Gibberellin Disrupts Hormonal Homeostasis and Anther Integrity to Trigger Sex Reversal in Spinach
Abstract
1. Introduction
2. Results
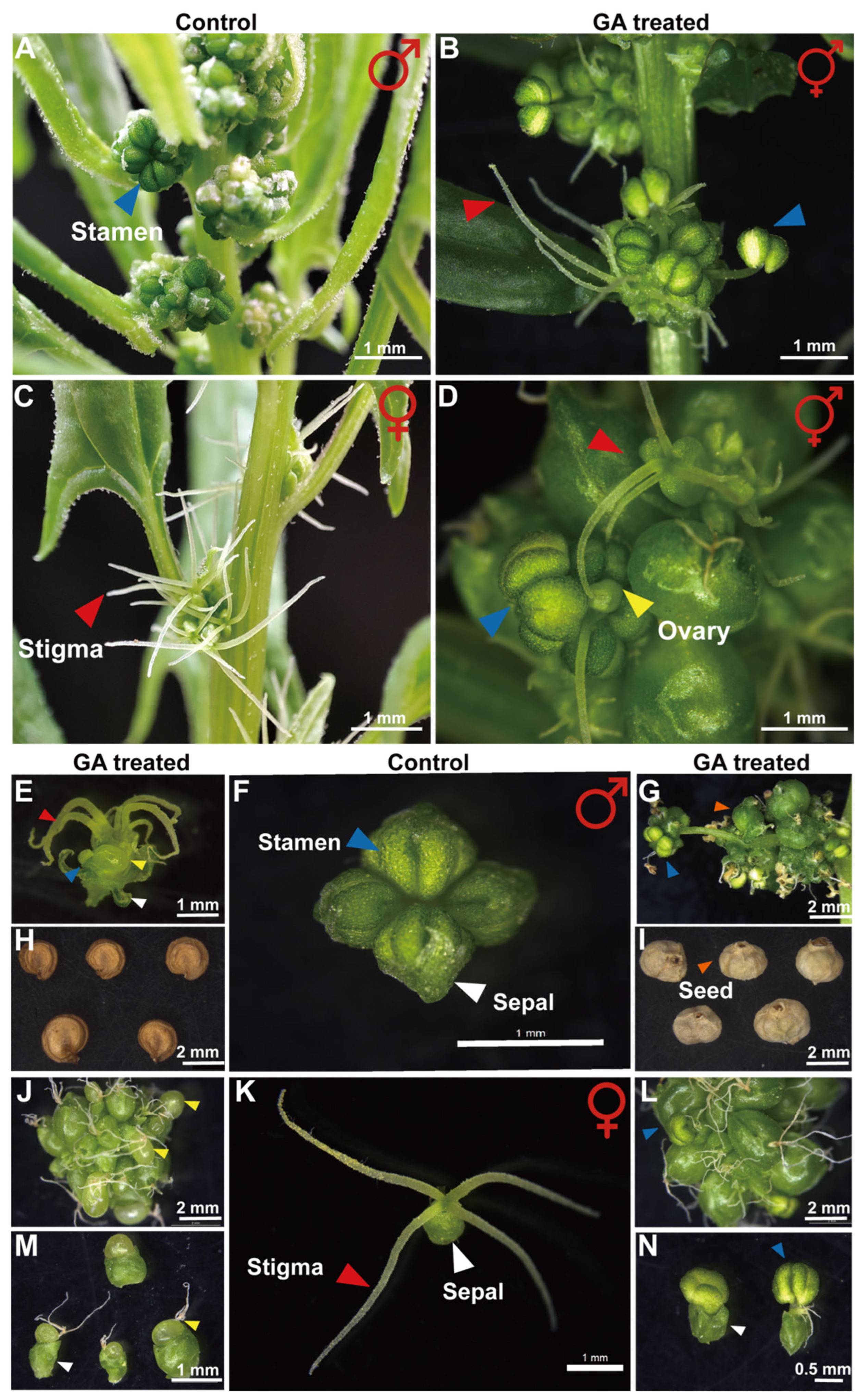
2.1. Gibberellin Plays a Dual Role in Regulating Spinach Flower Phenotype
2.2. Transcriptome Analysis and Endogenous Hormone Measurement to Identify Differentially Expressed Genes Associated with Stamen-to-Carpel Conversion in Dioecious Spinach
2.2.1. Specific Alterations in Gibberellin and Jasmonate Profiles in Feminized Flowers
2.2.2. GA Treatment Synergistically Enhances Cytokinin Metabolism
2.2.3. Activation of Auxin and Abscisic Acid Pathways and Suppression of Ethylene Biosynthesis
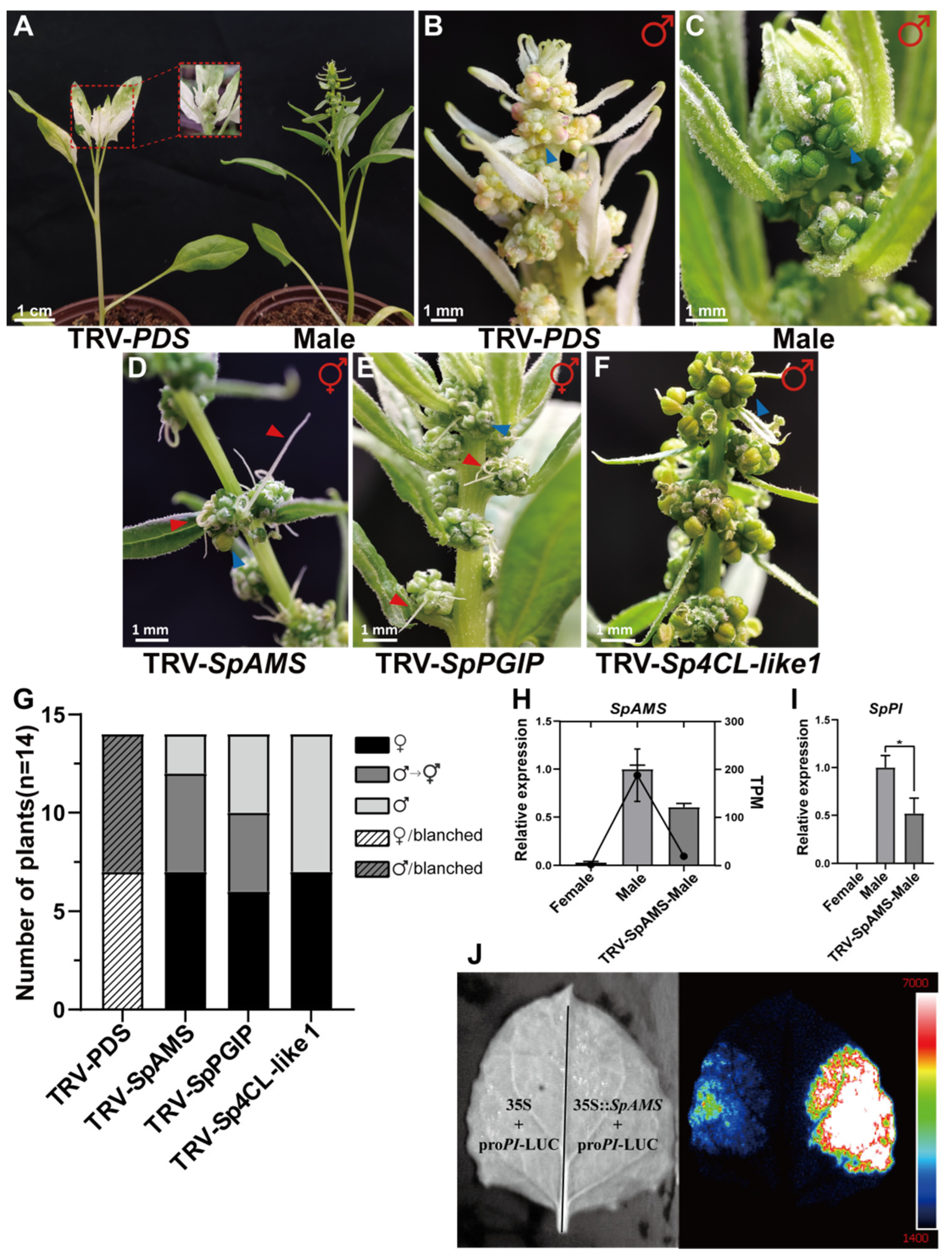
2.2.4. Functional Validation of Candidate Genes Through Virus-Induced Gene Silencing (VIGS)
2.3. In Situ Localization of SpAMS and SpPGIP Genes
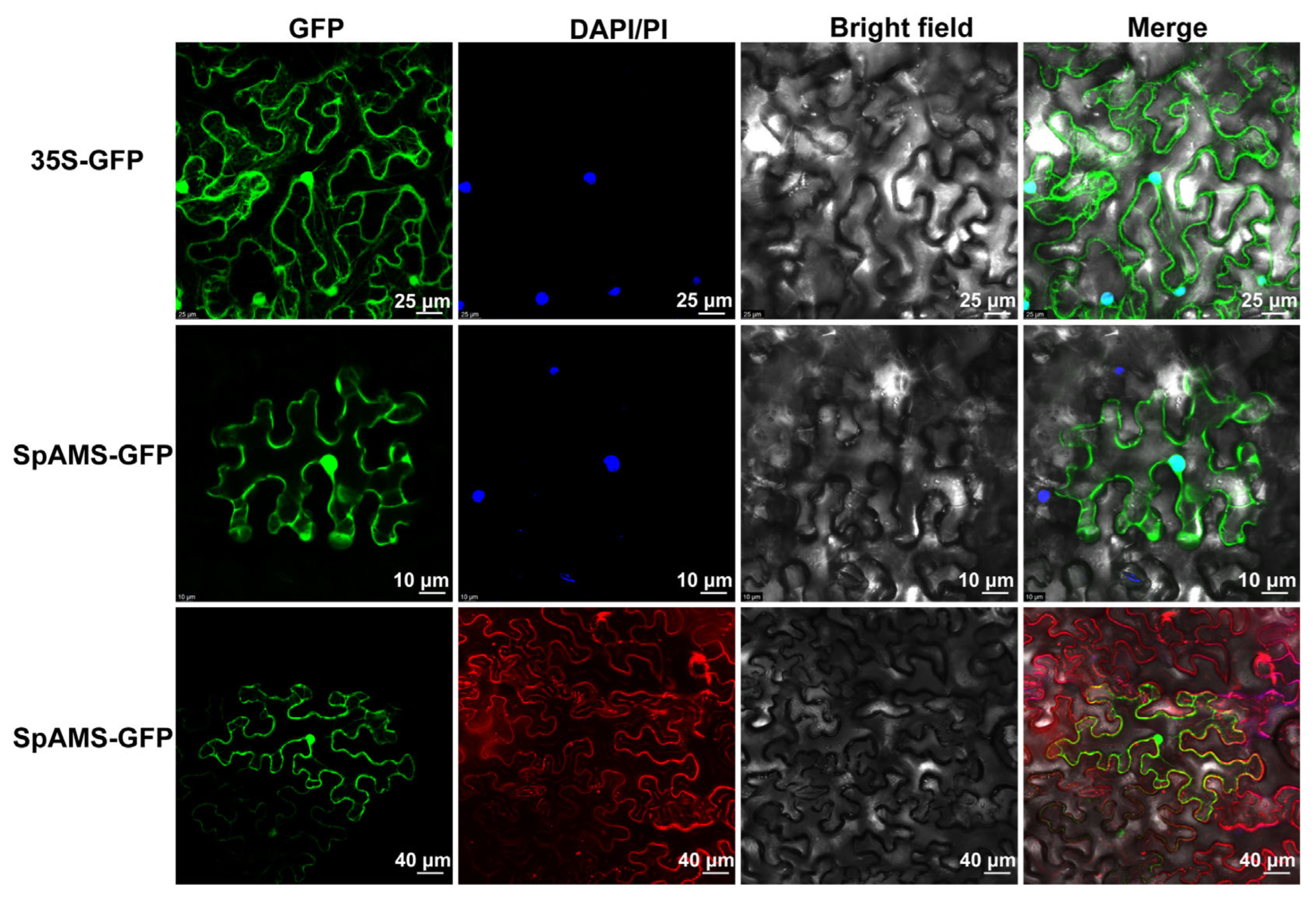
2.4. Subcellular Localization Analysis of SpAMS and SpPGIP
2.5. Proposed Regulatory Model of GA Induced Stamen Carpelization in Spinach
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Gibberellin Treatment and Tissues Collection
4.3. Molecular Sex Verification
4.4. RNA Extraction, cDNA Library Construction, Transcriptome Sequencing, and Data Quality Assessment
4.5. Endogenous Hormone Profiling
4.6. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-qPCR) Validation
4.7. In Situ Hybridization
4.8. Subcellular Localization
4.9. Virus-Induced Gene Silencing (VIGS)
4.10. Dual Luciferase In Vivo Imaging Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Renner, S.S. The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 2014, 101, 1588–1596. [Google Scholar] [CrossRef]
- Qin, L.; Chen, J.L.; Pan, C.T.; Ye, L.; Lu, G. Advances in the study of plant sex chromosome evolution and sex-determining genes. Chin. Bull. Bot. 2016, 51, 841–848. (In Chinese) [Google Scholar]
