Evaluation of Five Plasma miRNAs as Biomarkers for Minimally Invasive Staging of Liver Fibrosis in β-Thalassaemia Patients
Abstract
1. Introduction
2. Results
2.1. Patient Population, Demographics, and Clinical Information
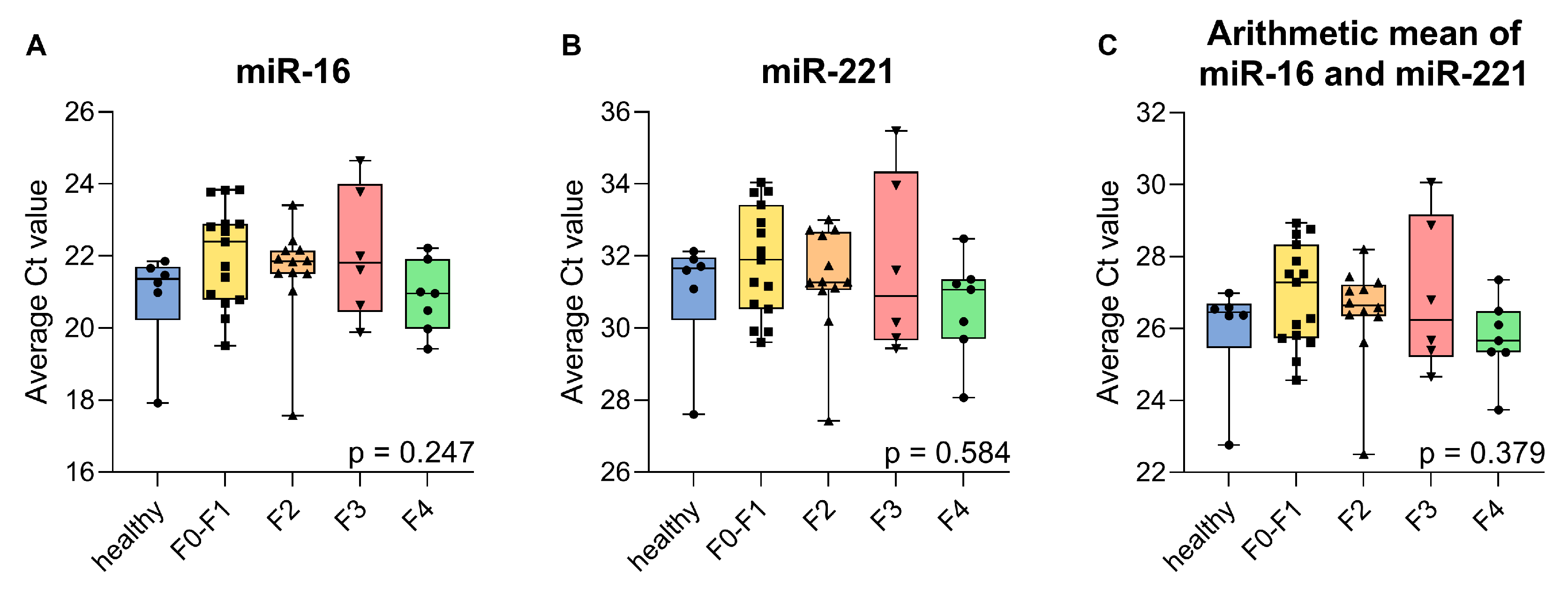
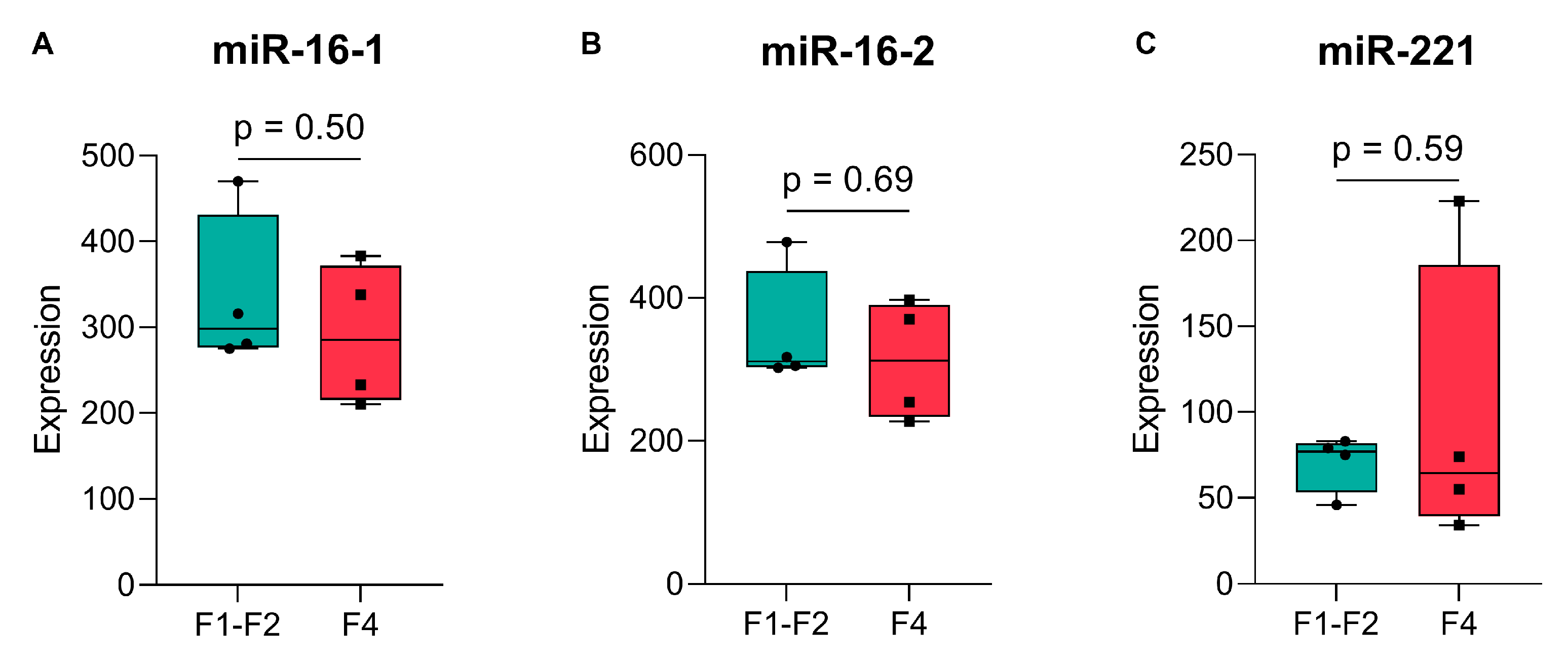
2.2. Validation of Endogenous Reference miRNAs, miR-16, and miR-221
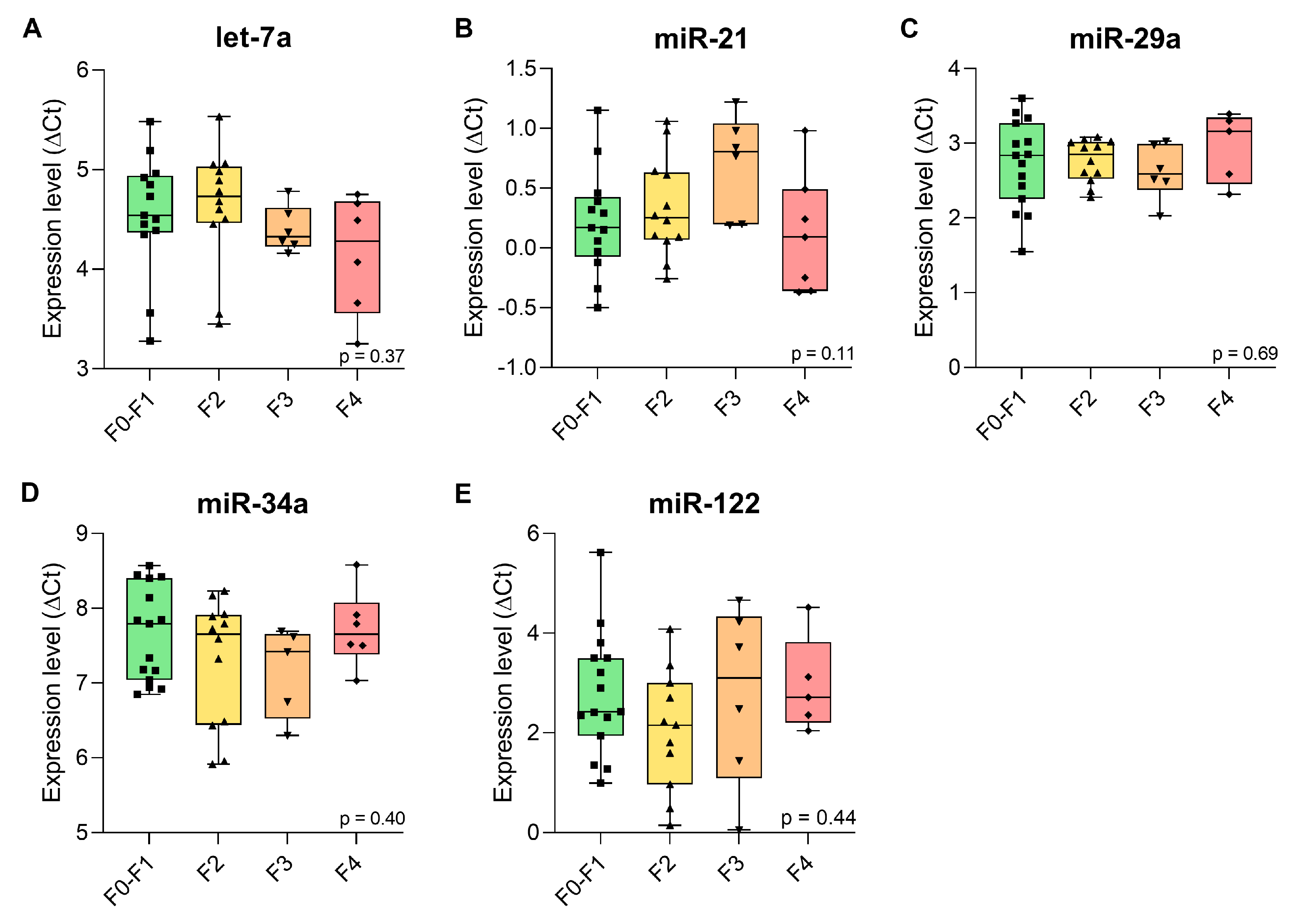
2.3. Expression Levels of the Candidate Plasma miRNAs in β-Thalassaemia Patients with Liver Fibrosis
2.4. Diagnostic Accuracy of Candidate miRNAs for Detection of Liver Fibrosis Stages in β-Thalassaemia Patients
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Blood Sampling
4.3. RNA Isolation
4.4. Reverse Transcription
4.5. Reverse-Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CI | Confidence interval |
| ECM | Extracellular matrix |
| GEO | Gene Expression Omnibus |
| HCC | Hepatocellular carcinoma |
| HMDD | Human MicroRNA Disease Database |
| IQR | Interquartile range |
| miRNA | microRNA |
| NAFLD | Non-alcoholic fatty liver disease |
| NPV | Negative predictive value |
| NTDT | Non-transfusion-dependent thalassaemia |
| OR | Odds ratio |
| PPV | Positive predictive value |
| ROC | Receiver operating characteristic |
| SWE | Shear wave elastography |
| TDT | Transfusion-dependent thalassaemia |
| TE | Transient elastography |
References
- Moukhadder, H.M.; Halawi, R.; Cappellini, M.D.; Taher, A.T. Hepatocellular Carcinoma as an Emerging Morbidity in the Thalassemia Syndromes: A Comprehensive Review. Cancer 2017, 123, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Maakaron, J.E.; Musallam, K.M.; Ayache, J.B.; Jabbour, M.; Tawil, A.N.; Taher, A.T. A Liver Mass in an Iron-Overloaded Thalassaemia Intermedia Patient. Br. J. Haematol. 2013, 161, 1. [Google Scholar] [CrossRef]
- Mancuso, A. Hepatocellular Carcinoma in Thalassemia: A Critical Review. World J. Hepatol. 2010, 2, 171–174. [Google Scholar] [CrossRef]
- Pinto, V.M.; Forni, G.L. Management of Iron Overload in Beta-Thalassemia Patients: Clinical Practice Update Based on Case Series. Int. J. Mol. Sci. 2020, 21, 8771. [Google Scholar] [CrossRef]
- Gressner, O.A.; Weiskirchen, R.; Gressner, A.M. Biomarkers of Liver Fibrosis: Clinical Translation of Molecular Pathogenesis or Based on Liver-Dependent Malfunction Tests. Clin. Chim. Acta 2007, 381, 107–113. [Google Scholar] [CrossRef]
- Bertolani, C.; Sancho-Bru, P.; Failli, P.; Bataller, R.; Aleffi, S.; DeFranco, R.; Mazzinghi, B.; Romagnani, P.; Milani, S.; Ginés, P.; et al. Resistin as an Intrahepatic Cytokine: Overexpression during Chronic Injury and Induction of Proinflammatory Actions in Hepatic Stellate Cells. Am. J. Pathol. 2006, 169, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.S.; Gaudio, E.; Miller, T.; Alvaro, D.; Alpini, G. Cholangiocyte Proliferation and Liver Fibrosis. Expert Rev. Mol. Med. 2009, 11, e7. [Google Scholar] [CrossRef] [PubMed]
- Mutimer, D.; Aghemo, A.; Diepolder, H.; Negro, F.; Robaeys, G.; Ryder, S.; Zoulim, F.; Peck, M.; Craxi, A.; Fried, M.; et al. EASL Clinical Practice Guidelines: Management of Hepatitis C Virus Infection. J. Hepatol. 2014, 60, 392–420. [Google Scholar] [CrossRef]
