Abstract
Inflammatory bowel disease (IBD) demonstrates chronic relapsing inflammation extending beyond adaptive immunity dysfunction. “Trained immunity”—the reprogramming of innate immune memory in myeloid cells and hematopoietic progenitors—maintains intestinal inflammation; however, the mechanism by which gut microbiome orchestration determines protective versus pathological outcomes remains unclear. Microbial metabolites demonstrate context-dependent dual effects along the gut–bone marrow axis. Short-chain fatty acids typically induce tolerogenic immune memory, whereas metabolites like succinate and polyamines exhibit dual roles: promoting inflammation in certain contexts while enhancing barrier integrity in others, influenced by cell-specific receptors and microenvironmental factors. Interventions include precision probiotics and postbiotics delivering specific metabolites, fecal microbiota transplantation addressing dysbiotic trained immunity, targeted metabolite supplementation, and pharmacologic reprogramming of pathological myeloid training states. Patient stratification based on microbiome composition and host genetics enhances therapeutic precision. Future research requires integration of non-coding RNAs regulating trained immunity, microbiome–immune–neuronal axis interactions, and host genetic variants modulating microbiome–immunity crosstalk. Priorities include developing companion diagnostics, establishing regulatory frameworks for microbiome therapeutics, and defining mechanistic switches for personalized interventions.
1. Introduction
Inflammatory bowel disease (IBD), encompassing Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic relapsing inflammatory disorder affecting the gastrointestinal mucosa. Despite therapeutic advances, IBD remains highly prevalent, causes substantial morbidity, and increases colorectal cancer risk [1,2]. Recurrent abdominal pain, diarrhea, and rectal bleeding compromise quality of life and impose significant healthcare costs [3]. Current pharmacological treatments, including biologics, demonstrate inconsistent efficacy with marked inter-patient variability and limited long-term remission rates [4,5,6,7]. Precision therapeutic strategies are therefore essential to prevent disease progression and complications.
Innate immunity is central to IBD pathogenesis and persistence. Genetic variants in NOD2 and CARD9 disrupt innate signaling, triggering aberrant microbial pattern recognition that compromises barrier integrity and activates adaptive immunity [8,9]. Sustained innate cell activation drives chronic inflammation and fibrogenesis.
Critically, innate cells acquire non-specific “trained” memory through epigenetic and metabolic reprogramming, which in IBD’s inflammatory environment perpetuates pro-inflammatory cytokine production and fibrosis [8,10,11]. Following initial insult, monocytes, macrophages, and innate lymphoid cells exhibit heightened cytokine responses upon re-challenge [12]. While trained immunity normally provides pathogen protection, its dysregulation sustains pathological inflammation. Understanding the signals establishing and maintaining this memory is therefore crucial for explaining IBD chronicity.
The gut microbiome provides many such signals. Dysbiosis depletes beneficial taxa while enriching pathobionts, altering luminal concentrations of short-chain fatty acids, secondary bile acids, and tryptophan metabolites [13,14]. These molecules function as epigenetic cofactors and metabolic substrates that program trained immunity in innate cells [12]. Modulating microbial composition or supplementing key metabolites thus offers a practical approach to recalibrate pathogenic innate memory.
Here, we synthesize current understanding of the innate immunity–microbiota axis in IBD and highlight therapeutic strategies targeting trained immunity for precise, durable disease control.
2. Mechanism and Plasticity of Trained Immunity
Trained immunity refers to the non-specific immune memory acquired by innate immune cells following an initial stimulus such as infection, vaccination, or inflammatory factors, first proposed by Netea et al. in 2011 [15]. Unlike adaptive immunity, which relies on T and B cells, trained immunity involves functional reprogramming of monocytes, macrophages, NK cells, and innate lymphoid cells [16]. This process is primarily driven by epigenetic reprogramming, including histone modifications and DNA methylation, which persist even after the stimulus is removed, allowing cells to respond more rapidly and robustly to future exposures [17,18,19]. Key epigenetic changes include histone lactylation (H3K18la) and DNA methylation (e.g., H3K4me3), regulated by immune-initiating lncRNAs, which guide chromatin modifications at immune-related gene promoters [20,21,22]. These “imprints” enable innate cells to initiate stronger immune responses upon subsequent encounters with similar or different stimuli, conferring a memory-like feature with broad adaptability [10,16]. Besides, trained immunity differs from adaptive immunity in IBD pathogenesis (Table 1). These differences not only explain the rapid nonspecific recurrence and treatment resistance of IBD that traditional adaptive immune theories cannot account for but also provide new therapeutic targets and time windows. Furthermore, they suggest that therapies capable of simultaneously targeting both forms of immune memory may prove more effective.

Table 1.
Similarities and differences between trained immunity and adaptive immunity in IBD pathogenesis.
Metabolic reprogramming is one of the key drivers of trained immunity (Figure 1), which provides energy to immune cells and participates in epigenetic modification and signaling through the production of specific metabolic intermediates to maintain the trained state at the functional level. It was found that during trained immunity, innate immune cells undergo significant metabolic pathway switching, which is manifested by enhanced aerobic glycolysis (Warburg effect), redistribution of the tricarboxylic acid cycle (TCA) and oxidative phosphorylation, and other metabolic dynamics. This reprogramming results in increased glucose uptake, significantly elevated levels of glycolysis, and rapid conversion of pyruvate to lactate, which provides rapid cellular energy and promotes activation of pro-inflammatory signaling [39]. At the same time, some TCA cycle intermediates have signaling functions of their own [40]. Succinate is a key metabolic signaling molecule that activates pro-inflammatory pathways and enhances the expression of inflammatory factors through its receptor SUCNR1 [41]. Succinate accumulation is closely associated with developing an inflammatory phenotype in innate immune cells (e.g., macrophages) and is an essential metabolic marker of trained immunity [42].
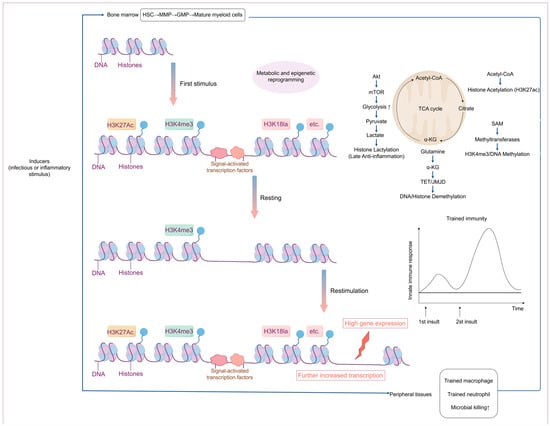
Figure 1.
Overview of the epigenetic and metabolic mechanisms of trained immunity.
Multiple infectious or inflammatory stimuli can trigger central trained immunity (TRIM) in the bone marrow. This mechanism encompasses metabolic and epigenetic reprogramming of hematopoietic stem and progenitor cells, including hematopoietic stem cells (HSCs), multipotent progenitors (MPPs), and granulocyte–macrophage progenitors (GMPs). This reprogramming leads to increased production of mature myeloid cells (neutrophils and monocytes) with heightened immune responsiveness to subsequent challenges encountered in the circulation or peripheral tissues. TRIM can also be directly induced in mature myeloid cells within the periphery (circulation or peripheral tissues and organs). Epigenetic marks—such as H3K4me3—can persist without the initial stimulus, facilitating more robust transcription of immune genes upon secondary stimulation and resulting in an amplified immune response [17].
Trained immunity is highly plastic. Established studies have shown that infection or vaccination induces durable epigenetic modifications in innate immune cells, which enhance their ability to respond to subsequent pathogenic or inflammatory stimuli. For example, trained immunity induced by the BCG vaccine (the primary vaccine against Mycobacterium tuberculosis worldwide) not only boosts immune protection against Mycobacterium tuberculosis but also offers cross-protection against heterologous infections [27,43,44]. This process might be achieved by inducing HIF1α and mTOR-mediated metabolic reprogramming of monocytes [45,46]. More importantly, trained immunity is not limited to peripheral innate immune cells, and its epigenetic reprogramming can also be established in immune progenitor cells in the bone marrow, explaining that some vaccines can maintain their protective effect against heterologous infections for years [19]. However, trained immunity, when excessive or dysregulated, can play a disease-promoting role in chronic inflammatory diseases. Following an initial infection or inflammatory challenge, trained immunity is epigenetically reprogrammed via bone marrow hematopoietic stem and progenitor cells (BM-HSPCs) to form long-term inflammatory memories in response to inflammatory signals, which amplify and delay chronic inflammatory pathologies [47]. Notably, trained immunity represents a double-edged sword. For example, in systemic sclerosis (SSc), BCG-trained macrophages exacerbate the fibrosis process. In contrast, low-dose LPS pre-trained macrophages attenuate the disease [48], which reveals that trained immunity can exhibit opposite functional effects under different stimulus conditions.
3. Microbiome Regulation of Trained Immunity
3.1. Exposure to Bacterial Flora Directly Regulates Trained Immunity
Commensal and pathogenic microbes, their components, vaccines, and microbial metabolites can all reprogram innate immune cells through epigenetic and metabolic mechanisms (Figure 2), producing a spectrum from trained immunity to tolerance. The specific outcome is shaped by the agent involved, the cell type, the context of the interaction, and the host tissue environment, which provides opportunities for both targeted mucosal protection and the maintenance of intestinal immune homeostasis (Table 2) [12,49,50,51,52,53,54,55,56,57,58,59,60,61,62]
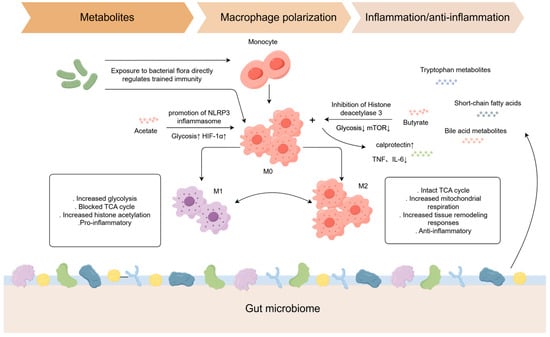
Figure 2.
How microbes and their metabolites induce different types of trained immunity.

Table 2.
Microbial agents, components, and metabolites that induce or modulate innate immune memory (trained immunity or tolerance) in experimental models.
