When Fat Talks: How Adipose-Derived Extracellular Vesicles Fuel Breast Cancer
Abstract
1. Introduction
2. Results
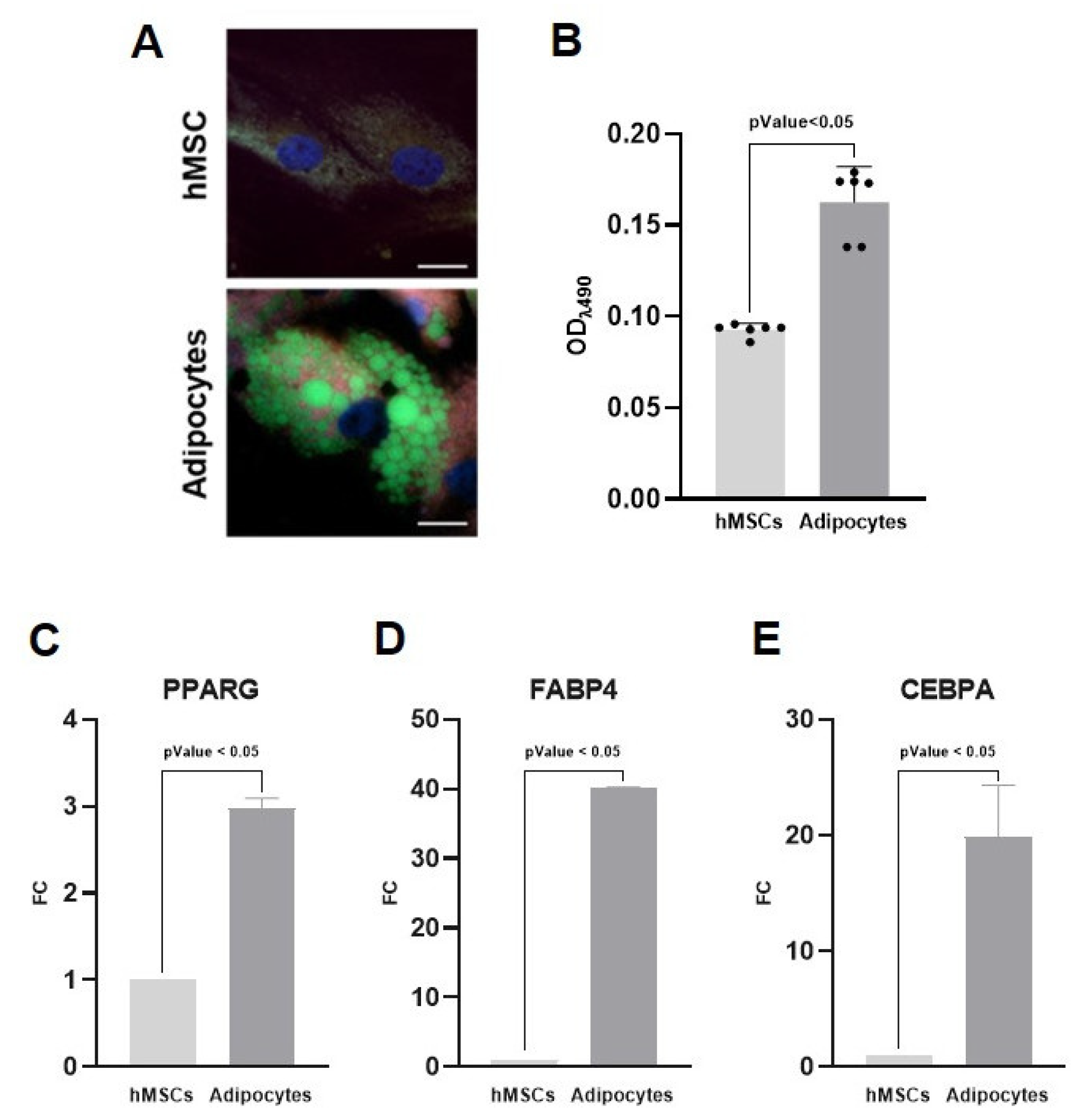
2.1. Adipose Tissue Reconstruction
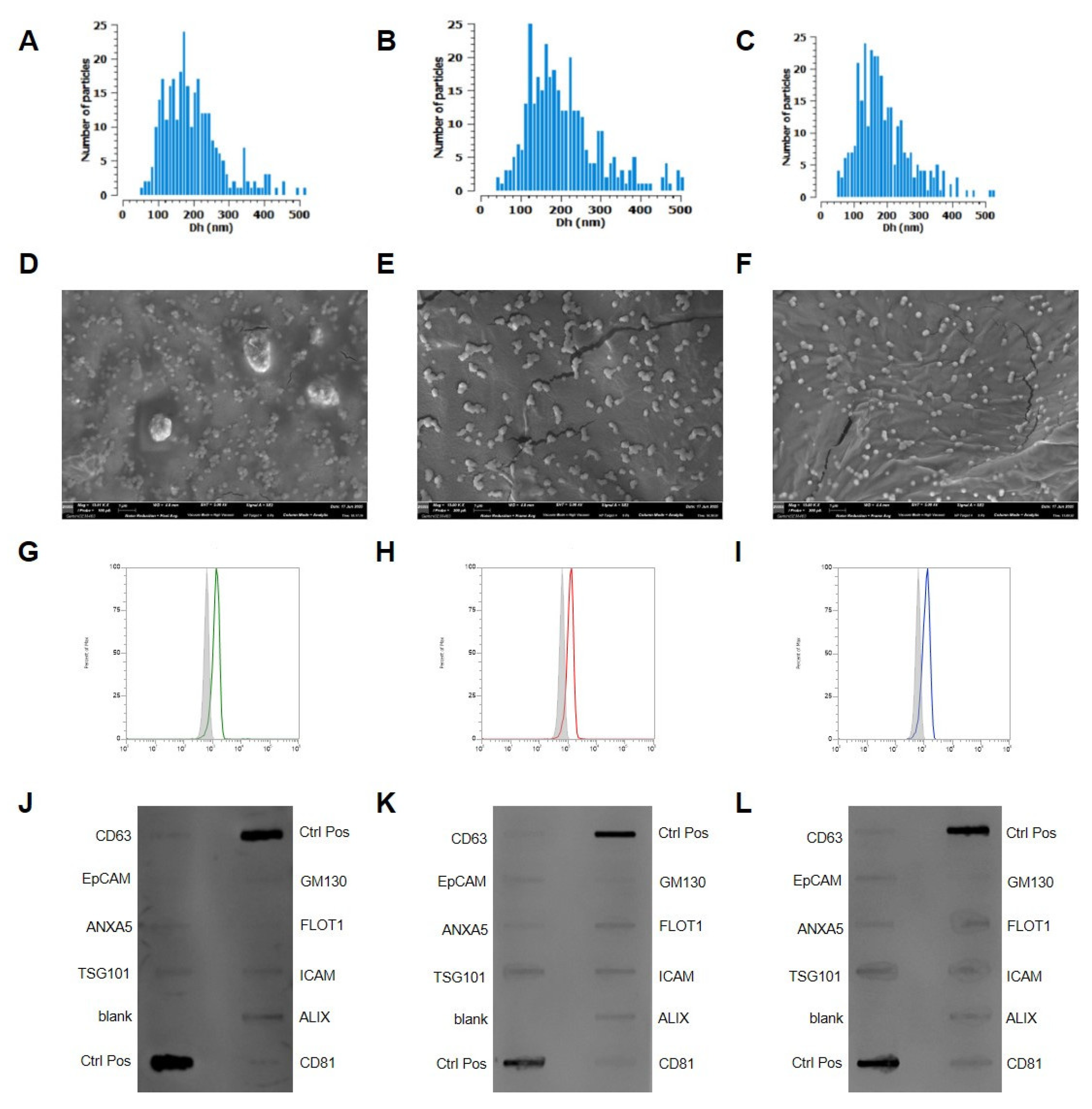
2.2. Extracellular Vesicles Isolation and Characterization
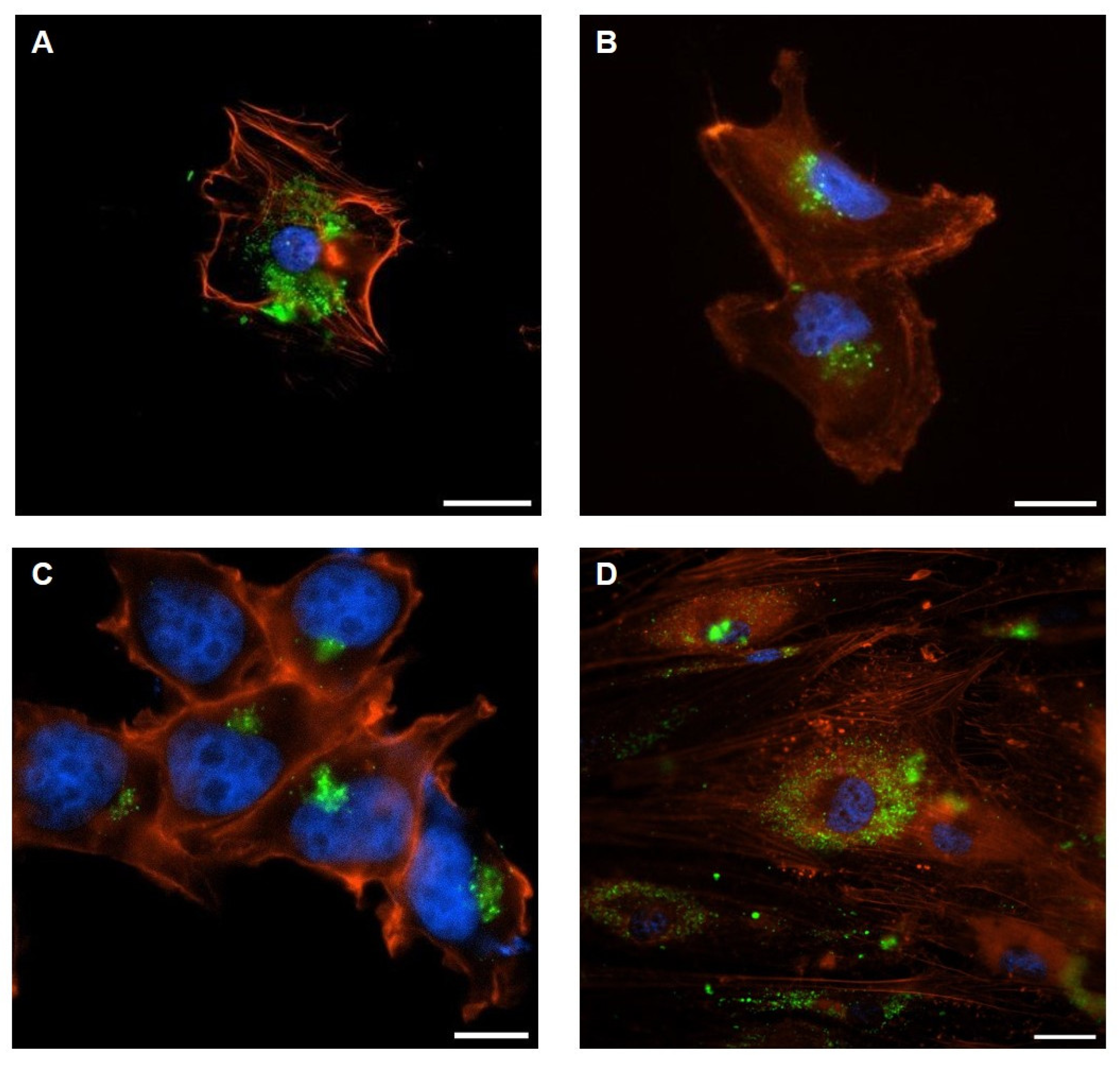
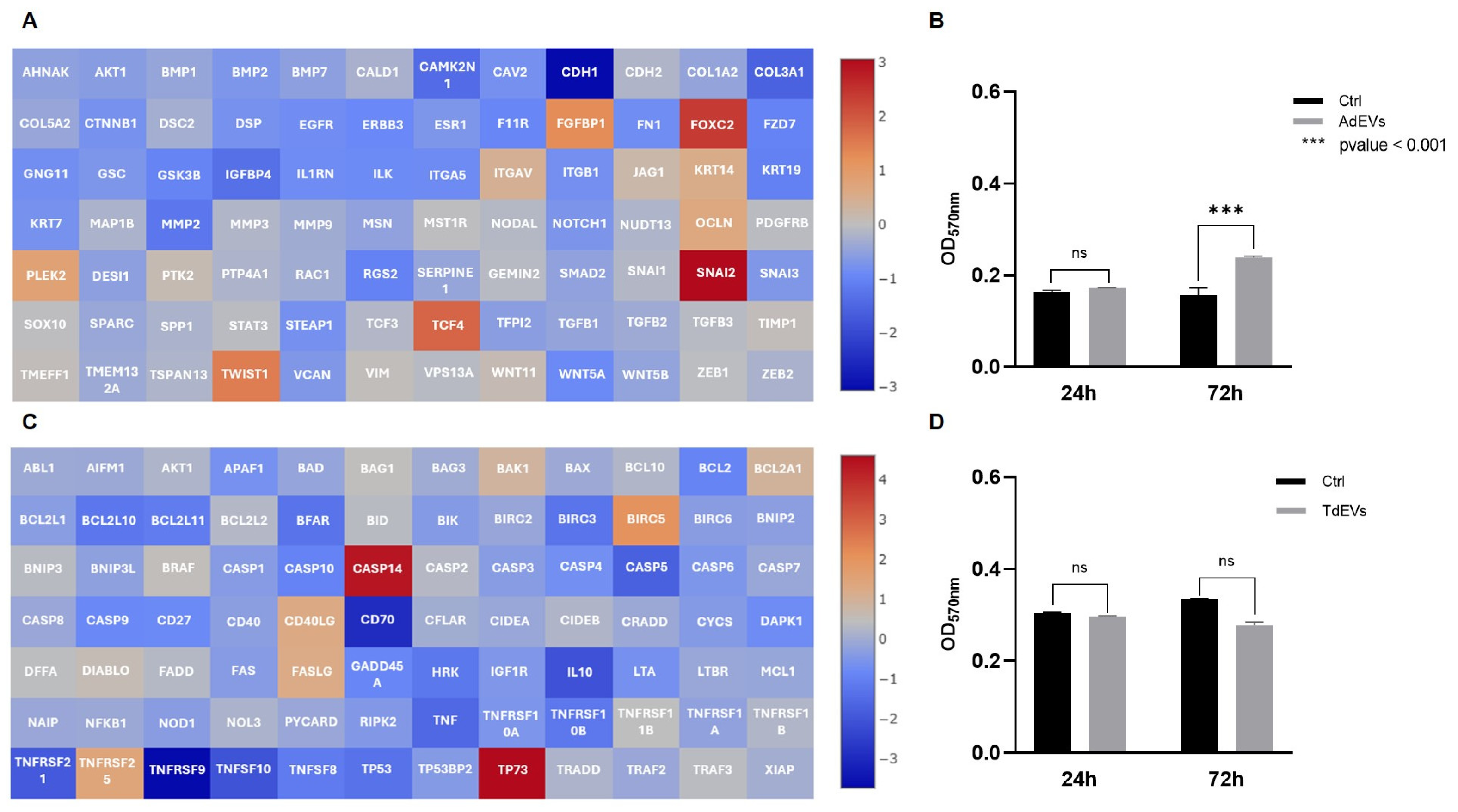
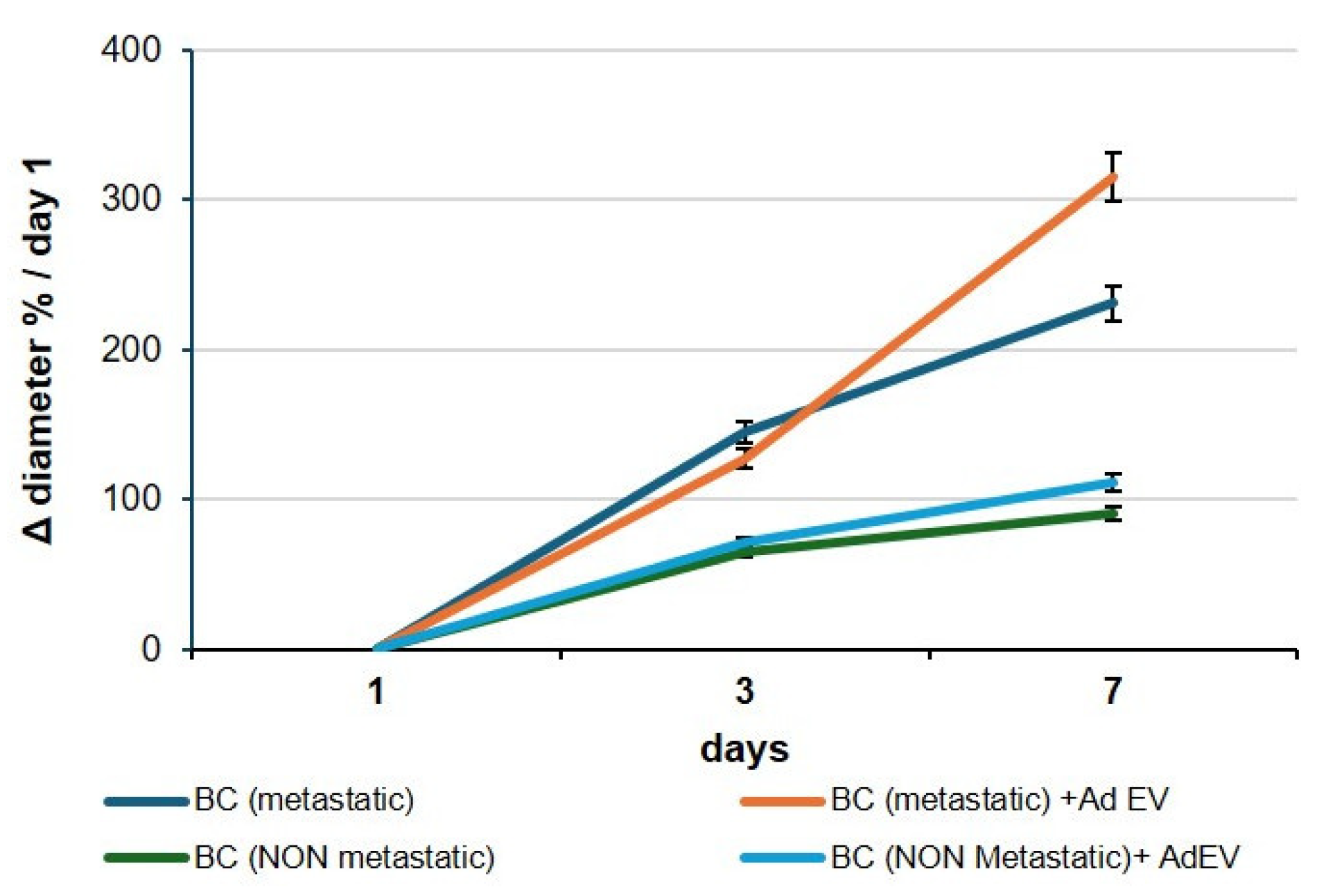
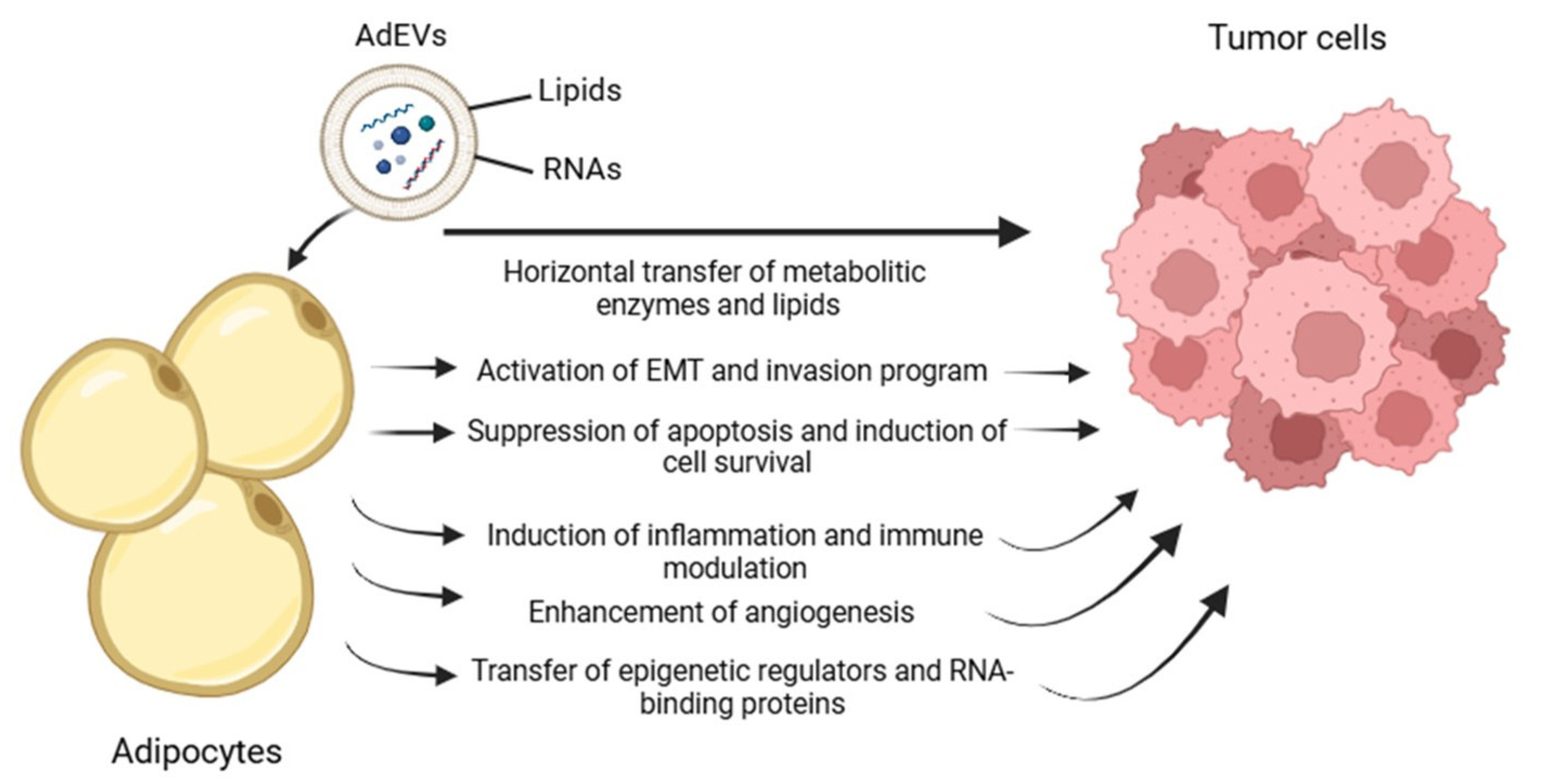
2.3. Bidirectional Communication Mediated by AdEV and TdEV
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Lipid Quantification with Oil Red O Staining
4.3. Isolation of Extracellular Vesicles
4.4. Quantification and Size Characterization of Extracellular Vesicles
4.5. Transmission Electron Microscopy of Extracellular Vesicles
4.6. Cell Treatments with Extracellular Vesicles
4.7. Cell Viability Assay
4.8. Fluorescence Microscopy
4.9. RNA Extraction and Purification
4.10. Organoid Production and Treatments
4.11. Gene Expression Analysis
4.12. Statistical and Bioinformatics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AdEVs | adipocyte-derived EVs |
| BC | breast cancer |
| ECM | extracellular matrix |
| EMT | epithelial-to-mesenchymal transition |
| EVs | extracellular vesicles |
