Spontaneous Seizure Outcomes in Mice Using an Improved Version of the Pilocarpine Model of Temporal Lobe Epilepsy
Abstract
1. Introduction
2. Results
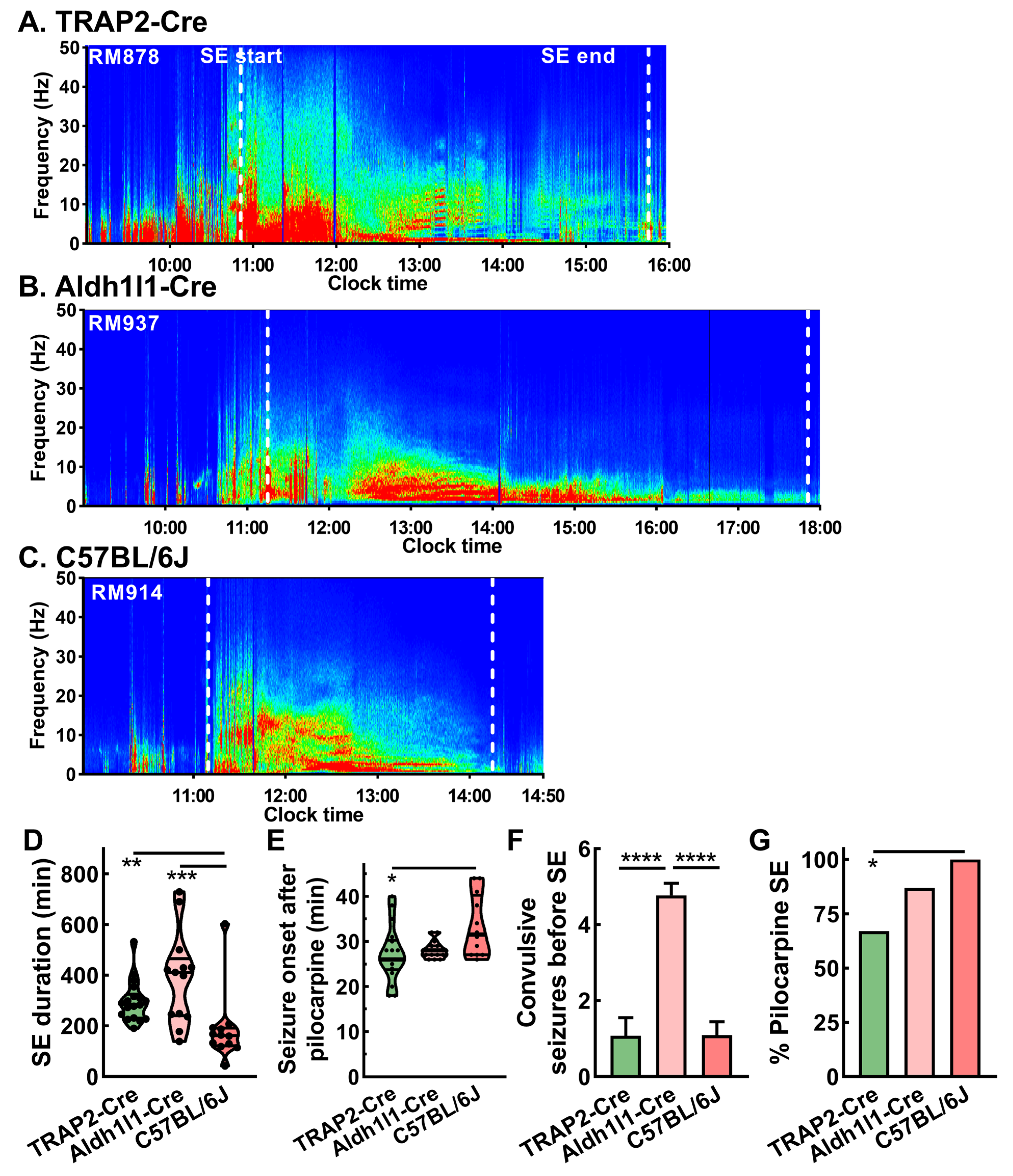
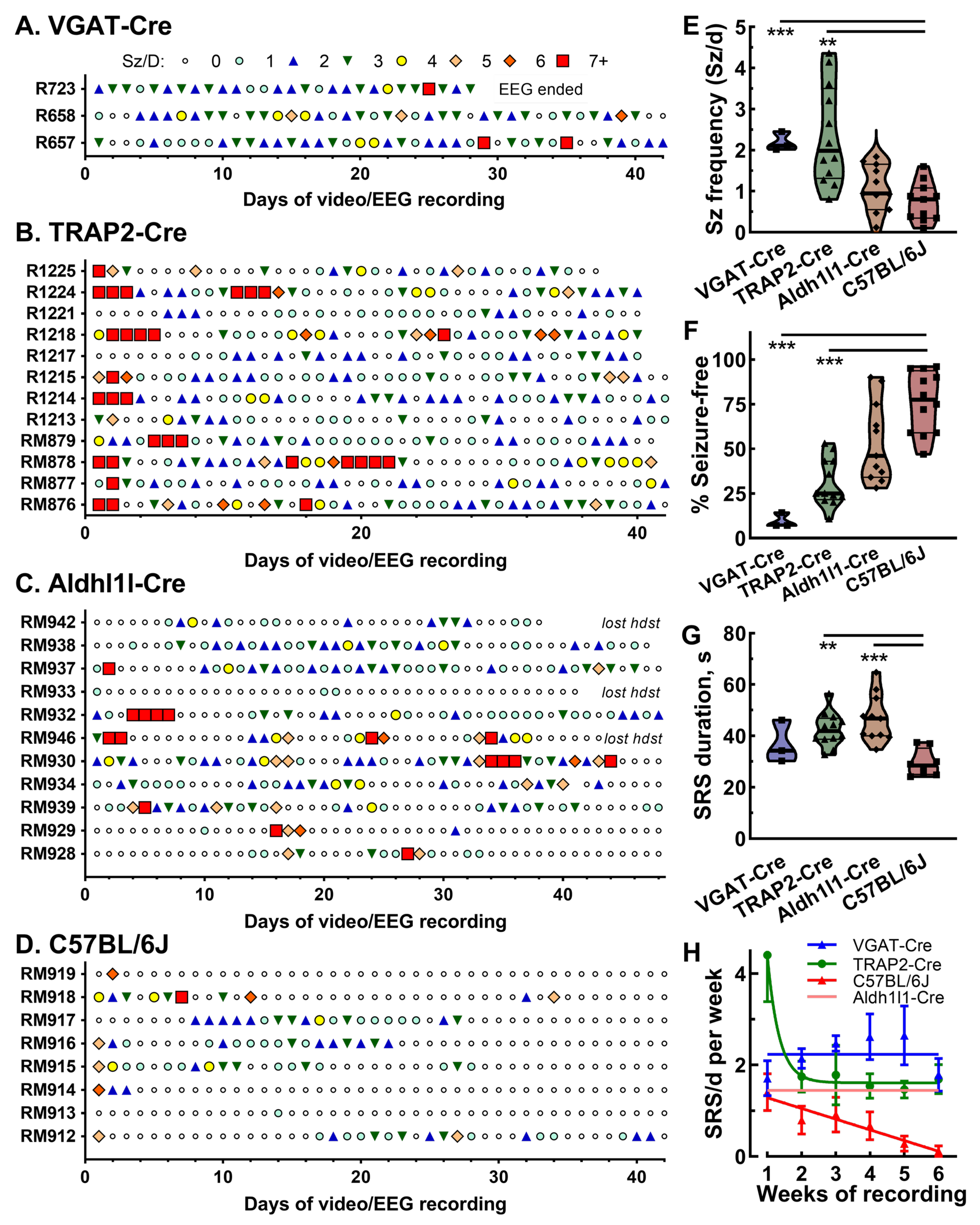
2.1. Seizure Patterns in Three Models of TLE
2.2. Development of Pharmacokinetic Model for Pilocarpine Dosing
2.3. Pilocarpine Induction with EEG
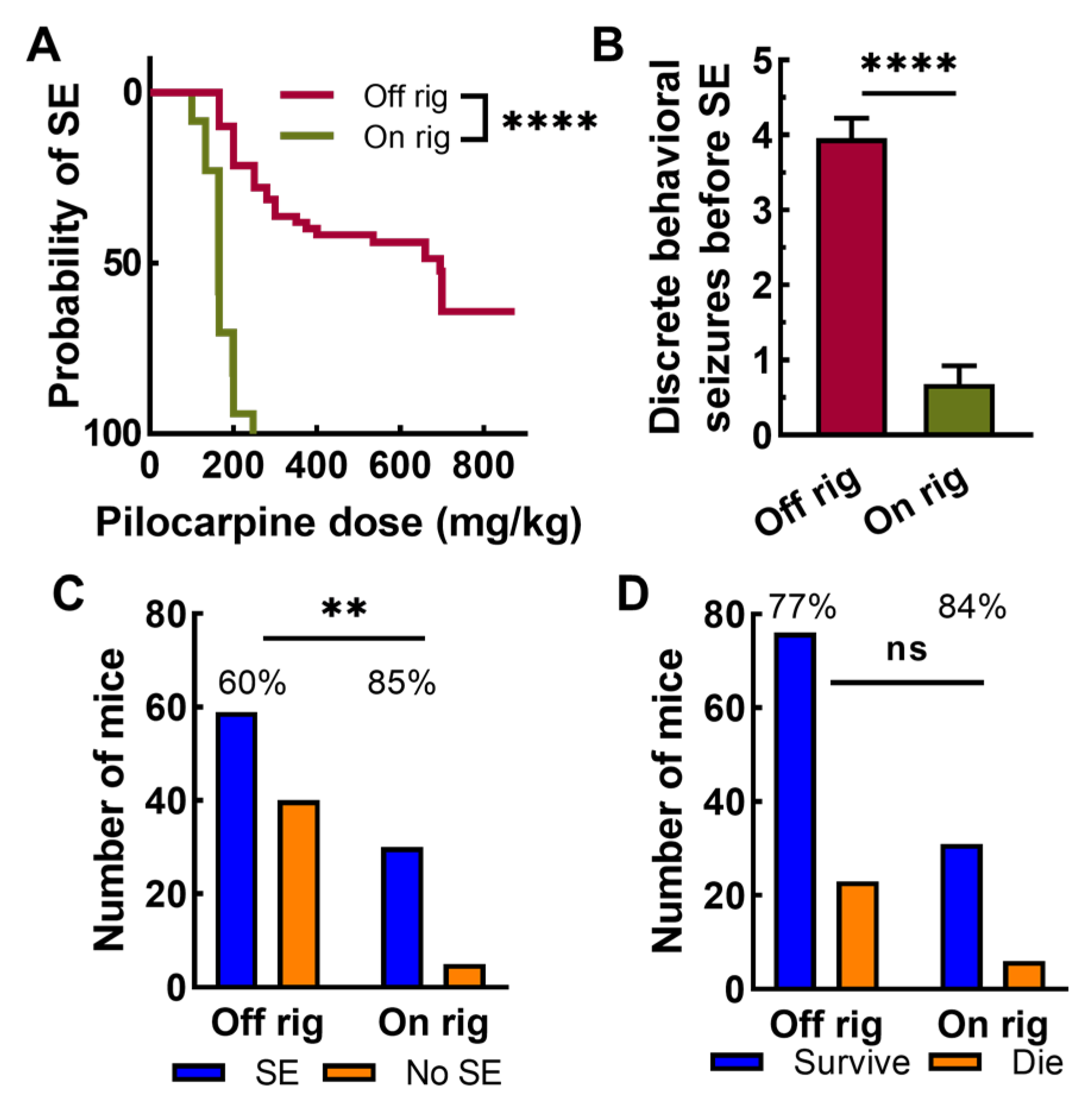
2.4. Strain Dependence of Pilocarpine Induction on Rig
2.5. Strain Dependence of Seizure Patterns in Epileptic Mice
3. Discussion
3.1. Animal Models Suitable for Drug Screening Are an Unmet Need
3.2. Pilocarpine Model in Rats
3.3. Shortcomings of Kindled VGAT-Cre Mice Models
3.4. Developing a Pharmacokinetic Model of Pilocarpine
3.5. Pilocarpine Induction on the EEG Rig
3.6. Strain-Dependent Seizure Outcomes of Pilocarpine-Induced SE
3.7. Limitations of Study
4. Materials and Methods
4.1. Animals
4.2. Surgery
4.3. Pilocarpine Induction
4.4. Kindled and Hybrid Kindled Models in VGAT-Cre Mice
4.5. EEG and Behavioral Monitoring
4.6. Statistical Analysis and Rigor
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zack, M.M.; Kobau, R. National and State Estimates of the Numbers of Adults and Children with Active Epilepsy—United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 821–825. [Google Scholar] [CrossRef]
- Boro, A.; Haut, S. Medical comorbidities in the treatment of epilepsy. Epilepsy Behav. 2003, 4 (Suppl. S2), S2–S12. [Google Scholar] [CrossRef] [PubMed]
- French, J.A.; Williamson, P.D.; Thadani, V.M.; Darcey, T.M.; Mattson, R.H.; Spencer, S.S.; Spencer, D.D. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann. Neurol. 1993, 34, 774–780. [Google Scholar] [CrossRef]
- Klein, P.; Dingledine, R.; Aronica, E.; Bernard, C.; Blumcke, I.; Boison, D.; Brodie, M.J.; Brooks-Kayal, A.R.; Engel, J., Jr.; Forcelli, P.A.; et al. Commonalities in epileptogenic processes from different acute brain insults: Do they translate? Epilepsia 2018, 59, 37–66. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshe, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Wiebe, S.; Blume, W.T.; Girvin, J.P.; Eliasziw, M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N. Engl. J. Med. 2001, 345, 311–318. [Google Scholar] [CrossRef]
