Unveiling the Microbiome’s Role in Hidradenitis Suppurativa: A Comprehensive Review of Pathogenetic Mechanisms
Abstract
1. Introduction
2. Microbiome
2.1. General Concepts
2.2. Dysbiosis
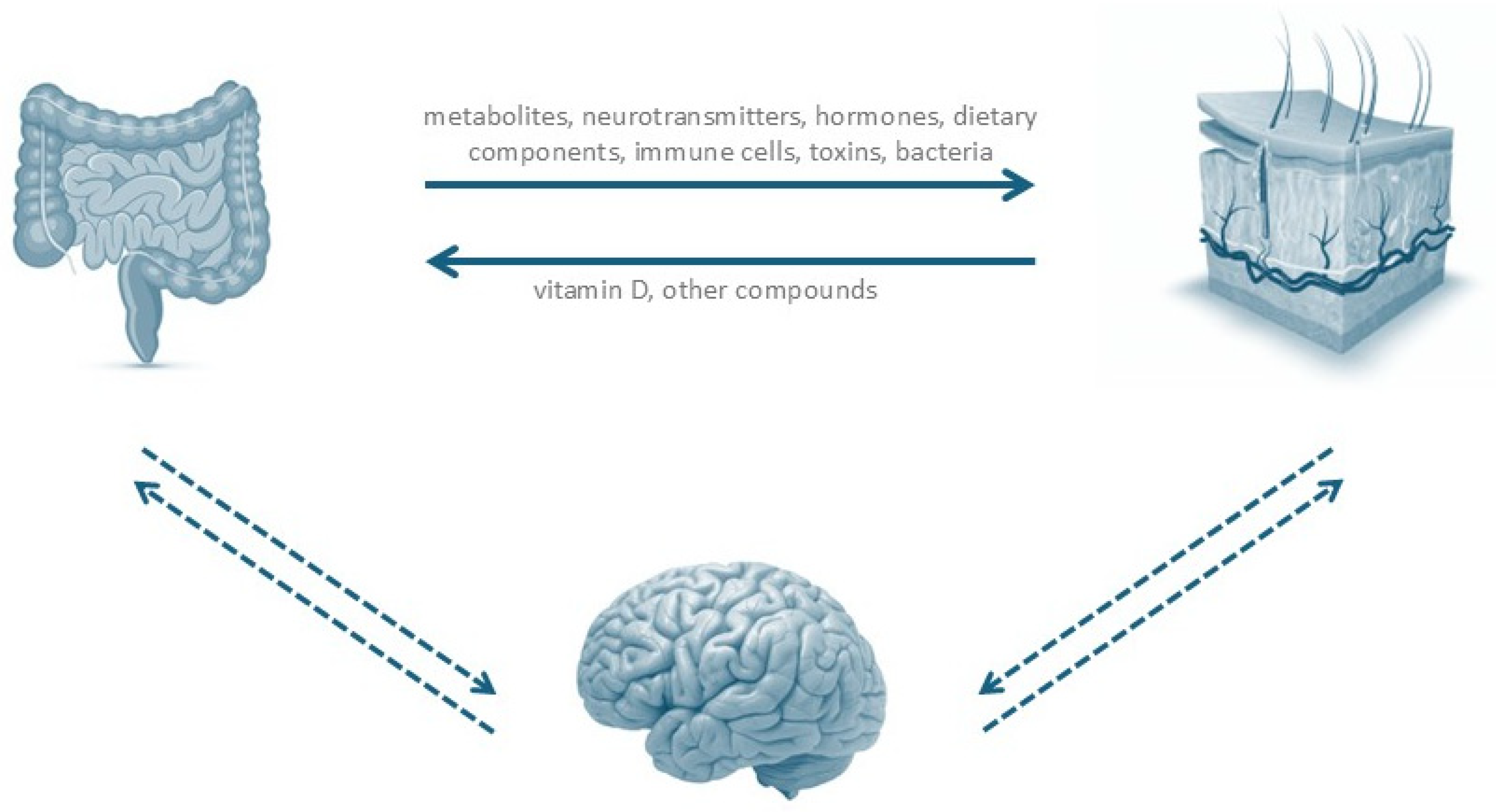
2.3. Gut–Skin Axis
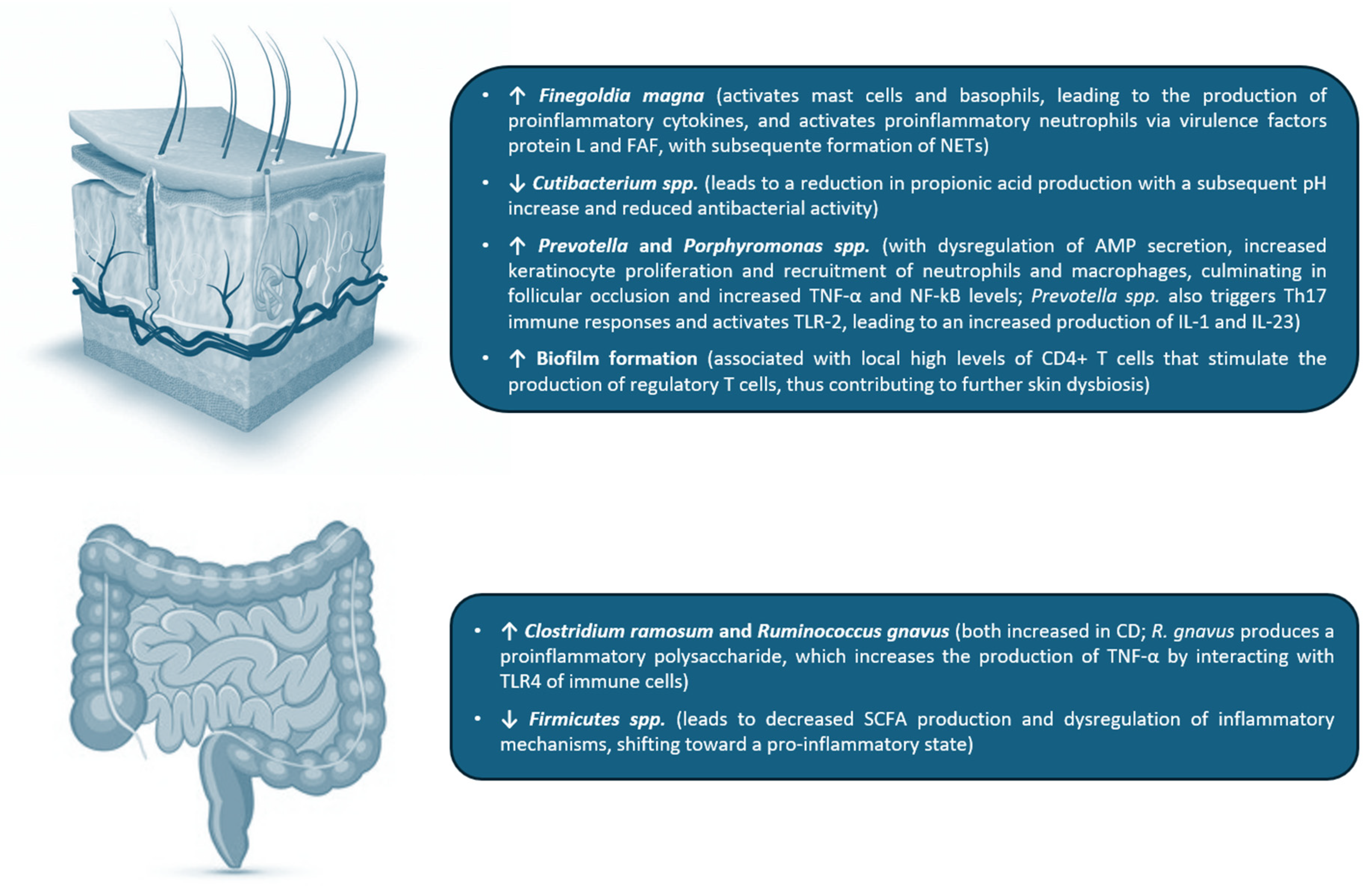
2.4. Cutaneous Microbiome in HS
2.5. Intestinal Microbiome in HS
2.6. Blood Microbiome in HS
2.7. Methodological Limitations
3. Comorbidities in HS and Microbiome
3.1. Obesity and Smoking
3.2. Inflammatory Bowel Disease
4. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agnese, E.R.; Tariche, N.; Sharma, A.; Gulati, R. The Pathogenesis and Treatment of Hidradenitis Suppurativa. Cureus 2023, 15, e49390. [Google Scholar] [CrossRef]
- Wark, K.J.L.; Cains, G.D. The Microbiome in Hidradenitis Suppurativa: A Review. Dermatol. Ther. 2021, 11, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Świerczewska, Z.; Lewandowski, M.; Surowiecka, A.; Barańska-Rybak, W. Microbiome in Hidradenitis Suppurativa—What We Know and Where We Are Heading. Int. J. Mol. Sci. 2022, 23, 11280. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, S.; Barrett, M.; Kirthi, S.; Pellanda, P.; Vlckova, K.; Tobin, A.M.; Murphy, M.; Shanahan, F.; O’Toole, P.W. Altered Skin and Gut Microbiome in Hidradenitis Suppurativa. J. Investig. Dermatol. 2022, 142, 459–468.e15. [Google Scholar] [CrossRef]
- Schell, S.L.; Schneider, A.M.; Nelson, A.M. Yin and Yang: A Disrupted Skin Microbiome and an Aberrant Host Immune Response in Hidradenitis Suppurativa. Exp. Dermatol. 2021, 30, 1453–1470. [Google Scholar] [CrossRef]
- Jiang, S.W.; Whitley, M.J.; Mariottoni, P.; Jaleel, T.; MacLeod, A.S. Hidradenitis Suppurativa: Host-Microbe and Immune Pathogenesis Underlie Important Future Directions. JID Innov. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Chung, M.G.; Preda-Naumescu, A.; Yusuf, N. Hidradenitis Suppurativa: Consequences of Microbiome Dysbiosis on Immune Dysregulation and Disease Severity. Indian J. Dermatol. 2022, 67, 699–704. [Google Scholar] [CrossRef]
- Rosi, E.; Guerra, P.; Silvi, G.; Nunziati, G.; Scandagli, I.; Di Cesare, A.; Prignano, F. Consistency of Bacterial Triggers in the Pathogenesis of Hidradenitis Suppurativa. Vaccines 2023, 11, 179. [Google Scholar] [CrossRef]
- Lelonek, E.; Bouazzi, D.; Jemec, G.B.E.; Szepietowski, J.C. Skin and Gut Microbiome in Hidradenitis Suppurativa: A Systematic Review. Biomedicines 2023, 11, 2277. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Benhadou, F.; Byrd, A.S.; Chandran, N.S.; Giamarellos-Bourboulis, E.J.; Fabbrocini, G.; Frew, J.W.; Fujita, H.; González-López, M.A.; Guillem, P.; et al. What Causes Hidradenitis Suppurativa?—15 Years After. Exp. Dermatol. 2020, 29, 1154–1170. [Google Scholar] [CrossRef]
