Molecular Adaptations to Repeated Radiation Exposure in Triple-Negative Breast Cancer: Dysregulation of Cell Adhesion, Mitochondrial Function, and Epithelial–Mesenchymal Transition
Abstract
1. Introduction
2. Results
2.1. Whole-Transcriptome Profiling Reveals Key Pathways Altered in Radiation-Adapted MDA-MB-231RR Cells
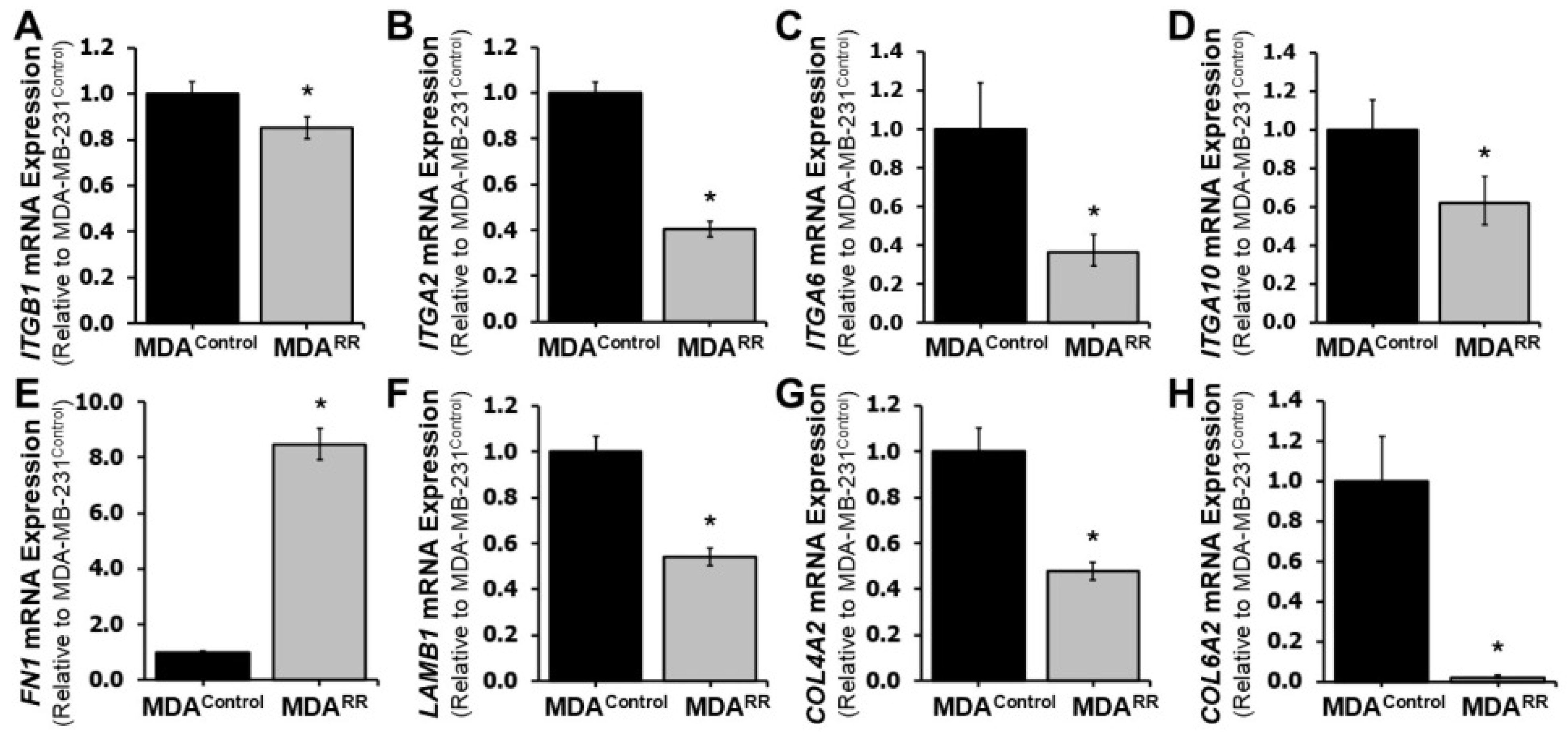
2.2. Radiation Adaptation Alters Expression of Cell Adhesion-Related Genes
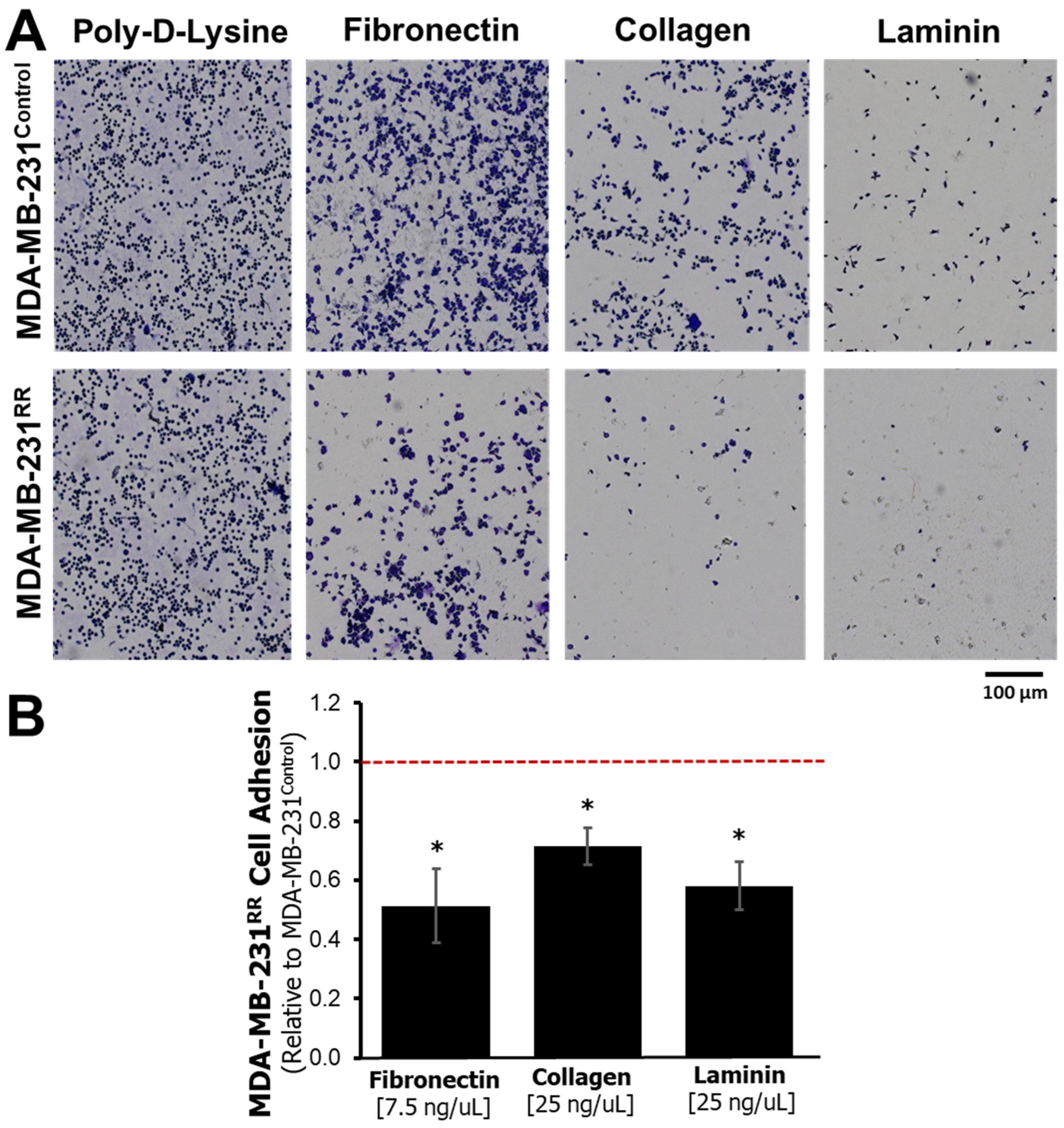
2.3. Functional Adhesion to ECM Proteins Is Reduced in MDA-MB-231RR Cells
2.4. EMT-Associated Transcriptional Changes in MDA-MB-231RR Cells
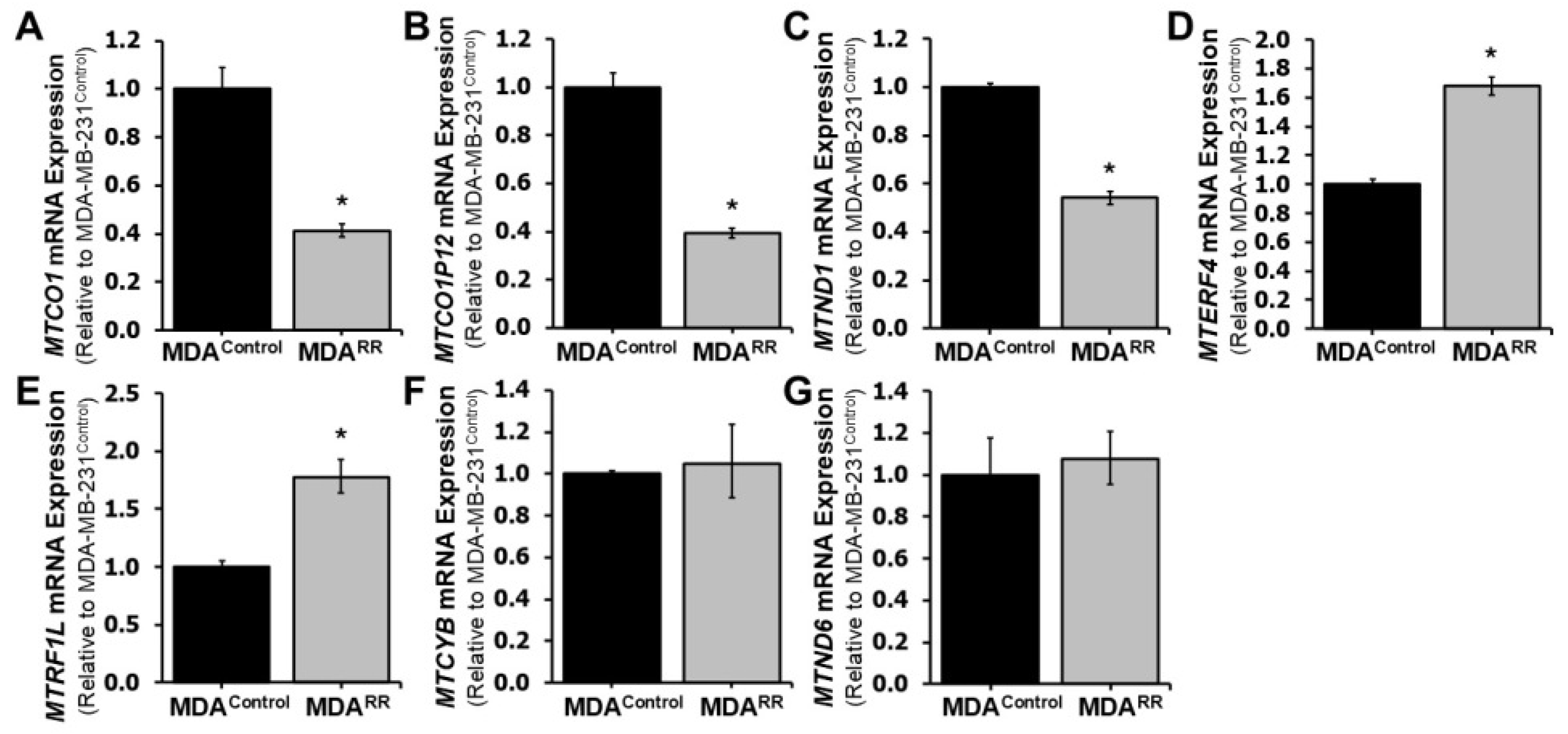
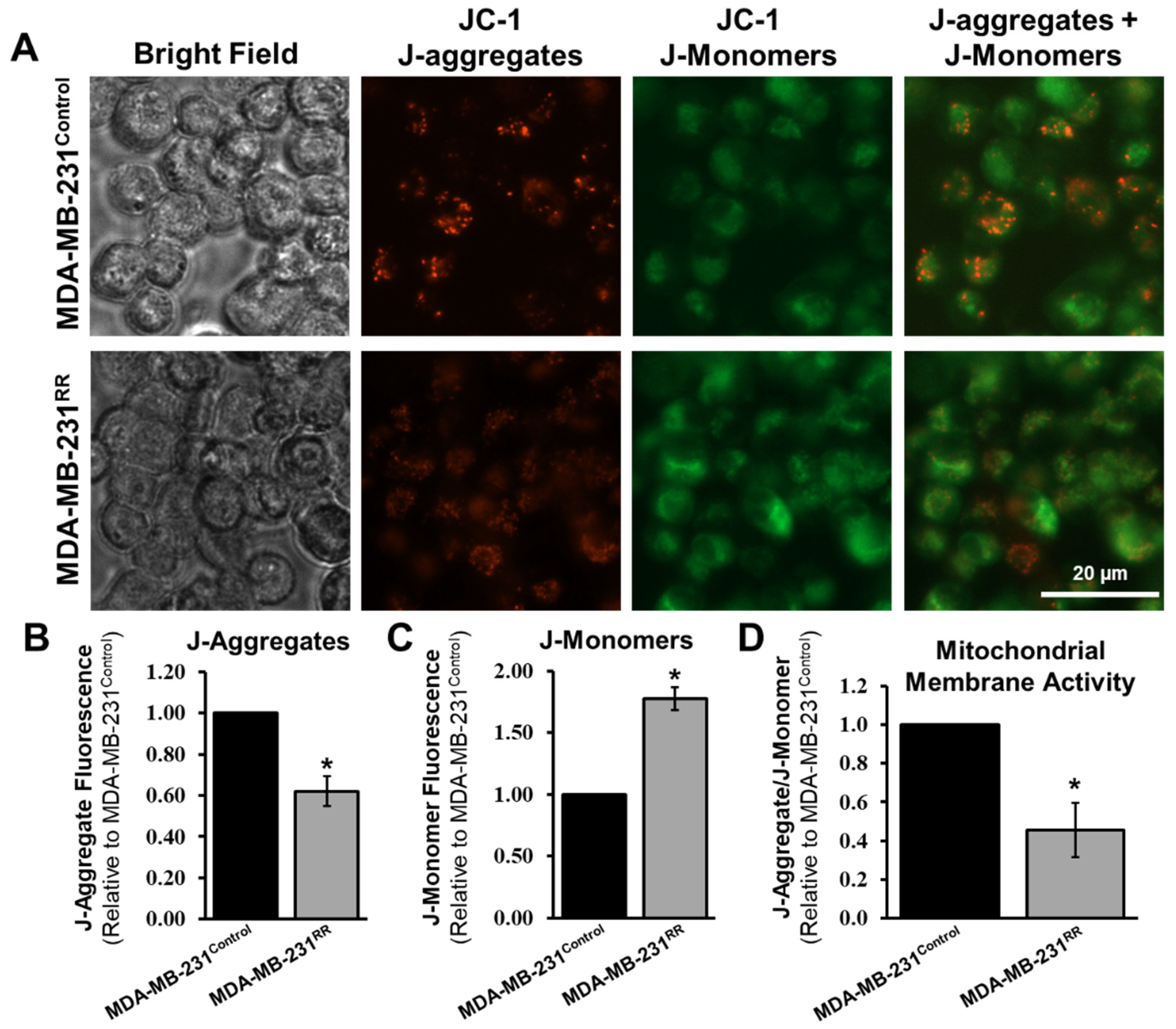
2.5. Radiation-Adapted Cells Exhibit Altered Expression of Mitochondrial Genes and Reduced Mitochondrial Membrane Potential
3. Discussion
3.1. Remodeling of Cell–ECM Engagement and Partial EMT Under Radiation Stress
3.2. Mitochondrial Dysfunction and Compensatory Nuclear Responses
3.3. Additional Networks Potentially Modulating Radiation Adaptation
3.4. Limitations and Future Directions
4. Methods
4.1. Cell Culture
4.2. Development of MDA-MB-231RR Cells
4.3. RNA Sample Preparation
4.4. RNA Sequencing and Gene Ontology
4.5. cDNA Synthesis and RT-qPCR
4.6. Cell Adhesion Assay
4.7. Mitochondrial Membrane Potential Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lewis, J.E.; Kemp, M.L. Integration of Machine Learning and Genome-Scale Metabolic Modeling Identifies Multi-Omics Biomarkers for Radiation Resistance. Nat. Commun. 2021, 12, 2700. [Google Scholar] [CrossRef]
- Xiong, N.; Wu, H.; Yu, Z. Advancements and Challenges in Triple-Negative Breast Cancer: A Comprehensive Review of Therapeutic and Diagnostic Strategies. Front. Oncol. 2024, 14, 1405491. [Google Scholar] [CrossRef]
- Jie, H.; Ma, W.; Huang, C. Diagnosis, Prognosis, and Treatment of Triple-Negative Breast Cancer: A Review. Breast Cancer Targets Ther. 2025, 17, 265–274. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Zheng, C.-C.; Huang, Y.-N.; He, M.-L.; Xu, W.W.; Li, B. Molecular Mechanisms of Chemo- and Radiotherapy Resistance and the Potential Implications for Cancer Treatment. MedComm 2021, 2, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Mageau, E.; Derbowka, R.; Dickinson, N.; Lefort, N.; Thomas, K.; Boreham, D.; Tai, T.C.; Thome, C.; Tharmalingam, S. Molecular Mechanisms of Radiation Resistance in Breast Cancer: A Systematic Review of Radiosensitization Strategies. Curr. Issues Mol. Biol. 2025, 47, 589. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Song, Y.; Wang, R.; Wang, T. Molecular Mechanisms of Tumor Resistance to Radiotherapy. Mol. Cancer 2023, 22, 96. [Google Scholar] [CrossRef]
- Li, J.; Jia, Z.; Dong, L.; Cao, H.; Huang, Y.; Xu, H.; Xie, Z.; Jiang, Y.; Wang, X.; Liu, J. DNA Damage Response in Breast Cancer and Its Significant Role in Guiding Novel Precise Therapies. Biomark. Res. 2024, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Oropeza, E.; Seker, S.; Carrel, S.; Mazumder, A.; Lozano, D.; Jimenez, A.; VandenHeuvel, S.N.; Noltensmeyer, D.A.; Punturi, N.B.; Lei, J.T.; et al. Molecular Portraits of Cell Cycle Checkpoint Kinases in Cancer Evolution, Progression, and Treatment Responsiveness. Sci. Adv. 2023, 9, eadf2860. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Wang, W.; Li, X.; Sun, X.; Zhao, Y.; Wang, Q.; Li, Y.; Hu, F.; Ren, H. Metabolic Reprogramming and Therapeutic Resistance in Primary and Metastatic Breast Cancer. Mol. Cancer 2024, 23, 261. [Google Scholar] [CrossRef]
- Hashemi, M.; Arani, H.Z.; Orouei, S.; Fallah, S.; Ghorbani, A.; Khaledabadi, M.; Kakavand, A.; Tavakolpournegari, A.; Saebfar, H.; Heidari, H.; et al. EMT Mechanism in Breast Cancer Metastasis and Drug Resistance: Revisiting Molecular Interactions and Biological Functions. Biomed. Pharmacother. 2022, 155, 113774. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Tharmalingam, S. The LNT Model for Cancer Induction Is Not Supported by Radiobiological Data. Chem. Biol. Interact. 2019, 301, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Nocquet, L.; Roul, J.; Lefebvre, C.C.; Duarte, L.; Campone, M.; Juin, P.P.; Souazé, F. Low BCL-xL Expression in Triple-Negative Breast Cancer Cells Favors Chemotherapy Efficacy, and This Effect Is Limited by Cancer-Associated Fibroblasts. Sci. Rep. 2024, 14, 14177. [Google Scholar] [CrossRef]
