Cerebellar Contributions to Spatial Learning and Memory: Effects of Discrete Immunotoxic Lesions
Abstract
1. Introduction
2. Results
2.1. General Observations
2.2. Effects of the Lesions on Spatial Navigation
2.2.1. Effects on Cued Navigation and Reference Memory
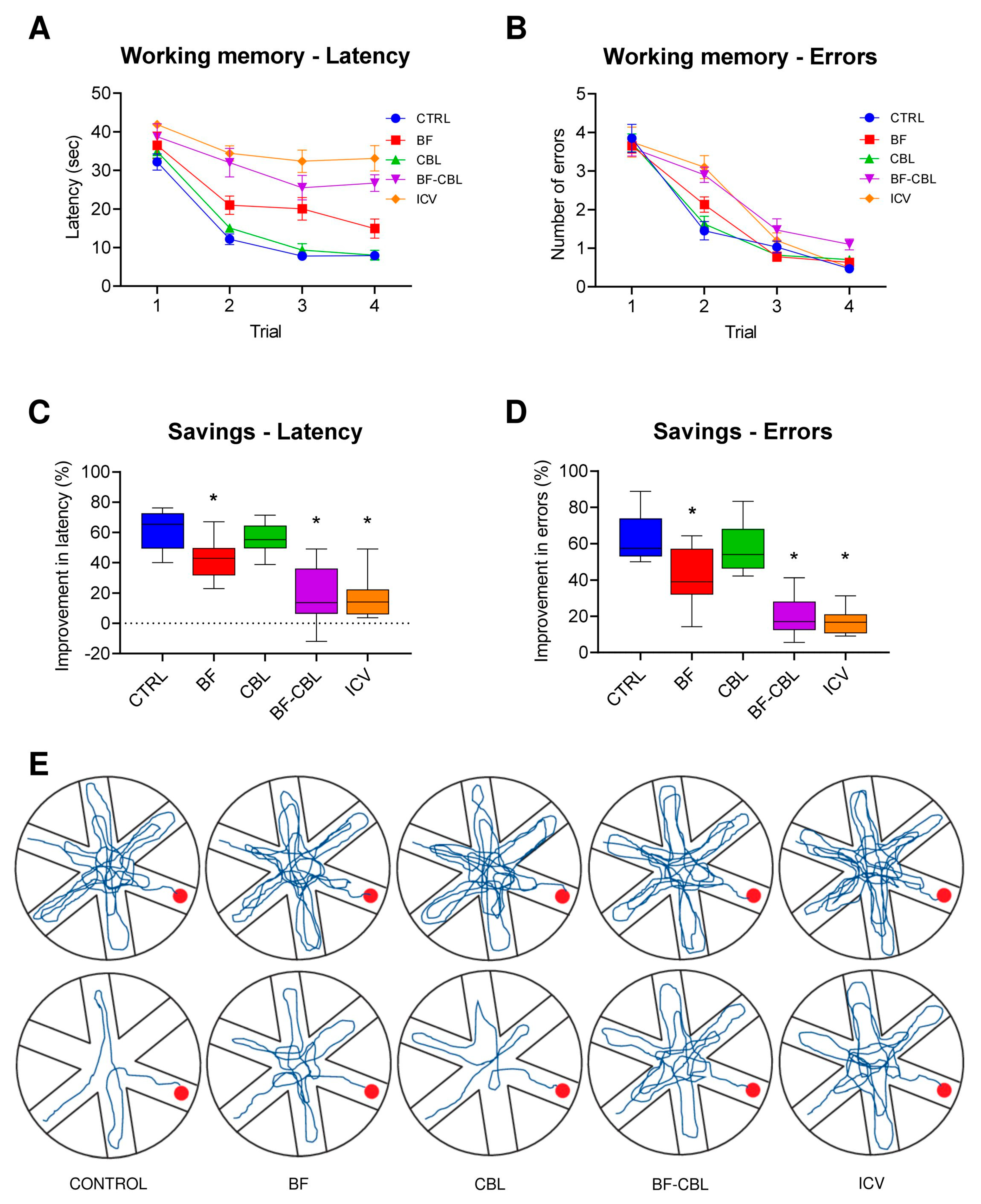
2.2.2. Effects on Working Memory
2.3. Morphological Analyses
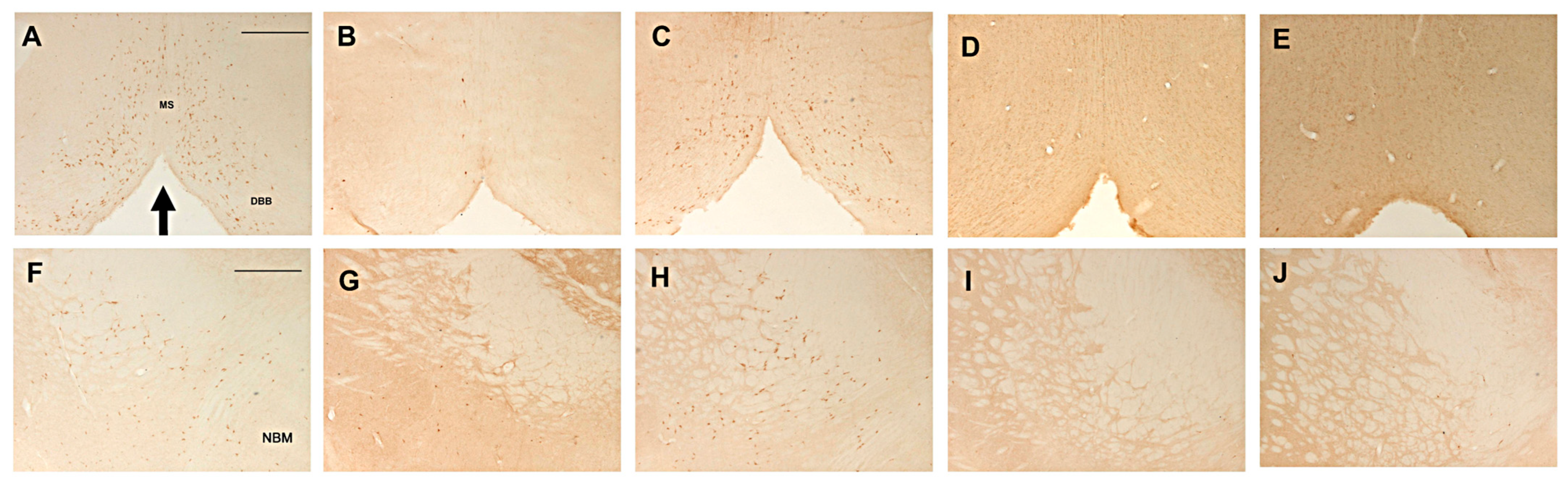
2.3.1. Effects on ChAT-Immunoreactive Neurons in the Basal Forebrain Nuclei
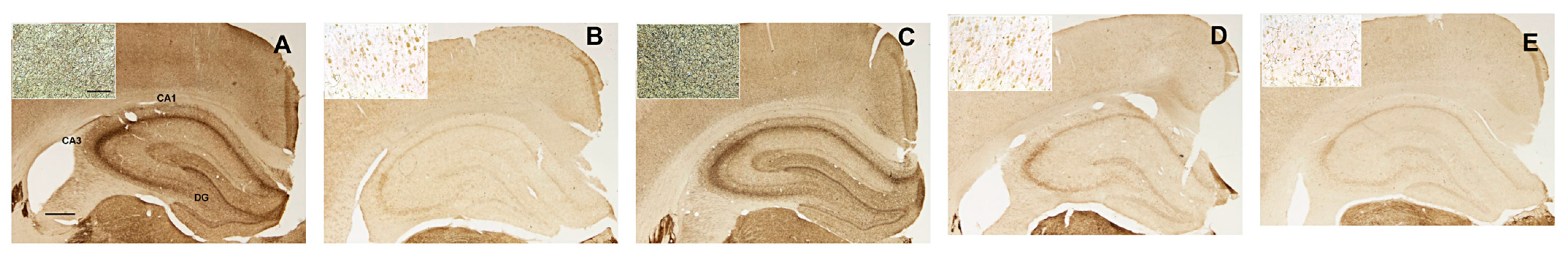
2.3.2. Effects on AChE-Positive Innervation in Neocortex and Hippocampus
2.3.3. Effects on Calbindin-Positive Cerebellar Neurons
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Lesion Surgery
4.3. Behavioral Analyses
4.3.1. Motor Tests
4.3.2. Morris Water Maze
4.3.3. Radial Arm Water Maze
4.4. Histology
4.5. Microscopical Analyses and Quantitative Estimations
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | acetylcholine |
| AChE | acetylcholinesterase |
| AD | Alzheimer’s disease |
| BF | basal forebrain |
| CBL | cerebellum |
| ChAT | choline acetyltransferase |
| HBSS | Hank’s balanced salt solution |
| KPBS | potassium phosphate-buffered saline |
| MWM | Morris water maze |
| NBM | nucleus basalis magnocellularis |
| NGF | nerve growth factor |
| NGS | normal goat serum |
| RAWM | radial arm water maze |
| Sept/vDBB | septum/vertical limb of the diagonal band of broca |
| SW | south-west |
References
- Dow, R.S.; Moruzzi, G. The Physiology and Pathology of the Cerebellum; University of Minnesota Press: Minneapolis, MN, USA, 1958. [Google Scholar]
- Allen, G.I.; Tsukahara, N. Cerebellar communication systems. Physiol. Rev. 1974, 54, 957–1006. [Google Scholar] [CrossRef]
- Ivry, R.B.; Baldo, J.V. Is the cerebellum involved in learning and cognition? Curr. Opin. Neurobiol. 1992, 2, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Leiner, H.C.; Leiner, A.L.; Dow, R.S. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993, 11, 444–447. [Google Scholar] [CrossRef]
- Raymond, J.L.; Lisberger, S.G.; Mauk, M.D. The cerebellum: A neuronal learning machine. Science 1996, 272, 1126–1131. [Google Scholar] [CrossRef]
- Leiner, H.C.; Leiner, A.L.; Dow, R.S. Does the cerebellum contribute to mental skills. Behav. Neurosci. 1986, 100, 443–454. [Google Scholar] [CrossRef]
- Leiner, H.C.; Leiner, A.L.; Dow, R.S. The human cerebro-cerebellar system: Its computing, cognitive and language skills. Behav. Brain Res. 1991, 44, 113–128. [Google Scholar] [CrossRef]
