Digestive Neurobiology in Autism: From Enteric and Central Nervous System Interactions to Shared Genetic Pathways
Abstract
1. Introduction
2. Methods
2.1. Data Collection
2.2. Pathway Analysis
3. The Gut-Microbiota-Brain Axis
4. Neuronal Factors of GI Disorders in ASD
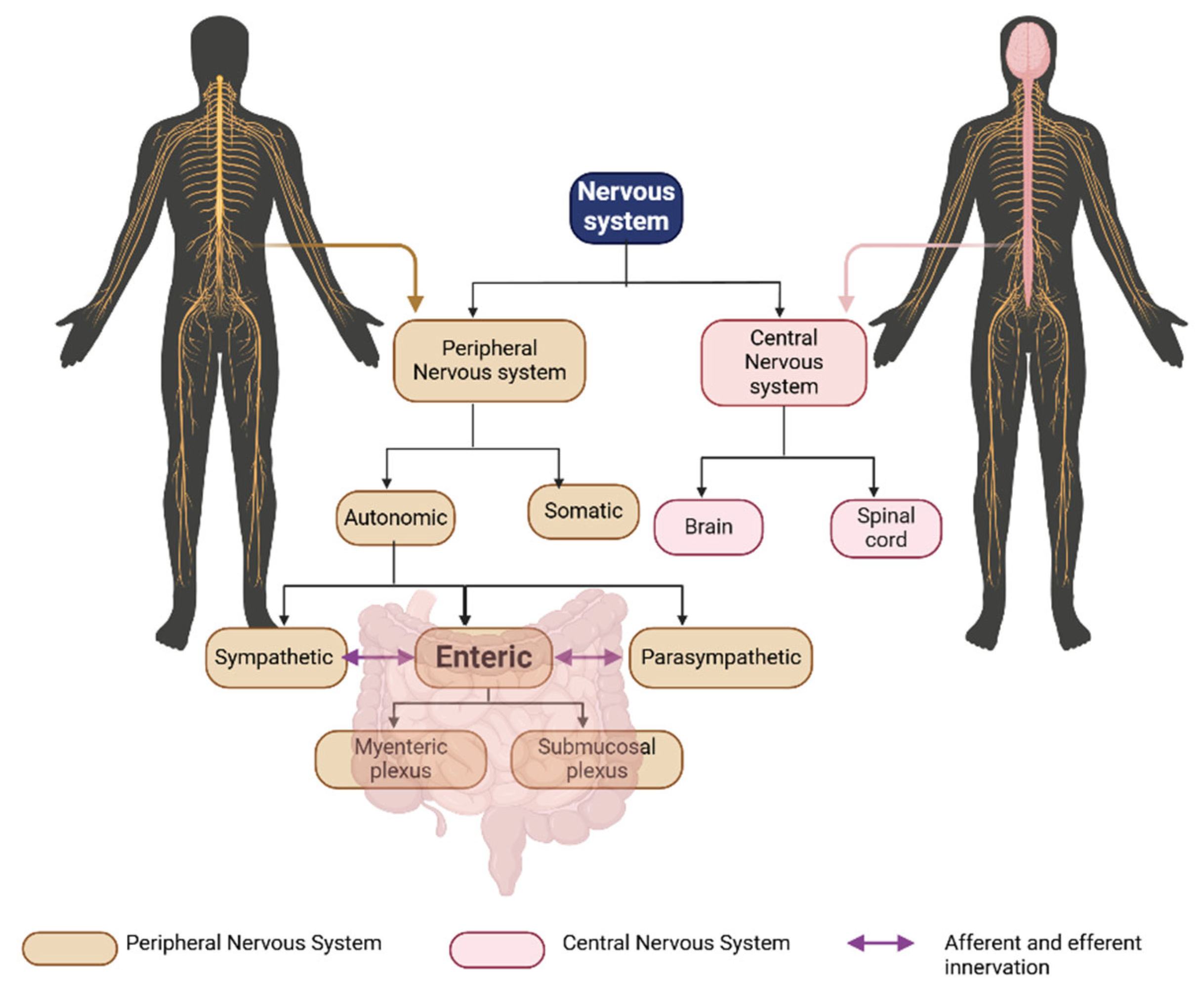
4.1. The Enteric Nervous System (ENS)
4.2. Sympathetic Nervous System Overactivation and Dysbiosis That Is Unrelated to Nutritional Habits
4.3. iPSC-Based Approaches to Study ASD and ENS Dysregulation
5. GI-Related Genetic Factors in ASD
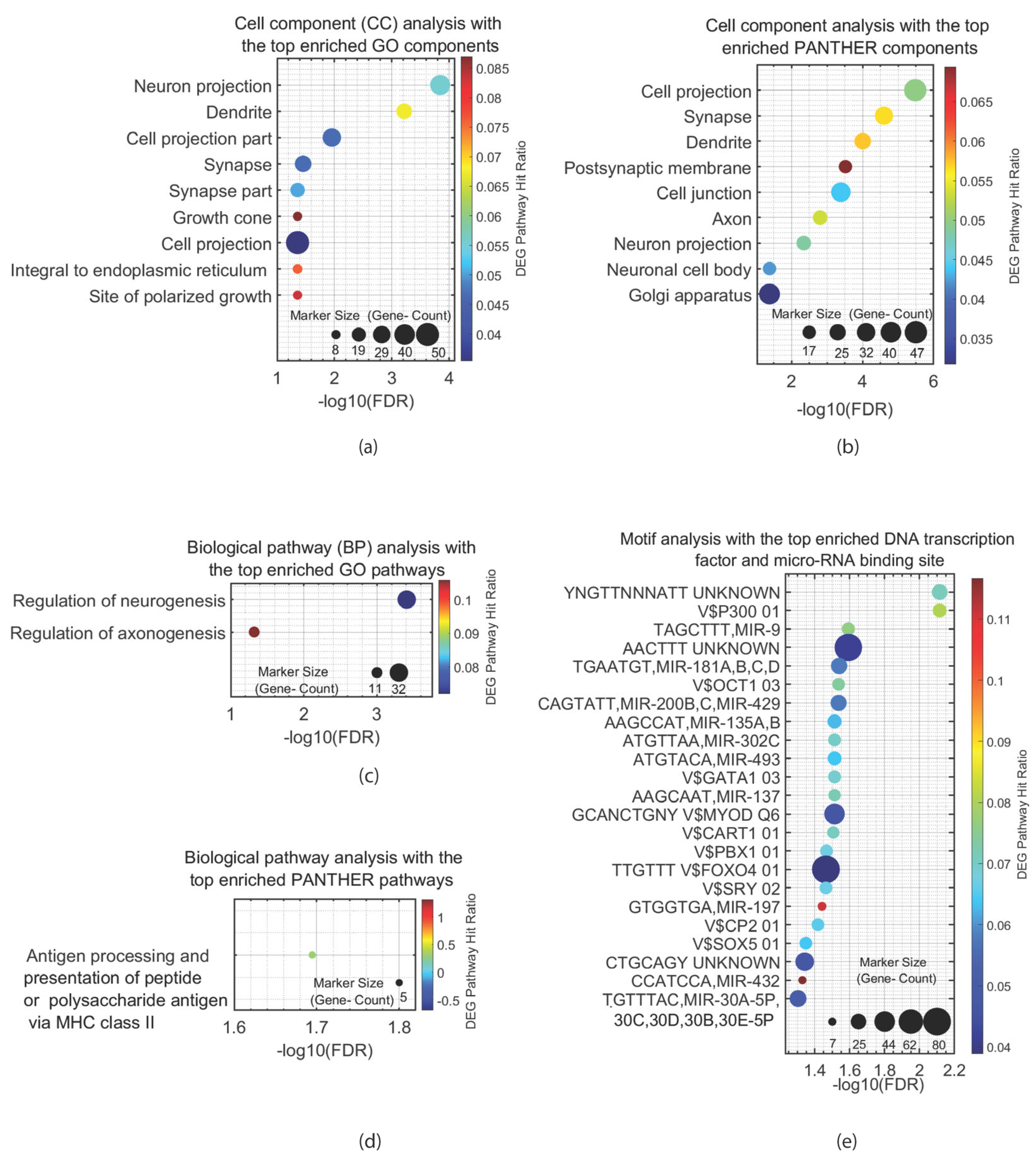
5.1. Pathway and Motif Analysis of ASD-Associated Genes Expressed in the ENS
5.2. Findings from Genetic Animal Models on GI and ENS Alterations in ASD
6. Metabolic and Dietary Factors That Influence GI Disorders in ASD
6.1. The Nutrition of Children with ASD and Its Influence on Gut Microbiota and GI Symptoms
6.2. Altered Metabolome in ASD
7. Immunological Factors of GI Disorders in ASD
7.1. Animal Research Immunological Maternal Models of the Autistic ENS: Strengths and Limitations
7.2. The Contribution of Gut Microbiota to ASD-Associated GI Issues
7.3. Differences in GI Inflammation Between Children with ASD and Control Peers
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hirota, T.; King, B.H. Autism Spectrum Disorder: A Review. JAMA 2023, 329, 157–168. [Google Scholar] [CrossRef]
- Wang, X.; Tang, R.; Wei, Z.; Zhan, Y.; Lu, J.; Li, Z. The enteric nervous system deficits in autism spectrum disorder. Front. Neurosci. 2023, 17, 1101071. [Google Scholar] [CrossRef]
- Rosen, N.E.; Lord, C.; Volkmar, F.R. The Diagnosis of Autism: From Kanner to DSM-III to DSM-5 and Beyond. J. Autism Dev. Disord. 2021, 51, 4253–4270. [Google Scholar] [CrossRef]
- Rutter, M. Genetic Studies of Autism: From the 1970s into the Millennium. J. Abnorm. Child Psychol. 2000, 28, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.S.; Geschwind, D.H. Advances in autism genetics: On the threshold of a new neurobiology. Nat. Rev. Genet. 2008, 9, 341–355, Erratum in: Nat. Rev. Genet. 2008, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Anney, R.; Klei, L.; Pinto, D.; Regan, R.; Conroy, J.; Magalhaes, T.R.; Correia, C.; Abrahams, B.S.; Sykes, N.; Pagnamenta, A.T.; et al. A genome-wide scan for common alleles affecting risk for autism. Hum. Mol. Genet. 2010, 19, 4072–4082. [Google Scholar] [CrossRef]
- Glessner, J.T.; Wang, K.; Cai, G.; Korvatska, O.; Kim, C.E.; Wood, S.; Zhang, H.; Estes, A.; Brune, C.W.; Bradfield, J.P.; et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 2009, 459, 569–572. [Google Scholar] [CrossRef] [PubMed]
- International Molecular Genetic Study of Autism Consortium. A Full Genome Screen for Autism with Evidence for Linkage to a Region on Chromosome 7q. 1998. Available online: http://www.well.ox.ac.uk/ (accessed on 1 March 1998).
- Weiss, L.A.; Arking, D.E.; The Gene Discovery Project of Johns Hopkins & the Autism Consortium. A genome-wide linkage and association scan reveals novel loci for autism. Nature 2009, 461, 802–808. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, H.; Ma, D.; Bucan, M.; Glessner, J.T.; Abrahams, B.S.; Salyakina, D.; Imielinski, M.; Bradfield, J.P.; Sleiman, P.M.A.; et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 2009, 459, 528–533. [Google Scholar] [CrossRef]
- Ma, D.; Salyakina, D.; Jaworski, J.M.; Konidari, I.; Whitehead, P.L.; Andersen, A.N.; Hoffman, J.D.; Slifer, S.H.; Hedges, D.J.; Cukier, H.N.; et al. A genome-wide association study of autism reveals a common novel risk locus at 5p14.1. Ann. Hum. Genet. 2009, 73, 263–273. [Google Scholar] [CrossRef]
- Pinto, D.; Pagnamenta, A.T.; Klei, L.; Anney, R.; Merico, D.; Regan, R.; Conroy, J.; Magalhaes, T.R.; Correia, C.; Abrahams, B.S.; et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 2010, 466, 368–372. [Google Scholar] [CrossRef]
- Romanovsky, E.; Choudhary, A.; Peles, D.; Abu-Akel, A.; Stern, S. Uncovering convergence and divergence between autism and schizophrenia using genomic tools and patients’ neurons. Mol. Psychiatry 2024, 30, 1019–1028. [Google Scholar] [CrossRef]
- Jolanta Wasilewska, J.; Klukowski, M. Gastrointestinal symptoms and autism spectrum disorder: Links and risks—A possible new overlap syndrome. Pediatr. Health Med. Ther. 2015, 6, 153–166. [Google Scholar] [CrossRef]
- Al-Beltagi, M.; Saeed, N.K.; Bediwy, A.S.; Elbeltagi, R.; Alhawamdeh, R. Role of gastrointestinal health in managing children with autism spectrum disorder. World J. Clin. Pediatr. 2023, 12, 171–196. [Google Scholar] [CrossRef]
- Frye, R.E. Metabolic and mitochondrial disorders associated with epilepsy in children with autism spectrum disorder. Epilepsy Behav. 2015, 47, 147–157. [Google Scholar] [CrossRef]
- Grochowska, M.; Wojnar, M.; Radkowski, M. The gut microbiota in neuropsychiatric disorders. Acta Neurobiol. Exp. 2018, 78, 69–81. [Google Scholar] [CrossRef]
- Xu, G.; Snetselaar, L.G.; Jing, J.; Liu, B.; Strathearn, L.; Bao, W. Association of Food Allergy and Other Allergic Conditions with Autism Spectrum Disorder in Children. JAMA Netw. Open 2018, 1, e180279. [Google Scholar] [CrossRef] [PubMed]
- Buie, T.; Campbell, D.B.; Fuchs, G.J.; Furuta, G.T.; Levy, J.; Van De Water, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics 2010, 125, S1–S18. [Google Scholar] [CrossRef]
- Maenner, M.J.; Arneson, C.L.; Levy, S.E.; Kirby, R.S.; Nicholas, J.S.; Durkin, M.S. Brief report: Association between behavioral features and gastrointestinal problems among children with autism spectrum disorder. J. Autism Dev. Disord. 2012, 42, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal problems in children with autism, developmental delays or typical development. J. Autism Dev. Disord. 2014, 44, 1117–1127. [Google Scholar] [CrossRef]
