Blood–Brain Barrier Penetration of Novel 4-Trifluoromethyl-Coumarin Hybrids with Antibacterial Properties as Potential Brain Therapeutics in the Context of Spatially Diverse Healthcare Systems
Abstract
1. Introduction
2. Results and Discussion
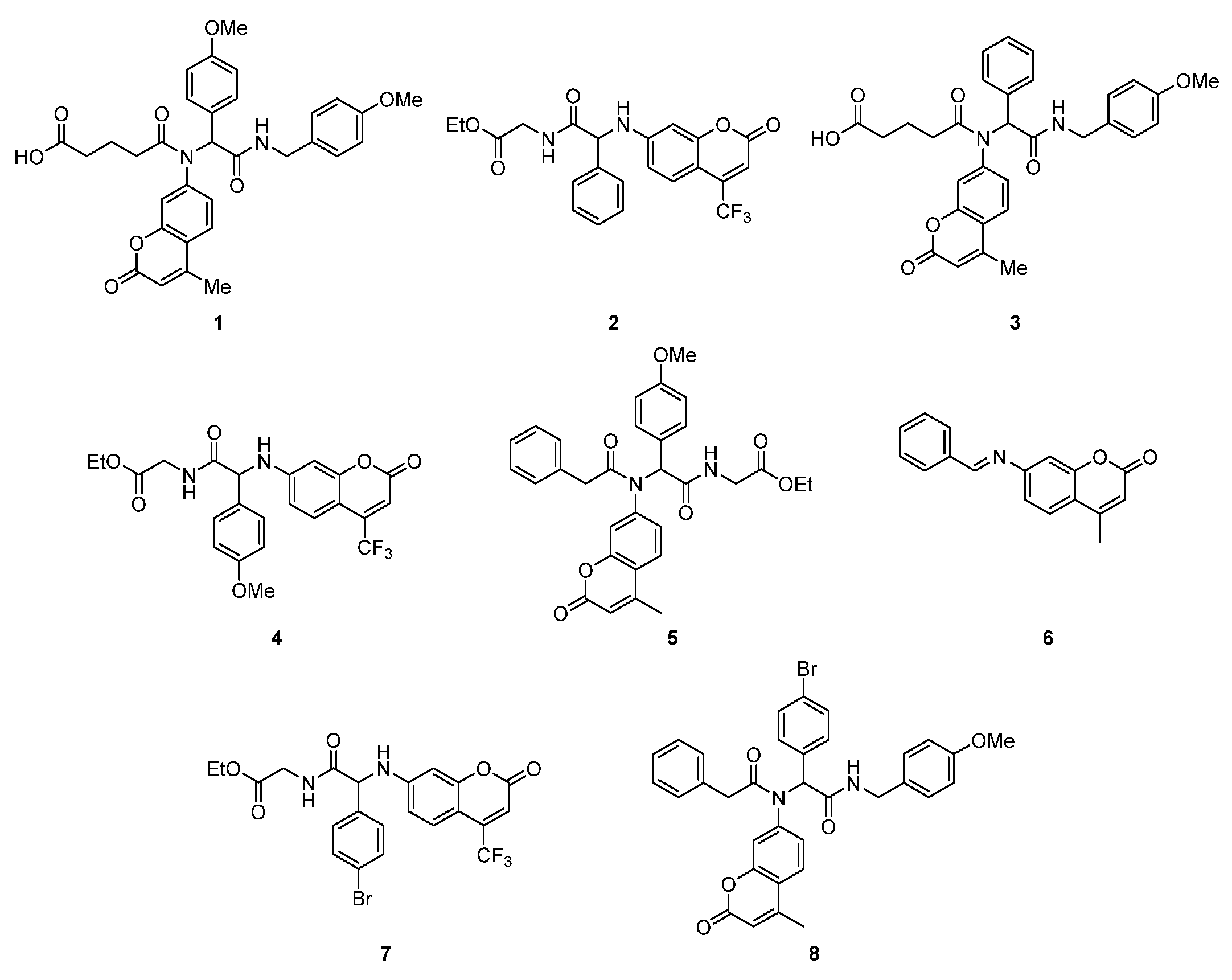
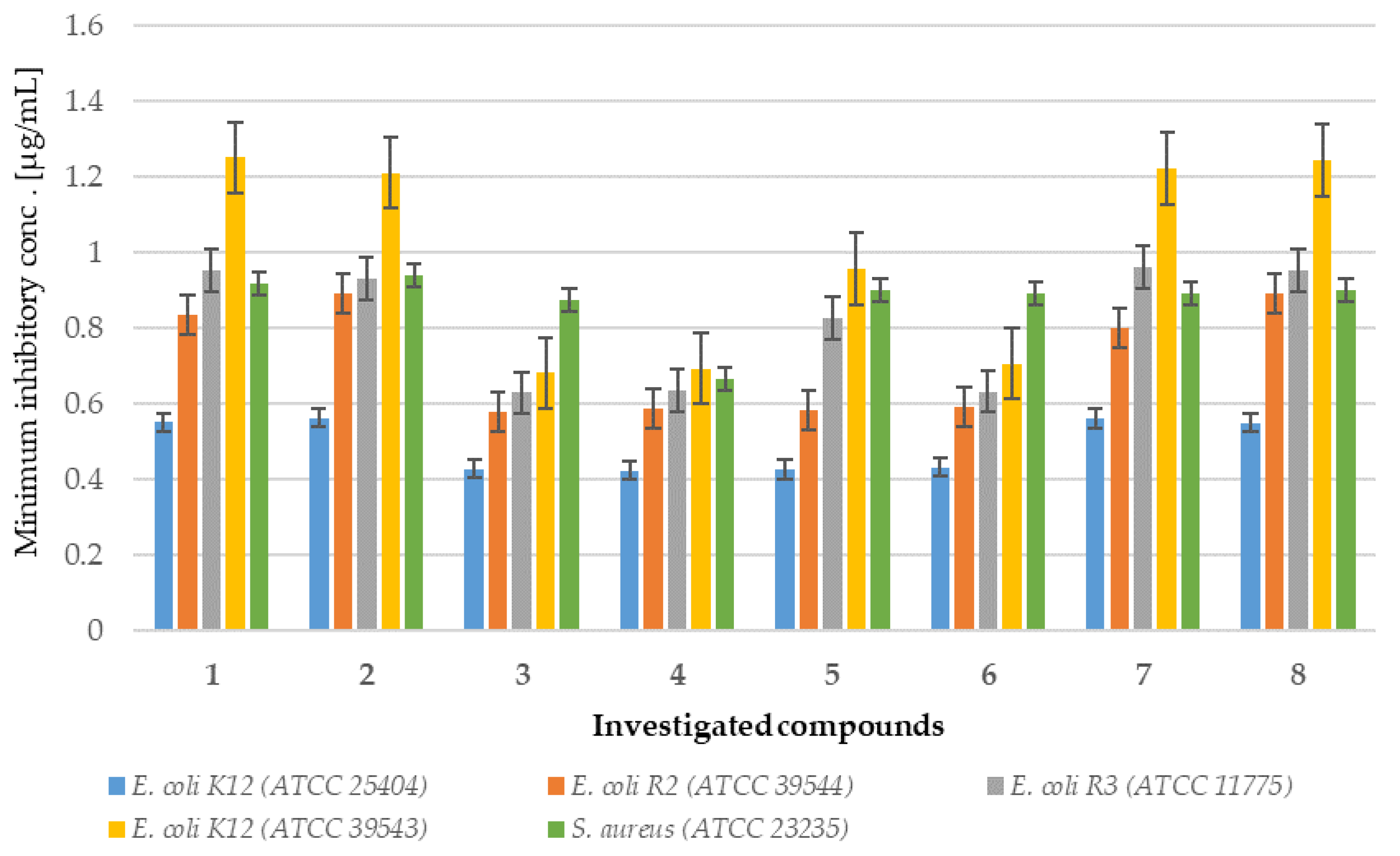
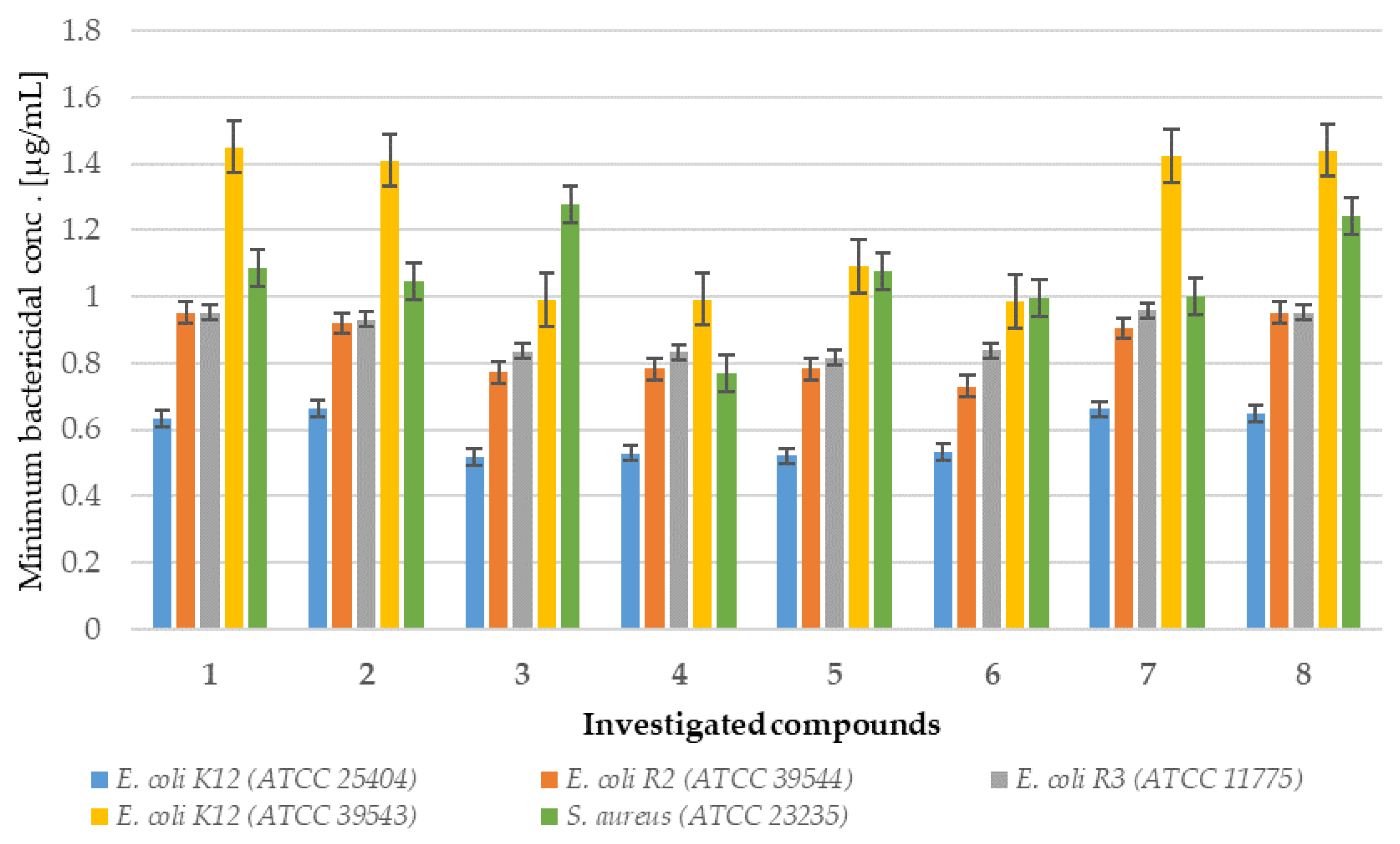
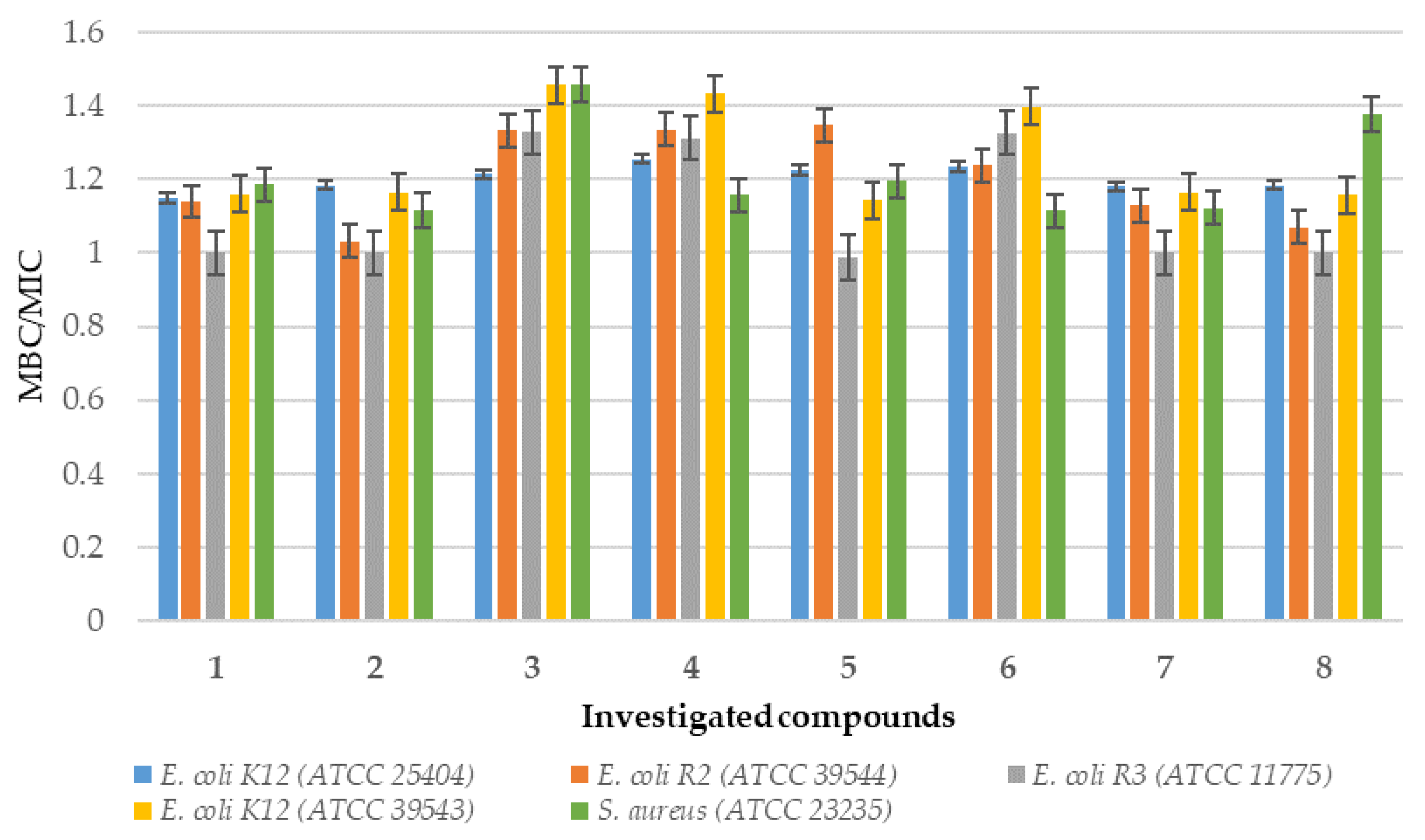
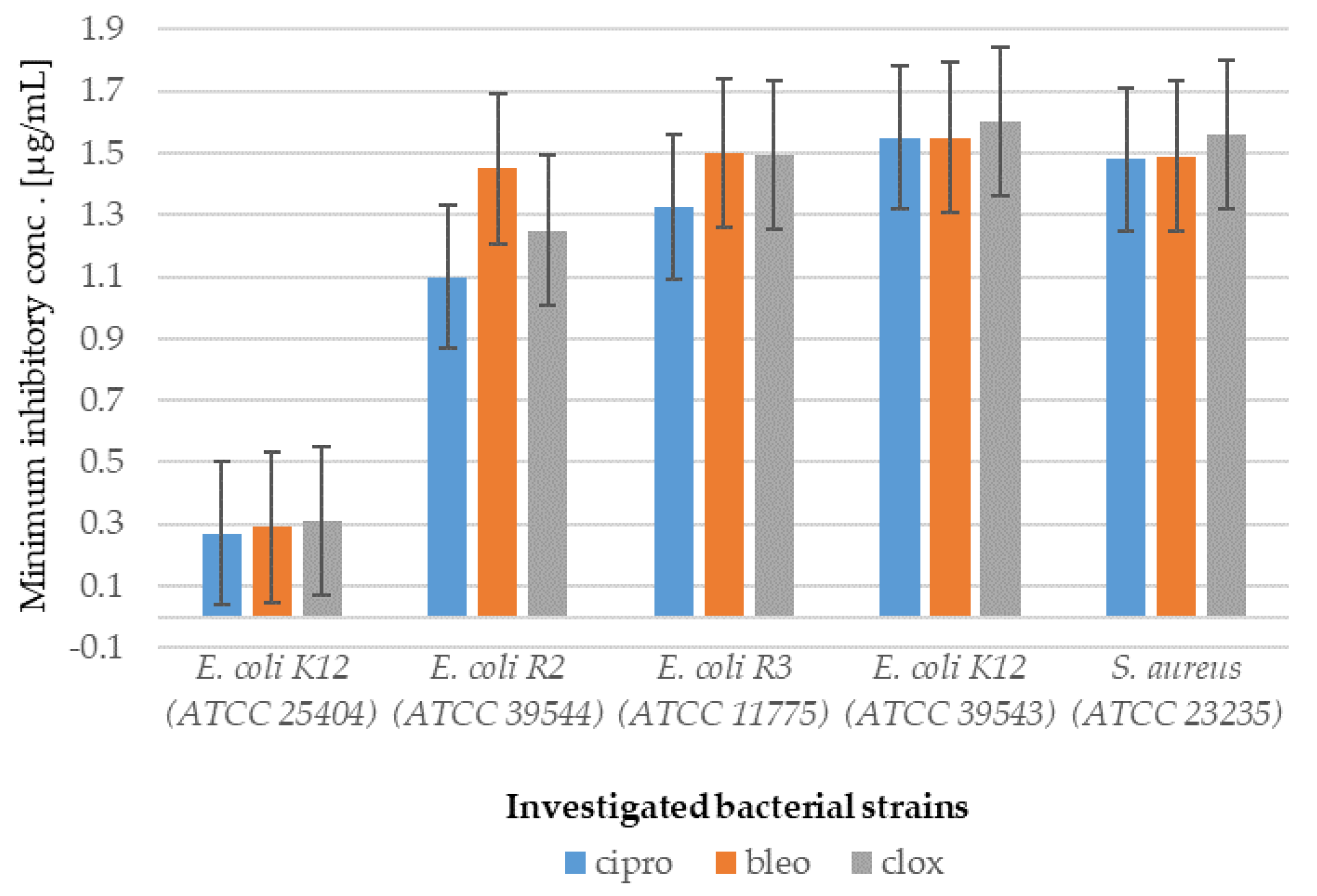
2.1. In Vitro Experiment—Bactericidal Effects
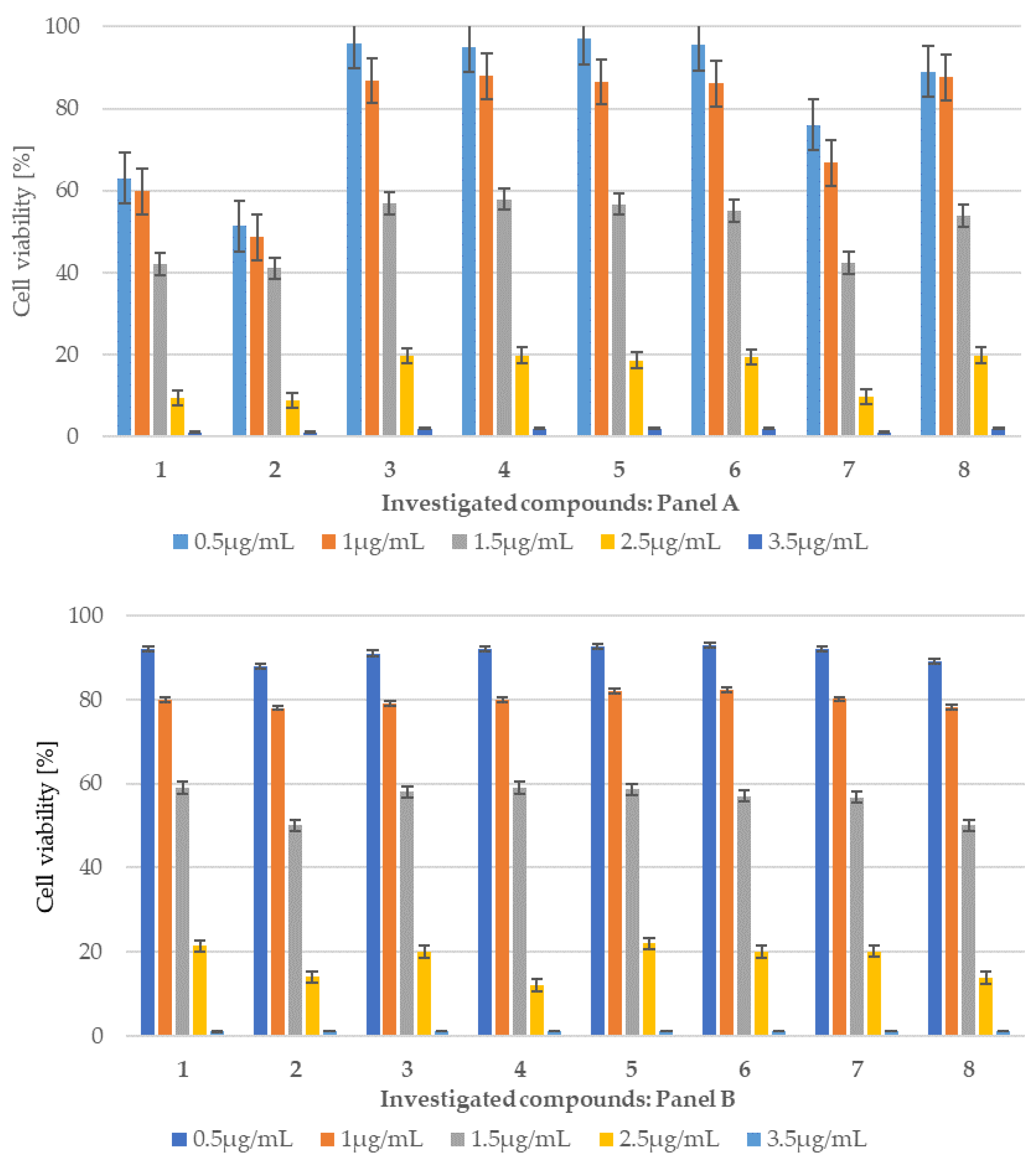
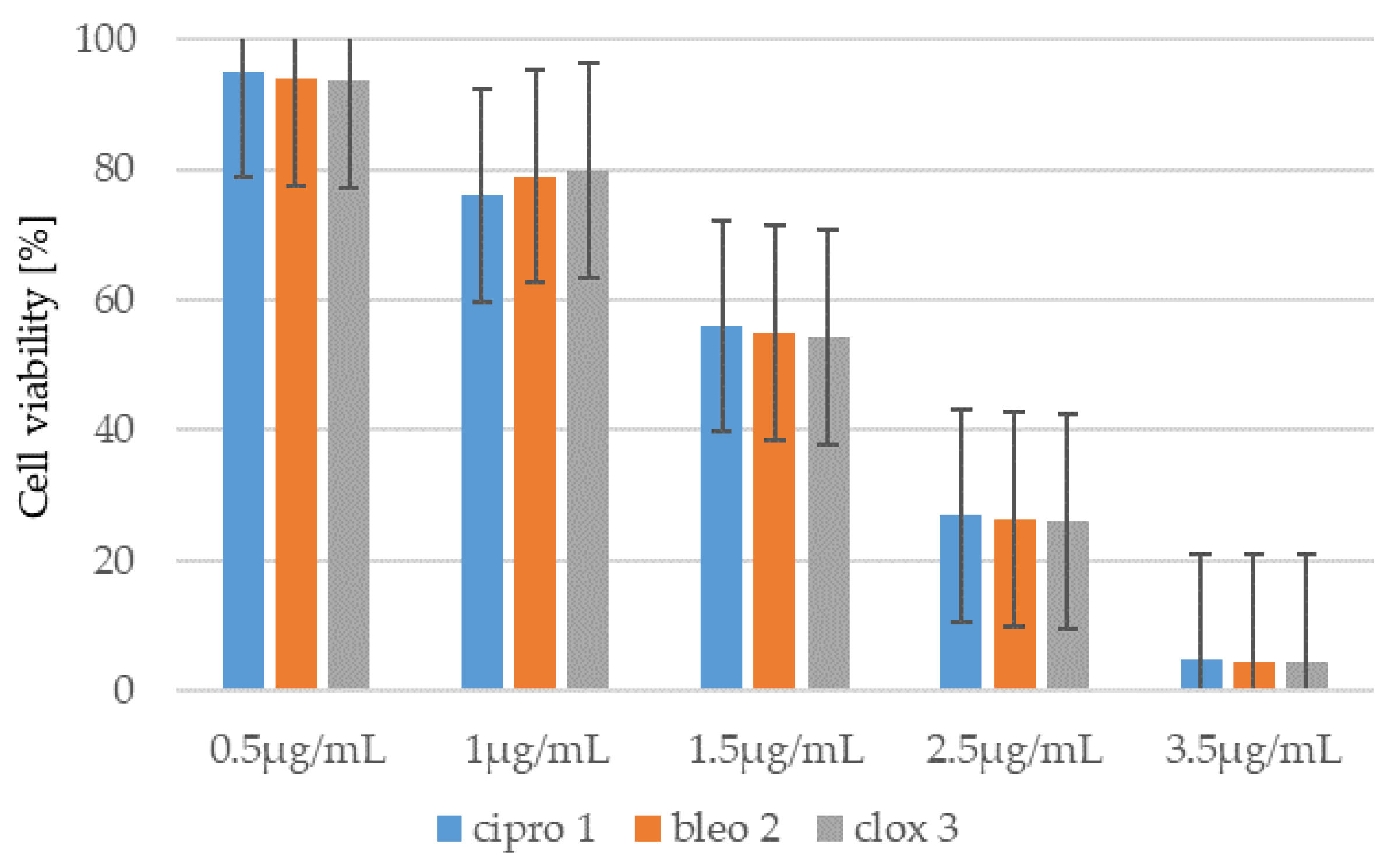
2.1.1. Cytotoxic Studies of the Library of Coumarin with Trifluoromethyl Group
2.1.2. Analysis of E. coli R2–R4 Strains Treated with Investigated Compounds 1–8
2.1.3. Cytotoxicity of Coumarin-Based Peptidomimetics 1–8
2.2. In Vivo Experiment—Transfer of Coumarins to the CSF in Sheep
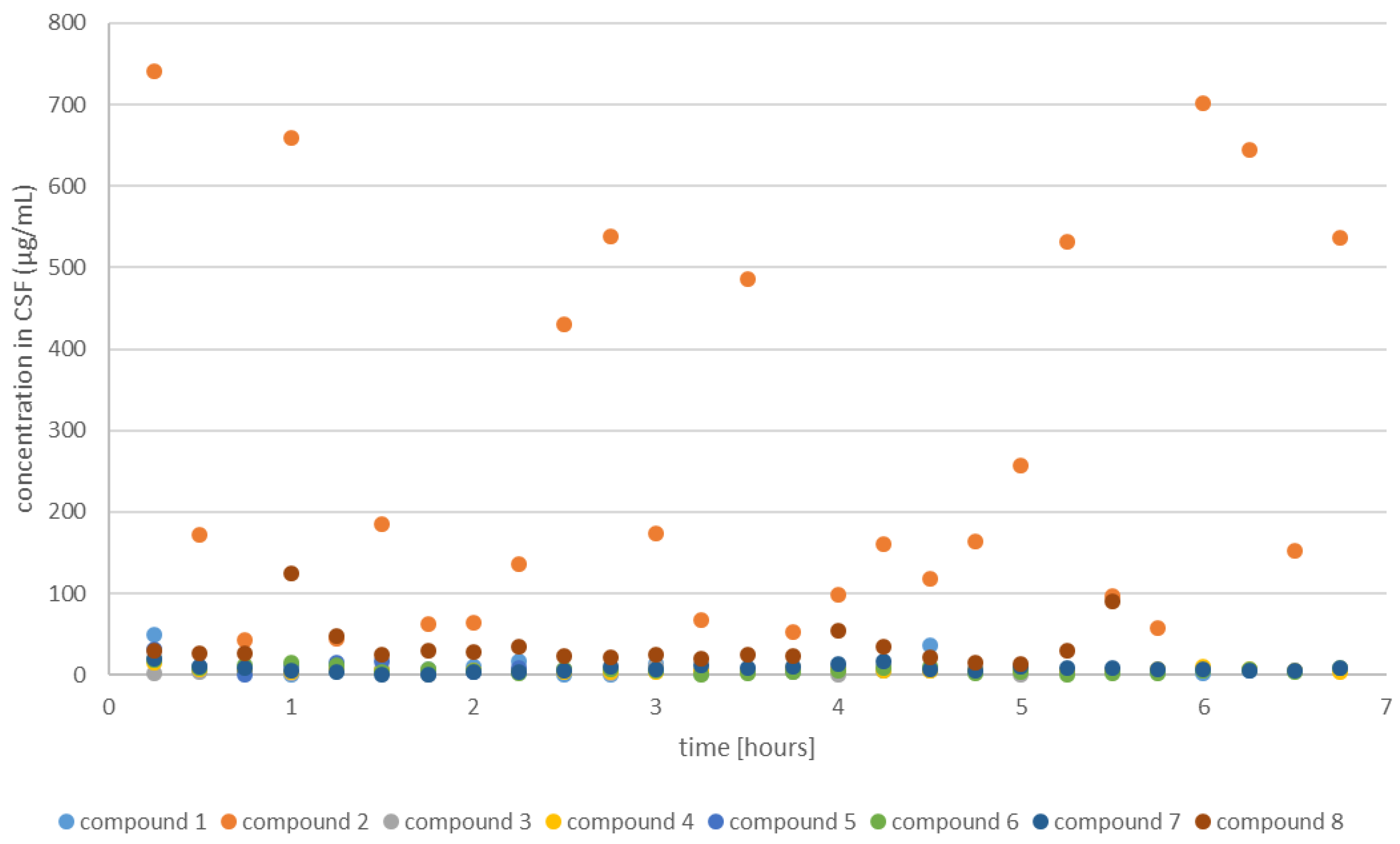
2.2.1. CSF Coumarins Concentration
2.2.2. Centrifugation of CSF Decreases the Coumarin Concentration
2.2.3. Change in Coumarin Concentration Levels over Time
3. Materials and Methods