- Manning, D.S.; Afdhal, N.H. Diagnosis and Quantitation of Fibrosis. Gastroenterology 2008, 134, 1670–1681. [Google Scholar] [CrossRef]
- Castera, L.; Foucher, J.; Bernard, P.H.; Carvalho, F.; Allaix, D.; Merrouche, W.; Couzigou, P.; De Ledinghen, V. Pitfalls of Liver Stiffness Measurement: A 5-Year Prospective Study of 13,369 Examinations. Hepatology 2010, 51, 828–835. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Krarup, H.; Sand, J.M.B.; Christensen, P.B.; Gerstoft, J.; Leeming, D.J.; Weis, N.; Schaffalitzky De Muckadell, O.B.; Krag, A. The Efficacy of Biomarkers in Chronic Fibroproliferative Diseases—Early Diagnosis and Prognosis, with Liver Fibrosis as an Exemplar. Aliment. Pharmacol. Ther. 2014, 40, 233–249. [Google Scholar] [CrossRef]
- Baranova, A.; Lal, P.; Birerdinc, A.; Younossi, Z.M. Non-Invasive Markers for Hepatic Fibrosis. BMC Gastroenterol. 2011, 11, 91. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Cai, J.; Sun, M.; Zeng, L.; Wu, F.; Zhang, Y.; Hu, M. Serum Biomarkers for Liver Fibrosis. Clin. Chim. Acta 2022, 537, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; McPhaul, M.J.; Caulfield, M.P.; Clark, V.C.; Soldevilla-Pico, C.; Firpi-Morell, R.J.; Lai, J.; Shiffman, D.; Rowland, C.M.; Cusi, K. Performance of Plasma Biomarkers and Diagnostic Panels for Nonalcoholic Steatohepatitis and Advanced Fibrosis in Patients with Type 2 Diabetes. Diabetes Care 2020, 43, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Mantovani, A.; Baldelli, E.; Lugari, S.; Maurantonio, M.; Nascimbeni, F.; Marrazzo, A.; Romagnoli, D.; Targher, G.; Lonardo, A. Liver Fibrosis Biomarkers Accurately Exclude Advanced Fibrosis and Are Associated with Higher Cardiovascular Risk Scores in Patients with NAFLD or Viral Chronic Liver Disease. Diagnostics 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Papasavva, P.L.; Papaioannou, N.Y.; Patsali, P.; Kurita, R.; Nakamura, Y.; Sitarou, M.; Christou, S.; Kleanthous, M.; Lederer, C.W. Distinct Mirna Signatures and Networks Discern Fetal from Adult Erythroid Differentiation and Primary from Immortalized Erythroid Cells. Int. J. Mol. Sci. 2021, 22, 3626. [Google Scholar] [CrossRef]
- Hammond, S.; Bernstein, E.; Beach, D.; Hannon, G. An RNA-Directed Nuclease Mediates-Transcriptional Gene silencing in Drosophila Cells. Nature 2000, 404, 293–296. [Google Scholar] [CrossRef]
- Wang, X.W.; Heegaard, N.H.H.; Orum, H. MicroRNAs in Liver Disease. Gastroenterology 2012, 142, 1431–1443. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of MicroRNA Biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; MacKowiak, B.; Gao, B. MicroRNAs as Regulators, Biomarkers and Therapeutic Targets in Liver Diseases. Gut 2021, 70, 784–795. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Li, Y.; Jiao, K.; Zhang, Y. Let-7a Suppresses Liver Fibrosis via TGFβ/SMAD Signaling Transduction Pathway. Exp. Ther. Med. 2019, 17, 3935–3942. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 Contributes to Myocardial Disease by Stimulating MAP Kinase Signalling in Fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Friggeri, A.; Yang, Y.; Milosevic, J.; Ding, Q.; Thannickal, V.J.; Kaminski, N.; Abraham, E. MiR-21 Mediates Fibrogenic Activation of Pulmonary Fibroblasts and Lung Fibrosis. J. Exp. Med. 2010, 207, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Marquez, R.T.; Bandyopadhyay, S.; Wendlandt, E.B.; Keck, K.; Hoffer, B.A.; Icardi, M.S.; Christensen, R.N.; Schmidt, W.N.; McCaffrey, A.P. Correlation between MicroRNA Expression Levels and Clinical Parameters Associated with Chronic Hepatitis C Viral Infection in Humans. Lab. Investig. 2010, 90, 1727–1736. [Google Scholar] [CrossRef]
- Varnholt, H. The Role of MicroRNAs in Primary Liver Cancer. Ann. Hepatol. 2008, 7, 104–113. [Google Scholar] [CrossRef]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 Regulates Expression of the PTEN Tumor Suppressor Gene in Human Hepatocellular Cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef]
- Zhang, Z.; Zha, Y.; Hu, W.; Huang, Z.; Gao, Z.; Zang, Y.; Chen, J.; Dong, L.; Zhang, J. The Autoregulatory Feedback Loop of MicroRNA-21/Programmed Cell Death Protein 4/Activation Protein-1 (MiR-21/PDCD4/AP-1) as a Driving Force for Hepatic Fibrosis Development. J. Biol. Chem. 2013, 288, 37082–37093. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Itami, S.; Kuroda, M.; Yoshizato, K.; Kawada, N.; Murakami, Y. MiR-29a Assists in Preventing the Activation of Human Stellate Cells and Promotes Recovery from Liver Fibrosis in Mice. Mol. Ther. 2016, 24, 1848–1859. [Google Scholar] [CrossRef]
- Jin, Y.; Wong, Y.S.; Goh, B.K.P.; Chan, C.Y.; Cheow, P.C.; Chow, P.K.H.; Lim, T.K.H.; Goh, G.B.B.; Krishnamoorthy, T.L.; Kumar, R.; et al. Circulating MicroRNAs as Potential Diagnostic and Prognostic Biomarkers in Hepatocellular Carcinoma. Sci. Rep. 2019, 9, 10464. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.F.; Ji, F.J.; Zang, H.L.; Cao, H. Activation of the MiR-34a/SIRT1/P53 Signaling Pathway Contributes to the Progress of Liver Fibrosis via Inducing Apoptosis in Hepatocytes but Not in HSCs. PLoS ONE 2016, 11, e0158657. [Google Scholar] [CrossRef]
- Girard, M.; Jacquemin, E.; Munnich, A.; Lyonnet, S.; Henrion-Caude, A. MiR-122, a Paradigm for the Role of MicroRNAs in the Liver. J. Hepatol. 2008, 48, 648–656. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of Tissue-Specific MicroRNAs from Mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, J.; Medico, L.; Wang, D.; Ambrosone, C.B.; Liu, S. A Pilot Study of Circulating MiRNAs as Potential Biomarkers of Early Stage Breast Cancer. PLoS ONE 2010, 5, e13735. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, H.M.; Miller, N.; Lowery, A.J.; Sweeney, K.J.; Newell, J.; Kerin, M.J. Circulating Micrornas as Novel Minimally Invasive Biomarkers for Breast Cancer. Ann. Surg. 2010, 251, 499–505. [Google Scholar] [CrossRef]
- Fang, R.; Zhu, Y.; Hu, L.; Khadka, V.S.; Ai, J.; Zou, H.; Ju, D.; Jiang, B.; Deng, Y.; Hu, X. Plasma MicroRNA Pair Panels as Novel Biomarkers for Detection of Early Stage Breast Cancer. Front. Physiol. 2019, 10, 1879. [Google Scholar] [CrossRef]