- Chopra, D.; Arens, R.A.; Amornpairoj, W.; Lowes, M.A.; Tomic-Canic, M.; Strbo, N.; Lev-Tov, H.; Pastar, I. Innate Immunity and Microbial Dysbiosis in Hidradenitis Suppurativa—Vicious Cycle of Chronic Inflammation. Front. Immunol. 2022, 13, 960488. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.; Mallonee, C.J.; Stanisic, D.; Homme, R.P.; George, A.K.; Singh, M.; Tyagi, S.C. Hidradenitis Suppurativa and 1-Carbon Metabolism: Role of Gut Microbiome, Matrix Metalloproteinases, and Hyperhomocysteinemia. Front. Immunol. 2020, 11, 1730. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the Human Microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef]
- Balato, A.; Cacciapuoti, S.; Di Caprio, R.; Marasca, C.; Masarà, A.; Raimondo, A.; Fabbrocini, G. Human Microbiome: Composition and Role in Inflammatory Skin Diseases. Arch. Immunol. Ther. Exp. 2019, 67, 1–18. [Google Scholar] [CrossRef]
- Mintoff, D.; Borg, I.; Pace, N.P. The Clinical Relevance of the Microbiome in Hidradenitis Suppurativa: A Systematic Review. Vaccines 2021, 9, 1076. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of Gut Microbiome on Skin Health: Gut-Skin Axis Observed through the Lenses of Therapeutics and Skin Diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef]
- Niemeyer-van der Kolk, T.; van der Wall, H.E.C.; Balmforth, C.; Van Doorn, M.B.A.; Rissmann, R. A Systematic Literature Review of the Human Skin Microbiome as Biomarker for Dermatological Drug Development. Br. J. Clin. Pharmacol. 2018, 84, 2178–2193. [Google Scholar] [CrossRef]
- Baglama, Š.Š.; Trčko, K. Skin and Gut Microbiota Dysbiosis in Autoimmune and Inflammatory Skin Diseases. Acta Dermatovenerol. Alp. Pannonica Adriat. 2022, 31, 105–109. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, X.; Cheng, G. Human Skin Bacterial Microbiota Homeostasis: A Delicate Balance between Health and Disease. mLife 2023, 2, 107–120. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Ravel, J. The Vocabulary of Microbiome Research: A Proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef]
- Olunoiki, E.; Rehner, J.; Bischoff, M.; Koshel, E.; Vogt, T.; Reichrath, J.; Becker, S.L. Characteristics of the Skin Microbiome in Selected Dermatological Conditions: A Narrative Review. Life 2022, 12, 1420. [Google Scholar] [CrossRef]
- Bay, L.; Ring, H.C. Human Skin Microbiota in Health and Disease: The Cutaneous Communities’ Interplay in Equilibrium and Dysbiosis. APMIS 2022, 130, 706–718. [Google Scholar] [CrossRef]
- Bay, L.; Jemec, G.B.; Ring, H.C. Microenvironmental Host-Microbe Interactions in Chronic Inflammatory Skin Diseases. APMIS 2024, 132, 974–984. [Google Scholar] [CrossRef]