- Nikolov, R.N.; Bearss, K.E.; Lettinga, J.; Erickson, C.; Rodowski, M.; Aman, M.G.; McCracken, J.T.; McDougle, C.J.; Tierney, E.; Vitiello, B.; et al. Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J. Autism Dev. Disord. 2009, 39, 405–413. [Google Scholar] [CrossRef]
- Alharthi, A.; Alhazmi, S.; Alburae, N.; Bahieldin, A. The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 1363. [Google Scholar] [CrossRef]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota–Gut–Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef] [PubMed]
- Taniya, M.A.; Chung, H.J.; Al Mamun, A.; Alam, S.; Aziz, M.A.; Emon, N.U.; Islam, M.; Hong, S.-T.S.; Podder, B.R.; Mimi, A.A.; et al. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 915701. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Lai, X.; Li, C.; Ding, D.; Wang, Y.; Zhu, Y. The Role of Gut Microbiota in Various Neurological and Psychiatric Disorders—An Evidence Mapping Based on Quantified Evidence. Mediat. Inflamm. 2023, 2023, 5127157. [Google Scholar] [CrossRef]
- Van Der Kleij, H.; O’mahony, C.; Shanahan, F.; O’mahony, L.; Bienenstock, J. Protective effects of Lactobacillus reuteri and Bifidobacterium infantis in murine models for colitis do not involve the vagus nerve. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Houlden, A.; Goldrick, M.; Brough, D.; Vizi, E.S.; Lénárt, N.; Martinecz, B.; Roberts, I.S.; Denes, A. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav. Immun. 2016, 57, 10–20. [Google Scholar] [CrossRef]
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut Microbe to Brain Signaling: What Happens in Vagus. Neuron 2019, 101, 998–1002. [Google Scholar] [CrossRef]
- Erny, D.; De Angelis, A.L.H.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Powell, N.; Walker, M.M.; Talley, N.J. The mucosal immune system: Master regulator of bidirectional gut-brain communications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 143–159. [Google Scholar] [CrossRef]
- Gutierrez, E.G.; Banks, W.A.; Kastin, A.J. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J. Neuroimmunol. 1993, 47, 169–176. [Google Scholar] [CrossRef]
- Garbett, K.; Ebert, P.J.; Mitchell, A.; Lintas, C.; Manzi, B.; Mirnics, K.; Persico, A.M. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol. Dis. 2008, 30, 303–311. [Google Scholar] [CrossRef]
- Morgan, J.T.; Chana, G.; Pardo, C.A.; Achim, C.; Semendeferi, K.; Buckwalter, J.; Courchesne, E.; Everall, I.P. Microglial Activation and Increased Microglial Density Observed in the Dorsolateral Prefrontal Cortex in Autism. Biol. Psychiatry 2010, 68, 368–376. [Google Scholar] [CrossRef]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.-C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M. Microbial Endocrinology in the Microbiome-Gut-Brain Axis: How Bacterial Production and Utilization of Neurochemicals Influence Behavior. PLoS Pathog. 2013, 9, e1003726. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, H.; Feng, Y.; Guo, R.; Wan, D. The impact of gut microbiota disorders on the blood–brain barrier. Infect. Drug Resist. 2020, 13, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; Jenkins, D.J.A. Carbohydrate Digestibility and Metabolic Effects. J. Nutr. 2007, 137, 2539S–2546S. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.H. Basics of autonomic nervous system function. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 160, pp. 407–418. [Google Scholar]
- Sasselli, V.; Pachnis, V.; Burns, A.J. The enteric nervous system. Dev. Biol. 2012, 366, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, J.D.; Martz, S.; Gil, V.; Wang, X.Y.; Jimenez, M.; Parsons, S. Two independent networks of interstitial cells of Cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front. Neurosci. 2011, 5, 93. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Sanders, K.M.; Koh, S.D.; Ward, S.M. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu. Rev. Physiol. 2006, 68, 307–343. [Google Scholar] [CrossRef]
- Bertrand, P.P.; Kunze, W.A.A.; Bornstein, J.C.; Furness, J.B.; Smith, M.L. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am. J. Physiol. 1997, 273, G422–G435. [Google Scholar] [CrossRef]
- Izumi, N.; Matsuyama, H.; Ko, M.; Shimizu, Y.; Takewaki, T. Role of intrinsic nitrergic neurones on vagally mediated striated muscle contractions in the hamster oesophagus. J. Physiol. 2003, 551, 287–294. [Google Scholar] [CrossRef]
- de Groat, W.C.; Nadelhaft, I.; Milne, R.J.; Booth, A.M.; Morgan, C.; Thor, K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J. Auton. Nerv. Syst. 1981, 3, 135–160. [Google Scholar] [CrossRef]
- Coury, D.L.; Ashwood, P.; Fasano, A.; Fuchs, G.; Geraghty, M.; Kaul, A.; Mawe, G.; Patterson, P.; Jones, N.E. Gastrointestinal conditions in children with autism spectrum disorder: Developing a research agenda. Pediatrics 2012, 130, S160–S168. [Google Scholar] [CrossRef] [PubMed]
- Horvath, K.; Perman, J.A. Autistic disorder and gastrointestinal disease. Curr. Opin. Pediatr. 2002, 14, 583–587. [Google Scholar] [CrossRef]
- Kong, X.; Liu, J.; Liu, K.; Koh, M.; Tian, R.; Hobbie, C.; Fong, M.; Chen, Q.; Zhao, M.; Budjan, C.; et al. Altered Autonomic Functions and Gut Microbiome in Individuals with Autism Spectrum Disorder (ASD): Implications for Assisting ASD Screening and Diagnosis. J. Autism Dev. Disord. 2020, 51, 144–157. [Google Scholar] [CrossRef]
- Wang, Y.; Hensley, M.K.; Tasman, A.; Sears, L.; Casanova, M.F.; Sokhadze, E.M. Heart Rate Variability and Skin Conductance During Repetitive TMS Course in Children with Autism. Appl. Psychophysiol. Biofeedback 2016, 41, 47–60. [Google Scholar] [CrossRef]