- Viedma-Rodriguez, R.; Baiza-Gutman, L.A.; García-Carrancá, A.; Moreno-Fierros, L.; Salamanca-Gómez, F.; Arenas-Aranda, D. Suppression of the Death Gene BIK Is a Critical Factor for Resistance to Tamoxifen in MCF-7 Breast Cancer Cells. Int. J. Oncol. 2013, 43, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayyat, W.; Pirkannen, J.; Dougherty, J.; Laframboise, T.; Dickinson, N.; Khaper, N.; Lees, S.J.; Mendonca, M.S.; Boreham, D.R.; Tai, T.C.; et al. Overexpression of FRA1 (FOSL1) Leads to Global Transcriptional Perturbations, Reduced Cellular Adhesion and Altered Cell Cycle Progression. Cells 2023, 12, 2344. [Google Scholar] [CrossRef]
- Buyuk, B.; Jin, S.; Ye, K. Epithelial-to-Mesenchymal Transition Signaling Pathways Responsible for Breast Cancer Metastasis. Cell. Mol. Bioeng. 2022, 15, 1–13. [Google Scholar] [CrossRef]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Popova, N.V.; Jücker, M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Tharmalingam, S.; Wu, C.; Hampson, D.R. The Calcium-Sensing Receptor and Integrins Modulate Cerebellar Granule Cell Precursor Differentiation and Migration. Dev. Neurobiol. 2016, 76, 375–389. [Google Scholar] [CrossRef]
- Tharmalingam, S.; Daulat, A.M.; Antflick, J.E.; Ahmed, S.M.; Nemeth, E.F.; Angers, S.; Conigrave, A.D.; Hampson, D.R. Calcium-Sensing Receptor Modulates Cell Adhesion and Migration via Integrins. J. Biol. Chem. 2011, 286, 40922–40933. [Google Scholar] [CrossRef]
- Tharmalingam, S.; Hampson, D.R. The Calcium-Sensing Receptor and Integrins in Cellular Differentiation and Migration. Front. Physiol. 2016, 7, 190. [Google Scholar] [CrossRef]
- Klabukov, I.; Smirnova, A.; Yakimova, A.; Kabakov, A.E.; Atiakshin, D.; Petrenko, D.; Shestakova, V.A.; Sulina, Y.; Yatsenko, E.; Stepanenko, V.N.; et al. Oncomatrix: Molecular Composition and Biomechanical Properties of the Extracellular Matrix in Human Tumors. J. Mol. Pathol. 2024, 5, 437–453. [Google Scholar] [CrossRef]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-Regulated FAK-Src Signaling in Normal and Cancer Cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef]
- Nguyen, C.D.K.; Yi, C. YAP/TAZ Signaling and Resistance to Cancer Therapy. Trends Cancer 2019, 5, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Biel, T.G.; Aryal, B.; Gerber, M.H.; Trevino, J.G.; Mizuno, N.; Rao, V.A. Mitochondrial Dysfunction Generates Aggregates That Resist Lysosomal Degradation in Human Breast Cancer Cells. Cell Death Dis. 2020, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z. Potential Mechanism Underlying the Role of Mitochondria in Breast Cancer Drug Resistance and Its Related Treatment Prospects. Front. Oncol. 2021, 11, 629614. [Google Scholar] [CrossRef] [PubMed]
- Kalezic, A.; Udicki, M.; Srdic Galic, B.; Aleksic, M.; Korac, A.; Jankovic, A.; Korac, B. Tissue-Specific Warburg Effect in Breast Cancer and Cancer-Associated Adipose Tissue-Relationship between AMPK and Glycolysis. Cancers 2021, 13, 2731. [Google Scholar] [CrossRef]
- Tharmalingam, S.; Sreetharan, S.; Brooks, A.L.; Boreham, D.R. Re-Evaluation of the Linear No-Threshold (LNT) Model Using New Paradigms and Modern Molecular Studies. Chem. Biol. Interact. 2019, 301, 54–67. [Google Scholar] [CrossRef]
- Peixoto, P.; Etcheverry, A.; Aubry, M.; Missey, A.; Lachat, C.; Perrard, J.; Hendrick, E.; Delage-Mourroux, R.; Mosser, J.; Borg, C.; et al. EMT Is Associated with an Epigenetic Signature of ECM Remodeling Genes. Cell Death Dis. 2019, 10, 205. [Google Scholar] [CrossRef]
