Abstract
Alzheimer’s disease (AD) is the most common cause of cognitive decline; currently, anti-amyloid monoclonal antibodies are available for clinical use as disease-modifying treatments, while many other substances are being tested in clinical trials. Molecular biomarkers for AD have been studied for more than two decades, and various guidelines and diagnostic recommendations have been published. However, there are still questions and controversies about the biomarker profile needed to confirm AD and the eligibility for such established treatments and clinical trials. Is amyloid positivity sufficient for eligibility, or is a biomarker for tau biochemistry/pathology also needed? What is the role of hybrid ratios combining amyloid and tau? Should we rely on plasma biomarkers alone? This review aimed to describe and discuss such questions and controversies.
1. Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease and cause of cognitive decline [1]. It accounts for ~60–70% of cases; by 2030, patients with AD are expected to reach 75 million worldwide, posing a major health and socioeconomic problem [2]. The main biochemical and pathological characteristics of AD include the extracellular polymerization of amyloid beta peptide with 42 amino acids (Aβ42) forming amyloid plaques [3] and the intracellular polymerization of hyperphosphorylated tau protein in the form of paired helical filaments evolving to neurofibrillary tangles [4]. The interplay between the two converging mechanisms [5] leads to AD [6].
Until a few years ago, AD therapeutics were based on symptomatic treatments introduced ~25 years ago [7]. Since the amyloid pathway is an early and key pathogenic mechanism, as well as a potential therapeutic target [8], anti-amyloid monoclonal antibodies have been recently introduced in AD therapeutics as the first disease-modifying treatments [7,8,9,10]. Currently, Lecanemab [11] and Donanemab [12] are the two anti-amyloid antibodies fully approved in the USA by the Food and Drug Administration and in Europe by the European Medicines Agency. They are approved for use in early AD, i.e., mild cognitive impairment (MCI) or mild dementia due to AD; appropriate use recommendations thought to be useful in clinical practice [13] have been published from authorities in various states, including the U.S.A. [14,15], France [16], Canada [17], and South Korea [18].
But how is AD diagnosed during life? It has been observed that clinical diagnosis may be accompanied by a high clinicopathological concordance (>90%) when typical cases without comorbidities, fulfilling even the older probable criteria of AD [19], are examined by specialists in tertiary/academic centers [20]. However, this is not always true; in early or atypical cases, in the presence of comorbidities, and in the community (approach by non-experts), diagnostic accuracy may decrease to <75% [21]. In general, it has been estimated that ~25–30% of patients clinically diagnosed with AD will suffer from a non-AD disorder at post-mortem pathological examination [22]. On the other hand, post-mortem examination will reveal the presence of AD (co)pathology in ~39% of cases of patients with a clinical diagnosis of non-AD disorder [23]. Thus, it is not surprising that the chance of in vivo misdiagnosis was ~1/3 in a more recent pathologically confirmed study from an academic center [24]. The newer disease-modifying anti-amyloid antibodies are (a) effective in cases with early AD, i.e., exactly in the population where clinical diagnostic accuracy may be relatively lower, and (b) not free of safety issues, including amyloid-related imaging abnormality (ARIA) [11,12,25]. Thus, the maximal possible diagnostic accuracy should be ensured before considering their administration. Over the last 25 years, classical cerebrospinal fluid (CSF) [26,27] and molecular biomarkers visualized via positron emission tomography (PET) [28] have become the gold standard in vivo diagnosis of AD. They have been incorporated into diagnostic criteria and guidelines [29,30,31,32,33,34], resulting in pathologically verified improvement of in vivo diagnostic accuracy [24]. Research on biomarker-based diagnosis has intensified in the context of anti-amyloid treatment approval [35,36,37].
There are many extensive, comprehensive, and very informative reviews and recommendations on the use of various biomarkers for AD, many of which are cited in this study. A search gave a total of 886, 626, and 1602 results for CSF [38], PET [39], and blood-based biomarkers [40], respectively. However, despite improved diagnostic abilities [37], there are still controversial issues and debates among various authorities that publish diagnostic and classification criteria for AD [41,42,43,44,45]. The aim of this work was not to present just another review but to present some critical points and questions related to the molecular biomarker-based approach in diagnosing AD and identifying patients eligible for approved or investigational disease-modifying treatments.
2. Classical Biomarkers with Molecular Specificity for AD
Classical biomarkers with molecular specificity for AD are related to the two fundamental biochemical and pathological hallmarks of AD: Aβ formation and deposition in amyloid plaques and tau hyperphosphorylation and the formation of paired helical filaments and neurofibrillary tangles.
2.1. CSF Markers
Aβ42 levels are decreased in the CSF of patients with AD, inversely related to amyloid plaque burden [46]. Amyloid beta peptide with 40 amino acids (Aβ40) can also be determined, and the Aβ42/Aβ40 ratio may be a better indicator of amyloid plaque formation [47], being preferable to Aβ42 alone [48]. Thus, Aβ42/Aβ40 shows molecular specificity for the amyloid component of AD.
Increased levels of hyperphosphorylated tau protein are considered a marker of the tauopathic component of AD [49]. Tau hyperphosphorylated at a threonine residue at position 181 (τP-181) is the most widely used form; however, other hyperphosphorylated tau forms can be quantified in the CSF, including τP-217 and τP-231 [50], with τP-217 having some possible advantages over τP-181 [51]. All of the above hyperphosphorylated tau forms become abnormal early in the disease process, when tau molecules and small aggregates are still soluble (before neurofibrillary tangle formation), as a result of the amyloid cascade, due to amyloid-triggered activation of kinases such as cyclin-dependent kinase-5 (CDK-5), glycogen synthase kinase-3β (GSK-3β), and mitogen-activated protein kinases (MAPKs) [52,53]. Thus, these forms may be viewed as markers of the amyloid-triggered initiation of the tauopathic process and as “indirect markers” of amyloidogenesis. In this context, CSF τP-217 (especially the ratio of the phosphorylated to total form) may be a slightly better indicator of amylogenesis than the Aβ42/Aβ40 ratio [54]. Recently, CSF τP-212, which may be related to the action of dual-specificity tyrosine phosphorylation-regulated kinase 1A, was found to be increased early in the course of AD, either sporadic or due to Down syndrome [55].
As tauopathy progresses, tau aggregates become insoluble, and tangle pathology emerges. At this time, other tau forms increase in the CSF, including the tau microtubule-binding region containing residue 243 (MDBR243) [56] and τP-205 [57], which enables AD staging [58].
2.2. PET Markers
PET for Aβ with various tracers, such as florbetaben, florbetapir, and flutemetamol, provides imaging evidence of amyloid deposition [59]. It can be interpreted visually or by Standardized Uptake Value Ratios (SUVRs) or centiloids for detecting neuritic plaque load corresponding to Thal phases 3–5 [60]. Thus, PET for Aβ generally correlates with the Thal phases; however, it cannot detect the very early phases of amyloidogenesis and early Thal phases, and a centiloid level of at least 28–30 should be reached for diagnostic purposes [61].
On the other hand, PET for tau deposition (neurofibrillary tangle formation) generally follows Braak stages, usually requiring a Braak stage of at least III [62], whilst for diagnostic purposes, 18F-flortaucipir PET detects stages ≥ IV [63]. The severity of tau PET abnormality correlates positively with the AD progression rate [64]. Furthermore, while in PET for Aβ, the amyloid distribution pattern is usually similar in all AD patients, the tau load distribution in tau PET is not the same and may be either typical (as expected by Braak staging) or atypical, showing a posterior, left predominant, or hippocampal sparing pattern [65]. Thus, PET for tau deposition may correlate with both the clinical picture (typical amnestic or atypical presentations) and AD severity.
3. Other Biomarkers Typically Used
3.1. Markers of Neurodegeneration
Cerebrospinal fluid τT [66,67] and NfL [68] are long-known markers of neuronal/axonal degeneration, and their levels are usually increased in AD [4]. However, they are not specific for AD and may be increased in other neurodegenerative disorders [69].
Positron emission tomography (PET) with 18F-fluoro-deoxy-glucose (FDG) can reveal hypometabolic brain areas. In AD, hypometabolism occurs typically in the parietotemporal association cortices, posterior cingulate cortex, and precuneus [70], and this helps in the differential diagnosis from other neurodegenerative disorders [33]. However, deviations from the typical topography occur in atypical clinical presentations of AD, posing difficulties in the diagnostic process, for example, between frontotemporal dementias and the frontal variant of AD [70,71]. Single-Photon Emission Computed Tomography (SPECT) with 99mTc-Hexamethylpropylen-amine Oxime (HMPAO) for assessing hypoperfusion may be a useful alternative, depending on availability issues [72]. Recently, 3-Tesla Magnetic Resonance Imaging (3T MRI) with arterial spin labeling (ASL) quantifying cerebral blood flow has emerged as another alternative [73], more affordable, and radiation-free imaging method, suitable for longitudinal follow-up and with a diagnostic accuracy that may be comparable to that of PET [74].
Brain atrophy observed in MRI or even CT is another useful marker of neurodegeneration, and the topography may be helpful in the (differential) diagnosis of AD. Volumetry may have some advantages [75], but visual scales assessing the atrophy of hippocampal formation, such as the long-known Medial Temporal Atrophy (MTA) scale [76] or, more recently, the Entorhinal Cortex Atrophy (ERICA) scale [77,78], are easy-to-use tools for everyday practice.
It is worth noting that the term neurodegeneration encompasses many components, including neuronal and/or axonal and/or neuropil dysfunction/loss, hypometabolism and hypoperfusion, and central and cortical atrophy [41]. Therefore, the above markers are not equivalent and may show different patterns of evolution during the disease process, with atrophy usually being the last to emerge [79].
3.2. Markers of Other Primary Co-Pathologies
For vascular co-pathology, MRI or CT are the usual imaging methods. In case of large vessels and/or lacunar infarcts, the vascular nature of the lesions is easily recognized. However, white matter hyperintensities should not always be attributed to cerebral small vessel disease in AD. It has been observed that anterior (frontal) white matter lesions may be associated with both vascular pathology and AD neurodegenerative changes (tau-related axonal loss and Aβ burden) [80,81]; thus, they may be related to mixed cognitive impairment (AD + vascular). On the contrary, posterior (parietal) periventricular lesions are related to cortical AD pathology [82] and, if associated with hippocampal and temporal atrophy, may even serve as early biomarkers of AD [81]. A juxtacortical multisport pattern of white matter MRI hyperintensities and lobar microbleeds points to cerebral amyloid angiopathy [81,83].
Various methods of assessing CSF α-synuclein (α-syn) have been tested [84,85,86], but the seeding amplification assay is the most promising [87,88,89]. PET for α-syn has also been studied [28]. Similarly, CSF and PET assessments of transactive-response DNA-binding protein-43 (TDP-43) have been studied [90,91], but it is unknown whether they can be used effectively to identify TDP-43 co-pathology in patients with AD [92]. TDP-43 accumulation is the hallmark of limbic predominant age-related TDP-43 encephalopathy (LATE), an entity characterized by AD-like symptoms mainly in patients over 80 years old, with distinct neuropathological stages initiated by the amygdala [91]. Co-pathology identification is significant since TDP-43 inclusions can occur up to 50% in AD and may be closely related to the disease’s pathogenesis and prognosis [91,92].