| hMSCs | human mesenchymal stem cells |
| TdEVs | tumor-derived EVs |
| TME | tumor microenvironment |
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, M.; Xu, Y. Understanding the mechanisms underlying obesity in remodeling the breast tumor immune microenvironment: From the perspective of inflammation. Cancer Biol. Med. 2023, 20, 268–286. [Google Scholar] [CrossRef]
- Li, J.; Han, X. Adipocytokines and breast cancer. Curr. Probl. Cancer 2018, 42, 208–214. [Google Scholar] [CrossRef]
- Blücher, C.; Stadler, S.C. Obesity and Breast Cancer: Current Insights on the Role of Fatty Acids and Lipid Metabolism in Promoting Breast Cancer Growth and Progression. Front. Endocrinol. 2017, 8, 293. [Google Scholar] [CrossRef]
- Zanolla, I.; Trentini, M.; Tiengo, E.; Zanotti, F.; Pusceddu, T.; Rubini, A.; Rubini, G.; Brugnoli, F.; Licastro, D.; Debortoli, M.; et al. Adipose-derived stem cell exosomes act as delivery vehicles of microRNAs in a dog model of chronic hepatitis. Nanotheranostics 2024, 8, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Huang, R.; Wumaier, R.; Lyu, J.; Huang, M.; Zhang, Y.; Chen, Q.; Liu, W.; Tao, M.; Li, J.; et al. Proteomic Profiling of Serum Extracellular Vesicles Identifies Diagnostic Signatures and