- Mukherjee, A.; Ha, P.; Wai, K.C.; Naara, S. The Role of ECM Remodeling, EMT, and Adhesion Molecules in Cancerous Neural Invasion: Changing Perspectives. Adv. Biol. 2022, 6, e2200039. [Google Scholar] [CrossRef]
- Mistry, D.S.; Chen, Y.; Wang, Y.; Zhang, K.; Sen, G.L. SNAI2 Controls the Undifferentiated State of Human Epidermal Progenitor Cells. Stem Cells 2014, 32, 3209–3218. [Google Scholar] [CrossRef]
- Castro-Muñozledo, F.; Meza-Aguilar, D.G.; Domínguez-Castillo, R.; Hernández-Zequinely, V.; Sánchez-Guzmán, E. Vimentin as a Marker of Early Differentiating, Highly Motile Corneal Epithelial Cells. J. Cell. Physiol. 2017, 232, 818–830. [Google Scholar] [CrossRef]
- van der Wal, T.; van Amerongen, R. Walking the Tight Wire between Cell Adhesion and WNT Signalling: A Balancing Act for β-Catenin. Open Biol. 2020, 10, 200267. [Google Scholar] [CrossRef]
- Eslami-S, Z.; Cortés-Hernández, L.E.; Alix-Panabières, C. Epithelial Cell Adhesion Molecule: An Anchor to Isolate Clinically Relevant Circulating Tumor Cells. Cells 2020, 9, 1836. [Google Scholar] [CrossRef]
- Deshmukh, A.P.; Vasaikar, S.V.; Tomczak, K.; Tripathi, S.; den Hollander, P.; Arslan, E.; Chakraborty, P.; Soundararajan, R.; Jolly, M.K.; Rai, K.; et al. Identification of EMT Signaling Cross-Talk and Gene Regulatory Networks by Single-Cell RNA Sequencing. Proc. Natl. Acad. Sci. USA 2021, 118, e2102050118. [Google Scholar] [CrossRef] [PubMed]
- Tharmalingam, S.; Sreetharan, S.; Kulesza, A.V.; Boreham, D.R.; Tai, T.C. Low-Dose Ionizing Radiation Exposure, Oxidative Stress and Epigenetic Programing of Health and Disease. Radiat. Res. 2017, 188, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio-Protocol 2019, 9, e3128. [Google Scholar] [CrossRef]
- Schober, M.; Raghavan, S.; Nikolova, M.; Polak, L.; Pasolli, H.A.; Beggs, H.E.; Reichardt, L.F.; Fuchs, E. Focal Adhesion Kinase Modulates Tension Signaling to Control Actin and Focal Adhesion Dynamics. J. Cell Biol. 2007, 176, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Cseh, B.; Fernandez-Sauze, S.; Grall, D.; Schaub, S.; Doma, E.; Van Obberghen-Schilling, E. Autocrine Fibronectin Directs Matrix Assembly and Crosstalk between Cell-Matrix and Cell-Cell Adhesion in Vascular Endothelial Cells. J. Cell Sci. 2010, 123, 3989–3999. [Google Scholar] [CrossRef]
- Haeger, A.; Alexander, S.; Vullings, M.; Kaiser, F.M.P.; Veelken, C.; Flucke, U.; Koehl, G.E.; Hirschberg, M.; Flentje, M.; Hoffman, R.M.; et al. Collective Cancer Invasion Forms an Integrin-Dependent Radioresistant Niche. J. Exp. Med. 2020, 217, e20181184. [Google Scholar] [CrossRef]
- Huttenlocher, A.; Horwitz, A.R. Integrins in Cell Migration. Cold Spring Harb. Perspect. Biol. 2011, 3, a005074. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Koo, K.H.; Sung, J.Y.; Yun, U.-J.; Kim, H. Anoikis Resistance: An Essential Prerequisite for Tumor Metastasis. Int. J. Cell Biol. 2012, 2012, 306879. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, X.; Ou, Y.; Zou, L.; Zhang, D.; Yang, Q.; Qin, Y.; Du, X.; Li, W.; Yuan, Z.; et al. Anoikis Resistance—Protagonists of Breast Cancer Cells Survive and Metastasize after ECM Detachment. Cell Commun. Signal. CCS 2023, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Graziani, V.; Rodriguez-Hernandez, I.; Maiques, O.; Sanz-Moreno, V. The Amoeboid State as Part of the Epithelial-to-Mesenchymal Transition Programme. Trends Cell Biol. 2022, 32, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Sawicka, K.M.; Seeliger, M.; Musaev, T.; Macri, L.K.; Clark, R.A.F. Fibronectin Interaction and Enhancement of Growth Factors: Importance for Wound Healing. Adv. Wound Care 2015, 4, 469–478. [Google Scholar] [CrossRef]
- Ishizuka, S.; Tsuchiya, S.; Ohashi, Y.; Terabe, K.; Askew, E.B.; Ishizuka, N.; Knudson, C.B.; Knudson, W. Hyaluronan Synthase 2 (HAS2) Overexpression Diminishes the Procatabolic Activity of Chondrocytes by a Mechanism Independent of Extracellular Hyaluronan. J. Biol. Chem. 2019, 294, 13562–13579. [Google Scholar] [CrossRef]