4. Plasma Biomarkers
The above-described biomarkers are either CSF-based (requiring lumbar puncture, a generally safe but minimally to moderately invasive procedure) or imaging-based (mainly PET). Blood-based (plasma) biomarkers have received significant attention recently due to their noninvasiveness and reduced costs compared to CSF and PET biomarkers [93,94].
Plasma Aβ42/Aβ40 ratio [95,96], plasma τP-181 [97,98], plasma τP-231 [99], plasma τP-212 [55], and especially plasma τP-217 [95,96,99,100,101,102] are early markers of AD; as in CSF, the above phosphorylated tau forms are good indicators of not only amyloid-triggered tau hyperphosphorylation but also amyloidogenesis [95,96,99], with a sensitivity comparable to that of CSF Aβ markers and better than CSF τP-181 [103]. It has been suggested that plasma levels of the endogenous cleaved tau microtubule-binding region containing residue 243 (eMBDR-tau243) increase when tangle pathology appears [104].
Plasma levels of NfL serve as a nonspecific marker of neurodegeneration in AD [105]. Therefore, NfL assessment could serve as a valuable prognostic marker in AD, considering its association with brain atrophy, especially if it were feasible to acquire repetitive values throughout the course of the disease [105].
Neuroinflammation markers, such as Glial Fibrillary Acidic Protein (GFAP), may be assessed in plasma [106], and it has been observed that plasma GFAP levels may perform diagnostically better than CSF levels [107]. Hence, the response of plasma GFAP levels to monoclonal antibodies has been studied in crucial randomized trials [11,12], and there is evidence that their increase, as a result of reactive astrocytosis, could accompany much earlier stages of AD pathology. More specifically, findings that contain uncertainty suggest a relation to the amyloid cascade, even in the preclinical phase of the disease [106,107].
5. Alternative Biomarkers
Some biomarkers have been introduced into research relatively recently or have been long known to have special advantages in studying various aspects of AD pathology [26]. They could have the potential to be helpful either clinically or to study (in clinical trials) the possible benefits of new treatments in aspects other than Aβ or tau proteinopathy.
5.1. Biomarkers of Amyloidopathy or Tauopathy
Soluble Aβ oligomers, formed very early in the amyloid cascade process, are thought to be neurotoxic through their ligand-like action [108]. They can be quantified in CSF [109] and plasma [110] and have the potential to offer very early diagnosis [111]. Recently, a portable apparatus detecting Aβ oligomers has been tested, promising easy diagnosis with low costs, even at the site of primary care [112]. In a similar context, CSF quantification of tau and α-syn oligomers is also being tested as a diagnostic tool, possibly useful in the differential diagnosis of dementia-related and Parkinsonian neurodegenerative disorders [113].
In addition to the only approved tracer for PET, 18F-flortaucipir, other tracers are being tested [114], and there is evidence that some may outperform flortaucipir, showing increased selectivity for affected brain areas [115].
5.2. Biomarkers of Microglia Activation and Other Parameters of Neuroinflammation
Microglia are activated early in Alzheimer’s disease in an attempt to remove aggregating Aβ but with ongoing increases in Aβ burden, impairment in microglial phagocytic activity, and chronic dysregulated neuroinflammation occur, leading to neurodegeneration [116]. However, this phenomenon is nonspecific and may occur in many other neurodegenerative conditions. Triggering Receptor Expressed on Myeloid cells 2 (TREM2) is a marker of microglia activation, which has received much attention recently due to its involvement in AD mechanisms, its potential as a diagnostic marker, and its role as a possible therapeutic target [106]. The CSF levels of soluble TREM2 (sTREM2) are increased in early AD [117,118] and could be related to discrepancies between Aβ42 levels and the τP-217/Aβ42 ratio [119]. sTREM2 can also be measured in plasma [120] and may be associated with concomitant cerebrovascular disease [121]. However, abnormal levels of sTREM2 may be observed in other neurodegenerative disorders [122].
CSF or plasma levels of chitinase 3-like protein 1 (YKL-40) [123,124,125] and numerous other markers, including Interleukin-1β (IL-1β), Tumor Necrosis Factor-alpha (TNF-α), and S100, involved in the interplay between microglia, astrocytes, and inflammation, are being extensively studied both as diagnostic biomarkers and possible targets of individualized therapies [126,127,128].
Recently, CSF levels of soluble α-Klotho (sαKl), a protein involved in antioxidant and neuroprotective mechanisms, have been reported to be lower in dementia due to AD compared to controls [129].
5.3. Biomarkers of Synaptic Loss
Synaptic dysfunction and loss are integral parts of Alzheimer’s neuropathology [130]. Among other molecules, Synaptotagmin (SYT-1) [131], Synaptosomal-Associated Protein 25 (SNAP-25) [132,133], Postsynaptic Density Protein 95 (PSD-95) [133], Neurogranin [133,134], and Vesicle-Associated Membrane Protein 2 (VAMP-2) [135] have been tested as possible markers of synaptic loss in AD.
Brain-Derived Neurotrophic Factor (BDNF) is a neurotrophin supporting neuronal and synaptic integrity and plasticity. Low levels have been reported in Alzheimer’s disease, associated with Aβ- and tau-related mechanisms, neuroinflammation, and apoptosis, but BDNF may be involved in many other neurological and psychiatric disorders [136].
It has been suggested that combining various classical biomarkers with markers of neuroinflammation and synaptic loss may prove diagnostically helpful and even outperform classical biomarkers alone [128]. However, in an umbrella review, only blood-based YKL-40 yielded convincing results, while many others were suggestive than established proof [137].
6. Genetic Biomarkers
Apolipoprotein E (APOE) genotyping is not uncommonly performed in cognitively affected patients. It is a susceptibility gene for AD, present in three allelic forms: ε2, ε3, and ε4 [138]. The presence of at least one ε4 allele is a major risk factor for AD [138], while the ε2 allele may be protective [139]. Recently, it has been suggested that although ε4 heterozygosity is a risk factor, ε4 homozygosity may represent a distinct genetic type of AD [140]. This notion has been challenged [44] since it has been observed that the concomitant presence of a loss-of-function mutation in the Sortilin-Related Receptor 1 gene (SORL1) is necessary for this to occur [141]. Thus, in addition to evaluating the risk of AD, APOE and/or SORL1 testing could be diagnostically helpful.
Nevertheless, APOE genotyping before initiating anti-amyloid treatment is considered significant for AD patients since the presence of ε4 increases the risk of side effects (amyloid-related imaging abnormalities), and in the case of ε4 homozygosity, these drugs may be contraindicated [14,15,16]. It is also significant in clinical trials for newer drugs targeting ε4 [142].
Mutations in the gene encoding TREM2 may be related to neurodegeneration and increase the risk of AD [143].
Down syndrome is a specific genetic type of AD [144] for which anti-Aβ antibody treatment is contraindicated due to the increased presence of cerebral amyloid angiopathy [14]. These treatments are also contraindicated in Amyloid Precursor Protein gene (APP) mutations associated with cerebral amyloid angiopathy [14].
7. Profiles and Ratios
The National Institute of Aging—Alzheimer’s Association (NIA-AA) research framework (2018) introduced the so-called AT(N) system for the diagnosis and classification of AD [41]. A stands for amyloid biomarkers, and A+ and A− indicate positive (abnormal) and negative (normal) results of the biomarker used for amyloids, respectively. T stands for tauopathy biomarkers (tau phosphorylation, tangle formation), with T+ and T− indicating abnormal and normal results of the biomarker tested, respectively. Finally, (N) stands for neurodegeneration (neuronal or axonal dysfunction or loss), with (N) and (N) indicating abnormal and normal results of the corresponding tests, respectively.
Biomarkers widely used (or suggested for use) currently in AD diagnosis and staging are summarized in Table 1, according to the 2018 NIA-AA research framework and the 2024 Alzheimer’s Association (AA) revised classification system [41,43]. Since biomarkers for neurodegeneration or neuroinflammation are nonspecific, and vascular biomarkers or α-syn indicate possible co-pathologies, only those indicating amyloid- and tau-related biochemistry/pathology are currently considered suitable for AD diagnosis and staging [27].

Table 1.
Biomarkers of various types of abnormal biochemistry/pathology, useful in the (differential) diagnosis of Alzheimer’s disease and other dementia-related disorders, based on the classifications presented in Refs. [41,43] 1.
Cumulative data from many studies [103,144,145,146,147,148] lead to the general notion that CSF or plasma Aβ42/Aβ40 are the first to become abnormal very early in the disease process and earlier than PET for Aβ up to ~19 years before symptom onset (Figure 1). The levels of CSF τP-181, τP-217, τP-231, and τP-212 may start to increase around the first indication of abnormality in Aβ PET, and they are usually already abnormal when Aβ PET reaches the diagnostic threshold (28–30 centiloids). Thus, the above phospho-tau (“T”) forms [41] are now termed “T1”, and, together with Aβ (A) markers, they are classified as “core 1” biomarkers [43]. With disease progression, insoluble forms of tau accumulate in the form of neurofibrillary tangles, at which point CSF or plasma τP-205 and MBDR-τ243 become abnormal, and tangle pathology becomes evident in PET for tau. These “T” biomarkers are now classified as “T2” and “core 2” biomarkers [43].
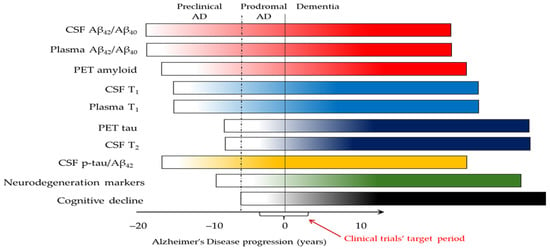
Figure 1.
Theoretical diagram of biomarker changes during Alzheimer’s disease evolution and progression [41,43,103,144,145,146,147,148]. The changes in biomarkers follow the smooth increase in color intensity throughout the course of AD. The red arrow shows the period that is the target of monoclonal antibodies’ clinical trials. Deviations may occur in some patients based on genetic or other factors, as well as the biomarker tests used. Cerebrospinal fluid (CSF) T1 refers to τP-181, τP-217, and τP-231. Plasma T1 refers to τP-217, τP-181, and τP-212. CSF T2 mainly refers to τP-205 and MDBR-τ243. For simplicity reasons, neurodegeneration markers are shown together in one bar, although they are not equivalent and may become abnormal at relatively different time points.
According to the 2018 NIA-AA criteria, AD can be diagnosed when the biomarker profile is A+T+, while the A+T− profile is classified as Alzheimer’s continuum since only amyloid biomarkers may be positive very early in the disease process, with T biomarkers remaining negative [41]. According to the 2024 AA revised criteria, an abnormal core 1 biomarker either A (PET for Aβ, or CSF Aβ42/Aβ40) or plasma T1 (τP-217, good indicator of Aβ pathology) is sufficient for AD diagnosis since the vast majority of A+ patients (~90% with positive PET for Aβ) will prove to have AD at autopsy [43]. Indeed, this is true, but it is not always the case.