- Khoo, A.; Tisi, J.; Mannan, S.; O’Keeffe, A.G.; Sander, J.W.; Duncan, J.S. Reasons for not having epilepsy surgery. Epilepsia 2021, 62, 2909–2919. [Google Scholar] [CrossRef]
- Samanta, D.; Ostendorf, A.P.; Willis, E.; Singh, R.; Gedela, S.; Arya, R.; Scott Perry, M. Underutilization of epilepsy surgery: Part I: A scoping review of barriers. Epilepsy Behav. 2021, 117, 107837. [Google Scholar] [CrossRef]
- Engel, J., Jr.; Wiebe, S.; French, J.; Sperling, M.; Williamson, P.; Spencer, D.; Gumnit, R.; Zahn, C.; Westbrook, E.; Enos, B. Practice parameter: Temporal lobe and localized neocortical resections for epilepsy: Report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology 2003, 60, 538–547. [Google Scholar] [CrossRef]
- de Tisi, J.; Bell, G.S.; Peacock, J.L.; McEvoy, A.W.; Harkness, W.F.; Sander, J.W.; Duncan, J.S. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: A cohort study. Lancet 2011, 378, 1388–1395. [Google Scholar] [CrossRef]
- Engel, J., Jr.; McDermott, M.P.; Wiebe, S.; Langfitt, J.T.; Stern, J.M.; Dewar, S.; Sperling, M.R.; Gardiner, I.; Erba, G.; Fried, I.; et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: A randomized trial. JAMA 2012, 307, 922–930. [Google Scholar] [CrossRef]
- Galanopoulou, A.S.; Buckmaster, P.S.; Staley, K.J.; Moshe, S.L.; Perucca, E.; Engel, J., Jr.; Loscher, W.; Noebels, J.L.; Pitkanen, A.; Stables, J.; et al. Identification of new epilepsy treatments: Issues in preclinical methodology. Epilepsia 2012, 53, 571–582. [Google Scholar] [CrossRef]
- Kehne, J.H.; Klein, B.D.; Raeissi, S.; Sharma, S. The National Institute of Neurological Disorders and Stroke (NINDS) Epilepsy Therapy Screening Program (ETSP). Neurochem. Res. 2017, 42, 1894–1903. [Google Scholar] [CrossRef]
- Pitkanen, A.; Buckmaster, P.S.; Galanopoulou, A.S.; Moshe, S.L. (Eds.) Models of Seizures and Epilepsy, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef]
- Buckmaster, P.S.; Haney, M.M. Factors affecting outcomes of pilocarpine treatment in a mouse model of temporal lobe epilepsy. Epilepsy Res. 2012, 102, 153–159. [Google Scholar] [CrossRef]
