YKL-40 in Virus-Associated Liver Disease: A Translational Biomarker Linking Fibrosis, Hepatocarcinogenesis, and Liver Transplantation
Abstract
1. Introduction
2. Mechanistic and Clinical Relevance of YKL-40 in Virus-Associated Fibrosis and Hepatocarcinogenesis
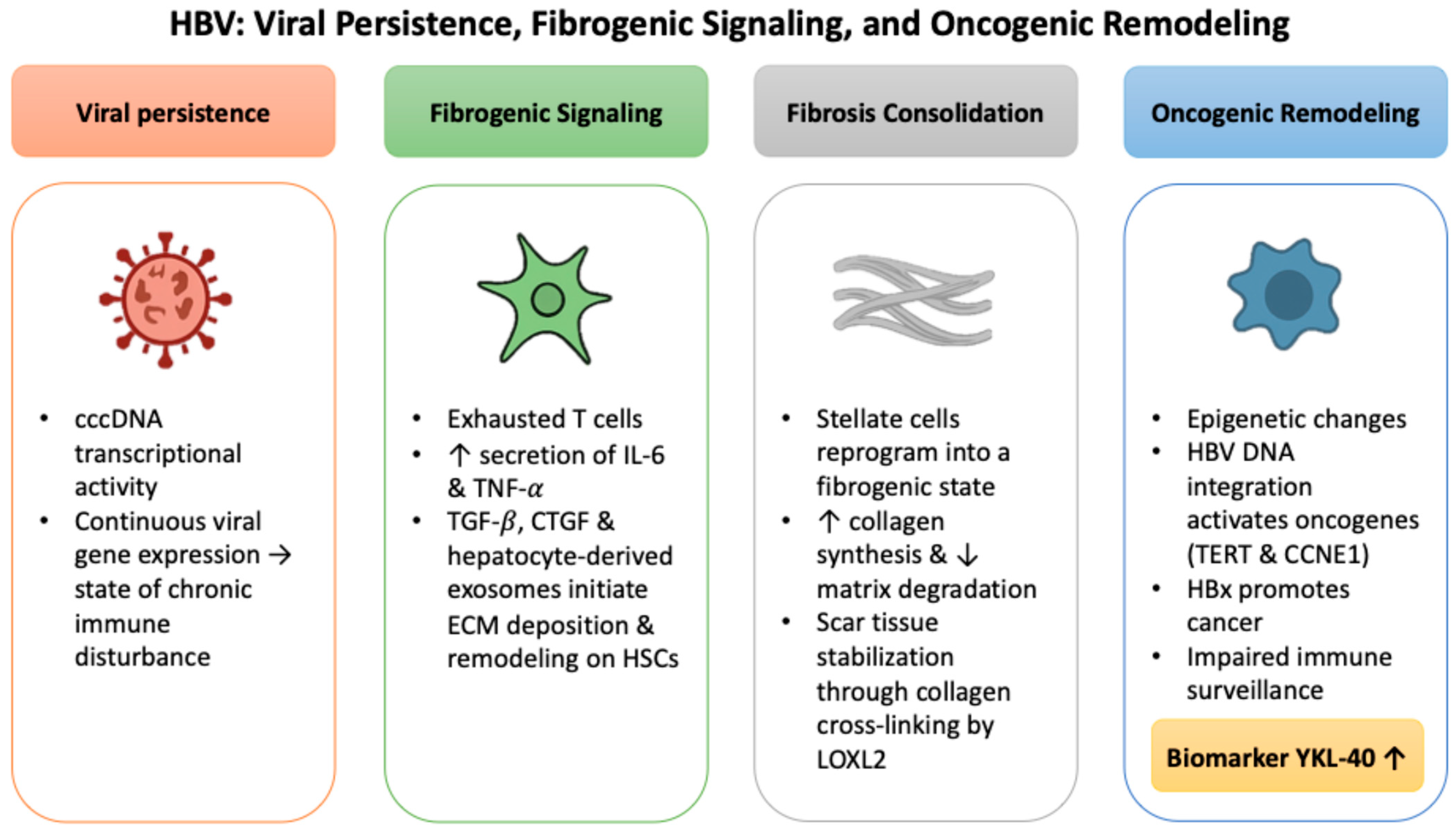
2.1. HBV: Viral Persistence, Fibrogenic Signaling, and Oncogenic Remodeling
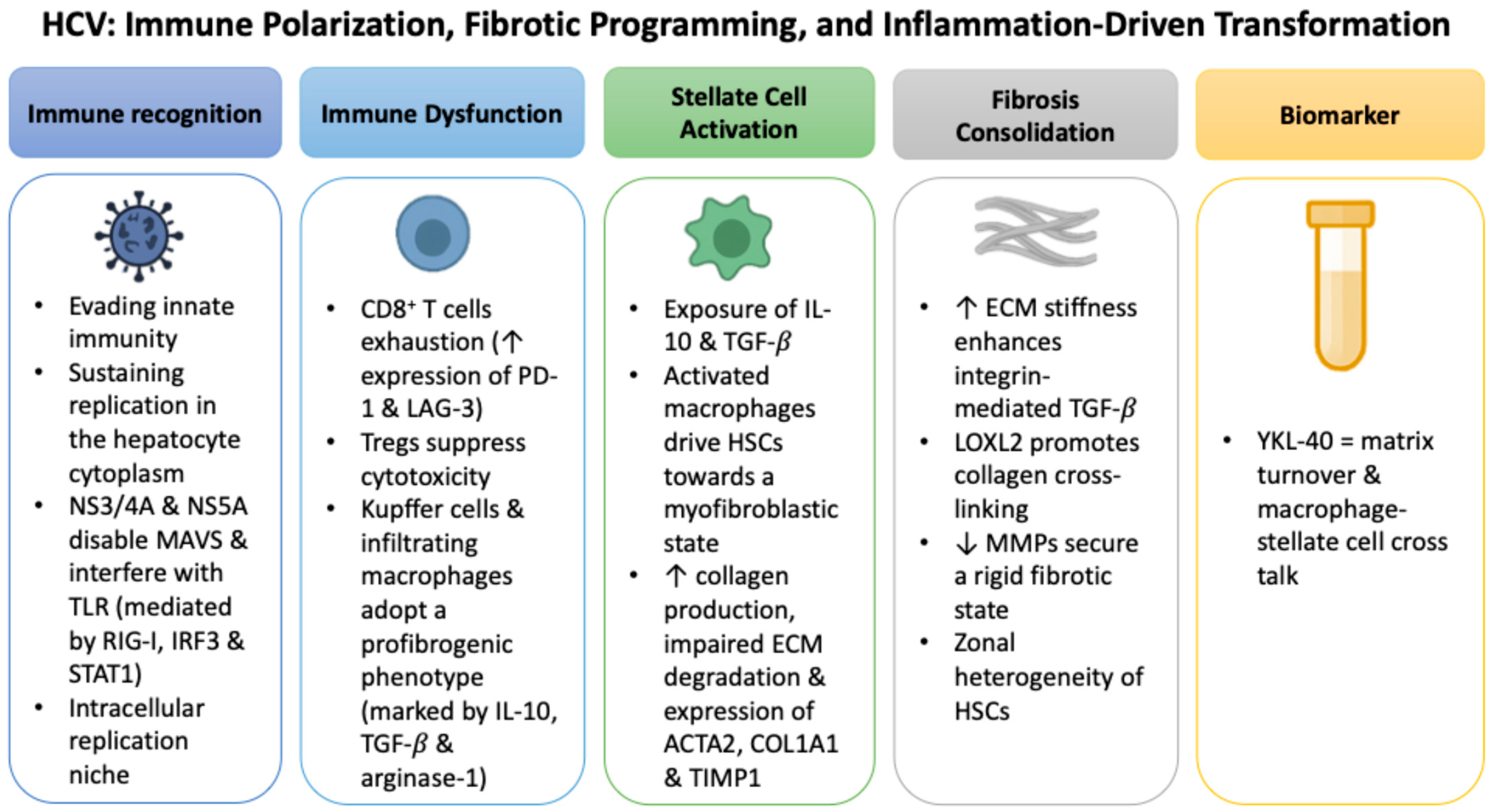
2.2. HCV: Immune Polarization, Fibrotic Programming, and Inflammation-Driven Transformation
2.3. HDV: Intensified Immunopathology and Fibrotic Escalation
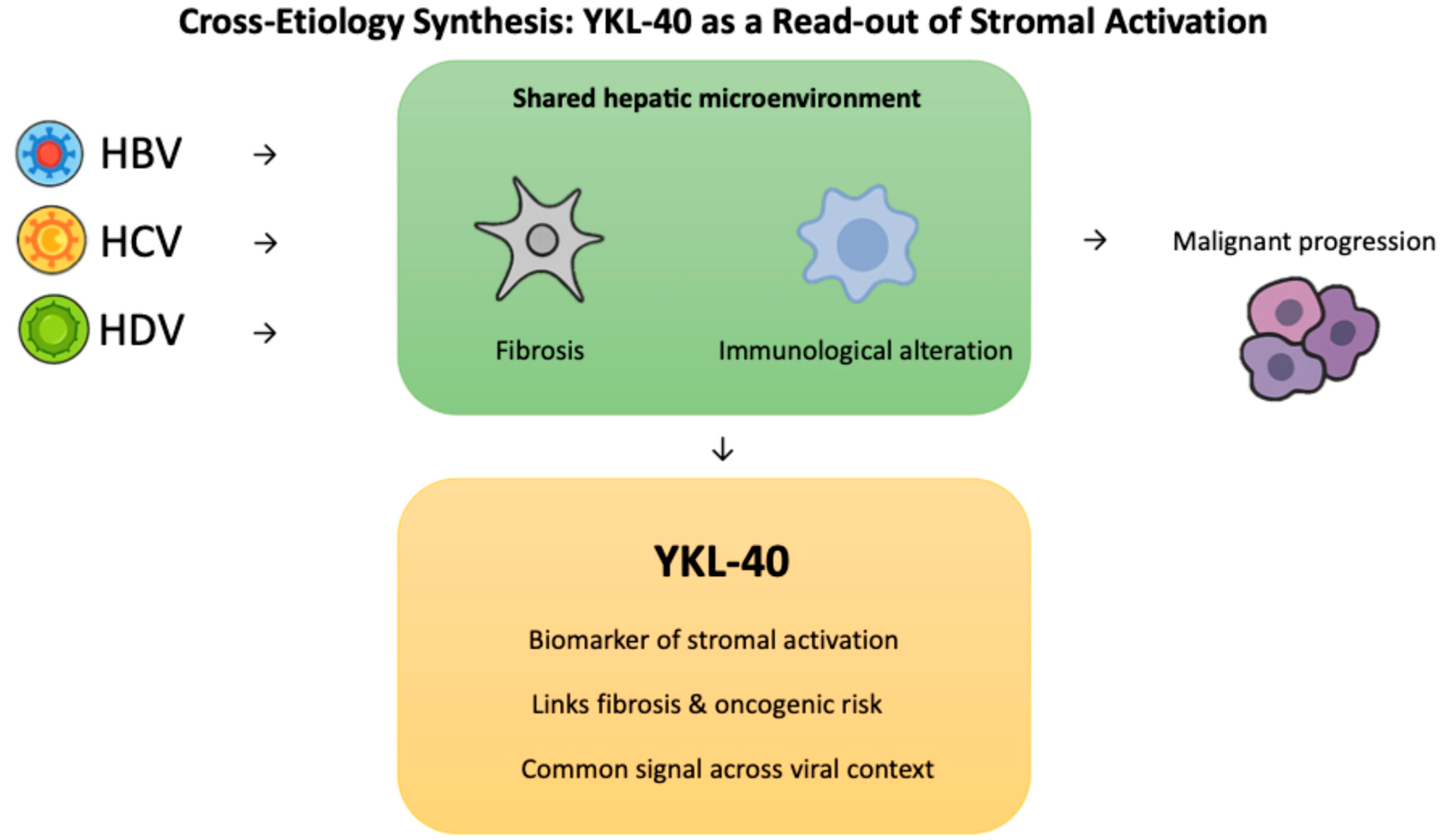
2.4. Cross-Etiology Synthesis: YKL-40 as a Read-Out of Stromal Activation
3. YKL-40 as a Translational Biomarker in Virus-Associated Liver Disease
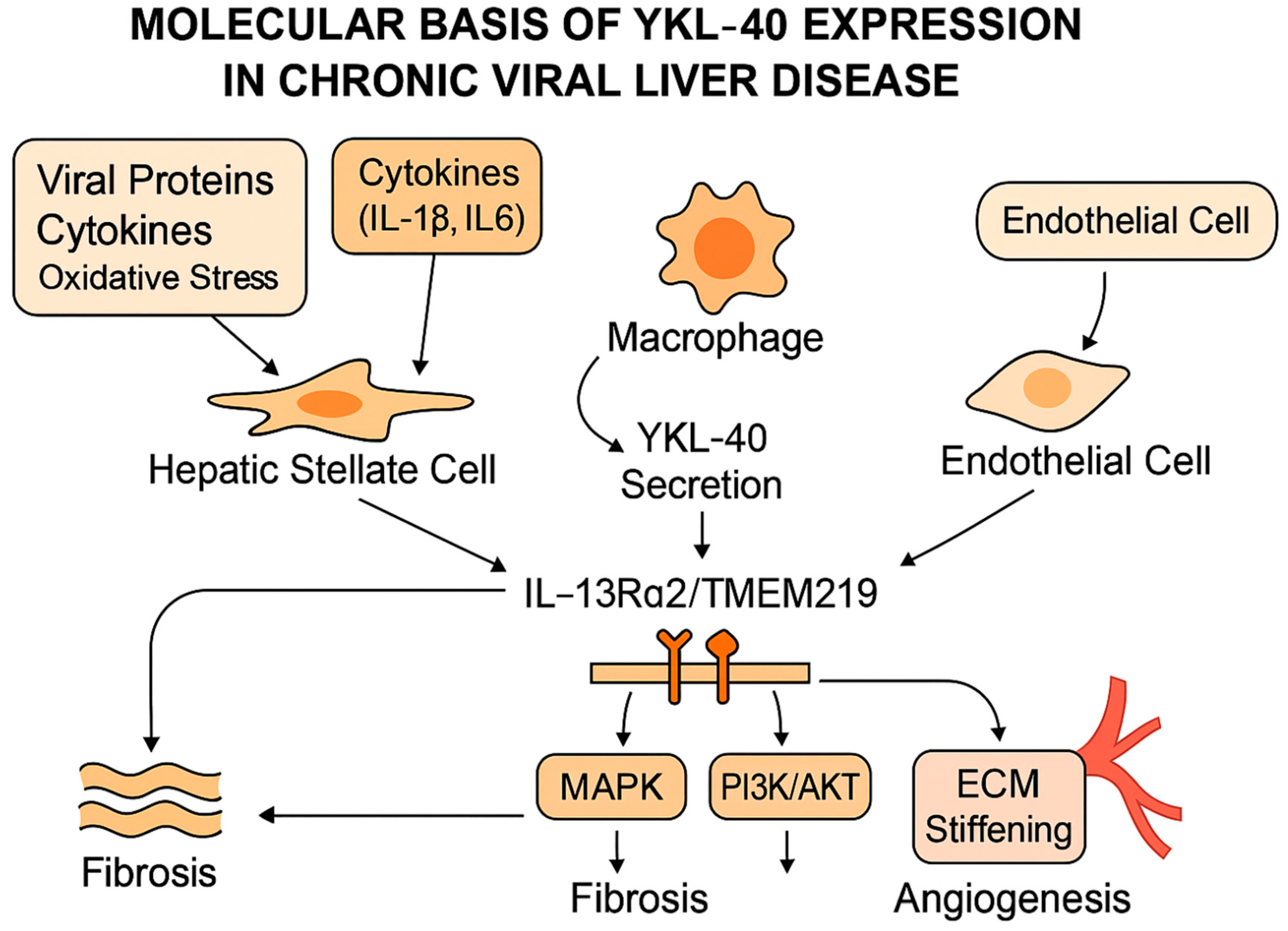
3.1. Molecular Basis of YKL-40 Expression
3.2. Clinical and Translational Applications of YKL-40
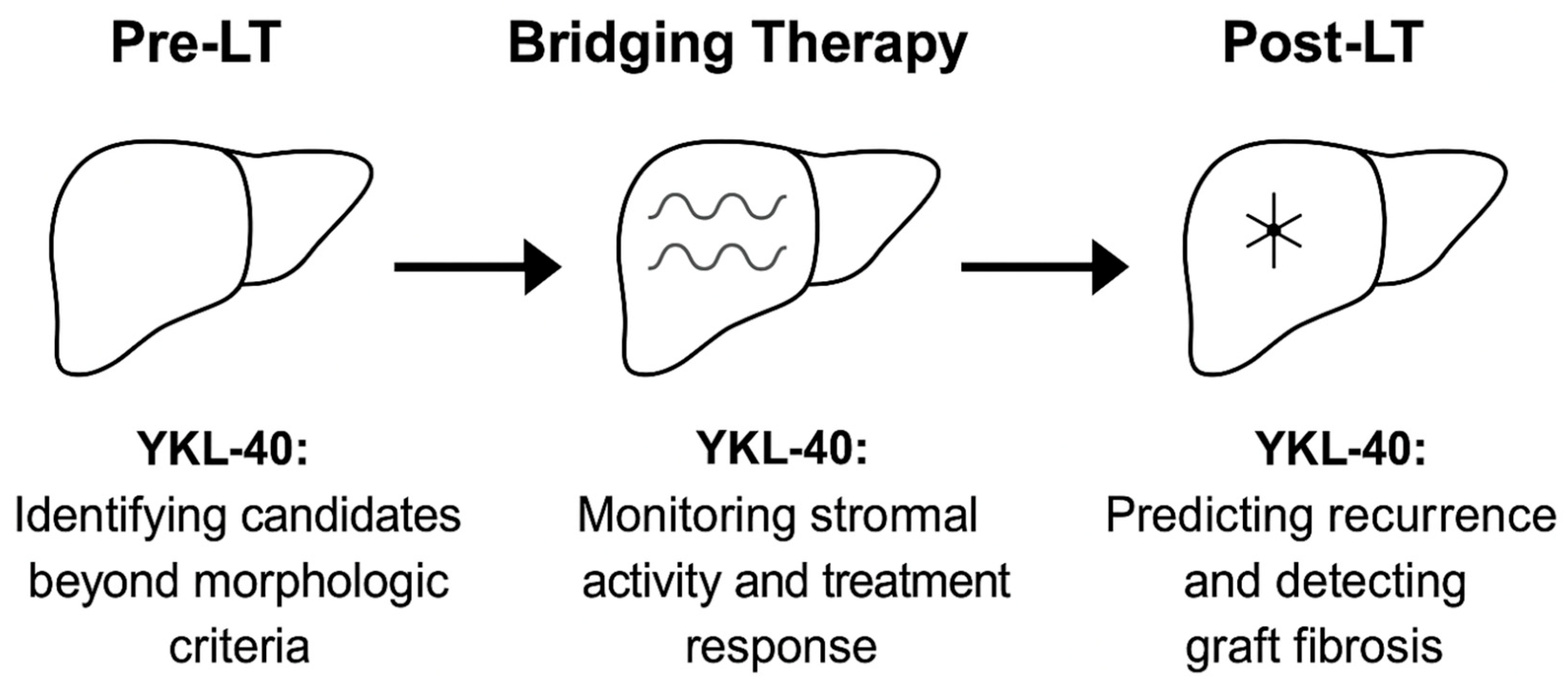
4. Pre- and Post-Transplant Applications of YKL-40 in HCC: From Eligibility to Risk Stratification
4.1. Informing Transplant Eligibility Beyond Morphology
4.2. Refining Pre-Transplant Risk Stratification
4.3. Post-Transplant Recurrence Prediction and Integration into Biomarker Panels
4.4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTA2 | Alpha-smooth muscle actin |
| AFP | Alpha-fetoprotein |
| AKT | Protein kinase B |
| ALT | Alanine aminotransferase |
| αvβ3 | Integrin alpha-V beta-3 |
| αvβ5 | Integrin alpha-V beta-5 |
| cccDNA | Covalently closed circular DNA |
| CCL2 | C-C motif chemokine ligand 2 |
| CCNE1 | Cyclin E1 |
| CD8+ T cells | Cluster of differentiation 8 positive T lymphocytes |
| cfDNA | Cell-free DNA |
| CHB | Chronic hepatitis B |
| CHC | Chronic hepatitis C |
| COL1A1 | Collagen type I alpha 1 chain |
| ctDNA | Circulating tumor DNA |
| CTGF | Connective tissue growth factor |
| CTL | Cytotoxic T lymphocyte |
| CXCL10 | Interferon gamma-induced protein 10 |
| DAA | Direct-acting antiviral |
| DCP | Des-gamma-carboxy prothrombin |
| DNA | Deoxyribonucleic acid |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| EVs | Extracellular vesicles |
| EV cargo | Extracellular vesicle cargo |
| FIB-4 | Fibrosis-4 Index |
| GPC3 | Glypican-3 |
| IFN | Interferon |
| IHC | Immunohistochemistry |
| HBsAg | Hepatitis B surface antigen |
| HBV | Hepatitis B virus |
| HBx | Hepatitis B X protein |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| HDV | Hepatitis D virus |
| HSC | Hepatic stellate cell |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-13Rα2 | Interleukin-13 receptor subunit alpha-2 |
| IRF3 | Interferon regulatory factor 3 |
| LAG-3 | Lymphocyte-activation gene 3 |
| L-HDAg | Large hepatitis D antigen |
| LOXL2 | Lysyl oxidase-like 2 |
| LT | Liver transplantation |
| M2 | Type 2 macrophage polarization |
| MAVS | Mitochondrial antiviral signaling protein |
| miRNA | MicroRNA |
| MMP | Matrix metalloproteinase |
| MoRAL | Model to predict tumor Recurrence After Living-donor liver transplantation |
| MRD | Minimal (molecular) residual disease |
| MVI | Microvascular invasion |
| NADPH | Nicotinamide adenine dinucleotide |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NR | Not reported |
| NS5A | Non-structural protein 5A |
| OPN | Osteopontin |
| PDGFRB | Platelet-derived growth factor receptor beta |
| PIVKA-II | Protein induced by vitamin K absence or antagonist-II |
| Pre-LT | Pre-liver transplantation |
| Post-LT | Post-liver transplantation |
| SMAD2/3 | Mothers against decapentaplegic homologs 2 and 3 |
| STAT3 | Signal transducer and activator of transcription 3 |
| SVR | Sustained virologic response |
| SWI/SNF | Switch/sucrose non-fermentable |
| PD-1 | Programmed cell death protein 1 |
| RIG-I | Retinoic acid-inducible gene I |
| RNA | Ribonucleic acid |
| TACE | Transarterial chemoembolization |
| TERT | Telomerase reverse transcriptase |
| TGF-β | Transforming growth factor-beta |
| TIMP1 | Tissue inhibitor of metalloproteinases-1 |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-alpha |
| Tregs | Regulatory T cells |
| UCSF | University of California, San Francisco |
| VEGF | Vascular endothelial growth factor |
| VET | Viable enhancing tumor |
| Wnt/β-catenin | Wingless-related integration site/β-catenin |
| YKL-40 | Chitinase-3-like protein 1 |
References
- Suzuki, H.; Mishra, S.; Paul, S.; Hoshida, Y. Molecular and immune landscape of hepatocellular carcinoma for therapeutic development. J. Liver Cancer 2025, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Capasso, M.; Cossiga, V.; Guarino, M.; Ranieri, L.; Morisco, F. The Role of Hepatitis Viruses as Drivers of Hepatocancerogenesis. Cancers 2024, 16, 1505. [Google Scholar] [CrossRef]
- Farci, P.; Niro, G.A.; Zamboni, F.; Diaz, G. Hepatitis D Virus and Hepatocellular Carcinoma. Viruses 2021, 13, 830. [Google Scholar] [CrossRef]
- Jiang, J.; Shiels, M.S.; Rivera, D.; Ghany, M.G.; Engels, E.A.; O’Brien, T.R. Trends in hepatocellular carcinoma and viral hepatitis treatment in older Americans. PLoS ONE 2024, 19, e0307746. [Google Scholar] [CrossRef]