Firstly, the A+ profile is heterogeneous, comprising different subgroups of patients with typical amnestic AD, AD mixed with other pathologies, or vascular cognitive decline [149]. The A+T− profile is also heterogeneous. Many such patients may suffer from early presymptomatic or even mildly symptomatic AD in which the T biomarkers have not become abnormal yet [145]. Additionally, AD with atypical clinical presentations [150,151,152] or mixed AD [153] may present with marginal or normal CSF τP-181 levels. The A+T− profile may also be observed in other non-AD disorders such as dementia with Lewy bodies (DLB) [154], subcortical small vessel disease, various tauopathies [155,156], Creutzfeldt–Jakob disease [157], limbic-predominant age-related TDP-43 encephalopathy [158], subcortical small vessel disease [159], and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) [160]. Ιn such clinical settings, with equivocal CSF profiles, the Aβ42/Aβ40 ratio, instead of Aβ42 alone, led to a reliable diagnosis in ~50% of cases [161], but not in all, and diagnostic uncertainty may remain. A proportion of A+T− patients may remain A+T− for at least 5 years, showing some clinical and genetic differences compared to those with A+T+ [162]. Recently, an amyloid predominant subtype of Alzheimer’s neuropathological change (~10%) has been described, with patients progressing to amyloid Thal phase 5 while remaining in tau Braak stages I–II and not progressing up to stage VI, as would be expected for AD; these patients may present with clinical and genetic differences compared to those with typical AD pathology [163]. Furthermore, some patients with amyloid pathology may never progress clinically [164]. Taken together, the above findings indicate that a percentage of A+ or A+T− patients, despite showing amyloid pathology, may deviate from what is expected in AD.
To increase the diagnostic performance of biomarkers, hybrid ratios of different biomarkers have been used, such as CSF τP-181/Aβ42, CSF τT/Aβ42, and plasma τP-217/Aβ42 [165,166,167,168,169,170]. Since the nominator increases and the denominator decreases, the ratio change is augmented, resulting in good discrimination between AD and non-AD disorders. The CSF τP-181/Aβ42 and τT/Aβ42 ratios may be helpful in cases with marginal or conflicting results from individual biomarkers [166] and in discriminating between likely AD and likely non-AD patients within the A+T− profile [171]. However, these ratios could sometimes become abnormal due to a significant reduction in only Aβ42, i.e., in the absence of any evidence of tau-related biochemistry. These CSF hybrid ratios and the CSF Aβ42/Aβ40 ratio, also included in Table 1, are classified independently as “core 1” biomarkers according to the 2024 AA revised criteria, reflecting a well-established AD neuropathological change, even in asymptomatic individuals [43]. The importance of the CSF Aβ42/Aβ40 ratio, which identifies amyloid pathology with adequate accuracy, has already been mentioned as a key initial change over the course of AD in relation to classic fluid and imaging biomarkers [147,148]. Ultimately, plasma τP-217/Aβ42, which has recently received FDA approval, established encouraging data in the cognitive impaired population using the two-cut-off approach, but it needs further investigation to strengthen its clinical utility [170].
In the approval study of Lecanemab (Clarity AD), evidence of amyloid positivity was required for recruitment (PET or CSF Aβ42) [11], while for Donanemab (TRAILBLAZER-ALZ 2), PET scans for both Aβ and tau were required [12]. According to appropriate use recommendations for Lecanemab, an abnormal PET for Aβ or CSF findings of increased p-tau and reduced Aβ42 (increased p-tau/Aβ42) is required [14], while for Donanemab, amyloid positivity by an abnormal PET for Aβ or by reduced CSF Aβ42/Aβ40 or, alternatively, by abnormal CSF τP-181/Aβ42 and τT/Aβ42 ratios are required [15]. Canadian [17] and Korean [18] guidelines are similar to the appropriate use recommendations for Lecanemab and Donanemab. However, the French appropriate use recommendations for Lecanemab show some deviations since they recommend the CSF A+T+ profile, and in cases with inconclusive CSF results or, alternatively, an abnormal PET for Aβ [16]. In fact, similar recommendations are advocated by the International Working Group (IWG) criteria for the diagnosis of AD [42,44].
Thus, there are still debates over the definition and biomarker profile of AD, i.e., amongst the 2018 NIA-AA criteria [41], 2024 AA revised criteria [43], and IWG criteria [42,44]. However, this may be very important since it has been observed that different criteria may result in discordant diagnoses in ~42% of patients, especially when only one biomarker is abnormal [172].
8. Discussion
Currently, the most widely used biomarkers in everyday practice for AD diagnosis are the classical or core biomarkers (CSF or PET). Depending on availability, familiarity, acceptance, contraindications, cost, and reimbursement issues, either CSF or PET may be preferred in various countries for confirming AD and eligibility for anti-amyloid treatments or enrolment in experimental trials. However, after roughly 25 years of research on classical biomarkers, there are still controversies.
- Should we use the AT(N) classification system according to the 2018 NIA-AA guidelines [41] or the 2024 AA revised criteria [43]?
- Should we use the AA revised guidelines [43] or the IWG recommendations [44]?
- Should the profile be both A+ and T+ (either T1+ or T2+), or is A+ sufficient?
- Should we rely on the τP-181/Aβ42 and τT/Aβ42 ratios and when?
In some countries, CSF studies are the first to be performed. An example of how we work with our patients (not a recommendation) is shown in Figure 2, based on different scenarios according to CSF findings. To maximize confidence, we prefer using the A+T+ profile for confirming AD presence [153]. In the case of inconclusive results, we use the τP-181/Aβ42 [171] or (if affordable due to high cost) a PET scan for Aβ42. By doing so, we try to combine the various criteria and guidelines.
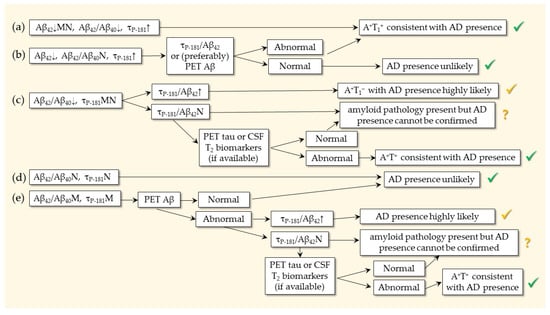
Figure 2.
Use of biomarkers for the confirmation of AD presence in 5 scenarios, starting from CSF analysis. N: normal; M: marginal. Green “O.K. symbols” indicate confirmation. Yellow “O.K. symbols” indicate the likely presence of AD. Question marks indicate the presence of amyloid pathology, but AD (additional occurrence of tau pathology) cannot be verified. In scenario (a), the typical CSF profile of AD is present (A+T+ or A+T1+). In scenario (b), Aβ42/Aβ40 is normal, raising questions about the presence of amyloid pathology, despite the abnormality of both Aβ42 and τP-181. In this case, a PET scan for Aβ or (if not available) the τP-181/Aβ42 ratio is required to reach a conclusion. In scenario (c), the A+T− profile is present with abnormality only in the Aβ42/Aβ40 ratio (or alternatively the amyloid PET). In this case, the τP-181/Aβ42 ratio can be used, but if normal, a T2 biomarker (if available) may be required. In scenario (d), the A−T− (A−T1−) profile is inconsistent with AD. In scenario (e), both molecular biomarkers are marginal. In such patients, the τP-181/Aβ42 ratio and/or PET scans for Aβ and/or tau may be needed according to availability and cost.
It is noteworthy to highlight that, accordingly to the AA revised guidelines, hybrid ratios encompass CSF τP-181/Aβ42, CSF τT/Aβ42, CSF Aβ42/Aβ40, and plasma %τP-217, without discrimination, even though the last two ratios represent distinct protein deposits, tau, and amyloid beta protein, respectively. Conversely, the first ones demonstrate the change in tau protein in relation to amyloid beta, assuming that τP-181/Aβ42 is more specific for AD, instead of total tau, which is a general indicator of neuronal damage. The nomenclature is of great importance, considering it may help both the comprehension of ratio implications and their accurate utilization.
An AD diagnosis based only on biomarkers may not suffice for treatment eligibility. Some patients may have mixed pathologies, and AD may not be the main pathology responsible for the clinical picture. This may be true in cases with DLB [154] or significant cerebrovascular disease [159], and evidence or suspicion of such co-pathologies is considered a contraindication to anti-amyloid treatment [14].
The clinical phenotype of AD may also be important. For the common AD phenotypes (typical amnestic, logopenic-type primary progressive aphasia, and posterior cortical atrophy) [42], there are no concerns [16,173].
However, for the uncommon AD phenotypes (frontal variant, non-logopenic primary progressive aphasia, and corticobasal syndrome), AD may not be the only pathology, and, since the presence of other co-pathologies cannot be excluded, anti-amyloid treatments may not be indicated [16].
From this perspective, it is questionable whether blood-based biomarkers could be a standalone tool for AD diagnosis and evaluation of anti-amyloid treatment eligibility, despite the adequate diagnostic accuracy of some of them. We need further investigation into the plasma biomarker changes in the aforementioned mixed and atypical pathologies, as well as in other pathologies that can affect the central nervous system, including secondary causes of dementia. Moreover, a recent approach to their assessment, also classified according to the performed immunoassays [174], enlightens a new era with realistic expectations and skepticism.
There are some limitations in this review. We did not perform a systematic or umbrella review with or without meta-analyses. But this was not our aim. We preferred a simple narrative review to show the current uncertainties in biomarker-based confirmation of AD, not for theoretical reasons but for implementing anti-amyloid treatments or for recruiting patients for clinical trials of new drugs. Furthermore, the data enclosed and analyzed primarily come from White populations, limiting the potential of generalizability in different ancestries.
9. Future Directions
Currently, there are many criteria and guidelines for AD diagnosis [33,34,41,42,43,44,45] and for eligibility for anti-amyloid treatments [13,14,15,16,17,18]. Among them, there are many common features, but also some differences. Every effort should be made to reach a consensus and achieve homogeneous criteria.
To increase diagnostic accuracy, alternative biomarker testing for synaptic loss, microglia activation, and neuroinflammation should become more widely available since, in the appropriate clinical setting, they have the potential to offer additional information and explain some discrepancies between core biomarkers [119,128].
To simplify the diagnostic procedure and potentially decrease the cost, plasma biomarkers, especially τP-217 and the τP-217/Aβ42, are promising and may enter everyday diagnostic algorithms in the near future [16]. However, the recently published AA Clinical Practice Guideline suggested caution since there may be significant variability in their diagnostic accuracy and moderate confidence [174]. The role of salivary, urinary, or ocular biomarkers in offering simple and accurate diagnosis remains to be established [137].
Author Contributions
Conceptualization, I.T., A.A., F.B., C.Z., V.C.C., S.G., J.S.T., E.K., G.T. and G.P.P.; data curation, I.T., A.A., F.B., A.T., A.M., P.-E.T., A.B., J.S.T., C.Z., S.G.P., S.G. and G.P.P.; formal analysis, I.T., V.C.C., A.M., F.B., A.T., A.A., C.Z., J.S.T., S.G.P., A.B., E.K., G.T. and G.P.P.; investigation, I.T., F.B., A.A., A.M., A.T., J.S.T., P.-E.T., C.Z., S.G.P., A.B., E.K., G.T. and G.P.P.; methodology, I.T., A.A., V.C.C., F.B., J.S.T., A.T., P.-E.T., S.G.P., S.G., E.K., G.T. and G.P.P. project administration, E.K., G.T. and G.P.P.; supervision, S.G., E.K., G.T. and G.P.P.; visualization, S.G., E.K., G.T. and G.P.P.; writing—original draft, I.T., V.C.C., P.-E.T., S.G.P., J.S.T., A.M., A.T. and G.P.P.; writing—review and editing, I.T., A.A., F.B., C.Z., A.B., S.G., J.S.T., E.K., G.T. and G.P.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that this study received no funding. G.T., G.P.P., I.T., A.A., C.Z. and A.B. are clinical investigators in the “EVOKE” and “EVOKE plus” trials of semaglutide for early Alzheimer’s disease (NovoNordisk, NCT04777396 and NCT04777409, respectively). G.P.P. received fees from Biogen International and from ITF Hellas as a consultant for advisory boards. These activities are not related to the present work and the funders were not involved in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to submit it for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this review article.