- Ryding, E.; Decety, J.; Sjoholm, H.; Stenberg, G.; Ingvar, D.H. Motor imagery activates the cerebellum regionally. A SPECT rCBF study with 99mTc-HMPAO. Cogn. Brain Res. 1993, 1, 94–99. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Dysmetria of thought: Clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn. Sci. 1998, 2, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Brodal, A. The Cerebellum in “Neurological Anatomy in Relation to Clinical Medicine”; Oxford Press: New York, NY, USA, 1981; pp. 294–393. [Google Scholar]
- Snider, R.S.; Maiti, A. Cerebellar contributions to the Papez circuit. J. Neurosci. 1976, 2, 133–146. [Google Scholar] [CrossRef]
- Azzarelli, B.; Muller, J.; Ghetti, B.; Dyken, M.; Conneally, P.M. Cerebellar plaques in familial Alzheimer’s disease (Gerstmann-Straussler-Scheinker variant)? Acta Neuropathol. 1985, 65, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Sjöbeck, M.; Englund, E. Alzheimer’s disease and the cerebellum: A morphologic study on neuronal and glial changes. Dement. Geriatr. Cogn. Disord. 2001, 12, 211–218. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Cerebellum in Alzheimer’s disease and frontotemporal dementia: Not a silent bystander. Brain 2016, 139, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, I.; Fotiou, D.F.; Adipepe, L.F.; Manani, M.G.; Njau, S.D.; Psaroulis, D.; Costa, V.G.; Baloyannis, S.J. Morphological changes of the human purkinje cells and deposition of neuritic plaques and neurofibrillary tangles on the cerebellar cortex of Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Dement. 2010, 25, 585–591. [Google Scholar] [CrossRef]
- Mavroudis, I.; Petridis, F.; Kazis, D.; Njau, S.N.; Costa, V.; Baloyannis, S.J. Purkinje Cells Pathology in Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement. 2019, 34, 439–449. [Google Scholar] [CrossRef]
- Goodlett, C.R.; Hamre, K.M.; West, J.R. Dissociation of spatial navigation and visual guidance performance in Purkinje cell degeneration (pcd) mutant mice. Behav. Brain Res. 1992, 47, 129–141. [Google Scholar] [CrossRef]
- Lalonde, R.; Strazielle, C. Motor coordination, exploration and spatial learning in a natural mouse mutation (nervous) with Purkinje cell degeneration. Behav. Genet. 2003, 33, 59–66. [Google Scholar] [CrossRef]
- Petrosini, L.; Leggio, M.G.; Molinari, M. The cerebellum in the spatial problem solving: A co-star or a guest-star? Prog. Neurobiol. 1998, 56, 191–210. [Google Scholar] [CrossRef]