- Shen, C.; Jiang, X.; Li, M.; Luo, Y. Hepatitis Virus and Hepatocellular Carcinoma: Recent Advances. Cancers 2023, 15, 533. [Google Scholar] [CrossRef]
- Shan, L.; Wang, F.; Xue, W.; Zhai, D.; Liu, J.; Lv, X. New insights into fibrotic signaling in hepatocellular carcinoma. Front. Oncol. 2023, 13, 1196298. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, Y.J. The evidence-based multifaceted roles of hepatic stellate cells in liver diseases: A concise review. Life Sci. 2024, 344, 122547. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, W.; Li, X.; Zhao, H.; Wang, S. The Diagnostic Performance of AFP, AFP-L3, DCP, CA199, and Their Combination for Primary Liver Cancer. J. Hepatocell. Carcinoma 2025, 12, 513–526. [Google Scholar] [CrossRef]
- Zhang, J.; Li, R.; Tan, X.; Wang, C. Targeting Glypican-3 for Liver Cancer Therapy: Clinical Applications and Detection Methods. J. Clin. Transl. Hepatol. 2025, 13, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Niu, K.; Yang, Z.; Song, J.; Wei, D.; Zhang, R.; Tao, K. Osteopontin: An indispensable component in common liver, pancreatic, and biliary related disease. J. Cancer Res. Clin. Oncol. 2024, 150, 508. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Jeong, S.W.; Jang, J.Y.; Eun, H.; Lee, Y.S.; Song, D.S.; Yu, S.J.; Lee, S.H.; Kim, W.; Lee, H.W.; et al. The diagnostic value of circulating tumor DNA in hepatitis B virus induced hepatocellular carcinoma: A systematic review and meta-analysis. J. Liver Cancer 2022, 22, 167–177. [Google Scholar] [CrossRef]
- Ramalingam, P.S.; Zhang, L.; Hussain, M.S.; Khan, G.; Mawkili, W.; Hanbashi, A.; Gupta, G.; Balakrishnan, P.; Arumugam, S. Non-coding RNAs as key regulators in hepatitis B virus-related hepatocellular carcinoma. Front. Immunol. 2025, 16, 1602252, Correction in: Front. Immunol. 2025, 16, 1653985. [Google Scholar] [CrossRef]
- Kouroumalis, E.; Tsomidis, I.; Voumvouraki, A. Extracellular Vesicles in Viral Liver Diseases. Viruses 2024, 16, 1785. [Google Scholar] [CrossRef]
- Patel, K.; Sebastiani, G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020, 2, 100067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, M.; Jiang, L. Potential Roles and Future Perspectives of Chitinase 3-like 1 in Macrophage Polarization and the Development of Diseases. Int. J. Mol. Sci. 2023, 24, 16149. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Meng, Y.; Hu, X.; Liu, J.; Qin, X. Uncovering novel mechanisms of chitinase-3-like protein 1 in driving inflammation-associated cancers. Cancer Cell. Int. 2024, 24, 268. [Google Scholar] [CrossRef]
- Boonstra, A.; Sari, G. HBV cccDNA: The Molecular Reservoir of Hepatitis B Persistence and Challenges to Achieve Viral Eradication. Biomolecules 2025, 15, 62. [Google Scholar] [CrossRef]
- Fisicaro, P.; Barili, V.; Rossi, M.; Montali, I.; Vecchi, A.; Acerbi, G.; Laccabue, D.; Zecca, A.; Penna, A.; Missale, G.; et al. Pathogenetic Mechanisms of T Cell Dysfunction in Chronic HBV Infection and Related Therapeutic Approaches. Front. Immunol. 2020, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xu, B.; Fan, Y.; Tuekprakhon, A.; Stamataki, Z.; Wang, F. The role of immune regulation in HBV infection and hepatocellular carcinogenesis. Front. Immunol. 2025, 16, 1506526. [Google Scholar] [CrossRef]
- Costa, J.P.; de Carvalho, A.; Paiva, A.; Borges, O. Insights into Immune Exhaustion in Chronic Hepatitis B: A Review of Checkpoint Receptor Expression. Pharmaceuticals 2024, 17, 964. [Google Scholar] [CrossRef]
- You, H.; Wang, X.; Ma, L.; Zhang, F.; Zhang, H.; Wang, Y.; Pan, X.; Zheng, K.; Kong, F.; Tang, R. Insights into the impact of hepatitis B virus on hepatic stellate cell activation. Cell Commun. Signal. 2023, 21, 70. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Yang, Y.; Liu, K.; Wu, J.; Gao, P.; Zhang, C. Exosomes in liver fibrosis: The role of modulating hepatic stellate cells and immune cells, and prospects for clinical applications. Front. Immunol. 2023, 14, 1133297. [Google Scholar] [CrossRef]
- Cheng, S.; Zou, Y.; Zhang, M.; Bai, S.; Tao, K.; Wu, J.; Shi, Y.; Wu, Y.; Lu, Y.; He, K.; et al. Single-cell RNA sequencing reveals the heterogeneity and intercellular communication of hepatic stellate cells and macrophages during liver fibrosis. MedComm 2023, 4, e378. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Y.; Liu, X.; Ma, H.; Liu, Y.; Lv, G.; Niu, J. Multi-omics profiling of primary hepatic stellate cells from advanced liver fibrosis patients reveals distinctive molecular signatures. J. Gastroenterol. Hepatol. 2023, 38, 1416–1425. [Google Scholar] [CrossRef]
- Rockey, D.C.; Du, Q.; Weymouth, N.D.; Shi, Z. Smooth Muscle α-Actin Deficiency Leads to Decreased Liver Fibrosis via Impaired Cytoskeletal Signaling in Hepatic Stellate Cells. Am. J. Pathol. 2019, 189, 2209–2220, Correction in: Am. J. Pathol. 2020, 190, 1580. [Google Scholar] [CrossRef]
- Du, K.; Jun, J.H.; Dutta, R.K.; Diehl, A.M. Plasticity, heterogeneity, and multifunctionality of hepatic stellate cells in liver pathophysiology. Hepatol. Commun. 2024, 8, e0411. [Google Scholar] [CrossRef]
