, SHANK3, SCN1A, MYT1L (neuronal differentiation), RELN (neuronal migration, cortical layering), and NRXN1.
Of the 22 dominantly inherited genes identified, 18 (81.8%) were classified as Category 1 (High Confidence) in the SFARI Gene database, 2 (9.1%) as Category 2 (Strong Candidate), and 1 (4.5%) as Category 3 (Suggestive Evidence). In addition, 12 genes (54.5%) were associated with genetic syndromes and therefore categorized as syndromic (S).
Among variants with unclear inheritance, we detected only one intronic variant in CACNA2D3 of maternal origin. CACNA2D3 encodes the α2δ3 subunit of voltage-dependent calcium channels, which plays a critical role in regulating calcium influx into neurons and modulating synaptic transmission. It should be noted that three out of four genes were classified as Category 1 (High Confidence) according to the SFARI Gene database, and in two cases, an association with genetic syndromes was identified.
In the case of variants inherited in a recessive manner, 14 were of maternal origin, located in ABCC8 (insulin regulation), TPH2 (serotonin synthesis), NSUN2 (RNA modification), SYNE1 (ataxia), STIL (cell division/brain development), SCN7A (sodium channel), TRAPPC9 (intellectual disability), and VPS13B (Cohen syndrome). Six were of paternal origin, identified in ABCC8, NSUN2, WFS1 (Wolfram syndrome), and STIL. Additionally, 12 were transmitted from both parents, including seven cases in TPH2, as well as variants in MCPH1 (DNA repair/brain size), STIL, and VPS13B. In this case, the vast majority of genes had no established association with ASD according to the SFARI Gene database. Among the 13 identified genes, 2 (15.4%) were classified as Category 1 (High Confidence), 3 (23.1%) as Category 2 (Strong Candidate), while 3 (23.1%) were linked to genetic syndromes.
The variants identified exclusively in the healthy siblings were intronic, three of which followed a recessive mode of inheritance. These occurred in MAGEL2 (neurodevelopment), CACNA1H (neuronal excitability), SLC9A9 (synaptic regulation), and ASPM (cortical development). Their intronic location and inheritance pattern indicate limited clinical relevance. According to the SFARI Gene classification, one gene was assigned to Category 1 (High Confidence) and was also linked to a genetic syndrome, while two genes were classified as Category 2 (Strong Candidate)
When considering all variants collectively, we observed a heterogeneous pattern of inheritance. In total, 23 maternally and 14 paternally inherited variants were identified, spanning both exonic and intronic regions as well as one located in the untranslated region (UTR). In two cases, the parental origin remained ambiguous. Furthermore, 12 de novo variants were detected, distributed across exonic and intronic regions. Of note, four intronic variants were found exclusively in healthy siblings, suggesting limited clinical relevance given their genomic context and inheritance pattern.
2.2. Variant of Uncertain Significance
Pathogenicity assessment using the Franklin database (
https://franklin.genoox.com/, accessed on 20 May 2025) classified five variants as VUS (Variant of Uncertain Significance). The results are summarized in
Table 5.
Two variants were identified in the ABCC8 gene: p.Ala726Thr, inherited from the father, and p.Ser1053Asn, inherited from the mother. Both fulfilled the PM2 criterion, defined as absence or extremely low frequency in large population databases (e.g., gnomAD), and were categorized as PP2, consistent with the low tolerance of ABCC8 to missense variation. In addition to these, a variant in the CUL23 gene (p.Gln343Arg) was identified. This variant also fulfilled PM2, was classified as PP3 by in silico predictions supporting a deleterious effect, and PP2 based on intolerance of the gene to missense changes. The same variant was also present in a healthy sibling. In the TSC2 gene, the variant p.Arg1529Gln was identified in one affected proband as well as in a healthy sibling. This variant was classified as PM2 and PP3, reflecting both rarity in population databases and in silico predictions supportive of a deleterious effect.
A synonymous variant in MCPH1 (p.Arg171=) was identified in three affected children in the homozygous state. This variant was also classified as PM2 based on its absence or extremely low frequency in population databases.