Therapeutic Targets in Breast Cancer. Cancer Res. 2024, 84, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Benito-Martin, A.; Pelissier Vatter, F.A.; Hanif, S.Z.; Liu, C.; Bhardwaj, P.; Sethupathy, P.; Farghli, A.R.; Piloco, P.; Paik, P.; et al. Breast adipose tissue-derived extracellular vesicles from obese women alter tumor cell metabolism. EMBO Rep. 2023, 24, e57339. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.C.; Pires, J.; Gonzalez, E.; Garcia-Vallicrosa, C.; Reis, C.A.; Falcon-Perez, J.M.; Freitas, D. Extracellular vesicles in tumor-adipose tissue crosstalk: Key drivers and therapeutic targets in cancer cachexia. Extracell. Vesicles Circ. Nucl. Acids 2024, 5, 371–396. [Google Scholar] [CrossRef]
- Caprara, G.; Pallavi, R.; Sanyal, S.; Pelicci, P.G. Dietary Restrictions and Cancer Prevention: State of the Art. Nutrients 2025, 17, 503. [Google Scholar] [CrossRef]
- Prakash, J.; Shaked, Y. The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov. 2024, 14, 1375–1388. [Google Scholar] [CrossRef]
- Popova, N.V.; Jücker, M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- La Camera, G.; Gelsomino, L.; Malivindi, R.; Barone, I.; Panza, S.; De Rose, D.; Giordano, F.; D’Esposito, V.; Formisano, P.; Bonofiglio, D.; et al. Adipocyte-derived extracellular vesicles promote breast cancer cell malignancy through HIF-1α activity. Cancer Lett. 2021, 521, 155–168. [Google Scholar] [CrossRef]