- Akkız, H.; Gieseler, R.K.; Canbay, A. Liver Fibrosis: From Basic Science towards Clinical Progress, Focusing on the Central Role of Hepatic Stellate Cells. Int. J. Mol. Sci. 2024, 25, 7873. [Google Scholar] [CrossRef] [PubMed]
- Radic, J.; Kozik, B.; Nikolic, I.; Kolarov-Bjelobrk, I.; Vasiljevic, T.; Vranjkovic, B.; Despotovic, S. Multiple Roles of LOXL2 in the Progression of Hepatocellular Carcinoma and Its Potential for Therapeutic Targeting. Int. J. Mol. Sci. 2023, 24, 11745. [Google Scholar] [CrossRef] [PubMed]
- Ben-Moshe, S.; Veg, T.; Manco, R.; Dan, S.; Papinutti, D.; Lifshitz, A.; Kolodziejczyk, A.A.; Bahar Halpern, K.; Elinav, E.; Itzkovitz, S. The spatiotemporal program of zonal liver regeneration following acute injury. Cell Stem Cell. 2022, 29, 973–989.e10. [Google Scholar] [PubMed]
- Dobie, R.; Wilson-Kanamori, J.R.; Henderson, B.E.P.; Smith, J.R.; Matchett, K.P.; Portman, J.R.; Wallenborg, K.; Picelli, S.; Zagorska, A.; Pendem, S.V.; et al. Single-Cell Transcriptomics Uncovers Zonation of Function in the Mesenchyme during Liver Fibrosis. Cell Rep. 2019, 29, 1832–1847.e8. [Google Scholar] [CrossRef]
- Bybee, G.; Moeun, Y.; Wang, W.; Kharbanda, K.K.; Poluektova, L.Y.; Kidambi, S.; Osna, N.A.; Ganesan, M. Increased liver stiffness promotes hepatitis B progression by impairing innate immunity in CCl4-induced fibrotic HBV+ transgenic mice. Front. Immunol. 2023, 14, 1166171. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, S.; Jin, J.; Ying, S.; Chen, Z.; Zhu, D.; Xiao, B.; Hu, Y.; Qian, Y.; Cai, T.; et al. The clinical significance of serum chitinase 3-like 1 in hepatitis B-related chronic liver diseases. J. Clin. Lab. Anal. 2020, 34, e23200. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, T.; Zhou, J.; You, H.; Jia, J. Changes in serum chitinase 3-like 1 levels correlate with changes in liver fibrosis measured by two established quantitative methods in chronic hepatitis B patients following antiviral therapy. Hepatol. Res. 2018, 48, E283–E290. [Google Scholar] [CrossRef]
- Lin, H.Y.; Jeon, A.J.; Chen, K.; Lee, C.J.M.; Wu, L.; Chong, S.L.; Anene-Nzelu, C.G.; Foo, R.S.; Chow, P.K. The epigenetic basis of hepatocellular carcinoma—Mechanisms and potential directions for biomarkers and therapeutics. Br. J. Cancer 2025, 132, 869–887. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, W.; Wang, S.; Zhang, L.; Li, X.; Zhang, Z.; Xie, Y.; Li, M. Epigenetic modification of hepatitis B virus infection and related hepatocellular carcinoma. Virulence 2024, 15, 2421231. [Google Scholar] [CrossRef]
- Quan, W.; Bello, K.E.; Shueb, R.H.; Mustaffa, N. Circular RNAs in hepatitis B virus-induced hepatocellular carcinoma: A comprehensive review and recent advances. Genes. Dis. 2025, 12, 101605. [Google Scholar] [CrossRef]
- Cui, X.; Li, Y.; Xu, H.; Sun, Y.; Jiang, S.; Li, W. Characteristics of Hepatitis B virus integration and mechanism of inducing chromosome translocation. NPJ Genom. Med. 2023, 8, 11. [Google Scholar] [CrossRef]
- Hu, G.; Huang, M.X.; Li, W.Y.; Gan, C.J.; Dong, W.X.; Peng, X.M. Liver damage favors the eliminations of HBV integration and clonal hepatocytes in chronic hepatitis B. Hepatol. Int. 2021, 15, 60–70. [Google Scholar] [CrossRef]
- Huang, M.; Wang, D.; Huang, J.; Bae, A.N.; Xia, Y.; Zhao, X.; Mortaja, M.; Azin, M.; Collier, M.R.; Semenov, Y.R.; et al. Hepatitis B virus promotes liver cancer by modulating the immune response to environmental carcinogens. Nat. Commun. 2025, 16, 5360. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Liu, Y.; Li, T.; Wang, C.; He, S.; Zhai, L.; Yang, Z.; Zhang, X.; Wu, Y.; Liu, Y. Viral oncogenesis in cancer: From mechanisms to therapeutics. Signal Transduct. Target. Ther. 2025, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hamadalnil, Y.; Tu, T. Hepatitis B Viral Protein HBx: Roles in Viral Replication and Hepatocarcinogenesis. Viruses 2024, 16, 1361. [Google Scholar] [CrossRef]
- Villanueva, R.A.; Loyola, A. The Intrinsically Disordered Region of HBx and Virus-Host Interactions: Uncovering New Therapeutic Approaches for HBV and Cancer. Int. J. Mol. Sci. 2025, 26, 3552. [Google Scholar] [CrossRef] [PubMed]
- Zoulim, F.; Chen, P.J.; Dandri, M.; Kennedy, P.T.; Seeger, C. Hepatitis B virus DNA integration: Implications for diagnostics, therapy, and outcome. J. Hepatol. 2024, 81, 1087–1099. [Google Scholar] [CrossRef]
- Zhong, J.; Li, Y.; Liu, Y.; Qiao, J.; Wu, Y.; Kong, X.; Qi, M.; Lin, Y.; Yao, Y.; Jin, Y.; et al. Hepatitis B virus X protein (HBx)-mediated immune modulation and prognostic model development in hepatocellular carcinoma. PLoS ONE 2025, 20, e0325363. [Google Scholar] [CrossRef]
- Bang, B.R.; Elmasry, S.; Saito, T. Organ system view of the hepatic innate immunity in HCV infection. J. Med. Virol. 2016, 88, 2025–2037. [Google Scholar] [PubMed]
- Lee, J.; Ou, J.J. HCV-induced autophagy and innate immunity. Front. Immunol. 2024, 15, 1305157. [Google Scholar] [CrossRef]