2.3. Segregation Analysis
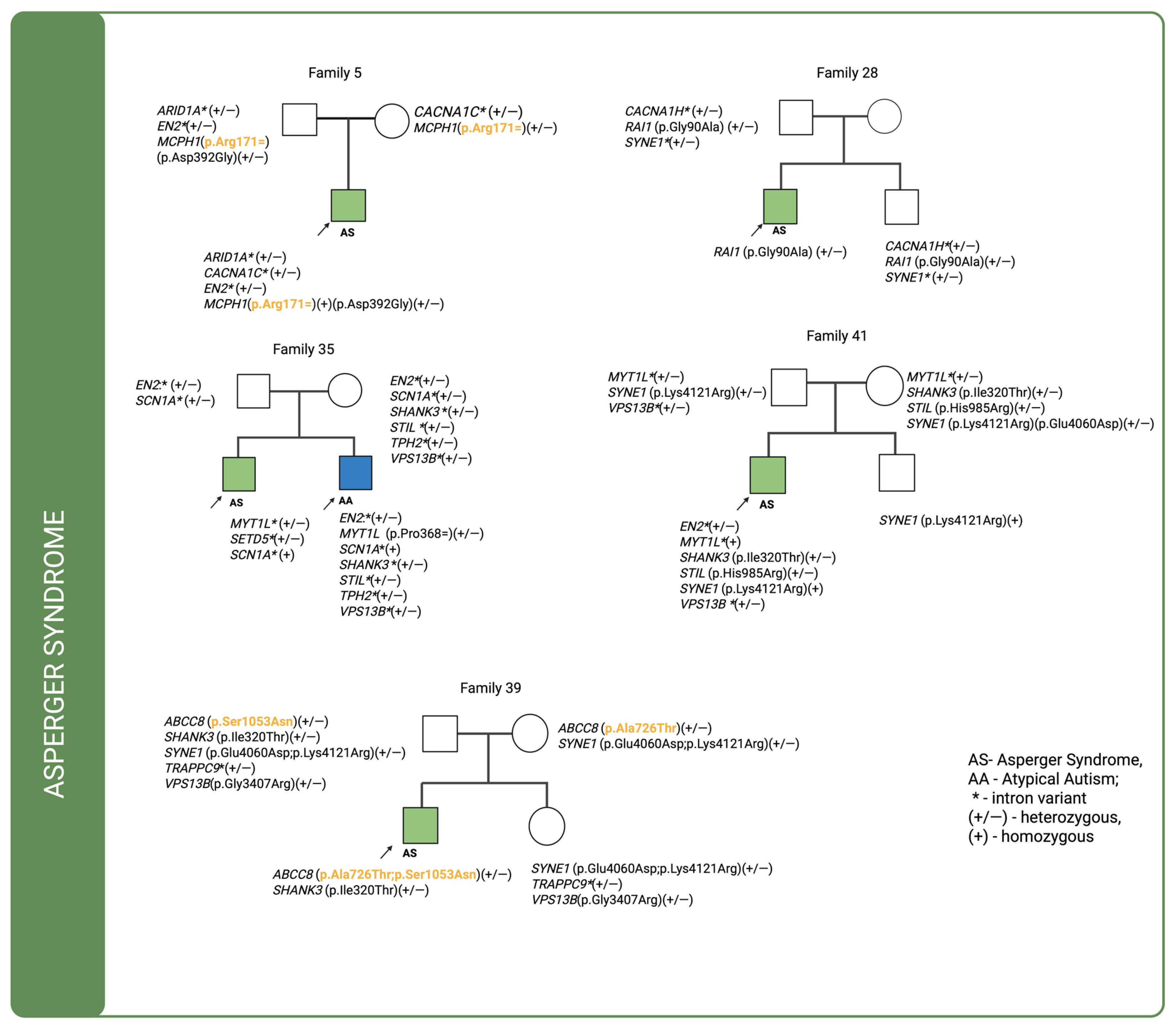
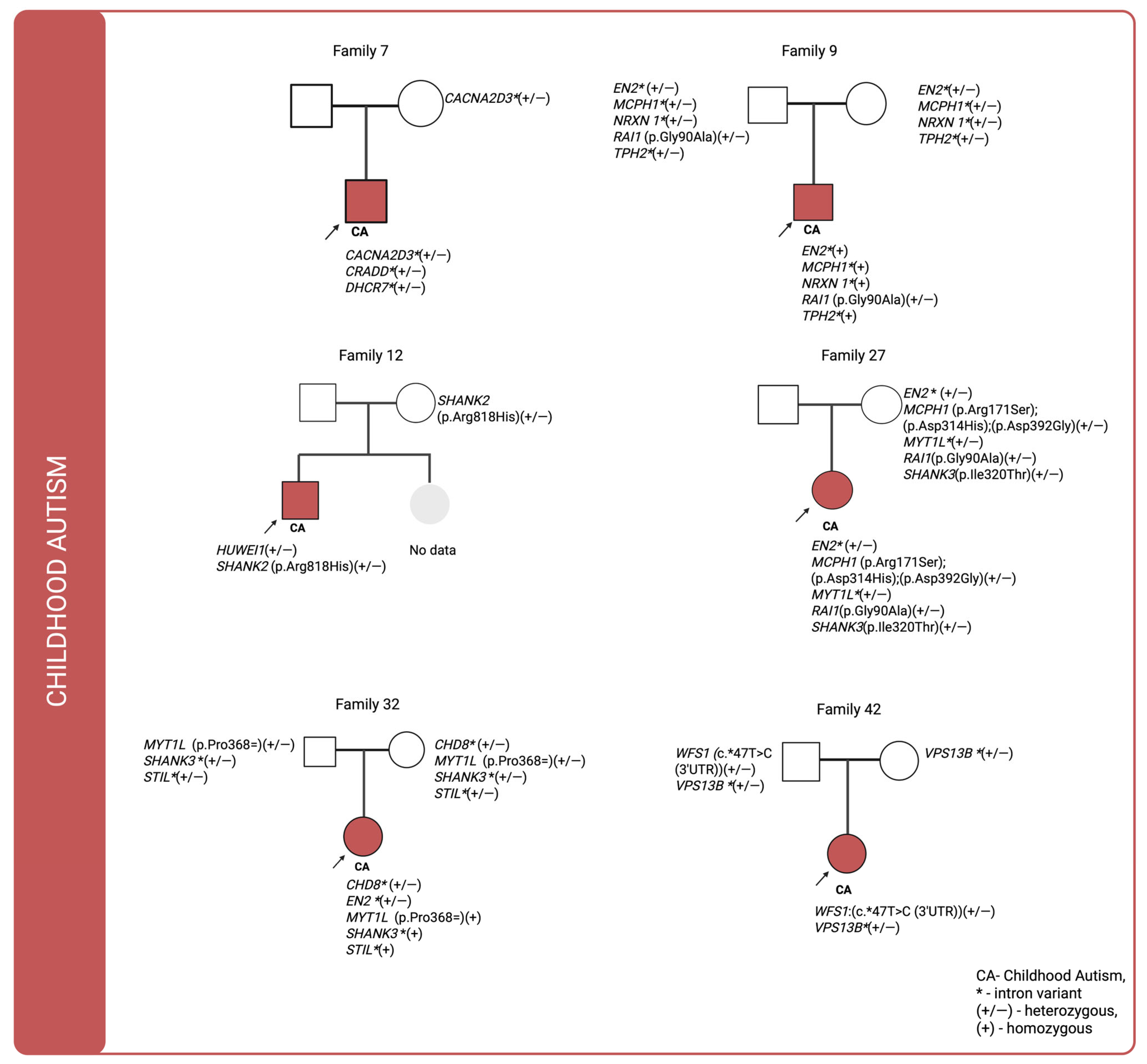
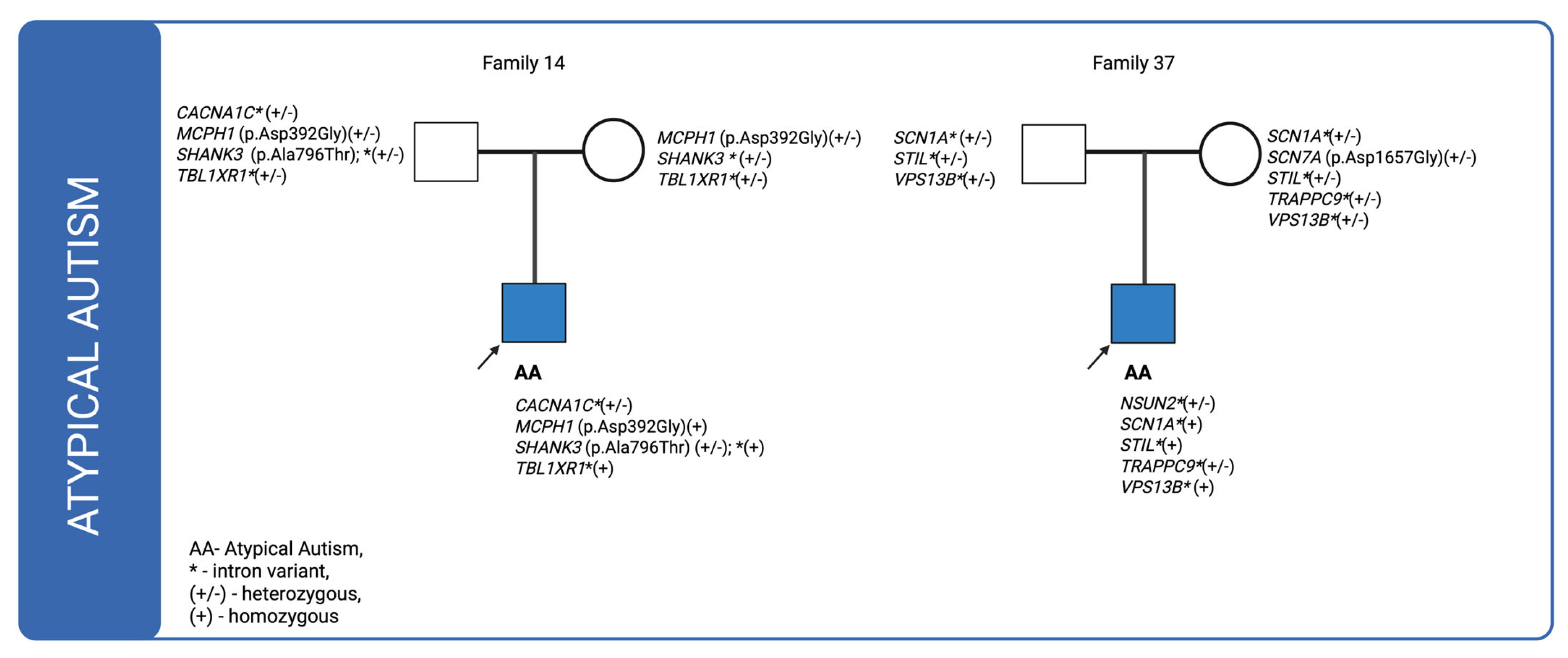
For cases in which multiple genetic variants were detected and sequencing data were available for both affected individuals and their parents, we investigated the co-segregation of these variants in the context of their inheritance modes. The corresponding pedigrees are shown in
Figure 1,
Figure 2 and
Figure 3. In the figures, variants classified as VUS in the Franklin database are highlighted in orange and bolded, whereas the remaining variants are shown as likely benign.
