The Biomarker Profile of Alzheimer’s Disease for Disease-Modifying Treatment Eligibility: Questions and Debates
Abstract
1. Introduction
2. Classical Biomarkers with Molecular Specificity for AD
2.1. CSF Markers
2.2. PET Markers
3. Other Biomarkers Typically Used
3.1. Markers of Neurodegeneration
3.2. Markers of Other Primary Co-Pathologies
4. Plasma Biomarkers
5. Alternative Biomarkers
5.1. Biomarkers of Amyloidopathy or Tauopathy
5.2. Biomarkers of Microglia Activation and Other Parameters of Neuroinflammation
5.3. Biomarkers of Synaptic Loss
6. Genetic Biomarkers
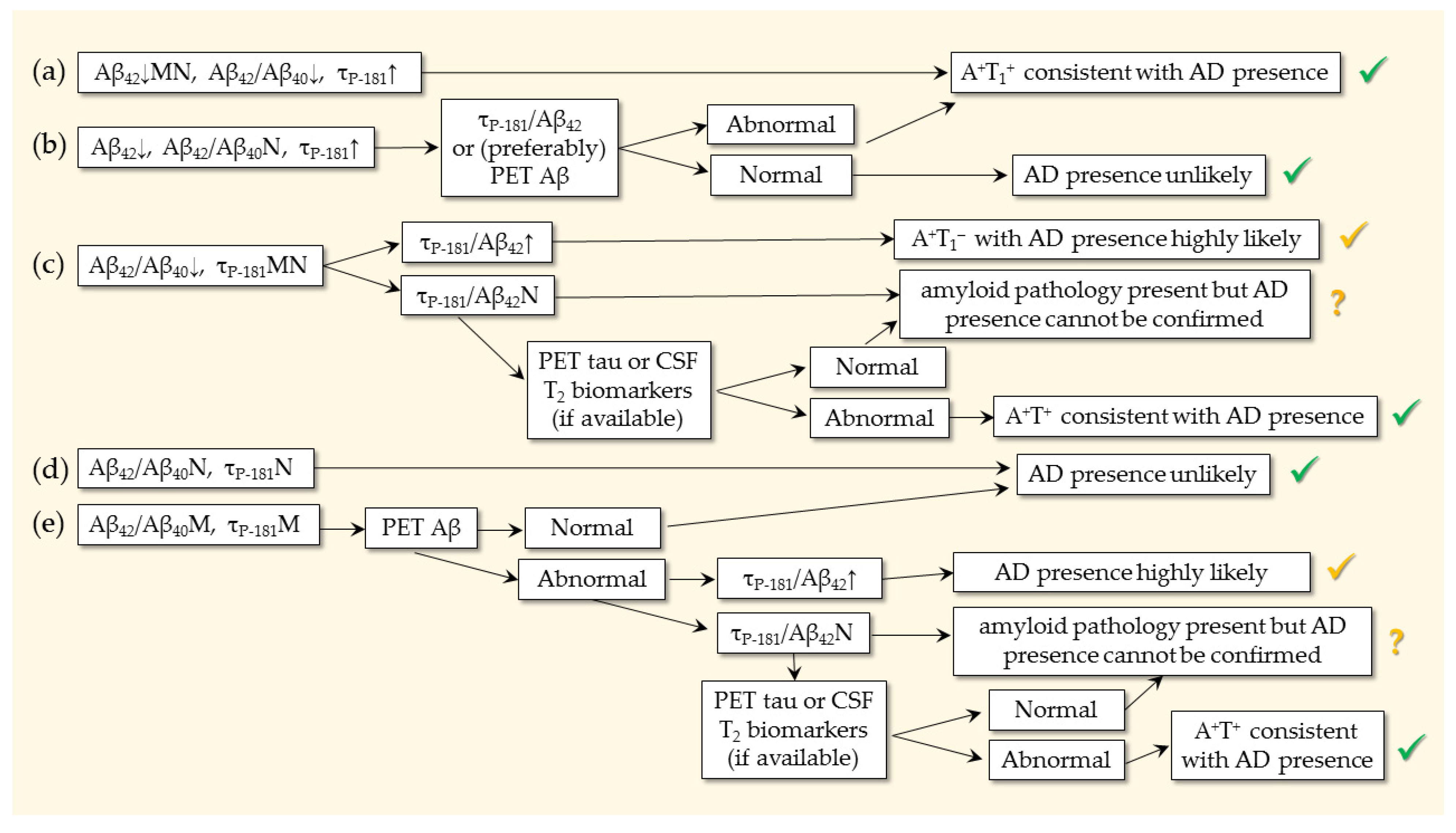
7. Profiles and Ratios
8. Discussion
- Should the profile be both A+ and T+ (either T1+ or T2+), or is A+ sufficient?
- Should we rely on the τP-181/Aβ42 and τT/Aβ42 ratios and when?
9. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025. Available online: https://iris.who.int/handle/10665/259615 (accessed on 6 July 2025).
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Jiang, G.; Xie, G.; Li, X.; Xiong, J. Cytoskeletal Proteins and Alzheimer’s Disease Pathogenesis: Focusing on the Interplay with Tau Pathology. Biomolecules 2025, 15, 831. [Google Scholar] [CrossRef]
- Koller, E.J.; Ibanez, K.R.; Vo, Q.; McFarland, K.N.; Gonzalez De La Cruz, E.; Zobel, L.; Williams, T.; Xu, G.; Ryu, D.; Patel, P.; et al. Combinatorial model of amyloid β and tau reveals synergy between amyloid deposits and tangle formation. Neuropathol. Appl. Neurobiol. 2022, 48, e12779. [Google Scholar] [CrossRef]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Serban, M.; Covache-Busuioc, R.A.; Ciurea, A.V.; Enyedi, M. Decoding Neurodegeneration: A Review of Molecular Mechanisms and Therapeutic Advances in Alzheimer’s, Parkinson’s, and ALS. Int. J. Mol. Sci. 2024, 25, 12613. [Google Scholar] [CrossRef]
- Rathee, S.; Pandey, V.; Soni, S.; Sen, D.; Jain, S.K. Comprehending Alzheimer’s Disease: Molecular Mechanisms and Treatment Strategies. Curr. Alzheimer Res. 2025, 22, 414–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Chundu, U.C.; Thiriveedhi, S.R.; Bhatti, C.; Mupparaju, J.S.; Otinashvili, N.; Gelishvili, E. A Systematic Review of the Efficacy and Safety of Anti-amyloid Monoclonal Antibodies in Alzheimer’s Disease. Cureus 2025, 7, e85377. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S. Are Appropriate Use Recommendations useful in clinical practice? J. Prev. Alzheimers Dis. 2025, 12, 100165. [Google Scholar] [CrossRef]
- Cummings, J.; Apostolova, L.; Rabinovici, G.D.; Atri, A.; Aisen, P.; Greenberg, S.; Hendrix, S.; Selkoe, D.; Weiner, M.; Petersen, R.C.; et al. Lecanemab: Appropriate Use Recommendations. J. Prev. Alzheimers Dis. 2023, 10, 362–377. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Selkoe, D.J.; Schindler, S.E.; Aisen, P.; Apostolova, L.G.; Atri, A.; Greenberg, S.M.; Hendrix, S.B.; Petersen, R.C.; Weiner, M.; et al. Donanemab: Appropriate use recommendations. J. Prev. Alzheimers Dis. 2025, 12, 100150. [Google Scholar] [CrossRef]
- Villain, N.; Planche, V.; Lilamand, M.; Cordonnier, C.; Soto-Martin, M.; Mollion, H.; Bombois, S.; Delrieu, J.; French Federation of Memory Clinics Work Group on Anti-Amyloid Immunotherapies. Lecanemab for early Alzheimer’s disease: Appropriate use recommendations from the French federation of memory clinics. J. Prev. Alzheimers Dis. 2025, 12, 100094. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Phillips, N.A.; Feldman, H.H.; Borrie, M.; Ganesh, A.; Henri-Bhargava, A.; Desmarais, P.; Frank, A.; Badhwar, A.; Barlow, L.; et al. Use of lecanemab and donanemab in the Canadian healthcare system: Evidence, challenges, and areas for future research. J. Prev. Alzheimers. Dis. 2025, 12, 100068. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, G.H.; Kim, C.H.; Koh, S.H.; Moon, S.Y.; Park, Y.H.; Seo, S.W.; Yoon, B.; Lim, J.S.; Kim, B.C.; et al. Lecanemab: Appropriate Use Recommendations by Korean Dementia Association. Dement. Neurocogn. Disord. 2024, 23, 165–187. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- Lopez, O.L.; Becker, J.T.; Klunk, W.; Saxton, J.; Hamilton, R.L.; Kaufer, D.I.; Sweet, R.A.; Cidis Meltzer, C.; Wisniewski, S.; Kamboh, M.I.; et al. Research evaluation and diagnosis of probable Alzheimer’s disease over the last two decades: I. Neurology 2000, 55, 1854–1862. [Google Scholar] [CrossRef]
- Mendez, M.; Mastri, A.R.; Sung, J.H.; Frey, W.H. Clinically diagnosed Alzheimer’s disease: Neuropathologic findings in 650 cases. Alzheimer Dis. Assoc. Disord. 1992, 6, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Head, E.; Schmitt, F.A.; Davis, P.R.; Neltner, J.H.; Jicha, G.A.; Abner, E.L.; Smith, C.D.; Van Eldik, L.J.; Kryscio, R.J.; et al. Alzheimer’s disease is not “brain aging”: Neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011, 121, 571–587. [Google Scholar] [CrossRef]
- Galasko, D.; Hansen, L.A.; Katzman, R.; Wiederholt, W.; Masliah, E.; Terry, R.; Hill, L.R.; Lessin, P.; Thal, L.J. Clinical-neuropathological correlations in Alzheimer’s disease and related dementias. Arch. Neurol. 1994, 51, 888–895. [Google Scholar] [CrossRef]
- Sarto, J.; Mayà, G.; Molina-Porcel, L.; Balasa, M.; Gelpi, E.; Aldecoa, I.; Borrego-Écija, S.; Contador, J.; Ximelis, T.; Vergara, M.; et al. Evolution of Clinical-Pathological Correlations in Early-Onset Alzheimer’s Disease Over a 25-Year Period in an Academic Brain Bank. J. Alzheimers Dis. 2022, 87, 1659–1669. [Google Scholar] [CrossRef]
- Xing, X.; Zhang, X.; Wang, K.; Wang, Z.; Feng, Y.; Li, X.; Hua, Y.; Zhang, L.; Dong, X. Post-marketing safety concerns with lecanemab: A pharmacovigilance study based on the FDA Adverse Event Reporting System database. Alzheimers Res. Ther. 2025, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- McGrowder, D.A.; Miller, F.; Vaz, K.; Nwokocha, C.; Wilson-Clarke, C.; Anderson-Cross, M.; Brown, J.; Anderson-Jackson, L.; Williams, L.; Latore, L.; et al. Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease: Current Evidence and Future Perspectives. Brain Sci. 2021, 11, 215. [Google Scholar] [CrossRef]