- de Vries, L.; Fouquaet, I.; Boets, B.; Naulaers, G.; Steyaert, J. Autism spectrum disorder and pupillometry: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 120, 479–508. [Google Scholar] [CrossRef]
- Ming, X.; Patel, R.; Kang, V.; Chokroverty, S.; Julu, P.O. Respiratory and autonomic dysfunction in children with autism spectrum disorders. Brain Dev. 2016, 38, 225–232. [Google Scholar] [CrossRef]
- Heneghan, A.F.; Pierre, J.F.; Tandee, K.; Shanmuganayagam, D.; Wang, X.; Reed, J.D.; Steele, J.L.; Kudsk, K.A. Parenteral nutrition decreases paneth cell function and intestinal bactericidal activity while increasing susceptibility to bacterial enteroinvasion. J. Parenter. Enter. Nutr. 2014, 38, 817–824. [Google Scholar] [CrossRef]
- Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Busch, R.A.; Heneghan, A.F.; Pierre, J.F.; Wang, X.; Kudsk, K.A. The enteric nervous system neuropeptide, bombesin, reverses innate immune impairments during parenteral nutrition. Ann. Surg. 2014, 260, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Induced pluripotent stem cells: Past, present, and future. Cell Stem Cell 2012, 10, 678–684. [Google Scholar] [CrossRef]
- Saade, M.; Rike, W.A.; Sharma, O.; Abu-Akel, A.; Stern, S. Prader-Willi syndrome: Genetics, clinical symptoms, and model systems. Genom. Psychiatry 2025, 1–21. [Google Scholar] [CrossRef]
- Nayak, R.; Sharma, O.; Mizrahi, L.; Shemen, A.; Tripathi, U.; Hussein, Y.; Amelo Rike, W.; Rosh, I.; Radzishevsky, I.; Mandel, H.; et al. Dysregulation of Multiple Solute Carrier genes and Metabolic Deficits in SLC1A4-Mutant Human iPSC-Derived Hippocampal Neurons. bioRxiv 2025. [Google Scholar] [CrossRef]
- Tumdam, R.; Hussein, Y.; Garin-Shkolnik, T.; Stern, S. NMDA Receptors in Neurodevelopmental Disorders: Pathophysiology and Disease Models. Int. J. Mol. Sci. 2024, 25, 12366. [Google Scholar] [CrossRef]
- Fischer, I.; Shohat, S.; Leichtmann-Bardoogo, Y.; Nayak, R.; Wiener, G.; Rosh, I.; Shemen, A.; Tripathi, U.; Rokach, M.; Bar, E.; et al. Shank3 mutation impairs glutamate signaling and myelination in ASD mouse model and human iPSC-derived OPCs. Sci. Adv. 2024, 10, eadl4573. [Google Scholar] [CrossRef]
- Schafer, S.T.; Paquola, A.C.M.; Stern, S.; Gosselin, D.; Ku, M.; Pena, M.; Kuret, T.J.M.; Liyanage, M.; Mansour, A.A.; Jaeger, B.N.; et al. Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons. Nat. Neurosci. 2019, 22, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Rosh, I.; Rabinski, T.; Falik, D.; Mendel Percia, M.; Stern, S. Generation and characterization of iPSC lines (UOHi003-A, UOHi002-A) from a patient with SHANK3 mutation and her healthy mother. Stem Cell Res. 2022, 64, 102899. [Google Scholar] [CrossRef]
- Hussein, Y.; Tripathi, U.; Choudhary, A.; Nayak, R.; Peles, D.; Rosh, I.; Rabinski, T.; Djamus, J.; Vatine, G.D.; Spiegel, R.; et al. Early maturation and hyperexcitability is a shared phenotype of cortical neurons derived from different ASD-associated mutations. Transl. Psychiatry 2023, 13, 246. [Google Scholar] [CrossRef]
- Quraishi, I.H.; Stern, S.; Mangan, K.P.; Zhang, Y.; Ali, S.R.; Mercier, M.R.; Marchetto, M.C.; McLachlan, M.J.; Jones, E.M.; Gage, F.H.; et al. An epilepsy-associated KCNT1 mutation enhances excitability of human iPSC-derived neurons by increasing slack KNa currents. J. Neurosci. 2019, 39, 7438–7449. [Google Scholar] [CrossRef]
- Brant, B.; Stern, T.; Shekhidem, H.A.; Mizrahi, L.; Rosh, I.; Stern, Y.; Ofer, P.; Asleh, A.; Umanah, G.K.E.; Jada, R.; et al. IQSEC2 mutation associated with epilepsy, intellectual disability, and autism results in hyperexcitability of patient-derived neurons and deficient synaptic transmission. Mol. Psychiatry 2021, 26, 7498–7508. [Google Scholar] [CrossRef]
- Bonaglia, M.C.; Giorda, R.; Mani, E.; Aceti, G.; Anderlid, B.M.; Baroncini, A.; Pramparo, T.; Zuffardi, O. Identification of a recurrent breakpoint within the SHANK3 gene in the 22q13.3 deletion syndrome. J. Med. Genet. 2006, 43, 822–828. [Google Scholar] [CrossRef]
- Pfaender, S.; Sauer, A.K.; Hagmeyer, S.; Mangus, K.; Linta, L.; Liebau, S.; Bockmann, J.; Huguet, G.; Bourgeron, T.; Boeckers, T.M.; et al. Zinc deficiency and low enterocyte zinc transporter expression in human patients with autism related mutations in SHANK3. Sci. Rep. 2017, 7, 45190. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, D. Modeling the Enteric Nervous System in Autism Spectrum Disorder Using Patient Specific Ipsc-Derived Innervated Gastrointestinal Organoids. Master’s Thesis, Wake Forest University, Winston-Salem, NC, USA, 2019. [Google Scholar]
- Casanova, M.F.; Casanova, E.L.; Frye, R.E.; Baeza-Velasco, C.; LaSalle, J.M.; Hagerman, R.J.; Scherer, S.W.; Natowicz, M.R. Editorial: Secondary vs. Idiopathic Autism. Front. Psychiatry 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Li, A.; Liu, H.; Zhang, W.; Cui, X.; Wang, K. Expression of neurexin and neuroligin in the enteric nervous system and their down-regulated expression levels in Hirschsprung disease. Mol. Biol. Rep. 2013, 40, 2969–2975. [Google Scholar] [CrossRef]
- Niesler, B.; Rappold, G.A. Emerging evidence for gene mutations driving both brain and gut dysfunction in autism spectrum disorder. Mol. Psychiatry 2021, 26, 1442–1444. [Google Scholar] [CrossRef]
- Lefter, R.; Ciobica, A.; Timofte, D.; Stanciu, C.; Trifan, A. A descriptive review on the prevalence of gastrointestinal disturbances and their multiple associations in autism spectrum disorder. Medicina 2020, 56, 11. [Google Scholar] [CrossRef]
- Hayot, G.; Massonot, M.; Keime, C.; Faure, E.; Golzio, C. Loss of autism-candidate CHD8 perturbs neural crest development and intestinal homeostatic balance. Life Sci. Alliance 2023, 6, e202201456. [Google Scholar] [CrossRef]
