Beyond Static Cold Storage: Toward the Next Generation of Tailored Organ Preservation Solutions
Abstract
1. Introduction
2. Historical Evolution of Preservation Solutions

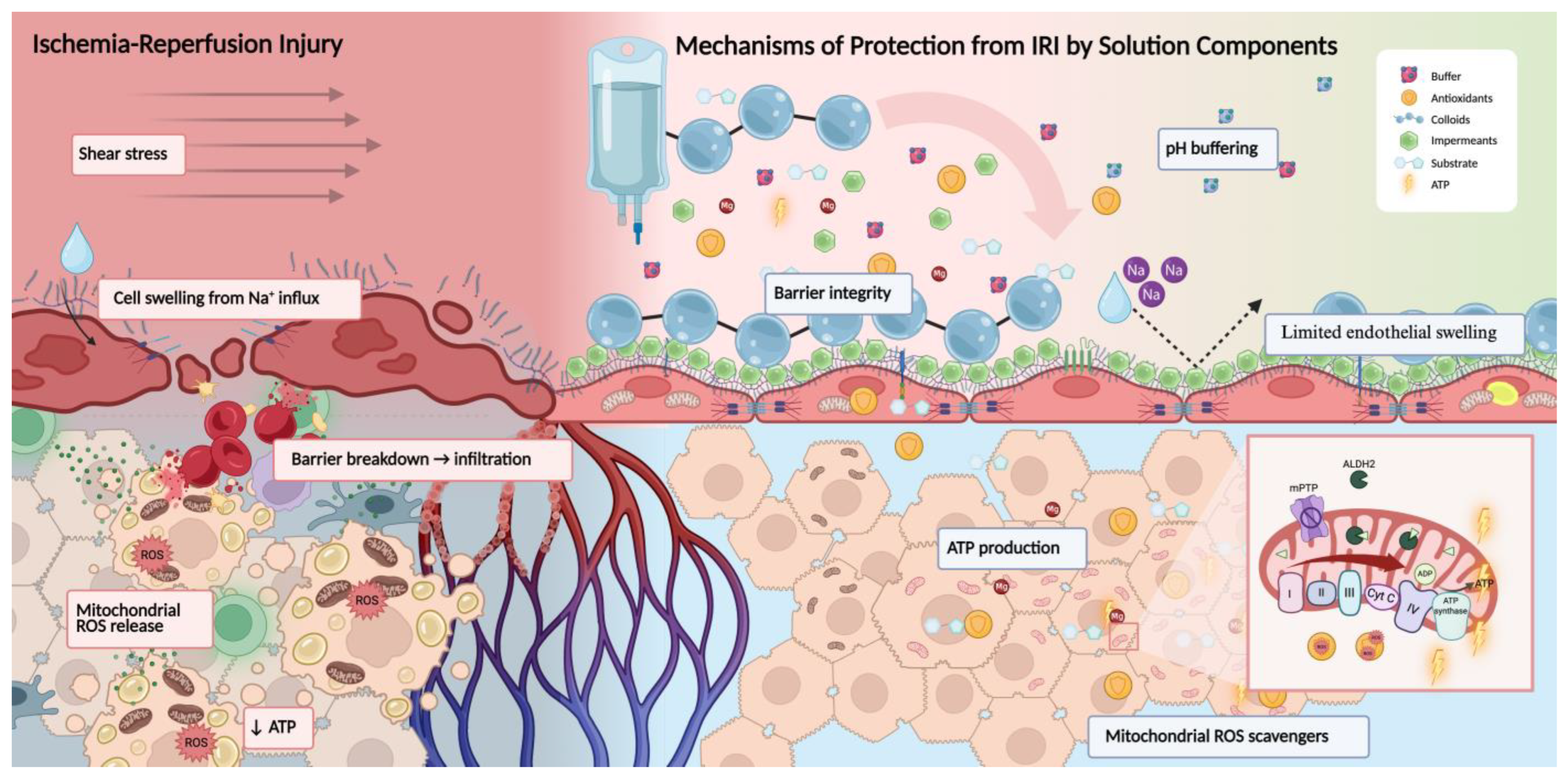
3. Mechanisms of Injury and Protection: Systems-Based Framework
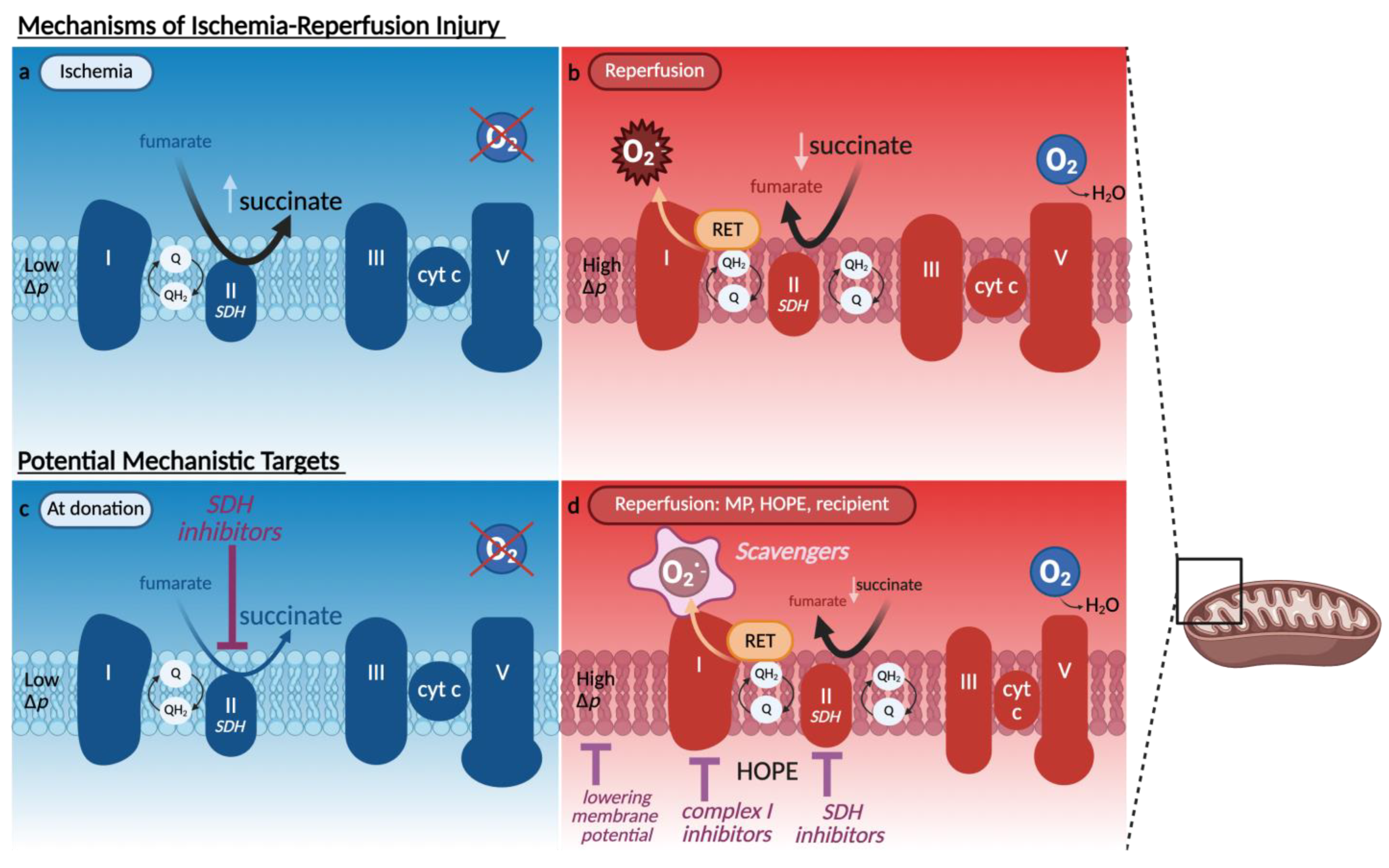
3.1. Mitochondrial Dysfunction and Redox Imbalance
3.1.1. Glutathione
3.1.2. MitoQ
3.1.3. Itaconate
3.1.4. Quercetin
3.1.5. Hydrogen Sulfide Donors
3.1.6. PrC-210
3.2. Endothelial Barrier Breakdown and Vascular Dysfunction
3.3. Inflammatory Signaling and Immune Activation
3.3.1. Nitric Oxide and Immune Modulation
3.3.2. Prostaglandins and Cyclooxygenase Inhibitors
3.3.3. Immune Modulation via Perfusion Platforms
3.4. Energy Collapse and Metabolic Support
3.5. Pharmacologic Modulators and Emerging Additives
4. Organ-Specific Preservation and the Case for Unification
4.1. Variability in Injury Profiles Across Organs
4.2. Limitations of the One-Size-Fits-All Approach
4.3. Towards Unified or Modular Strategies
4.4. Divergence of Storage and Perfusion Solutions
4.5. Temperature Shift in Cardiothoracic Preservation and Implications
5. Preservation Modalities: Modulating Injury Through Storage and Perfusion
6. Summary and Future Outlooks
Funding
Data Availability Statement
Conflicts of Interest
References
- Jing, L.; Yao, L.; Zhao, M.; Peng, L.P.; Liu, M. Organ preservation: From the past to the future. Acta Pharmacol. Sin. 2018, 39, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Williams, P.; Salinas, J.; Vengohechea, J.; Lodge, J.P.A.; Fondevila, C.; Hessheimer, A.J. Abdominal Organ Preservation Solutions in the Age of Machine Perfusion. Transplantation 2023, 107, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, J.; Xia, T.C.; Xu, R.; He, X.; Xia, Y. Preservation Solutions for Kidney Transplantation: History, Advances and Mechanisms. Cell Transplant. 2019, 28, 1472–1489. [Google Scholar] [CrossRef]
- Micó-Carnero, M.; Zaouali, M.A.; Rojano-Alfonso, C.; Maroto-Serrat, C.; ben Abdennebi, H.; Peralta, C. A Potential Route to Reduce Ischemia/Reperfusion Injury in Organ Preservation. Cells 2022, 11, 2763. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation-from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Tingle, S.J.; Dobbins, J.J.; Thompson, E.R.; Figueiredo, R.S.; Mahendran, B.; Pandanaboyana, S.; Wilson, C. Machine perfusion in liver transplantation. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Qin, G.; Jernryd, V.; Sjöberg, T.; Steen, S.; Nilsson, J. Machine Perfusion for Human Heart Preservation: A Systematic Review. Transpl. Int. 2022, 35, 10258. [Google Scholar] [CrossRef]
- Ran, Q.; Zhang, J.; Zhong, J.; Lin, J.; Zhang, S.; Li, G.; You, B. Organ preservation: Current limitations and optimization approaches. Front. Med. 2025, 12, 1566080. [Google Scholar] [CrossRef]
- Mangus, A.E.; Kubal, C.A.; Ekser, B.; Mihaylov, P.; Lutz, A.J.; Fridell, J.A.; Mangus, R.S. Deceased Donor Flush Volume Similar for Histidine-Tryptophan-Ketoglutarate and University of Wisconsin at a Single US Organ Procurement Organization: Adult and Pediatric Data. Transpl. Proc. 2023, 55, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, C.A.; Nicely, B.; Mattice, B.J.; Teaster, R. Comparative analysis of clinical efficacy and cost between University of Wisconsin solution and histidine-tryptophan-ketoglutarate. Prog. Transplant. 2008, 18, 166–171; quiz 172. [Google Scholar] [CrossRef] [PubMed]