- Chen, L.L.; Feng, H.F.; Mao, X.X.; Ye, Q.; Zeng, L.H. One hour of pilocarpine-induced status epilepticus is sufficient to develop chronic epilepsy in mice, and is associated with mossy fiber sprouting but not neuronal death. Neurosci. Bull. 2013, 29, 295–302. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.H.; Huang, Y.G.; Chen, L.W. Time-course of neuronal death in the mouse pilocarpine model of chronic epilepsy using Fluoro-Jade C staining. Brain Res. 2008, 1241, 157–167. [Google Scholar] [CrossRef]
- Dey, D.; Eckle, V.-S.; Vitko, I.; Sullivan, K.A.; Lasiecka, Z.M.; Winckler, B.; Stornetta, R.L.; Williamson, J.M.; Kapur, J.; Perez-Reyes, E. A potassium leak channel silences hyperactive neurons and ameliorates status epilepticus. Epilepsia 2014, 55, 203–213. [Google Scholar] [CrossRef]
- Lim, J.A.; Moon, J.; Kim, T.J.; Jun, J.S.; Park, B.; Byun, J.I.; Sunwoo, J.S.; Park, K.I.; Lee, S.T.; Jung, K.H.; et al. Clustering of spontaneous recurrent seizures separated by long seizure-free periods: An extended video-EEG monitoring study of a pilocarpine mouse model. PLoS ONE 2018, 13, e0194552, Correction in: PLoS ONE 2020, 15, e0240544. https://doi.org/10.1371/journal.pone.0240544. [Google Scholar] [CrossRef]
- Goffin, K.; Nissinen, J.; Van Laere, K.; Pitkanen, A. Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp. Neurol. 2007, 205, 501–505. [Google Scholar] [CrossRef]
- West, P.J.; Thomson, K.; Billingsley, P.; Pruess, T.; Rueda, C.; Saunders, G.W.; Smith, M.D.; Metcalf, C.S.; Wilcox, K.S. Spontaneous recurrent seizures in an intra-amygdala kainate microinjection model of temporal lobe epilepsy are differentially sensitive to antiseizure drugs. Exp. Neurol. 2022, 349, 113954. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.A.; Vitko, I.; Blair, K.; Gaykema, R.P.; Failor, M.J.; San Pietro, J.M.; Dey, D.; Williamson, J.M.; Stornetta, R.L.; Kapur, J.; et al. Drug-Inducible Gene Therapy Effectively Reduces Spontaneous Seizures in Kindled Rats but Creates Off-Target Side Effects in Inhibitory Neurons. Int. J. Mol. Sci. 2023, 24, 11347. [Google Scholar] [CrossRef]
- De Deyn, P.; Marescau, B.; MacDonald, R. Epilepsy and the GABA-hypothesis a brief review and some examples. Acta Neurol. Belg. 1990, 90, 65–81. [Google Scholar]