- Gao, W.J.; Ji, Y.K.; Xiao, L.H.; Hong, D.; Deng, C.; Lu, L. Advances in the study of functional genes related to sex determination in dioecious plants. Plant Genet. Resour. 2008, 9, 125–129. (In Chinese) [Google Scholar] [CrossRef]
- Bai, S.-N. Plant sex: The evolutionary consequence of a dynamic interaction between genes, hormones, and environment. Front. Plant Sci. 2021, 12, 667858. [Google Scholar]
- Zhang, W.; Wang, X.; Yu, R.; Ming, R. Divergent flowering time and floral morphology genes underlie the evolution of dioecy in kiwifruit. Plant J. 2022, 110, 1453–1466. [Google Scholar] [CrossRef]
- Ma, X.; Yu, L.; Fatima, M.; Wadlington, W.H.; Hulse-Kemp, A.M.; Zhang, X.; Zhang, S.; Xu, X.; Wang, J.; Huang, H.; et al. The spinach YY genome reveals sex chromosome evolution, domestication, and introgression history of the species. Genome Biol. 2022, 23, 75. [Google Scholar] [CrossRef]
- Ming, R.; Bendahmane, A.; Renner, S.S. Sex Chromosomes in Land Plants. Annu. Rev. Plant Biol. 2011, 62, 485–514. [Google Scholar] [CrossRef]
- Charlesworth, D. Plant sex chromosome evolution. J. Exp. Bot. 2013, 64, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, D. Plant sex determination and sex chromosomes. Heredity 2002, 88, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Theißen, G.; Melzer, R.; Rümpler, F. MADS-domain transcription factors and the floral quartet model of flower development: Linking plant development and evolution. Development 2016, 143, 3259–3271. [Google Scholar] [CrossRef]