- Smith, A.; Dumbrava, R.; Ghori, N.U.H.; Foster, R.; Campbell, J.; Duthie, A.; Hoyne, G.; Rademaker, M.; Bowen, A.C. An Overview of the Skin Microbiome, the Potential for Pathogen Shift, and Dysbiosis in Common Skin Pathologies. Microorganisms 2025, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Langan, E.A.; Recke, A.; Bokor-billmann, T.; Billmann, F.; Kahle, B.K.; Zillikens, D. The Role of the Cutaneous Microbiome in Hidradenitis Suppurativa—Light at the End of the Microbiological Tunnel. Int. J. Mol. Sci. 2020, 21, 1205. [Google Scholar] [CrossRef]
- Williams, S.C.; Frew, J.W.; Krueger, J.G. A Systematic Review and Critical Appraisal of Metagenomic and Culture Studies in Hidradenitis Suppurativa. Exp. Dermatol. 2021, 30, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Prast-Nielsen, S.; Tobin, A.M.; Adamzik, K.; Powles, A.; Hugerth, L.W.; Sweeney, C.; Kirby, B.; Engstrand, L.; Fry, L. Investigation of the Skin Microbiome: Swabs vs. Biopsies. Br. J. Dermatol. 2019, 181, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Naik, H.B.; Piguet, V. Standardizing Hidradenitis Suppurativa Skin Microbiome Research: The Methods Matter. J. Investig. Dermatol. 2020, 140, 1688–1690. [Google Scholar] [CrossRef]
- Ring, H.C.; Sigsgaard, V.; Thorsen, J.; Fuursted, K.; Fabricius, S.; Saunte, D.M.; Jemec, G.B. The Microbiome of Tunnels in Hidradenitis Suppurativa Patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1775–1780. [Google Scholar] [CrossRef]
- Taha, M.R.; Tyring, S.K. A Review of the Role and Treatment of Biofilms in Skin Disorders. Ski. Ther. Lett. 2024, 29, 6–9. [Google Scholar]
- Lousada, M.B.; Lachnit, T.; Edelkamp, J.; Rouillé, T.; Ajdic, D.; Uchida, Y.; Di Nardo, A.; Bosch, T.C.G.; Paus, R. Exploring the Human Hair Follicle Microbiome. Br. J. Dermatol. 2021, 184, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.C.; Saborowski, V.; Lange, S.; Kern, W.V.; Bruckner-Tuderman, L.; Rieg, S. Expression of Innate Defense Antimicrobial Peptides in Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 2012, 66, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Thomi, R.; Schlapbach, C.; Yawalkar, N.; Simon, D.; Yerly, D.; Hunger, R.E. Elevated Levels of the Antimicrobial Peptide LL-37 in Hidradenitis Suppurativa Are Associated with a Th1/Th17 Immune Response. Exp. Dermatol. 2018, 27, 172–177. [Google Scholar] [CrossRef]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The Gut-Skin Axis in Health and Disease: A Paradigm with Therapeutic Implications. BioEssays 2016, 38, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Cannistrà, C.; Finocchi, V.; Trivisonno, A.; Tambasco, D. New Perspectives in the Treatment of Hidradenitis Suppurativa: Surgery and Brewer’s Yeast-Exclusion Diet. Surgery 2013, 154, 1126–1130. [Google Scholar] [CrossRef]