- Vong, L.; Ye, C.; Yang, Z.; Choi, B.; Chua, S., Jr.; Lowell, B.B. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 2011, 71, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Straub, J.; Gawda, A.; Ravichandran, P.; McGrew, B.; Nylund, E.; Kang, J.; Burke, C.; Vitko, I.; Scott, M.; Williamson, J.; et al. Characterization of kindled VGAT-Cre mice as a new animal model of temporal lobe epilepsy. Epilepsia 2020, 61, 11. [Google Scholar] [CrossRef]
- Vigier, A.; Partouche, N.; Michel, F.J.; Crépel, V.; Marissal, T. Substantial outcome improvement using a refined pilocarpine mouse model of temporal lobe epilepsy. Neurobiol. Dis. 2021, 161, 105547. [Google Scholar] [CrossRef]
- Arshad, M.; Naegele, J. Induction of Temporal Lobe Epilepsy in Mice with Pilocarpine. Bio-Protocol 2020, 10, e3533. [Google Scholar] [CrossRef]
- Wenker, I.C.; Teran, F.A.; Wengert, E.R.; Wagley, P.K.; Panchal, P.S.; Blizzard, E.A.; Saraf, P.; Wagnon, J.L.; Goodkin, H.P.; Meisler, M.H.; et al. Postictal Death Is Associated with Tonic Phase Apnea in a Mouse Model of Sudden Unexpected Death in Epilepsy. Ann. Neurol. 2021, 89, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Mazzuferi, M.; Kumar, G.; Rospo, C.; Kaminski, R.M. Rapid epileptogenesis in the mouse pilocarpine model: Video-EEG, pharmacokinetic and histopathological characterization. Exp. Neurol. 2012, 238, 156–167. [Google Scholar] [CrossRef]
- Guo, D.; Zeng, L.; Brody, D.L.; Wong, M. Rapamycin Attenuates the Development of Posttraumatic Epilepsy in a Mouse Model of Traumatic Brain Injury. PLoS ONE 2013, 8, e64078. [Google Scholar] [CrossRef]
- Turski, W.A.; Cavalheiro, E.A.; Bortolotto, Z.A.; Mello, L.M.; Schwarz, M.; Turski, L. Seizures produced by pilocarpine in mice: A behavioral, electroencephalographic and morphological analysis. Brain Res. 1984, 321, 237–253. [Google Scholar] [CrossRef]
- Toyoda, I.; Bower, M.R.; Leyva, F.; Buckmaster, P.S. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J. Neurosci. 2013, 33, 11100–11115. [Google Scholar] [CrossRef]