- Sather, D.N.; York, A.; Pobursky, K.J.; Golenberg, E.M. Sequence evolution and sex-specific expression patterns of the C class floral identity gene, SpAGAMOUS, in dioecious Spinacia oleracea L. Planta 2005, 222, 284–292. Planta 2005, 222, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Pfent, C.; Pobursky, K.J.; Sather, D.N.; Golenberg, E.M. Characterization of SpAPETALA3 and SpPISTILLATA, B class floral identity genes in Spinacia oleracea, and their relationship to sexual dimorphism. Dev. Genes Evol. 2005, 215, 132–142. [Google Scholar] [CrossRef]
- Qian, W.; Fan, G.; Liu, D.; Zhang, H.; Wang, X.; Wu, J.; Xu, Z. Construction of a high-density genetic map and the X/Y sex-determining gene mapping in spinach based on large-scale markers developed by specific-locus amplified fragment sequencing (SLAF-seq). BMC Genom. 2017, 18, 276. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Sun, X.; Xu, C.; Zhang, Y.; Estep, M.; Li, Z.; Wang, F.; Li, Y.; Chen, X.; Zhang, X.; et al. Genomic analyses provide insights into spinach domestication and the genetic basis of agronomic traits. Nat. Commun. 2021, 12, 7246, Erratum in Nat. Commun. 2022, 13, 665. [Google Scholar] [CrossRef]
- She, H.; Liu, Z.; Li, S.; Xu, Z.; Zhang, H.; Cheng, F.; Wu, J.; Wang, X.; Deng, C.; Charlesworth, D.; et al. Evolution of the spinach sex-linked region within a rarely recombining pericentromeric region. Plant Physiol. 2023, 193, 1263–1280. [Google Scholar] [CrossRef]
- Chailakhyan, M.K.; Khryanin, V.N. Effect of growth regulators and role of roots in sex expression in spinach. Planta 1978, 142, 207–210. [Google Scholar] [CrossRef]
- Cao, Z.X. Control of spinach sex expression by gibberellin and ethephon and its relationship with isozymes. Acta Phytophysiol. Sin. 1980, 6, 149–156. (In Chinese) [Google Scholar]