- Martineau, C.A.; Rivard, N.; Bisaillon, M. From viruses to cancer: Exploring the role of the hepatitis C virus NS3 protein in carcinogenesis. Infect. Agent. Cancer 2024, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. Suppression of Innate Immunity by the Hepatitis C Virus (HCV): Revisiting the Specificity of Host-Virus Interactive Pathways. Int. J. Mol. Sci. 2023, 24, 16100. [Google Scholar]
- Cheng, D.; Zhu, C.; Liao, F.; Zhao, L.; Shen, L.; Jiang, W. Reciprocal induction of hepatitis C virus replication and stimulation of hepatic profibrogenic cytokine release and cellular viability by YKL-40. Ann. Transl. Med. 2021, 9, 1649. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Yester, J.W.; Singh, S.K.; Biswas, D.D.; Surace, M.J.; Waters, M.R.; Hauser, K.F.; Yao, Z.; Boyce, B.F.; Kordula, T. RelB/p50 complexes regulate cytokine-induced YKL-40 expression. J. Immunol. 2015, 194, 2862–2870. [Google Scholar] [CrossRef]
- Osuch, S.; Laskus, T.; Perlejewski, K.; Berak, H.; Bukowska-Ośko, I.; Pollak, A.; Zielenkiewicz, M.; Radkowski, M.; Caraballo Cortés, K. CD8+ T-Cell Exhaustion Phenotype in Chronic Hepatitis C Virus Infection Is Associated With Epitope Sequence Variation. Front. Immunol. 2022, 13, 832206. [Google Scholar] [CrossRef]
- Bility, M.T.; Nio, K.; Li, F.; McGivern, D.R.; Lemon, S.M.; Feeney, E.R.; Chung, R.T.; Su, L. Chronic hepatitis C infection-induced liver fibrogenesis is associated with M2 macrophage activation. Sci. Rep. 2016, 6, 39520. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, M.U.; Ottolini, S.; Oliviero, B.; Mantovani, S.; Cerino, A.; Mele, D.; Varchetta, S. Hepatitis C Virus and the Host: A Mutual Endurance Leaving Indelible Scars in the Host’s Immunity. Int. J. Mol. Sci. 2023, 25, 268. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, J.; Xu, R.; Yang, K.; Qu, R.; Liu, S.; Zhang, Y.; Xiang, M. The spatiotemporal heterogeneity of reactive oxygen species in the malignant transformation of viral hepatitis to hepatocellular carcinoma: A new insight. Cell. Mol. Biol. Lett. 2025, 30, 70. [Google Scholar] [CrossRef] [PubMed]
- Saitou, Y.; Shiraki, K.; Yamanaka, Y.; Yamaguchi, Y.; Kawakita, T.; Yamamoto, N.; Sugimoto, K.; Murata, K.; Nakano, T. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J. Gastroenterol. 2005, 11, 476–481. [Google Scholar] [CrossRef]
- Wang, L.T.; Wang, S.N.; Chiou, S.S.; Tsai, J.P.; Chai, C.Y.; Tseng, L.W.; Lee, J.C.; Lin, M.H.; Huang, S.K.; Hsu, S.H. HCV Core Protein-ISX Axis Promotes Chronic Liver Disease Progression via Metabolic Remodeling and Immune Suppression. Adv. Sci. 2023, 10, e2300644. [Google Scholar] [CrossRef]
- Cao, Y.; Mai, W.; Li, R.; Deng, S.; Li, L.; Zhou, Y.; Qin, Q.; Zhang, Y.; Zhou, X.; Han, M.; et al. Macrophages evoke autophagy of hepatic stellate cells to promote liver fibrosis in NAFLD mice via the PGE2/EP4 pathway. Cell. Mol. Life Sci. 2022, 79, 303. [Google Scholar] [CrossRef]
- Du, Q.; Dickinson, A.; Nakuleswaran, P.; Maghami, S.; Alagoda, S.; Hook, A.L.; Ghaemmaghami, A.M. Targeting Macrophage Polarization for Reinstating Homeostasis following Tissue Damage. Int. J. Mol. Sci. 2024, 25, 7278. [Google Scholar] [CrossRef]
- Di Stasio, D.; Guida, A.; Romano, A.; Petruzzi, M.; Marrone, A.; Fiori, F.; Lucchese, A. Hepatitis C Virus (HCV) Infection: Pathogenesis, Oral Manifestations, and the Role of Direct-Acting Antiviral Therapy: A Narrative Review. J. Clin. Med. 2024, 13, 4012. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, R.; Huang, Y.; Zhu, L.; Xiao, L.; Wang, C.; Wang, L. Macrophages in organ fibrosis: From pathogenesis to therapeutic targets. Cell Death Discov. 2024, 10, 487. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Deng, X.W.; Xu, G.Q.; Lin, J.; Lu, H.Z.; Chen, J. Mechanical homeostasis imbalance in hepatic stellate cells activation and hepatic fibrosis. Front. Mol. Biosci. 2023, 10, 1183808. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, A.; Zhang, W.; Li, H.; Xu, A.; Yan, X.; Han, Q.; Wang, B.; You, H.; Chen, W. Crosstalk of lysyl oxidase-like 1 and lysyl oxidase prolongs their half-lives and regulates liver fibrosis through Notch signal. Hepatol. Commun. 2024, 8, e0391. [Google Scholar] [CrossRef]
- Kazemi, A.; Fathy, M.; Jahanian, A.; Khanali, J.; Ostadi, Y.; Babajani, A.; Tayebi, T.; Niknejad, H. The role of MMPs and TIMPs in regenerative medicine: From pathological ECM remodeling to therapeutic applications. Biomed. Pharmacother. 2025, 191, 118457. [Google Scholar] [CrossRef]
- Merens, V.; Knetemann, E.; Gürbüz, E.; De Smet, V.; Messaoudi, N.; Reynaert, H.; Verhulst, S.; van Grunsven, L.A. Hepatic stellate cell single cell atlas reveals a highly similar activation process across liver disease aetiologies. JHEP Rep. 2024, 7, 101223. [Google Scholar] [CrossRef]
- Su, T.; Yang, Y.; Lai, S.; Jeong, J.; Jung, Y.; McConnell, M.; Utsumi, T.; Iwakiri, Y. Single-Cell Transcriptomics Reveals Zone-Specific Alterations of Liver Sinusoidal Endothelial Cells in Cirrhosis. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1139–1161. [Google Scholar] [CrossRef]