In four out of five Asperger syndrome families, variants were inherited from both parents, with intronic variants being the most prevalent. In family 5, a missense variant in MCPH1 (p.Asp392Gly) was inherited exclusively from the father, together with a synonymous variant (p.Arg171=). The synonymous variant is classified as a VUS (Variant of Uncertain Significance). DOMINO analysis predicts a recessive mode of inheritance, consistent with the segregation pattern in this family, where two altered alleles were transmitted by healthy parents to the affected child. Furthermore, the SFARI Gene database assigns MCPH1 to category 2, indicating strong evidence for its implication in autism spectrum disorder from multiple independent genetic studies, although additional replication and functional validation are warranted to confirm its pathogenic role. In family 28, no maternally inherited variants were identified in the analyzed genes; a missense variant in the RAI1 gene was inherited from the father but was also present in the proband’s unaffected brother. In family 35, which included two affected boys—one diagnosed with Asperger syndrome and the other with atypical autism—only intronic variants were found to be inherited. In the child with Asperger syndrome, no missense variant were identified in the analyzed genes. In contrast, the child with atypical autism carried three de novo variants: a synonymous variant in the MYT1L (p.Pro368=) gene. In family 39, two missense variants in ABCC8 (p.Ala726Thr and p.Ser1053Asn) were identified, each inherited from a different parent, resulting in a compound heterozygous genotype in the proband. Both variants are classified as VUS, yet their combined effect may contribute to the disease phenotype. Consistent with this observation, DOMINO analysis predicts a recessive mode of inheritance for ABCC8, supporting the interpretation that the presence of two deleterious alleles, transmitted independently from healthy parents, may underlie the proband’s condition. Notably, ABCC8 is not listed among autism risk genes in the SFARI Gene database, underscoring the novelty of this finding in the context of neurodevelopmental disorders. Additionally, a paternally inherited variant was detected in the SHANK3 (p.Ile320Thr) gene. None of these variants was present in the proband’s unaffected sister. In family 41, two missense variants—STIL (p.His985Arg) and SHANK3 (p.Ile320Thr)—were inherited from the mother and were absent in the unaffected brother. A SYNE1 (p.Lys4121Arg), variant inherited from the father, was present in both siblings.
Pedigree analysis of individuals diagnosed with childhood autism (ICD-10: F84.0) revealed that in three of six cases, no paternally inherited variants were detected, whereas maternally inherited variants were present in all of them. These variants were predominantly intronic. De novo variants identified in families 7, 12, and 32 were primarily located within intronic loci. In family 9, a missense variant in the RAI1 (p.Gly90Ala) gene was inherited from the father, while only intronic variants were transmitted from the mother. In family 12, no variants were identified in the father’s genome that were passed on to the offspring. A variant potentially affecting amino acid synthesis in SHANK2 (p.Arg818His) was inherited from the mother. Notably, in family 27 there were three maternally inherited missense variants in MCPH1(p.Arg171Ser;p.Asp314His;p.Asp392Gly). In family 42 a variant located in the untranslated region (UTR) of the WFS1 gene and inherited from the father was identified.
In family 14, a missense variant in the
SHANK3 (p.Ala796Thr) gene was inherited from the father, while a variant in
MCPH1 (p.Asp392Gly) was transmitted from both parents, resulting in a homozygous genotype in the affected individual. Case 35, illustrated in
Figure 1, also involves a patient with atypical autism who has an affected sibling. The patient inherited intronic variants from the father and three de novo variants were identified: one synonymous variant in
MYT1(p.Pro368=) and two missense variants in
NIPBL (p.Ala179Thr; p.Asn968Ser). In the case of family 37 only intronic variants were found to be inherited. Notably, one of these, located in
NSUN2, was classified as de novo.
2.4. Variant Segregation Among Siblings
In our cohort, we identified a heterogeneous spectrum of variants across different family structures; the data are shown in
Table 6. The table presents results from families in which sequencing was of high quality and available for both the affected proband and their siblings.