- Qin, R.Y.; Wang, S.J.; Liu, X.X.; Du, Y.; Wang, B.; Li, Z.; Yan, N.; Li, S.; Gao, W.; Deng, C. Effect of gibberellin (GA3) on sex differentiation and molecular mechanism in spinach. J. Jiangsu Agric. Sci. 2017, 45, 133–135. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, L.Y.; Yang, Y.; Zhao, X.; Zhu, H.-W.; You, C.; Chen, N.; Wei, S.-J.; Li, S.-F.; Gao, W.-J. Gibberellins regulate masculinization through the SpGAI-SpSTM module in dioecious spinach. Plant J. 2024, 118, 1907–1921. [Google Scholar] [CrossRef]
- Li, N.; Wang, B.; Shang, X.; Yang, Q.; Yang, L.; Tao, M.; Muhammad, S.; Shi, A.; Deng, C. SpMS1, a Male Sterility Factor, Interacts with SpAP1 to Regulate Unisexual Flower Development in Dioecious Spinach. Plant Cell Physiol. 2025, 66, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Sather, D.N.; Jovanovic, M.; Golenberg, E.M. Functional analysis of B and C class floral organ genes in spinach demonstrates their role in sexual dimorphism. BMC Plant Biol. 2010, 10, 46. [Google Scholar] [CrossRef]
- Yu, L.; Ma, X.; Wadlington, W.; Ming, R. Identification of structural variation and polymorphisms of a sex co-segregating scaffold in spinach. Plant Reprod. 2022, 35, 19–30. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Wan, S.Y.; Yao, Y.; Zhao, Z.; Dong, F.; Ming, R.; Zhang, W. Development of sex-associated KASP markers in monoecious spinach. J. Shanxi Agric. Univ. (Nat. Sci. Ed.) 2022, 42, 29–36. (In Chinese) [Google Scholar] [CrossRef]
- Sherry, R.A.; Eckard, K.J.; Lord, E.M. Flower development in dioecious spinacia oleracea (chenopodiaceae). Am. J. Bot. 1993, 80, 283–291. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Zhang, L. Cloning, Bioinformatics Analysis, and Expression of Polygalacturonase-Inhibiting Protein Gene (Bnpgip2-1) in Brassica napus. Master’s Thesis, Yangzhou University, Yangzhou, China, 2012. (In Chinese). [Google Scholar]