- Guet-Revillet, H.; Coignard-Biehler, H.; Jais, J.P.; Quesne, G.; Frapy, E.; Poirée, S.; Le Guern, A.S.; Le Flèche-Matéos, A.; Hovnanian, A.; Consigny, P.H.; et al. Bacterial Pathogens Associated with Hidradenitis Suppurativa, France. Emerg. Infect. Dis. 2014, 20, 1990–1998. [Google Scholar] [CrossRef]
- Ring, H.C.; Bay, L.; Kallenbach, K.; Miller, I.M.; Prens, E.; Saunte, D.M.; Bjarnsholt, T.; Jemec, G.B.E. Normal Skin Microbiota Is Altered in Pre-Clinical Hidradenitis Suppurativa. Acta Derm. Venereol. 2017, 97, 208–213. [Google Scholar] [CrossRef]
- Ring, H.C.; Thorsen, J.; Saunte, D.M.; Lilje, B.; Bay, L.; Riis, P.T.; Larsen, N.; Andersen, L.O.; Nielsen, H.V.; Miller, I.M.; et al. The Follicular Skin Microbiome in Patients with Hidradenitis Suppurativa and Healthy Controls. JAMA Dermatol. 2017, 153, 897–905. [Google Scholar] [CrossRef]
- Ring, H.C.; Bay, L.; Nilsson, M.; Kallenbach, K.; Miller, I.M.; Saunte, D.M.; Bjarnsholt, T.; Tolker-Nielsen, T.; Jemec, G.B. Bacterial Biofilm in Chronic Lesions of Hidradenitis Suppurativa. Br. J. Dermatol. 2017, 176, 993–1000. [Google Scholar] [CrossRef]
- Guet-Revillet, H.; Jais, J.-P.; Ungeheuer, M.-N.; Coignard-Biehler, H.; Duchatelet, S.; Delage, M.; Lam, T.; Hovnanian, A.; Lortholary, O.; Nassif, X.; et al. The Microbiological Landscape of Anaerobic Infections in Hidradenitis Suppurativa: A Prospective Metagenomic Study. Clin. Infect. Dis. 2017, 65, 282–291. [Google Scholar] [CrossRef]
- Riverain-Gillet, É.; Guet-Revillet, H.; Jais, J.P.; Ungeheuer, M.N.; Duchatelet, S.; Delage, M.; Lam, T.; Hovnanian, A.; Nassif, A.; Join-Lambert, O. The Surface Microbiome of Clinically Unaffected Skinfolds in Hidradenitis Suppurativa: A Cross-Sectional Culture-Based and 16S RRNA Gene Amplicon Sequencing Study in 60 Patients. J. Investig. Dermatol. 2020, 140, 1847–1855.e6. [Google Scholar] [CrossRef]
- Schneider, A.M.; Cook, L.C.; Zhan, X.; Banerjee, K.; Cong, Z.; Imamura-Kawasawa, Y.; Gettle, S.L.; Longenecker, A.L.; Kirby, J.S.; Nelson, A.M. Loss of Skin Microbial Diversity and Alteration of Bacterial Metabolic Function in Hidradenitis Suppurativa. J. Investig. Dermatol. 2020, 140, 716–720. [Google Scholar] [CrossRef]
- Ring, H.C.; Thorsen, J.; Jørgensen, A.H.; Bay, L.; Bjarnsholt, T.; Fuursted, K.; Thomsen, S.F.; Jemec, G.B. Predictive Metagenomic Analysis Reveals a Role of Cutaneous Dysbiosis in the Development of Hidradenitis Suppurativa. J. Investig. Dermatol. 2020, 140, 1473–1476. [Google Scholar] [CrossRef]