- Degasperi, E.; Sandmann, L.; Wedemeyer, H.; Lampertico, P.; Delta Cure 2024 Working Group. Hepatitis D Virus Infection: Pathophysiology, Epidemiology and Treatment. Report From the Third Delta Cure Meeting 2024. Liver Int. 2025, 45, e70189. [Google Scholar] [CrossRef]
- Gish, R.G.; Wong, R.J.; Di Tanna, G.L.; Kaushik, A.; Kim, C.; Smith, N.J.; Kennedy, P.T.F. Association of hepatitis delta virus with liver morbidity and mortality: A systematic literature review and meta-analysis. Hepatology 2024, 79, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Sadler, M.; Patel, N.H.; Osiowy, C.; Fonseca, K.; Coffin, C.S. Systemic cytokine and viral antigen-specific responses in hepatitis D virus RNA positive versus HDV RNA negative patients. Front. Med. 2023, 10, 1125139. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, O.A.; Ivanova, O.N.; Mukhtarov, F.; Valuev-Elliston, V.T.; Fedulov, A.P.; Rubtsov, P.M.; Zakirova, N.F.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Hepatitis Delta Virus Antigens Trigger Oxidative Stress, Activate Antioxidant Nrf2/ARE Pathway, and Induce Unfolded Protein Response. Antioxidants 2023, 12, 974. [Google Scholar] [CrossRef]
- Wranke, A.; Heidrich, B.; Deterding, K.; Hupa-Breier, K.L.; Kirschner, J.; Bremer, B.; Cornberg, M.; Wedemeyer, H. Clinical long-term outcome of hepatitis D compared to hepatitis B monoinfection. Hepatol. Int. 2023, 17, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Kefalakes, H.; Koh, C.; Sidney, J.; Amanakis, G.; Sette, A.; Heller, T.; Rehermann, B. Hepatitis D Virus-Specific CD8+ T Cells Have a Memory-Like Phenotype Associated With Viral Immune Escape in Patients With Chronic Hepatitis D Virus Infection. Gastroenterology 2019, 156, 1805–1819.e9. [Google Scholar] [CrossRef]
- Luxenburger, H.; Neumann-Haefelin, C. Liver-resident CD8+ T cells in viral hepatitis: Not always good guys. J. Clin. Investig. 2023, 133, e165033. [Google Scholar] [CrossRef] [PubMed]
- Quirino, A.; Marascio, N.; Branda, F.; Ciccozzi, A.; Romano, C.; Locci, C.; Azzena, I.; Pascale, N.; Pavia, G.; Matera, G.; et al. Viral Hepatitis: Host Immune Interaction, Pathogenesis and New Therapeutic Strategies. Pathogens 2024, 13, 766. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Pinzani, M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology 2022, 75, 473–488. [Google Scholar] [CrossRef]
- Altalbawy, F.M.A.; Zwamel, A.H.; Sanghvi, G.; Roopashree, R.; Kumari, M.; Kashyap, A.; Gayathri, S.; Panigrahi, R. Interactions in hepatic tumor microenvironment: Potential targets and modulations for effective therapy. Pathol. Res. Pract. 2025, 272, 156074. [Google Scholar] [CrossRef]
- Lulic, I.; Lulic, D.; Pavicic Saric, J.; Bacak Kocman, I.; Rogic, D. Personalized translational medicine: Investigating YKL-40 as early biomarker for clinical risk stratification in hepatocellular carcinoma recurrence post-liver transplantation. World J. Transplant. 2025, 15, 103036. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, E.; Sadanaga, T.; Nanni, L.; Wang, S.; Mizoguchi, A. Recently Updated Role of Chitinase 3-like 1 on Various Cell Types as a Major Influencer of Chronic Inflammation. Cells 2024, 13, 678. [Google Scholar] [CrossRef]
- Yu, J.E.; Yeo, I.J.; Han, S.B.; Yun, J.; Kim, B.; Yong, Y.J.; Lim, Y.S.; Kim, T.H.; Son, D.J.; Hong, J.T. Significance of chitinase-3-like protein 1 in the pathogenesis of inflammatory diseases and cancer. Exp. Mol. Med. 2024, 56, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; De Battista, D.; McGivern, D.R.; Engle, R.E.; Tice, A.; Fares-Gusmao, R.; Kabat, J.; Pomerenke, A.; Nguyen, H.; Sato, S.; et al. Chitinase 3-like 1 is a profibrogenic factor overexpressed in the aging liver and in patients with liver cirrhosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2019633118. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, L.; Tian, Y.; Guo, X.; Wei, F.; Zhang, Y. Signaling pathways that activate hepatic stellate cells during liver fibrosis. Front. Med. 2024, 11, 1454980. [Google Scholar] [CrossRef]
- Hu, X.; Liu, W.; Liu, J.; Wang, B.; Qin, X. Research advances in serum chitinase-3-like protein 1 in liver fibrosis. Front. Med. 2024, 11, 1372434. [Google Scholar] [CrossRef]
- He, C.H.; Lee, C.G.; Dela Cruz, C.S.; Lee, C.M.; Zhou, Y.; Ahangari, F.; Ma, B.; Herzog, E.L.; Rosenberg, S.A.; Li, Y.; et al. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor α2. Cell Rep. 2013, 4, 830–841. [Google Scholar] [CrossRef]
- Shao, R.; Hamel, K.; Petersen, L.; Cao, Q.J.; Arenas, R.B.; Bigelow, C.; Bentley, B.; Yan, W. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 2009, 28, 4456–4468. [Google Scholar] [CrossRef]
- Chang, M.C.; Chen, C.T.; Chiang, P.F.; Chiang, Y.C. The Role of Chitinase-3-like Protein-1 (YKL40) in the Therapy of Cancer and Other Chronic-Inflammation-Related Diseases. Pharmaceuticals 2024, 17, 307. [Google Scholar] [CrossRef]
- Zhao, T.; Su, Z.; Li, Y.; Zhang, X.; You, Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct. Target. Ther. 2020, 5, 201. [Google Scholar] [CrossRef]
- Li, H.; Xu, X.; Yan, T. Serum YKL-40 Levels as a Non-Invasive Potential Biomarker for Liver Fibrosis Patients: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2025, 54, 939–950. [Google Scholar] [CrossRef]