- Wei, S.C.; Yang-Yen, H.F.; Tsao, P.N.; Weng, M.T.; Tung, C.C.; Yu, L.C.H.; Lai, L.-C.; Hsiao, J.-H.; Chuang, E.Y.; Shun, C.-T.; et al. SHANK3 Regulates Intestinal Barrier Function Through Modulating ZO-1 Expression Through the PKCϵ-dependent Pathway. Inflamm. Bowel Dis. 2017, 23, 1730–1740. [Google Scholar] [CrossRef]
- Lewis, J.D.; Meehan, R.R.; Henzel, W.J.; Maurer-Fogy, I.; Jeppesen, P.; Klein, F.; Bird, A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to Methylated DNA. Cell 1992, 69, 905–914. [Google Scholar] [CrossRef]
- Amir, R.E.; Van Den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- The Rett Syndrome Diagnostic Criteria Work Group. Diagnostic criteria for rett syndrome. Ann. Neurol. 1988, 23, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vaart, M.; Svoboda, O.; Weijts, B.G.; Espín-Palazón, R.; Sapp, V.; Pietri, T.; Bagnat, M.; Muotri, A.R.; Traver, D. Mecp2 regulates tnfa during zebrafish embryonic development and acute inflammation. DMM Dis. Models Mech. 2017, 10, 1439–1451. [Google Scholar]
- Valor, L.M.; Viosca, J.; Lopez-Atalaya, J.P.; Barco, A. Lysine Acetyltransferases CBP and p300 as Therapeutic Targets in Cognitive and Neurodegenerative Disorders. Curr. Pharm. Des. 2013, 19, 5051–5064. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, H.; Kollmeyer, M.L.; Linz, V.C.; Stuhlinger, M.; Groneberg, D.; Reigl, A.; Zizer, E.; Friebe, A.; Niesler, B.; Rappold, G. Gastrointestinal dysfunction in autism displayed by altered motility and Achalasia in Foxp1+/− mice. Proc. Natl. Acad. Sci. USA 2019, 116, 22237–22245. [Google Scholar] [CrossRef]
- Pasterkamp, R.J. Getting neural circuits into shape with semaphorins. Nat. Rev. Neurosci. 2012, 13, 605–618. [Google Scholar] [CrossRef]
- Melin, M.; Carlsson, B.; Anckarsater, H.; Rastam, M.; Betancur, C.; Isaksson, A.; Gillberg, C.; Dahl, N. Constitutional downregulation of SEMA5A expression in autism. Neuropsychobiology 2006, 54, 64–69. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, S.-H.; Song, J.; Mironova, Y.; Ming, G.; Kolodkin, A.L.; Gillberg, C.; Dahl, N. Semaphorin 5A inhibits synaptogenesis in early postnatal- and adult-born hippocampal dentate granule cells. elife 2014, 3, e04390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Le Dréan, M.E.; Le Berre-Scoul, C.; Paillé, V.; Caillaud, M.; Oullier, T.; Gonzales, J.; Hulin, P.; Neunlist, M.; Talon, S.; Boudin, H. The regulation of enteric neuron connectivity by semaphorin 5A is affected by the autism-associated S956G missense mutation. iScience 2024, 27, 109638. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, M.; Hammer, R.E.; Südhof, T.C. A Phospho-Switch Controls the Dynamic Association of Synapsins with Synaptic Vesicles. Neuron 1999, 24, 377–387. [Google Scholar] [CrossRef]
- Van Bon, B.W.M.; Coe, B.P.; Bernier, R.; Green, C.; Gerdts, J.; Witherspoon, K.; Kleefstra, T.; Willemsen, M.H.; Kumar, R.; Bosco, P.; et al. Disruptive de novo mutations of DYRK1A lead to a syndromic form of autism and ID. Mol. Psychiatry 2016, 21, 126–132. [Google Scholar] [CrossRef]
- McCluskey, K.E.; Stovell, K.M.; Law, K.; Kostyanovskaya, E.; Schmidt, J.D.; Exner, C.R.T.; Dea, J.; Brimble, E.; State, M.W.; Willsey, A.J.; et al. Autism gene variants disrupt enteric neuron migration and cause gastrointestinal dysmotility. Nat. Commun. 2025, 16, 2238. [Google Scholar] [CrossRef]
- Wiltrout, K.; Brimble, E.; Poduri, A. Comprehensive phenotypes of patients with SYNGAP1-related disorder reveals high rates of epilepsy and autism. Epilepsia 2024, 65, 1428–1438. [Google Scholar] [CrossRef]
- Ivashko-Pachima, Y.; Ganaiem, M.; Ben-Horin-Hazak, I.; Lobyntseva, A.; Bellaiche, N.; Fischer, I.; Levy, G.; Sragovich, S.; Karmon, G.; Giladi, E.; et al. SH3- and actin-binding domains connect ADNP and SHANK3, revealing a fundamental shared mechanism underlying autism. Mol. Psychiatry 2022, 27, 3316–3327. [Google Scholar] [CrossRef]
- Eberly, G.L.; Manthey, M.; Pang, K.K.L.; Hussein, H.; Vargas Paniagua, E.; Machen, S.; Klingensmith, S.M.; Anikeeva, P. Shank3 mutation manifests in abnormal gastrointestinal morphology and function in mice. Front. Neurosci. 2025, 19, 1552369. [Google Scholar] [CrossRef]
- James, D.M.; Kozol, R.A.; Kajiwara, Y.; Wahl, A.L.; Storrs, E.C.; Buxbaum, J.D.; Klein, M.; Moshiree, B.; Dallman, J.E. Intestinal dysmotility in a zebrafish (Danio rerio) shank3a;shank3b mutant model of autism. Mol. Autism 2019, 10, 3. [Google Scholar] [CrossRef]
- Robinson, B.G.; Oster, B.A.; Robertson, K.; Kaltschmidt, J.A. Loss of ASD-related molecule Cntnap2 affects colonic motility in mice. Front. Neurosci. 2023, 17, 1287057. [Google Scholar] [CrossRef]
- Leembruggen, A.J.L.; Balasuriya, G.K.; Zhang, J.; Schokman, S.; Swiderski, K.; Bornstein, J.C.; Nithianantharajah, J.; Hill-Yardin, E.L. Colonic dilation and altered ex vivo gastrointestinal motility in the neuroligin-3 knockout mouse. Autism Res. 2020, 13, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Hosie, S.; Ellis, M.; Swaminathan, M.; Ramalhosa, F.; Seger, G.O.; Balasuriya, G.K.; Gillberg, C.; Råstam, M.; Churilov, L.; McKeown, S.J.; et al. Gastrointestinal dysfunction in patients and mice expressing the autism-associated R451C mutation in neuroligin-3. Autism Res. 2019, 12, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Karmon, G.; Sragovich, S.; Hacohen-Kleiman, G.; Ben-Horin-Hazak, I.; Kasparek, P.; Schuster, B.; Sedlacek, R.; Pasmanik-Chor, M.; Theotokis, P.; Touloumi, O.; et al. Novel ADNP Syndrome Mice Reveal Dramatic Sex-Specific Peripheral Gene Expression With Brain Synaptic and Tau Pathologies. Biol. Psychiatry 2022, 92, 81–95. [Google Scholar] [CrossRef]
- Provenzano, G.; Zunino, G.; Genovesi, S.; Sgadó, P.; Bozzi, Y. Mutant mouse models of autism spectrum disorders. Dis. Markers 2012, 33, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, J.W. In Search of an Animal Model of Autism Spectrum Disorders. Open Access J. Neurol. Neurosurg. 2020, 13, 555863. [Google Scholar] [CrossRef]