- Henriet, E.; Sala, M.; Abou Hammoud, A.; Tuariihionoa, A.; Di Martino, J.; Ros, M.; Saltel, F. Multitasking Discoidin Domain Receptors Are Involved in Several and Specific Hallmarks of Cancer. Cell Adhes. Migr. 2018, 12, 363–377. [Google Scholar] [CrossRef]
- Lin, S.; Wang, J.; Mukherjee, P.K.; Mao, R.; West, G.; Czarnecki, D.; Zhao, S.; Nguyen, Q.T.; Elias, M.; Massey, W.J.; et al. Milk Fat Globule-Epidermal Growth Factor 8 (MFGE8) Prevents Intestinal Fibrosis. Gut 2024, 73, 1110–1123. [Google Scholar] [CrossRef] [PubMed]
- Maltseva, D.; Nersisyan, A.; Tonevitsky, A. Interplay of Integrins and Selectins in Metastasis. Mol. Oncol. 2025, 19, 1582–1611. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 Pathways in Cancer. Cytokine Growth Factor. Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, C.; Damato, M.; Stanca, E.; Aboulouard, S.; Zito, F.A.; De Summa, S.; Traversa, D.; Schirosi, L.; Bravaccini, S.; Pirini, F.; et al. Unraveling the Protein Kinase C/NDRG1 Signaling Network in Breast Cancer. Cell Biosci. 2024, 14, 156. [Google Scholar] [CrossRef]
- Ju, G.; Zeng, K.; Lu, L.; Diao, H.; Wang, H.; Li, X.; Zhou, T. Identification and Validation of the Cellular Senescence-Related Molecular Subtypes of Triple Negative Breast Cancer via Integrating Bulk and Single-Cell RNA Sequencing Data. Am. J. Cancer Res. 2023, 13, 569–588. [Google Scholar]
- Ortiz-Montero, P.; Londoño-Vallejo, A.; Vernot, J.-P. Senescence-Associated IL-6 and IL-8 Cytokines Induce a Self- and Cross-Reinforced Senescence/Inflammatory Milieu Strengthening Tumorigenic Capabilities in the MCF-7 Breast Cancer Cell Line. Cell Commun. Signal. CCS 2017, 15, 17. [Google Scholar] [CrossRef]
- Tang, P.-X.; Li, J.-X.; Guo, C.; Xia, Z.; Zheng, X.-Y.; Zhu, S.-Y.; Zheng, C.; Kang, S.-N.; Xu, W.-F.; Li, X.-H. A Novel Polypeptide Inhibitor of MMP-1 Attenuates the UVA-Mediated Skin Aging. Biochem. Biophys. Res. Commun. 2025, 152681. [Google Scholar] [CrossRef]
- Picard, M.; McEwen, B.S.; Epel, E.S.; Sandi, C. An Energetic View of Stress: Focus on Mitochondria. Front. Neuroendocrinol. 2018, 49, 72–85. [Google Scholar] [CrossRef]
- Bar-Ziv, R.; Bolas, T.; Dillin, A. Systemic Effects of Mitochondrial Stress. EMBO Rep. 2020, 21, e50094. [Google Scholar] [CrossRef]
- Clémençon, B.; Babot, M.; Trézéguet, V. The Mitochondrial ADP/ATP Carrier (SLC25 Family): Pathological Implications of Its Dysfunction. Mol. Aspects Med. 2013, 34, 485–493. [Google Scholar] [CrossRef]
- Gouriou, Y.; Alam, M.R.; Harhous, Z.; Crola Da Silva, C.; Baetz, D.B.; Badawi, S.; Lefai, E.; Rieusset, J.; Durand, A.; Harisseh, R.; et al. ANT2-Mediated ATP Import into Mitochondria Protects against Hypoxia Lethal Injury. Cells 2020, 9, 2542. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Y.; Meisenhelder, J.; Yang, W.; Hawke, D.H.; Zheng, Y.; Xia, Y.; Aldape, K.; He, J.; Hunter, T.; et al. Mitochondria-Translocated PGK1 Functions as a Protein Kinase to Coordinate Glycolysis and the TCA Cycle in Tumorigenesis. Mol. Cell 2016, 61, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg Effect: Essential Part of Metabolic Reprogramming and Central Contributor to Cancer Progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, F.M.; Torelli, N.Q.; Kowaltowski, A.J. Mitochondrial Retrograde Signaling: Triggers, Pathways, and Outcomes. Oxid. Med. Cell. Longev. 2015, 2015, 482582. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Pioso, M.S.; Olesinski, E.A.; Yilma, B.; Ryan, J.A.; Mashaka, T.; Leutz, B.; Adamia, S.; Zhu, H.; Kuang, Y.; et al. Reduced Mitochondrial Apoptotic Priming Drives Resistance to BH3 Mimetics in Acute Myeloid Leukemia. Cancer Cell 2020, 38, 872–890.e6. [Google Scholar] [CrossRef]
- Bian, Z.-M.; Elner, S.G.; Elner, V.M. Dual Involvement of Caspase-4 in Inflammatory and ER Stress-Induced Apoptotic Responses in Human Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2009, 50, 6006–6014. [Google Scholar] [CrossRef]