- Jope, R.S.; Morrisett, R.A.; Snead, O.C. Characterization of lithium potentiation of pilocarpine-induced status epilepticus in rats. Exp. Neurol. 1986, 91, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Sun, H.; Rajasekaran, K.; Williamson, J.; Perez-Reyes, E.; Kapur, J. A novel therapeutic approach for treatment of catamenial epilepsy. Neurobiol. Dis. 2017, 111, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.; Martin, B.S.; Sun, C.; Williamson, J.; Kapur, J. Endogenous neurosteroid synthesis modulates seizure frequency. Ann. Neurol. 2010, 67, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.J.; Bankstahl, M.; Groticke, I.; Loscher, W. Pilocarpine vs. lithium-pilocarpine for induction of status epilepticus in mice: Development of spontaneous seizures, behavioral alterations and neuronal damage. Eur. J. Pharmacol. 2009, 619, 15–24. [Google Scholar] [CrossRef]
- Wilcox, K.S.; West, P.J.; Metcalf, C.S. The current approach of the Epilepsy Therapy Screening Program contract site for identifying improved therapies for the treatment of pharmacoresistant seizures in epilepsy. Neuropharmacology 2020, 166, 107811. [Google Scholar] [CrossRef]
- Bertram, E.H. Seizure Monitoring in Rodents. In Experimental and Translational Methods to Screen Drugs Effective Against Seizures and Epilepsy; Springer: New York, NY, USA, 2021; pp. 145–163. [Google Scholar]
- Tse, K.; Beamer, E.; Simpson, D.; Beynon, R.J.; Sills, G.J.; Thippeswamy, T. The Impacts of Surgery and Intracerebral Electrodes in C57BL/6J Mouse Kainate Model of Epileptogenesis: Seizure Threshold, Proteomics, and Cytokine Profiles. Front. Neurol. 2021, 12, 625017. [Google Scholar] [CrossRef]
- Balzekas, I.; Hernandez, J.; White, J.; Koh, S. Confounding effect of EEG implantation surgery: Inadequacy of surgical control in a two hit model of temporal lobe epilepsy. Neurosci. Lett. 2016, 622, 30–36. [Google Scholar] [CrossRef]
- Turski, L.; Ikonomidou, C.; Turski, W.A.; Bortolotto, Z.A.; Cavalheiro, E.A. Review: Cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: A novel experimental model of intractable epilepsy. Synapse 1989, 3, 154–171. [Google Scholar] [CrossRef]
- Gibbs-Shelton, S.; Benderoth, J.; Gaykema, R.P.; Straub, J.; Okojie, K.A.; Uweru, J.O.; Lentferink, D.H.; Rajbanshi, B.; Cowan, M.N.; Patel, B.; et al. Microglia play beneficial roles in multiple experimental seizure models. Glia 2023, 71, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Karoly, P.J.; Rao, V.R.; Gregg, N.M.; Worrell, G.A.; Bernard, C.; Cook, M.J.; Baud, M.O. Cycles in epilepsy. Nat. Rev. Neurol. 2021, 17, 267–284. [Google Scholar] [CrossRef]