- Solje, E.; Benussi, A.; Buratti, E.; Remes, A.M.; Haapasalo, A.; Borroni, B. State-of-the-Art Methods and Emerging Fluid Biomarkers in the Diagnostics of Dementia-A Short Review and Diagnostic Algorithm. Diagnostics 2021, 11, 788. [Google Scholar] [CrossRef]
- Wilson, H.; Pagano, G.; Politis, M. Dementia spectrum disorders: Lessons learnt from decades with PET research. J. Neural Transm. 2019, 126, 233–251. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Festari, C.; Massa, F.; Cotta Ramusino, M.; Orini, S.; Aarsland, D.; Agosta, F.; Babiloni, C.; Borroni, B.; Cappa, S.F.; et al. European intersocietal recommendations for the biomarker-based diagnosis of neurocognitive disorders. Lancet Neurol. 2024, 23, 302–312. [Google Scholar] [CrossRef]
- Agnello, L.; Gambino, C.M.; Ciaccio, A.M.; Masucci, A.; Vassallo, R.; Tamburello, M.; Scazzone, C.; Lo Sasso, B.; Ciaccio, M. Molecular Biomarkers of Neurodegenerative Disorders: A Practical Guide to Their Appropriate Use and Interpretation in Clinical Practice. Int. J. Mol. Sci. 2024, 25, 4323. [Google Scholar] [CrossRef] [PubMed]
- Grabher, B.J. Amyloid Imaging Update: How the Amyloid Landscape Is Changing in Light of the Recent Food and Drug Administration Approval of Antiamyloid Therapeutics. J. Nucl. Med. Technol. 2024, 52, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Decaix, T.; Mouton-Liger, F.; Dumurgier, J.; Cognat, E.; Vrillon, A.; Hugon, J.; Hourregue, C.; Bouaziz-Amar, E.; Wallon, D.; Muraine, M.Q.; et al. Usefulness of Cerebrospinal Fluid Alzheimer’s disease biomarkers in older patients: Evidence from a national multicenter prospective study. J. Prev. Alzheimers Dis. 2025, 12, 100009. [Google Scholar] [CrossRef]
- Leuzy, A.; Bollack, A.; Pellegrino, D.; Teunissen, C.E.; La Joie, R.; Rabinovici, G.D.; Franzmeier, N.; Johnson, K.; Barkhof, F.; Shaw, L.M.; et al. Considerations in the clinical use of amyloid PET and CSF biomarkers for Alzheimer’s disease. Alzheimers Dement. 2025, 21, e14528. [Google Scholar] [CrossRef] [PubMed]
- Niemantsverdriet, E.; Valckx, S.; Bjerke, M.; Engelborghs, S. Alzheimer’s disease CSF biomarkers: Clinical indications and rational use. Acta Neurol. Belg. 2017, 117, 591–602. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=alzheimer+CSF+biomarkers&filter=pubt.review&sort=date (accessed on 13 August 2025). [CrossRef]
- Jack, C.R.; Wiste, H.J.; Algeciras-Schimnich, A.; Weigand, S.D.; Figdore, D.J.; Lowe, V.J.; Vemuri, P.; Graff-Radford, J.; Ramanan, V.K.; Knopman, D.S.; et al. Comparison of plasma biomarkers and amyloid PET for predicting memory decline in cognitively unimpaired individuals. Alzheimers Dement. 2024, 20, 2143–2154. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=alzheimer+PET+biomarkers&filter=pubt.review&sort=date (accessed on 13 August 2025). [CrossRef]
- Mandal, P.K.; Maroon, J.C.; Garg, A.; Arora, N.K.; Bansal, R.; Kaushik, A.; Samkaria, A.; Kumaran, G.; Arora, Y. Blood Biomarkers in Alzheimer’s Disease. ACS Chem. Neurosci. 2023, 14, 3975–3978. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=alzheimer+blood+biomarkers&filter=pubt.review&sort=date (accessed on 13 August 2025). [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Villain, N.; Schneider, L.; Fox, N.; Campbell, N.; Galasko, D.; Kivipelto, M.; Jessen, F.; Hanseeuw, B.; Boada, M.; et al. Alzheimer Disease as a Clinical-Biological Construct-An International Working Group Recommendation. JAMA Neurol. 2024, 81, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Planche, V.; Schindler, S.; Knopman, D.S.; Frisoni, G.; Galasko, D.; Grill, J.D.; Schneider, L.; Karlawish, J.; Villain, N. The science does not yet support regulatory approval of amyloid-targeting therapies for Alzheimer’s disease based solely on biomarker evidence. Alzheimers Dement. 2025, 21, e70068. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, M.; Minthon, L.; Davidsson, P.; Granérus, A.K.; Clarberg, A.; Vanderstichele, H.; Vanmechelen, E.; Wallin, A.; Blennow, K. CSF levels of tau, beta-amyloid(1-42) and GAP-43 in frontotemporal dementia, other types of dementia and normal aging. J. Neural Transm. 2000, 107, 563–579. [Google Scholar]
- Niemantsverdriet, E.; Ottoy, J.; Somers, C.; De Roeck, E.; Struyfs, H.; Soetewey, F.; Verhaeghe, J.; Van den Bossche, T.; Van Mossevelde, S.; Goeman, J.; et al. The Cerebrospinal Fluid Aβ1-42/Aβ1-40 Ratio Improves Concordance with Amyloid-PET for Diagnosing Alzheimer’s Disease in a Clinical Setting. J. Alzheimers Dis. 2017, 60, 561–576. [Google Scholar] [CrossRef]
- Constantinides, V.C.; Paraskevas, G.P.; Boufidou, F.; Bourbouli, M.; Pyrgelis, E.S.; Stefanis, L.; Kapaki, E. CSF Aβ42 and Aβ42/Aβ40 Ratio in Alzheimer’s Disease and Frontotemporal Dementias. Diagnostics 2023, 13, 783. [Google Scholar] [CrossRef]
- Vanderstichele, H.; De Vreese, K.; Blennow, K.; Andreasen, N.; Sindic, C.; Ivanoiu, A.; Hampel, H.; Bürger, K.; Parnetti, L.; Lanari, A.; et al. Analytical performance and clinical utility of the INNOTEST PHOSPHO-TAU181P assay for discrimination between Alzheimer’s disease and dementia with Lewy bodies. Clin. Chem. Lab. Med. 2006, 44, 1472–1480. [Google Scholar] [CrossRef]
- Suárez-Calvet, M.; Karikari, T.K.; Ashton, N.J.; Lantero Rodríguez, J.; Milà-Alomà, M.; Gispert, J.D.; Salvadó, G.; Minguillon, C.; Fauria, K.; Shekari, M.; et al. Novel Tau Biomarkers Phosphorylated at T181, T217 or T231 Rise in the Initial Stages of the Preclinical Alzheimer’s Continuum When Only Subtle Changes in Aβ Pathology Are Detected. EMBO Mol. Med. 2020, 12, e12921. [Google Scholar] [CrossRef]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.C.; Proctor, N.K.; Chai, X.; Shcherbinin, S.; Sims, J.R.; et al. Cerebrospinal Fluid P-Tau217 Performs Better than p-Tau181 as a Biomarker of Alzheimer’s Disease. Nat. Commun. 2020, 11, 1683. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Abeta and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef]