- Panayotova, G.G.; Lunsford, K.E.; Quillin, R.C., 3rd; Rana, A.; Agopian, V.G.; Lee-Riddle, G.S.; Markovic, D.; Paterno, F.; Griesemer, A.D.; Amin, A.; et al. Portable hypothermic oxygenated machine perfusion for organ preservation in liver transplantation: A randomized, open-label, clinical trial. Hepatology 2024, 79, 1033–1047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus. Med. Hemother. 2011, 38, 125–142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, S.; Saner, F.H.; Bezinover, D. A brief history of liver transplantation and transplant anesthesia. BMC Anesthesiol. 2022, 22, 363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oto, T.; Griffiths, A.P.; Rosenfeldt, F.; Levvey, B.J.; Williams, T.J.; Snell, G.I. Early outcomes comparing Perfadex, Euro-Collins, and Papworth solutions in lung transplantation. Ann. Thorac. Surg. 2006, 82, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Fitzpatrick, A.M.; Haykal, S. Preservation solutions for attenuation of ischemia-reperfusion injury in vascularized composite allotransplantation. SAGE Open Med. 2021, 9, 20503121211034924. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Southard, J.H.; Belzer, F.O. Organ preservation. Annu. Rev. Med. 1995, 46, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdennebi, H.; Steghens, J.P.; Hadj-Aïssa, A.; Barbieux, A.; Ramella-Virieux, S.; Gharib, C.; Boillot, O. A preservation solution with polyethylene glycol and calcium: A possible multiorgan liquid. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2002, 15, 348–354. [Google Scholar] [CrossRef]
- Bardallo, R.G.; da Silva, R.T.; Carbonell, T.; Folch-Puy, E.; Palmeira, C.; Roselló-Catafau, J.; Pirenne, J.; Adam, R.; Panisello-Roselló, A. Role of peg35, mitochondrial aldh2, and glutathione in cold fatty liver graft preservation: An igl-2 approach. Int. J. Mol. Sci. 2021, 22, 5332. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.T.; Bardallo, R.G.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.M.; Fondevila, C.; Adam, R.; Roselló-Catafau, J.; Panisello-Roselló, A. IGL-2 as a Unique Solution for Cold Static Preservation and Machine Perfusion in Liver and Mitochondrial Protection. Transplant. Proc. 2022, 54, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Nakamura, T.; Wada, H. Development of new organ preservation solutions in Kyoto University. Yonsei Med. J. 2004, 45, 1107–1114. [Google Scholar] [CrossRef]
- Tingle, S.; Figueiredo, R.; Moir, J.; Goodfellow, M.; Talbot, D.; Wilson, C. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Sun, K.; Jiao, C.; Panconesi, R.; Satish, S.; Karakaya, O.F.; de Goeij, F.H.C.; Diwan, T.; Ali, K.; Cadinu, L.A.; Cazzaniga, B.; et al. Quantifying Flavin mononucleotide: An internationally validated methodological approach for enhanced decision making in organ transplantation. EBioMedicine 2025, 116, 105761. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 2018, 17, 85–886. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Loscalzo, J. Metabolic Responses to Reductive Stress. Antioxid. Redox Signal. 2020, 32, 1330–1347. [Google Scholar] [CrossRef]
- Chen, T.H.; Wang, H.C.; Chang, C.J.; Lee, S.Y. Mitochondrial Glutathione in Cellular Redox Homeostasis and Disease Manifestation. Int. J. Mol. Sci. 2024, 25, 1314. [Google Scholar] [CrossRef]
- Prag, H.A.; Aksentijevic, D.; Dannhorn, A.; Giles, A.v.; Mulvey, J.F.; Sauchanka, O.; Du, L.; Bates, G.; Reinhold, J.; Kula-Alwar, D.; et al. Ischemia-Selective Cardioprotection by Malonate for Ischemia/Reperfusion Injury. Circ. Res. 2022, 131, 528–541. [Google Scholar] [CrossRef]