- Platzer, K.; Yuan, H.; Schütz, H.; Winschel, A.; Chen, W.; Hu, C.; Kusumoto, H.; O Heyne, H.; Helbig, K.L.; Tang, S.; et al. GRIN2B encephalopathy: Novel findings on phenotype, variant clustering, functional consequences and treatment aspects. J. Med. Genet. 2017, 54, 460–470. [Google Scholar] [CrossRef]
- Joseph, L.; Thurm, A.; Farmer, C.; Shumway, S. Repetitive behavior and restricted interests in young children with autism: Comparisons with controls and stability over 2 years. Autism Res. 2013, 6, 584–595. [Google Scholar] [CrossRef]
- Cherif, L.; Boudabous, J.; Khemekhem, K.; Mkawer, S.; Ayadi, H.; Moalla, Y. Feeding Problems in Children with Autism Spectrum Disorders. J. Fam. Med. 2018, 1, 30–39. [Google Scholar] [CrossRef]
- Prosperi, M.; Santocchi, E.; Balboni, G.; Narzisi, A.; Bozza, M.; Fulceri, F.; Apicella, F.; Igliozzi, R.; Cosenza, A.; Tancredi, R.; et al. Behavioral Phenotype of ASD Preschoolers with Gastrointestinal Symptoms or Food Selectivity. J. Autism Dev. Disord. 2017, 47, 3574–3588. [Google Scholar] [CrossRef]
- Babinska, K.; Celusakova, H.; Belica, I.; Szapuova, Z.; Waczulikova, I.; Nemcsicsova, D.; Tomova, A.; Ostatnikova, D. Gastrointestinal symptoms and feeding problems and their associations with dietary interventions, food supplement use, and behavioral characteristics in a sample of children and adolescents with autism spectrum disorders. Int. J. Environ. Res. Public Health 2020, 17, 6372. [Google Scholar] [CrossRef] [PubMed]
- Huke, V.; Turk, J.; Saeidi, S.; Kent, A.; Morgan, J.F. Autism spectrum disorders in eating disorder populations: A systematic review. Eur. Eat. Disord. Rev. 2013, 21, 345–351. [Google Scholar] [CrossRef]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food Selectivity and Sensory Sensitivity in Children with Autism Spectrum Disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Zamora, A.F.; Ramírez-Valenzuela, D.G.; Ramos-Jiménez, A. Food Selectivity and Its Implications Associated with Gastrointestinal Disorders in Children with Autism Spectrum Disorders. Nutrients 2022, 14, 2660. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, A.; Hen, L.; Fluss, R.; Cermak, S.A.; Engel-Yeger, B.; Gal, E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 1–11. [Google Scholar] [CrossRef]
- Dellapiazza, F.; Michelon, C.; Vernhet, C.; Muratori, F.; Blanc, N.; Picot, M.C.; Baghdadli, A. Sensory processing related to attention in children with ASD, ADHD, or typical development: Results from the ELENA cohort. Eur. Child Adolesc. Psychiatry 2021, 30, 283–291. [Google Scholar] [CrossRef]
- Dellapiazza, F.; Michelon, C.; Oreve, M.J.; Robel, L.; Schoenberger, M.; Chatel, C.; Vesperini, S.; Maffre, T.; Schmidt, R.; Blanc, N.; et al. The Impact of Atypical Sensory Processing on Adaptive Functioning and Maladaptive Behaviors in Autism Spectrum Disorder During Childhood: Results From the ELENA Cohort. J. Autism Dev. Disord. 2020, 50, 2142–2152. [Google Scholar] [CrossRef]
- Thye, M.D.; Bednarz, H.M.; Herringshaw, A.J.; Sartin, E.B.; Kana, R.K. The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev. Cogn. Neurosci. 2018, 29, 151–167. [Google Scholar] [CrossRef]
- Page, S.D.; Souders, M.C.; Kral, T.V.E.; Chao, A.M.; Pinto-Martin, J. Correlates of Feeding Difficulties Among Children with Autism Spectrum Disorder: A Systematic Review. J. Autism Dev. Disord. 2022, 52, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Martins, Y.; Young, R.L.; Robson, D.C. Feeding and eating behaviors in children with autism and typically developing children. J. Autism Dev. Disord. 2008, 38, 1878–1887. [Google Scholar] [CrossRef]
- Holingue, C.; Newill, C.; Lee, L.C.; Pasricha, P.J.; Daniele Fallin, M. Gastrointestinal symptoms in autism spectrum disorder: A review of the literature on ascertainment and prevalence. Autism Res. 2018, 11, 24–36. [Google Scholar] [CrossRef]
- Leader, G.; Forde, J.; Naughton, K.; Maher, L.; Arndt, S.; Mannion, A. Relationships among gastrointestinal symptoms, sleep problems, challenging behaviour, comorbid psychopathology and autism spectrum disorder symptoms in children and adolescents with 15q duplication syndrome. J. Intellect. Disabil. Res. 2021, 65, 32–46. [Google Scholar] [CrossRef]
- Vela, G.; Stark, P.; Socha, M.; Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Zinc in gut-brain interaction in autism and neurological disorders. Neural Plast. 2015, 2015, 972791. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.K.; Neil, J.R.; Simmons, S.D.; Mansab, F.; Benjamin, S.; Pitfield, V.; Boulet, S.; Miyan, J.A. Zinc Deficiency in Autism: A Controlled Study. Insights Biomed 2019, 4, 12. [Google Scholar]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef]
- Zhou, W.; Li, S. Decreased levels of serum retinoic acid in chinese children with autism spectrum disorder. Psychiatry Res. 2018, 269, 469–473. [Google Scholar] [CrossRef]
- Cheng, B.; Zhu, J.; Yang, T.; Guo, M.; Lai, X.; Li, Q.; Chen, J.; Li, T. Vitamin A deficiency increases the risk of gastrointestinal comorbidity and exacerbates core symptoms in children with autism spectrum disorder. Pediatr. Res. 2021, 89, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Zhu, J.; Yang, T.; Wang, S.; Liu, H.; Wu, Q.; Zhang, X.; Chen, J.; Li, T. Vitamin A deficiency exacerbates autism-like behaviors and abnormalities of the enteric nervous system in a valproic acid-induced rat model of autism. NeuroToxicology 2020, 79, 184–190. [Google Scholar] [CrossRef]
- Horvath, K.; Papadimitriou, J.C.; Rabsztyn, A.; Drachenberg, C.; Tyson Tildon, J. Gastrointestinal abnormalities in children with autistic disorder. J. Pediatr. 1999, 135, 559–563. [Google Scholar] [CrossRef]