- Ribarič, S. Detecting Early Cognitive Decline in Alzheimer’s Disease with Brain Synaptic Structural and Functional Evaluation. Biomedicines 2023, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, N.R.; Saef, B.; Li, Y.; Gordon, B.A.; He, Y.; Horie, K.; Stomrud, E.; Salvadó, G.; Janelidze, S.; Sato, C.; et al. CSF tau phosphorylation occupancies at T217 and T205 represent improved biomarkers of amyloid and tau pathology in Alzheimer’s disease. Nat. Aging. 2023, 3, 391–401. [Google Scholar] [CrossRef]
- Kac, P.R.; Alcolea, D.; Montoliu-Gaya, L.; Fernández, S.; Rodriguez, J.L.; Maure, L.; González-Ortiz, F.; Benejam, B.; Turton, M.; Barroeta, I.; et al. Plasma p-tau212 as a biomarker of sporadic and Down syndrome Alzheimer’s disease. Alzheimers Dement. 2025, 21, e70172. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Salvadó, G.; Barthélemy, N.R.; Janelidze, S.; Li, Y.; He, Y.; Saef, B.; Chen, C.D.; Jiang, H.; Strandberg, O.; et al. CSF MTBR-tau243 is a specific biomarker of tau tangle pathology in Alzheimer’s disease. Nat. Med. 2023, 29, 1954–1963. [Google Scholar] [CrossRef]
- Strain, J.F.; Barthelemy, N.; Horie, K.; Gordon, B.A.; Kilgore, C.; Aschenbrenner, A.; Cruchaga, C.; Xiong, C.; Joseph-Mathurin, N.; Hassenstab, J.; et al. CSF Tau phosphorylation at Thr205 is associated with loss of white matter integrity in autosomal dominant Alzheimer disease. Neurobiol. Dis. 2022, 168, 105714. [Google Scholar] [CrossRef]
- Lantero-Rodriguez, J.; Montoliu-Gaya, L.; Ashton, N.J.; Pola, I.; Therriault, J.; Rahmouni, N.; Brum, W.S.; Servaes, S.; Stevenson, J.; Di Molfetta, G.; et al. Biofluid-based staging of Alzheimer’s disease. Acta Neuropathol. 2025, 149, 27. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Herholz, K. Amyloid imaging for dementia in clinical practice. BMC Med. 2015, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Beach, T.G.; Zanette, M.; Lilja, J.; Heurling, K.; Chakrabarty, A.; Ismail, A.; Farrar, G.; Buckley, C.; Smith, A.P.L. Estimation of amyloid distribution by [(18)F]flutemetamol PET predicts the neuropathological phase of amyloid beta-protein deposition. Acta Neuropathol. 2018, 136, 557–567. [Google Scholar] [CrossRef]
- Collij, L.E.; Bollack, A.; La Joie, R.; Shekari, M.; Bullich, S.; Roé-Vellvé, N.; Koglin, N.; Jovalekic, A.; Garciá, D.V.; Drzezga, A.; et al. Centiloid recommendations for clinical context-of-use from the AMYPAD consortium. Alzheimers Dement. 2024, 20, 9037–9048. [Google Scholar] [CrossRef]
- Schwarz, A.J.; Yu, P.; Miller, B.B.; Shcherbinin, S.; Dickson, J.; Navitsky, M.; Joshi, A.D.; Devous, M.D., Sr.; Mintun, M.S. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain 2016, 139, 1539–1550. [Google Scholar] [CrossRef]
- Lowe, V.J.; Lundt, E.S.; Albertson, S.M.; Min, H.K.; Fang, P.; Przybelski, S.A.; Senjem, M.L.; Schwarz, C.G.; Kantarci, K.; Boeve, B.; et al. Tau-positron emission tomography correlates with neuropathology findings. Alzheimers Dement. 2020, 16, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Pichet Binette, A.; Groot, C.; Smith, R.; Strandberg, O.; Palmqvist, S.; Stomrud, E.; Tideman, P.; Ohlsson, T.; Jogi, J.; et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat. Med. 2022, 28, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.W.; Young, A.L.; Oxtoby, N.P.; Smith, R.; Ossenkoppele, R.; Strandberg, O.T.; La Joie, R.; Aksman, L.M.; Grothe, M.J.; Iturria-Medina, Y.; et al. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med. 2021, 27, 871–881. [Google Scholar] [CrossRef]
- Blennow, K.; Wallin, A.; Agren, H.; Spenger, C.; Siegfried, J.; Vanmechelen, E. Tau protein in cerebrospinal fluid: A biochemical marker for axonal degeneration in Alzheimer disease? Mol. Chem. Neuropathol. 1995, 26, 231–245. [Google Scholar] [CrossRef]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Nixon, R.A. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef]
- Chatziefstathiou, A.; Canaslan, S.; Kanata, E.; Vekrellis, K.; Constantinides, V.C.; Paraskevas, G.P.; Kapaki, E.; Schmitz, M.; Zerr, I.; Xanthopoulos, K.; et al. SIMOA Diagnostics on Alzheimer’s Disease and Frontotemporal Dementia. Biomedicines 2024, 12, 1253. [Google Scholar] [CrossRef]
- Minoshima, S.; Cross, D.; Thientunyakit, T.; Foster, N.L.; Drzezga, A. 18F-FDG PET Imaging in Neurodegenerative Dementing Disorders: Insights into Subtype Classification, Emerging Disease Categories, and Mixed Dementia with Copathologies. J. Nucl. Med. 2022, 63 (Suppl. S1), 2S–12S. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Singleton, E.H.; Groot, C.; Dijkstra, A.A.; Eikelboom, W.S.; Seeley, W.W.; Miller, B.; Laforce, R.J.; Scheltens, P.; Papma, J.M.; et al. Research Criteria for the Behavioral Variant of Alzheimer Disease: A Systematic Review and Meta-analysis. JAMA Neurol. 2022, 79, 48–60. [Google Scholar] [CrossRef]
- Swan, A.; Waddell, B.; Holloway, G.; Bak, T.; Colville, S.; Khan, Z.; Pal, S. The diagnostic utility of 99mTc-HMPAO SPECT imaging: A retrospective case series from a tertiary referral early-onset cognitive disorders clinic. Dement. Geriatr. Cogn. Disord. 2015, 39, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Thropp, P.; Phillips, E.; Jung, Y.; Thomas, D.L.; Tosun, D.; Alzheimer’s Disease Neuroimaging Initiative. Arterial spin labeling perfusion MRI in the Alzheimer’s Disease Neuroimaging Initiative: Past, present, and future. Alzheimers Dement. 2024, 20, 8937–8952. [Google Scholar] [CrossRef]
- Bernetti, C.; D’Andrea, V.; Buoso, A.; Barbalace, I.; Greco, F.; Pilato, F.; Calandrelli, R.; Di Lazzaro, V.; Zobel, B.B.; Mallio, C.A. Arterial Spin Labeling MRI in Alzheimer’s Disease: A Systematic Review of Cerebral Perfusion Biomarkers. J. Neuroimaging 2025, 35, e70035. [Google Scholar] [CrossRef]
- Bosco, P.; Redolfi, A.; Bocchetta, M.; Ferrari, C.; Mega, A.; Galluzzi, S.; Austin, M.; Chincarini, A.; Collins, D.L.; Duchesne, S.; et al. The impact of automated hippocampal volumetry on diagnostic confidence in patients with suspected Alzheimer’s disease: A European Alzheimer’s Disease Consortium study. Alzheimers Dement. 2017, 13, 1013–1023. [Google Scholar] [CrossRef]