- Naik, H.B.; Jo, J.H.; Paul, M.; Kong, H.H. Skin Microbiota Perturbations Are Distinct and Disease Severity–Dependent in Hidradenitis Suppurativa. J. Investig. Dermatol. 2020, 140, 922–925.e3. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, N.; Muirhead, G.; Prast-Nielsen, S.; Jeanmougin, M.; Olah, P.; Skoog, T.; Jules-Clement, G.; Feld, M.; Barrientos-Somarribas, M.; Sinkko, H.; et al. Microbe-Host Interplay in Atopic Dermatitis and Psoriasis. Nat. Commun. 2019, 10, 4703. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.S.; Carmona-Rivera, C.; O’Neil, L.J.; Carlucci, P.M.; Cisar, C.; Rosenberg, A.Z.; Kerns, M.L.; Caffrey, J.A.; Milner, S.M.; Sacks, J.M.; et al. Neutrophil Extracellular Traps, B Cells, and Type I Interferons Contribute to Immune Dysregulation in Hidradenitis Suppurativa. Sci. Transl. Med. 2019, 11, eaav5908. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Björck, L.; Frick, I.M. Finegoldia Magna, an Anaerobic Gram-Positive Bacterium of the Normal Human Microbiota, Induces Inflammation by Activating Neutrophils. Front. Microbiol. 2020, 11, 65. [Google Scholar] [CrossRef]
- Hsu, T.J.; Yeh, H.H.; Lee, C.H.; Tseng, H.C. The Temporal Evolution of Distinct Skin Surface Microbiome in Asian Patients with Severe Hidradenitis Suppurativa during Effective Adalimumab Treatment. J. Investig. Dermatol. 2022, 142, 740–743.e2. [Google Scholar] [CrossRef]
- Pardo, L.M.; Wang, C.; Ardon, C.B.; Kraaij, R.; Prens, E.P.; Van Straalen, K.R. Bacterial Microbiota Composition in Hidradenitis Suppurativa Differs per Skin Layer. J. Investig. Dermatol. 2024, 144, 426–430.e5. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, W.; Wang, M.; Wang, X.; Wang, S. Causal Roles of Skin and Gut Microbiota in Skin Appendage Disorders Suggested by Genetic Study. Front. Immunol. 2024, 15, 1427276. [Google Scholar] [CrossRef]
- Guo, S.; Li, P.; Lu, J.; Zhou, P.; Sun, B.; Wang, J. Causal Relationship between Skin Microbiota and Hidradenitis Suppurativa: A Two-Sample Mendelian Randomization Study. Arch. Dermatol. Res. 2025, 317, 238. [Google Scholar] [CrossRef]
- Ring, H.C.; Thorsen, J.; Fuursted, K.; Bjarnsholt, T.; Bay, L.; Egeberg, A.; Ingham, A.C.; Vedel Nielsen, H.; Frew, J.W.; Saunte, D.M.L.; et al. Amplicon Sequencing Demonstrates Comparable Follicular Mycobiomes in Patients with Hidradenitis Suppurativa Compared with Healthy Controls. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e580–e583. [Google Scholar] [CrossRef]
- Jørgensen, A.H.R.; Thomsen, S.F.; Karmisholt, K.E.; Ring, H.C. Clinical, Microbiological, Immunological and Imaging Characteristics of Tunnels and Fistulas in Hidradenitis Suppurativa and Crohn’s Disease. Exp. Dermatol. 2020, 29, 118–123. [Google Scholar] [CrossRef]