3.1. Chemistry of Newly Synthesized Coumarin Derivatives
3.2. General Method for Imine Preparation
3.3. General Procedure for Synthesis of 4U-MCR Compounds
3.4. General Procedure for Synthesis of 3U-MCR Compounds
3.5. Compound 1
3.6. Compound 2
3.7. Compound 4
3.8. Compound 5
3.9. Compound 7
3.10. Microorganisms and Media—In Vitro Experiment Number 1-Bactericidal Effects
3.11. The Determination of the Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (MBC)
3.12. Experiment No. 2—MTT Assay
3.13. Experiment No. 3—Transfer of Coumarins to the CSF in Sheep In Vivo-Animal Management
3.14. Third Ventricle (IIIv) Cannulation
3.15. Drug Preparation
3.16. Experimental Design and Sample Collection
3.17. Measurement of Coumarin Concentration in the CSF of Sheep
3.18. Mass Spectrometric Determination of Coumarin 2 in Cerebrospinal Fluid (CSF) Collected from Sheep
3.19. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gong, X.; Wang, N.; Zhu, H.; Tang, N.; Wu, K.; Meng, Q. Anti-NMDAR antibodies, the blood-brain barrier, and anti-NMDAR encephalitis. Front. Neurol. 2023, 14, 1283511. [Google Scholar] [CrossRef]