- Williams, B.L.; Hornig, M.; Buie, T.; Bauman, M.L.; Cho Paik, M.; Wick, I.; Bennett, A.; Jabado, O.; Hirschberg, D.L.; Lipkin, W.I. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS ONE 2011, 6, e24585. [Google Scholar] [CrossRef]
- Likhitweerawong, N.; Thonusin, C.; Boonchooduang, N.; Louthrenoo, O.; Nookaew, I.; Chattipakorn, N.; Chattipakorn, S.C. Profiles of urine and blood metabolomics in autism spectrum disorders. Metab. Brain Dis. 2021, 36, 1641–1671. [Google Scholar] [CrossRef]
- Novarino, G.; El-Fishawy, P.; Kayserili, H.; Meguid, N.A.; Scott, E.M.; Schroth, J.; Silhavy, J.L.; Kara, M.; Khalil, R.O.; Ben-Omran, T.; et al. Mutations in BCKD-kinase Lead to a Potentially Treatable Form of Autism with Epilepsy. Science 2021, 338, 394–397. [Google Scholar] [CrossRef]
- Liang, Y.; Ke, X.; Xiao, Z.; Zhang, Y.; Chen, Y.; Li, Y.; Wang, Z.; Lin, L.; Yao, P.; Lu, J. Untargeted Metabolomic Profiling Using UHPLC-QTOF/MS Reveals Metabolic Alterations Associated with Autism. BioMed Res. Int. 2020, 2020, 6105608. [Google Scholar] [CrossRef] [PubMed]
- Yui, K.; Tanuma, N.; Yamada, H.; Kawasaki, Y. Decreased total antioxidant capacity has a larger effect size than increased oxidant levels in urine in individuals with autism spectrum disorder. Environ. Sci. Pollut. Res. 2017, 24, 9635–9644. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Stein, T.P.; Barnes, V.; Rhodes, N.; Guo, L. Metabolic perturbance in autism spectrum disorders: A metabolomics study. J. Proteome Res. 2012, 11, 5856–5862. [Google Scholar] [CrossRef]
- Liu, A.; Zhou, W.; Qu, L.; He, F.; Wang, H.; Wang, Y.; Cai, C.; Li, X.; Zhou, W.; Wang, M. Altered urinary amino acids in children with autism spectrum disorders. Front. Cell Neurosci. 2019, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Olesova, D.; Galba, J.; Piestansky, J.; Celusakova, H.; Repiska, G.; Babinska, K.; Ostatnikova, D.; Katina, S.; Kovac, A. A novel uhplc-ms method targeting urinary metabolomic markers for autism spectrum disorder. Metabolites 2020, 10, 443. [Google Scholar] [CrossRef]
- Delwing, D.; Delwing, D.; Bavaresco, C.S.; Wyse, A.T.S. Protective effect of nitric oxide synthase inhibition or antioxidants on brain oxidative damage caused by intracerebroventricular arginine administration. Brain Res. 2008, 1193, 120–127. [Google Scholar] [CrossRef]
- Diémé, B.; Mavel, S.; Blasco, H.; Tripi, G.; Bonnet-Brilhault, F.; Malvy, J.; Bocca, C.; Andres, C.R.; Nadal-Desbarats, L.; Emond, P. Metabolomics Study of Urine in Autism Spectrum Disorders Using a Multiplatform Analytical Methodology. J. Proteome Res. 2015, 14, 5273–5282. [Google Scholar] [CrossRef]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef]
- Meguid, N.A.; Dardir, A.A.; Abdel-Raouf, E.R.; Hashish, A. Evaluation of oxidative stress in autism: Defective antioxidant enzymes and increased lipid peroxidation. Biol. Trace Elem. Res. 2011, 143, 58–65. [Google Scholar] [CrossRef]
- Devnani, P.A.; Hegde, A.U. Autism and sleep disorders. J. Pediatr. Neurosci. 2015, 10, 304–307. [Google Scholar] [CrossRef]
- Vernia, F.; Di Ruscio, M.; Ciccone, A.; Viscido, A.; Frieri, G.; Stefanelli, G.; Latella, G. Sleep disorders related to nutrition and digestive diseases: A neglected clinical condition. Int. J. Med. Sci. 2021, 18, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Mazahery, H.; Stonehouse, W.; Delshad, M.; Kruger, M.C.; Conlon, C.A.; Beck, K.L.; Von Hurst, P.R. Relationship between long chain n-3 polyunsaturated fatty acids and autism spectrum disorder: Systematic review and meta-analysis of case-control and randomised controlled trials. Nutrients 2017, 2, 155. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, S.; Wang, M.; Gao, J.; Sun, C.; Wang, J.; Xia, W.; Wu, S.; Sumner, S.J.; Zhang, F.; et al. Potential serum biomarkers from a metabolomics study of autism. J. Psychiatry Neurosci. 2016, 41, 27–37. [Google Scholar] [CrossRef]
- Anwar, A.; Abruzzo, P.M.; Pasha, S.; Rajpoot, K.; Bolotta, A.; Ghezzo, A.; Marini, M.; Posar, A.; Visconti, P.; Thornalley, P.J.; et al. Advanced glycation endproducts, dityrosine and arginine transporter dysfunction in autism—A source of biomarkers for clinical diagnosis. Mol. Autism 2018, 9, 3. [Google Scholar] [CrossRef]
- Bae, Y.S.; Choi, M.K.; Lee, W.J. Dual oxidase in mucosal immunity and host–microbe homeostasis. Trends Immunol. 2010, 31, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; Mcallister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Knuesel, I.; Chicha, L.; Britschgi, M.; Schobel, S.A.; Bodmer, M.; Hellings, J.A.; Toovey, S.; Prinssen, E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014, 10, 643–660. [Google Scholar] [CrossRef]
- Canetta, S.; Bolkan, S.; Padilla-Coreano, N.; Song, L.J.; Sahn, R.; Harrison, N.L.; Gordon, J.A.; Brown, A.; Kellendonk, C. Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol. Psychiatry 2016, 21, 956–968. [Google Scholar] [CrossRef]
- Meyer, U. Prenatal Poly(I:C) Exposure and Other Developmental Immune Activation Models in Rodent Systems. Biol. Psychiatry 2014, 75, 307–315. [Google Scholar] [CrossRef]
- Schwartzer, J.J.; Careaga, M.; Onore, C.E.; Rushakoff, J.A.; Berman, R.F.; Ashwood, P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl. Psychiatry 2013, 3, e240. [Google Scholar] [CrossRef] [PubMed]
- Bronson, S.L.; Ahlbrand, R.; Horn, P.S.; Kern, J.R.; Richtand, N.M. Individual differences in maternal response to immune challenge predict offspring behavior: Contribution of environmental factors. Behav. Brain Res. 2011, 220, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, S.; Khan, D.; Kong, E.; Berger, A.; Pollak, A.; Pollak, D.D. The Poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol. Ther. 2015, 149, 213–226. [Google Scholar] [CrossRef]