- Zenlander, R.; Salter, H.; Gilg, S.; Eggertsen, G.; Stål, P. MicroRNAs as Plasma Biomarkers of Hepatocellular Carcinoma in Patients with Liver Cirrhosis—A Cross-Sectional Study. Int. J. Mol. Sci. 2024, 25, 2414. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.K.K.; Lu, Y.; Qiu, J.; Yang, Z.; Zhou, X.; Xie, H.; Zhou, Z.; Yang, M. Establishing a MiRNA Panel for Hepatocellular Carcinoma Screening through a Multicenter Study. iScience 2025, 28, 112986. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.T.; Dong, Q.Z.; Wang, G.; Zhou, H.J.; Ren, N.; Jia, H.L.; Ye, Q.H.; Qin, L.X. Identification of Suitable Reference Genes for QRT-PCR Analysis of Circulating MicroRNAs in Hepatitis B Virus-Infected Patients. Mol. Biotechnol. 2012, 50, 49–56. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, G.M.; Liu, L.L.; Liu, C.; Liu, F.; Jiang, D.N.; Pu, X.Y. Assessment of Endogenous Reference Gene Suitability for Serum Exosomal MicroRNA Expression Analysis in Liver Carcinoma Resection Studies. Mol. Med. Rep. 2015, 12, 4683–4691. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Liu, F.; Xiang, G.; Jiang, D.; Pu, X. Identification of Endogenous Controls for Analyzing Serum Exosomal MiRNA in Patients with Hepatitis B or Hepatocellular Carcinoma. Dis. Markers 2015, 2015, 893594. [Google Scholar] [CrossRef]
- Cai, P.; Mu, Y.; Olveda, R.M.; Ross, A.G.; Olveda, D.U.; McManus, D.P. Circulating MiRNAs as Footprints for Liver Fibrosis Grading in Schistosomiasis. eBioMedicine 2018, 37, 334–343. [Google Scholar] [CrossRef]
- Cisilotto, J.; do Amaral, A.E.; Rosolen, D.; Rode, M.P.; Silva, A.H.; Winter, E.; da Silva, T.E.; Fischer, J.; Matiollo, C.; de Morais Rateke, E.C.; et al. MicroRNA Profiles in Serum Samples from Acute-On-Chronic Liver Failure Patients and MiR-25-3p as a Potential Biomarker for Survival Prediction. Sci. Rep. 2020, 10, 100. [Google Scholar] [CrossRef]
- Matsuura, K.; Aizawa, N.; Enomoto, H.; Nishiguchi, S.; Toyoda, H.; Kumada, T.; Iio, E.; Ito, K.; Ogawa, S.; Isogawa, M.; et al. Circulating Let-7 Levels in Serum Correlate with the Severity of Hepatic Fibrosis in Chronic Hepatitis C. Open Forum Infect. Dis. 2018, 5, ofy268. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; De Giorgi, V.; Schechterly, C.; Wang, R.Y.; Farci, P.; Tanaka, Y.; Alter, H.J. Circulating Let-7 Levels in Plasma and Extracellular Vesicles Correlate With Hepatic Fibrosis Progression in Chronic Hepatitis C. Hepatology 2016, 64, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Tomimaru, Y.; Eguchi, H.; Nagano, H.; Wada, H.; Kobayashi, S.; Marubashi, S.; Tanemura, M.; Tomokuni, A.; Takemasa, I.; Umeshita, K.; et al. Circulating MicroRNA-21 as a Novel Biomarker for Hepatocellular Carcinoma. J. Hepatol. 2012, 56, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, I.; Thum, T.; Baumann, U. Circulating MiR-21 and MiR-29a as Markers of Disease Severity and Etiology in Cholestatic Pediatric Liver Disease. J. Clin. Med. 2016, 5, 28. [Google Scholar] [CrossRef]
- da Silva Freire, A.K.; Furtado de Mendonça Belmont, T.; Pinto Santiago, E.J.; Cristina Cordeiro Farias, I.; Palmeira do Ó, K.; Soares da Silva, A.; Richardson Silva Vasconcelos, L. Potential Role of Circulating MiR-21 in the Diagnosis of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Expert Rev. Mol. Diagn. 2022, 22, 1037–1052. [Google Scholar] [CrossRef]