- Sorensen, A.-M.; Kröber, S.; Unte, U.S.; Huijser, P.; Dekker, K.; Saedler, H. The arabidopsis ABORTED MICROSPORES (AMS) gene encodes a MYC class transcription factor. Plant J. 2003, 33, 413–423. [Google Scholar] [CrossRef] [PubMed]
- West, N.W.; Golenberg, E.M. Gender-specific expression of GIBBERELLIC ACID INSENSITIVE is critical for unisexual organ initiation in dioecious Spinacia oleracea. New Phytol. 2018, 217, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Chen, Y.; Yang, W.; Li, M.; Sun, B.; Song, H.; Tang, W.; Zhang, Y.; Gong, R. Joint metabolome and transcriptome analysis of the effects of exogenous GA3 on endogenous hormones in sweet cherry and mining of potential regulatory genes. Front. Plant Sci. 2022, 13, 1041068. [Google Scholar] [CrossRef]
- Davoudpour, Y.; Schmidt, M.; Calabrese, F.; Richnow, H.H.; Musat, N. High resolution microscopy to evaluate the efficiency of surface sterilization of zea mays seeds. PLoS ONE 2020, 15, e0242247. [Google Scholar] [CrossRef]
- Wadlington, W.H.; Ming, R. Development of an X-specific marker and identification of YY individuals in spinach. Theor. Appl. Genet. 2018, 131, 1987–1994. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Shou, L. Modified CTAB method for extracting genomic DNA from wheat leaf. Agric. Sci. Technol. 2013, 14, 946–949. [Google Scholar]
- He, L.; Liu, X.; Wu, Z.; Teng, N. Transcriptome and Metabolome Analyses Provide Insights into the Stomium Degeneration Mechanism in Lily. Int. J. Mol. Sci. 2021, 22, 12124. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kovaka, S.; Zimin, A.V.; Pertea, G.M.; Razaghi, R.; Salzberg, S.L.; Pertea, M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019, 20, 278. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jackson, D. In situ hybridization in plants. In Molecular Plant Pathology: A Practical Approach; Bowles, D.J., Gurr, S.J., McPherson, M., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 163–174. [Google Scholar]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 2005, 1, 13. [Google Scholar] [CrossRef]