- Zhang, L.; Nan, X.; Zhou, D.; Wang, X.; Zhu, S.; Li, Q.; Jia, F.; Zhu, B.; Si, Y.; Cao, S.; et al. Japanese encephalitis virus NS1 and NS1’ protein disrupts the blood-brain barrier through macrophage migration inhibitory factor-mediated autophagy. J. Virol. 2024, 98, e0011624. [Google Scholar] [CrossRef]
- Oevermann, A.; Di Palma, S.; Doherr, M.G.; Abril, C.; Zurbriggen, A.; Vandevelde, V.M. Neuropathogenesis of naturally occurring encephalitis caused by Listeria monocytogenes in ruminants. Brain Pathol. 2010, 20, 378–390. [Google Scholar] [CrossRef]
- Fecteau, G.; George, L.W. Bacterial meningitis and encephalitis in ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Filioussis, G.; Petridou, E.; Karavanis, E.; Giadinis, N.D.; Xexaki, A.; Govaris, A.; Kritas, S.K.J. An outbreak of caprine meningoencephalitis due to Escherichia coli O157:H7. J. Vet. Diagn. Investig. 2013, 25, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cai, M.; Hu, J.; Zhang, Z.; Wang, X.; Chang, X.; Zhang, F.; Guo Ch Wang, X. Mechanism of blood-brain barrier disruption by an Escherichia coli from lambs with severe diarrhea and meningoencephalitis. Microb. Pathog. 