- Liu, S.; Benito-Martin, A.; Vatter, F.A.P.; Hanif, S.Z.; Liu, C.; Bhardwaj, P.; Sethupathy, P.; Farghli, A.R.; Piloco, P.; Paik, P.; et al. Breast adipose tissue-derived extracellular vesicles from women with obesity stimulate mitochondrial-induced dysregulated tumor cell metabolism. bioRxiv 2023. [Google Scholar] [CrossRef]
- Jafari, N.; Kolla, M.; Meshulam, T.; Shafran, J.S.; Qiu, Y.; Casey, A.N.; Pompa, I.R.; Ennis, C.S.; Mazzeo, C.S.; Rabhi, N.; et al. Adipocyte-derived exosomes may promote breast cancer progression in type 2 diabetes. Sci. Signal 2021, 14, eabj2807. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, Q.; Rajasekaran, S.; Wu, R. MMP3 at the crossroads: Linking molecular pathways to disease diagnosis and therapy. Pharmacol. Res. 2025, 216, 107750. [Google Scholar] [CrossRef]
- Spada, S.; Tocci, A.; Di Modugno, F.; Nisticò, P. Fibronectin as a multiregulatory molecule crucial in tumor matrisome: From structural and functional features to clinical practice in oncology. J. Exp. Clin. Cancer Res. 2021, 40, 102. [Google Scholar] [CrossRef]