Microbiota metabolites regulate macrophage polarization through metabolic reprogramming [63]. Butyrate, a short-chain fatty acid (SCFA), modulates the epigenetic landscape of intestinal macrophages by inhibiting histone deacetylase (HDAC), enhancing antimicrobial capacity (e.g., upregulating calprotectin expression) while suppressing pro-inflammatory cytokine release (e.g., TNF and IL-6), thereby promoting intestinal homeostasis [64,65]. Acetate enhances macrophage bactericidal activity via NLRP3 inflammasome activation and glycolysis-mediated HIF-1α induction [66].
3.2. Microbial Metabolite-Mediated Regulation of Trained Immunity
Microbial metabolites are pivotal modulators of trained immunity. The establishment and maintenance of trained immunity rely on the involvement of various metabolic pathways, including aerobic glycolysis, glutamine catabolism, cholesterol metabolism, and fatty acid synthesis. All these pathways are intimately connected to the metabolic activities of intestinal microbes. These microbial metabolites influence innate immune cells both directly (Figure 2), by altering their epigenetic landscape, and indirectly, through activation of immunoregulatory pathways in intestinal epithelial cells.
Recent years have witnessed substantial advances in understanding how specific microbial metabolites regulate trained immunity [63]. Notably, short-chain fatty acids (SCFAs) have been repeatedly shown to induce trained immunity via epigenetic and metabolic reprogramming of innate immune cells [12]. Beyond SCFAs, other metabolites such as lactic acid can serve as metabolic fuels for the tricarboxylic acid cycle and, through histone lactylation, increase chromatin accessibility, thus enhancing immune responsiveness to secondary stimuli [67]. The aromatic compound resorcinol, via AhR pathway activation, epigenetically reprograms hematopoietic stem and progenitor cells (HSPCs) to confer central trained immunity [32]. Furthermore, tryptophan-derived metabolites (e.g., p-cresol sulfate and indole-3-glyoxalate) amplify inflammatory responses in the CNS by activating AhR and promoting IL-17 production from γδ T cells and Th17 cells [68].
Other microbial products, including γ-aminobutyric acid (GABA), polyamines, and histamine, have also been implicated in immune regulation, partly through epigenetic modifications. Additionally, intermediates from the mevalonate pathway promote trained immunity maintenance by activating the IGF1-mTOR signaling axis and driving histone modification [69].
Notably, host–microbe interactions are bidirectional. For example, apolipoprotein L (APOL9a/b) secreted by intestinal epithelial cells can enhance the release of bacterial outer membrane vesicles (OMVs), thereby activating TLR2 signaling in dendritic cells and facilitating IFN-γ secretion and MHC-II upregulation, which together promote intraepithelial lymphocyte differentiation [70]. These findings underscore the intricate interplay between microbial metabolites, host cells, and immune function.
3.3. Host Genetics: A Key Modulator of the Microbiome–Immunity Axis
While the gut microbiome regulates trained immunity, this regulation is fundamentally shaped by host genetic susceptibility. IBD pathogenesis involves over 200 genetic risk loci, many encoding proteins essential for innate immunity, microbial recognition, and autophagy, including NOD2, CARD9, and ATG16L1 [71,72,73]. These genetic variants predispose to dysregulated host–microbe interactions. Loss-of-function NOD2 variants impair muramyl dipeptide sensing, causing defective Paneth cell antimicrobial function and subsequent dysbiosis characterized by increased Enterobacteriaceae abundance [74]. Similarly, CARD9 polymorphisms alter gut microbiome composition and anti-fungal immune responses [75,76]. Individual genetic profiles thus determine microbial composition and immune setpoints. This genetically driven dysbiosis provides persistent aberrant stimuli, priming innate immunity for maladaptive trained responses that contribute to IBD chronicity. Additionally, non-coding RNAs, particularly long non-coding RNAs (lncRNAs), increasingly influence trained immunity. LncRNAs regulate histone modifications (H3K4me3 and H3K27me3) and DNA methylation through chromatin modification complex binding, while modulating immune cell metabolic pathways—glycolysis and oxidative phosphorylation—by controlling metabolic enzyme expression and activity [77,78]. Studies demonstrate non-coding RNA’s roles in IBD pathogenesis; inhibiting lncRNA NEAT1 attenuates IBD inflammatory responses [79]. These findings suggest non-coding RNAs shape trained immunity in IBD, though this emerging field requires further definitive research.
4. Tolerogenic Trained Immunity and Bidirectional Regulation by Microbial Metabolites
Although trained immunity is typically linked to pro-inflammatory memory, recent studies demonstrate that specific commensal and probiotic bacteria can induce a tolerogenic form, characterised by dampened inflammatory responses and promotion of immune homeostasis. For instance, Lactiplantibacillus plantarum imprints tolerogenic memory in mononuclear phagocytes by downregulating ROS production and reprogramming metabolic pathways, thereby reducing TNF-α and IL-1β upon restimulation [50]. Similarly, Akkermansia muciniphila modulates macrophage training through mitochondrial and lysosomal signaling, enhancing immune tolerance and barrier protection [51]. These effects, which depend on viable bacteria and are influenced by environmental cues such as retinoic acid and mucosal oxygen, involve distinct epigenetic marks (e.g., H3K27me3 enrichment) and suppression of NF-κB signaling mechanistically [51].
Notably, the influence of microbial metabolites on intestinal inflammation via trained immunity is complex. Clinical and preclinical evidence suggests that the anti-inflammatory effects of certain short-chain fatty acids (SCFAs) are linked to trained immunity [80,81,82]. For example, the SCFA butyrate inhibits histone deacetylases, particularly HDAC3, in intestinal macrophages, thereby promoting an anti-inflammatory phenotype, enhancing epithelial barrier integrity, and limiting excessive type 2 immunity to support mucosal homeostasis [64,65,83,84]. In contrast, succinate exhibits context-dependent effects: chronic elevation in IBD can drive pro-inflammatory pathways through SUCNR1 signaling and Th17 activation, whereas controlled dietary supplementation activates the IL-4Rα–Hif-1α axis to induce anti-inflammatory M2 macrophages and promote tissue repair [31,85,86]. Polyamines demonstrate similar duality. In IBD, physiological levels of spermidine promote macrophage polarisation toward an anti-inflammatory M2 phenotype, a protection dependent on epithelial and myeloid PTPN2 [87], whereas dysregulated polyamine levels and metabolism are associated with persistent inflammation and tissue damage [88,89]. Given that both excessive and insufficient immune activation can exacerbate intestinal pathology, precise modulation of these metabolite levels may offer a promising approach to restoring immune balance in chronic inflammatory conditions such as IBD.
5. Critical Pathways of the Flora–Immune Memory Axis Driving IBD Chronicity
5.1. The Gut–Bone Marrow Axis
In recent years, the concept of the “gut–bone marrow axis” has been proposed, suggesting that signals from the gut flora may act remotely on the bone marrow hematopoietic system to induce trained immunity, which is involved in the maintenance and amplification of chronic inflammation in IBD [32,90]. In the DSS-induced mouse colitis model, Mincle-mediated trained immunity significantly enhances the body’s protective immune response to subsequent bacterial and viral infections (Figure 3). However, it may also exacerbate the pathologic course of the associated disease by enhancing the inflammatory response in the presence of intestinal barrier damage [36]. Upon intestinal barrier damage, ZO-1 degradation leads to translocation of enterococci to the bone marrow, triggering activation of bone marrow immune cells and transmission of immune memory [91]. Simultaneously, CX3CR1+ monocytes act as “sentinels” that capture LPS and transport it to the bone marrow niche, activating immune cells in the bone marrow and transmitting immune memory signals [91]. In contrast to the traditional “bone marrow → periphery” linear hematopoietic model, this “gut–bone marrow–periphery” pathway reveals the dynamic capacity of gut signals to shape the fate of bone marrow immune cells. Trained immunity is thus no longer merely an activation of the end-cell state, but a functional reprogramming of early hematopoietic progenitors. Their “memory” alterations can maintain a pro-inflammatory phenotype over time, providing a deeper driving mechanism for the chronicity of IBD. In another study, a constitutively activated mouse model of STING (N153s) revealed that intestinal dysbiosis induces K63 ubiquitination modification of STING through the bacterial metabolite c-di-GMP, leading to the accumulation of innate immune junction protein STING in myeloid cells (macrophage/monocyte), activation of the TBK1/IRF3/NF-κB pathway, driving the release of pro-inflammatory factors (e.g., TNF-α and CXCL10) and promoting T cell-dependent colitis [92].
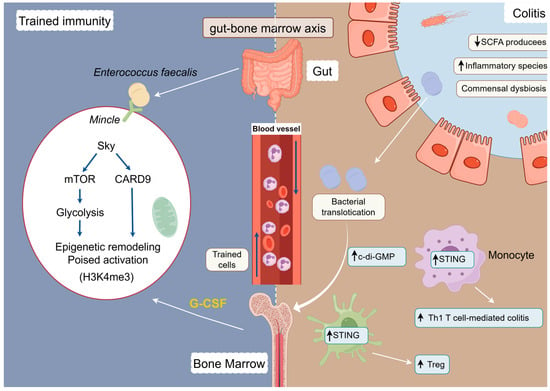
Figure 3.
Modelling of the ‘gut–bone marrow axis’ tele-training immune pathway.
The trans-barrier translocation of gut microbiota, particularly Enterococcus fecalis, induces a long-term state of trained immunity by activating the Mincle receptor on bone marrow myeloid progenitor cells [36]. And intestinal dysbiosis induces K63 ubiquitination modification of STING through the bacterial metabolite c-di-GMP [92].
5.2. Deficiency of Protective Metabolites and Pathological Training of Pro-Inflammatory Metabolites
Microbial metabolites not only exert remote effects but also directly modulate trained immunity within the intestinal microenvironment, thereby influencing IBD pathogenesis [85]. In IBD patients, the loss of SCFA-producing bacteria and tryptophan-metabolising bacteria leads to reduced levels of key metabolites such as butyrate and indole derivatives [63,93]. These metabolites are essential for the regulation of innate immune cell metabolic programming and epigenetic remodelling, both of which underpin trained immunity. For example, butyrate has been shown to inhibit histone deacetylase (HDAC) activity in monocytes and neutrophils, suppressing excessive inflammatory responses and modulating trained immunity [93]. Moreover, colonisation experiments in germ-free mice indicate that the gut microbiota from IBD patients can enhance glycolytic activity in bone marrow CD11b+ cells, further supporting the role of microbial-driven metabolic reprogramming in shaping trained immunity [94,95]. These findings collectively highlight how altered microbial composition and metabolite availability in IBD contribute to dysregulated trained immunity and the maintenance of chronic intestinal inflammation.