2020, 147, 104288. [Google Scholar] [CrossRef]
- Banks, W.A. From blood–brain barrier to blood–brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Peluffo, H.; Unzueta, U.; Negro-Demontel, M.L. BBB-targeting, protein-based nanomedicines for drug and nucleic acid delivery to the CNS. Biotechnol. Adv. 2015, 33, 277–287. [Google Scholar] [CrossRef]
- Abbott, N.J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef]
- Elmeliegy, M.A.; Carcaboso, A.M.; Tagen, M.; Bai, F.; Stewart, C.F. Role of ATP-Binding Cassette and Solute Carrier Transporters in Erlotinib CNS Penetration and Intracellular Accumulation. Clin. Cancer Res. 2011, 17, 89–99. [Google Scholar] [CrossRef]
- Pérez-Hernández, M.; Fernández-Valle, M.E.; Rubio-Araiz, A.; Vidal, R.; Gutiérrez-López, M.D.; O’Shea, E.; Colado, M.I. 3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) produces edema due to BBB disruption induced by MMP-9 activation in rat hippocampus. Neuropharmacology 2017, 118, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 1–14, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Ayrton, A.; Morgan, P. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica 2001, 31, 469–497. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug and gene targeting to the brain with molecular Trojan horses. Nat. Rev. Drug Discov. 2002, 1, 131–139. [Google Scholar] [CrossRef]
- Yan, E.; Castillo-Meléndez, M.; Nicholls, T.; Hirst, J.; Walker, D. Cerebrovascular responses in the fetal sheep brain to low-dose endotoxin. Pediatr. Res. 2004, 55, 855–863. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Sakka, A.L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef]
- Laterra, J.; Keep, R.; Betz, L.A.; Goldstein, G.W. Blood-cerebrospinal fluid barrier. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Siegel, G.J., Agranoff, B.W., Albers, R.W., Fisher, S.K., Uhler, M.D., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999. Available online: https://www.ncbi.nlm.nih.gov/books/NBK27998/ (accessed on 6 November 1999).