- Sun, N.; Zhao, X. Therapeutic Implications of FABP4 in Cancer: An Emerging Target to Tackle Cancer. Front. Pharmacol. 2022, 13, 948610. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef]
- Hetmanski, J.H.R.; Schwartz, J.M.; Caswell, P.T. Rationalizing Rac1 and RhoA GTPase signaling: A mathematical approach. Small GTPases 2018, 9, 224–229. [Google Scholar] [CrossRef]
- Błachnio-Zabielska, A.U.; Sadowska, P.; Chlabicz, U.; Pogodzińska, K.; Le Stunff, H.; Laudański, P.; Szamatowicz, J.; Kuźmicki, M. Differential Effects of Sphingolipids on Cell Death and Antioxidant Defenses in Type 1 and Type 2 Endometrial Cancer Cells. Int. J. Mol. Sci. 2025, 26, 4472. [Google Scholar] [CrossRef] [PubMed]
- Rambold, A.S.; Cohen, S.; Lippincott-Schwartz, J. Fatty acid trafficking in starved cells: Regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 2015, 32, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Zhang, D.; Sun, X.; Wu, Y.; Wang, J.; Li, Q.; Jiang, G. The macrophage polarization by miRNAs and its potential role in the treatment of tumor and inflammation (Review). Oncol. Rep. 2023, 50, 190. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.; Liang, H.; Fan, Q.; Zhu, R.; Cui, J.; Zhang, W.; Zen, K.; Zhang, C.-Y.; Hou, D.; et al. miR-23a/b promote tumor growth and suppress apoptosis by targeting PDCD4 in gastric cancer. Cell Death Dis. 2017, 8, e3059. [Google Scholar] [CrossRef]