- Scheltens, P.; Leys, D.; Barkhof, F.; Huglo, D.; Weinstein, H.C.; Vermersch, P.; Kuiper, M.; Steinling, M.; Wolters, E.C.; Valk, J. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: Diagnostic value and neuropsychological correlates. J. Neurol. Neurosurg. Psychiatry 1992, 55, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Enkirch, S.J.; Traschütz, A.; Müller, A.; Widmann, C.N.; Gielen, G.H.; Heneka, M.T.; Jurcoane, A.; Schild, H.H.; Hattingen, E. The ERICA Score: An MR Imaging-based Visual Scoring System for the Assessment of Entorhinal Cortex Atrophy in Alzheimer Disease. Radiology 2018, 288, 226–233. [Google Scholar] [CrossRef]
- Roberge, X.; Brisson, M.; Laforce, R.J. Specificity of Entorhinal Atrophy MRI Scale in Predicting Alzheimer’s Disease Conversion. Can. J. Neurol. Sci. 2023, 50, 112–114. [Google Scholar] [CrossRef]
- Alexopoulos, P.; Kriett, L.; Haller, B.; Klupp, E.; Gray, K.; Grimmer, T.; Laskaris, N.; Förster, S.; Perneczky, R.; Kurz, A.; et al. Limited agreement between biomarkers of neuronal injury at different stages of Alzheimer’s disease. Alzheimers Dement. 2014, 10, 684–689. [Google Scholar] [CrossRef]
- McAleese, K.E.; Miah, M.; Graham, S.; Hadfield, G.M.; Walker, L.; Johnson, M.; Colloby, S.J.; Thomas, A.J.; DeCarli, C.; Koss, D.; et al. Frontal white matter lesions in Alzheimer’s disease are associated with both small vessel disease and AD-associated cortical pathology. Acta Neuropathol. 2021, 142, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Liu, S.; Zhang, Y.; Zhao, Z.; Li, H.; Li, Y.; Lan, X.; Wang, L.; Liu, J.; Ji, C. Phenotypes of white matter hyperintensities, mechanisms and aetiological differentiation: Future targets pre-dementia–a systematic review. Discov. Neurosci. 2025, 20, 6. [Google Scholar] [CrossRef]
- McAleese, K.E.; Walker, L.; Graham, S.; Moya, E.L.J.; Johnson, M.; Erskine, D.; Colloby, S.J.; Dey, M.; Martin-Ruiz, C.; Taylor, J.P.; et al. Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 2017, 134, 459–473. [Google Scholar] [CrossRef]
- Theodorou, A.; Palaiodimou, L.; Papagiannopoulou, G.; Kargiotis, O.; Psychogios, K.; Safouris, A.; Bakola, E.; Chondrogianni, M.; Kotsali-Peteinelli, V.; Melanis, K.; et al. Clinical Characteristics, Neuroimaging Markers, and Outcomes in Patients with Cerebral Amyloid Angiopathy: A Prospective Cohort Study. J. Clin. Med. 2023, 12, 5591. [Google Scholar] [CrossRef]
- Kapaki, E.; Paraskevas, P.G.; Emmanouilidou, E.; Vekrellis, K. The diagnostic value of CSF α-synuclein in the differential diagnosis of dementia with Lewy bodies vs. normal subjects and patients with Alzheimer’s disease. PLoS ONE 2013, 8, e81654. [Google Scholar] [CrossRef]
- Anagnostou, D.; Sfakianaki, G.; Melachroinou, K.; Soutos, M.; Constantinides, V.; Vaikath, N.; Tsantzali, I.; Paraskevas, G.P.; Agnaf, O.E.; Vekrellis, K.; et al. Assessment of Aggregated and Exosome-Associated α-Synuclein in Brain Tissue and Cerebrospinal Fluid Using Specific Immunoassays. Diagnostics 2023, 13, 2192. [Google Scholar] [CrossRef] [PubMed]
- Kapsali, I.; Brinia, M.E.; Constantinides, V.C. Cerebrospinal Fluid Total, Phosphorylated and Oligomeric A-Synuclein in Parkinson’s Disease: A Systematic Review, Meta-Analysis and Meta-Regression Study. Biomedicines 2024, 12, 2266. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, G.; De Luca, C.M.G.; Paoletti, F.P.; Gaetani, L.; Moda, F.; Parnetti, L. alpha-Synuclein Seed Amplification Assays for Diagnosing Synucleinopathies: The Way Forward. Neurology 2022, 99, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Bang, J.I.; Ahn, C.; Nyaga, V.N.; Kim, Y.E.; Kang, M.J.; Ahn, T.B. Diagnostic value of alpha-synuclein seeding amplification assays in alpha-synucleinopathies: A systematic review and meta-analysis. Park. Relat. Disord. 2022, 104, 99–109. [Google Scholar] [CrossRef]
- Srivastava, A.; Wang, Q.; Orrù, C.D.; Fernandez, M.; Compta, Y.; Ghetti, B.; Zanusso, G.; Zou, W.Q.; Caughey, B.; Beauchemin, C.A.A. Enhanced quantitation of pathological alpha-synuclein in patient biospecimens by RT-QuIC seed amplification assays. PLoS Pathog. 2024, 20, e1012554. [Google Scholar] [CrossRef]
- Kapaki, E.; Boufidou, F.; Bourbouli, M.; Pyrgelis, E.S.; Constantinides, V.C.; Anastassopoulou, C.; Paraskevas, G.P. Cerebrospinal Fluid Biomarker Profile in TDP-43-Related Genetic Frontotemporal Dementia. J. Pers. Med. 2022, 12, 1747. [Google Scholar] [CrossRef]
- Duong, M.T.; Wolk, D.A. Limbic-Predominant Age-Related TDP-43 Encephalopathy: LATE-Breaking Updates in Clinicopathologic Features and Biomarkers. Curr. Neurol. Neurosci. Rep. 2022, 22, 689–698. [Google Scholar] [CrossRef]
- Youssef, H.; Weissmann, C.; Uruk, G.; Gatto, R.G. Looking into Abnormal Co-Expressions of Tau and TDP-43 in the Realm of Mixed Dementia Types: A Double-Punch Scenario. Brain Sci. 2025, 15, 716. [Google Scholar] [CrossRef]
- Zabala-Findlay, A.; Penny, L.K.; Lofthouse, R.A.; Porter, A.J.; Palliyil, S.; Harrington, C.R.; Wischik, C.M.; Arastoo, M. Utility of Blood-Based Tau Biomarkers for Mild Cognitive Impairment and Alzheimer’s Disease: Systematic Review and Meta-Analysis. Cells 2023, 12, 1184. [Google Scholar] [CrossRef]
- Nunkoo, V.S.; Jurcau, A.; Les, M.; Cristian, A.; Militaru, M.; Marge, C.; Iovanovici, D.C.; Jurcau, M.C. Circulating Biomarkers for the Early Diagnosis of Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 7268. [Google Scholar] [CrossRef]
- Rissman, R.A.; Langford, O.; Raman, R.; Donohue, M.C.; Abdel-Latif, S.; Meyer, M.R.; Wente-Roth, T.; Kirmess, K.M.; Ngolab, J.; Winston, C.N.; et al. Plasma Aβ42/Aβ40 and phospho-tau217 concentration ratios increase the accuracy of amyloid PET classification in preclinical Alzheimer’s disease. Alzheimers Dement. 2024, 20, 1214–1224. [Google Scholar] [CrossRef]
- Janelidze, S.; Barthélemy, N.R.; Salvadó, G.; Schindler, S.E.; Palmqvist, S.; Mattsson-Carlgren, N.; Braunstein, J.B.; Ovod, V.; Bollinger, J.G.; He, Y.; et al. Plasma Phosphorylated Tau 217 and Aβ42/40 to Predict Early Brain Aβ Accumulation in People Without Cognitive Impairment. JAMA Neurol. 2024, 81, 947–957. [Google Scholar] [CrossRef]