- Amuthan, G.; Biswas, G.; Zhang, S.Y.; Klein-Szanto, A.; Vijayasarathy, C.; Avadhani, N.G. Mitochondria-to-Nucleus Stress Signaling Induces Phenotypic Changes, Tumor Progression and Cell Invasion. EMBO J. 2001, 20, 1910–1920. [Google Scholar] [CrossRef]
- Murai, J.; Zhang, H.; Pongor, L.; Tang, S.-W.; Jo, U.; Moribe, F.; Ma, Y.; Tomita, M.; Pommier, Y. Chromatin Remodeling and Immediate Early Gene Activation by SLFN11 in Response to Replication Stress. Cell Rep. 2020, 30, 4137–4151.e6. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Chen, R.A.; Huang, M.; Fung, T.S.; Liu, D.X. Activation of the MKK3-P38-MK2-ZFP36 Axis by Coronavirus Infection Restricts the Upregulation of AU-Rich Element-Containing Transcripts in Proinflammatory Responses. J. Virol. 2022, 96, e0208621. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, W.; Ge, J.; Zhang, Z. Prostaglandin E2 Receptor EP4 Is Involved in the Cell Growth and Invasion of Prostate Cancer via the cAMP-PKA/PI3K-Akt Signaling Pathway. Mol. Med. Rep. 2018, 17, 4702–4712. [Google Scholar] [CrossRef]
- Walker, O.L.; Dahn, M.L.; Power Coombs, M.R.; Marcato, P. The Prostaglandin E2 Pathway and Breast Cancer Stem Cells: Evidence of Increased Signaling and Potential Targeting. Front. Oncol. 2022, 11, 791696. [Google Scholar] [CrossRef]
- Jin, K.; Qian, C.; Lin, J.; Liu, B. Cyclooxygenase-2-Prostaglandin E2 Pathway: A Key Player in Tumor-Associated Immune Cells. Front. Oncol. 2023, 13, 1099811. [Google Scholar] [CrossRef]
- Margraf, A.; Perretti, M. Immune Cell Plasticity in Inflammation: Insights into Description and Regulation of Immune Cell Phenotypes. Cells 2022, 11, 1824. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Hussein, O.; Sastry, K.S.; Bougarn, S.; Gopinath, N.; Chin-Smith, E.; Sinha, Y.; Korashy, H.M.; Maccalli, C. Deciphering the Complexities of Cancer Cell Immune Evasion: Mechanisms and Therapeutic Implications. Adv. Cancer Biol.-Metastasis 2023, 8, 100107. [Google Scholar] [CrossRef]
- Majumder, R.; Nguyen, T. Protein S: Function, Regulation, and Clinical Perspectives. Curr. Opin. Hematol. 2021, 28, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-M.; Kim, J.-Y.; Cho, H.J.; Lee, W.J.; Nguyen, D.; Kim, S.S.; Oh, Y.T.; Kim, H.-J.; Jung, C.-W.; Pinero, G.; et al. Tissue Factor Is a Critical Regulator of Radiation Therapy-Induced Glioblastoma Remodeling. Cancer Cell 2023, 41, 1480–1497.e9. [Google Scholar] [CrossRef]
- Handelsman, S.; Overbey, J.; Chen, K.; Lee, J.; Haj, D.; Li, Y. PD-L1’s Role in Preventing Alloreactive T Cell Responses Following Hematopoietic and Organ Transplant. Cells 2023, 12, 1609. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.M.; Trivedi, S.; Concha-Benavente, F.; Hyun-Bae, J.; Wang, L.; Seethala, R.R.; Branstetter, B.F.; Ferrone, S.; Ferris, R.L. STAT1-Induced HLA Class I Upregulation Enhances Immunogenicity and Clinical Response to Anti-EGFR mAb Cetuximab Therapy in HNC Patients. Cancer Immunol. Res. 2015, 3, 936–945. [Google Scholar] [CrossRef]
- Cambier, S.; Gouwy, M.; Proost, P. The Chemokines CXCL8 and CXCL12: Molecular and Functional Properties, Role in Disease and Efforts towards Pharmacological Intervention. Cell. Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef]
- Jaradeh, M.; Baig, N.; Bontekoe, E.; Mitrovic, M.; Antic, D.; Hoppensteadt, D.; Kantarcioglu, B.; Fareed, J. The Relationship Between Thrombo-Inflammatory Biomarkers and Cellular Indices of Inflammation in Lymphoma Patients. Clin. Appl. Thromb. 2021, 27, 10760296211050358. [Google Scholar] [CrossRef]
- Strasenburg, W.; Jóźwicki, J.; Durślewicz, J.; Kuffel, B.; Kulczyk, M.P.; Kowalewski, A.; Grzanka, D.; Drewa, T.; Adamowicz, J. Tumor Cell-Induced Platelet Aggregation as an Emerging Therapeutic Target for Cancer Therapy. Front. Oncol. 2022, 12, 909767. [Google Scholar] [CrossRef]