- Malik, J.; Klammer, M.; Rolny, V.; Chan, H.L.Y.; Piratvisuth, T.; Tanwandee, T.; Thongsawat, S.; Sukeepaisarnjaroen, W.; Esteban, J.I.; Bes, M.; et al. Comprehensive Evaluation of MicroRNA as a Biomarker for the Diagnosis of Hepatocellular Carcinoma. World J. Gastroenterol. 2022, 28, 3917–3933. [Google Scholar] [CrossRef]
- Eldosoky, M.A.; Hammad, R.; Elmadbouly, A.A.; Aglan, R.B.; Abdel-Hamid, S.G.; Alboraie, M.; Hassan, D.A.; Shaheen, M.A.; Rushdi, A.; Ahmed, R.M.; et al. Diagnostic Significance of Hsa-MiR-21-5p, Hsa-MiR-192-5p, Hsa-MiR-155-5p, Hsa-MiR-199a-5p Panel and Ratios in Hepatocellular Carcinoma on Top of Liver Cirrhosis in HCV-Infected Patients. Int. J. Mol. Sci. 2023, 24, 3157. [Google Scholar] [CrossRef]
- Lin, X.J.; Chong, Y.; Guo, Z.W.; Xie, C.; Yang, X.J.; Zhang, Q.; Li, S.P.; Xiong, Y.; Yuan, Y.; Min, J.; et al. A Serum MicroRNA Classifier for Early Detection of Hepatocellular Carcinoma: A Multicentre, Retrospective, Longitudinal Biomarker Identification Study with a Nested Case-Control Study. Lancet Oncol. 2015, 16, 804–815. [Google Scholar] [CrossRef]
- Shehab-Eldeen, S.; Metwaly, M.F.; Saber, S.M.; El-Kousy, S.M.; Badr, E.A.E.; Essa, A. MicroRNA-29a and MicroRNA-124 as Novel Biomarkers for Hepatocellular Carcinoma. Dig. Liver Dis. 2023, 55, 283–290, Erratum in Dig. Liver Dis. 2023, 55, 1164. [Google Scholar] [CrossRef]
- Liu, X.L.; Pan, Q.; Zhang, R.N.; Shen, F.; Yan, S.Y.; Sun, C.; Xu, Z.J.; Chen, Y.W.; Fan, J.G. Disease-Specific MiR-34a as Diagnostic Marker of Non-Alcoholic Steatohepatitis in a Chinese Population. World J. Gastroenterol. 2016, 22, 9844. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Mao, Y.; Chen, W.; Liu, C.; Wu, H.; Zhang, J.; Wang, S.; Wang, C.; Lin, Y.; Lv, Y. Serum Exosomal MiR-34a as a Potential Biomarker for the Diagnosis and Prognostic of Hepatocellular Carcinoma. J. Cancer 2022, 13, 1410–1417. [Google Scholar] [CrossRef]
- Salvoza, N.C.; Klinzing, D.C.; Gopez-Cervantes, J.; Baclig, M.O. Association of Circulating Serum MIR-34a and MIR-122 with Dyslipidemia among Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0153497. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, L.; Gao, X.; Hu, J.; Wang, J.; Dai, Z.; Wang, J.F.; Zhang, Z.; Lu, S.; Huang, X.; et al. Plasma MicroRNA Panel to Diagnose Hepatitis B Virus-Related Hepatocellular Carcinoma. J. Clin. Oncol. 2011, 29, 4781–4788. [Google Scholar] [CrossRef] [PubMed]
- Jehn, J.; Trudzinski, F.; Horos, R.; Schenz, J.; Uhle, F.; Weigand, M.A.; Frank, M.; Kahraman, M.; Heuvelman, M.; Sikosek, T.; et al. MiR-Blood—A Small RNA Atlas of Human Blood Components. Sci. Data 2024, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Cabral, B.C.A.; Hoffmann, L.; Bottaro, T.; Costa, P.F.; Ramos, A.L.A.; Coelho, H.S.M.; Villela-Nogueira, C.A.; Ürményi, T.P.; Faffe, D.S.; Silva, R. Circulating MicroRNAs Associated with Liver Fibrosis in Chronic Hepatitis C Patients. Biochem. Biophys. Rep. 2020, 24, 100814. [Google Scholar] [CrossRef]