- Huang, R.; Liu, J.; Wang, J.; Qiu, Y.; Zhu, L.; Li, Y.; Liu, Y.; Zhan, J.; Xue, R.; Jiang, S.; et al. Histological features of chronic hepatitis B patients with normal alanine aminotransferase according to different criteria. Hepatol. Commun. 2024, 8, e0357. [Google Scholar] [CrossRef]
- Duan, M.; Xiao, H.; Shi, M.; Xie, Y.; Zhao, P.; Li, S.; Chi, X.; Liu, X.; Zhuang, H. Significant liver histological change is common in HBeAg-positive chronic hepatitis B with normal ALT. BMC Infect. Dis. 2024, 24, 723. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.; Zhang, L.; Hu, W.; Luo, H.; Li, J.; Qiu, S.; Zhu, S. Serum CHI3L1 as a diagnostic marker and risk factor for liver fibrosis in HBeAg-negative chronic hepatitis B. Am. J. Transl. Res. 2022, 14, 4090–4096. [Google Scholar] [PubMed]
- Kang, Q.; Chen, J.; Luo, H.; Tan, N.; Gao, H.; Zhang, X.; Yu, M.; Liu, D.; Xi, H.; An, Y.; et al. Decrease in Chitinase 3-Like Protein 1 Levels Reflects Improvement in Liver Fibrosis after HCV Eradication. Dis. Markers 2020, 2020, 8539804. [Google Scholar] [CrossRef] [PubMed]
- Mangoud, N.O.M.; Ali, S.A.; El Kassas, M.; Soror, S.H. Chitinase 3-like-1, Tolloid-like protein 1, and intergenic gene polymorphisms are predictors for hepatocellular carcinoma development after hepatitis C virus eradication by direct-acting antivirals. IUBMB Life 2021, 73, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Bian, B.; Li, L.; Yang, J.; Liu, Y.; Xie, G.; Zheng, Y.; Zeng, L.; Zeng, J.; Shen, L. Prognostic value of YKL-40 in solid tumors: A meta-analysis of 41 cohort studies. Cancer Cell Int. 2019, 19, 259. [Google Scholar] [CrossRef]
- Jiang, Y.; Gong, W.; Liu, Y.; Zhou, Z.; Liang, X.; Lin, Q.; Qiu, M.; Lin, B.; Qiu, X.; Yu, H. Serum CHI3L1 Levels Predict Overall Survival of Hepatocellular Carcinoma Patients after Hepatectomy. J. Cancer 2024, 15, 6315–6325. [Google Scholar] [CrossRef] [PubMed]
- Blazevic, N.; Rogic, D.; Pelajic, S.; Miler, M.; Glavcic, G.; Ratkajec, V.; Vrkljan, N.; Bakula, D.; Hrabar, D.; Pavic, T. YKL-40 as a biomarker in various inflammatory diseases: A review. Biochem. Med. 2024, 34, 010502. [Google Scholar]
- Schultz, N.A.; Johansen, J.S. YKL-40-A Protein in the Field of Translational Medicine: A Role as a Biomarker in Cancer Patients? Cancers 2010, 2, 1453–1491. [Google Scholar] [CrossRef]
- Tırnova, I.; Kanmaz, T. Liver transplantation for HCC within and beyond Milan Criteria: Single center experience with literature review. Front. Surg. 2025, 12, 1594361. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Ou, Y.; Li, H.; Xu, W. Optimizing Treatment Selection for Early Hepatocellular Carcinoma Based on Tumor Biology, Liver Function, and Patient Status. J. Hepatocell. Carcinoma 2025, 12, 777–790. [Google Scholar] [CrossRef]
- Kokudo, T.; Kokudo, N. Evolving Indications for Liver Transplantation for Hepatocellular Carcinoma Following the Milan Criteria. Cancers 2025, 17, 507. [Google Scholar] [CrossRef]
- Núñez, K.G.; Sandow, T.; Fort, D.; Patel, J.; Hibino, M.; Carmody, I.; Cohen, A.J.; Thevenot, P. Baseline Alpha-Fetoprotein, Alpha-Fetoprotein-L3, and Des-Gamma-Carboxy Prothrombin Biomarker Status in Bridge to Liver Transplant Outcomes for Hepatocellular Carcinoma. Cancers 2021, 13, 4765. [Google Scholar] [CrossRef]
- Kjaergaard, A.D.; Johansen, J.S.; Bojesen, S.E.; Nordestgaard, B.G. Role of inflammatory marker YKL-40 in the diagnosis, prognosis and cause of cardiovascular and liver diseases. Crit. Rev. Clin. Lab. Sci. 2016, 53, 396–408. [Google Scholar] [CrossRef]
- Zhu, C.B.; Wang, C.; Chen, L.L.; Ma, G.L.; Zhang, S.C.; Su, L.; Tian, J.J.; Gai, Z.T. Serum YKL-40 independently predicts outcome after transcatheter arterial chemoembolization of hepatocellular carcinoma. PLoS ONE 2012, 7, e44648. [Google Scholar] [CrossRef]
- Ogawa, K.; Takada, Y. Role of Pretransplant Treatments for Patients with Hepatocellular Carcinoma Waiting for Liver Transplantation. Cancers 2022, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Li, X.; Mu, R.; Zheng, W.; Yang, P.; Huang, B.; Liu, F.; Liu, X.; Song, Z.; Qin, X.; et al. Comparison of the diagnostic efficacy between imaging features and iodine density values for predicting microvascular invasion in hepatocellular carcinoma. Front. Oncol. 2024, 14, 1437347. [Google Scholar] [CrossRef]
- Yoon, J.K.; Choi, J.Y. MRI Imaging Biomarkers for Prognostication of Hepatocellular Carcinoma. J. Korean Soc. Radiol. 2025, 86, 364–380. [Google Scholar] [CrossRef]
- Qiu, Q.C.; Wang, L.; Jin, S.S.; Liu, G.F.; Liu, J.; Ma, L.; Mao, R.F.; Ma, Y.Y.; Zhao, N.; Chen, M.; et al. CHI3L1 promotes tumor progression by activating TGF-β signaling pathway in hepatocellular carcinoma. Sci. Rep. 2018, 8, 15029. [Google Scholar] [CrossRef]
- D’souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef]
- Bolkvadze, R.; Kashibadze, K.; Nakashidze, M.; Mikeladze, L.; Beridze, D.; Beridze, S. Incidence and Risk Factor of Hepatocellular Carcinoma Recurrence After Liver Transplant: Results From a Single Clinic. Exp. Clin. Transplant. 2024, 22, 256–262. [Google Scholar]