- Tsantzali, I.; Foska, A.; Sideri, E.; Routsi, E.; Tsomaka, E.; Kitsos, D.K.; Zompola, C.; Bonakis, A.; Giannopoulos, S.; Voumvourakis, K.I.; et al. Plasma Phospho-Tau-181 as a Diagnostic Aid in Alzheimer’s Disease. Biomedicines 2022, 10, 1879. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Boufidou, F.; Stergiou, C.; Kuhle, J.; Willemse, E.; Palaiodimou, L.; Tsantzali, I.; Sideri, E.; Bonakis, A.; Giannopoulos, S.; et al. Plasma P-Tau181 for the Discrimination of Alzheimer’s Disease from Other Primary Dementing and/or Movement Disorders. Biomolecules 2022, 12, 1099. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Pascoal, T.A.; Karikari, T.K.; Benedet, A.L.; Lantero-Rodriguez, J.; Brinkmalm, G.; Snellman, A.; Schöll, M.; Troakes, C.; Hye, A.; et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021, 141, 709–724. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Salvadó, G.; Janelidze, S.; Pichet Binette, A.; Bali, D.; Karlsson, L.; Palmqvist, S.; Mattsson-Carlgren, N.; Stomrud, E.; Therriault, J.; et al. Plasma p-tau217 and tau-PET predict future cognitive decline among cognitively unimpaired individuals: Implications for clinical trials. Nat. Aging 2025, 5, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Dubarbie, F.; Guerra-Ruiz, A.; López-García, S.; Lage, C.; Fernández-Matarrubia, M.; Nevado-Cáceres, Á.; Rivera-Sánchez, M.; Valera-Barrero, A.; Pozueta-Cantudo, A.; García-Martínez, M.; et al. Diagnostic performance of plasma p-tau217 in a memory clinic cohort using the Lumipulse automated platform. Alzheimers Res. Ther. 2025, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Schraen-Maschke, S.; Vidal, J.S.; Delaby, C.; Buee, L.; Blanc, F.; Paquet, C.; Allinquant, B.; Bombois, S.; Gabelle, A.; et al. Clinical value of plasma ALZpath pTau217 immunoassay for assessing mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2024, 95, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Poesen, K.; Vandenberghe, R.; De Meyer, S. Alzheimer’s disease neuropathology and its estimation with fluid and imaging biomarkers. Mol. Neurodegener. 2025, 20, 33. [Google Scholar] [CrossRef]
- Horie, K.; Salvadó, G.; Koppisetti, R.K.; Janelidze, S.; Barthélemy, N.R.; He, Y.; Sato, C.; Gordon, B.A.; Jiang, H.; Benzinger, T.L.S.; et al. Plasma MTBR-tau243 biomarker identifies tau tangle pathology in Alzheimer’s disease. Nat. Med. 2025, 31, 2044–2053. [Google Scholar] [CrossRef]
- Jung, Y.; Damoiseaux, J.S. The potential of blood neurofilament light as a marker of neurodegeneration for Alzheimer’s disease. Brain 2024, 147, 12–25. [Google Scholar] [CrossRef]
- Jin, Z.; Lu, Y.; Tang, H.; Cui, H. Integrating neuroinflammation biomarkers into the ATN(X) framework: Advances in Alzheimer’s pathogenesis, diagnosis, and insights from non-human primate models. Alzheimers Dement. 2025, 21, 70472. [Google Scholar] [CrossRef]
- Benedet, A.L.; Milà-Alomà, M.; Vrillon, A.; Ashton, N.J.; Pascoal, T.A.; Lussier, F.; Karikari, T.K.; Hourregue, C.; Cognat, E.; Dumurgier, J.; et al. Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA Neurol. 2021, 78, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The Amyloid-β Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimers Dis. 2018, 64, S567–S610. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.R.; Jin, S.; Ramalingam, N.; Bellier, J.P.; Lish, A.M.; Ostaszewski, B.L.; Young-Pearse, T.; Liu, L.; Yang, H.S.; et al. An improved immunoassay detects Abeta oligomers in human biofluids: Their CSF levels rise with tau and phosphotau levels. Alzheimers Res. Ther. 2025, 17, 153. [Google Scholar] [CrossRef] [PubMed]
- Blömeke, L.; Rehn, F.; Pils, M.; Kraemer-Schulien, V.; Cousin, A.; Kutzsche, J.; Bujnicki, T.; Freiesleben, S.D.; Schneider, L.S.; Preis, L.; et al. Blood-based quantification of Aβ oligomers indicates impaired clearance from brain in ApoE ε4 positive subjects. Commun. Med. 2024, 4, 262. [Google Scholar] [CrossRef]
- An, J.; Kim, K.; Lim, H.J.; Kim, H.Y.; Shin, J.; Park, I.; Cho, I.; Kim, H.Y.; Kim, S.; McLean, C.; et al. Early onset diagnosis in Alzheimer’s disease patients via amyloid-beta oligomers-sensing probe in cerebrospinal fluid. Nat. Commun. 2024, 15, 1004. [Google Scholar] [CrossRef]
- Mao, H.; Ding, L. Ultrasensitive portable aptamer-electrochemical sensor for point-of-care testing of Alzheimer’s biomarker amyloid-beta oligomers. Bioelectrochemistry 2025, 167, 109079. [Google Scholar]
- Blömeke, L.; Pils, M.; Kraemer-Schulien, V.; Dybala, A.; Schaffrath, A.; Kulawik, A.; Rehn, F.; Cousin, A.; Nischwitz, V.; Willbold, J.; et al. Quantitative detection of α-Synuclein and Tau oligomers and other aggregates by digital single particle counting. npj Parkinsons Dis. 2022, 8, 68. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Alizadeh, H.; Marton, J.; Cumming, P. The Sensitivity of Tau Tracers for the Discrimination of Alzheimer’s Disease Patients and Healthy Controls by PET. Biomolecules 2023, 13, 290. [Google Scholar] [CrossRef]
- Aliaga, A.; Therriault, J.; Quispialaya, K.M.; Aliaga, A.; Hopewell, R.; Rahmouni, N.; Macedo, A.C.; Kunach, P.; Soucy, J.P.; Massarweh, G.; et al. Comparison Between Brain and Cerebellar Autoradiography Using [18F]Flortaucipir, [18F]MK6240, and [18F]PI2620 in Postmortem Human Brain Tissue. J. Nucl. Med. 2025, 66, 123–129. [Google Scholar] [CrossRef]
- Kang, X.; Tian, J.; Shu, Q.; Cheng, T.; Wang, S.; Hu, Y. Microglia-neuron crosstalk in Alzheimer’s disease: An exploration of molecular mechanisms and pathological implications. Neuroscience 2025, 583, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calvet, M.; Kleinberger, G.; Araque Caballero, M.Á.; Brendel, M.; Rominger, A.; Alcolea, D.; Fortea, J.; Lleó, A.; Blesa, R.; Gispert, J.D.; et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol. Med. 2016, 8, 466–476. [Google Scholar] [CrossRef]