Among nine two-child families with one affected and one unaffected sibling, most variants were observed exclusively in the affected child, including ABCC8 (p.Ser1053Asn), SHANK3 (p.Ile320Thr), and STIL (p.His985Arg). In several families, variants were shared between siblings, either in both (RAI1 p.Gly90Ala) or restricted to the unaffected child (ANKRD11 p.Arg840Gln, SLC9A9 p.Asn43Lys, SYNE1 p.Ser4596Thr, p.Lys4121Arg). In two three-child families with a single affected proband, variants showed partial segregation, with examples present in both the proband and one unaffected sibling (CUL3 p.Gln343Arg, POGZ p.His1363Gln, NRXN1 c.*98A>G), while others were confined to the proband (ANK2, HDAC4, KCNJ11). Finally, in one two-child family with both children affected, we identified a shared MYT1L synonymous variant (c.1104C>A, p.Pro368=). Notably, several of the detected variants represent VUS, including ABCC8 (p.Ala726Thr), CUL3 (p.Gln343Arg), VPS13B (p.Val3780Leu), and TSC2 (p.Arg1529Gln). These VUS were identified both in affected and, in some cases, unaffected siblings, reflecting incomplete segregation within families. The presence of VUS in genes implicated in neurodevelopment highlights potential relevance but precludes definitive interpretation without additional functional or segregation data.
2.5. Family History of Psychiatric Disorders
We additionally examined the prevalence of psychiatric disorders among family members, focusing on schizophrenia (ICD-10 code F20) and bipolar affective disorder (ICD-10 code F31). Evidence of these conditions was identified in 16,7% (7 of 42) of families assessed. To explore potential genetic correlations, we compared the distribution of genetic variants between individuals with and without a documented family history of these psychiatric disorders (de novo variants were excluded from the analysis). The results are shown in
Figure 4. Sequencing data were available for six families with a positive family history and for twenty-six families without such a history.
We found no evidence of an association between family history and the distribution of genetic variants in the analyzed groups.
3. Discussion
Despite decades of research, the genetic basis of autism remains largely unresolved. De novo variants and large CNVs explain only a small subset of cases, while inherited variants of modest effect may interact with these events to influence risk. Such de novo variants, often highly penetrant in males, are thought to drive a considerable fraction of sporadic autism [
9,
17,
18]. This pattern further supports the notion of a sex-specific liability threshold in ASD, whereby females may require a greater cumulative genetic burden to manifest the disorder. In our cohort of 43 ASD cases, 39 were male (90.7%) and 4 were female (9.3%), a distribution consistent with the pronounced male predominance reported across numerous studies. Inherited variation, while generally of lower penetrance, can interact with spontaneous changes to influence disease expression, especially in simplex families. By our family-based genomic investigation into the genetic underpinnings of ASD, we successfully identified a diverse landscape of variants across 47 candidate genes, allowing for a detailed examination of their inheritance patterns. Among these, we identified five variants classified as VUS (Variants of Uncertain Significance), distributed across four genes, as well as additional variants classified as likely benign and observed at varying frequencies in the population. Together, these findings highlight both the complexity of variant interpretation and the challenges in delineating their contribution to disease risk.
While the use of non-invasive buccal swabs presented challenges in DNA quality that precluded the definitive assignment of inheritance for every detected variant, our analysis of high-confidence calls revealed distinct and informative patterns of genetic transmission. A significant number of variants were traced to a maternal origin, affecting genes implicated in biological processes fundamental to neurodevelopment. Notably, we identified maternally inherited missense variants in genes crucial for synaptic signaling and structure, such as
SHANK2,
SHANK3, and
NRXN1, which are well-established risk factors for ASD [
19,
20]. Further variants were identified in critical neurodevelopmental regulators, including
CHD8 and
RAI1, as well as in genes involved in ion channel function (
ABCC8,
SCN7A) and key metabolic or structural pathways (
VPS13B,
STIL,
SYNE1). The DOMINO classification predicted a dominant mode of inheritance for the core synaptic genes
SHANK2,
SHANK3, and
NRXN1, indicating that even a single altered allele could contribute to ASD liability. It is worth noting that 9 out of 17 maternally inherited genes (52.9%) were classified as Category 1 (High Confidence) in the SFARI Gene database, while 3 (17.6%) were assigned to Category 2 (Strong Candidate). Four genes (23.5%) were associated with genetic syndromes. Although SFARI classification applies to genes rather than individual variants, it provides a useful framework for selecting molecular targets for panel-based studies. In our cohort, the respective variants were classified as likely benign; however, this does not exclude their potential contribution to disease through interaction with other genetic alterations or environmental factors.
Paternally inherited variants overlapped with maternally implicated genes, with missense changes observed in
SHANK3,
RAI1, and
ABCC8, suggesting that these loci may represent points of vulnerability independent of parental origin. Consistent with this, heterozygous
SHANK2 variants disrupt synapse formation and neuronal morphology, contributing to autistic traits [
20], while SHANK3 and NRXN1 variants have also been reported in ASD-affected children [
19]. Similarly,
CHD8 has emerged as a central regulator of neurogenesis, with de novo variants strongly associated with autism [
21].