5.3. The Microbiome–Immune–Neuronal Axis
The microbiota–gut–brain axis has been demonstrated to play a pivotal role in the pathogenesis of IBD [96]. Indeed, the immune system is also involved, forming the microbiota–immune–neural axis—another complex bidirectional communication system [97]. Within this framework, microbial metabolites serve as key hubs for neuro-immune coupling, acting on sensory neurons (TRPV1/TRPA1, P2X3), G protein-coupled receptors (GPR41/43/109A), nuclear receptors/transcription factors (AhR, FXR/TGR5), and immune receptors (TLR2/4/5), collectively shaping intestinal inflammation, pain sensitization, and behavioral/cognitive alterations [98,99,100]. As previously described, microbial metabolites can regulate host inflammatory memory through epigenetic and metabolic reprogramming. Indeed, beyond influencing neuronal signaling and immune memory in IBD, microbial metabolites also affect extraintestinal manifestations of IBD. When intestinal barrier function is compromised, associated protective mechanisms fail. Circulating proinflammatory cytokines (e.g., IL-6 and TNF-α) can then cross the damaged blood–brain barrier (BBB), activating resident brain immune cells such as microglia and inducing neuroinflammation. This may provide a pathological explanation for the psychiatric and neurological symptoms observed in IBD patients [101]. Current animal studies have demonstrated the beneficial effects of targeting the microbiome–immune–neural axis on IBD remission [100,102].
5.4. Transgenerational Transmission
More notably, several studies in recent years have suggested that the synergistic effect of gut flora and epigenetic mechanisms may be involved in the transgenerational transmission of trained immunity [103,104]. It has been shown that in granulopoietic mice with established trained immunity, granulocyte–monocyte progenitors (GMPs) exhibit a strong type I interferon signaling signature that correlates with type I interferon-dependent epigenetic imprinting in the progeny’s neutrophils, ultimately leading to a significant enhancement of oxidative burst and phagocytosis in these cells in response to subsequent stimulation [105]. In addition, changes in maternal diet and gut flora can influence the training status of immune cells in the offspring through epigenetic mechanisms such as miRNAs in the sperm or egg, which in turn affects their health [106]. Moreover, IBD familial aggregation may partly arise from vertical transmission of colony–immune memory [107]. These findings emphasise the profound influence of maternal microecological and epigenetic status on the risk of IBD in the offspring and provide new ideas for early intervention of the disease.
6. Intervention Strategies Targeting the Flora–Immune Memory Axis
The intricate interactions between the intestinal microbiota and the immune system offer promising therapeutic avenues for IBD. The “diet–gut microbiota–innate immunity” theoretical framework provides systematic guidance for treating IBD. Below, we outline intervention strategies at the levels of primary prevention, secondary treatment, and tertiary (long-term) management, highlighting evidence-based approaches supported by recent literature.
6.1. Primary Prevention
Maintaining intestinal flora homeostasis through dietary interventions in healthy populations or at-risk individuals can help reduce the risk of developing IBD [108]. This goes beyond generic healthy eating to specific dietary components that foster intestinal flora homeostasis and beneficial immune training.
6.1.1. Dietary Fibre and Whole Grains
A high intake of diverse dietary fibres and whole grains is associated with a lower risk of developing IBD [109]. These complex carbohydrates are indigestible by human enzymes but fermentable by gut bacteria, leading to the production of short-chain fatty acids (SCFAs) such as butyrate [110]. SCFAs play a vital role in maintaining gut barrier integrity, upregulating tight junction proteins, and modulating immune responses towards an anti-inflammatory profile. In a large prospective cohort study, individuals with the highest fibre intake (especially from cereals and whole grains) had a significantly reduced incidence of Crohn’s disease and ulcerative colitis [111]. This evidence reinforces dietary fibre as a cornerstone of IBD prevention. SCFA-mediated mechanisms further explain this benefit: butyrate serves as an energy source for colonic epithelial cells and promotes regulatory T-cell development, thereby enhancing mucosal tolerance.
6.1.2. Prebiotics
Beyond general fibre, specific prebiotic compounds support beneficial gut microbes like Bifidobacterium and Lactobacillus. Fructans (e.g., inulin from onions, garlic, and bananas) and galactooligosaccharides are selectively fermented by these microbes, boosting their growth and increasing SCFA production. Prebiotic supplementation has been shown to strengthen the intestinal mucus layer, reduce pro-inflammatory cytokines, and improve microbiota composition in experimental colitis. For example, oral inulin reduced disease severity in mouse colitis models by inhibiting IL-6/STAT3 signaling and elevating mucin (MUC2) levels. Such findings suggest that prebiotics actively contribute to intestinal homeostasis and immune regulation, acting as a proactive defence against dysbiosis that can precede IBD. Indeed, a recent review highlights that prebiotic fibre interventions modulate gut immunity—for instance, inulin increased anti-inflammatory Th2 responses and lowered pro-inflammatory cytokines in preclinical studies [112].
6.1.3. Omega-3 Polyunsaturated Fatty Acids (PUFAs)
Diets rich in omega-3 PUFAs (particularly eicosapentaenoic acid, EPA, and docosahexaenoic acid, DHA, found in fatty fish) appear to protect against IBD development. These fatty acids are precursors to specialised pro-resolving mediators that actively resolve inflammation rather than simply suppress it. Epidemiological studies and genetic analyses have observed that higher omega-3 intake (or circulating levels) is associated with a significantly decreased risk of both Crohn’s disease and ulcerative colitis. In contrast, high omega-6 intake (e.g., linoleic acid) has been linked to increased risk of UC. A recent Mendelian randomisation study confirmed a causal protective effect of omega-3 (especially DHA) on IBD: individuals genetically predisposed to higher DHA had ~19% lower odds of IBD. By shifting the balance of eicosanoids toward less pro-inflammatory and more pro-resolving mediators, a diet enriched in omega-3 fatty acids may mitigate early immune triggers for IBD [113].
6.1.4. Limiting Processed Foods and Additives
Conversely, Western-style diets high in ultra-processed foods (UPFs) are associated with heightened IBD risk. Processed foods often contain emulsifiers, artificial sweeteners, and high saturated fat, which can adversely affect the gut microbiome and barrier. A recent prospective study of over 240,000 adults found that those in the highest quartile of UPF consumption had a 70% higher risk of developing Crohn’s disease compared to those in the lowest quartile [92]. Mechanistic research offers insight: emulsifier additives (like polysorbate-80 and carboxymethylcellulose) can erode the mucus layer and promote bacterial encroachment, triggering colonic inflammation. Likewise, certain artificial sweeteners and a high-sugar/high-fat diet can favour pro-inflammatory microbial species and increase intestinal permeability. Reducing these dietary components—especially in early life—may thus lower IBD susceptibility. Notably, early-life exposure to unhealthy diets during critical immune development windows is thought to “program” a more inflammation-prone microbiota–immune setpoint. In a pooled analysis of Scandinavian birth cohorts (over 80,000 children), a high-quality diet in infancy (characterised by greater fish and vegetable intake and less sugary beverages) was associated with a significantly reduced risk of IBD later in childhood and adolescence. For example, children at 1 year old with high fish intake had about a 30–50% lower risk of IBD (particularly ulcerative colitis) compared to those with low fish intake. These findings underscore that fostering healthy eating patterns from infancy—and minimising early exposure to Western diet elements—can build a more resilient flora–immune axis.
6.2. Secondary Treatment
6.2.1. Fecal Microbiota Transplantation
Fecal microbiota transplantation (FMT), as a method of directly re-establishing intestinal flora, aims to restore the intestinal microbial ecology of IBD patients by transplanting faeces from healthy donors. However, while FMT has shown high remission rates in UC, its efficacy in CD remains controversial [85]. Next-generation FMT technology should require SCFA producer abundance > 40% for donor screening to ensure the quality and functionality of the transplanted flora. Engineered bacterial design is an emerging technology for ecological remodelling of flora in IBD patients; e.g., Nissle 1917, an engineered E. coli that secretes IL-2, modulates immune response and improves intestinal inflammation [114].
6.2.2. Probiotics
Probiotics, live microorganisms conferring health benefits when administered in adequate amounts, represent a targeted approach to microbial modulation [115]. Regarding immune memory, certain probiotic strains bidirectionally influence trained immunity. Lactiplantibacillus plantarum induces tolerogenic trained immunity by reprogramming mononuclear phagocytes to reduce pro-inflammatory cytokine release upon restimulation, promoting immune homeostasis [50]. However, conventional probiotics demonstrate limited therapeutic efficacy in IBD due to poor gastrointestinal survival and transient, inefficient mucosal colonization [115,116]. To overcome the limitations of conventional probiotics, research has shifted towards “next-generation” engineered probiotics, a frontier of synthetic biology. These “smart” microbes are rationally designed to function as living medicines, capable of sensing environmental cues and delivering therapeutic payloads with high precision directly at the site of inflammation. A prominent strategy involves engineering safe bacterial chassis, such as E. coli Nissle 1917, to produce and secrete specific immunomodulatory molecules. For example, strains have been developed to release Interleukin-10 (IL-10) or other anti-inflammatory cytokines [117], which can locally suppress aberrant immune responses and promote mucosal healing. More advanced designs incorporate sensor–actuator circuits, enabling bacteria to detect specific inflammatory markers in the gut, such as tetrathionate or nitric oxide [118], and trigger the production of a therapeutic agent only when and where it is needed. This “sense and respond” approach minimises systemic exposure and off-target effects, representing a significant leap towards personalized and dynamic IBD treatment.