- Chong, D.; Jones, N.C.; Schittenhelm, R.B.; Anderson, A.; Casillas-Espinosa, P.M. Multi-omics integration and epilepsy: Towards a better understanding of biological mechanisms. Prog. Neurobiol. 2023, 227, 102480. [Google Scholar] [CrossRef]
- Metcalf, C.S.; Vanegas, F.; Underwood, T.; Johnson, K.; West, P.J.; Smith, M.D.; Wilcox, K.S. Screening of prototype antiseizure and anti-inflammatory compounds in the Theiler’s murine encephalomyelitis virus model of epilepsy. Epilepsia Open 2022, 7, 46–58. [Google Scholar] [CrossRef]
- Srinivasan, R.; Lu, T.-Y.; Chai, H.; Xu, J.; Huang, B.S.; Golshani, P.; Coppola, G.; Khakh, B.S. New Transgenic Mouse Lines for Selectively Targeting Astrocytes and Studying Calcium Signals in Astrocyte Processes In Situ and In Vivo. Neuron 2016, 92, 1181–1195. [Google Scholar] [CrossRef]
- Burke, C.T.; Vitko, I.; Straub, J.; Nylund, E.O.; Gawda, A.; Blair, K.; Sullivan, K.A.; Ergun, L.; Ottolini, M.; Patel, M.K.; et al. EpiPro, a Novel, Synthetic, Activity-Regulated Promoter That Targets Hyperactive Neurons in Epilepsy for Gene Therapy Applications. Int. J. Mol. Sci. 2023, 24, 14467. [Google Scholar] [CrossRef] [PubMed]
- Straub, J.; Vitko, I.; Gaykema, R.P.; Perez-Reyes, E. Preparation and Implantation of Electrodes for Electrically Kindling VGAT-Cre Mice to Generate a Model for Temporal Lobe Epilepsy. J. Vis. Exp. 2021, 174, e62929. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Pakarinen, E.D.; Moerschbaecher, J.M. Comparison of the effects of scopolamine and methylscopolamine on the performance of a fixed-ratio discrimination in squirrel monkeys. Pharmacol. Biochem. Behav. 1993, 44, 815–819. [Google Scholar] [CrossRef] [PubMed]
- George, A.G.; Federico, A.; Gom, R.C.; Harris, S.A.; Teskey, G.C. Caffeine exacerbates seizure-induced death via postictal hypoxia. Sci. Rep. 2023, 13, 14150. [Google Scholar] [CrossRef] [PubMed]