The functional role of
ABCC8, which encodes the sulfonylurea receptor 1 (SUR1)—a regulatory subunit of ATP-sensitive potassium (
KATP) channels—further underscores the potential importance of ion channel genes. By coupling cellular metabolism to membrane excitability in both pancreatic β-cells and neurons, disruptions of
KATP channel function may alter excitability and signaling, a mechanism that could be relevant in neurodevelopmental disorders such as ASD [
22,
23].
Variants were also detected in
WFS1, including one in the 3′ untranslated regions (3′UTR), potentially affecting post-transcriptional regulation. While no conclusive evidence links
WFS1 directly to ASD, this gene is well established as the cause of Wolfram syndrome—a recessive neurodegenerative disorder characterized by diabetes mellitus, optic atrophy, and hearing loss—as well as autosomal dominant forms of non-syndromic deafness [
24]. Although isolated reports have described autism-like traits in individuals with
WFS1 variants, the evidence remains limited and inconclusive [
25]. According to DOMINO,
WFS1 variants are typically predicted to follow a recessive inheritance pattern; however, clinical observations, particularly in DFNA6 (a non-syndromic form of low-frequency sensorineural hearing loss), demonstrate that certain
WFS1 alleles can act dominantly. This duality in inheritance underscores the allele-specific nature of
WFS1 pathogenicity, likely shaped by dosage sensitivity and tissue-specific expression, and raises the possibility that dominant-acting variants could, under certain conditions, contribute to neuropsychiatric phenotypes. Perhaps one of the most compelling findings of our study was the identification of homozygous missense variants, which points to a biparental inheritance pattern, in four critical neurodevelopmental genes:
SHANK3,
MYT1L,
MCPH1, and
VPS13B. A particularly heavy burden was observed in
MCPH1, a gene encoding a centrosomal protein vital for neurogenesis, where we documented seven distinct missense changes. The classification of
MCPH1 as a strong candidate gene for ASD by the SFARI database, combined with the recessive inheritance model predicted by DOMINO, strongly aligns with our findings and supports the pathogenic potential of these homozygous variants. In our study, we identified a synonymous variant in
MCPH1(p.Arg171=), classified as a VUS, which was present in three affected children in the homozygous state, further underscoring its potential relevance in disease etiology. We also identified, homozygous variants in
MYT1L, a high-confidence ASD risk gene that encodes a transcription factor essential for establishing and maintaining neuronal identity, further underscore the theme of disrupted core neurodevelopmental processes. Beyond these clearer patterns, our analysis also uncovered more complex instances of inheritance that highlight the multifaceted nature of genetic risk. For example, in one proband from Family 39, we identified two different missense variants within the
ABCC8 gene (p.Ala726Thr; p.Ser1053Asn). One was inherited from the mother and the other from the father, representing a classic biallelic inheritance pattern often associated with recessive conditions or synergistic effects. Importantly, these variants were not identified in the unaffected sibling, further supporting their potential relevance to disease manifestation. Both variants were classified as VUS and occur at very low frequencies in population databases, consistent with potential pathogenic relevance. The co-occurrence of two rare missense variants in trans is particularly noteworthy, as it highlights a possible cumulative effect on protein function and underscores the importance of considering compound heterozygosity in the genetic architecture of ASD Collectively, these findings paint a detailed picture of a complex genetic architecture in ASD, where maternal, paternal, and biparental inheritance patterns all contribute to the landscape of risk, acting through genes critical to synaptic function and brain development. The potential involvement of some genes, such as
WFS1,
ABCC8, and
SCN7A, remains speculative and should be regarded as hypotheses requiring further validation rather than definitive associations.
In addition, we identified homozygous variants in
MYT1L, which encodes a neuron-specific transcription factor essential for maintaining neuronal identity and regulating neurodevelopmental gene expression. Loss-of-function variants in
MYT1L have been recurrently described in individuals with neurodevelopmental disorders, including ASD, intellectual disability, and obesity. Supporting its causal role, Weigl et al. (2023) demonstrated that
MYT1L haploinsufficiency in mice leads to disrupted cortical development, altered synaptic gene expression, and ASD-like phenotypes such as impaired social interactions and increased neuronal excitability [
26]. Collectively, these findings underscore
MYT1L as a high-confidence neurodevelopmental risk gene with direct relevance to ASD.
3.1. De Novo Variants
Multiple studies have demonstrated that de novo variants contribute substantially to ASD risk, as evidenced by comparisons between probands and unaffected siblings showing an excess burden of protein-altering de novo variants in affected individuals. These variants often occur in genes with established roles in neurodevelopment, synaptic architecture, and neuronal signaling. For example, O’Roak et al. (2014) identified recurrent de novo variants in
CHD8,
DYRK1A, and
SCN2A in simplex families, where only one child is affected by ASD [
18]. Such findings support a model in which highly penetrant de novo events in genes critical to brain development act as primary drivers of sporadic cases.