6.2.3. Postbiotics
The challenges associated with live probiotics have led to a growing interest in postbiotics, defined as a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host”. As a therapeutic strategy for IBD, postbiotics offer significant advantages over live microbes, including enhanced stability, a superior safety profile, and the capacity for precise dose standardisation. Clinical evidence supporting the efficacy of postbiotics in IBD, while still emerging, is growing. The most robust data comes from trials of topical butyrate administration in UC. In a multicenter trial, Vernia et al. demonstrated that the combination of butyrate and 5-ASA enemas significantly improved clinical parameters such as bowel movements and urgency compared to 5-ASA alone, suggesting a potential benefit in refractory distal UC [119]. Furthermore, studies using heat-inactivated probiotics have demonstrated potential. In an acute colitis mouse model, heat-inactivated Lactiplantibacillus plantarum L-137 treatment improved intestinal barrier integrity by upregulating ZO-1 and modulating cytokine levels, leading to an anti-inflammatory effect [120]; similar benefits are reported for inactivated lactobacilli and bacterial lysates that reduce pro-inflammatory cytokines and increase IL-10 [121]. Complementing these findings, oral administration of a composite postbiotic derived from Lacticaseibacillus casei Zhang, Lactiplantibacillus plantarum P-8, and Bifidobacterium animalis subsp. lactis V9 ameliorated DSS-induced colitis in rats in a dose-dependent manner, lowering TNF-α and IL-1β, increasing IL-10, and shifting the microbiota and fecal metabolome toward anti-inflammatory profiles [122].
Additionally, metabolic and epigenetically targeted therapies offer new avenues for the treatment of IBD chronicity. The innate immune cell-expressed G protein-coupled receptor GPR84 is significantly increased in response to acute inflammatory stimuli such as LPS and TNFα, and its antagonists inhibit colitis by decreasing the polarisation and function of pro-inflammatory microphages [123]. AhR agonists enhance polyamine biosynthesis, modulate immune responses, and maintain intestinal immune homeostasis [124].
6.3. Tertiary Management
In the long-term management stage, the dietary management of IBD patients is crucial. Previous studies have concluded that environmental factors (e.g., high-fat, high-sugar WD, and antibiotic use), rather than genetic factors, drive changes in flora structure and exacerbate intestinal permeability and inflammation [86,125,126]. However, some animal studies have shown that brief WD intake leads to beneficial immune training via the mevalonate pathway and macrophage training, which is protective against DSS-induced colitis [127]. In addition, modulation of the intestinal biological clock through diet is beneficial in ameliorating persistent inflammation due to dysbiosis [95].
Periodisation of treatment is important to prevent the recurrence of IBD. In addition, dynamic detection of flora-related markers is important. By monitoring the dynamic changes in clinical markers, we can understand the progress of patients’ conditions in real time, adjust the treatment strategy in time, improve the therapeutic effect, and improve patients’ prognosis.
7. Challenges and Future Directions
Despite significant advances in understanding the gut microbiota–immunity axis, key challenges persist across clinical practice, therapeutic translation, and fundamental science. Clinically, the disconnect between patient-reported symptoms and objective inflammatory activity, combined with limitations of current assessment tools, complicates therapeutic decision-making and evaluation of novel interventions [128,129,130]. These challenges are magnified when translating microbiome-based therapies from laboratory to clinic.
Future research must integrate multi-omics data to elucidate precise interactions between host genetics, microbiome, and immune memory [131]. Personalized IBD therapy requires understanding how genetic variants shape both microbiome composition and immune responses to specific therapies. For instance, patients with certain risk alleles may exhibit unique microbial metabolic signatures that, in turn, influence the state of trained immunity and the efficacy of a given treatment [132]. Integrating genotype, microbiome profile, and immune phenotype enables development of predictive biomarkers for treatment response and disease progression, facilitating true precision medicine through a “genetics–microbiota–immunity” axis approach.
For FMT, major clinical obstacles include unstandardised donor screening and administration protocols, leading to variable outcomes and unpredictable engraftment, alongside persistent long-term safety concerns over pathogen transmission [133,134,135,136]. Next-generation Live Biotherapeutic Products face manufacturing challenges in ensuring batch consistency and stability, compounded by regulatory complexities due to ambiguous classification and demanding Chemistry, Manufacturing, and Controls requirements that slow clinical translation.
While current postbiotic evidence in IBD is promising, larger randomised controlled trials are essential to confirm efficacy and establish standardised protocols. Future research should optimise formulations, determine dosing regimens, and identify responsive patient subgroups. The demonstrated mechanisms—mitochondrial modulation, microbiome–metabolome reprogramming, and targeted anti-inflammatory effects [121,122]—position postbiotics as precision therapeutics with superior safety and stability profiles. As mechanistic understanding and clinical evidence expand, postbiotics offer significant promise for transforming IBD treatment and achieving sustained remission.
Scientifically, causal relationships between dysbiosis and IBD remain incompletely defined [137]. More specifically, direct links between microbial community shifts and the balance of anti- versus pro-inflammatory microbial metabolites are not yet resolved; for example, IBD has been associated with increased succinate-producing taxa and with excessive polyamine levels linked to Bacteroides enrichment [138,139,140,141]. Although there is suggestive evidence that pro-inflammatory metabolites such as succinate can modulate trained immunity in inflammatory settings, robust experimental and clinical studies confirming causality remain limited in IBD [142]. Additionally, certain non-immune cell types, such as epithelial cells, endothelial cells, smooth muscle cells, and fibroblasts, can also establish inflammatory memory in a manner similar to monocytes and macrophages [23]. The definition of trained immunity may be further expanded, and whether inflammatory memory in non-immune cells exists in IBD warrants further exploration.
Addressing these challenges requires interdisciplinary innovation integrating personalized multi-omics analysis, real-time mucosal metabolic imaging, and digital twin models to dissect patient-specific mechanisms. Next-generation precision interventions show promise, including synthetic biology-modified probiotics engineered for inflammation sensing, organoid-based immune models for personalized therapy screening, and nanocarrier-mediated therapeutic delivery [63,143]. Furthermore, microbiota-targeted vaccines and gene editing technologies represent emerging frontiers, though their efficacy, safety, and translational pathways require validation [70,144,145,146]. Overcoming these barriers is essential for developing effective, personalized IBD therapeutics.
8. Conclusions
The traditional conceptualization of inflammatory bowel disease (IBD) as a simple dysregulation between pro- and anti-inflammatory mediators is undergoing fundamental revision. Our comprehensive review elucidates a more sophisticated paradigm, wherein maladaptive trained immunity, orchestrated and sustained by gut microbiome dysbiosis, constitutes a central pathogenic axis driving IBD chronicity. This “gut microbiome–immune memory” circuit provides mechanistic insight into the persistent, relapsing nature of IBD and, critically, unveils numerous novel therapeutic targets.
The future management of IBD is positioned to transition from broad-spectrum immunosuppression toward an era of precision, mechanism-based therapeutics. The targeted recalibration of maladaptive trained immunity—through strategic deployment of postbiotics to restore metabolic and epigenetic homeostasis, rationally engineered probiotics functioning as “living medicines”, or genetically informed nutritional interventions—represents the next therapeutic frontier in IBD management. By addressing the fundamental pathophysiological drivers rather than merely suppressing downstream inflammatory cascades, these precision strategies offer the potential not only for symptomatic control but for the restoration of mucosal homeostasis and achievement of sustained, treatment-free remission.
This paradigm shift from reactive immunosuppression to proactive immune reprogramming heralds a transformative approach to IBD therapeutics, with the ultimate goal of disease modification rather than mere disease management.
Author Contributions
Conceptualisation, B.Y., J.W. and T.B.; writing—original draft preparation, B.Y. and J.W.; writing—review and editing, B.Y., J.W., X.H., T.B. and S.L.; supervision, T.B. and S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the National Key Research and Development Program of China (2022YFC2504005).