- Wu, R.; Roy, A.; Tokumaru, Y.; Gandhi, S.; Asaoka, M.; Oshi, M.; Yan, L.; Ishikawa, T.; Takabe, K. NR2F1, a Tumor Dormancy Marker, Is Expressed Predominantly in Cancer-Associated Fibroblasts and Is Associated with Suppressed Breast Cancer Cell Proliferation. Cancers 2022, 14, 2962. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, X.; Wu, D.; Xu, Y. ROR Nuclear Receptors: Structures, Related Diseases, and Drug Discovery. Acta Pharmacol. Sin. 2015, 36, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Schaller, N.; Cardner, M.; Diepenbruck, M.; Saxena, M.; Tiede, S.; Lüönd, F.; Ivanek, R.; Beerenwinkel, N.; Christofori, G. A Hierarchical Regulatory Landscape during the Multiple Stages of EMT. Dev. Cell 2019, 48, 539–553.e6. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Okada, K.; Takakura, Y.; Takano, H.; Yamaguchi, N.; Yamaguchi, N. Vestigial-like Family Member 3 (VGLL3), a Cofactor for TEAD Transcription Factors, Promotes Cancer Cell Proliferation by Activating the Hippo Pathway. J. Biol. Chem. 2020, 295, 8798–8807. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, M.R.; Miri, S.R.; Zarandi, P.K.; Chalbatani, G.M.; Rapôso, C.; Mirzaei, H.R.; Akbari, M.E.; Mahmoodzadeh, H. The Hippo Tumor Suppressor Pathway (YAP/TAZ/TEAD/MST/LATS) and EGFR-RAS-RAF-MEK in Cancer Metastasis. Genes Dis. 2021, 8, 48–60. [Google Scholar] [CrossRef]
- Liu, S.; Imani, S.; Deng, Y.; Pathak, J.L.; Wen, Q.; Chen, Y.; Wu, J. Targeting IFN/STAT1 Pathway as a Promising Strategy to Overcome Radioresistance. OncoTargets Ther. 2020, 13, 6037–6050. [Google Scholar] [CrossRef]
- Seifert, L.L.; Si, C.; Saha, D.; Sadic, M.; de Vries, M.; Ballentine, S.; Briley, A.; Wang, G.; Valero-Jimenez, A.M.; Mohamed, A.; et al. The ETS Transcription Factor ELF1 Regulates a Broadly Antiviral Program Distinct from the Type I Interferon Response. PLoS Pathog. 2019, 15, e1007634. [Google Scholar] [CrossRef]
- Sawai, C.M.; Sisirak, V.; Ghosh, H.S.; Hou, E.Z.; Ceribelli, M.; Staudt, L.M.; Reizis, B. Transcription Factor Runx2 Controls the Development and Migration of Plasmacytoid Dendritic Cells. J. Exp. Med. 2013, 210, 2151–2159. [Google Scholar] [CrossRef]
- Shi, D.; Ao, L.; Yu, H.; Xia, Y.; Li, J.; Zhong, W.; Xia, H. Chromobox Homolog 8 (CBX8) in Human Tumor Carcinogenesis and Prognosis: A Pancancer Analysis Using Multiple Databases. Front. Genet. 2021, 12, 745277. [Google Scholar] [CrossRef]
- Gray, M.; Turnbull, A.K.; Ward, C.; Meehan, J.; Martínez-Pérez, C.; Bonello, M.; Pang, L.Y.; Langdon, S.P.; Kunkler, I.H.; Murray, A.; et al. Development and Characterisation of Acquired Radioresistant Breast Cancer Cell Lines. Radiat. Oncol. 2019, 14, 64. [Google Scholar] [CrossRef]
- Ye, M.; Wilhelm, M.; Gentschev, I.; Szalay, A. A Modified Limiting Dilution Method for Monoclonal Stable Cell Line Selection Using a Real-Time Fluorescence Imaging System: A Practical Workflow and Advanced Applications. Methods Protoc. 2021, 4, 16. [Google Scholar] [CrossRef]
- Peterson, J.; McTiernan, C.D.; Thome, C.; Khaper, N.; Lees, S.J.; Boreham, D.R.; Tai, T.C.; Tharmalingam, S. Identification of Radiation-Induced miRNA Biomarkers Using the CGL1 Cell Model System. Bioengineering 2022, 9, 214. [Google Scholar] [CrossRef]
- Tharmalingam, S.; Khurana, S.; Murray, A.; Lamothe, J.; Tai, T.C. Whole Transcriptome Analysis of Adrenal Glands from Prenatal Glucocorticoid Programmed Hypertensive Rodents. Sci. Rep. 2020, 10, 18755. [Google Scholar] [CrossRef] [PubMed]
- Pirkkanen, J.; Tharmalingam, S.; Morais, I.H.; Lam-Sidun, D.; Thome, C.; Zarnke, A.M.; Benjamin, L.V.; Losch, A.C.; Borgmann, A.J.; Sinex, H.C.; et al. Transcriptomic Profiling of Gamma Ray Induced Mutants from the CGL1 Human Hybrid Cell System Reveals Novel Insights into the Mechanisms of Radiation-Induced Carcinogenesis. Free Radic. Biol. Med. 2019, 145, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Pirkkanen, J.; Tharmalingam, S.; Thome, C.; Sinex, H.C.; Benjamin, L.V.; Losch, A.C.; Borgmann, A.J.; Dhaemers, R.M.; Gordon, C.; Boreham, D.R.; et al. Genomic Loss and Epigenetic Silencing of the FOSL1 Tumor Suppressor Gene in Radiation-Induced Neoplastic Transformation of Human CGL1 Cells Alters the Tumorigenic Phenotype In Vitro and In Vivo. Radiat. Res. 2023, 200, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Alhasawi, A.A.; Thomas, S.C.; Tharmalingam, S.; Legendre, F.; Appanna, V.D. Isocitrate Lyase and Succinate Semialdehyde Dehydrogenase Mediate the Synthesis of α-Ketoglutarate in Pseudomonas Fluorescens. Front. Microbiol. 2019, 10, 1929. [Google Scholar] [CrossRef]