- Cordes, T.; Metallo, C.M. Itaconate alters succinate and coenzyme a metabolism via inhibition of mitochondrial complex II and methylmalonyl-coa mutase. Metabolites 2021, 11, 117. [Google Scholar] [CrossRef]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.C.; Griss, T.; et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Thompson, E.; Bates, L.; Pither, T.L.; Hosgood, S.A.; Nicholson, M.L.; Watson, C.J.E.; Wilson, C.; Fisher, A.J.; Ali, S.; et al. Flavin Mononucleotide as a Biomarker of Organ Quality—A Pilot Study. Transplant. Direct 2020, 6, E600. [Google Scholar] [CrossRef]
- Eden, J.; Thorne, A.M.; Bodewes, S.B.; Patrono, D.; Roggio, D.; Breuer, E.; Lonati, C.; Dondossola, D.; Panayotova, G.; Boteon, A.P.C.S.; et al. Assessment of liver graft quality during hypothermic oxygenated perfusion: The first international validation study. J. Hepatol. 2025, 82, 523–534. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 2020, 60, 103014. [Google Scholar] [CrossRef]
- Bejaoui, M.; Slim, C.; Peralta, C.; ben Abdennebi, H. Effect of PERLA®, a new cold-storage solution, on oxidative stress injury and early graft function in rat kidney transplantation model. BMC Nephrol. 2024, 25, 62. [Google Scholar] [CrossRef]
- Vreugdenhil, P.K.; Belzer, F.O.; Southard, J.H. Effect of cold storage on tissue and cellular glutathione. Cryobiology 1991, 28, 143–149. [Google Scholar] [CrossRef]
- van Breussegem, A.; van Pelt, J.; Wylin, T.; Heedfeld, V.; Zeegers, M.; Monbaliu, D.; Pirenne, J.; Vekemans, K. Presumed and Actual Concentrations of Reduced Glutathione in Preservation Solutions. Transplant. Proc. 2011, 43, 3451–3454. [Google Scholar] [CrossRef]
- Polyak, M.M.; Arrington, B.O.; Kapur, S.; Stubenbord, W.T.; Kinkhabwala, M. Glutathione supplementation during cold ischemia does not confer early functional advantage in renal transplantation. Transplantation 2000, 70, 202–205. [Google Scholar]
- Tambyraja, A.L.; Mitchell, R.; Driscoll, P.J.; Deans, C.; Parks, R.W.; Rahman, I.; Megson, I.L. Glutathione supplementation to University of Wisconsin solution causes endothelial dysfunction. Transpl. Immunol. 2007, 18, 146–150. [Google Scholar] [CrossRef]
- van Rijn, R.; Schurink, I.J.; de Vries, Y.; van den Berg, A.P.; Cortes Cerisuelo, M.; Darwish Murad, S.; Erdmann, J.I.; Gilbo, N.; de Haas, R.J.; Heaton, N.; et al. Hypothermic Machine Perfusion in Liver Transplantation—A Randomized Trial. N. Engl. J. Med. 2021, 384, 1391–1401. [Google Scholar] [CrossRef]
- Azarkish, F.; Nematbakhsh, M.; Fazilati, M.; Talebi, A.; Asghar Pilehvarian, A.; Pezeshki, Z.; Moeini, M.; Mansouri, A.; Safari, T. N-acetylcysteine Prevents Kidney and Lung Disturbances in Renal Ischemia/Reperfusion Injury in Rat. Int. J. Prev. Med. 2013, 4, 1139–1146. [Google Scholar] [PubMed]
- Jia, D.; Guo, S.; Jia, Z.; Gao, Z.; You, K.; Gong, J.; Li, S. N-acetylcysteine in the Donor, Recipient, or Both Donor and Recipient in Liver Transplantation: A Systematic Review With Meta-analysis and Trial Sequential Analysis. Transplantation 2023, 107, 1976–1990. [Google Scholar] [CrossRef] [PubMed]
- Radajewska, A.; Szyller, J.; Krzywonos-Zawadzka, A.; Olejnik, A.; Sawicki, G.; Bil-Lula, I. Mitoquinone Alleviates Donation after Cardiac Death Kidney Injury during Hypothermic Machine Perfusion in Rat Model. Int. J. Mol. Sci. 2023, 24, 14772. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, N.; Campbell, L.H.; Marine, A.; Brockbank, K.G.M.; Macmillan-Crow, L.A. MitoQ blunts mitochondrial and renal damage during cold preservation of porcine kidneys. PLoS ONE 2012, 7, e48590. [Google Scholar] [CrossRef]