Acknowledgments
We thank all the authors for their contributions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IBD | Inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| TCA | Tricarboxylic acid cycle |
| NOD2 | Nucleotide-binding oligomerisation domain-containing protein 2 |
| CARD9 | Caspase recruitment domain-containing protein 9 |
| NK | Natural killer |
| lncRNAs | Long non-coding RNAs |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| mTOR | Mechanistic target of rapamycin |
| HSPCs | Hematopoietic stem and progenitor cells |
| SSc | Systemic sclerosis |
| HDAC | Histone deacetylase |
| SCFA | Short-chain fatty acid |
| AhR | Aryl hydrocarbon receptor |
| CNS | Central nervous system |
| γδ T | Gamma delta T cells |
| Th17 | T helper 17 |
| GABA | Gamma-aminobutyric acid |
| APOL9a/b | Apolipoprotein L9a/b |
| OMVs | Outer membrane vesicles |
| TLR | Toll-like receptor |
| IFN-γ | Interferon gamma |
| MHC-II | Major histocompatibility complex class II |
| NF-κB | Nuclear factor kappa B |
| MCI | Monocytes with enhanced inflammatory properties |
| MC | Monocytes with anti-inflammatory properties |
| GWASs | Genome-wide association studies |
| DSS | Dextran sulfate sodium |
| Mincle | Macrophage-inducible C-type lectin (CLEC4E) |
| ZO-1 | Zonula occludens-1 |
| LPS | Lipopolysaccharide |
| CX3CR1 | C-X3-C motif chemokine receptor 1 |
| STING | Stimulator of interferon genes |
| TBK1 | TANK-binding kinase 1 |
| IRF3 | Interferon regulatory factor 3 |
| GMPs | Granulocyte–monocyte progenitors |
| WD | Western diet |
| FMT | Fecal microbiota transplantation |
| IL-2 | Interleukin-2 |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| UPFs | Ultra-processed foods |
| STAT3 | Signal transducer and activator of transcription 3 |
| MUC2 | Mucin 2 |
| Th | T helper cell |
| Treg | Regulatory T cell |
| PAMPs | Pathogen-associated molecular patterns |
| DAMPs | Damage-associated molecular patterns |
| APC | Antigen-presenting cell |
References
- Xu, L.; He, B.; Sun, Y.; Li, J.; Shen, P.; Hu, L.; Liu, G.; Wang, J.; Duan, L.; Zhan, S.; et al. Incidence of Inflammatory Bowel Disease in Urban China: A Nationwide Population-Based Study. Clin. Gastroenterol. Hepatol. 2023, 21, 3379–3386.e29. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef]
- Jairath, V.; Feagan, B.G. Global Burden of Inflammatory Bowel Disease. Lancet Gastroenterol. Hepatol. 2020, 5, 2–3. [Google Scholar] [CrossRef]
- Zhang, H.; Mu, C.; Gu, Y.; Meng, F.; Qin, X.; Cao, H. Selection Strategy of Second-Line Biologic Therapies in Adult Patients with Ulcerative Colitis Following Prior Biologic Treatment Failure: Systematic Review and Meta-Analysis. Pharmacol. Res. 2024, 202, 107108. [Google Scholar] [CrossRef]
- Antonioli, L.; Fornai, M.; Romano, B.; Pellegrini, C.; Blandizzi, C. Editorial: IBD Management-Novel Targets and Therapeutic Perspectives. Front. Pharmacol. 2020, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, B.; Jin, T.; Ocansey, D.K.W.; Jiang, J.; Mao, F. Intestinal Fibrosis in Inflammatory Bowel Disease and the Prospects of Mesenchymal Stem Cell Therapy. Front. Immunol. 2022, 13, 835005. [Google Scholar] [CrossRef] [PubMed]
- Roberti, R.; Iannone, L.F.; Palleria, C.; De Sarro, C.; Spagnuolo, R.; Barbieri, M.A.; Vero, A.; Manti, A.; Pisana, V.; Fries, W.; et al. Safety Profiles of Biologic Agents for Inflammatory Bowel Diseases: A Prospective Pharmacovigilance Study in Southern Italy. Curr. Med. Res. Opin. 2020, 36, 1457–1463. [Google Scholar] [CrossRef]
- Danne, C.; Skerniskyte, J.; Marteyn, B.; Sokol, H. Neutrophils: From IBD to the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Cheon, J.H. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw. 2017, 17, 25–40. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Zhang, B.; Moorlag, S.J.; Dominguez-Andres, J.; Bulut, Ö.; Kilic, G.; Liu, Z.; van Crevel, R.; Xu, C.-J.; Joosten, L.A.; Netea, M.G.; et al. Single-Cell RNA Sequencing Reveals Induction of Distinct Trained-Immunity Programs in Human Monocytes. J. Clin. Invest. 2022, 132, e147719. [Google Scholar] [CrossRef]
- Pellon, A.; Palacios, A.; Abecia, L.; Rodríguez, H.; Anguita, J. Friends to Remember: Innate Immune Memory Regulation by the Microbiota. Trends Microbiol. 2025, 33, 510–520. [Google Scholar] [CrossRef]
- Guggeis, M.A.; Harris, D.M.; Welz, L.; Rosenstiel, P.; Aden, K. Microbiota-Derived Metabolites in Inflammatory Bowel Disease. Semin. Immunopathol. 2025, 47, 19. [Google Scholar] [CrossRef]
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W.M. Trained Immunity: A Memory for Innate Host Defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained Immunity, Tolerance, Priming and Differentiation: Distinct Immunological Processes. Nat. Immunol. 2021, 22, 2–6, Erratum in Nat. Immunol. 2021, 22, 928. [Google Scholar] [CrossRef] [PubMed]
- Fanucchi, S.; Domínguez-Andrés, J.; Joosten, L.A.B.; Netea, M.G.; Mhlanga, M.M. The Intersection of Epigenetics and Metabolism in Trained Immunity. Immunity 2021, 54, 32–43. [Google Scholar] [CrossRef]
- Klemm, S.L.; Shipony, Z.; Greenleaf, W.J. Chromatin Accessibility and the Regulatory Epigenome. Nat. Rev. Genet. 2019, 20, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Ochando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained Immunity—Basic Concepts and Contributions to Immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, A.; Novakovic, B.; Ventriglia, L.; Galang, N.; Tran, K.A.; Li, W.; Matzaraki, V.; van Unen, N.; Schlüter, T.; Ferreira, A.V.; et al. Long-Term Histone Lactylation Connects Metabolic and Epigenetic Rewiring in Innate Immune Memory. Cell 2025, 188, 2992–3012.e16. [Google Scholar] [CrossRef]
- Bannister, S.; Kim, B.; Domínguez-Andrés, J.; Kilic, G.; Ansell, B.R.E.; Neeland, M.R.; Moorlag, S.J.C.F.M.; Matzaraki, V.; Vlahos, A.; Shepherd, R.; et al. Neonatal BCG Vaccination Is Associated with a Long-Term DNA Methylation Signature in Circulating Monocytes. Sci. Adv. 2022, 8, eabn4002. [Google Scholar] [CrossRef]
- Novakovic, B.; Habibi, E.; Wang, S.-Y.; Arts, R.J.W.; Davar, R.; Megchelenbrink, W.; Kim, B.; Kuznetsova, T.; Kox, M.; Zwaag, J.; et al. β-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. Cell 2016, 167, 1354–1368.e14. [Google Scholar] [CrossRef]
- Domínguez-Andrés, J.; dos Santos, J.C.; Bekkering, S.; Mulder, W.J.M.; van der Meer, J.W.M.; Riksen, N.P.; Joosten, L.A.B.; Netea, M.G. Trained Immunity: Adaptation Within Innate Immune Mechanisms. Physiol. Rev. 2023, 103, 313–346. [Google Scholar] [CrossRef]
- Mitsialis, V.; Wall, S.; Liu, P.; Ordovas-Montanes, J.; Parmet, T.; Vukovic, M.; Spencer, D.; Field, M.; McCourt, C.; Toothaker, J.; et al. Single-Cell Analyses of Colon and Blood Reveal Distinct Immune Cell Signatures of Ulcerative Colitis and Crohn’s Disease. Gastroenterology 2020, 159, 591–608.e10. [Google Scholar] [CrossRef]
- Warrick, K.A.; Vallez, C.N.; Meibers, H.E.; Pasare, C. Bidirectional Communication Between the Innate and Adaptive Immune Systems. Annu. Rev. Immunol. 2025, 43, 489–514. [Google Scholar] [CrossRef]
- Pan, X.; Zhu, Q.; Pan, L.-L.; Sun, J. Macrophage Immunometabolism in Inflammatory Bowel Diseases: From Pathogenesis to Therapy. Pharmacol. Ther. 2022, 238, 108176. [Google Scholar] [CrossRef]
- Dharmasiri, S.; Garrido-Martin, E.M.; Harris, R.J.; Bateman, A.C.; Collins, J.E.; Cummings, J.R.F.; Sanchez-Elsner, T. Human Intestinal Macrophages Are Involved in the Pathology of Both Ulcerative Colitis and Crohn Disease. Inflamm. Bowel Dis. 2021, 27, 1641–1652. [Google Scholar] [CrossRef]
- Padoan, A.; Musso, G.; Contran, N.; Basso, D. Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases. Curr. Issues Mol. Biol. 2023, 45, 5534–5557. [Google Scholar] [CrossRef] [PubMed]
- Kuttke, M.; Hromadová, D.; Yildirim, C.; Brunner, J.S.; Vogel, A.; Paar, H.; Peters, S.; Weber, M.; Hofmann, M.; Kerndl, M.; et al. PI3K Signaling in Dendritic Cells Aggravates DSS-Induced Colitis. Front. Immunol. 2022, 13, 695576. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; Vázquez, A.; González, S.; Sacedón, R.; Fernández-Sevilla, L.M.; Varas, A.; Subiza, J.L.; Valencia, J.; Vicente, Á. Mucosal Bacterial Immunotherapy Attenuates the Development of Experimental Colitis by Reducing Inflammation Through the Regulation of Myeloid Cells. Int. J. Mol. Sci. 2024, 25, 13629. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Dang, B.; Ouyang, X.; Zhao, X.; Huang, Y.; Lin, Y.; Cheng, X.; Xie, G.; Lin, J.; Mi, P.; et al. Dietary Succinate Supplementation Alleviates DSS-Induced Colitis via the IL-4Rα/Hif-1α Axis. Int. Immunopharmacol. 2025, 152, 114408. [Google Scholar] [CrossRef]
- Castelo, J.; Araujo-Aris, S.; Barriales, D.; Pasco, S.T.; Seoane, I.; Peña-Cearra, A.; Palacios, A.; Simó, C.; Garcia-Cañas, V.; Khamwong, M.; et al. The Microbiota Metabolite, Phloroglucinol, Confers Long-Term Protection against Inflammation. Gut Microbes 2024, 16, 2438829. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, P.; Su, D.; Zhao, Y.; Zhang, M. Therapeutic Inhibition of the JAK-STAT Pathway in the Treatment of Inflammatory Bowel Disease. Cytokine Growth Factor. Rev. 2024, 79, 1–15. [Google Scholar] [CrossRef]
- Liu, J.; Di, B.; Xu, L.-L. Recent Advances in the Treatment of IBD: Targets, Mechanisms and Related Therapies. Cytokine Growth Factor. Rev. 2023, 71–72, 1–12. [Google Scholar] [CrossRef]
- Perez-Gracia, J.L.; Labiano, S.; Rodriguez-Ruiz, M.E.; Sanmamed, M.F.; Melero, I. Orchestrating Immune Check-Point Blockade for Cancer Immunotherapy in Combinations. Curr. Opin. Immunol. 2014, 27, 89–97. [Google Scholar] [CrossRef]
- Viola, M.F.; Mass, E. Bacterial Translocation Promotes Trained Immunity. Immunity 2025, 58, 268–270. [Google Scholar] [CrossRef]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and Adaptive Immunity in Inflammatory Bowel Disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, M.; Dai, Z.; Luo, S.; Shi, Y.; He, Z.; Chen, Y. Salidroside Alleviates Ulcerative Colitis via Inhibiting Macrophage Pyroptosis and Repairing the Dysbacteriosis-Associated Th17/Treg Imbalance. Phytother. Res. 2023, 37, 367–382. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.A.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1α-Mediated Aerobic Glycolysis as Metabolic Basis for Trained Immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed]
- Llibre, A.; Mauro, C. Lactate Trains Immunity. Trends Immunol. 2025, 46, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Winther, S.; Trauelsen, M.; Schwartz, T.W. Protective Succinate-SUCNR1 Metabolic Stress Signaling Gone Bad. Cell Metab. 2021, 33, 1276–1278. [Google Scholar] [CrossRef] [PubMed]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Gang of 3: How the Krebs Cycle-Linked Metabolites Itaconate, Succinate, and Fumarate Regulate Macrophages and Inflammation. Cell Metab. 2025, 37, 1049–1059. [Google Scholar] [CrossRef]
- Aaby, P.; Netea, M.G.; Benn, C.S. Beneficial Non-Specific Effects of Live Vaccines Against COVID-19 and Other Unrelated Infections. Lancet Infect. Dis. 2023, 23, e34–e42. [Google Scholar] [CrossRef] [PubMed]
- Tsilika, M.; Taks, E.; Dolianitis, K.; Kotsaki, A.; Leventogiannis, K.; Damoulari, C.; Kostoula, M.; Paneta, M.; Adamis, G.; Papanikolaou, I.; et al. ACTIVATE-2: A Double-Blind Randomized Trial of BCG Vaccination Against COVID-19 in Individuals at Risk. Front. Immunol. 2022, 13, 873067, Erratum in Front. Immunol. 2022, 13, 1018384. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Tang, M.; Wu, Q.; Wang, Y.; Liu, Q.; Zhu, P.; Xue, X.; Liu, Y.; Chai, X.; Hou, Y.; et al. NMAAP1 Regulated Macrophage Polarizion into M1 Type Through Glycolysis Stimulated with BCG. Int. Immunopharmacol. 2024, 126, 111257. [Google Scholar] [CrossRef]
- Xu, J.-C.; Wu, K.; Ma, R.-Q.; Li, J.-H.; Tao, J.; Hu, Z.; Fan, X.-Y. Establishment of an In Vitro Model of Monocyte-like THP-1 Cells for Trained Immunity Induced by Bacillus Calmette-Guérin. BMC Microbiol. 2024, 24, 130. [Google Scholar] [CrossRef]
- Trained Immunity in Chronic Inflammatory Diseases and Cancer | Nature Reviews Immunology. Available online: https://www.nature.com/articles/s41577-025-01132-x (accessed on 9 May 2025).