- Fernández-Matarrubia, M.; Valera-Barrero, A.; Renuncio-García, M.; Aguilella, M.; Lage, C.; López-García, S.; Ocejo-Vinyals, J.G.; Martínez-Dubarbie, F.; Molfetta, G.D.; Pozueta-Cantudo, A.; et al. Early microglial and astrocyte reactivity in preclinical Alzheimer’s disease. Alzheimers Dement. 2025, 21, e70502. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mantyh, W.G.; Men, L.; Jain, I.; Glittenberg, M.; An, B.; Zhang, L.; Li, L.; Alzheimer’s Disease Neuroimaging Initiative. sTREM2 in discordant CSF Abeta(42) and p-tau181. Alzheimers Dement. 2025, 17, e70072. [Google Scholar]
- Lan, G.; Li, A.; Gonzalez-Ortiz, F.; Lv, J.; Ran, W.; Cai, Y.; Sun, P.; Liu, L.; Yang, J.; Zhang, L.; et al. Higher plasma soluble TREM2 correlates with reduced cerebral tau accumulation in Alzheimer’s disease. Mol. Psychiatry 2025, 30, 3988–3997. [Google Scholar] [CrossRef]
- Rodriguez-Vieitez, E.; Ashton, N.J. Plasma sTREM2: A potential marker of cerebrovascular injury in neurodegenerative disorders. Brain 2021, 144, 3283–3285. [Google Scholar] [CrossRef]
- Jiao, L.; Yang, J.; Wang, W.; Liu, X.; Fu, Y.; Fan, D. sTREM2 cerebrospinal fluid levels are a potential biomarker in amyotrophic lateral sclerosis and associate with UMN burden. Front. Neurol. 2024, 15, 1515252. [Google Scholar] [CrossRef]
- Mavroudis, I.; Chowdhury, R.; Petridis, F.; Karantali, E.; Chatzikonstantinou, S.; Balmus, I.M.; Luca, I.S.; Ciobica, A.; Kazis, D. YKL-40 as a Potential Biomarker for the Differential Diagnosis of Alzheimer’s Disease. Medicina 2021, 58, 60. [Google Scholar] [CrossRef]
- Pelkmans, W.; Shekari, M.; Brugulat-Serrat, A.; Sánchez-Benavides, G.; Minguillón, C.; Fauria, K.; Molinuevo, J.L.; Grau-Rivera, O.; González Escalante, A.; Kollmorgen, G.; et al. Astrocyte biomarkers GFAP and YKL-40 mediate early Alzheimer’s disease progression. Alzheimers Dement. 2024, 20, 483–493. [Google Scholar] [CrossRef]
- Pase, M.P.; Himali, J.J.; Puerta, R.; Beiser, A.S.; Gonzales, M.M.; Satizabal, C.L.; Yang, Q.; Aparicio, H.J.; Kojis, D.J.; Decarli, C.S.; et al. Association of Plasma YKL-40 with MRI, CSF, and Cognitive Markers of Brain Health and Dementia. Neurology 2024, 102, e208075. [Google Scholar] [CrossRef]
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef]
- Lista, S.; Imbimbo, B.P.; Grasso, M.; Fidilio, A.; Emanuele, E.; Minoretti, P.; López-Ortiz, S.; Martín-Hernández, J.; Gabelle, A.; Caruso, G.; et al. Tracking neuroinflammatory biomarkers in Alzheimer’s disease: A strategy for individualized therapeutic approaches? J. Neuroinflammation 2024, 21, 187. [Google Scholar] [CrossRef] [PubMed]
- Argiris, G.; Akinci, M.; Peña-Gómez, C.; Palpatzis, E.; Garcia-Prat, M.; Shekari, M.; Blennow, K.; Zetterberg, H.; Kollmorgen, G.; Quijano-Rubio, C.; et al. Data-driven CSF biomarker profiling: Imaging and clinical outcomes in a cohort at risk of Alzheimer’s disease. Alzheimers Res. Ther. 2024, 16, 274. [Google Scholar] [CrossRef] [PubMed]
- Katonova, A.; Andel, R.; Jurasova, V.; Veverova, K.; Angelucci, F.; Matoska, V.; Hort, J. Associations of KLOTHO-VS heterozygosity and α-Klotho protein with cerebrospinal fluid Alzheimer’s disease biomarkers. J. Alzheimers Dis. 2025, 105, 159–171. [Google Scholar] [CrossRef]
- Griffiths, J.; Grant, S.G.N. Synapse pathology in Alzheimer’s disease. Semin. Cell Dev. Biol. 2023, 139, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.J.; Sekula, N.M.; Svirsky, S.; Maesako, M.; Zoltowska, K.M.; Berezovska, O. Presenilin 1 increases association with synaptotagmin 1 during normal aging. Neurobiol. Aging 2020, 86, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xie, S.; Malek, M. SNAP-25: A biomarker of synaptic loss in neurodegeneration. Clin. Chim. Acta 2025, 571, 120236. [Google Scholar] [CrossRef]
- Kivisäkk, P.; Carlyle, B.C.; Sweeney, T.; Quinn, J.P.; Ramirez, C.E.; Trombetta, B.A.; Mendes, M.; Brock, M.; Rubel, C.; Czerkowicz, J.; et al. Increased levels of the synaptic proteins PSD-95, SNAP-25, and neurogranin in the cerebrospinal fluid of patients with Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 58. [Google Scholar] [CrossRef]
- Jurasova, V.; Andel, R.; Katonova, A.; Veverova, K.; Zuntychova, T.; Horakova, H.; Vyhnalek, M.; Kolarova, T.; Matoska, V.; Blennow, K.; et al. CSF neurogranin levels as a biomarker in Alzheimer’s disease and frontotemporal lobar degeneration: A cross-sectional analysis. Alzheimers Res. Ther. 2024, 16, 199. [Google Scholar] [CrossRef]
- Goossens, J.; Cervantes González, A.; Dewit, N.; Lidón, L.; Fortea, J.; Alcolea, D.; Lleó, A.; Belbin, O.; Vanmechelen, E. Evaluation of cerebrospinal fluid levels of synaptic vesicle protein, VAMP-2, across the sporadic Alzheimer’s disease continuum. Alzheimers Res. Ther. 2023, 15, 186. [Google Scholar] [CrossRef]
- Gliwińska, A.; Czubilińska-Łada, J.; Więckiewicz, G.; Świętochowska, E.; Badeński, A.; Dworak, M.; Szczepańska, M. The Role of Brain-Derived Neurotrophic Factor (BDNF) in Diagnosis and Treatment of Epilepsy, Depression, Schizophrenia, Anorexia Nervosa and Alzheimer’s Disease as Highly Drug-Resistant Diseases: A Narrative Review. Brain Sci. 2023, 13, 163. [Google Scholar] [CrossRef]
- Kang, J.; Son, Y.; Yim, Y.; Cho, H.; Kim, J.; Lark, A.R.S.; Mir, F.A.; Kim, H.J.; Park, J.; Lee, H.; et al. Biomarkers for Alzheimer’s disease across diverse biological domains: An umbrella review and evidence map. J. Adv. Res 2025, S2090-1232(25)00547-8, in press. [Google Scholar] [CrossRef] [PubMed]
- Genin, E.; Hannequin, D.; Wallon, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Bullido, M.J.; Engelborghs, S.; De Deyn, P.; Berr, C.; et al. APOE and Alzheimer disease: A major gene with semi-dominant inheritance. Mol. Psychiatry 2011, 16, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shue, F.; Zhao, N.; Shinohara, M.; Bu, G. APOE2: Protective mechanism and therapeutic implications for Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Fortea, J.; Pegueroles, J.; Alcolea, D.; Belbin, O.; Dols-Icardo, O.; Vaqué-Alcázar, L.; Videla, L.; Gispert, J.D.; Suárez-Calvet, M.; Johnson, S.C.; et al. APOE4 homozygozity represents a distinct genetic form of Alzheimer’s disease. Nat. Med. 2024, 30, 1284–1291. [Google Scholar] [CrossRef]