In our cohort, several compelling de novo missense variants were identified in probands within genes strongly implicated in neurodevelopmental disorders, including
NIPBL,
TSC2,
MYT1L,
CRADD, and
HUWE1. The clinical relevance of these findings is underscored by the established roles of these genes in related syndromes:
NIPBL is the major causal gene in Cornelia de Lange syndrome, frequently associated with autistic features; pathogenic variants in
TSC2 cause Tuberous Sclerosis Complex, which is highly comorbid with ASD [
27,
28,
29,
30,
31,
32]; and
HUWE1 is an X-linked gene associated with intellectual disability and ASD-like phenotypes [
33]. Notably, within TSC2, we identified a variant classified as VUS (p.Arg1529Gln), which, despite its current uncertain significance, is of particular interest given the critical role of TSC2 in mTOR pathway regulation and neurodevelopment. The presence of de novo variants in these functionally essential genes strongly supports their potential pathogenic contribution to ASD in our cohort.
Beyond cohort-level observations, our study also enabled a detailed intra-familial comparison in a multiplex family with two siblings diagnosed on the autism spectrum. Here, we observed both shared inherited and unique de novo variants contributing to divergent phenotypes. The first sibling, diagnosed with Asperger syndrome, carried two de novo intronic variants in MYT1L and SETD5, together with a homozygous intronic variant in SCN1A inherited from both parents. The second sibling, diagnosed with atypical autism, presented four distinct de novo variants—an intronic change in EN2, a synonymous variant in MYT1L, in addition to the same homozygous SCN1A variant shared with the brother. Although these variants were individually classified as benign or likely benign, this does not diminish their potential importance, as ASD is increasingly regarded as a multifactorial condition in which such variants may exert a cumulative effect or interact with additional genetic and environmental factors. These findings highlight the coexistence of common inherited risk factors and distinct de novo events within the same family, underscoring the complex interplay between background genetic susceptibility and individual mutational landscapes in shaping ASD phenotypes.
3.2. Family History of Mental Disorders
The investigation of family history in relation to ASD risk supports a significant association between these factors. Lin et al. reported that the prevalence of schizophrenia or bipolar disorder was 1.8% among typically developing children, compared with 5.2% in those diagnosed with ASD [
30]. In our cohort, the proportion was even higher, with 16.67% of children having a positive family history.
Consistent with these observations, Lin and colleagues further noted that a family history of schizophrenia is associated with nearly a threefold increase in the risk of ASD, while bipolar disorder confers a more moderate, though still elevated, risk. Findings from the large cross-disorder genome-wide meta-analysis conducted by the Cross-Disorder Group of the Psychiatric Genomics Consortium provide compelling evidence that genetic risk factors for major psychiatric conditions transcend traditional diagnostic categories. In that study, nearly 75% of genome-wide significant loci exhibited pleiotropic effects, with the strongest genetic correlation (rg, i.e., the genome-wide genetic correlation coefficient) observed between schizophrenia (SCZ) and bipolar disorder (BD), estimated at approximately 0.70. Autism spectrum disorder (ASD) also demonstrated significant, albeit more moderate, overlap with both SCZ and BD. Recurrent copy-number variants, including [
30,
31] those at 15q11.2 and 17q12, were implicated across ASD and SCZ, highlighting convergent molecular pathways involved in neurodevelopment and synaptic regulation [
31].
Evidence from large population-based studies underscores the role of shared genetic susceptibility across these disorders [
30,
31].
3.3. Study Limitations
This study has several limitations. First, the biological material obtained from affected children (buccal swabs) was frequently of suboptimal quality and often yielded insufficient cellular content for high-confidence analysis. Second, the available family history data on psychiatric disorders lacked clinical specificity and detail, limiting their utility for stratified genetic comparisons. Third, although whole-genome sequencing (WGS) provides a comprehensive assessment of genetic variation, its full potential cannot be realized due to sample quality constraints. Fourth, the sample size was modest, limiting statistical power and generalizability, and should be expanded in future work. Finally, while DOMINO offers valuable predictions of inheritance patterns, its accuracy depends on previously annotated genes and may be less reliable for poorly characterized loci. These constraints highlight the importance of cautious interpretation and underscore the need for independent experimental validation.
In summary, our study highlights the significant contribution of both de novo and inherited genetic variation to autism spectrum disorder (ASD), with particular emphasis on genes implicated in synaptic function and early neurodevelopment. Variants exhibited diverse inheritance patterns—maternally, paternally, biparentally, or arising de novo. Maternally inherited variants were more frequently located within intronic regions, whereas de novo variants occurred across both coding and non-coding sequences. Paternally inherited variants included both exonic and regulatory changes, with several affecting genes previously associated with neurodevelopmental phenotypes, suggesting a potential pathogenic role. Biparentally inherited variants, often identified in a homozygous state, were enriched in genes predicted to follow a recessive mode of inheritance, supporting their possible involvement through compound heterozygosity or dosage effects.