- Larsen, J.M. The Immune Response to Prevotella Bacteria in Chronic Inflammatory Disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Eppinga, H.; Sperna Weiland, C.J.; Thio, H.B.; van der Woude, C.J.; Nijsten, T.E.C.; Peppelenbosch, M.P.; Konstantinov, S.R. Similar Depletion of Protective Faecalibacterium Prausnitzii in Psoriasis and Inflammatory Bowel Disease, but Not in Hidradenitis Suppurativa. J. Crohns Colitis 2016, 10, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Kam, S.; Collard, M.; Lam, J.; Alani, R.M. Gut Microbiome Perturbations in Patients with Hidradenitis Suppurativa: A Case Series. J. Investig. Dermatol. 2021, 141, 225–228.e2. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.Y.; Radjabzadeh, D.; Eppinga, H.; Nossent, Y.R.A.; van der Zee, H.H.; Kraaij, R.; Konstantinov, S.R.; Fuhler, G.M.; Prens, E.P.; Thio, H.B.; et al. A Microbiome Study to Explore the Gut-Skin Axis in Hidradenitis Suppurativa. J. Dermatol. Sci. 2021, 101, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.B.; Das, A.; O’Herlihy, E.; Coughlan, S.; Cisek, K.; Moore, M.; Bradley, F.; Carty, T.; Pradhan, M.; Dwibedi, C.; et al. Differences in Fecal Microbiomes and Metabolomes of People With vs Without Irritable Bowel Syndrome and Bile Acid Malabsorption. Gastroenterology 2020, 158, 1016–1028.e8. [Google Scholar] [CrossRef]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the Faecal Microbiota in Patients with Crohn’s Disease and Their Unaffected Relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef]
- Breban, M.; Tap, J.; Leboime, A.; Said-Nahal, R.; Langella, P.; Chiocchia, G.; Furet, J.P.; Sokol, H. Faecal Microbiota Study Reveals Specific Dysbiosis in Spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 1614–1622. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus Gnavus, a Member of the Human Gut Microbiome Associated with Crohn’s Disease, Produces an Inflammatory Polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Clooney, A.G.; Eckenberger, J.; Laserna-Mendieta, E.; Sexton, K.A.; Bernstein, M.T.; Vagianos, K.; Sargent, M.; Ryan, F.J.; Moran, C.; Sheehan, D.; et al. Ranking Microbiome Variance in Inflammatory Bowel Disease: A Large Longitudinal Intercontinental Study. Gut 2021, 70, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Öğüt, N.D.; Hasçelik, G.; Atakan, N. Alterations of the Human Gut Microbiome in Patients with Hidradenitis Suppurativa: A Case-Control Study and Review of the Literature. Dermatol. Pract. Concept. 2022, 12, e2022191. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; McCarthy, S.; Hurley, C.; Ghosh, T.S.; Cooney, J.C.; Tobin, A.M.; Murphy, M.; O’Connor, E.M.; Shanahan, F.; O’Toole, P.W. Comparative Diet-Gut Microbiome Analysis in Crohn’s Disease and Hidradenitis Suppurativa. Front. Microbiol. 2023, 14, 1289374. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, X.; Li, X. Causal Relationship between Gut Microbiota and Hidradenitis Suppurativa: A Two-Sample Mendelian Randomization Study. Front. Microbiol. 2024, 15, 1302822. [Google Scholar] [CrossRef]