- Taher, A.T.; Musallam, K.M.; Cappellini, M.D. β-Thalassemias. N. Engl. J. Med. 2021, 384, 727–743. [Google Scholar] [CrossRef]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on Non-Invasive Tests for Evaluation of Liver Disease Severity and Prognosis–2021 Update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Szabo, G.; Bala, S. MicroRNAs in Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 542–552. [Google Scholar] [CrossRef]
- Do Amaral, A.E.; Rode, M.P.; Cisilotto, J.; Da Silva, T.E.; Fischer, J.; Matiollo, C.; De Morais Rateke, E.C.; Narciso-Schiavon, J.L.; Schiavon, L.L.; Creczynski-Pasa, T.B. MicroRNA Profiles in Serum Samples from Patients with Stable Cirrhosis and MiRNA-21 as a Predictor of Transplant-Free Survival. Pharmacol. Res. 2018, 134, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Choi, S.S.; Diehl, A.M.; Jung, Y. Potential Role of Hedgehog Signaling and MicroRNA-29 in Liver Fibrosis of IKKβ-Deficient Mouse. J. Mol. Histol. 2014, 45, 103–112. [Google Scholar] [CrossRef]
- Cui, C.; Zhong, B.; Fan, R.; Cui, Q. HMDD v4.0: A Database for Experimentally Supported Human MicroRNA-Disease Associations. Nucleic Acids Res. 2024, 52, D1327–D1332. [Google Scholar] [CrossRef]
- Cermelli, S.; Ruggieri, A.; Marrero, J.A.; Ioannou, G.N.; Beretta, L. Circulating MicroRNAs in Patients with Chronic Hepatitis C and Non-Alcoholic Fatty Liver Disease. PLoS ONE 2011, 6, e23937. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Luedde, T. Circulating MicroRNAs as Markers of Liver Inflammation, Fibrosis and Cancer. J. Hepatol. 2014, 61, 1434–1437. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, X.; Zhou, X. Association between Diagnostic MicroRNAs of Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes: A Meta-Analysis. Indian J. Pharm. Sci. 2023, 85, 211–222. [Google Scholar] [CrossRef]
- Erceg, S.; Munjas, J.; Sopić, M.; Tomašević, R.; Mitrović, M.; Kotur-Stevuljević, J.; Mamić, M.; Vujčić, S.; Klisic, A.; Ninić, A. Expression Analysis of Circulating MiR-21, MiR-34a and MiR-122 and Redox Status Markers in Metabolic Dysfunction-Associated Steatotic Liver Disease Patients with and Without Type 2 Diabetes. Int. J. Mol. Sci. 2025, 26, 2392. [Google Scholar] [CrossRef]
- Heraclides, A.; Bashiardes, E.; Fernández-Domínguez, E.; Bertoncini, S.; Chimonas, M.; Christofi, V.; King, J.; Budowle, B.; Manoli, P.; Cariolou, M.A. Y-Chromosomal Analysis of Greek Cypriots Reveals a Primarily Common Pre-Ottoman Paternal Ancestry with Turkish Cypriots. PLoS ONE 2017, 12, e0179474. [Google Scholar] [CrossRef]
- Das, S.S.; Das, S.; Byram, P.K.; Rahaman, M.; Dolai, T.K.; Chatterjee, A.; Chakravorty, N. MicroRNA Expression Patterns in HbE/β-Thalassemia Patients: The Passwords to Unlock Fetal Hemoglobin Expression in β-Hemoglobinopathies. Blood Cells Mol. Dis. 2021, 87, 102523. [Google Scholar] [CrossRef]
- Wang, H.; Chen, M.; Xu, S.; Pan, Y.; Zhang, Y.; Huang, H.; Xu, L. Abnormal Regulation of MicroRNAs and Related Genes in Pediatric β-Thalassemia. J. Clin. Lab. Anal. 2021, 35, e23945. [Google Scholar] [CrossRef] [PubMed]