- Schramm, C.; Charbonnier, C.; Zaréa, A.; Lacour, M.; Wallon, D.; CNRMAJ Collaborators; Boland, A.; Deleuze, J.F.; Olaso, R.; ADES Consortium; et al. Penetrance estimation of Alzheimer disease in SORL1 loss-of-function variant carriers using a family-based strategy and stratification by APOE genotypes. Genome Med. 2022, 14, 69. [Google Scholar] [CrossRef]
- Poblano, J.; Castillo-Tobías, I.; Berlanga, L.; Tamayo-Ordoñez, M.C.; Del Carmen Rodríguez-Salazar, M.; Silva-Belmares, S.Y.; Aguayo-Morales, H.; Cobos-Puc, L.E. Drugs targeting APOE4 that regulate beta-amyloid aggregation in the brain: Therapeutic potential for Alzheimer’s disease. Basic Clin. Pharmacol. Toxicol. 2024, 135, 237–249. [Google Scholar] [CrossRef]
- Yang, H.; Kim, D.; Yang, Y.; Bagyinszky, E.; An, S.S.A. TREM2 in Neurodegenerative Disorders: Mutation Spectrum, Pathophysiology, and Therapeutic Targeting. Int. J. Mol. Sci. 2025, 26, 7057. [Google Scholar] [CrossRef] [PubMed]
- Fortea, J.; Zaman, S.H.; Hartley, S.; Rafii, M.S.; Head, E.; Carmona-Iragui, M. Alzheimer’s disease associated with Down syndrome: A genetic form of dementia. Lancet Neurol. 2021, 20, 930–942. [Google Scholar] [CrossRef]
- Chipi, E.; Salvadori, N.; Farotti, L.; Parnetti, L. Biomarker-Based Signature of Alzheimer’s Disease in Pre-MCI Individuals. Brain Sci. 2019, 9, 213. [Google Scholar] [CrossRef]
- Greenberg, B.D.; Pettigrew, C.; Soldan, A.; Wang, J.; Wang, M.C.; Darrow, J.A.; Albert, M.S.; Moghekar, A. CSF Alzheimer Disease Biomarkers: Time-Varying Relationships with MCI Symptom Onset and Associations with Age, Sex, and ApoE4. Neurology 2022, 99, e1640–e1650. [Google Scholar] [CrossRef]
- Li, Y.; Yen, D.; Hendrix, R.D.; Gordon, B.A.; Dlamini, S.; Barthélemy, N.R.; Aschenbrenner, A.J.; Henson, R.L.; Herries, E.M.; Volluz, K.; et al. Timing of Biomarker Changes in Sporadic Alzheimer’s Disease in Estimated Years from Symptom Onset. Ann. Neurol. 2024, 95, 951–965. [Google Scholar] [CrossRef]
- Milà-Alomà, M.; Tosun, D.; Schindler, S.E.; Hausle, I.; Petersen, K.K.; Li, Y.; Dage, J.L.; Du-Cuny, L.; Saad, Z.S.; Saef, B.; et al. Timing of Changes in Alzheimer’s Disease Plasma Biomarkers as Assessed by Amyloid and Tau PET Clocks. Ann. Neurol. 2025, 98, 508–523. [Google Scholar] [CrossRef]
- Prosser, L.; Sudre, C.H.; Oxtoby, N.P.; Young, A.L.; Malone, I.B.; Manning, E.N.; Pemberton, H.; Walsh, P.; Barkhof, F.; Biessels, G.J.; et al. Biomarker pathway heterogeneity of amyloid-positive individuals. Alzheimers Dement. 2024, 20, 8503–8515. [Google Scholar] [CrossRef]
- Paterson, R.W.; Toombs, J.; Slattery, C.F.; Nicholas, J.M.; Andreasson, U.; Magdalinou, N.K.; Blennow, K.; Warren, J.D.; Mummery, C.J.; Rossor, M.N.; et al. Dissecting IWG-2 typical and atypical Alzheimer’s disease: Insights from cerebrospinal fluid analysis. J. Neurol. 2015, 262, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; von Arnim, C.A.F.; Burnie, N.; Bozeat, S.; Cummings, J. Biomarkers in Alzheimer’s disease: Role in early and differential diagnosis and recognition of atypical variants. Alzheimers Res. Ther. 2023, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Salvioni Chiabotti, P.; Nasuti, M.; Rouaud, O.; Allali, G. Posterior cortical atrophy in the age of anti-amyloid treatments: An 11-year retrospective study of eligible patients from the Leenaards Memory Center. J. Alzheimers Dis. 2025, 106, 512–517. [Google Scholar] [CrossRef]
- Tsantzali, I.; Athanasaki, A.; Boufidou, F.; Constantinides, V.C.; Stefanou, M.I.; Moschovos, C.; Zompola, C.; Paraskevas, S.G.; Bonakis, A.; Giannopoulos, S.; et al. Cerebrospinal Fluid Classical Biomarker Levels in Mixed vs. Pure A+T+ (A+T1+) Alzheimer’s Disease. Biomedicines 2024, 12, 2904. [Google Scholar] [CrossRef]
- Bousiges, O.; Blanc, F. Biomarkers of Dementia with Lewy Bodies: Differential Diagnostic with Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 6371. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef]
- Kurihara, M.; Matsubara, T.; Morimoto, S.; Arakawa, A.; Ohse, K.; Kanemaru, K.; Iwata, A.; Murayama, S.; Saito, Y. Neuropathological changes associated with aberrant cerebrospinal fluid p-tau181 and Aβ42 in Alzheimer’s disease and other neurodegenerative diseases. Acta Neuropathol. Commun. 2024, 12, 48. [Google Scholar] [CrossRef]
- Kapaki, E.; Kilidireas, K.; Paraskevas, G.P.; Michalopoulou, M.; Patsouris, E. Highly increased CSF tau protein and decreased beta-amyloid (1–42) in sporadic CJD: A discrimination from Alzheimer’s disease? J. Neurol. Neurosurg. Psychiatry 2001, 71, 401–403. [Google Scholar] [CrossRef]
- Wolk, D.A.; Nelson, P.T.; Apostolova, L.; Arfanakis, K.; Boyle, P.A.; Carlsson, C.M.; Corriveau-Lecavalier, N.; Dacks, P.; Dickerson, B.C.; Domoto-Reilly, K.; et al. Clinical criteria for limbic-predominant age-related TDP-43 encephalopathy. Alzheimers Dement. 2025, 21, e14202. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.; Román, G.C.; Esiri, M.; Kettunen, P.; Svensson, J.; Paraskevas, G.P.; Kapaki, E. Update on Vascular Cognitive Impairment Associated with Subcortical Small-Vessel Disease. J. Alzheimers Dis. 2018, 62, 1417–1441. [Google Scholar] [CrossRef] [PubMed]
- Formichi, P.; Parnetti, L.; Radi, E.; Cevenini, G.; Dotti, M.T.; Federico, A. CSF Biomarkers Profile in CADASIL-A Model of Pure Vascular Dementia: Usefulness in Differential Diagnosis in the Dementia Disorder. Int. J. Alzheimers Dis. 2010, 2010, 959257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dumurgier, J.; Schraen, S.; Gabelle, A.; Vercruysse, O.; Bombois, S.; Laplanche, J.L.; Peoc’h, K.; Sablonnière, B.; Kastanenka, K.V.; Delaby, C.; et al. Cerebrospinal fluid amyloid-β 42/40 ratio in clinical setting of memory centers: A multicentric study. Alzheimers Res. Ther. 2015, 7, 30. [Google Scholar] [CrossRef]