Conflicts of Interest
None.
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025. Available online: https://iris.who.int/handle/10665/259615 (accessed on 6 July 2025).
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Jiang, G.; Xie, G.; Li, X.; Xiong, J. Cytoskeletal Proteins and Alzheimer’s Disease Pathogenesis: Focusing on the Interplay with Tau Pathology. Biomolecules 2025, 15, 831. [Google Scholar] [CrossRef]
- Koller, E.J.; Ibanez, K.R.; Vo, Q.; McFarland, K.N.; Gonzalez De La Cruz, E.; Zobel, L.; Williams, T.; Xu, G.; Ryu, D.; Patel, P.; et al. Combinatorial model of amyloid β and tau reveals synergy between amyloid deposits and tangle formation. Neuropathol. Appl. Neurobiol. 2022, 48, e12779. [Google Scholar] [CrossRef]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Serban, M.; Covache-Busuioc, R.A.; Ciurea, A.V.; Enyedi, M. Decoding Neurodegeneration: A Review of Molecular Mechanisms and Therapeutic Advances in Alzheimer’s, Parkinson’s, and ALS. Int. J. Mol. Sci. 2024, 25, 12613. [Google Scholar] [CrossRef]
- Rathee, S.; Pandey, V.; Soni, S.; Sen, D.; Jain, S.K. Comprehending Alzheimer’s Disease: Molecular Mechanisms and Treatment Strategies. Curr. Alzheimer Res. 2025, 22, 414–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Chundu, U.C.; Thiriveedhi, S.R.; Bhatti, C.; Mupparaju, J.S.; Otinashvili, N.; Gelishvili, E. A Systematic Review of the Efficacy and Safety of Anti-amyloid Monoclonal Antibodies in Alzheimer’s Disease. Cureus 2025, 7, e85377. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S. Are Appropriate Use Recommendations useful in clinical practice? J. Prev. Alzheimers Dis. 2025, 12, 100165. [Google Scholar] [CrossRef]
- Cummings, J.; Apostolova, L.; Rabinovici, G.D.; Atri, A.; Aisen, P.; Greenberg, S.; Hendrix, S.; Selkoe, D.; Weiner, M.; Petersen, R.C.; et al. Lecanemab: Appropriate Use Recommendations. J. Prev. Alzheimers Dis. 2023, 10, 362–377. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Selkoe, D.J.; Schindler, S.E.; Aisen, P.; Apostolova, L.G.; Atri, A.; Greenberg, S.M.; Hendrix, S.B.; Petersen, R.C.; Weiner, M.; et al. Donanemab: Appropriate use recommendations. J. Prev. Alzheimers Dis. 2025, 12, 100150. [Google Scholar] [CrossRef]
- Villain, N.; Planche, V.; Lilamand, M.; Cordonnier, C.; Soto-Martin, M.; Mollion, H.; Bombois, S.; Delrieu, J.; French Federation of Memory Clinics Work Group on Anti-Amyloid Immunotherapies. Lecanemab for early Alzheimer’s disease: Appropriate use recommendations from the French federation of memory clinics. J. Prev. Alzheimers Dis. 2025, 12, 100094. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Phillips, N.A.; Feldman, H.H.; Borrie, M.; Ganesh, A.; Henri-Bhargava, A.; Desmarais, P.; Frank, A.; Badhwar, A.; Barlow, L.; et al. Use of lecanemab and donanemab in the Canadian healthcare system: Evidence, challenges, and areas for future research. J. Prev. Alzheimers. Dis. 2025, 12, 100068. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, G.H.; Kim, C.H.; Koh, S.H.; Moon, S.Y.; Park, Y.H.; Seo, S.W.; Yoon, B.; Lim, J.S.; Kim, B.C.; et al. Lecanemab: Appropriate Use Recommendations by Korean Dementia Association. Dement. Neurocogn. Disord. 2024, 23, 165–187. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- Lopez, O.L.; Becker, J.T.; Klunk, W.; Saxton, J.; Hamilton, R.L.; Kaufer, D.I.; Sweet, R.A.; Cidis Meltzer, C.; Wisniewski, S.; Kamboh, M.I.; et al. Research evaluation and diagnosis of probable Alzheimer’s disease over the last two decades: I. Neurology 2000, 55, 1854–1862. [Google Scholar] [CrossRef]
- Mendez, M.; Mastri, A.R.; Sung, J.H.; Frey, W.H. Clinically diagnosed Alzheimer’s disease: Neuropathologic findings in 650 cases. Alzheimer Dis. Assoc. Disord. 1992, 6, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Head, E.; Schmitt, F.A.; Davis, P.R.; Neltner, J.H.; Jicha, G.A.; Abner, E.L.; Smith, C.D.; Van Eldik, L.J.; Kryscio, R.J.; et al. Alzheimer’s disease is not “brain aging”: Neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011, 121, 571–587. [Google Scholar] [CrossRef]
- Galasko, D.; Hansen, L.A.; Katzman, R.; Wiederholt, W.; Masliah, E.; Terry, R.; Hill, L.R.; Lessin, P.; Thal, L.J. Clinical-neuropathological correlations in Alzheimer’s disease and related dementias. Arch. Neurol. 1994, 51, 888–895. [Google Scholar] [CrossRef]
- Sarto, J.; Mayà, G.; Molina-Porcel, L.; Balasa, M.; Gelpi, E.; Aldecoa, I.; Borrego-Écija, S.; Contador, J.; Ximelis, T.; Vergara, M.; et al. Evolution of Clinical-Pathological Correlations in Early-Onset Alzheimer’s Disease Over a 25-Year Period in an Academic Brain Bank. J. Alzheimers Dis. 2022, 87, 1659–1669. [Google Scholar] [CrossRef]
- Xing, X.; Zhang, X.; Wang, K.; Wang, Z.; Feng, Y.; Li, X.; Hua, Y.; Zhang, L.; Dong, X. Post-marketing safety concerns with lecanemab: A pharmacovigilance study based on the FDA Adverse Event Reporting System database. Alzheimers Res. Ther. 2025, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- McGrowder, D.A.; Miller, F.; Vaz, K.; Nwokocha, C.; Wilson-Clarke, C.; Anderson-Cross, M.; Brown, J.; Anderson-Jackson, L.; Williams, L.; Latore, L.; et al. Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease: Current Evidence and Future Perspectives. Brain Sci. 2021, 11, 215. [Google Scholar] [CrossRef]
- Solje, E.; Benussi, A.; Buratti, E.; Remes, A.M.; Haapasalo, A.; Borroni, B. State-of-the-Art Methods and Emerging Fluid Biomarkers in the Diagnostics of Dementia-A Short Review and Diagnostic Algorithm. Diagnostics 2021, 11, 788. [Google Scholar] [CrossRef]
- Wilson, H.; Pagano, G.; Politis, M. Dementia spectrum disorders: Lessons learnt from decades with PET research. J. Neural Transm. 2019, 126, 233–251. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Festari, C.; Massa, F.; Cotta Ramusino, M.; Orini, S.; Aarsland, D.; Agosta, F.; Babiloni, C.; Borroni, B.; Cappa, S.F.; et al. European intersocietal recommendations for the biomarker-based diagnosis of neurocognitive disorders. Lancet Neurol. 2024, 23, 302–312. [Google Scholar] [CrossRef]
- Agnello, L.; Gambino, C.M.; Ciaccio, A.M.; Masucci, A.; Vassallo, R.; Tamburello, M.; Scazzone, C.; Lo Sasso, B.; Ciaccio, M. Molecular Biomarkers of Neurodegenerative Disorders: A Practical Guide to Their Appropriate Use and Interpretation in Clinical Practice. Int. J. Mol. Sci. 2024, 25, 4323. [Google Scholar] [CrossRef] [PubMed]
- Grabher, B.J. Amyloid Imaging Update: How the Amyloid Landscape Is Changing in Light of the Recent Food and Drug Administration Approval of Antiamyloid Therapeutics. J. Nucl. Med. Technol. 2024, 52, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Decaix, T.; Mouton-Liger, F.; Dumurgier, J.; Cognat, E.; Vrillon, A.; Hugon, J.; Hourregue, C.; Bouaziz-Amar, E.; Wallon, D.; Muraine, M.Q.; et al. Usefulness of Cerebrospinal Fluid Alzheimer’s disease biomarkers in older patients: Evidence from a national multicenter prospective study. J. Prev. Alzheimers Dis. 2025, 12, 100009. [Google Scholar] [CrossRef]
- Leuzy, A.; Bollack, A.; Pellegrino, D.; Teunissen, C.E.; La Joie, R.; Rabinovici, G.D.; Franzmeier, N.; Johnson, K.; Barkhof, F.; Shaw, L.M.; et al. Considerations in the clinical use of amyloid PET and CSF biomarkers for Alzheimer’s disease. Alzheimers Dement. 2025, 21, e14528. [Google Scholar] [CrossRef] [PubMed]
- Niemantsverdriet, E.; Valckx, S.; Bjerke, M.; Engelborghs, S. Alzheimer’s disease CSF biomarkers: Clinical indications and rational use. Acta Neurol. Belg. 2017, 117, 591–602. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=alzheimer+CSF+biomarkers&filter=pubt.review&sort=date (accessed on 13 August 2025). [CrossRef]
- Jack, C.R.; Wiste, H.J.; Algeciras-Schimnich, A.; Weigand, S.D.; Figdore, D.J.; Lowe, V.J.; Vemuri, P.; Graff-Radford, J.; Ramanan, V.K.; Knopman, D.S.; et al. Comparison of plasma biomarkers and amyloid PET for predicting memory decline in cognitively unimpaired individuals. Alzheimers Dement. 2024, 20, 2143–2154. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=alzheimer+PET+biomarkers&filter=pubt.review&sort=date (accessed on 13 August 2025). [CrossRef]
- Mandal, P.K.; Maroon, J.C.; Garg, A.; Arora, N.K.; Bansal, R.; Kaushik, A.; Samkaria, A.; Kumaran, G.; Arora, Y. Blood Biomarkers in Alzheimer’s Disease. ACS Chem. Neurosci. 2023, 14, 3975–3978. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=alzheimer+blood+biomarkers&filter=pubt.review&sort=date (accessed on 13 August 2025). [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Villain, N.; Schneider, L.; Fox, N.; Campbell, N.; Galasko, D.; Kivipelto, M.; Jessen, F.; Hanseeuw, B.; Boada, M.; et al. Alzheimer Disease as a Clinical-Biological Construct-An International Working Group Recommendation. JAMA Neurol. 2024, 81, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Planche, V.; Schindler, S.; Knopman, D.S.; Frisoni, G.; Galasko, D.; Grill, J.D.; Schneider, L.; Karlawish, J.; Villain, N. The science does not yet support regulatory approval of amyloid-targeting therapies for Alzheimer’s disease based solely on biomarker evidence. Alzheimers Dement. 2025, 21, e70068. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, M.; Minthon, L.; Davidsson, P.; Granérus, A.K.; Clarberg, A.; Vanderstichele, H.; Vanmechelen, E.; Wallin, A.; Blennow, K. CSF levels of tau, beta-amyloid(1-42) and GAP-43 in frontotemporal dementia, other types of dementia and normal aging. J. Neural Transm. 2000, 107, 563–579. [Google Scholar]