- Dare, A.J.; Bolton, E.A.; Pettigrew, G.J.; Bradley, J.A.; Saeb-Parsy, K.; Murphy, M.P. Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 2015, 5, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Dare, A.J.; Logan, A.; Prime, T.A.; Rogatti, S.; Goddard, M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J.; Murphy, M.P.; Saeb-Parsy, K. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J. Heart Lung Transplant. 2015, 34, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, N.; Križanac, M.; Kumrić, M.; Vukojević, K.; Božić, J. Mitochondrial Dysfunction: The Silent Catalyst of Kidney Disease Progression. Cells 2025, 14, 794. [Google Scholar] [CrossRef]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Lu, S.; Wang, C.; Ke, S.; Li, X.; Yin, B.; Yu, H.; Zhou, M.; Pan, S.; et al. Integrative analysis of the roles of lncrnas and mrnas in itaconate-mediated protection against liver ischemia-reperfusion injury in mice. J. Inflamm. Res. 2021, 14, 4519–4536. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cai, J.; Li, C.; Yang, M.; Duan, T.; Zhao, Q.; Xi, Y.; Sun, L.; He, L.; Tang, C.; et al. 4-Octyl itaconate attenuates LPS-induced acute kidney injury by activating Nrf2 and inhibiting STAT3 signaling. Mol. Med. 2023, 29, 58. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Yang, W.; Yin, Y.; Li, C.; Ma, X.; Shi, L.; Li, R.; Tao, K. 4-Octyl itaconate regulates immune balance by activating Nrf2 and negatively regulating PD-L1 in a mouse model of sepsis. Int. J. Biol. Sci. 2022, 18, 6189–6209. [Google Scholar] [CrossRef]
- Yang, J.; Duan, C.; Wang, P.; Zhang, S.; Gao, Y.; Lu, S.; Ji, Y. 4-Octyl Itaconate Alleviates Myocardial Ischemia-Reperfusion Injury Through Promoting Angiogenesis via ERK Signaling Activation. Adv. Sci. 2025, 12, 2411554. [Google Scholar] [CrossRef]
- Carrillo-Garmendia, A.; Madrigal-Perez, L.A.; Regalado-Gonzalez, C. The multifaceted role of quercetin derived from its mitochondrial mechanism. Mol. Cell. Biochem. 2024, 479, 1985–1997. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Samarghandian, S.; Hushmandi, K.; Zabolian, A.; Shahinozzaman, M.; Saleki, H.; Esmaeili, H.; Raei, M.; Entezari, M.; Zarrabi, A.; et al. Quercetin in Attenuation of Ischemic/Reperfusion Injury: A Review. Curr. Mol. Pharmacol. 2020, 14, 537–558. [Google Scholar] [CrossRef]
- Marcolin, E.; San-Miguel, B.; Vallejo, D.; Tieppo, J.; Marroni, N.; González-Gallego, J.; Tuñón, M.J. Quercetin treatment ameliorates inflammation and fibrosis in mice with nonalcoholic steatohepatitis. J. Nutr. 2012, 142, 1821–1828. [Google Scholar] [CrossRef]
- Otani, M.; Ishii, D.; Iwata, H.; Satake, Y.; Okada, Y.; Toriumi, A.; Imamura, M.; Nishikawa, Y.; Matsuno, N. Preservation Efficacy of a Quercetin and Sucrose Solution for Warm Ischemically Damaged Porcine Liver Grafts. Transplant. Proc. 2023, 55, 2212–2217. [Google Scholar] [CrossRef]
- Ishii, D.; Toriumi, A.; Obara, H.; Satake, Y.; Okada, Y.; Dewa, H.; Nishikawa, Y.; Matsuno, N. Protective effects of quercetin and sucrose on cold-preservation 2 injury in porcine kidneys donated after cardiac death 3. Preprints 2024. [Google Scholar] [CrossRef]
- Gochi, M.; Kato, F.; Toriumi, A.; Kawagoe, T.; Yotsuya, S.; Ishii, D.; Otani, M.; Nishikawa, Y.; Furukawa, H.; Matsuno, N. A Novel Preservation Solution Containing Quercetin and Sucrose for Porcine Kidney Transplantation. Transplant. Direct 2020, 6, E624. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Hardenberg, M.C.; Kok, W.F.; Boerema, A.S.; Carey, H.v.; Staples, J.F.; Henning, R.H.; Bouma, H.R. Renal Mitochondrial Response to Low Temperature in Non-Hibernating and Hibernating Species. Antioxid. Redox Signal. 2017, 27, 599–617. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Bornbaum, J.; Boengler, K.; Torregrossa, R.; Whiteman, M.; Wood, M.E.; Schulz, R.; Baxter, G.F. AP39, a mitochondria-targeting hydrogen sulfide (H2 S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. Br. J. Pharmacol. 2017, 174, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, G.J.; Juriasingani, S.; Richard-Mohamed, M.; Rasmussen, A.; Levine, M.; Liu, W.; Haig, A.; Whiteman, M.; Arp, J.; Luke, P.P.W.; et al. Static Cold Storage with Mitochondria-Targeted Hydrogen Sulfide Donor Improves Renal Graft Function in an Ex Vivo Porcine Model of Controlled Donation-after-Cardiac-Death Kidney Transplantation. Int. J. Mol. Sci. 2023, 24, 14017. [Google Scholar] [CrossRef]