- Josephs, K.A.; Weigand, S.D.; Whitwell, J.L. Characterizing Amyloid-Positive Individuals with Normal Tau PET Levels After 5 Years: An ADNI Study. Neurology 2022, 98, e2282–e2292. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Katsumata, Y.; Wu, X.; Aung, K.Z.; Fsardo, D.W.; Forrest, S.L.; Alzheimer’s Disease Genetics Consortium; Nelson, P.T. Amyloid-β predominant Alzheimer’s disease neuropathologic change. Brain 2025, 148, 401–407. [Google Scholar] [CrossRef]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Amyloid Biomarker Study Group. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- Rivero-Santana, A.; Ferreira, D.; Perestelo-Perez, L.; Westman, E.; Wahlund, L.O.; Sarria, A.; Serrano-Aguilar, P. Cerebrospinal Fluid Biomarkers for the Differential Diagnosis between Alzheimer’s Disease and Frontotemporal Lobar Degeneration: Systematic Review, HSROC Analysis, and Confounding Factors. J. Alzheimer’s Dis. 2017, 55, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, G.P.; Kasselimis, D.; Kourtidou, E.; Constantinides, V.; Bougea, A.; Potagas, C.; Evdokimidis, I.; Kapaki, E. Cerebrospinal Fluid Biomarkers as a Diagnostic Tool of the Underlying Pathology of Primary Progressive Aphasia. J. Alzheimers Dis. 2017, 55, 1453–31461. [Google Scholar] [CrossRef] [PubMed]
- Casoli, T.; Paolini, S.; Fabbietti, P.; Fattoretti, P.; Paciaroni, L.; Fabi, K.; Gobbi, B.; Galeazzi, R.; Rossi, R.; Lattanzio, F.; et al. Cerebrospinal fluid biomarkers and cognitive status in differential diagnosis of frontotemporal dementia and Alzheimer’s disease. J. Int. Med. Res. 2019, 47, 4968–4980. [Google Scholar] [CrossRef]
- Constantinides, V.C.; Boufidou, F.; Bourbouli, M.; Pyrgelis, E.S.; Ghika, A.; Koros, C.; Liakakis, G.; Papageorgiou, S.; Stefanis, L.; Paraskevas, G.P.; et al. Application of the AT(N) and Other CSF Classification Systems in Behavioral Variant Frontotemporal Dementia. Diagnostics 2023, 13, 332. [Google Scholar] [CrossRef]
- Smith, R.; Shaw, L.; Palmqvist, S.; Mattsson-Carlgren, N.; Klein, G.; Tonietto, M.; Alzheimer’s Disease Neuroimaging Initiative; Quijano-Rubio, C.; Rank, C.M.; Andreadou, M.; et al. Clinical Performance of the Elecsys CSF pTau181/Aβ42 Ratio for Concordance with Tau-PET in Two Independent Cohorts. Neurol. Ther. 2025, 14, 2011–2031. [Google Scholar] [CrossRef]
- Lehmann, S.; Gabelle, A.; Duchiron, M.; Busto, G.; Morchikh, M.; Delaby, C.; Hirtz, C.; Mondesert, E.; Cristol, J.P.; Barnier-Figue, G.; et al. Comparative performance of plasma pTau181/Aβ42, pTau217/Aβ42 ratios, and individual measurements in detecting brain amyloidosis. EBioMedicine 2025, 117, 105805. [Google Scholar] [CrossRef]
- Athanasaki, A.; Tsantzali, I.; Kroupis, C.; Theodorou, A.; Boufidou, F.; Constantinides, V.C.; Tzartos, J.S.; Tzartos, S.J.; Velonakis, G.; Zompola, C.; et al. APOE Genotyping in Cognitive Disorders: Preliminary Observations from the Greek Population. Int. J. Mol. Sci. 2025, 26, 7410. [Google Scholar] [CrossRef] [PubMed]
- Bieger, A.; Brum, W.S.; Borelli, W.V.; Therriault, J.; De Bastiani, M.A.; Moreira, A.G.; Benedet, A.L.; Ferrari-Souza, J.P.; Da Costa, J.C.; Souza, D.O.; et al. Influence of Different Diagnostic Criteria on Alzheimer Disease Clinical Research. Neurology 2024, 103, e209753. [Google Scholar] [CrossRef] [PubMed]
- Bouteloup, V.; Villain, N.; Vidal, J.S.; Gonzalez-Ortiz, F.; Yuksekel, I.; Santos, C.; Schraen-Maschken, S.; Pellegrin, I.; Lehmann, S.; Blennow, K.; et al. Cognitive Phenotyping and Interpretation of Alzheimer Blood Biomarkers. JAMA Neurol. 2025, 82, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, S.; Whitson, H.E.; Allen, L.A.; Suarez-Calvet, M.; Galasko, D.; Karikari, T.K.; Okrahvi, H.R.; Paczynski, M.; Schindler, S.E.; Teunissen, C.E.; et al. Alzheimer’s Association Clinical Practice Guideline on the use of blood-based biomarkers in the diagnostic workup of suspected Alzheimer’s disease within specialized care settings. Alzheimers Dement. 2025, 21, e70535. [Google Scholar] [CrossRef] [PubMed]

| A (Amyloid) | T (Tau) | N (Neurodegeneration) | V (Vascular) | S (α-syn) | I (Inflammation) | |
|---|---|---|---|---|---|---|
| T1 | T2 | |||||
| Core 1 | Core 2 | |||||
| CSF Aβ42 | CSF τP-181 | CSF τP-205 | CSF τT | CSF α-syn | CSF GFAP | |
| CSF Aβ42/Aβ40 | CSF τP-217 | CSF MDBR-τ243 | CSF NfL | |||
| CSF τP-231 | Other tau forms | |||||
| PET Aβ | PET tau | PET FDG | ||||
| SPECT HMPAO | ||||||
| MRI ASL | ||||||
| Atrophy (MRI, CT) | MRI, CT | |||||
| Plasma Aβ42 | Plasma τP-217 | Plasma eMDBR-τ243 | Plasma NfL | Plasma GFAP | ||
| Plasma Aβ42/Aβ40 | Plasma τP-181 | Plasma τT | ||||
| Plasma τP-212 | ||||||
| Hybrid ratios or combinations of different markers, including CSF τP-181/Aβ42, CSF τT/Aβ42, and plasma τP-217/Aβ42, are also in use. | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasaki, A.; Tsantzali, I.; Theodorou, A.; Michalopoulou, A.; Constantinides, V.C.; Boufidou, F.; Tzartos, J.S.; Tsalouchidou, P.-E.; Zompola, C.; Paraskevas, S.G.; et al. The Biomarker Profile of Alzheimer’s Disease for Disease-Modifying Treatment Eligibility: Questions and Debates. Int. J. Mol. Sci. 2025, 26, 9531. https://doi.org/10.3390/ijms26199531
Athanasaki A, Tsantzali I, Theodorou A, Michalopoulou A, Constantinides VC, Boufidou F, Tzartos JS, Tsalouchidou P-E, Zompola C, Paraskevas SG, et al. The Biomarker Profile of Alzheimer’s Disease for Disease-Modifying Treatment Eligibility: Questions and Debates. International Journal of Molecular Sciences. 2025; 26(19):9531. https://doi.org/10.3390/ijms26199531
Chicago/Turabian StyleAthanasaki, Athanasia, Ioanna Tsantzali, Aikaterini Theodorou, Amalia Michalopoulou, Vasilios C. Constantinides, Fotini Boufidou, John S. Tzartos, Panagiota-Eleni Tsalouchidou, Christina Zompola, Sotirios G. Paraskevas, and et al. 2025. "The Biomarker Profile of Alzheimer’s Disease for Disease-Modifying Treatment Eligibility: Questions and Debates" International Journal of Molecular Sciences 26, no. 19: 9531. https://doi.org/10.3390/ijms26199531
APA StyleAthanasaki, A., Tsantzali, I., Theodorou, A., Michalopoulou, A., Constantinides, V. C., Boufidou, F., Tzartos, J. S., Tsalouchidou, P.-E., Zompola, C., Paraskevas, S. G., Bonakis, A., Giannopoulos, S., Tsivgoulis, G., Kapaki, E., & Paraskevas, G. P. (2025). The Biomarker Profile of Alzheimer’s Disease for Disease-Modifying Treatment Eligibility: Questions and Debates. International Journal of Molecular Sciences, 26(19), 9531. https://doi.org/10.3390/ijms26199531