- Niemantsverdriet, E.; Ottoy, J.; Somers, C.; De Roeck, E.; Struyfs, H.; Soetewey, F.; Verhaeghe, J.; Van den Bossche, T.; Van Mossevelde, S.; Goeman, J.; et al. The Cerebrospinal Fluid Aβ1-42/Aβ1-40 Ratio Improves Concordance with Amyloid-PET for Diagnosing Alzheimer’s Disease in a Clinical Setting. J. Alzheimers Dis. 2017, 60, 561–576. [Google Scholar] [CrossRef]
- Constantinides, V.C.; Paraskevas, G.P.; Boufidou, F.; Bourbouli, M.; Pyrgelis, E.S.; Stefanis, L.; Kapaki, E. CSF Aβ42 and Aβ42/Aβ40 Ratio in Alzheimer’s Disease and Frontotemporal Dementias. Diagnostics 2023, 13, 783. [Google Scholar] [CrossRef]
- Vanderstichele, H.; De Vreese, K.; Blennow, K.; Andreasen, N.; Sindic, C.; Ivanoiu, A.; Hampel, H.; Bürger, K.; Parnetti, L.; Lanari, A.; et al. Analytical performance and clinical utility of the INNOTEST PHOSPHO-TAU181P assay for discrimination between Alzheimer’s disease and dementia with Lewy bodies. Clin. Chem. Lab. Med. 2006, 44, 1472–1480. [Google Scholar] [CrossRef]
- Suárez-Calvet, M.; Karikari, T.K.; Ashton, N.J.; Lantero Rodríguez, J.; Milà-Alomà, M.; Gispert, J.D.; Salvadó, G.; Minguillon, C.; Fauria, K.; Shekari, M.; et al. Novel Tau Biomarkers Phosphorylated at T181, T217 or T231 Rise in the Initial Stages of the Preclinical Alzheimer’s Continuum When Only Subtle Changes in Aβ Pathology Are Detected. EMBO Mol. Med. 2020, 12, e12921. [Google Scholar] [CrossRef]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.C.; Proctor, N.K.; Chai, X.; Shcherbinin, S.; Sims, J.R.; et al. Cerebrospinal Fluid P-Tau217 Performs Better than p-Tau181 as a Biomarker of Alzheimer’s Disease. Nat. Commun. 2020, 11, 1683. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Abeta and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef]
- Ribarič, S. Detecting Early Cognitive Decline in Alzheimer’s Disease with Brain Synaptic Structural and Functional Evaluation. Biomedicines 2023, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, N.R.; Saef, B.; Li, Y.; Gordon, B.A.; He, Y.; Horie, K.; Stomrud, E.; Salvadó, G.; Janelidze, S.; Sato, C.; et al. CSF tau phosphorylation occupancies at T217 and T205 represent improved biomarkers of amyloid and tau pathology in Alzheimer’s disease. Nat. Aging. 2023, 3, 391–401. [Google Scholar] [CrossRef]
- Kac, P.R.; Alcolea, D.; Montoliu-Gaya, L.; Fernández, S.; Rodriguez, J.L.; Maure, L.; González-Ortiz, F.; Benejam, B.; Turton, M.; Barroeta, I.; et al. Plasma p-tau212 as a biomarker of sporadic and Down syndrome Alzheimer’s disease. Alzheimers Dement. 2025, 21, e70172. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Salvadó, G.; Barthélemy, N.R.; Janelidze, S.; Li, Y.; He, Y.; Saef, B.; Chen, C.D.; Jiang, H.; Strandberg, O.; et al. CSF MTBR-tau243 is a specific biomarker of tau tangle pathology in Alzheimer’s disease. Nat. Med. 2023, 29, 1954–1963. [Google Scholar] [CrossRef]
- Strain, J.F.; Barthelemy, N.; Horie, K.; Gordon, B.A.; Kilgore, C.; Aschenbrenner, A.; Cruchaga, C.; Xiong, C.; Joseph-Mathurin, N.; Hassenstab, J.; et al. CSF Tau phosphorylation at Thr205 is associated with loss of white matter integrity in autosomal dominant Alzheimer disease. Neurobiol. Dis. 2022, 168, 105714. [Google Scholar] [CrossRef]
- Lantero-Rodriguez, J.; Montoliu-Gaya, L.; Ashton, N.J.; Pola, I.; Therriault, J.; Rahmouni, N.; Brum, W.S.; Servaes, S.; Stevenson, J.; Di Molfetta, G.; et al. Biofluid-based staging of Alzheimer’s disease. Acta Neuropathol. 2025, 149, 27. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Herholz, K. Amyloid imaging for dementia in clinical practice. BMC Med. 2015, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Beach, T.G.; Zanette, M.; Lilja, J.; Heurling, K.; Chakrabarty, A.; Ismail, A.; Farrar, G.; Buckley, C.; Smith, A.P.L. Estimation of amyloid distribution by [(18)F]flutemetamol PET predicts the neuropathological phase of amyloid beta-protein deposition. Acta Neuropathol. 2018, 136, 557–567. [Google Scholar] [CrossRef]
- Collij, L.E.; Bollack, A.; La Joie, R.; Shekari, M.; Bullich, S.; Roé-Vellvé, N.; Koglin, N.; Jovalekic, A.; Garciá, D.V.; Drzezga, A.; et al. Centiloid recommendations for clinical context-of-use from the AMYPAD consortium. Alzheimers Dement. 2024, 20, 9037–9048. [Google Scholar] [CrossRef]
- Schwarz, A.J.; Yu, P.; Miller, B.B.; Shcherbinin, S.; Dickson, J.; Navitsky, M.; Joshi, A.D.; Devous, M.D., Sr.; Mintun, M.S. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain 2016, 139, 1539–1550. [Google Scholar] [CrossRef]
- Lowe, V.J.; Lundt, E.S.; Albertson, S.M.; Min, H.K.; Fang, P.; Przybelski, S.A.; Senjem, M.L.; Schwarz, C.G.; Kantarci, K.; Boeve, B.; et al. Tau-positron emission tomography correlates with neuropathology findings. Alzheimers Dement. 2020, 16, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Pichet Binette, A.; Groot, C.; Smith, R.; Strandberg, O.; Palmqvist, S.; Stomrud, E.; Tideman, P.; Ohlsson, T.; Jogi, J.; et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat. Med. 2022, 28, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.W.; Young, A.L.; Oxtoby, N.P.; Smith, R.; Ossenkoppele, R.; Strandberg, O.T.; La Joie, R.; Aksman, L.M.; Grothe, M.J.; Iturria-Medina, Y.; et al. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med. 2021, 27, 871–881. [Google Scholar] [CrossRef]
- Blennow, K.; Wallin, A.; Agren, H.; Spenger, C.; Siegfried, J.; Vanmechelen, E. Tau protein in cerebrospinal fluid: A biochemical marker for axonal degeneration in Alzheimer disease? Mol. Chem. Neuropathol. 1995, 26, 231–245. [Google Scholar] [CrossRef]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Nixon, R.A. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef]
- Chatziefstathiou, A.; Canaslan, S.; Kanata, E.; Vekrellis, K.; Constantinides, V.C.; Paraskevas, G.P.; Kapaki, E.; Schmitz, M.; Zerr, I.; Xanthopoulos, K.; et al. SIMOA Diagnostics on Alzheimer’s Disease and Frontotemporal Dementia. Biomedicines 2024, 12, 1253. [Google Scholar] [CrossRef]
- Minoshima, S.; Cross, D.; Thientunyakit, T.; Foster, N.L.; Drzezga, A. 18F-FDG PET Imaging in Neurodegenerative Dementing Disorders: Insights into Subtype Classification, Emerging Disease Categories, and Mixed Dementia with Copathologies. J. Nucl. Med. 2022, 63 (Suppl. S1), 2S–12S. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Singleton, E.H.; Groot, C.; Dijkstra, A.A.; Eikelboom, W.S.; Seeley, W.W.; Miller, B.; Laforce, R.J.; Scheltens, P.; Papma, J.M.; et al. Research Criteria for the Behavioral Variant of Alzheimer Disease: A Systematic Review and Meta-analysis. JAMA Neurol. 2022, 79, 48–60. [Google Scholar] [CrossRef]
- Swan, A.; Waddell, B.; Holloway, G.; Bak, T.; Colville, S.; Khan, Z.; Pal, S. The diagnostic utility of 99mTc-HMPAO SPECT imaging: A retrospective case series from a tertiary referral early-onset cognitive disorders clinic. Dement. Geriatr. Cogn. Disord. 2015, 39, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Thropp, P.; Phillips, E.; Jung, Y.; Thomas, D.L.; Tosun, D.; Alzheimer’s Disease Neuroimaging Initiative. Arterial spin labeling perfusion MRI in the Alzheimer’s Disease Neuroimaging Initiative: Past, present, and future. Alzheimers Dement. 2024, 20, 8937–8952. [Google Scholar] [CrossRef]
- Bernetti, C.; D’Andrea, V.; Buoso, A.; Barbalace, I.; Greco, F.; Pilato, F.; Calandrelli, R.; Di Lazzaro, V.; Zobel, B.B.; Mallio, C.A. Arterial Spin Labeling MRI in Alzheimer’s Disease: A Systematic Review of Cerebral Perfusion Biomarkers. J. Neuroimaging 2025, 35, e70035. [Google Scholar] [CrossRef]
- Bosco, P.; Redolfi, A.; Bocchetta, M.; Ferrari, C.; Mega, A.; Galluzzi, S.; Austin, M.; Chincarini, A.; Collins, D.L.; Duchesne, S.; et al. The impact of automated hippocampal volumetry on diagnostic confidence in patients with suspected Alzheimer’s disease: A European Alzheimer’s Disease Consortium study. Alzheimers Dement. 2017, 13, 1013–1023. [Google Scholar] [CrossRef]
- Scheltens, P.; Leys, D.; Barkhof, F.; Huglo, D.; Weinstein, H.C.; Vermersch, P.; Kuiper, M.; Steinling, M.; Wolters, E.C.; Valk, J. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: Diagnostic value and neuropsychological correlates. J. Neurol. Neurosurg. Psychiatry 1992, 55, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Enkirch, S.J.; Traschütz, A.; Müller, A.; Widmann, C.N.; Gielen, G.H.; Heneka, M.T.; Jurcoane, A.; Schild, H.H.; Hattingen, E. The ERICA Score: An MR Imaging-based Visual Scoring System for the Assessment of Entorhinal Cortex Atrophy in Alzheimer Disease. Radiology 2018, 288, 226–233. [Google Scholar] [CrossRef]
- Roberge, X.; Brisson, M.; Laforce, R.J. Specificity of Entorhinal Atrophy MRI Scale in Predicting Alzheimer’s Disease Conversion. Can. J. Neurol. Sci. 2023, 50, 112–114. [Google Scholar] [CrossRef]