- Sreetharan, S.; Stoa, L.; Cybulski, M.E.; Jones, D.E.; Lee, A.H.; Kulesza, A.V.; Tharmalingam, S.; Boreham, D.R.; Tai, T.C.; Wilson, J.Y. Cardiovascular and Growth Outcomes of C57Bl/6J Mice Offspring Exposed to Maternal Stress and Ionizing Radiation during Pregnancy. Int. J. Radiat. Biol. 2019, 95, 1085–1093. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rosati, D.; Palmieri, M.; Brunelli, G.; Morrione, A.; Iannelli, F.; Frullanti, E.; Giordano, A. Differential Gene Expression Analysis Pipelines and Bioinformatic Tools for the Identification of Specific Biomarkers: A Review. Comput. Struct. Biotechnol. J. 2024, 23, 1154–1168. [Google Scholar] [CrossRef]


| Gene Ontology ID | Biological Process | DEGs | Total Genes | FDR |
|---|---|---|---|---|
| GO:0022610 | Biological Adhesion | 214 | 805 | 3.81 × 10−18 |
| GO:0007155 | Cell Adhesion | 212 | 802 | 6.50 × 10−18 |
| GO:0032501 | Multicellular Organismal Process | 722 | 3968 | 4.62 × 10−16 |
| GO:0051239 | Regulation of Multicellular Organismal Process | 323 | 1515 | 4.11 × 10−13 |
| GO:0009653 | Anatomical Structure Morphogenesis | 336 | 1621 | 4.91 × 10−12 |
| GO:0098609 | Cell–Cell Adhesion | 130 | 468 | 4.91 × 10−12 |
| GO:0023052 | Signaling | 627 | 3509 | 5.32 × 10−11 |
| GO:0007154 | Cell Communication | 632 | 3552 | 8.66 × 10−11 |
| GO:0007165 | Signal Transduction | 580 | 5258 | 2.02 × 10−9 |
| GO:0050896 | Response to Stimulus | 830 | 4973 | 3.12 × 10−9 |
| Pathway (ID) | FDR | Upregulated DEGs | Downregulated DEGs |
|---|---|---|---|
| Complement and Coagulation Cascades (04610) | 0.008 | FGB, F2RL2, SERPINB2, F3, CD55, ITGB2, PROS1 | SERPINA1, CFD, C1R, PLAU, C4BPB, F12 |
| Neuroactive Ligand-Receptor Interaction (04080) | 0.010 | HTR2C, GABRA3, P2RY10, GABBR2, F2RL2, ADM, S1PR3, GRPR, PRLR, KISS1, PTGER4, HRH1 | VIPR1, SSTR2, NMB, GRIK4, ADORA2A, S1PR5, PTGER2, HTR7, P2RY2, LPAR2 |
| Cell Adhesion Molecules (04514) | 0.027 | VCAN, NEO1, CD22, CD274, ITGB2, CDH4 | CD40, L1CAM, CLDN2, ICAM1, HLA-DRB1, ITGA6, NRCAM, HLA-A, HLA-DRA, CLDN3, NCAM2, VSIR, HLA-F, ICOSLG, HLA-C, HLA-B |
| ABC Transporters (02010) | 0.033 | ABCB7, ABCA3 | ABCC3, ABCB9, ABCG2, TAP1, ABCA2, ABCD1, TAP2, ABCA1, ABCC4 |
| Pathways in Cancer (05200) | 0.033 | CCND2, MMP1, PTGS2, LAMA1, HEYL, FN1, PLCB1, IL7R, FGF5, COL4A5, FGF1, ESR2, PTGER4, IL13RA1, CAMK2D, HHIP, MGST3, WNT7B, MITF, EGF, GADD45B, GNAS, GADD45A, CXCL8 | PDGFRB, EGLN3, FGFR4, JAG2, FHH, GSTM2, SUFU, JUP, PTGER2, PGF, FLT3LG, ITGA6, LAMA5, TERT, IL15RA, CDKN1A, ADCY6, FRAT1, NCOA1, TGFBR2, CCND3, IL15, ADCY7, CCNA1, PLCG1, DDB2, PLCG2, NCOA3, ITGA2, GSTM4, HES1, COL4A1, COL4A2, GNG11, RALB, PLD2, DVL2, LPAR2, LRP5, FZD1, TRAF5, EML4, TRAF3, AKT1, LAMB1, EPOR, STAT1 |
| Protein Digestion and Absorption (04974) | 0.033 | CPA3, ATP1A3, COL8A1, COL4A5 | COL5A1, COL6A2, COL6A3, COL27A1, COL7A1, COL13A1, COL4A1, COL4A2, KCNN4 |
| ECM-Receptor Interaction (04512) | 0.033 | LAMA1, FN1, TNC, COL4A5 | COL6A2, COL6A3, ITGB4, ITGA6, LAMA5, FREM2, ITGA2, COL4A1, COL4A2, ITGA10, DAG1, LAMB1 |
| AGE-RAGE Signaling Pathway in Diabetic Complications (04933) | 0.033 | FN1, PLCB1, COL4A5, F3, PRKCZ, EGR1, CXCL8 | NFATC1, ICAM1, PLCD1, MAPK13, TGFBR2, PLCG1, PLCG2, COL4A2, PRKCE, PLCD3, AKT1, STAT1 |
| Cytokine-Cytokine Receptor Interaction (04060) | 0.034 | IL7R, TNFRSF10D, PRLR, IL13RA1, IL1RL2, CXCL8 | CD40, INHBB, TNFRSF1B, NGFR, CX3CL1, IL11, GDF5, IFNLR1, TNFSF12, IL24, TNFRSF11B, IL15RA, CRLF2, TGFBR2, IL15, IL17RE, TNFRSF19, EPOR |
| Insulin Secretion (04911) | 0.040 | RYR2, ATP1A3, SNAP25, PLCB1, CAMK2D, KCNMB4, RIMS2, GNAS | KCNMB3, ADCY6, ADCY7, PCLO, ATF6B, KCNN4 |