- El-Khazragy, N.; Matbouly, S.; Hanna, D.H.; Mahran, N.A.; Mostafa, S.A.; Abdelrehim, B.A.; Farouk, Y.K.; Abuelela, S. Circulating MiRNAs and Tissue Iron Overload in Transfusion-Dependent β-Thalassemia Major: Novel Predictors and Follow-up Guide. Ann. Hematol. 2021, 100, 2909–2917. [Google Scholar] [CrossRef]
- Alsop, E.; Meechoovet, B.; Kitchen, R.; Sweeney, T.; Beach, T.G.; Serrano, G.E.; Hutchins, E.; Ghiran, I.; Reiman, R.; Syring, M.; et al. A Novel Tissue Atlas and Online Tool for the Interrogation of Small RNA Expression in Human Tissues and Biofluids. Front. Cell Dev. Biol. 2022, 10, 804164. [Google Scholar] [CrossRef] [PubMed]
- Fehlmann, T.; Ludwig, N.; Backes, C.; Meese, E.; Keller, A. Distribution of MicroRNA Biomarker Candidates in Solid Tissues and Body Fluids. RNA Biol. 2016, 13, 1084–1088. [Google Scholar] [CrossRef]
- Hamza, M.; Sankhyan, D.; Shukla, S.; Pandey, P. Advances in Body Fluid Identification: MiRNA Markers as Powerful Tool. Int. J. Legal Med. 2024, 138, 1223–1232. [Google Scholar] [CrossRef]
- Wolenski, F.S.; Shah, P.; Sano, T.; Shinozawa, T.; Bernard, H.; Gallacher, M.J.; Wyllie, S.D.; Varrone, G.; Cicia, L.A.; Carsillo, M.E.; et al. Identification of MicroRNA Biomarker Candidates in Urine and Plasma from Rats with Kidney or Liver Damage. J. Appl. Toxicol. 2017, 37, 278–286. [Google Scholar] [CrossRef]
- Zhou, G.; Zeng, Y.; Luo, Y.; Guo, S.; Bao, L.; Zhang, Q. Urine MiR-93-5p Is a Promising Biomarker for Early Detection of HBV-Related Hepatocellular Carcinoma. Eur. J. Surg. Oncol. 2022, 48, 95–102. [Google Scholar] [CrossRef]
- Su, J.; Wang, M.; Lin, P.; Huang, Z.; Li, G.; Chen, X.; Yan, H.; Zhou, L. Trigger-Activated Autonomous DNA Machine for Amplified Liver Cancer Biomarker MicroRNA21 Imaging. Anal. Sci. 2023, 39, 1661–1667. [Google Scholar] [CrossRef]
- Cimmino, W.; Migliorelli, D.; Singh, S.; Miglione, A.; Generelli, S.; Cinti, S. Design of a Printed Electrochemical Strip towards MiRNA-21 Detection in Urine Samples: Optimization of the Experimental Procedures for Real Sample Application. Anal. Bioanal. Chem. 2023, 415, 4511–4520. [Google Scholar] [CrossRef] [PubMed]
- Záveský, L.; Slanař, O. Discovery and Evaluation of Extracellular MicroRNA Biomarkers in Plasma, Ascites, and Urine. Methods Mol. Biol. 2023, 2630, 135–143. [Google Scholar] [CrossRef]
- Yang, X.; Greenhaw, J.; Shi, Q.; Su, Z.; Qian, F.; Davis, K.; Mendrick, D.L.; Salminen, W.F. Identification of Urinary MicroRNA Profiles in Rats That May Diagnose Hepatotoxicity. Toxicol. Sci. 2012, 125, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Poynard, T. An Algorithm for the Grading of Activity in Chronic Hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef] [PubMed]

| miRNA | miRNA Sequence (5′ to 3′) | Reason for Inclusion (Reported Abundance *) | References |
|---|---|---|---|
| hsa-miR-16-5p | UAGCAGCACGUAAAUAUUGGCG | Reported as endogenous reference miRNA in plasma-based assays (16.7 [15.4, 17.9]) | [34,35,36,37,38] |
| hsa-miR-221-3p | UGAGGUAGUAGGUUGUAUAGUU | Reported as endogenous reference miRNA in plasma-based assays (5.9 [3.9, 6.9]) | [39,40,41] |
| hsa-let-7a-5p | UAGCUUAUCAGACUGAUGUUGA | Downregulated circulating hepatic biomarker (7.25 [5.91, 8.39]) | [42,43,44,45] |