- Skipor, J.; Thiery, J.C. The choroid plexus—Cerebrospinal fluid system: Undervaluated pathway of neuroendocrine signaling into the brain. Acta Neurobiol. Exp. 2008, 68, 414–428. [Google Scholar] [CrossRef]
- Wang, D.; Guan, S.; Lu, P.; Li, Y.; Xu, H. Extracellular vesicles: Critical bilateral communicators in periphery-brain crosstalk in central nervous system disorders. Biomed. Pharmacother. 2023, 160, 114354. [Google Scholar] [CrossRef]
- Goncalves, A.R.A.; De Felice, F.G. The crosstalk between brain and periphery: Implications for brain health and disease. Neuropharmacology 2021, 197, 108728. [Google Scholar] [CrossRef]
- Murray, S.J.; Mitchell, N.L. The Translational Benefits of Sheep as Large Animal Models of Human Neurological Disorders. Front. Vet. Sci. 2022, 9, 831838. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Qu, S.; Wang, J.; He, X.; Lin, W.; Zhen, H.; Zhang, X. Neuroprotective effects of osthole pretreatment against traumatic brain injury in rats. Brain Res. 2012, 1433, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Galea, I. The blood-brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef] [PubMed]
- McCallum, N.; Berger-Bachi, B.; Senn, M.M. Regulation of antibiotic resistance in Staphylococcus aureus. Int. J. Med. Microbiol. 2010, 300, 118–129. [Google Scholar] [CrossRef]
- Kadeřábková, N.; Mahmood, A.J.S.; Mavridou, D.A.I. Antibiotic susceptibility testing using minimum inhibitory concentration (MIC) assays. Npj Antimicrob. Resist. 2024, 2, 37. [Google Scholar] [CrossRef]
- Takahashi, Y.; Takahashi, T.; Usuda, H.; Carter, S.; Fee, E.L.; Furfaro, L.; Chemtob, S.; Olson, D.M.; Keelan, J.A.; Kallapur, S.; et al. Pharmacological blockade of the interleukin-1 receptor suppressed Escherichia coli lipopolysaccharide-induced neuroinflammation in preterm fetal sheep. Am. J. Obstet. Gynecol. MFM. 2023, 5, 101124. [Google Scholar] [CrossRef]
- Matsushima, S.; Maeda, K.; Hayashi, H.; Debori, Y.; Schinkel, A.H.; Schuetz, J.D.; Kusuhara, H.; Sugiyama, Y. Involvement of Multiple Efflux Transporters in Hepatic Disposition of Fexofenadine. Mol. Pharmacol. 2008, 73, 1474–1483. [Google Scholar] [CrossRef]
- Patra, A.; Chen, X.; Sadowska, G.B.; Zhang, J.; Lim, Y.-P.; Padbury, J.F.; Banks, W.A.; Stonestreet, B.S. Neutralizing anti-interleukin-1β antibodies reduce ischemia-related interleukin-1β transport across the blood-brain barrier in fetal sheep. Neuroscience 2017, 346, 113–125. [Google Scholar] [CrossRef]
- Chen, X.; Sadowska, G.B.; Zhang, J.; Kim, J.E.; Cummings, E.E.; Bodge, C.A.; Lim, Y.P.; Makeyev, O.; Besio, W.G.; Gaitanis, J.; et al. Neutralizing anti-interleukin-1beta antibodies modulate fetal blood-brain barrier function after ischemia. Neurobiol. Dis. 2015, 73, 118–129. [Google Scholar] [CrossRef]
- Peng, X.M.; Damu, G.L.V.; Zhou, C.H. Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. Des. 2013, 19, 3884–3930. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, A.; Yang, Y.; Yang, P. Coumarin-containing hybrids and their antibacterial activities. Arch. Pharm. 2020, 353, 1900380. [Google Scholar] [CrossRef]
- Qin, H.L.; Zhang, Z.W.; Ravindar, L.; Rakesh, K.P. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020, 207, 112832. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Madej, A.; Paprocki, D.; Szymczak, M.; Ostaszewski, R. Coumarin Derivatives as New Toxic Compounds to Selected K12, R1-R4 E. coli Strains. Materials 2020, 13, 2499. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Wilk, M.; Parul, P.; Szymczak, M.; Kramkowski, K.; Raj, S.; Skiba, G.; Sulejczak, D.; Kleczkowska, P.; Ostaszewski, R. The Synthesis and Evaluation of Aminocoumarin Peptidomimetics as Cytotoxic Agents on Model Bacterial, E. coli Strains. Materials 2021, 14, 5725. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Koszelewski, D.; Brodzka, A.; Kramkowski, K.; Ostaszewski, R. Evaluation of Antibacterial Activity against Nosocomial Pathogens of an Enzymatically Derived α-Aminophosphonates Possessing Coumarin Scaffold. Int. J. Mol. Sci. 2023, 24, 14886. [Google Scholar] [CrossRef] [PubMed]
- Kalkhambkar, R.G.; Kulkarni, G.M.; Kamanavalli, C.M.; Premkumar, N.; Asdaq, S.M.B.; Sun, C.M. Synthesis and biological activities of some new fluorinated coumarins and 1-aza coumarins. Europ. J. Med. Chem. 2008, 43, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- LShukla, L.W.K.; Moodie, T.; Kindahl, C.J. Hedberg, Synthesis and Spectroscopic Properties of Fluorinated Coumarin Lysine Derivatives. Org. Chem. 2018, 83, 4792–4799. [Google Scholar] [CrossRef]
- Hornick, A.; Lieb, A.; Vo, N.P.; Rollinger, J.M.; Stuppner, H.; Prast, H. The coumarin scopoletin potentiates acetylcholine release from synaptosomes, amplifies hippocampal long-term potentiation and ameliorates anticholinergic- and age-impaired memory. Neuroscience 2011, 197, 280–292. [Google Scholar] [CrossRef]
- Solarz, A.; Majcher-Maślanka, I.; Chocyk, A. Effects of early-life stress and sex on blood-brain barrier permeability and integrity in juvenile and adult rats. Dev. Neurobiol. 2021, 7, 861–876. [Google Scholar] [CrossRef]
- Ronad, P.; Dharbamalla, S.; Hunshal, R.; Maddi, V. Synthesis of novel substituted 7-(benzylideneamino)-4-methyl-2H-chromen-2-one derivatives as anti-inflammatory and analgesic agents. Arch. Pharm. 2008, 341, 696–700. [Google Scholar] [CrossRef]
- Żołek, T.; Maciejewska, D. Theoretical evaluation of ADMET properties for coumarin derivatives as compounds with therapeutic potential. Eur. J. Pharm. Sci. 2017, 15, 486–502. [Google Scholar] [CrossRef]
- Wang, H.; Su, M.; Shi, X.; Li, X.; Zhang, X.; Yang, A.; Shen, R. Design, Synthesis, Calculation and Biological Activity Studies Based on Privileged Coumarin Derivatives as Multifunctional Anti-AD Lead Compound. Chem. Biodivers. 2023, 20, e202200867. [Google Scholar] [CrossRef]
- Orioli, R.; Belluti, F.; Gobbi, S.; Rampa, A.; Bisi, A. Naturally Inspired Coumarin Derivatives in Alzheimer’s Disease Drug Discovery: Latest Advances and Current Challenges. Molecules 2024, 29, 3514. [Google Scholar] [CrossRef]