- Mandolesi, L.; Leggio, M.G.; Spirito, F.; Petrosini, L. Cerebellar contribution to spatial event processing: Do spatial procedures contribute to formation of spatial declarative knowledge? Eur. J. Neurosci. 2003, 18, 2618–2626. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, C.C.; Kelly, R.M.; Wiley, R.G.; Walsh, T.J. Impaired acquisition of a Morris water maze task following selective destruction of cerebellar Purkinje cells with OX7-saporin. Behav. Brain Res. 2000, 109, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Wrenn, C.C.; Wiley, R.G. Lack of effect of moderate Purkinje cell loss on working memory. Neuroscience 2001, 107, 433–445. [Google Scholar] [CrossRef]
- Yan, Q.; Johnson, E.M.J. Immunohistochemical localization and biochemical characterization of nerve growth factor receptor in adult rat brain. J. Comp. Neurol. 1989, 290, 585–598. [Google Scholar] [CrossRef]
- Cuello, A.C.; Pioro, E.P.; Ribiero-da-Silva, A. Cellular and subcellular localization of nerve growth factor receptor-like immunoreactivity in the rat CNS. Neurochem. Int. 1990, 17, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Heckers, D.; Ohtake, T.; Wiley, R.G.; Lappi, D.A.; Geula, C.; Mesulman, M.M. Complete and selective cholinergic denervation of rat neocortex and hippocampus but not amygdala by an immunotoxin against the p75 NGF receptor. J. Neurosci. 1994, 14, 1271–1289. [Google Scholar] [CrossRef] [PubMed]
- Leanza, G.; Nilsson, O.G.; Wiley, R.G.; Björklund, A. A selective lesioning of the basal forebrain cholinergic system by intraventricular 192 IgG-saporin: Behavioural, biochemical and stereological studies in the rat. Eur. J. Neurosci. 1995, 7, 329–343. [Google Scholar] [CrossRef]
- Waite, J.J.; Chen, A.D.; Wardlow, M.L.; Wiley, R.G.; Lappi, D.A.; Thal, L.J. 192 Immunoglobulin G-saporin produces graded behavioural and biochemical changes accompanying the loss of cholinergic neurons of the basal forebrain and cerebellar Purkinje cells. Neuroscience 1995, 65, 463–476. [Google Scholar] [CrossRef]
- Berger-Sweeney, J.; Heckers, S.; Mesulam, M.M.; Wiley, R.G.; Lappi, D.A.; Sharma, M. Differential effects on spatial navigation of immunotoxin-induced cholinergic lesions of the medial septal area and nucleus basalis magnocellularis. J. Neurosci. 1994, 14, 4507–4519. [Google Scholar] [CrossRef]
- Torres, E.M.; Perry, T.A.; Blockland, A.; Wilkinson, L.S.; Wiley, R.G.; Lappi, D.A.; Dunnett, S.B. Behavioural, histochemical and biochemical consequences of selective immunolesions in discrete regions of the basal forebrain cholinergic system. Neuroscience 1994, 63, 95–122. [Google Scholar] [CrossRef]