- Alexopoulos, P.; Kriett, L.; Haller, B.; Klupp, E.; Gray, K.; Grimmer, T.; Laskaris, N.; Förster, S.; Perneczky, R.; Kurz, A.; et al. Limited agreement between biomarkers of neuronal injury at different stages of Alzheimer’s disease. Alzheimers Dement. 2014, 10, 684–689. [Google Scholar] [CrossRef]
- McAleese, K.E.; Miah, M.; Graham, S.; Hadfield, G.M.; Walker, L.; Johnson, M.; Colloby, S.J.; Thomas, A.J.; DeCarli, C.; Koss, D.; et al. Frontal white matter lesions in Alzheimer’s disease are associated with both small vessel disease and AD-associated cortical pathology. Acta Neuropathol. 2021, 142, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Liu, S.; Zhang, Y.; Zhao, Z.; Li, H.; Li, Y.; Lan, X.; Wang, L.; Liu, J.; Ji, C. Phenotypes of white matter hyperintensities, mechanisms and aetiological differentiation: Future targets pre-dementia–a systematic review. Discov. Neurosci. 2025, 20, 6. [Google Scholar] [CrossRef]
- McAleese, K.E.; Walker, L.; Graham, S.; Moya, E.L.J.; Johnson, M.; Erskine, D.; Colloby, S.J.; Dey, M.; Martin-Ruiz, C.; Taylor, J.P.; et al. Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 2017, 134, 459–473. [Google Scholar] [CrossRef]
- Theodorou, A.; Palaiodimou, L.; Papagiannopoulou, G.; Kargiotis, O.; Psychogios, K.; Safouris, A.; Bakola, E.; Chondrogianni, M.; Kotsali-Peteinelli, V.; Melanis, K.; et al. Clinical Characteristics, Neuroimaging Markers, and Outcomes in Patients with Cerebral Amyloid Angiopathy: A Prospective Cohort Study. J. Clin. Med. 2023, 12, 5591. [Google Scholar] [CrossRef]
- Kapaki, E.; Paraskevas, P.G.; Emmanouilidou, E.; Vekrellis, K. The diagnostic value of CSF α-synuclein in the differential diagnosis of dementia with Lewy bodies vs. normal subjects and patients with Alzheimer’s disease. PLoS ONE 2013, 8, e81654. [Google Scholar] [CrossRef]
- Anagnostou, D.; Sfakianaki, G.; Melachroinou, K.; Soutos, M.; Constantinides, V.; Vaikath, N.; Tsantzali, I.; Paraskevas, G.P.; Agnaf, O.E.; Vekrellis, K.; et al. Assessment of Aggregated and Exosome-Associated α-Synuclein in Brain Tissue and Cerebrospinal Fluid Using Specific Immunoassays. Diagnostics 2023, 13, 2192. [Google Scholar] [CrossRef] [PubMed]
- Kapsali, I.; Brinia, M.E.; Constantinides, V.C. Cerebrospinal Fluid Total, Phosphorylated and Oligomeric A-Synuclein in Parkinson’s Disease: A Systematic Review, Meta-Analysis and Meta-Regression Study. Biomedicines 2024, 12, 2266. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, G.; De Luca, C.M.G.; Paoletti, F.P.; Gaetani, L.; Moda, F.; Parnetti, L. alpha-Synuclein Seed Amplification Assays for Diagnosing Synucleinopathies: The Way Forward. Neurology 2022, 99, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Bang, J.I.; Ahn, C.; Nyaga, V.N.; Kim, Y.E.; Kang, M.J.; Ahn, T.B. Diagnostic value of alpha-synuclein seeding amplification assays in alpha-synucleinopathies: A systematic review and meta-analysis. Park. Relat. Disord. 2022, 104, 99–109. [Google Scholar] [CrossRef]
- Srivastava, A.; Wang, Q.; Orrù, C.D.; Fernandez, M.; Compta, Y.; Ghetti, B.; Zanusso, G.; Zou, W.Q.; Caughey, B.; Beauchemin, C.A.A. Enhanced quantitation of pathological alpha-synuclein in patient biospecimens by RT-QuIC seed amplification assays. PLoS Pathog. 2024, 20, e1012554. [Google Scholar] [CrossRef]
- Kapaki, E.; Boufidou, F.; Bourbouli, M.; Pyrgelis, E.S.; Constantinides, V.C.; Anastassopoulou, C.; Paraskevas, G.P. Cerebrospinal Fluid Biomarker Profile in TDP-43-Related Genetic Frontotemporal Dementia. J. Pers. Med. 2022, 12, 1747. [Google Scholar] [CrossRef]
- Duong, M.T.; Wolk, D.A. Limbic-Predominant Age-Related TDP-43 Encephalopathy: LATE-Breaking Updates in Clinicopathologic Features and Biomarkers. Curr. Neurol. Neurosci. Rep. 2022, 22, 689–698. [Google Scholar] [CrossRef]
- Youssef, H.; Weissmann, C.; Uruk, G.; Gatto, R.G. Looking into Abnormal Co-Expressions of Tau and TDP-43 in the Realm of Mixed Dementia Types: A Double-Punch Scenario. Brain Sci. 2025, 15, 716. [Google Scholar] [CrossRef]
- Zabala-Findlay, A.; Penny, L.K.; Lofthouse, R.A.; Porter, A.J.; Palliyil, S.; Harrington, C.R.; Wischik, C.M.; Arastoo, M. Utility of Blood-Based Tau Biomarkers for Mild Cognitive Impairment and Alzheimer’s Disease: Systematic Review and Meta-Analysis. Cells 2023, 12, 1184. [Google Scholar] [CrossRef]
- Nunkoo, V.S.; Jurcau, A.; Les, M.; Cristian, A.; Militaru, M.; Marge, C.; Iovanovici, D.C.; Jurcau, M.C. Circulating Biomarkers for the Early Diagnosis of Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 7268. [Google Scholar] [CrossRef]
- Rissman, R.A.; Langford, O.; Raman, R.; Donohue, M.C.; Abdel-Latif, S.; Meyer, M.R.; Wente-Roth, T.; Kirmess, K.M.; Ngolab, J.; Winston, C.N.; et al. Plasma Aβ42/Aβ40 and phospho-tau217 concentration ratios increase the accuracy of amyloid PET classification in preclinical Alzheimer’s disease. Alzheimers Dement. 2024, 20, 1214–1224. [Google Scholar] [CrossRef]
- Janelidze, S.; Barthélemy, N.R.; Salvadó, G.; Schindler, S.E.; Palmqvist, S.; Mattsson-Carlgren, N.; Braunstein, J.B.; Ovod, V.; Bollinger, J.G.; He, Y.; et al. Plasma Phosphorylated Tau 217 and Aβ42/40 to Predict Early Brain Aβ Accumulation in People Without Cognitive Impairment. JAMA Neurol. 2024, 81, 947–957. [Google Scholar] [CrossRef]
- Tsantzali, I.; Foska, A.; Sideri, E.; Routsi, E.; Tsomaka, E.; Kitsos, D.K.; Zompola, C.; Bonakis, A.; Giannopoulos, S.; Voumvourakis, K.I.; et al. Plasma Phospho-Tau-181 as a Diagnostic Aid in Alzheimer’s Disease. Biomedicines 2022, 10, 1879. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Boufidou, F.; Stergiou, C.; Kuhle, J.; Willemse, E.; Palaiodimou, L.; Tsantzali, I.; Sideri, E.; Bonakis, A.; Giannopoulos, S.; et al. Plasma P-Tau181 for the Discrimination of Alzheimer’s Disease from Other Primary Dementing and/or Movement Disorders. Biomolecules 2022, 12, 1099. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Pascoal, T.A.; Karikari, T.K.; Benedet, A.L.; Lantero-Rodriguez, J.; Brinkmalm, G.; Snellman, A.; Schöll, M.; Troakes, C.; Hye, A.; et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021, 141, 709–724. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Salvadó, G.; Janelidze, S.; Pichet Binette, A.; Bali, D.; Karlsson, L.; Palmqvist, S.; Mattsson-Carlgren, N.; Stomrud, E.; Therriault, J.; et al. Plasma p-tau217 and tau-PET predict future cognitive decline among cognitively unimpaired individuals: Implications for clinical trials. Nat. Aging 2025, 5, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Dubarbie, F.; Guerra-Ruiz, A.; López-García, S.; Lage, C.; Fernández-Matarrubia, M.; Nevado-Cáceres, Á.; Rivera-Sánchez, M.; Valera-Barrero, A.; Pozueta-Cantudo, A.; García-Martínez, M.; et al. Diagnostic performance of plasma p-tau217 in a memory clinic cohort using the Lumipulse automated platform. Alzheimers Res. Ther. 2025, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Schraen-Maschke, S.; Vidal, J.S.; Delaby, C.; Buee, L.; Blanc, F.; Paquet, C.; Allinquant, B.; Bombois, S.; Gabelle, A.; et al. Clinical value of plasma ALZpath pTau217 immunoassay for assessing mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2024, 95, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Poesen, K.; Vandenberghe, R.; De Meyer, S. Alzheimer’s disease neuropathology and its estimation with fluid and imaging biomarkers. Mol. Neurodegener. 2025, 20, 33. [Google Scholar] [CrossRef]
- Horie, K.; Salvadó, G.; Koppisetti, R.K.; Janelidze, S.; Barthélemy, N.R.; He, Y.; Sato, C.; Gordon, B.A.; Jiang, H.; Benzinger, T.L.S.; et al. Plasma MTBR-tau243 biomarker identifies tau tangle pathology in Alzheimer’s disease. Nat. Med. 2025, 31, 2044–2053. [Google Scholar] [CrossRef]
- Jung, Y.; Damoiseaux, J.S. The potential of blood neurofilament light as a marker of neurodegeneration for Alzheimer’s disease. Brain 2024, 147, 12–25. [Google Scholar] [CrossRef]
- Jin, Z.; Lu, Y.; Tang, H.; Cui, H. Integrating neuroinflammation biomarkers into the ATN(X) framework: Advances in Alzheimer’s pathogenesis, diagnosis, and insights from non-human primate models. Alzheimers Dement. 2025, 21, 70472. [Google Scholar] [CrossRef]
- Benedet, A.L.; Milà-Alomà, M.; Vrillon, A.; Ashton, N.J.; Pascoal, T.A.; Lussier, F.; Karikari, T.K.; Hourregue, C.; Cognat, E.; Dumurgier, J.; et al. Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA Neurol. 2021, 78, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The Amyloid-β Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimers Dis. 2018, 64, S567–S610. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.R.; Jin, S.; Ramalingam, N.; Bellier, J.P.; Lish, A.M.; Ostaszewski, B.L.; Young-Pearse, T.; Liu, L.; Yang, H.S.; et al. An improved immunoassay detects Abeta oligomers in human biofluids: Their CSF levels rise with tau and phosphotau levels. Alzheimers Res. Ther. 2025, 17, 153. [Google Scholar] [CrossRef] [PubMed]