- Garas, M.G.; Calzadilla-Bertot, L.; Smith, B.W.; Delriviere, L.; Jaques, B.; Mou, L.; Adams, L.A.; MacQuillan, G.C.; Garas, G.; Jeffrey, G.P.; et al. Hepatocellular carcinoma recurrence after liver transplant: An Australian single-centre study. World J. Transplant. 2025, 15, 99004. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Z.; Lin, Z.; Chen, H.; Cao, C.; Chen, J.; Yang, X.; Li, H.; Shen, W.; Wei, X.; et al. Chitinase-3 like-protein-1, a prognostic biomarker in patients with hepatocellular carcinoma and concomitant myosteatosis. BMC Cancer 2024, 24, 1042. [Google Scholar] [CrossRef]
- Pungpapong, S.; Nunes, D.P.; Krishna, M.; Nakhleh, R.; Chambers, K.; Ghabril, M.; Dickson, R.C.; Hughes, C.B.; Steers, J.; Nguyen, J.H.; et al. Serum fibrosis markers can predict rapid fibrosis progression after liver transplantation for hepatitis C. Liver Transpl. 2008, 14, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Eurich, D.; Neumann, U.P.; Boas-Knoop, S.; Neuhaus, R.; Kiessling, A.; Yahyazadeh, A.; Trautwein, C.; Wasmuth, H.; Puhl, G.; Neuhaus, P.; et al. YKL-40-gene polymorphism affects acute cellular rejection and fibrosis progression after transplantation for hepatitis C virus-induced liver disease. J. Gastroenterol. Hepatol. 2013, 28, 153–160. [Google Scholar] [CrossRef]
- Liao, Y.; Peng, S.; Huang, L.; Li, Z.; Hu, J.; Xu, R.; Tang, W.; Zhuang, J. Analytical and Clinical Evaluation of a Chemiluminescent Immunoassay to Detect Serum Chitinase-3-like Protein 1 in HBV-Related Liver Diseases. Int. J. Anal. Chem. 2024, 2024, 6688819. [Google Scholar] [CrossRef]
- Li, P.J.; Tabrizian, P.; Daher, D.; Gaviria, F.; Ajmera, V.; Montalvan-Sanchez, E.E.; Gutierrez, J.A.; Zhou, K.; Delebecque, F.; Garcia, N.; et al. A prospective multicenter validation of RETREAT for posttransplantation HCC recurrence prediction. Hepatology 2025, 10, 1097. [Google Scholar] [CrossRef]
- Faibish, M.; Francescone, R.; Bentley, B.; Yan, W.; Shao, R. A YKL-40-neutralizing antibody blocks tumor angiogenesis and progression: A potential therapeutic agent in cancers. Mol. Cancer Ther. 2011, 10, 742–751. [Google Scholar] [CrossRef]
- Lee, C.M.; He, C.H.; Nour, A.M.; Zhou, Y.; Ma, B.; Park, J.W.; Kim, K.H.; Dela Cruz, C.; Sharma, L.; Nasr, M.L.; et al. IL-13Rα2 uses TMEM219 in chitinase 3-like-1-induced signalling and effector responses. Nat. Commun. 2016, 7, 12752, Correction in: Nat. Commun. 2016, 7, 13541. [Google Scholar] [CrossRef] [PubMed]
- Su, P.C.; Chen, C.Y.; Yu, M.H.; Kuo, I.Y.; Yang, P.S.; Hsu, C.H.; Hou, Y.C.; Hsieh, H.T.; Chang, C.P.; Shan, Y.S.; et al. Fully human chitinase-3 like-1 monoclonal antibody inhibits tumor growth, fibrosis, angiogenesis, and immune cell remodeling in lung, pancreatic, and colorectal cancers. Biomed. Pharmacother. 2024, 176, 116825. [Google Scholar] [CrossRef] [PubMed]

| Biomarker Class | Biology/Principal Source | What the Marker Captures | Evidence in Viral Hepatitis and HCC (HBV/HCV/HDV) | Where It Can Inform the LT Pathway * | Key Caveats |
|---|---|---|---|---|---|
| AFP | Tumor-derived glycoprotein (oncofetal) | Tumor burden/secretory phenotype | Widely used in HBV/HCV HCC; imperfect sensitivity in early/non-secretory tumors | Pre-LT: selection models (e.g., AFP-based); Bridging: response tracking; Post-LT: recurrence surveillance adjunct | False positives (active hepatitis, pregnancy); non-secretors |
| DCP (PIVKA-II) | Abnormal prothrombin (des-γ-carboxylated) | Tumor biology; angiogenesis/ vitamin-K axis | Complements AFP in HBV/HCV; prognostic value | Pre-LT: MoRAL-type scores; Bridging: biology signal; Post-LT: recurrence risk adjunct | Affected by vitamin-K status/ warfarin; assay variability |
| GPC3 | Oncofetal proteoglycan (tumor membrane/serum) | Tumor presence/ aggressiveness | Overexpressed in viral-related HCC; IHC and serum utility | Pre-LT: biology beyond size/number; Bridging: residual activity | Serum assays not standardized; not expressed in all HCC |
| OPN | Matricellular protein (hepatocytes, stroma, immune) | Inflammation, fibrosis, invasion | Elevated in HBV/HCV HCC; prognostic associations | Pre-LT: risk enrichment; Bridging: limited but plausible; Post-LT: prognosis (exploratory) | Non-specific; influenced by systemic inflammation |
| ctDNA | Tumor-derived cfDNA (mutational/ epigenetic) | Genomic/epigenetic alterations; MRD | HBV-HCC meta-analyses; increasing early-detection/ monitoring data | Pre-LT: biology beyond imaging (VET, MVI risk); Bridging: MRD after downstaging; Post-LT: molecular recurrence | Low tumor fraction in early disease; high-complexity assays |
| microRNA (cell-free/EV cargo) | Regulatory RNAs (hepatocytes, tumor, immune) | Pathway dysregulation; injury/oncogenic programs | HBV/HCV signatures (e.g., miR-122/21 panels) | Pre-LT: risk phenotyping; Post-LT: recurrence/ rejection (emerging) | Normalization, platform and pre-analytics; heterogeneity |