- Baxter, M.G.; Bucci, D.J.; Gorman, L.K.; Wiley, R.G.; Gallagher, M. Selective immunotoxic lesions of basal forebrain cholinergic cells: Effects on learning and memory in rats. Behav. Neurosci. 1995, 109, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Wisman, L.A.B.; Sahin, G.; Maingay, M.; Leanza, G.; Kirik, D. Functional convergence of dopaminergic and cholinergic input is critical for hippocampus-dependent working memory. J. Neurosci. 2008, 28, 7797–7807. [Google Scholar] [CrossRef]
- Antonini, V.; Marrazzo, A.; Kleiner, G.; Coradazzi, M.; Ronsisvalle, S.; Prezzavento, O.; Ronsisvalle, G.; Leanza, G. Anti-amnesic and neuroprotective actions of the sigma-1 receptor agonist (-)-MR22 in rats with selective cholinergic lesion and amyloid infusion. J. Alzheimer’s Dis. 2011, 24, 569–586. [Google Scholar] [CrossRef]
- Leanza, G.; Nilsson, O.G.; Nikkhah, G.; Wiley, R.G.; Björklund, A. Effects of neonatal lesions of the basal forebrain cholinergic system by 192 immunoglobulin G-saporin: Biochemical, behavioural and morphological characterization. Neuroscience 1996, 74, 119–141. [Google Scholar] [CrossRef]
- Pappas, B.A.; Davidson, C.M.; Fortin, T.; Nallathamby, S.; Park, G.A.; Mohr, E.; Wiley, R.G. 192 IgG-saporin lesion of basal forebrain cholinergic neurons in neonatal rats. Brain Res. Dev. Brain Res. 1996, 96, 52–61. [Google Scholar] [CrossRef] [PubMed]
- de Leo, G.; Gulino, R.; Coradazzi, M.; Leanza, G. Acetylcholine and noradrenaline differentially regulate hippocampus-dependent spatial learning and memory. Brain Commun. 2023, 5, fcac338. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.J.; Wardlow, M.L.; Power, A.E. Deficit in selective and divided attention associated with cholinergic basal forebrain immunotoxic lesion produced be 192-saporin; motoric/sensory deficit associated with Purkinje cell immunotoxic lesion produced by OX7-saporin. Neurobiol. Learn. Mem. 1999, 71, 325–352. [Google Scholar] [CrossRef]
- Leanza, G.; Martìnez-Serrano, A.; Björklund, A. Amelioration of spatial navigation and short-term memory deficits by grafts of foetal basal forebrain tissue placed into the hippocampus and cortex of rats with selective cholinergic lesions. Eur. J. Neurosci. 1998, 10, 2353–2370. [Google Scholar] [CrossRef]
- Ferencz, I.; Leanza, G.; Nanobashvili, A.; Kokaia, M.; Lindvall, O. Basal forebrain neurons suppress amygdala kindling via cortical but not hippocampal cholinergic projections in rats. Eur. J. Neurosci. 2000, 12, 2107–2116. [Google Scholar] [CrossRef]