- Zhao, Y.K.; Zhu, X.D.; Liu, R.; Yang, X.; Liang, Y.L.; Wang, Y. The Role of PPARγ Gene Polymorphisms, Gut Microbiota in Type 2 Diabetes: Current Progress and Future Prospects. Diabetes Metab. Syndr. Obes. 2023, 16, 3557–3566. [Google Scholar] [CrossRef]
- Pedroza-Torres, A.; Romero-Córdoba, S.L.; Justo-Garrido, M.; Salido-Guadarrama, I.; Rodríguez-Bautista, R.; Montaño, S.; Muñiz-Mendoza, R.; Arriaga-Canon, C.; Fragoso-Ontiveros, V.; Álvarez-Gómez, R.M.; et al. MicroRNAs in Tumor Cell Metabolism: Roles and Therapeutic Opportunities. Front. Oncol. 2019, 9, 1404. [Google Scholar] [CrossRef]
- Janderová, L.; McNeil, M.; Murrell, A.N.; Mynatt, R.L.; Smith, S.R. Human mesenchymal stem cells as an in vitro model for human adipogenesis. Obes. Res. 2003, 11, 65–74. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; Bellin, G.; Vindigni, V.; Pavan, C.; Zavan, B. Effects of novel antidepressant drugs on mesenchymal stem cell physiology. Biomed. Pharmacother. 2019, 114, 108853. [Google Scholar] [CrossRef]