- Kentner, A.C.; Bilbo, S.D.; Brown, A.S.; Hsiao, E.Y.; McAllister, A.K.; Meyer, U.; Pearce, B.D.; Pletnikov, M.V.; Yolken, R.H.; Bauman, M.D. Maternal immune activation: Reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology 2019, 44, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, S.M.; Gagnidze, K.; Reyes, A.; Norstedt, N.; Månsson, K.; Francis, K.; Pfaff, D.W. Sex-specific gene-environment interactions underlying ASD-like behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, 1383–1388. [Google Scholar] [CrossRef]
- Ehninger, D.; Sano, Y.; de Vries, P.J.; Dies, K.; Franz, D.; Geschwind, D.H.; Kaur, M.; Lee, Y.-S.; Li, W.; Lowe, J.K.; et al. Gestational immune activation and Tsc2 haploinsufficiency cooperate to disrupt fetal survival and may perturb social behavior in adult mice. Mol. Psychiatry 2012, 17, 62–70, Erratum in: Mol. Psychiatry 2012, 17, 469. [Google Scholar] [CrossRef]
- Wu, W.L.; Adams, C.E.; Stevens, K.E.; Chow, K.H.; Freedman, R.; Patterson, P.H. The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain Behav. Immun. 2015, 46, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Karatas, H.; Erdener, S.E.; Gursoy-Ozdemir, Y.; Lule, S.; Eren-Koçak, E.; Sen, Z.D.; Dalkara, T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 2013, 339, 1092–1095, Erratum in: Science 2015, 20, 921. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Nyffeler, M.; Schwendener, S.; Knuesel, I.; Yee, B.K.; Feldon, J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology 2008, 33, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Schwendener, S.; Meyer, U.; Feldon, J. Deficient maternal care resulting from immunological stress during pregnancy is associated with a sex-dependent enhancement of conditioned fear in the offspring. J. Neurodev. Disord. 2009, 1, 15–32. [Google Scholar] [CrossRef]
- Richetto, J.; Calabrese, F.; Meyer, U.; Riva, M.A. Prenatal versus postnatal maternal factors in the development of infection-induced working memory impairments in mice. Brain Behav. Immun. 2013, 33, 190–200. [Google Scholar] [CrossRef]
- Johannessen, C.U.; Johannessen, S.I. Valproate: Past, present, and future. CNS Drug Rev. 2003, 9, 199–216. [Google Scholar] [CrossRef]
- Löscher, W. Valproate: A reappraisal of its pharmacodynamic properties and mechanisms of action. Prog. Neurobiol. 1999, 58, 31–59. [Google Scholar] [CrossRef]
- Gobbi, G.; Janiri, L. Sodium- and magnesium-valproate in vivo modulate glutamatergic and GABAergic synapses in the medial prefrontal cortex. Psychopharmacology 2006, 185, 255–262. [Google Scholar] [CrossRef]
- Phiel, C.J.; Zhang, F.; Huang, E.Y.; Guenther, M.G.; Lazar, M.A.; Klein, P.S. Histone Deacetylase Is a Direct Target of Valproic Acid, a Potent Anticonvulsant, Mood Stabilizer, and Teratogen. J. Biol. Chem. 2001, 276, 36734–36741. [Google Scholar] [CrossRef]
- Rasalam, A.D.; Hailey, H.; Williams, J.H.G.; Moore, S.J.; Turnpenny, P.D.; Lloyd, D.J.; Dean, J.C.S. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev. Med. Child Neurol. 2005, 47, 551–555. [Google Scholar] [CrossRef]
- Meador, K.J.; Baker, G.A.; Browning, N.; Clayton-Smith, J.; Combs-Cantrell, D.T.; Cohen, M.; Kalayjian, L.A.; Kanner, A.; Liporace, J.D.; Pennell, P.B.; et al. Cognitive Function at 3 Years of Age after Fetal Exposure to Antiepileptic Drugs. N. Engl. J. Med. 2009, 360, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Shallcross, R.; Bromley, B.R.L.; Irwin, B.; Bonnett, R.L.J.; Morrow, M.J.; Baker, G.A. Child development following in utero exposure Levetiracetam vs sodium valproate. Neurology 2011, 76, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Nau, H.; Hauck, R.-S.; Ehlers, K. Valproic Acid-Induced Neural Tube Defects in Mouse and Human: Aspects of Chirality, Alternative Drug Development, Pharmacokinetics and Possible Mechanisms. Pharmacol. Toxicol. 1991, 69, 310–321. [Google Scholar] [CrossRef]
- Nadebaum, C.; Anderson, V.; Vajda, F.; Reutens, D.; Barton, S.; Wood, A. The australian brain and cognition and antiepileptic drugs study: Iq in school-aged children exposed to sodium valproate and polytherapy. J. Int. Neuropsychol. Soc. 2011, 17, 133–142. [Google Scholar] [CrossRef]
- Schneider, T.; Przewłocki, R. Behavioral alterations in rats prenatally to valproic acid: Animal model of autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef]
- Markram, K.; Rinaldi, T.; La Mendola, C.; Markram, H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology 2008, 33, 901–912. [Google Scholar] [CrossRef]
- Schneider, T.; Ziòłkowska, B.; Gieryk, A.; Tyminska, A.; Przewłocki, R. Prenatal exposure to valproic acid disturbs the enkephalinergic system functioning, basal hedonic tone, and emotional responses in an animal model of autism. Psychopharmacology 2007, 193, 547–555. [Google Scholar] [CrossRef]
- Schneider, T.; Turczak, J.; Przewłocki, R. Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: Issues for a therapeutic approach in autism. Neuropsychopharmacology 2006, 31, 36–46. [Google Scholar] [CrossRef]
- Kataoka, S.; Takuma, K.; Hara, Y.; Maeda, Y.; Ago, Y.; Matsuda, T. Autism-like behaviours with transient histone hyperacetylation in mice treated prenatally with valproic acid. Int. J. Neuropsychopharmacol. 2013, 16, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Seung, H.; Kwon, K.J.; Ko, M.J.; Lee, E.J.; Oh, H.A.; Choi, C.S.; Kim, K.C.; Gonzales, E.L.; You, J.S.; et al. Subchronic treatment of donepezil rescues impaired social, hyperactive, and stereotypic behavior in valproic acid-induced animal model of autism. PLoS ONE 2014, 9, e104927. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Lee, D.K.; Go, H.S.; Kim, P.; Choi, C.S.; Kim, J.W.; Jeon, S.J.; Song, M.-R.; Shin, C.Y. Pax6-dependent cortical glutamatergic neuronal differentiation regulates autism-like behavior in prenatally valproic acid-exposed rat offspring. Mol. Neurobiol. 2014, 49, 512–528. [Google Scholar] [CrossRef]