- Wu, D.; Wang, J.; Li, H.; Xue, M.; Ji, A.; Li, Y. Role of hydrogen sulfide in ischemia-reperfusion injury. Oxidative Med. Cell. Longev. 2015, 2015, 186908. [Google Scholar] [CrossRef]
- Goesch, T.R.; Wilson, N.A.; Zeng, W.; Verhoven, B.M.; Zhong, W.; Coumbe Gitter, M.M.; Fahl, W.E. Suppression of inflammation-associated kidney damage post-transplant using the new prc-210 free radical scavenger in rats. Biomolecules 2021, 11, 1054. [Google Scholar] [CrossRef]
- Verhoven, B.M.; Karim, A.S.; Bath, N.M.; Sarabia Fahl, C.J.; Wilson, N.A.; Redfield, R.R.; Fahl, W.E. Significant Improvement in Rat Kidney Cold Storage Using UW Organ Preservation Solution Supplemented with the Immediate-Acting PrC-210 Free Radical Scavenger. Transplant. Direct 2020, 6, e578. [Google Scholar] [CrossRef]
- Bath, N.M.; Fahl, W.E.; Redfield, R.R. Significant reduction of murine renal ischemia-reperfusion cell death using the immediate-acting PrC-210 reactive oxygen species scavenger. Transplant. Direct 2019, 5, e469. [Google Scholar] [CrossRef]
- Ries, W.P.; Marie, Y.; Patel, K.; Turnbull, C.; Smith, T.B.; Jamil, N.S.M.; Caldwell, H.; Telfer, R.; Neil, D.A.H.; Nath, J.; et al. A simple ex vivo model of human renal allograft preservation using the gonadal vein. Ann. R. Coll. Surg. Engl. 2019, 101, 609–616. [Google Scholar] [CrossRef]
- Chen-Yoshikawa, T.F. Ischemia–reperfusion injury in lung transplantation. Cells 2021, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Cartier, R.; Hollmann, C.; Dagenais, F.; Buluran, J.; Pellerin, M.; Leclerc, Y. Effects of University of Wisconsin solution on endothelium-dependent coronary artery relaxation in the rat. Ann. Thorac. Surg. 1993, 55, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Eberl, T.; Salvenmoser, W.; Rieger, G.; Gorny, I.; Heiß, V.; Kumpitsch, B.; Gnaiger, E.; Margreiter, R. Ultrastructural Analysis of Human Endothelial Cells after Hypothermic Storage in Organ Preservation Solutions. J. Surg. Res. 1999, 82, 253–260. [Google Scholar] [CrossRef]
- Bhogal, R.H.; Mergental, H.; Mirza, D.F.; Afford, S.C. The Emerging Importance of Liver Sinusoidal Endothelial Cells in Regulating Injury during Machine Perfusion of Deceased Liver Donors. Semin. Liver Dis. 2018, 38, 252–259. [Google Scholar] [CrossRef]
- Layton, G.R.; Ladak, S.S.; Abbasciano, R.; McQueen, L.W.; George, S.J.; Murphy, G.J.; Zakkar, M. The Role of Preservation Solutions upon Saphenous Vein Endothelial Integrity and Function: Systematic Review and UK Practice Survey. Cells 2023, 12, 815. [Google Scholar] [CrossRef]
- Houtzager, J.H.E.; van Stalborch, A.-M.; Hofstee, C.; van Gulik, T.M.; van Buul, J.D. Protection of liver sinusoidal endothelial cells using different preservation solutions. Vasc. Biol. 2023, 7, e240004. [Google Scholar] [CrossRef] [PubMed]
- Ćurko-Cofek, B.; Jenko, M.; Taleska Stupica, G.; Batičić, L.; Krsek, A.; Batinac, T.; Ljubačev, A.; Zdravković, M.; Knežević, D.; Šoštarič, M.; et al. The Crucial Triad: Endothelial Glycocalyx, Oxidative Stress, and Inflammation in Cardiac Surgery-Exploring the Molecular Connections. Int. J. Mol. Sci. 2024, 25, 10891. [Google Scholar] [CrossRef]