- Lelonek, E.; Szepietowski, J.C. Insights into Gut Microbiome Composition in Hidradenitis Suppurativa: A Comprehensive Examination of Dietary Habits and Environmental Influences. Nutrients 2024, 16, 1776. [Google Scholar] [CrossRef]
- Seetan, K.; Eldos, B.; Saraireh, M.; Omari, R.; Rubbai, Y.; Jayyusi, A.; Abu Jubran, M. Prevalence of Low Vitamin D Levels in Patients with Hidradenitis Suppurativa in Jordan: A Comparative Cross-Sectional Study. PLoS ONE 2022, 17, e0265672. [Google Scholar] [CrossRef]
- Bora, S.A.; Kennett, M.J.; Smith, P.B.; Patterson, A.D.; Cantorna, M.T. The Gut Microbiota Regulates Endocrine Vitamin D Metabolism through Fibroblast Growth Factor 23. Front. Immunol. 2018, 9, 408. [Google Scholar] [CrossRef]
- Ring, H.C.; Thorsen, J.; Saunte, D.M.; Lilje, B.; Bay, L.; Theut Riis, P.; Larsen, N.; O’Brien Andersen, L.; Vedel Nielsen, H.; Miller, I.M.; et al. Moderate to Severe Hidradenitis Suppurativa Patients Do Not Have an Altered Bacterial Composition in Peripheral Blood Compared to Healthy Controls. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 125–128. [Google Scholar] [CrossRef]
- Hispán, P.; Murcia, O.; Gonzalez-Villanueva, I.; Francés, R.; Giménez, P.; Riquelme, J.; Betlloch, I.; Pascual, J.C. Identification of Bacterial DNA in the Peripheral Blood of Patients with Active Hidradenitis Suppurativa. Arch. Dermatol. Res. 2020, 312, 159–163. [Google Scholar] [CrossRef]
- van der Zee, H.H.; Horvath, B.; Jemec, G.B.E.; Prens, E.P. The Association between Hidradenitis Suppurativa and Crohn’s Disease: In Search of the Missing Pathogenic Link. J. Investig. Dermatol. 2016, 136, 1747–1748. [Google Scholar] [CrossRef]
- Fleshner, L.; Roster, K.; Farabi, B.; Hirani, R.; Tepper, K.; Pitchumoni, C.S.; Safai, B.; Marmon, S. Follicular Skin Disorders, Inflammatory Bowel Disease, and the Microbiome: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10203. [Google Scholar] [CrossRef]
- Deckers, I.E.; Benhadou, F.; Koldijk, M.J.; del Marmol, V.; Horváth, B.; Boer, J.; van der Zee, H.H.; Prens, E.P. Inflammatory Bowel Disease Is Associated with Hidradenitis Suppurativa: Results from a Multicenter Cross-Sectional Study. J. Am. Acad. Dermatol. 2017, 76, 49–53. [Google Scholar] [CrossRef]
- Van Der Zee, H.H.; De Winter, K.; Van Der Woude, C.J.; Prens, E.P. The Prevalence of Hidradenitis Suppurativa in 1093 Patients with Inflammatory Bowel Disease. Br. J. Dermatol. 2014, 171, 673–675. [Google Scholar] [CrossRef]