- Hamuľaková, S.; Gucký, A.; Mezencev, R.; Kožurková, M.; Bednáriková, Z.; Marek, J.; Soukup, O.; Janoušek, J.; Gažová, Z. Inhibition of amyloid fibrillization of amyloid β peptide by 4,7-disubstituted coumarin derivatives. Bioorg. Med. Chem. 2025, 129, 118302. [Google Scholar] [CrossRef]
- Sarhan, M.O.; Abd El-Karim, S.S.; Anwar, M.M.; Gouda, R.H.; Zaghary, W.A.; Khedr, M.A. Discovery of New Coumarin-Based Lead with Potential Anticancer, CDK4 Inhibition and Selective Radiotheranostic Effect: Synthesis, 2D & 3D QSAR, Molecular Dynamics, In Vitro Cytotoxicity, Radioiodination, and Biodistribution Studies. Molecules 2021, 26, 2273. [Google Scholar] [CrossRef]
- Sharma, A.; Bharate, S.B. Synthesis and Biological Evaluation of Coumarin Triazoles as Dual Inhibitors of Cholinesterases and β-Secretase. ACS Omega 2023, 12, 11161–11176. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Chang, K.-H.; Chiu, Y.-J.; Chen, Y.-R.; Lung, T.-H.; Hsieh-Li, H.M.; Su, M.-T.; Sun, Y.-C.; Chen, C.-M.; Lin, W.; et al. Multi-Target Effects of Novel Synthetic Coumarin Derivatives Protecting Aβ-GFP SH-SY5Y Cells against Aβ Toxicity. Cells 2021, 10, 3095. [Google Scholar] [CrossRef] [PubMed]
- Młotkowska, P.; Marciniak, E.; Misztal, A.; Misztal, T. Effect of Neurosteroids on Basal and Stress-Induced Oxytocin Secretion in Luteal-Phase and Pregnant Sheep. Animals 2023, 13, 1658. [Google Scholar] [CrossRef]
- Welento, J.; Szteyn, S.; Milart, Z. Observations on the stereotaxic configuration of the hypothalamus nuclei in the sheep. Anat. Anz. 1969, 124, 1–27. [Google Scholar]
- Haziak, K.; Herman, A.P.; Wojtulewicz, K.; Pawlina, B.; Paczesna, K.; Bochenek, J.; Tomaszewska-Zaremba, D. Effect of CD14/TLR4 antagonist on GnRH/LH secretion in ewe during central inflammation induced by intra cerebro ventricular administration of LPS. J. Anim. Sci. Biotechnol. 2018, 9, 52. [Google Scholar] [CrossRef]
- Curley, P.; Rajoli, R.K.R.; Moss, D.M.; Liptrott, N.J.; Letendre, S.; Owen, A.; Siccardi, M. Efavirenz Is Predicted To Accumulate in Brain Tissue: An In Silico, In Vitro, and In Vivo Investigation. Antimicrob. Agents Chemother. 2017, 61, e01841-16. [Google Scholar] [CrossRef]
- Visentin, M.; Chang, M.-H.; Romero, M.F.; Zhao, R.; Goldman, I.D. Substrate- and PH-Specific Antifolate Transport Mediated by Organic Anion-Transporting Polypeptide 2B1 (OATP2B1-SLCO2B1). Mol. Pharmacol. 2012, 81, 134–142. [Google Scholar] [CrossRef]
- Glaeser, H.; Bailey, D.G.; Dresser, G.K.; Gregor, J.C.; Schwarz, U.I.; McGrath, J.S.; Jolicoeur, E.; Lee, W.; Leake, B.F.; Tirona, R.G.; et al. Intestinal Drug Transporter Expression and the Impact of Grapefruit Juice in Humans. Clin. Pharmacol. Ther. 2007, 81, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, V.; Minic, R.; Vakic, J.; Ivanovic, S.; Cupic, V.; Borozan, S.; Nesic, A.; Zivkovic, I. MTT based L-aminoacid oxidase activity test for determination of antivenom potency against Vipera ammodytes envenomation. Toxicon 2021, 192, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Tataringa, G.; Zbancioc, A.M.; Rao, A.V. (Eds.) Phytochemicals in Human Health; Intech Open: London, UK, 2020. [Google Scholar] [CrossRef]
- Bandelj, P.; Jamnikar-Ciglenecki, U.; Ocepek, M.; Blagus, R.; Vengust, M. Risk factors associated with fecal shedding of Listeria monocytogenes by dairy cows and calves. J. Vet. Intern. Med. 2018, 32, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, S.; Gudbjarnason, S. Inhibition of acetylcholinesterase by extracts and constituents from Angelica archangelica and Geranium sylvaticum. Z. Naturforsch C J. Biosci. 2007, 62, 689–693. [Google Scholar] [CrossRef]
- Wenjie, L.; Limeng, W.; Wenwu, L.; Liting, T.; Chen Ch, H.; Wu, Z.; Wang, N.; Liu, X.; Qiu, J.; Feng, X.; et al. Design, synthesis and biological evaluation of novel coumarin derivatives as multifunctional ligands for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2022, 242, 114689. [Google Scholar] [CrossRef]
- van der Waterbeemd, H.; Camenisch, G.; Folkers, G.; Chretien, J.R.; Raevsky, O.A. Estimation of blood-brain barrier crossing of drugs using molecular size and shape, and H-bonding descriptors. J. Drug Target. 1998, 6, 151–165. [Google Scholar] [CrossRef]
- Solarz, A.; Majcher-Maślanka, I.; Kryst, J.; Chocyk, A. Early-life stress affects peripheral, blood-brain barrier, and brain responses to immune challenge in juvenile and adult rats. Brain Behav. Immun. 2023, 108, 1–15. [Google Scholar] [CrossRef]
- Pluimer, B.R.; Colt, M.; Zhao, Z. G Protein-Coupled Receptors in the Mammalian Blood-Brain Barrier. Front. Cell. Neurosci. 2020, 14, 139. [Google Scholar] [CrossRef]
- Parvez, M.M.; Sadighi, A.; Ahn, Y.; Keller, S.F.; Enoru, J.O. Uptake Transporters at the Blood–Brain Barrier and Their Role in Brain Drug Disposition. Pharmaceutics 2023, 15, 2473. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, N.; Lankadeva, Y.R.; Peiris, R.M.; Birchall, I.E.; May, C.N. Rapid and persistent decrease in brain tissue oxygenation in ovine gram-negative sepsis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R990–R996. [Google Scholar] [CrossRef] [PubMed]
- Disdier, C.; Awa, F.; Chen, X.; Dhillon, S.K.; Galinsky, R.; Davidson, J.O.; Lear, C.A.; Bennet, L.; Gunn, A.J.; Stonestreet, B.S. Lipopolysaccharide-induced changes in the neurovascular unit in the preterm fetal sheep brain. J. Neuroinflamm. 2020, 28, 167. [Google Scholar] [CrossRef] [PubMed]
- Garnier, Y.; Berger, R.; Alm, S.; von Duering, M.U.; Coumans, A.B.; Michetti, F.; Bruschettini, M.; Lituania, M.; Hasaart, T.H.; Gazzolo, D. Systemic endotoxin administration results in increased S100B protein blood levels and periventricular brain white matter injury in the preterm fetal sheep. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 124, 15–22. [Google Scholar] [CrossRef]
- Finnie, J.W. Pathogenesis of brain damage produced in sheep by Clostridium perfringens type D epsilon toxin: A review. Aust. Vet. J. 2003, 81, 219–221. [Google Scholar] [CrossRef]
- Pérez-Fernández, R.; Cazanga, V.; Jeldres, J.A.; Silva, P.P.; Riquelme, J.; Quiroz, F.; Palma, C.; Carretta, M.D.; Burgos, R.A. Plasma and tissue disposition of florfenicol in Escherichia coli lipopolysaccharide-induced endotoxaemic sheep. Xenobiotica 2017, 47, 408–415. [Google Scholar] [CrossRef]