- Kojima, H.; Hirao, H.; Kadono, K.; Ito, T.; Yao, S.; Torgerson, T.; Dery, K.J.; Kitajima, H.; Ogawa, T.; Kaldas, F.M.; et al. Cold stress-induced ferroptosis in liver sinusoidal endothelial cells determines liver transplant injury and outcomes. JCI Insight. 2024, 9, e174354. [Google Scholar] [CrossRef]
- Muller, X.; Rossignol, G.; Couillerot, J.; Breton, A.; Hervieu, V.; Lesurtel, M.; Mohkam, K.; Mabrut, J.Y. A Single Preservation Solution for Static Cold Storage and Hypothermic Oxygenated Perfusion of Marginal Liver Grafts: A Preclinical Study. Transplantation 2024, 108, 175–183. [Google Scholar] [CrossRef]
- Parente, A.; Flores Carvalho, M.; Schlegel, A. Endothelial Cells and Mitochondria: Two Key Players in Liver Transplantation. Int. J. Mol. Sci. 2023, 24, 10091. [Google Scholar] [CrossRef]
- Panconesi, R.; Flores Carvalho, M.; Dondossola, D.; Muiesan, P.; Dutkowski, P.; Schlegel, A. Impact of Machine Perfusion on the Immune Response After Liver Transplantation–A Primary Treatment or Just a Delivery Tool. Front. Immunol. 2022, 13, 855263. [Google Scholar] [CrossRef]
- Nannelli, G.; Terzuoli, E.; Giorgio, V.; Donnini, S.; Lupetti, P.; Giachetti, A.; Bernardi, P.; Ziche, M. ALDH2 Activity Reduces Mitochondrial Oxygen Reserve Capacity in Endothelial Cells and Induces Senescence Properties. Oxidative Med. Cell. Longev. 2018, 2018, 9765027. [Google Scholar] [CrossRef]
- Vekemans, K.; van Pelt, J.; Komuta, M.; Wylin, T.; Heedfeld, V.; Detry, O.; Monbaliu, D.; Pirenne, J. Attempt to rescue discarded human liver grafts by end ischemic hypothermic oxygenated machine perfusion. Transplant. Proc. 2011, 43, 3455–3459. [Google Scholar] [CrossRef]
- Parris, C.L.; Liu, C.; Rani, A.; Tran, M.H.; Li, M.; Esquivel, C.; Oropeza, A.M.; Wang, L. Macula Densa Nitric Oxide Synthase 1β Restoration by Kidney Alkalization Enhances Renal Graft Outcomes. Am. J. Physiol. Ren. Physiol. 2025, 329, F347–F361. [Google Scholar] [CrossRef] [PubMed]
- Maida, K.; Akamatsu, Y.; Hara, Y.; Tokodai, K.; Miyagi, S.; Kashiwadate, T.; Miyazawa, K.; Kawagishi, N.; Ohuchi, N. Short oxygenated warm perfusion with prostaglandin e1 administration before cold preservation as a novel resuscitation method for liver grafts from donors after cardiac death in a rat in vivo model. Transplantation 2016, 100, 1052–1058. [Google Scholar] [CrossRef]
- Polyak, M.M.R.; Phil, M.; O’, B.; Arrington, M.; Stubenbord, W.T.; Kapur, S.; Kinkhabwala, M. Prostaglandin E 1 Influences Pulsatile Preservation Characteristics and Early Graft Function in Expanded Criteria Donor Kidneys. J. Surg. Res. 1999, 85, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Risbey, C.W.G.; Thomas, C.; Niu, A.; Liu, K.; Crawford, M.; Pulitano, C. Hypothermic Oxygenated machine PErfusion for high-risk liver grafts for transplantation: A systematic review and meta-analysis. Artif. Organs. 2024, 48, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Boteon, Y.L.; Martins, P.N.; Muiesan, P.; Schlegel, A. Machine perfusion of the liver: Putting the puzzle pieces together. World J. Gastroenterol. 2021, 27, 5727–5736. [Google Scholar] [CrossRef] [PubMed]
- Padma, A.M.; Truong, M.L.; Jar-Allah, T.; Song, M.J.; Oltean, M.; Brännström, M.; Hellström, M. The development of an extended normothermic ex vivo reperfusion model of the sheep uterus to evaluate organ quality after cold ischemia in relation to uterus transplantation. Acta Obstet. Et Gynecol. Scand. 2019, 98, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, Y.; Zhang, X.; Zhang, Y.; Hua, K. Morphologic assessment of hypertonic citrate adenine, histidine–tryptophan–ketoglutarate, and university of Wisconsin solutions for hypothermic uterus preservation in rats. J. Obstet. Gynaecol. Res. 2021, 47, 1097–1109. [Google Scholar] [CrossRef]