- Marasca, C.; Donnarumma, M.; Annunziata, M.C.; Fabbrocini, G. Homocysteine Plasma Levels in Patients Affected by Hidradenitis Suppurativa: An Italian Experience. Clin. Exp. Dermatol. 2019, 44, e28–e29. [Google Scholar] [CrossRef]
| HS PATIENTS | NORMAL SKIN |
|---|---|
| Decrease microbiome diversity | Increased microbiome diversity |
| Increased abundance of anaerobic bacteria and opportunistic pathogens, (such as Porphyromonas, Peptoniphilus, Bacteroides, Peptostreptococcus, Pseudomonas and Prevotella spp.) | Decreased abundance of anaerobic bacteria and opportunistic pathogens, ubiquitous presence of Staphylococcus aureus and Streptococcus pyogenes |
| Loss of skin commensal species, such as Cutibacterium | Presence of skin commensal species, such as Cutibacterium |
| Bacterial biofilms commonly present | Bacterial biofilms usually absent |
| HS PATIENTS | NORMAL GUT |
|---|---|
| Lower overall microbial diversity and richness | Increased microbial diversity and richness |
| Higher abundance of pro-inflammatory bacteria, such as Proteobacteria and Actinobacteria | Lower abundance of pro-inflammatory bacteria, such as Proteobacteria and Actinobacteria |
| Lower abundance of anti-inflammatory bacteria, such as Firmicutes and Bacteroidetes | Higher abundance of anti-inflammatory bacteria, such as Firmicutes and Bacteroidetes |
| Presence of certain bacterial species potential implied in disease pathogenesis (such as Robinsoniella peoriensis, Bilophila, Holdemania, and Ruminococcus callidus) | Absence of these species |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queirós, C.; Lisboa, C.; Magina, S. Unveiling the Microbiome’s Role in Hidradenitis Suppurativa: A Comprehensive Review of Pathogenetic Mechanisms. Int. J. Mol. Sci. 2025, 26, 9542. https://doi.org/10.3390/ijms26199542
Queirós C, Lisboa C, Magina S. Unveiling the Microbiome’s Role in Hidradenitis Suppurativa: A Comprehensive Review of Pathogenetic Mechanisms. International Journal of Molecular Sciences. 2025; 26(19):9542. https://doi.org/10.3390/ijms26199542
Chicago/Turabian StyleQueirós, Catarina, Carmen Lisboa, and Sofia Magina. 2025. "Unveiling the Microbiome’s Role in Hidradenitis Suppurativa: A Comprehensive Review of Pathogenetic Mechanisms" International Journal of Molecular Sciences 26, no. 19: 9542. https://doi.org/10.3390/ijms26199542
APA StyleQueirós, C., Lisboa, C., & Magina, S. (2025). Unveiling the Microbiome’s Role in Hidradenitis Suppurativa: A Comprehensive Review of Pathogenetic Mechanisms. International Journal of Molecular Sciences, 26(19), 9542. https://doi.org/10.3390/ijms26199542