- Jeljeli, M.; Riccio, L.G.C.; Doridot, L.; Chêne, C.; Nicco, C.; Chouzenoux, S.; Deletang, Q.; Allanore, Y.; Kavian, N.; Batteux, F. Trained Immunity Modulates Inflammation-Induced Fibrosis. Nat. Commun. 2019, 10, 5670. [Google Scholar] [CrossRef]
- Nenciarini, S.; Rivero, D.; Ciccione, A.; Amoriello, R.; Cerasuolo, B.; Pallecchi, M.; Bartolucci, G.L.; Ballerini, C.; Cavalieri, D. Impact of Cooperative or Competitive Dynamics Between the Yeast Saccharomyces Cerevisiae and Lactobacilli on the Immune Response of the Host. Front. Immunol. 2024, 15, 1399842. [Google Scholar] [CrossRef]
- Pellon, A.; Barriales, D.; Peña-Cearra, A.; Castelo-Careaga, J.; Palacios, A.; Lopez, N.; Atondo, E.; Pascual-Itoiz, M.A.; Martín-Ruiz, I.; Sampedro, L.; et al. The Commensal Bacterium Lactiplantibacillus Plantarum Imprints Innate Memory-like Responses in Mononuclear Phagocytes. Gut Microbes 2021, 13, 1939598. [Google Scholar] [CrossRef]
- Peña-Cearra, A.; Palacios, A.; Pellon, A.; Castelo, J.; Pasco, S.T.; Seoane, I.; Barriales, D.; Martin, J.E.; Pascual-Itoiz, M.Á.; Gonzalez-Lopez, M.; et al. Akkermansia Muciniphila-Induced Trained Immune Phenotype Increases Bacterial Intracellular Survival and Attenuates Inflammation. Commun. Biol. 2024, 7, 192. [Google Scholar] [CrossRef]
- Lasaviciute, G.; Barz, M.; van der Heiden, M.; Arasa, C.; Tariq, K.; Quin, J.; Östlund Farrants, A.-K.; Sverremark-Ekström, E. Gut Commensal Limosilactobacillus Reuteri Induces Atypical Memory-like Phenotype in Human Dendritic Cells In Vitro. Gut Microbes 2022, 14, 2045046. [Google Scholar] [CrossRef] [PubMed]
- Serafini, N.; Jarade, A.; Surace, L.; Goncalves, P.; Sismeiro, O.; Varet, H.; Legendre, R.; Coppee, J.-Y.; Disson, O.; Durum, S.K.; et al. Trained ILC3 Responses Promote Intestinal Defense. Science 2022, 375, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Uhr, T. Induction of Protective IgA by Intestinal Dendritic Cells Carrying Commensal Bacteria. Science 2004, 303, 1662–1665. [Google Scholar] [CrossRef]
- Fung, T.C.; Bessman, N.J.; Hepworth, M.R.; Kumar, N.; Shibata, N.; Kobuley, D.; Wang, K.; Ziegler, C.G.K.; Goc, J.; Shima, T.; et al. Lymphoid-Tissue-Resident Commensal Bacteria Promote Members of the IL-10 Cytokine Family to Establish Mutualism. Immunity 2016, 44, 634–646. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Moorlag, S.J.C.F.M.; Novakovic, B.; Li, Y.; Wang, S.-Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B.; et al. BCG Vaccination Protects Against Experimental Viral Infection in Humans Through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100.e5. [Google Scholar] [CrossRef] [PubMed]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.-J.; Wijmenga, C.; et al. Candida Albicans Infection Affords Protection against Reinfection via Functional Reprogramming of Monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction Between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Dang, B.; Gao, Q.; Zhang, L.; Zhang, J.; Cai, H.; Zhu, Y.; Zhong, Q.; Liu, J.; Niu, Y.; Mao, K.; et al. The Glycolysis/HIF-1α Axis Defines the Inflammatory Role of IL-4-Primed Macrophages. Cell Rep. 2023, 42, 112471. [Google Scholar] [CrossRef]
- Hiengrach, P.; Visitchanakun, P.; Finkelman, M.A.; Chancharoenthana, W.; Leelahavanichkul, A. More Prominent Inflammatory Response to Pachyman than to Whole-Glucan Particle and Oat-β-Glucans in Dextran Sulfate-Induced Mucositis Mice and Mouse Injection through Proinflammatory Macrophages. Int. J. Mol. Sci. 2022, 23, 4026. [Google Scholar] [CrossRef]
- Debisarun, P.A.; Gössling, K.L.; Bulut, O.; Kilic, G.; Zoodsma, M.; Liu, Z.; Oldenburg, M.; Rüchel, N.; Zhang, B.; Xu, C.-J.; et al. Induction of Trained Immunity by Influenza Vaccination—Impact on COVID-19. PLoS Pathog. 2021, 17, e1009928. [Google Scholar] [CrossRef]
- Brandi, P.; Conejero, L.; Cueto, F.J.; Martínez-Cano, S.; Dunphy, G.; Gómez, M.J.; Relaño, C.; Saz-Leal, P.; Enamorado, M.; Quintas, A.; et al. Trained Immunity Induction by the Inactivated Mucosal Vaccine MV130 Protects Against Experimental Viral Respiratory Infections. Cell Rep. 2022, 38, 110184. [Google Scholar] [CrossRef]
- Michaels, M.; Madsen, K.L. Immunometabolism and Microbial Metabolites at the Gut Barrier: Lessons for Therapeutic Intervention in Inflammatory Bowel Disease. Mucosal Immunol. 2023, 16, 72–85. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The Microbial Metabolite Butyrate Regulates Intestinal Macrophage Function via Histone Deacetylase Inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.G.; Patente, T.A.; Rouillé, Y.; Heumel, S.; Melo, E.M.; Deruyter, L.; Pourcet, B.; Sencio, V.; Teixeira, M.M.; Trottein, F. Acetate Improves the Killing of Streptococcus Pneumoniae by Alveolar Macrophages via NLRP3 Inflammasome and Glycolysis-HIF-1α Axis. Front. Immunol. 2022, 13, 773261. [Google Scholar] [CrossRef]
- Cai, H.; Chen, X.; Liu, Y.; Chen, Y.; Zhong, G.; Chen, X.; Rong, S.; Zeng, H.; Zhang, L.; Li, Z.; et al. Lactate Activates Trained Immunity by Fueling the Tricarboxylic Acid Cycle and Regulating Histone Lactylation. Nat. Commun. 2025, 16, 3230. [Google Scholar] [CrossRef]
- Montgomery, T.L.; Eckstrom, K.; Lile, K.H.; Caldwell, S.; Heney, E.R.; Lahue, K.G.; D’Alessandro, A.; Wargo, M.J.; Krementsov, D.N. Lactobacillus Reuteri Tryptophan Metabolism Promotes Host Susceptibility to CNS Autoimmunity. Microbiome 2022, 10, 198. [Google Scholar] [CrossRef]
- Bekkering, S.; Arts, R.J.W.; Novakovic, B.; Kourtzelis, I.; van der Heijden, C.D.C.C.; Li, Y.; Popa, C.D.; Ter Horst, R.; van Tuijl, J.; Netea-Maier, R.T.; et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 2018, 172, 135–146.e9. [Google Scholar] [CrossRef]
- Yang, T.; Hu, X.; Cao, F.; Yun, F.; Jia, K.; Zhang, M.; Kong, G.; Nie, B.; Liu, Y.; Zhang, H.; et al. Targeting Symbionts by Apolipoprotein L Proteins Modulates Gut Immunity. Nature 2025, 643, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-Microbe Interactions Have Shaped the Genetic Architecture of Inflammatory Bowel Disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Xavier, R.J. Pathway Paradigms Revealed from the Genetics of Inflammatory Bowel Disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef]
- Knights, D.; Silverberg, M.S.; Weersma, R.K.; Gevers, D.; Dijkstra, G.; Huang, H.; Tyler, A.D.; van Sommeren, S.; Imhann, F.; Stempak, J.M.; et al. Complex Host Genetics Influence the Microbiome in Inflammatory Bowel Disease. Genome Med. 2014, 6, 107. [Google Scholar] [CrossRef]
- Couturier-Maillard, A.; Secher, T.; Rehman, A.; Normand, S.; De Arcangelis, A.; Haesler, R.; Huot, L.; Grandjean, T.; Bressenot, A.; Delanoye-Crespin, A.; et al. NOD2-Mediated Dysbiosis Predisposes Mice to Transmissible Colitis and Colorectal Cancer. J. Clin. Invest. 2013, 123, 700–711. [Google Scholar] [CrossRef]
- Ji, C.; Yang, Z.; Zhong, X.; Xia, J. The Role and Mechanism of CARD9 Gene Polymorphism in Diseases. Biomed. J. 2021, 44, 560–566. [Google Scholar] [CrossRef]
- Lam, S.; Zuo, T.; Ho, M.; Chan, F.K.L.; Chan, P.K.S.; Ng, S.C. Review Article: Fungal Alterations in Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2019, 50, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, M.W. Editorial: The Role of Oxidative Stress, Epigenetics and Non-Coding RNA in Regulating Trained Immunity. Front. Immunol. 2020, 11, 2114. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Arsh, A.M.; Rathore, J.S. Trained Innate Immunity and Diseases: Bane with the Boon. Clin. Immunol. Commun. 2022, 2, 118–129. [Google Scholar] [CrossRef]