- Sousa, C.H.; Mercier, M.; Rioux-Leclercq, N.; Flecher, E.; Bendavid, C.; Val-Laillet, D.; Ferrant, J.; Jaillard, S.; Loiseau, E.; Branchereau, J.; et al. Hypothermic machine perfusion in uterus transplantation in a porcine model: A proof of concept and the first results in graft preservation. Acta Obstet. Et Gynecol. Scand. 2025, 104, 461–473. [Google Scholar] [CrossRef]
- Fontes, P.A. The Evolution of Oxygen Carrier Solutions for Machine Perfusion. Transplantation 2017, 101, 2657–2658. [Google Scholar] [CrossRef]
- Saemann, L.; Wächter, K.; Gharpure, N.; Pohl, S.; Hoorn, F.; Korkmaz-Icöz, S.; Karck, M.; Veres, G.; Simm, A.; Szabó, G. HTK vs. HTK-N for Coronary Endothelial Protection during Hypothermic, Oxygenated Perfusion of Hearts Donated after Circulatory Death. Int. J. Mol. Sci. 2024, 25, 2262. [Google Scholar] [CrossRef]
- Elzawahry, M.A.; Reichman, T.; Sutherland, A. New methods for improving pancreas preservation. Curr. Opin. Organ Transplant. 2025, 30, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Matar, A.J.; Rao, J.S.; Kandaswamy, R. Novel techniques of pancreas and islet preservation. Curr. Opin. Organ Transplant. 2025, 30, 330–336. [Google Scholar] [CrossRef]
- Oltean, M.; Churchill, T.A. Organ-specific solutions and strategies for the intestinal preservation. Int. Rev. Immunol. 2014, 33, 234–244. [Google Scholar] [CrossRef]
- Bardallo, R.G.; Chullo, G.; Alva, N.; Rosello-Catafau, J.; Fundora-Suárez, Y.; Carbonell, T.; Panisello-Rosello, A. Mitigating Cold Ischemic Injury: HTK, UW and IGL-2 Solution’s Role in Enhancing Antioxidant Defence and Reducing Inflammation in Steatotic Livers. Int. J. Mol. Sci. 2024, 25, 9318. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yang, J.; Fu, S.; Zhao, H. Application of ex vivo lung perfusion (EVLP) in lung transplantation. J. Thorac. Dis. 2018, 10, 4637–4642. [Google Scholar] [CrossRef]
- Mesnard, B.; Ogbemudia, E.; Bruneau, S.; le Bas-Bernardet, S.; Minault, D.; Hervouet, J.; Kervella, D.; Masset, C.; Cantarovich, D.; Rigaud, J.; et al. Pancreas Preservation: Hypothermic Oxygenated Perfusion to Improve Graft Reperfusion. Transplantation 2025, 109, e1–e10. [Google Scholar] [CrossRef]
- Hou, W.; Yang, S.; Lu, J.; Shi, Y.; Chen, J.; Chen, D.; Wang, F.; Liu, L. Hypothermic machine perfusion alleviates ischemia-reperfusion injury of intestinal transplantation in pigs. Front. Immunol. 2023, 14, 1117292. [Google Scholar] [CrossRef]
- Roskott, A.M.C.; Nieuwenhuijs, V.B.; Dijkstra, G.; Koudstaal, L.G.; Leuvenink, H.G.D.; Ploeg, R.J. Small bowel preservation for intestinal transplantation: A review. Transpl. Int. 2011, 24, 107–131. [Google Scholar] [CrossRef]
- Galeone, A.; Grano, M.; Brunetti, G. Tumor Necrosis Factor Family Members and Myocardial Ischemia-Reperfusion Injury: State of the Art and Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 4606. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.W.; Bhogal, R.H.; Wallace, L.; Boteon, Y.; Neil, D.A.H.; Smith, A.; Stephenson, B.T.F.; Schlegel, A.; Hübscher, S.G.; Mirza, D.F.; et al. The Use of an Acellular Oxygen Carrier in a Human Liver Model of Normothermic Machine Perfusion. Transplantation 2017, 101, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Swaminathan, S.; Bachman, L.A.; Croatt, A.J.; Nath, K.A.; Griffin, M.D. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007, 71, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.Y.; Cheng, J.Y.; Ge, W.Y.; Tong, Y.M.; Yin, D.C. Revolution in Organ Preservation: Technological Exploration. Acta Biomater. 2025, 199, 50–73. [Google Scholar] [CrossRef]