| EVs | Vesicles from liver/tumor/immune cells | Intercellular crosstalk; composite cargo (miRNA/protein) | Viral hepatitis cohorts; fibrosis/HCC signals | Pre-LT/Bridging: disease activity; Post-LT: rejection/ fibrosis (exploratory) | Isolation/ quantification not harmonized; specificity |
| YKL-40 | HSCs, macrophages, endothelium, stressed hepatocytes | Stromal activation/fibrogenesis/angiogenic tone | Elevated with advanced fibrosis; prognostic in HCC; biologically coherent in HBV/HCV | Pre-LT: selection beyond morphology; Bridging: trajectory vs. biology; Post-LT: recurrence and graft fibrosis signals | Non-specific (inflammation, infection); age/genetic effects; assay variability |
| Pathogenic Axis | HBV-Associated Mechanisms | HCV-Associated Mechanisms | Shared Features |
|---|---|---|---|
| Viral trigger | HBx activates TGF-β/SMAD and IL-6/STAT3 signaling | Core and NS5A proteins induce ROS and NF-κB activation | Upregulation of IL-6, TGF-β, and VEGF |
| Fibrogenesis | cccDNA persistence sustains HSC activation, even in inactive carriers | ROS-driven HSC activation and progressive fibrotic remodeling | Collagen I and fibronectin accumulation with impaired ECM turnover |
| Immune modulation | Expansion of M2 macrophages and IL-10, dominant immune tolerance | T-cell exhaustion with PD-1+ CD8+ subsets | Recruitment of Tregs and MDSCs; promotion of angiogenesis |
| YKL-40 expression | Elevated even at low viremia, correlates with necroinflammatory activity | Persists in a subset after SVR, associated with residual fibrosis | Reflects active stromal remodeling and immunosuppressive microenvironment |
| Biomarker potential | Sensitive marker of fibrogenic activity in HCC-naïve patients | Identifies a high-risk phenotype post-SVR | Applicable to early HCC risk stratification and supportive in pre-transplant decision-making pathways |
| Viral Etiology | First Author (Year) | Cohort/Setting | Diagnostic Endpoint * | AUC | Cut-Off (ng/mL) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| HBV | Jiang (2020) [32] | CHB; biopsy-verified fibrosis | Significant fibrosis (F0-F1 vs. F2-F3) | 0.970 | 68.75 | 95.2 | 89.7 |
| HBV | Wang (2018) [33] | CHB; baseline biopsy cohort | Ishak ≥ F2 | 0.86 | 60.9 | 82 | 83 |
| Ishak ≥ F3 | — (NR) | 73.8 | 53 | 70 | |||
| Ishak ≥ F4 | — (NR) | 91.9 | 69 | 67 | |||
| Ishak ≥ F5 | — (NR) | 106.9 | 61 | 70 | |||
| HCV | Saitou (2005) [55] | CHC; biopsy cohort | Significant fibrosis (F0-F1 vs. F2-F4) | 0.809 | 186.4 | 78 | 81 |
| Cirrhosis (F4 vs. F0-F3) | 0.795 | 284.8 | 80 | 71 |
| Tumor-Promoting Mechanism | YKL-40-Associated Effect | Clinical Implication |
|---|---|---|
| Activation of invasive signaling | Activates PI3K/AKT and TGF-β pathways in cancer models | Indicates high-risk tumor phenotype beyond AFP/morphology |
| ECM remodeling | Upregulates MMP-9 expression, promoting ECM degradation | Reflects stromal remodeling undetectable by imaging |
| Loss of epithelial adhesion | Suppresses E-cadherin, facilitating epithelial–mesenchymal transition (EMT) | Correlates with dedifferentiation and early metastatic potential |
| Angiogenesis | Induces endothelial cell migration and tube formation independently of VEGF | May predict microvascular invasion and post-LT recurrence |
| Resistance to bridging therapy | Persistently elevated levels despite radiologic response suggest stromal or residual tumor activity | Supports re-evaluation of treatment response and LT candidacy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavicic Saric, J.; Lulic, D.; Rogic, D.; Jadrijevic, S.; Mikulic, D.; Filipec Kanizaj, T.; Prpic, N.; Bozic, L.K.; Adamovic, I.; Bacak Kocman, I.; et al. YKL-40 in Virus-Associated Liver Disease: A Translational Biomarker Linking Fibrosis, Hepatocarcinogenesis, and Liver Transplantation. Int. J. Mol. Sci. 2025, 26, 9584. https://doi.org/10.3390/ijms26199584
Pavicic Saric J, Lulic D, Rogic D, Jadrijevic S, Mikulic D, Filipec Kanizaj T, Prpic N, Bozic LK, Adamovic I, Bacak Kocman I, et al. YKL-40 in Virus-Associated Liver Disease: A Translational Biomarker Linking Fibrosis, Hepatocarcinogenesis, and Liver Transplantation. International Journal of Molecular Sciences. 2025; 26(19):9584. https://doi.org/10.3390/ijms26199584
Chicago/Turabian StylePavicic Saric, Jadranka, Dinka Lulic, Dunja Rogic, Stipislav Jadrijevic, Danko Mikulic, Tajana Filipec Kanizaj, Nikola Prpic, Laura Karla Bozic, Ivona Adamovic, Iva Bacak Kocman, and et al. 2025. "YKL-40 in Virus-Associated Liver Disease: A Translational Biomarker Linking Fibrosis, Hepatocarcinogenesis, and Liver Transplantation" International Journal of Molecular Sciences 26, no. 19: 9584. https://doi.org/10.3390/ijms26199584
APA StylePavicic Saric, J., Lulic, D., Rogic, D., Jadrijevic, S., Mikulic, D., Filipec Kanizaj, T., Prpic, N., Bozic, L. K., Adamovic, I., Bacak Kocman, I., Sarec, Z., Erceg, G., Adanic, M., Ozegovic Zuljan, P., Jadrijevic, F., & Lulic, I. (2025). YKL-40 in Virus-Associated Liver Disease: A Translational Biomarker Linking Fibrosis, Hepatocarcinogenesis, and Liver Transplantation. International Journal of Molecular Sciences, 26(19), 9584. https://doi.org/10.3390/ijms26199584