4. Materials and Methods
Design: A total of 42 ASD-affected families were included in the study. Among the 43 affected children, 39 were boys (90.7%) and 4 were girls (9.3%). Family structures were distributed as follows: among the 43 families included in the study, 31 (72.1%) had a single affected child with ASD, 9 (20.9%) comprised one affected child and one unaffected sibling, 2 (4.7%) had three children of whom only one was affected, and 1 family (2.3%) had two children diagnosed with ASD. In two cases the DNA concentration was insufficient, and these samples were excluded from analysis. In one family the biological father was absent, and in another one father refused a contribution in research. Finally, we collected 139 samples. The diagnosis of ASD was made by a specialist of child and adolescent psychiatrist according to DSM-5 rules.
Sample collection and DNA isolation: The genomic DNA was collected from volunteers according to buccal swab collection protocol. The patient rinses the mouth with water for at least 30 s, to remove any food particles or contaminates that might affect sample quality. At least 1 min was required to collect desquamated epithelial cells from the oral mucosa. The collected swab was put into the probe with a lysis buffer immediately. The isolation was conducted using gravity-flow method (Genomic Micro AX Swab Gravity Plus, A&ABiotechnology, Gdansk, Poland) according to manufacturers. DNA concentration was determined using spectrophotometric method (DeNovix DS-11+, DeNovix, Wilmington, DE, USA) and fluorometric method (QuantiFluo
® ONE dsDNA System (Promega, Madison, WI, USA). Good quality DNA (min. 10 ng/µL) was further analyzed. The identification of genetic variants in 236 genes associated with autism was conducted using NGS method (Illumina Autism Research Panel) The full list of analyzed amplicons is available on manufacturer’s website (
https://emea.illumina.com/products/by-brand/ampliseq/community-panels/autism.html, accessed on 1 September 2022).
Library preparation and Sequencing Libraries were constructed using the AmpliSeq for Illumina Library Prep (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. Validation and Quantification of the libraries were performed using quantitative PCR (qPCR) protocol. Library normalization and pooling were prepared according to AmpliSeq for Illumina Autism Research Panel. Sequencing was carried out using the MiSeq sequencing platform (Illumina, Inc., San Diego, CA, USA). Using MiSeq Reagent Kits v2, (Illumina, Inc., San Diego, CA, USA).
Data analysis: The quality analysis of raw FASTQ data was performed using FastQC version 0.12.0 [
https://www.bioinformatics.babraham.ac.uk/projects/fastqc/] (accessed on 12 December 2024). Based on the analysis, data quality was assessed, and criteria were established for discarding low-quality reads. Additionally, a decision was made to trim the first 17 nucleotides due to poor sequencing quality using the fastx-Toolkit software [
https://github.com/agordon/fastx_toolkit] (accessed on 12 December 2024). The report did not indicate any overrepresented sequences. Read mapping was carried out using Bowtie2 version 2.5.4 [
32] to the hg19 reference genome provided by the UCSC Genome Browser [
hgdownload.soe.ucsc.edu/goldenPath/hg19/bigZips] (accessed on 12 December 2024). Duplicate marking and removal were then performed using Picard:MarkDuplicates [broadinstitute.github.io/picard] (accessed on 12 December 2024).
SNP and INDEL Variant Calling and Annotation: To call germline variants, a pileup file was first generated using Samtools: samtools mpileup -B -f [reference sequence] [BAM file] >myData.pileup [
34]. Genetic variants were called using VarScan 2 [
https://varscan.sourceforge.net/, accessed on 12 December 2024] with the mpileup2 protocol for variants with at least x8 coverage --min-coverage 8, minimum frequency 0.01 --min-var-freq 0.01, and statistical significance greater than 0.05 --
p-value 0.05. The filtering criteria were the same for single nucleotide variants and structural variants. The detected variants were annotated using the web-based version of wAnnovar in relation to the hg19 reference genome [
35]. Annotated variants were cross-referenced with established databases including VarSome, ClinVar, gnomAD, dbSNP, SnpEff, and LiftVar. Variant classification was assigned according to the American College of Medical Genetics and Genomics Secondary Findings Version 3.1 (ACMG SF v3.1) guidelines. All identified variants were additionally verified using the Franklin platform (Genoox;
https://franklin.genoox.com/) (accessed on 20 May 2025).
Statistical analysis and data visualization: Statistical analysis was conducted using Statistica software [Statsoft Poland, v.13.3.7] and R [v 4.1.2, R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL
https://www.R-project.org/, accessed on 12 December 2024]. The probability of inheritance was estimated using DOMINO, a tool assessing the likelihood for a gene to harbor dominant changes. The method is based on linear discriminant analysis (LDA) trained on a set of genes with known inheritance mode on series of specific features, and finally validated with an independent group of genes [
36]. The figures were created using BioRender (
https://biorender.com) (accessed on 30 May 2025).