- Wiley, R.G.; Oeltmann, T.N.; Lappi, D.A. Immunolesioning: Selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res. 1991, 562, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Dean, R.L.; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Dornan, W.A.; McCampbell, A.R.; Tinkler, G.P.; Hickman, L.J.; Bannon, A.W.; Decker, M.W.; Gunther, K.L. Comparison of site specific injections into the basal forebrain on water maze and radial arm maze performance in the male rat after immunolesioning with 192 IgG saporin. Behav. Brain Res. 1997, 86, 181–189. [Google Scholar] [CrossRef]
- Pizzo, D.P.; Thal, L.J.; Winkler, J. Mnemonic deficits in animals depend upon the degree of cholinergic deficit and task complexity. Exp. Neurol. 2002, 177, 292–305. [Google Scholar] [CrossRef]
- Lehmann, O.; Grottick, A.J.; Cassel, J.C.; Higgins, G.A. A double dissociation between serial reaction time and radial maze performance in rats subjected to 192 IgG-saporin lesions of the nucleus basalis and/or the septal region. Eur. J. Neurosci. 2003, 18, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.E.; Dietrichs, E. An HRP study of hypothalamo-cerebellar and cerebello-hypothalamic connections in squirrel monkey (Saimiri sciureus). J. Comp. Neurol. 1984, 229, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Siwek, D.F.; Pandya, D.N. Prefrontal projections to the mediodorsal nucleus of the thalamus in the rhesus monkey. J. Comp. Neurol. 1991, 312, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Habas, C.; Kamdar, N.; Nguyen, D.; Prater, K.; Beckmann, C.F.; Menon, V.; Greicius, M.D. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009, 29, 8586–8594. [Google Scholar] [CrossRef]
- Buckner, R.; Krienen, F.; Castellanos, A.; Diaz, J.C.; Yeo, B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 2322–2345. [Google Scholar] [CrossRef]
- Buckner, R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 2013, 80, 807–815. [Google Scholar] [CrossRef]
- Guo, C.C.; Tan, R.; Hodges, J.R.; Hu, X.; Saber, S.; Hornberger, M. Network-selective vulnerability of the human cerebellum to Alzheimer’s disease and frontotemporal dementia. Brain 2016, 139, 1532–1543. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: San Diego, CA, USA, 1986. [Google Scholar]
- Morris, R.G.M. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Buresova, O.; Bolhuis, J.J.; Bures, J. Differential effects of cholinergic blockade on performances of rats in the water tank navigation task and in a radial water maze. Behav. Neurosci. 1986, 100, 476–482. [Google Scholar] [CrossRef]