- del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
| Additive | Mechanism of Action | Model & Context | EC-Specific Effects | References |
|---|---|---|---|---|
| PEG-35 | Oncotic agent, stabilizes membrane and glycocalyx | IGL-1; Liver and Kidney SCS/HMP | Preserves endothelial ultrastructure and glycocalyx integrity | [2,8] |
| Quercetin | Antioxidant, anti-inflammatory (NF-κB, ICAM-1 inhibition), ERG upregulation | Kidney SCS | ↓ ICAM-1, ↑ ERG, ↓ platelet adhesion | [55] |
| NO donors | Vasodilation, anti-inflammatory, ICAM-1/eNOS modulation | Hypothermic machine perfusion (HMP) | ↓ ICAM-1, stabilized sinusoidal perfusion | [32,78] |
| A. Static Cold Storage (SCS): Solutions Mapped Across Organs | ||||||||
| Organ | UW | HTK | IGL-1 | IGL-2 | Celsior | Custodiol-N | Perfadex | Key Considerations |
| Liver | ✓ | ✓ | ✓ | ✓ | ✓ | High metabolic rate, RET-sensitive; bile duct injury risk [19,32] | ||
| Kidney | ✓ | ✓ | ✓ | Mitochondrial IRI, edema sensitivity [3] | ||||
| Heart | ✓ | ✓ | ✓ | High O2 demand, edema, redox stress [88] | ||||
| Lung | ✓ | Barrier disruption, edema; Perfadex standard [66] | ||||||
| Pancreas | ✓ | ✓ | ✓ | Islet preservation, acidosis risk [89,90] | ||||
| Intestine | ✓ | ✓ | Barrier & mucosal integrity critical [91] | |||||
| Uterus | ✓ | ✓ | ✓ | Cold ischemia impairs contractility [84,85] | ||||
| B. Machine Perfusion (MP): Solutions Mapped Across Organs | ||||||||
| Organ | Belzer MPS | Custodiol-MP | Steen | RBC-Based | Key Considerations | |||
| Liver | ✓ | ✓ | HOPE slows RET; NMP for bile viability [92] | |||||
| Kidney | ✓ | ✓ | ✓ | FMN-guided HOPE; DGF reduction [3] | ||||
| Heart | ✓ | ✓ | Contractility + lactate clearance [7] | |||||
| Lung | ✓ | ✓ | EVLP for microbe clearance, function [93] | |||||
| Pancreas | ✓ | ✓ | Islet yield & acinar injury studies [94] | |||||
| Intestine | ✓ (exp.) | ✓ (exp.) | Lactate trend & mucosal barrier [95,96] | |||||
| Uterus | ✓ (exp.) | ✓ (exp.) | Vasoreactivity under investigation [58] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, F.W.; Yildirim, F.S.; Horie, H.; Karakaya, O.F.; Jiao, C.; Crasta, G.S.; Eshraghi, N.; Takase, K.; Diwan, T.; Batista de Oliveira, L.; et al. Beyond Static Cold Storage: Toward the Next Generation of Tailored Organ Preservation Solutions. Int. J. Mol. Sci. 2025, 26, 9515. https://doi.org/10.3390/ijms26199515
Fernandes FW, Yildirim FS, Horie H, Karakaya OF, Jiao C, Crasta GS, Eshraghi N, Takase K, Diwan T, Batista de Oliveira L, et al. Beyond Static Cold Storage: Toward the Next Generation of Tailored Organ Preservation Solutions. International Journal of Molecular Sciences. 2025; 26(19):9515. https://doi.org/10.3390/ijms26199515
Chicago/Turabian StyleFernandes, Fernanda W., Fatma Selin Yildirim, Hiroshi Horie, Omer F. Karakaya, Chunbao Jiao, Geofia S. Crasta, Nasim Eshraghi, Koki Takase, Tobias Diwan, Laura Batista de Oliveira, and et al. 2025. "Beyond Static Cold Storage: Toward the Next Generation of Tailored Organ Preservation Solutions" International Journal of Molecular Sciences 26, no. 19: 9515. https://doi.org/10.3390/ijms26199515
APA StyleFernandes, F. W., Yildirim, F. S., Horie, H., Karakaya, O. F., Jiao, C., Crasta, G. S., Eshraghi, N., Takase, K., Diwan, T., Batista de Oliveira, L., Miller, C., Wehrle, C. J., Satish, S., Sun, K., Matsuno, N., & Schlegel, A. (2025). Beyond Static Cold Storage: Toward the Next Generation of Tailored Organ Preservation Solutions. International Journal of Molecular Sciences, 26(19), 9515. https://doi.org/10.3390/ijms26199515