- Mehta, M.V.; Gandal, M.J.; Siegel, S.J. mGluR5-antagonist mediated reversal of elevated stereotyped, repetitive behaviors in the VPA model of autism. PLoS ONE 2011, 6, e26077. [Google Scholar] [CrossRef]
- Kim, K.C.; Kim, P.; Go, H.S.; Choi, C.S.; Yang, S.-I.; Cheong, J.H.; Shin, C.Y.; Ko, K.H. The critical period of valproate exposure to induce autistic symptoms in Sprague–Dawley rats. Toxicol. Lett. 2011, 201, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Roman, A.; Basta-Kaim, A.; Kubera, M.; Budziszewska, B.; Schneider, K.; Przewłocki, R. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology 2008, 33, 728–740. [Google Scholar] [CrossRef]
- Moldrich, R.X.; Leanage, G.; She, D.; Dolan-Evans, E.; Nelson, M.; Reza, N.; Reutens, D.C. Inhibition of histone deacetylase in utero causes sociability deficits in postnatal mice. Behav. Brain Res. 2013, 257, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8836–8847. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, A.M.; Villani, A.; Ajmone-Cat, M.A.; Minghetti, L.; Ricceri, L.; Pazienza, V.; De Simone, R.; Calamandrei, G. Maternal immune activation induces autism-like changes in behavior, neuroinflammatory profile and gut microbiota in mouse offspring of both sexes. Transl. Psychiatry 2022, 12, 384. [Google Scholar] [CrossRef]
- Lim, J.S.; Lim, M.Y.; Choi, Y.; Ko, G. Modeling environmental risk factors of autism in mice induces IBD-related gut microbial dysbiosis and hyperserotonemia. Mol. Brain 2017, 10, 14. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.A.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Lammert, C.R.; Frost, E.L.; Bolte, A.C.; Paysour, M.J.; Shaw, M.E.; Bellinger, C.E.; Weigel, T.K.; Zunder, E.R.; Lukens, J.R. Cutting Edge: Critical Roles for Microbiota-Mediated Regulation of the Immune System in a Prenatal Immune Activation Model of Autism. J. Immunol. 2018, 201, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism—Comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 2012, 57, 2096–2102. [Google Scholar] [CrossRef]
- Settanni, C.R.; Bibbò, S.; Ianiro, G.; Rinninella, E.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A. Gastrointestinal involvement of autism spectrum disorder: Focus on gut microbiota. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 599–622. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef]
- Gonzales, J.; Marchix, J.; Aymeric, L.; Le Berre-Scoul, C.; Zoppi, J.; Bordron, P.; Burel, M.; Davidovic, L.; Richard, J.-R.; Gaman, A.; et al. Fecal supernatant from adult with autism spectrum disorder alters digestive functions, intestinal epithelial barrier, and enteric nervous system. Microorganisms 2021, 9, 1723. [Google Scholar] [CrossRef]
- Teskey, G.; Anagnostou, E.; Mankad, D.; Smile, S.; Roberts, W.; Brian, J.; Bowdish, D.M.; Foster, J.A. Intestinal permeability correlates with behavioural severity in very young children with ASD: A preliminary study. J. Neuroimmunol. 2021, 357, 577607. [Google Scholar] [CrossRef]
- Fiorentino, M.; Sapone, A.; Senger, S.; Camhi, S.S.; Kadzielski, S.M.; Buie, T.M.; Kelly, D.L.; Cascella, N.; Fasano, A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. Autism 2016, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Pachnis, V. The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology 2016, 151, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, A.J.; Ashwood, P.; Limb, K.; Anthony, A.; Wakefield, A. The significance of ileo-colonic lymphoid nodular hyperplasia in children with autistic spectrum disorder. Eur. J. Gastroenterol. Hepatol. 2005, 17, 827–836. [Google Scholar] [CrossRef]
- Furlano, R.I.; Anthony, A.; Day, R.; Brown, A.; McGarvey, L.; Thomson, M.A.; Davies, S.E.; Berelowitz, M.; Forbes, A.; Wakefield, A.J.; et al. Colonic CD8 and γδ T-cell infiltration with epithelial damage in children with autism. J. Pediatr. 2001, 138, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Anthony, A.; Pellicer, A.A.; Torrente, F.; Walker-Smith, J.A.; Wakefield, A.J. Intestinal Lymphocyte Populations in Children with Regressive Autism: Evidence for Extensive Mucosal Immunopathology. J. Clin. Immunol. 2003, 23, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.J.; Fortunato, J.; Gonzalez, L.G.; Krigsman, A. Identification of Unique Gene Expression Profile in Children with Regressive Autism Spectrum Disorder (ASD) and Ileocolitis. PLoS ONE 2013, 8, e58058. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robas, R.; Tripathi, U.; Rike, W.A.; Sharma, O.; Stern, S. Digestive Neurobiology in Autism: From Enteric and Central Nervous System Interactions to Shared Genetic Pathways. Int. J. Mol. Sci. 2025, 26, 9580. https://doi.org/10.3390/ijms26199580
Robas R, Tripathi U, Rike WA, Sharma O, Stern S. Digestive Neurobiology in Autism: From Enteric and Central Nervous System Interactions to Shared Genetic Pathways. International Journal of Molecular Sciences. 2025; 26(19):9580. https://doi.org/10.3390/ijms26199580
Chicago/Turabian StyleRobas, Raz, Utkarsh Tripathi, Wote Amelo Rike, Omveer Sharma, and Shani Stern. 2025. "Digestive Neurobiology in Autism: From Enteric and Central Nervous System Interactions to Shared Genetic Pathways" International Journal of Molecular Sciences 26, no. 19: 9580. https://doi.org/10.3390/ijms26199580
APA StyleRobas, R., Tripathi, U., Rike, W. A., Sharma, O., & Stern, S. (2025). Digestive Neurobiology in Autism: From Enteric and Central Nervous System Interactions to Shared Genetic Pathways. International Journal of Molecular Sciences, 26(19), 9580. https://doi.org/10.3390/ijms26199580