- Ferroni, L.; D’Amora, U.; Gardin, C.; Leo, S.; Dalla Paola, L.; Tremoli, E.; Giuliani, A.; Calzà, L.; Ronca, A.; Ambrosio, L.; et al. Stem cell-derived small extracellular vesicles embedded into methacrylated hyaluronic acid wound dressings accelerate wound repair in a pressure model of diabetic ulcer. J. Nanobiotechnology 2023, 21, 469. [Google Scholar] [CrossRef]
- Ferroni, L.; Rubini, A.; Bargellini, P.; Tremoli, E.; Cappucci, I.P.; D’Amora, U.; Ronca, A.; Calogero, G.; Panini, P.C.; Bettini, G.; et al. Apple vescicles: Revolutionary gut microbiota treatment for Inflammatory Bowel Disease. Food Biosci. 2024, 62, 105052. [Google Scholar] [CrossRef]
- Gardin, C.; Bosco, G.; Ferroni, L.; Quartesan, S.; Rizzato, A.; Tatullo, M.; Zavan, B. Hyperbaric Oxygen Therapy Improves the Osteogenic and Vasculogenic Properties of Mesenchymal Stem Cells in the Presence of Inflammation In Vitro. Int. J. Mol. Sci. 2020, 21, E1452. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| PPARG | CAGGAGATCACAGAGTATGCCAA | TCCCTTGTCATGAAGCCTTGG |
| FABP4 | TGACCTGGACTGAAGTTCGC | AAGCACAATGAATACATCATTACATCACC |
| CEBPA | GGACTTGGTGCGTCTAAGATGAG | GCATTGGAGCGGTGAGTTTG |