- Diamond, D.M.; Park, C.R.; Heman, K.L.; Rose, G.M. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus 1999, 9, 542–552. [Google Scholar] [CrossRef]
- Hedreen, J.C.; Bacon, S.J.; Price, D.L. A modified hystochemical technique to visualize acetylcholinesterase-containing axons. J. Histochem. Cytochem. 1985, 33, 134–140. [Google Scholar] [CrossRef]
- West, M.J.; Slomianka, L.; Gundersen, H.J. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991, 231, 482–497. [Google Scholar] [CrossRef]
- Aztiria, E.; Cataudella, T.; Spampinato, S.; Leanza, G. Septal grafts restore cognitive abilities and amyloid precursor protein metabolism. Neurobiol. Aging 2009, 30, 1614–1625. [Google Scholar] [CrossRef]
- Rasband, W.S.; Bright, D.S. NIH Image: A public domain image processing program for Macintosh. Microbeam Anal. Soc. J. 1995, 4, 137–149. [Google Scholar]
- Fiez, J.A. Cerebellar contributions to cognition. Neuron 1996, 16, S0896–S6273. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Sherman, J.C. The cerebellar cognitive affective syndrome. Brain 1998, 121, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D. The cerebrocerebellar system: Anatomic substrates of the cerebellar contribution to cognition and emotion. Int. Rev. Psychiatry 2001, 13, 247–260. [Google Scholar] [CrossRef]
- Baumann, O.; Borra, R.J.; Bower, J.M.; Cullen, K.E.; Habas, C.; Ivry, R.B.; Leggio, M.; Mattingley, J.B.; Molinari, M.; Moulton, E.A.; et al. Consensus paper: The role of the cerebellum in perceptual processes. Cerebellum 2014, 14, 197–220. [Google Scholar] [CrossRef]
- Tomlinson, B.E. Plaques, tangles and Alzheimer’s disease. Psychol. Med. 1982, 12, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E.; Bohl, J.; Lang, W. Alzheimer’s disease: Amyloid plaques in the cerebellum. J. Neurol. Sci. 1989, 93, 277–287. [Google Scholar] [CrossRef]
- Cole, G.; Neal, J.W.; Singhrao, S.K.; Jasani, B.; Newman, G.R. The distribution of amyloid plaques in the cerebellum and brainstem in Down’s syndrome and Alzheimer’s disease: A light microscopical analysis. Acta Neuropathol. 1993, 85, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef]
- Samstag, C.L.; Chapman, N.H.; Gibbons, L.E.; Geller, J.; Loeb, N.; Dharap, S.; Yagi, M.; Cook, D.G.; Pagulayan, K.F.; Crane, P.K.; et al. Neuropathological correlates of vulnerability and resilience in the cerebellum in Alzheimer’s disease. Alzheimer’s Dement. 2025, 21, e14428. [Google Scholar] [CrossRef]
- Andersen, K.; Andersen, B.B.; Pakkenberg, B. Stereological quantification of the cerebellum in patients with Alzheimer’s disease. Neurobiol. Aging 2012, 33, 197.e11–197.e20. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [PubMed]