| hsa-miR-21-5p | ACUGAUUUCUUUUGGUGUUCAG | Upregulated circulating hepatic biomarker (9.4 [8.5, 10.4]) | [37,38,46,47,48,49,50] |
| hsa-miR-29a-5p | UGGCAGUGUCUUAGCUGGUUGU | Upregulated circulating hepatic biomarker (0 [0, 0.67]) | [51,52] |
| hsa-miR-34a-5p | UGGAGUGUGACAAUGGUGUUUG | Upregulated circulating hepatic biomarker (2.4 [0, 3.6]) | [37,53,54,55] |
| hsa-miR-122-5p | AGCUACAUUGUCUGCUGGGUUUC | Upregulated circulating hepatic biomarker (10.1 [8.1, 12.0]) | [38,55,56] |
| Fibrosis Stage | F0–F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Age: mean ± SD | 48.4 ± 9.2 | 51.7 ± 7.3 | 50.5 ± 3.9 | 57.7 ± 10.3 |
| Number of patients, n | 15 | 12 | 6 | 7 |
| Patients | ||||
| Male | 5 (33.3%) | 8 (66.7%) | 4 (66.7%) | 4 (57.1%) |
| Female | 10 (66.6%) | 4 (33.3%) | 2 (33.3%) | 3 (42.9%) |
| LSM (kPa): mean ± SD | 5.84 ± 1.07 | 6.84 ± 0.89 | 10.35 ± 1.27 | 14.96 ± 3.91 |
| Fibrosis Stage (n **) | let-7a | miR-21 | miR-29a | miR-34a | miR-122 |
|---|---|---|---|---|---|
| F0–F1 (15) | 4.52 (4.15–4.93) | 0.17 (−0.075–0.43) | 2.84 (2.26–3.27) | 7.79 (7.04–8.40) | 2.42 (1.94–3.50) |
| F2 (12) | 4.73 (4.46–5.03) | 0.25 (0.068–0.63) | 2.85 (2.53–3.02) | 7.66 (6.44–7.91) | 2.15 (0.96–3.00) |
| F3 (6) | 4.33 (4.23–4.62) | 0.81 (0.20–1.04) | 2.59 (2.38–2.99) | 7.42 (6.53–7.66) | 3.10 (1.09–4.34) |
| F4 (7) | 4.49 (3.66–4.75) | 0.09 (−0.36–0.49) | 3.23 (2.52–4.33) | 7.66 (7.38–8.08) | 2.71 (2.20–3.82) |
| p-value | 0.37 | 0.11 | 0.69 | 0.40 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özkaramehmet, S.; Andreou, S.; Yiangou, K.; Christou, S.; Hadjigavriel, M.; Sitarou, M.; Pyrovolaki, K.; Papanicolaou, E.; Flourou, C.; Savvidou, I.; et al. Evaluation of Five Plasma miRNAs as Biomarkers for Minimally Invasive Staging of Liver Fibrosis in β-Thalassaemia Patients. Int. J. Mol. Sci. 2025, 26, 9543. https://doi.org/10.3390/ijms26199543
Özkaramehmet S, Andreou S, Yiangou K, Christou S, Hadjigavriel M, Sitarou M, Pyrovolaki K, Papanicolaou E, Flourou C, Savvidou I, et al. Evaluation of Five Plasma miRNAs as Biomarkers for Minimally Invasive Staging of Liver Fibrosis in β-Thalassaemia Patients. International Journal of Molecular Sciences. 2025; 26(19):9543. https://doi.org/10.3390/ijms26199543
Chicago/Turabian StyleÖzkaramehmet, Sevgi, Savanna Andreou, Kristia Yiangou, Soteroula Christou, Michalis Hadjigavriel, Maria Sitarou, Katerina Pyrovolaki, Eleni Papanicolaou, Christina Flourou, Irene Savvidou, and et al. 2025. "Evaluation of Five Plasma miRNAs as Biomarkers for Minimally Invasive Staging of Liver Fibrosis in β-Thalassaemia Patients" International Journal of Molecular Sciences 26, no. 19: 9543. https://doi.org/10.3390/ijms26199543
APA StyleÖzkaramehmet, S., Andreou, S., Yiangou, K., Christou, S., Hadjigavriel, M., Sitarou, M., Pyrovolaki, K., Papanicolaou, E., Flourou, C., Savvidou, I., Boutsikos, P., Mendoni, A., Kleanthous, M., Phylactides, M., & Lederer, C. W. (2025). Evaluation of Five Plasma miRNAs as Biomarkers for Minimally Invasive Staging of Liver Fibrosis in β-Thalassaemia Patients. International Journal of Molecular Sciences, 26(19), 9543. https://doi.org/10.3390/ijms26199543