- Blömeke, L.; Rehn, F.; Pils, M.; Kraemer-Schulien, V.; Cousin, A.; Kutzsche, J.; Bujnicki, T.; Freiesleben, S.D.; Schneider, L.S.; Preis, L.; et al. Blood-based quantification of Aβ oligomers indicates impaired clearance from brain in ApoE ε4 positive subjects. Commun. Med. 2024, 4, 262. [Google Scholar] [CrossRef]
- An, J.; Kim, K.; Lim, H.J.; Kim, H.Y.; Shin, J.; Park, I.; Cho, I.; Kim, H.Y.; Kim, S.; McLean, C.; et al. Early onset diagnosis in Alzheimer’s disease patients via amyloid-beta oligomers-sensing probe in cerebrospinal fluid. Nat. Commun. 2024, 15, 1004. [Google Scholar] [CrossRef]
- Mao, H.; Ding, L. Ultrasensitive portable aptamer-electrochemical sensor for point-of-care testing of Alzheimer’s biomarker amyloid-beta oligomers. Bioelectrochemistry 2025, 167, 109079. [Google Scholar]
- Blömeke, L.; Pils, M.; Kraemer-Schulien, V.; Dybala, A.; Schaffrath, A.; Kulawik, A.; Rehn, F.; Cousin, A.; Nischwitz, V.; Willbold, J.; et al. Quantitative detection of α-Synuclein and Tau oligomers and other aggregates by digital single particle counting. npj Parkinsons Dis. 2022, 8, 68. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Alizadeh, H.; Marton, J.; Cumming, P. The Sensitivity of Tau Tracers for the Discrimination of Alzheimer’s Disease Patients and Healthy Controls by PET. Biomolecules 2023, 13, 290. [Google Scholar] [CrossRef]
- Aliaga, A.; Therriault, J.; Quispialaya, K.M.; Aliaga, A.; Hopewell, R.; Rahmouni, N.; Macedo, A.C.; Kunach, P.; Soucy, J.P.; Massarweh, G.; et al. Comparison Between Brain and Cerebellar Autoradiography Using [18F]Flortaucipir, [18F]MK6240, and [18F]PI2620 in Postmortem Human Brain Tissue. J. Nucl. Med. 2025, 66, 123–129. [Google Scholar] [CrossRef]
- Kang, X.; Tian, J.; Shu, Q.; Cheng, T.; Wang, S.; Hu, Y. Microglia-neuron crosstalk in Alzheimer’s disease: An exploration of molecular mechanisms and pathological implications. Neuroscience 2025, 583, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calvet, M.; Kleinberger, G.; Araque Caballero, M.Á.; Brendel, M.; Rominger, A.; Alcolea, D.; Fortea, J.; Lleó, A.; Blesa, R.; Gispert, J.D.; et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol. Med. 2016, 8, 466–476. [Google Scholar] [CrossRef]
- Fernández-Matarrubia, M.; Valera-Barrero, A.; Renuncio-García, M.; Aguilella, M.; Lage, C.; López-García, S.; Ocejo-Vinyals, J.G.; Martínez-Dubarbie, F.; Molfetta, G.D.; Pozueta-Cantudo, A.; et al. Early microglial and astrocyte reactivity in preclinical Alzheimer’s disease. Alzheimers Dement. 2025, 21, e70502. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mantyh, W.G.; Men, L.; Jain, I.; Glittenberg, M.; An, B.; Zhang, L.; Li, L.; Alzheimer’s Disease Neuroimaging Initiative. sTREM2 in discordant CSF Abeta(42) and p-tau181. Alzheimers Dement. 2025, 17, e70072. [Google Scholar]
- Lan, G.; Li, A.; Gonzalez-Ortiz, F.; Lv, J.; Ran, W.; Cai, Y.; Sun, P.; Liu, L.; Yang, J.; Zhang, L.; et al. Higher plasma soluble TREM2 correlates with reduced cerebral tau accumulation in Alzheimer’s disease. Mol. Psychiatry 2025, 30, 3988–3997. [Google Scholar] [CrossRef]
- Rodriguez-Vieitez, E.; Ashton, N.J. Plasma sTREM2: A potential marker of cerebrovascular injury in neurodegenerative disorders. Brain 2021, 144, 3283–3285. [Google Scholar] [CrossRef]
- Jiao, L.; Yang, J.; Wang, W.; Liu, X.; Fu, Y.; Fan, D. sTREM2 cerebrospinal fluid levels are a potential biomarker in amyotrophic lateral sclerosis and associate with UMN burden. Front. Neurol. 2024, 15, 1515252. [Google Scholar] [CrossRef]
- Mavroudis, I.; Chowdhury, R.; Petridis, F.; Karantali, E.; Chatzikonstantinou, S.; Balmus, I.M.; Luca, I.S.; Ciobica, A.; Kazis, D. YKL-40 as a Potential Biomarker for the Differential Diagnosis of Alzheimer’s Disease. Medicina 2021, 58, 60. [Google Scholar] [CrossRef]
- Pelkmans, W.; Shekari, M.; Brugulat-Serrat, A.; Sánchez-Benavides, G.; Minguillón, C.; Fauria, K.; Molinuevo, J.L.; Grau-Rivera, O.; González Escalante, A.; Kollmorgen, G.; et al. Astrocyte biomarkers GFAP and YKL-40 mediate early Alzheimer’s disease progression. Alzheimers Dement. 2024, 20, 483–493. [Google Scholar] [CrossRef]
- Pase, M.P.; Himali, J.J.; Puerta, R.; Beiser, A.S.; Gonzales, M.M.; Satizabal, C.L.; Yang, Q.; Aparicio, H.J.; Kojis, D.J.; Decarli, C.S.; et al. Association of Plasma YKL-40 with MRI, CSF, and Cognitive Markers of Brain Health and Dementia. Neurology 2024, 102, e208075. [Google Scholar] [CrossRef]
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef]
- Lista, S.; Imbimbo, B.P.; Grasso, M.; Fidilio, A.; Emanuele, E.; Minoretti, P.; López-Ortiz, S.; Martín-Hernández, J.; Gabelle, A.; Caruso, G.; et al. Tracking neuroinflammatory biomarkers in Alzheimer’s disease: A strategy for individualized therapeutic approaches? J. Neuroinflammation 2024, 21, 187. [Google Scholar] [CrossRef] [PubMed]
- Argiris, G.; Akinci, M.; Peña-Gómez, C.; Palpatzis, E.; Garcia-Prat, M.; Shekari, M.; Blennow, K.; Zetterberg, H.; Kollmorgen, G.; Quijano-Rubio, C.; et al. Data-driven CSF biomarker profiling: Imaging and clinical outcomes in a cohort at risk of Alzheimer’s disease. Alzheimers Res. Ther. 2024, 16, 274. [Google Scholar] [CrossRef] [PubMed]
- Katonova, A.; Andel, R.; Jurasova, V.; Veverova, K.; Angelucci, F.; Matoska, V.; Hort, J. Associations of KLOTHO-VS heterozygosity and α-Klotho protein with cerebrospinal fluid Alzheimer’s disease biomarkers. J. Alzheimers Dis. 2025, 105, 159–171. [Google Scholar] [CrossRef]
- Griffiths, J.; Grant, S.G.N. Synapse pathology in Alzheimer’s disease. Semin. Cell Dev. Biol. 2023, 139, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.J.; Sekula, N.M.; Svirsky, S.; Maesako, M.; Zoltowska, K.M.; Berezovska, O. Presenilin 1 increases association with synaptotagmin 1 during normal aging. Neurobiol. Aging 2020, 86, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xie, S.; Malek, M. SNAP-25: A biomarker of synaptic loss in neurodegeneration. Clin. Chim. Acta 2025, 571, 120236. [Google Scholar] [CrossRef]
- Kivisäkk, P.; Carlyle, B.C.; Sweeney, T.; Quinn, J.P.; Ramirez, C.E.; Trombetta, B.A.; Mendes, M.; Brock, M.; Rubel, C.; Czerkowicz, J.; et al. Increased levels of the synaptic proteins PSD-95, SNAP-25, and neurogranin in the cerebrospinal fluid of patients with Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 58. [Google Scholar] [CrossRef]
- Jurasova, V.; Andel, R.; Katonova, A.; Veverova, K.; Zuntychova, T.; Horakova, H.; Vyhnalek, M.; Kolarova, T.; Matoska, V.; Blennow, K.; et al. CSF neurogranin levels as a biomarker in Alzheimer’s disease and frontotemporal lobar degeneration: A cross-sectional analysis. Alzheimers Res. Ther. 2024, 16, 199. [Google Scholar] [CrossRef]
- Goossens, J.; Cervantes González, A.; Dewit, N.; Lidón, L.; Fortea, J.; Alcolea, D.; Lleó, A.; Belbin, O.; Vanmechelen, E. Evaluation of cerebrospinal fluid levels of synaptic vesicle protein, VAMP-2, across the sporadic Alzheimer’s disease continuum. Alzheimers Res. Ther. 2023, 15, 186. [Google Scholar] [CrossRef]
- Gliwińska, A.; Czubilińska-Łada, J.; Więckiewicz, G.; Świętochowska, E.; Badeński, A.; Dworak, M.; Szczepańska, M. The Role of Brain-Derived Neurotrophic Factor (BDNF) in Diagnosis and Treatment of Epilepsy, Depression, Schizophrenia, Anorexia Nervosa and Alzheimer’s Disease as Highly Drug-Resistant Diseases: A Narrative Review. Brain Sci. 2023, 13, 163. [Google Scholar] [CrossRef]
- Kang, J.; Son, Y.; Yim, Y.; Cho, H.; Kim, J.; Lark, A.R.S.; Mir, F.A.; Kim, H.J.; Park, J.; Lee, H.; et al. Biomarkers for Alzheimer’s disease across diverse biological domains: An umbrella review and evidence map. J. Adv. Res 2025, S2090-1232(25)00547-8, in press. [Google Scholar] [CrossRef] [PubMed]
- Genin, E.; Hannequin, D.; Wallon, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Bullido, M.J.; Engelborghs, S.; De Deyn, P.; Berr, C.; et al. APOE and Alzheimer disease: A major gene with semi-dominant inheritance. Mol. Psychiatry 2011, 16, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shue, F.; Zhao, N.; Shinohara, M.; Bu, G. APOE2: Protective mechanism and therapeutic implications for Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Fortea, J.; Pegueroles, J.; Alcolea, D.; Belbin, O.; Dols-Icardo, O.; Vaqué-Alcázar, L.; Videla, L.; Gispert, J.D.; Suárez-Calvet, M.; Johnson, S.C.; et al. APOE4 homozygozity represents a distinct genetic form of Alzheimer’s disease. Nat. Med. 2024, 30, 1284–1291. [Google Scholar] [CrossRef]
- Schramm, C.; Charbonnier, C.; Zaréa, A.; Lacour, M.; Wallon, D.; CNRMAJ Collaborators; Boland, A.; Deleuze, J.F.; Olaso, R.; ADES Consortium; et al. Penetrance estimation of Alzheimer disease in SORL1 loss-of-function variant carriers using a family-based strategy and stratification by APOE genotypes. Genome Med. 2022, 14, 69. [Google Scholar] [CrossRef]
- Poblano, J.; Castillo-Tobías, I.; Berlanga, L.; Tamayo-Ordoñez, M.C.; Del Carmen Rodríguez-Salazar, M.; Silva-Belmares, S.Y.; Aguayo-Morales, H.; Cobos-Puc, L.E. Drugs targeting APOE4 that regulate beta-amyloid aggregation in the brain: Therapeutic potential for Alzheimer’s disease. Basic Clin. Pharmacol. Toxicol. 2024, 135, 237–249. [Google Scholar] [CrossRef]