- Traczyk, W.; Przekop, F. A method for testing the function of the hypothalamus and pituitary in the sheep in chronic experiments. Acta Physiol. Pol. 1963, 14, 227–236. [Google Scholar]
- Adan, H.; Guy, S.; Arulanandam, R.; Geletu, M.; Daniel, J.; Raptis, L. Activated Src requires Cadherin-11, Rac, and gp130 for Stat3 activation and survival of mouse Balb/c3T3 fibroblasts. Cancer Gene Ther. 2022, 29, 1502–1513. [Google Scholar] [CrossRef]
- Orlandini, M.; Oliviero, S. In fibroblasts Vegf-D expression is induced by cell-cell contact mediated by cadherin-11. J. Biol. Chem. 2001, 276, 6576–6581. [Google Scholar] [CrossRef]
- Windle, J.J.; Weiner, R.I.; Mellon, P.L. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol. Endocrinol. 1990, 4, 597–603. [Google Scholar] [CrossRef]
- van Kuppeveld, F.J.; Johansson, K.E.; Galama, J.M.; Kissing, J.; Bölske, G.; van der Logt, J.T.; Melchers, W.J. Detection of mycoplasma contamination in cell cultures by a mycoplasma group-specific PCR. Appl. Environ. Microbiol. 1994, 60, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Shokrzadeh, M.; Modanloo, M. An overview of the most common methods for assessing cell viability. J. Res. Med. Dent. Sci. 2017, 5, 33–41. [Google Scholar] [CrossRef]
- Alarid, E.T.; Windle, J.J.; Whyte, D.B.; Mellon, P.L. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 1996, 122, 3319–3329. [Google Scholar] [CrossRef] [PubMed]
- Strzetelski, J.; Brzóska, F.; Kowalski, Z.; Osięgłowski, S. IZ PIB–INRA Feeding Recommendations for Ruminants and Feed Tables; Foundation of the National Research Institute of Animal Production Patronus Animalium: Krakow, Poland, 2014; ISBN 978-83-938377-0-0. (In Polish) [Google Scholar]
- Albrecht, P.; Hryniewicz, W.; Kuch, A.; Skoczyńska, A.; Zajkowska, J. Rekomendacje Postępowania w Zakażeniach Ośrodkowego Układu Nerwowego—Leczenie, Profilaktyka, Nadzór Nad Zakażeniami; Narodowy Instytut Leków: Warszawa, Poland, 2024; ISBN 978-83-949636-9-9. [Google Scholar]
- Koper-Lenkiewicz, O.; Kamińska, J.; Lewoniewska, S.; Wilińska, E. Selektywność Transportu Przez Barierę Krew-Mózg Polski Przegląd Neurologiczny; VIA Medica: Abu Dhabi, United Arab Emirates, 2018; Volume 14, pp. 200–208. ISSN 1734–5251. [Google Scholar]
- Brzezińska, K.; Ziaja, M. The structure and role of blood-brain barier. Postępy Biol. Komórki 2012, 39, 84–99. [Google Scholar]
- Caveney, N.A.; Li, F.K.K.; Strynadka, N.C.J. Enzyme structures of the bacterial peptidoglycan and wall teichoic acid biogenesis pathways. Curr. Opin. Structur. Biol. 2018, 53, 45–58. [Google Scholar] [CrossRef]
- Kessell, A.E.; Finnieb, J.W.; Windsorc, P.A. Neurological diseases of ruminant livestock in Australia. III: Bacterial and protozoal infections. Aust. Vet. J. 2011, 89, 289–296. [Google Scholar] [CrossRef]
- MHerian Wojtas, A.; Maćkowiak, M.; Wawrzczak-Bargiela, A.; Solarz, A.; Bysiek, A.; Madej, K.; Gołembiowska, K. Neurotoxicological profile of the hallucinogenic compound 25I-NBOMe. Sci. Rep. 2022, 12, 2939. [Google Scholar] [CrossRef]
- Shohami, E.; Novikov, M.; Mechoulam, R. A nonpsychotropic cannabinoid, HU-211, has cerebroprotective effects after closed head injury in the rat. J. Neurotrauma. 1993, 10, 109–119. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/adolescents-health-risks-and-solutions (accessed on 26 November 2024).
| No. of Samples | 1, 2, 3, 4 | 5, 6, 7, 8 | Type of Test |
|---|---|---|---|
| E.coli K12 | ** | * | MIC |
| E.coli R2 | ** | * | MIC |
| E.coli R3 | ** | * | MIC |
| E.coli R4 | ** | * | MIC |
| E.coli K12 | * | ** | MBC |
| E.coli R2 | * | ** | MBC |
| E.coli R3 | * | ** | MBC |
| E.coli R4 | * | ** | MBC |
| E.coli K12 | ** | * | MBC/MIC |
| E.coli R2 | ** | * | MBC/MIC |
| E.coli R3 | ** | * | MBC/MIC |
| E.coli R4 | ** | * | MBC/MIC |
| Staphylococcus aureus | * | * | MIC |
| Staphylococcus aureus | * | * | MBC |
| Staphylococcus aureus | ** | ** | MBC/MIC |
| Compound | Value (μg/mL) Without Centrifugation | Mean (μg/mL) Value with Centrifugation |
|---|---|---|
| 1 | 10 (+/−5) | 2 (+/−5) |
| 2 | 350 (+/−5) | 2 (+/−5) |
| 3 | 2.5 (+/−5) | nd |
| 4 | 7 (+/−5) | nd |
| 5 | 5 (+/−5) | nd |
| 6 | 8 (+/−5) | nd |
| 7 | 6 (+/−5) | 6 (+/−5) |
| 8 | 30 (+/−5) | 20 (+/−5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, P.; Koszelewski, D.; Misztal, T.; Szlis, M.; Młotkowska, P.; Gołębiewski, M.; Głowacz, K.; Kocot, M.; Marczyk, M.; Wypych, A.; et al. Blood–Brain Barrier Penetration of Novel 4-Trifluoromethyl-Coumarin Hybrids with Antibacterial Properties as Potential Brain Therapeutics in the Context of Spatially Diverse Healthcare Systems. Int. J. Mol. Sci. 2025, 26, 9655. https://doi.org/10.3390/ijms26199655
Kowalczyk P, Koszelewski D, Misztal T, Szlis M, Młotkowska P, Gołębiewski M, Głowacz K, Kocot M, Marczyk M, Wypych A, et al. Blood–Brain Barrier Penetration of Novel 4-Trifluoromethyl-Coumarin Hybrids with Antibacterial Properties as Potential Brain Therapeutics in the Context of Spatially Diverse Healthcare Systems. International Journal of Molecular Sciences. 2025; 26(19):9655. https://doi.org/10.3390/ijms26199655
Chicago/Turabian StyleKowalczyk, Paweł, Dominik Koszelewski, Tomasz Misztal, Michał Szlis, Patrycja Młotkowska, Marcin Gołębiewski, Krzysztof Głowacz, Malwina Kocot, Michał Marczyk, Aleksandra Wypych, and et al. 2025. "Blood–Brain Barrier Penetration of Novel 4-Trifluoromethyl-Coumarin Hybrids with Antibacterial Properties as Potential Brain Therapeutics in the Context of Spatially Diverse Healthcare Systems" International Journal of Molecular Sciences 26, no. 19: 9655. https://doi.org/10.3390/ijms26199655
APA StyleKowalczyk, P., Koszelewski, D., Misztal, T., Szlis, M., Młotkowska, P., Gołębiewski, M., Głowacz, K., Kocot, M., Marczyk, M., Wypych, A., Kurylczyk, A., Krajewska-Pędzik, A., & Ostaszewski, R. (2025). Blood–Brain Barrier Penetration of Novel 4-Trifluoromethyl-Coumarin Hybrids with Antibacterial Properties as Potential Brain Therapeutics in the Context of Spatially Diverse Healthcare Systems. International Journal of Molecular Sciences, 26(19), 9655. https://doi.org/10.3390/ijms26199655