- Liu, R.; Tang, A.; Wang, X.; Chen, X.; Zhao, L.; Xiao, Z.; Shen, S. Inhibition of lncRNA NEAT1 Suppresses the Inflammatory Response in IBD by Modulating the Intestinal Epithelial Barrier and by Exosome-Mediated Polarization of Macrophages. Int. J. Mol. Med. 2018, 42, 2903–2913. [Google Scholar] [CrossRef]
- Xu, X.; Huang, Z.; Huang, Z.; Lv, X.; Jiang, D.; Huang, Z.; Han, B.; Lin, G.; Liu, G.; Li, S.; et al. Butyrate Attenuates Intestinal Inflammation in Crohn’s Disease by Suppressing Pyroptosis of Intestinal Epithelial Cells via the cGSA-STING-NLRP3 Axis. Int. Immunopharmacol. 2024, 143, 113305. [Google Scholar] [CrossRef]
- Chen, H.; Qian, Y.; Jiang, C.; Tang, L.; Yu, J.; Zhang, L.; Dai, Y.; Jiang, G. Butyrate Ameliorated Ferroptosis in Ulcerative Colitis through Modulating Nrf2/GPX4 Signal Pathway and Improving Intestinal Barrier. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166984. [Google Scholar] [CrossRef]
- Lührs, H.; Gerke, T.; Müller, J.G.; Melcher, R.; Schauber, J.; Boxberge, F.; Scheppach, W.; Menzel, T. Butyrate Inhibits NF-kappaB Activation in Lamina Propria Macrophages of Patients with Ulcerative Colitis. Scand. J. Gastroenterol. 2002, 37, 458–466. [Google Scholar] [CrossRef]
- Santis, S.D.; Pizarro, T.T. Host-Microbial Crosstalk Relies on “Tuft” Love. Immunity 2024, 57, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, E.M.; Rice, T.; Potter, C.; Waddell, A.; Hashimoto-Hill, S.; Woo, V.; Field, S.; Engleman, L.; Lim, H.-W.; Schumacher, M.A.; et al. Microbiota-Derived Butyrate Restricts Tuft Cell Differentiation via Histone Deacetylase 3 to Modulate Intestinal Type 2 Immunity. Immunity 2024, 57, 319–332.e6. [Google Scholar] [CrossRef]
- Wu, R.; Xiong, R.; Li, Y.; Chen, J.; Yan, R. Gut Microbiome, Metabolome, Host Immunity Associated with Inflammatory Bowel Disease and Intervention of Fecal Microbiota Transplantation. J. Autoimmun. 2023, 141, 103062. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Niechcial, A.; Schwarzfischer, M.; Wawrzyniak, M.; Atrott, K.; Laimbacher, A.; Morsy, Y.; Katkeviciute, E.; Häfliger, J.; Westermann, P.; Akdis, C.A.; et al. Spermidine Ameliorates Colitis via Induction of Anti-Inflammatory Macrophages and Prevention of Intestinal Dysbiosis. J. Crohns Colitis 2023, 17, 1489–1503. [Google Scholar] [CrossRef]
- Weiss, T.S.; Herfarth, H.; Obermeier, F.; Ouart, J.; Schölmerich, J.; Jauch, K.-W.; Rogler, G. Intracellular Polyamine Levels of Intestinal Epithelial Cells in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2004, 10, 529–535. [Google Scholar] [CrossRef]
- Li, X.; Xiao, X.; Wang, S.; Wu, B.; Zhou, Y. Uncovering de Novo Polyamine Biosynthesis in the Gut Microbiome and Its Alteration in Inflammatory Bowel Disease. Gut Microbes 2025, 17, 2464225. [Google Scholar] [CrossRef]
- Weaver, L.K.; Minichino, D.; Biswas, C.; Chu, N.; Lee, J.-J.; Bittinger, K.; Albeituni, S.; Nichols, K.E.; Behrens, E.M. Microbiota-Dependent Signals Are Required to Sustain TLR-Mediated Immune Responses. JCI Insight 2019, 4, e124370. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; Jarit-Cabanillas, A.; Brandi, P.; Martínez-López, M.; Martínez-Cano, S.; Rodrigo-Tapias, M.; Femenía-Muiña, M.; Redondo-Urzainqui, A.; Nuñez, V.; González-Correa, C.; et al. Microbiota Translocation Following Intestinal Barrier Disruption Promotes Mincle-Mediated Training of Myeloid Progenitors in the Bone Marrow. Immunity 2025, 58, 381–396.e9. [Google Scholar] [CrossRef] [PubMed]
- Shmuel-Galia, L.; Humphries, F.; Lei, X.; Ceglia, S.; Wilson, R.; Jiang, Z.; Ketelut-Carneiro, N.; Foley, S.E.; Pechhold, S.; Houghton, J.; et al. Dysbiosis Exacerbates Colitis by Promoting Ubiquitination and Accumulation of the Innate Immune Adaptor STING in Myeloid Cells. Immunity 2021, 54, 1137–1153.e8. [Google Scholar] [CrossRef]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota Metabolite Butyrate Constrains Neutrophil Functions and Ameliorates Mucosal Inflammation in Inflammatory Bowel Disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- Ruple, H.K.; Haasis, E.; Bettenburg, A.; Maier, C.; Fritz, C.; Schüle, L.; Löcker, S.; Soltow, Y.; Schintgen, L.; Schmidt, N.S.; et al. The Gut Microbiota Predicts and Time-Restricted Feeding Delays Experimental Colitis. Gut Microbes 2025, 17, 2453019. [Google Scholar] [CrossRef]
- Niu, Y.; Heddes, M.; Altaha, B.; Birkner, M.; Kleigrewe, K.; Meng, C.; Haller, D.; Kiessling, S. Targeting the Intestinal Circadian Clock by Meal Timing Ameliorates Gastrointestinal Inflammation. Cell Mol. Immunol. 2024, 21, 842–855. [Google Scholar] [CrossRef]
- Banfi, D.; Moro, E.; Bosi, A.; Bistoletti, M.; Cerantola, S.; Crema, F.; Maggi, F.; Giron, M.C.; Giaroni, C.; Baj, A. Impact of Microbial Metabolites on Microbiota-Gut-Brain Axis in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 1623. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Jain, S.; Yadav, H. Age-Related Cognitive Decline and Dementia: Interface of Microbiome–Immune–Neuronal Interactions. J. Gerontol. A Biol. Sci. Med. Sci. 2025, 80, glaf038. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Cao, L.-S.; Xia, W.-Y.; Wang, J.-M.; Wu, Q.-F. Gut Sensory Neurons as Regulators of Neuro-Immune-Microbial Interactions: From Molecular Mechanisms to Precision Therapy for IBD/IBS. J. Neuroinflamm. 2025, 22, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, T.; Chen, G.; Liang, Y.; Nie, X.; Gu, W.; Li, L.; Tong, H.; Huang, W.; Lu, T.; et al. Vinegar-Processed Schisandra Chinensis Enhanced Therapeutic Effects on Colitis-Induced Depression Through Tryptophan Metabolism. Phytomedicine 2024, 135, 156057. [Google Scholar] [CrossRef]
- Zheng, S.-Y.; Li, H.-X.; Xu, R.-C.; Miao, W.-T.; Dai, M.-Y.; Ding, S.-T.; Liu, H.-D. Potential Roles of Gut Microbiota and Microbial Metabolites in Parkinson’s Disease. Ageing Res. Rev. 2021, 69, 101347. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, C.; Lu, H.; Feng, C.; He, P.; Yang, X.; Xiang, C.; Zuo, J.; Tang, W. Protective Role of Berberine on Ulcerative Colitis through Modulating Enteric Glial Cells–Intestinal Epithelial Cells–Immune Cells Interactions. Acta Pharm. Sin. B 2020, 10, 447–461. [Google Scholar] [CrossRef]
- Mitroulis, I.; Ruppova, K.; Wang, B.; Chen, L.-S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I.; et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 2018, 172, 147–161.e12. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Yu, X.; Saha, G.; Kalafati, L.; Ioannidis, C.; Mitroulis, I.; Netea, M.G.; Chavakis, T.; Hajishengallis, G. Maladaptive Innate Immune Training of Myelopoiesis Links Inflammatory Comorbidities. Cell 2022, 185, 1709–1727.e18. [Google Scholar] [CrossRef]
- Kalafati, L.; Kourtzelis, I.; Schulte-Schrepping, J.; Li, X.; Hatzioannou, A.; Grinenko, T.; Hagag, E.; Sinha, A.; Has, C.; Dietz, S.; et al. Innate Immune Training of Granulopoiesis Promotes Anti-Tumor Activity. Cell 2020, 183, 771–785.e12. [Google Scholar] [CrossRef]
- Katzmarski, N.; Domínguez-Andrés, J.; Cirovic, B.; Renieris, G.; Ciarlo, E.; Le Roy, D.; Lepikhov, K.; Kattler, K.; Gasparoni, G.; Händler, K.; et al. Transmission of Trained Immunity and Heterologous Resistance to Infections across Generations. Nat. Immunol. 2021, 22, 1382–1390. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Xu, J.; Zhang, L.; Huang, C.; Nie, Y.; Zhou, Y. Gut Microbiota and Epigenetic Inheritance: Implications for the Development of IBD. Gut Microbes 2025, 17, 2490207. [Google Scholar] [CrossRef] [PubMed]
- Damianos, J.; Perumareddi, P. Gut Microbiome and Dietar Considerations. Prim. Care 2023, 50, 493–505. [Google Scholar] [CrossRef]
- Dietary Fiber in Inflammatory Bowel Disease: Are We Ready to Change the Paradigm? Available online: https://www.mdpi.com/2072-6643/16/8/1108 (accessed on 15 July 2025).
- Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10609902/ (accessed on 15 July 2025).
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A Prospective Study of Long-Term Intake of Dietary Fiber and Risk of Crohn’s Disease and Ulcerative Colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Jung, S.-C.; Kwak, K.; Kim, J.-S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, Z. Unraveling the Causal Link: Fatty Acids and Inflammatory Bowel Disease. Front. Immunol. 2024, 15, 1405790. [Google Scholar] [CrossRef]
- Li, M.; Liu, N.; Zhu, J.; Wu, Y.; Niu, L.; Liu, Y.; Chen, L.; Bai, B.; Miao, Y.; Yang, Y.; et al. Engineered Probiotics with Sustained Release of Interleukin-2 for the Treatment of Inflammatory Bowel Disease After Oral Delivery. Biomaterials 2024, 309, 122584. [Google Scholar] [CrossRef]
- Mishra, J.; Stubbs, M.; Kuang, L.; Vara, N.; Kumar, P.; Kumar, N. Inflammatory Bowel Disease Therapeutics: A Focus on Probiotic Engineering. Mediators Inflamm. 2022, 2022, 9621668. [Google Scholar] [CrossRef]
- Han, M.; Lei, W.; Liang, J.; Li, H.; Hou, M.; Gao, Z. The Single-Cell Modification Strategies for Probiotics Delivery in Inflammatory Bowel Disease: A Review. Carbohydr. Polym. 2024, 324, 121472. [Google Scholar] [CrossRef] [PubMed]
- Steidler, L.; Hans, W.; Schotte, L.; Neirynck, S.; Obermeier, F.; Falk, W.; Fiers, W.; Remaut, E. Treatment of Murine Colitis by Lactococcus lactis Secreting Interleukin-10. Science 2000, 289, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Daeffler, K.N.; Galley, J.D.; Sheth, R.U.; Ortiz-Velez, L.C.; Bibb, C.O.; Shroyer, N.F.; Britton, R.A.; Tabor, J.J. Engineering Bacterial Thiosulfate and Tetrathionate Sensors for Detecting Gut Inflammation. Mol. Syst. Biol. 2017, 13, 923. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Annese, V.; Bresci, G.; D’Albasio, G.; D’Incà, R.; Giaccari, S.; Ingrosso, M.; Mansi, C.; Riegler, G.; Valpiani, D.; et al. Topical Butyrate Improves Efficacy of 5—ASA in Refractory Distal Ulcerative Colitis: Results of a Multicentre Trial. Eur. J. Clin. Investig. 2003, 33, 244–248. [Google Scholar] [CrossRef]
- Wong, W.-Y.; Chan, B.D.; Cho, P.-T.; Leung, T.-W.; Tai, W.C.-S. Beneficial and Immunomodulatory Effects of Heat-Killed Lactobacillus plantarum L137 in Normal and Acute Colitis Mice. J. Funct. Foods 2024, 116, 106167. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Yadav, D.; Katiyar, S.; Jain, S.; Yadav, H. Postbiotics as Mitochondrial Modulators in Inflammatory Bowel Disease: Mechanistic Insights and Therapeutic Potential. Biomolecules 2025, 15, 954. [Google Scholar] [CrossRef]
- Feng, C.; Peng, C.; Zhang, W.; Zhang, T.; He, Q.; Kwok, L.-Y.; Zhang, H. Postbiotic Administration Ameliorates Colitis and Inflammation in Rats Possibly Through Gut Microbiota Modulation. J. Agric. Food Chem. 2024, 72, 9054–9066. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, L.-H.; Yang, H.; Fang, Y.-C.; Wang, S.-W.; Wang, M.; Yuan, Q.-T.; Wu, W.; Zhang, Y.-M.; Liu, Z.-J.; et al. GPR84 Signaling Promotes Intestinal Mucosal Inflammation via Enhancing NLRP3 Inflammasome Activation in Macrophages. Acta Pharmacol. Sin. 2022, 43, 2042–2054. [Google Scholar] [CrossRef]
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl Hydrocarbon Receptor and Intestinal Immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef]
- Willing, B.P.; Dicksved, J.; Halfvarson, J.; Andersson, A.F.; Lucio, M.; Zheng, Z.; Järnerot, G.; Tysk, C.; Jansson, J.K.; Engstrand, L. A Pyrosequencing Study in Twins Shows That Gastrointestinal Microbial Profiles Vary with Inflammatory Bowel Disease Phenotypes. Gastroenterology 2010, 139, 1844–1854.e1. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Yang, X.; Gu, L.; McGeachy, M.J.; Liu, X. Temporary Consumption of Western Diet Trains the Immune System to Reduce Future Gut Inflammation. iScience 2023, 26, 106915. [Google Scholar] [CrossRef]
- Ungaro, R.C.; Yzet, C.; Bossuyt, P.; Baert, F.J.; Vanasek, T.; D’Haens, G.R.; Joustra, V.W.; Panaccione, R.; Novacek, G.; Reinisch, W.; et al. Deep Remission at 1 Year Prevents Progression of Early Crohn’s Disease. Gastroenterology 2020, 159, 139–147. [Google Scholar] [CrossRef]
- Neurath, M.F.; Travis, S.P.L. Mucosal Healing in Inflammatory Bowel Diseases: A Systematic Review. Gut 2012, 61, 1619–1635. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-C.; Stappenbeck, T.S. Genetics and Pathogenesis of Inflammatory Bowel Disease. Annu. Rev. Pathol. 2016, 11, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chu, C.-Q. Prediction of Treatment Response: Personalized Medicine in the Management of Rheumatoid Arthritis. Best. Pract. Res. Clin. Rheumatol. 2022, 36, 101741. [Google Scholar] [CrossRef]
- van Lingen, E.; Nooij, S.; Terveer, E.M.; Crossette, E.; Prince, A.L.; Bhattarai, S.K.; Watson, A.; Galazzo, G.; Menon, R.; Szabady, R.L.; et al. Faecal Microbiota Transplantation Engraftment After Budesonide or Placebo in Patients with Active Ulcerative Colitis Using Pre-Selected Donors: A Randomized Pilot Study. J. Crohns Colitis 2024, 18, 1381–1393. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Fischer, M. Fecal Microbiota Transplantation for Ulcerative Colitis. Are We Ready for Primetime? Gastroenterol. Clin. North. Am. 2020, 49, 739–752. [Google Scholar] [CrossRef]
- Kuhnen, A. Genetic and Environmental Considerations for Inflammatory Bowel Disease. Surg. Clin. North. Am. 2019, 99, 1197–1207. [Google Scholar] [CrossRef]
- Drewes, J.L.; Corona, A.; Sanchez, U.; Fan, Y.; Hourigan, S.K.; Weidner, M.; Sidhu, S.D.; Simner, P.J.; Wang, H.; Timp, W.; et al. Transmission and Clearance of Potential Procarcinogenic Bacteria During Fecal Microbiota Transplantation for Recurrent Clostridioides Difficile. JCI Insight 2019, 4, e130848. [Google Scholar] [CrossRef]
- Fritsch, J.; Abreu, M.T. The Microbiota and the Immune Response: What Is the Chicken and What Is the Egg? Gastrointest. Endosc. Clin. N. Am. 2019, 29, 381–393. [Google Scholar] [CrossRef]
- Anthamatten, L.; von Bieberstein, P.R.; Menzi, C.; Zünd, J.N.; Lacroix, C.; de Wouters, T.; Leventhal, G.E. Stratification of Human Gut Microbiomes by Succinotype Is Associated with Inflammatory Bowel Disease Status. Microbiome 2024, 12, 186. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.J.; Gan, H.Y.; Li, X.; Huang, Y.; Li, Z.C.; Deng, H.M.; Chen, S.Z.; Zhou, Y.; Wang, L.S.; Han, Y.P.; et al. Correlation of Diet, Microbiota and Metabolite Networks in Inflammatory Bowel Disease. J. Dig. Dis. 2019, 20, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Ryan, F.J.; Ahern, A.M.; Fitzgerald, R.S.; Laserna-Mendieta, E.J.; Power, E.M.; Clooney, A.G.; O’Donoghue, K.W.; McMurdie, P.J.; Iwai, S.; Crits-Christoph, A.; et al. Colonic Microbiota Is Associated with Inflammation and Host Epigenomic Alterations in Inflammatory Bowel Disease. Nat. Commun. 2020, 11, 1512. [Google Scholar] [CrossRef] [PubMed]
- Keiran, N.; Ceperuelo-Mallafré, V.; Calvo, E.; Hernández-Alvarez, M.I.; Ejarque, M.; Núñez-Roa, C.; Horrillo, D.; Maymó-Masip, E.; Rodríguez, M.M.; Fradera, R.; et al. SUCNR1 Controls an Anti-Inflammatory Program in Macrophages to Regulate the Metabolic Response to Obesity. Nat. Immunol. 2019, 20, 581–592. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Z.; Zhao, X.; Xu, L.; Teng, C.; Liu, S.; Huang, W.; Li, Y. Advanced Targeted Curcumin Delivery Using Biodegradable Hierarchical Microspheres with Calcium Pectinate Matrix and Hyaluronic Acid Moieties for Enhancing Colitis Amelioration. Carbohydr. Polym. 2025, 353, 123273. [Google Scholar] [CrossRef]
- Ziogas, A.; Bruno, M.; van der Meel, R.; Mulder, W.J.M.; Netea, M.G. Trained Immunity: Target for Prophylaxis and Therapy. Cell Host Microbe 2023, 31, 1776–1791. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhu, F.; Cheng, K.; Ma, N.; Ma, X.; Feng, Q.; Xu, C.; Gao, X.; Wang, X.; Shi, J.; et al. Outer Membrane Vesicle-Based Nanohybrids Target Tumor-Associated Macrophages to Enhance Trained Immunity-Related Vaccine-Generated Antitumor Activity. Adv. Mater. 2023, 35, e2306158. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.M.; Cox, D.J.; Connolly, S.A.; Breen, E.P.; Brugman, A.A.; Phelan, J.J.; Keane, J.; Basdeo, S.A. Trained Immunity Is Induced in Humans after Immunization with an Adenoviral Vector COVID-19 Vaccine. J. Clin. Invest. 2023, 133, e162581. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).