| Gene ID | Adhesion Genes | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|
| 3688 | ITGB1 | GCCGCGCGGAAAAGATGAAT | CACAATTTGGCCCTGCTTGTA |
| 3673 | ITGA2 | TTAGCGCTCAGTCAAGGCAT | TGCACTGCATAGCCAAACTG |
| 3655 | ITGA6 | GCAGCCTTCAACTTGGACAC | ACGAGCAACAGCCGCTT |
| 8515 | ITGA10 | GGACAGAAACCGATCAGGCA | CCAGGTTAAAGGGGGAGCAG |
| 2335 | FN1 | CGGGACTCAATCCAAATGCC | TTCCAGGAACCCTGAACTGT |
| 3912 | LAMB1 | TGGTTACTGTTGCACACAACG | TTTTGCTTCAGAGACCATCTTGG |
| 1284 | COL4A2 | TGCTTCTGGAAGGGCCAATG | GTCAGTCCCACTTAGCCTCG |
| 1292 | COL6A2 | CCTGCCAAACAGAGCTGTCC | GTGGAAGTTCTGCTCACCCA |
| 3371 | TNC | TCTCGCCCATCGGAAAGAAAA | GGCTCTAGGGCTCTAGGGTAT |
| 5818 | NECTIN1 | AGCATCCTGCTGGTGTTGAT | TACACGTGCTTCTTGGTGCT |
| Gene ID | Mitochondrial Genes | Forward Primer Sequence | Reverse Primer Sequence |
| 4512 | MTCO1 | CTTTTCACCGTAGGTGGCCT | AGTGGAAGTGGGCTACAACG |
| 107075141 | MTCO1P12 | CGCCGACCGTTGACTATTCT | TGCCTAGGACTCCAGCTCAT |
| 4535 | MTND1 | TACAACTACGCAAGGCCCC | TGGTAGATGTGGCGGGTTTT |
| 51537 | MTFP1 | AAGAAGGCTGGAGAGGTGCC | TTGATGGTGAAGCCCGGAAT |
| 4519 | MTCYB | CGCCTTTTCACTAATCGCCC | CGTAATATAGGCCTCGCCCG |
| 4541 | MTND6 | GATCCTCCCGAATCAACCCT | GGGTTAGCGATGGAGGTAGG |
| Gene ID | EMT Genes | Forward Primer Sequence | Reverse Primer Sequence |
| 59 | ACTA2 | GAGGGAAGGTCCTAACAGCC | TAGTCCCGGGGATAGGCAAA |
| 208 | AKT2 | TGCAGAGATTGTCTCGGCTC | CCGTCACTGATGCCCTCTTT |
| 960 | CD44 | GCACAGACAGAATCCCTGCT | TCTTGCCTCTTGGTTGCTGT |
| 1000 | CDH2 | TGGATGAAAGACCCATCCACG | CAGGGAGTCATATGGTGGAGC |
| 3880 | CK19 | GGGCAACGAGAAGCTAACCA | GGTACCAGTCGCGGATCTTC |
| 1499 | CTNNB1 | AATCAGCTGGCCTGGTTTGA | GCTTGGTTAGTGTGTCAGGC |
| 6615 | SNAI1 | GTTTACCTTCCAGCAGCCCT | TCCCAGATGAGCATTGGCAG |
| 6591 | SNAI2 | GGACCACAGTGGCTCAGAAA | CTTCAATGGCATGGGGGTCT |
| 7431 | VIM | TCCGCACATTCGAGCAAAGA | AACTTACAGCTGGGCCATCG |
| Gene ID | Housekeeping Genes | Forward Primer Sequence | Reverse Primer Sequence |
| 3329 | HSPD1 | CGCCGCCGACGACCT | GTGGGTAACCGAAGCATTTCTGC |
| 10376 | TUBA1B | AGGCCCGTGAAGATATGGCT | AGCTGAAATTCTGGGAGCATGA |
| 506 | ATP5F1B | TGTCGATCTGCTAGCTCCCT | ACAGAGTAACCACCATGGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dickinson, N.; Murray, A.; Davis, M.; Marshall-Bergeron, K.; Dougherty, J.; Al-Khayyat, W.; Narendrula, R.; Lavoie, M.; Mageau, E.; Derbowka, R.; et al. Molecular Adaptations to Repeated Radiation Exposure in Triple-Negative Breast Cancer: Dysregulation of Cell Adhesion, Mitochondrial Function, and Epithelial–Mesenchymal Transition. Int. J. Mol. Sci. 2025, 26, 9611. https://doi.org/10.3390/ijms26199611
Dickinson N, Murray A, Davis M, Marshall-Bergeron K, Dougherty J, Al-Khayyat W, Narendrula R, Lavoie M, Mageau E, Derbowka R, et al. Molecular Adaptations to Repeated Radiation Exposure in Triple-Negative Breast Cancer: Dysregulation of Cell Adhesion, Mitochondrial Function, and Epithelial–Mesenchymal Transition. International Journal of Molecular Sciences. 2025; 26(19):9611. https://doi.org/10.3390/ijms26199611
Chicago/Turabian StyleDickinson, Noah, Alyssa Murray, Megan Davis, Kaitlyn Marshall-Bergeron, Jessica Dougherty, Wuroud Al-Khayyat, Ramya Narendrula, Maggie Lavoie, Emma Mageau, Ronan Derbowka, and et al. 2025. "Molecular Adaptations to Repeated Radiation Exposure in Triple-Negative Breast Cancer: Dysregulation of Cell Adhesion, Mitochondrial Function, and Epithelial–Mesenchymal Transition" International Journal of Molecular Sciences 26, no. 19: 9611. https://doi.org/10.3390/ijms26199611
APA StyleDickinson, N., Murray, A., Davis, M., Marshall-Bergeron, K., Dougherty, J., Al-Khayyat, W., Narendrula, R., Lavoie, M., Mageau, E., Derbowka, R., Kovala, A. T., Boreham, D. R., Lefort, N., Thome, C., Tai, T. C., & Tharmalingam, S. (2025). Molecular Adaptations to Repeated Radiation Exposure in Triple-Negative Breast Cancer: Dysregulation of Cell Adhesion, Mitochondrial Function, and Epithelial–Mesenchymal Transition. International Journal of Molecular Sciences, 26(19), 9611. https://doi.org/10.3390/ijms26199611