- Umpierre, A.D.; Bennett, I.V.; Nebeker, L.D.; Newell, T.G.; Tian, B.B.; Thomson, K.E.; White, H.S.; White, J.A.; Wilcox, K.S. Repeated low-dose kainate administration in C57BL/6J mice produces temporal lobe epilepsy pathology but infrequent spontaneous seizures. Exp. Neurol. 2016, 279, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, E.; Joshi, S.; Williamson, J.; Penmetsa, M.; Shan, S.; Kapur, J. Electroencephalography and behavior patterns during experimental status epilepticus. Epilepsia 2018, 59, 369–380. [Google Scholar] [CrossRef] [PubMed]


| Variable | Protocol | Reference |
|---|---|---|
| 8–12 weeks | [16] |
| 1.75 mg/kg | [28] |
| 20 min | this study |
| 166 mg/kg | this study |
| 1/2 initial dose | [27] |
| 30 min | [27] |
| 10 mg/kg | [20] |
| 60’ after SE start | [17] |
| ON rig | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaykema, R.P.; Failor, M.J.; Maciejczuk, A.; Pikus, M.; Oliinyk, M.; Ellison, M.B.; Behrooz, A.A.; Singh, K.; Williamson, J.M.; Perez-Reyes, E. Spontaneous Seizure Outcomes in Mice Using an Improved Version of the Pilocarpine Model of Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2025, 26, 9540. https://doi.org/10.3390/ijms26199540
Gaykema RP, Failor MJ, Maciejczuk A, Pikus M, Oliinyk M, Ellison MB, Behrooz AA, Singh K, Williamson JM, Perez-Reyes E. Spontaneous Seizure Outcomes in Mice Using an Improved Version of the Pilocarpine Model of Temporal Lobe Epilepsy. International Journal of Molecular Sciences. 2025; 26(19):9540. https://doi.org/10.3390/ijms26199540
Chicago/Turabian StyleGaykema, Ronald P., Madison J. Failor, Aleksandra Maciejczuk, Magda Pikus, Mariia Oliinyk, Maggie B. Ellison, Amir A. Behrooz, Kiran Singh, John M. Williamson, and Edward Perez-Reyes. 2025. "Spontaneous Seizure Outcomes in Mice Using an Improved Version of the Pilocarpine Model of Temporal Lobe Epilepsy" International Journal of Molecular Sciences 26, no. 19: 9540. https://doi.org/10.3390/ijms26199540
APA StyleGaykema, R. P., Failor, M. J., Maciejczuk, A., Pikus, M., Oliinyk, M., Ellison, M. B., Behrooz, A. A., Singh, K., Williamson, J. M., & Perez-Reyes, E. (2025). Spontaneous Seizure Outcomes in Mice Using an Improved Version of the Pilocarpine Model of Temporal Lobe Epilepsy. International Journal of Molecular Sciences, 26(19), 9540. https://doi.org/10.3390/ijms26199540