| B2M | CTGGTCTTTCTATCTCTTGTACTACACTG | CAAACCTCCATGATGCTGCTTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaleri, M.P.; Pusceddu, T.; Sileo, L.; Ardondi, L.; Vitali, I.; Cappucci, I.P.; Basile, L.; Pezzotti, G.; Fiorica, F.; Ferroni, L.; et al. When Fat Talks: How Adipose-Derived Extracellular Vesicles Fuel Breast Cancer. Int. J. Mol. Sci. 2025, 26, 9666. https://doi.org/10.3390/ijms26199666
Cavaleri MP, Pusceddu T, Sileo L, Ardondi L, Vitali I, Cappucci IP, Basile L, Pezzotti G, Fiorica F, Ferroni L, et al. When Fat Talks: How Adipose-Derived Extracellular Vesicles Fuel Breast Cancer. International Journal of Molecular Sciences. 2025; 26(19):9666. https://doi.org/10.3390/ijms26199666
Chicago/Turabian StyleCavaleri, Maria Pia, Tommaso Pusceddu, Lucia Sileo, Luna Ardondi, Ilaria Vitali, Ilenia Pia Cappucci, Laura Basile, Giuseppe Pezzotti, Francesco Fiorica, Letizia Ferroni, and et al. 2025. "When Fat Talks: How Adipose-Derived Extracellular Vesicles Fuel Breast Cancer" International Journal of Molecular Sciences 26, no. 19: 9666. https://doi.org/10.3390/ijms26199666
APA StyleCavaleri, M. P., Pusceddu, T., Sileo, L., Ardondi, L., Vitali, I., Cappucci, I. P., Basile, L., Pezzotti, G., Fiorica, F., Ferroni, L., & Zavan, B. (2025). When Fat Talks: How Adipose-Derived Extracellular Vesicles Fuel Breast Cancer. International Journal of Molecular Sciences, 26(19), 9666. https://doi.org/10.3390/ijms26199666