| Equilibrium Time on Ramp (%) | Latency to Cross Ramp (s) | Latency to Reverse on Grids (s) | Number of Falls in Grids | |
|---|---|---|---|---|
| Control (12) | 97.8 ± 10.8 | 7.0 ± 0.4 | 6.6 ± 0.6 | 2.4 ± 0.6 |
| BF (12) | 99.0 ± 9.9 | 7.2 ± 0.3 | 5.9 ± 0.6 | 3.3 ± 0.4 |
| CBL (12) | 98.6 ± 7.2 | 6.9 ± 0.4 | 6.2 ± 0.7 | 2.4 ± 0.5 |
| BF-CBL (12) | 97.4 ± 7.5 | 7.1 ± 0.4 | 6.3 ± 0.8 | 2.6 ± 0.6 |
| ICV (12) | 97.7 ± 7.1 | 6.7 ± 0.4 | 6.3 ± 0.7 | 2.9 ± 0.4 |
| ChAT MS/DBB | ChAT NBM | AChE Fr-ParCx | AChE HPC | CALB Vermis | CALB Hemisph | |
|---|---|---|---|---|---|---|
| Control (12) | 9552 ± 204 | 2924 ± 186 | 97.1 ± 3.7 | 103.7 ± 5.1 | 10,117 ± 360 | 9824 ± 402 |
| BF (12) | 254 ± 31 * | 179 ± 8 * | 25.7 ± 2.5 * | 31.6 ± 1.1 * | 9532 ± 324 | 9239 ± 340 |
| CBL (12) | 9384 ± 241 | 2784 ± 110 | 94.4 ± 1.9 | 99.5 ± 4.0 | 3794 ± 285 * | 3775 ± 166 * |
| BF-CBL (12) | 367 ± 63 * | 174 ± 19 * | 25.3 ± 2.2 * | 30.6 ± 1.5 * | 3416 ± 443 * | 3813 ± 125 * |
| ICV (12) | 233 ± 35 * | 176 ± 32 * | 21.2 ± 2.5 * | 24.5 ± 2.1 * | 3945 ± 281 * | 3671 ± 183 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leanza, M.H.; Storelli, E.; D’Arco, D.; de Leo, G.; Kleiner, G.; Arancio, L.; Capodieci, G.; Gulino, R.; Bava, A.; Leanza, G. Cerebellar Contributions to Spatial Learning and Memory: Effects of Discrete Immunotoxic Lesions. Int. J. Mol. Sci. 2025, 26, 9553. https://doi.org/10.3390/ijms26199553
Leanza MH, Storelli E, D’Arco D, de Leo G, Kleiner G, Arancio L, Capodieci G, Gulino R, Bava A, Leanza G. Cerebellar Contributions to Spatial Learning and Memory: Effects of Discrete Immunotoxic Lesions. International Journal of Molecular Sciences. 2025; 26(19):9553. https://doi.org/10.3390/ijms26199553
Chicago/Turabian StyleLeanza, Martina Harley, Elisa Storelli, David D’Arco, Gioacchino de Leo, Giulio Kleiner, Luciano Arancio, Giuseppe Capodieci, Rosario Gulino, Antonio Bava, and Giampiero Leanza. 2025. "Cerebellar Contributions to Spatial Learning and Memory: Effects of Discrete Immunotoxic Lesions" International Journal of Molecular Sciences 26, no. 19: 9553. https://doi.org/10.3390/ijms26199553
APA StyleLeanza, M. H., Storelli, E., D’Arco, D., de Leo, G., Kleiner, G., Arancio, L., Capodieci, G., Gulino, R., Bava, A., & Leanza, G. (2025). Cerebellar Contributions to Spatial Learning and Memory: Effects of Discrete Immunotoxic Lesions. International Journal of Molecular Sciences, 26(19), 9553. https://doi.org/10.3390/ijms26199553