- Yang, H.; Kim, D.; Yang, Y.; Bagyinszky, E.; An, S.S.A. TREM2 in Neurodegenerative Disorders: Mutation Spectrum, Pathophysiology, and Therapeutic Targeting. Int. J. Mol. Sci. 2025, 26, 7057. [Google Scholar] [CrossRef] [PubMed]
- Fortea, J.; Zaman, S.H.; Hartley, S.; Rafii, M.S.; Head, E.; Carmona-Iragui, M. Alzheimer’s disease associated with Down syndrome: A genetic form of dementia. Lancet Neurol. 2021, 20, 930–942. [Google Scholar] [CrossRef]
- Chipi, E.; Salvadori, N.; Farotti, L.; Parnetti, L. Biomarker-Based Signature of Alzheimer’s Disease in Pre-MCI Individuals. Brain Sci. 2019, 9, 213. [Google Scholar] [CrossRef]
- Greenberg, B.D.; Pettigrew, C.; Soldan, A.; Wang, J.; Wang, M.C.; Darrow, J.A.; Albert, M.S.; Moghekar, A. CSF Alzheimer Disease Biomarkers: Time-Varying Relationships with MCI Symptom Onset and Associations with Age, Sex, and ApoE4. Neurology 2022, 99, e1640–e1650. [Google Scholar] [CrossRef]
- Li, Y.; Yen, D.; Hendrix, R.D.; Gordon, B.A.; Dlamini, S.; Barthélemy, N.R.; Aschenbrenner, A.J.; Henson, R.L.; Herries, E.M.; Volluz, K.; et al. Timing of Biomarker Changes in Sporadic Alzheimer’s Disease in Estimated Years from Symptom Onset. Ann. Neurol. 2024, 95, 951–965. [Google Scholar] [CrossRef]
- Milà-Alomà, M.; Tosun, D.; Schindler, S.E.; Hausle, I.; Petersen, K.K.; Li, Y.; Dage, J.L.; Du-Cuny, L.; Saad, Z.S.; Saef, B.; et al. Timing of Changes in Alzheimer’s Disease Plasma Biomarkers as Assessed by Amyloid and Tau PET Clocks. Ann. Neurol. 2025, 98, 508–523. [Google Scholar] [CrossRef]
- Prosser, L.; Sudre, C.H.; Oxtoby, N.P.; Young, A.L.; Malone, I.B.; Manning, E.N.; Pemberton, H.; Walsh, P.; Barkhof, F.; Biessels, G.J.; et al. Biomarker pathway heterogeneity of amyloid-positive individuals. Alzheimers Dement. 2024, 20, 8503–8515. [Google Scholar] [CrossRef]
- Paterson, R.W.; Toombs, J.; Slattery, C.F.; Nicholas, J.M.; Andreasson, U.; Magdalinou, N.K.; Blennow, K.; Warren, J.D.; Mummery, C.J.; Rossor, M.N.; et al. Dissecting IWG-2 typical and atypical Alzheimer’s disease: Insights from cerebrospinal fluid analysis. J. Neurol. 2015, 262, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; von Arnim, C.A.F.; Burnie, N.; Bozeat, S.; Cummings, J. Biomarkers in Alzheimer’s disease: Role in early and differential diagnosis and recognition of atypical variants. Alzheimers Res. Ther. 2023, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Salvioni Chiabotti, P.; Nasuti, M.; Rouaud, O.; Allali, G. Posterior cortical atrophy in the age of anti-amyloid treatments: An 11-year retrospective study of eligible patients from the Leenaards Memory Center. J. Alzheimers Dis. 2025, 106, 512–517. [Google Scholar] [CrossRef]
- Tsantzali, I.; Athanasaki, A.; Boufidou, F.; Constantinides, V.C.; Stefanou, M.I.; Moschovos, C.; Zompola, C.; Paraskevas, S.G.; Bonakis, A.; Giannopoulos, S.; et al. Cerebrospinal Fluid Classical Biomarker Levels in Mixed vs. Pure A+T+ (A+T1+) Alzheimer’s Disease. Biomedicines 2024, 12, 2904. [Google Scholar] [CrossRef]
- Bousiges, O.; Blanc, F. Biomarkers of Dementia with Lewy Bodies: Differential Diagnostic with Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 6371. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef]
- Kurihara, M.; Matsubara, T.; Morimoto, S.; Arakawa, A.; Ohse, K.; Kanemaru, K.; Iwata, A.; Murayama, S.; Saito, Y. Neuropathological changes associated with aberrant cerebrospinal fluid p-tau181 and Aβ42 in Alzheimer’s disease and other neurodegenerative diseases. Acta Neuropathol. Commun. 2024, 12, 48. [Google Scholar] [CrossRef]
- Kapaki, E.; Kilidireas, K.; Paraskevas, G.P.; Michalopoulou, M.; Patsouris, E. Highly increased CSF tau protein and decreased beta-amyloid (1–42) in sporadic CJD: A discrimination from Alzheimer’s disease? J. Neurol. Neurosurg. Psychiatry 2001, 71, 401–403. [Google Scholar] [CrossRef]
- Wolk, D.A.; Nelson, P.T.; Apostolova, L.; Arfanakis, K.; Boyle, P.A.; Carlsson, C.M.; Corriveau-Lecavalier, N.; Dacks, P.; Dickerson, B.C.; Domoto-Reilly, K.; et al. Clinical criteria for limbic-predominant age-related TDP-43 encephalopathy. Alzheimers Dement. 2025, 21, e14202. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.; Román, G.C.; Esiri, M.; Kettunen, P.; Svensson, J.; Paraskevas, G.P.; Kapaki, E. Update on Vascular Cognitive Impairment Associated with Subcortical Small-Vessel Disease. J. Alzheimers Dis. 2018, 62, 1417–1441. [Google Scholar] [CrossRef] [PubMed]
- Formichi, P.; Parnetti, L.; Radi, E.; Cevenini, G.; Dotti, M.T.; Federico, A. CSF Biomarkers Profile in CADASIL-A Model of Pure Vascular Dementia: Usefulness in Differential Diagnosis in the Dementia Disorder. Int. J. Alzheimers Dis. 2010, 2010, 959257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dumurgier, J.; Schraen, S.; Gabelle, A.; Vercruysse, O.; Bombois, S.; Laplanche, J.L.; Peoc’h, K.; Sablonnière, B.; Kastanenka, K.V.; Delaby, C.; et al. Cerebrospinal fluid amyloid-β 42/40 ratio in clinical setting of memory centers: A multicentric study. Alzheimers Res. Ther. 2015, 7, 30. [Google Scholar] [CrossRef]
- Josephs, K.A.; Weigand, S.D.; Whitwell, J.L. Characterizing Amyloid-Positive Individuals with Normal Tau PET Levels After 5 Years: An ADNI Study. Neurology 2022, 98, e2282–e2292. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Katsumata, Y.; Wu, X.; Aung, K.Z.; Fsardo, D.W.; Forrest, S.L.; Alzheimer’s Disease Genetics Consortium; Nelson, P.T. Amyloid-β predominant Alzheimer’s disease neuropathologic change. Brain 2025, 148, 401–407. [Google Scholar] [CrossRef]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Amyloid Biomarker Study Group. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- Rivero-Santana, A.; Ferreira, D.; Perestelo-Perez, L.; Westman, E.; Wahlund, L.O.; Sarria, A.; Serrano-Aguilar, P. Cerebrospinal Fluid Biomarkers for the Differential Diagnosis between Alzheimer’s Disease and Frontotemporal Lobar Degeneration: Systematic Review, HSROC Analysis, and Confounding Factors. J. Alzheimer’s Dis. 2017, 55, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, G.P.; Kasselimis, D.; Kourtidou, E.; Constantinides, V.; Bougea, A.; Potagas, C.; Evdokimidis, I.; Kapaki, E. Cerebrospinal Fluid Biomarkers as a Diagnostic Tool of the Underlying Pathology of Primary Progressive Aphasia. J. Alzheimers Dis. 2017, 55, 1453–31461. [Google Scholar] [CrossRef] [PubMed]
- Casoli, T.; Paolini, S.; Fabbietti, P.; Fattoretti, P.; Paciaroni, L.; Fabi, K.; Gobbi, B.; Galeazzi, R.; Rossi, R.; Lattanzio, F.; et al. Cerebrospinal fluid biomarkers and cognitive status in differential diagnosis of frontotemporal dementia and Alzheimer’s disease. J. Int. Med. Res. 2019, 47, 4968–4980. [Google Scholar] [CrossRef]
- Constantinides, V.C.; Boufidou, F.; Bourbouli, M.; Pyrgelis, E.S.; Ghika, A.; Koros, C.; Liakakis, G.; Papageorgiou, S.; Stefanis, L.; Paraskevas, G.P.; et al. Application of the AT(N) and Other CSF Classification Systems in Behavioral Variant Frontotemporal Dementia. Diagnostics 2023, 13, 332. [Google Scholar] [CrossRef]
- Smith, R.; Shaw, L.; Palmqvist, S.; Mattsson-Carlgren, N.; Klein, G.; Tonietto, M.; Alzheimer’s Disease Neuroimaging Initiative; Quijano-Rubio, C.; Rank, C.M.; Andreadou, M.; et al. Clinical Performance of the Elecsys CSF pTau181/Aβ42 Ratio for Concordance with Tau-PET in Two Independent Cohorts. Neurol. Ther. 2025, 14, 2011–2031. [Google Scholar] [CrossRef]
- Lehmann, S.; Gabelle, A.; Duchiron, M.; Busto, G.; Morchikh, M.; Delaby, C.; Hirtz, C.; Mondesert, E.; Cristol, J.P.; Barnier-Figue, G.; et al. Comparative performance of plasma pTau181/Aβ42, pTau217/Aβ42 ratios, and individual measurements in detecting brain amyloidosis. EBioMedicine 2025, 117, 105805. [Google Scholar] [CrossRef]
- Athanasaki, A.; Tsantzali, I.; Kroupis, C.; Theodorou, A.; Boufidou, F.; Constantinides, V.C.; Tzartos, J.S.; Tzartos, S.J.; Velonakis, G.; Zompola, C.; et al. APOE Genotyping in Cognitive Disorders: Preliminary Observations from the Greek Population. Int. J. Mol. Sci. 2025, 26, 7410. [Google Scholar] [CrossRef] [PubMed]
- Bieger, A.; Brum, W.S.; Borelli, W.V.; Therriault, J.; De Bastiani, M.A.; Moreira, A.G.; Benedet, A.L.; Ferrari-Souza, J.P.; Da Costa, J.C.; Souza, D.O.; et al. Influence of Different Diagnostic Criteria on Alzheimer Disease Clinical Research. Neurology 2024, 103, e209753. [Google Scholar] [CrossRef] [PubMed]
- Bouteloup, V.; Villain, N.; Vidal, J.S.; Gonzalez-Ortiz, F.; Yuksekel, I.; Santos, C.; Schraen-Maschken, S.; Pellegrin, I.; Lehmann, S.; Blennow, K.; et al. Cognitive Phenotyping and Interpretation of Alzheimer Blood Biomarkers. JAMA Neurol. 2025, 82, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, S.; Whitson, H.E.; Allen, L.A.; Suarez-Calvet, M.; Galasko, D.; Karikari, T.K.; Okrahvi, H.R.; Paczynski, M.; Schindler, S.E.; Teunissen, C.E.; et al. Alzheimer’s Association Clinical Practice Guideline on the use of blood-based biomarkers in the diagnostic workup of suspected Alzheimer’s disease within specialized care settings. Alzheimers Dement. 2025, 21, e70535. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).