- Floková, K.; Tarkowská, D.; Miersch, O.; Strnad, M.; Wasternack, C.; Novák, O. UHPLC-MS/MS based target profiling of stress-induced phytohormones. Phytochemistry 2014, 105, 147–157. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.; Yan, X.; Zhang, J.; Xu, J. Simultaneous analysis of ten phytohormones in sargassum horneri by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1804–1813. [Google Scholar] [CrossRef]
- Cai, B.D.; Zhu, J.X.; Gao, Q.; Luo, D.; Yuan, B.F.; Feng, Y.Q. Rapid and high-throughput determination of endogenous cytokinins in oryza sativa by bare Fe3O4 nanoparticles-based magnetic solid-phase extraction. J. Chromatogr. 2014, 1340, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Zong, Y.; Qian, M.; Yang, F.; Teng, Y. Simultaneous quantitative determination of major plant hormones in pear flowers and fruit by UPLC/ESI-MS/MS. Anal. Methods 2014, 6, 1766–1773. [Google Scholar] [CrossRef]
- Xiao, H.M.; Cai, W.J.; Ye, T.T.; Ding, J.; Feng, Y.Q. Spatio-temporal profiling of abscisic acid, indoleacetic acid and jasmonic acid in single rice seed during seed germination. Anal. Chim. Acta 2018, 1031, 119–127. [Google Scholar] [CrossRef]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant hormonomics: Multiple phytohormone profiling by targeted metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, J.; Han, W. Comparison of pretreatment, preservation and determination methods for foliar pH of plant samples. J. Plant Ecol. 2022, 15, 673–682. [Google Scholar] [CrossRef]
- Zhang, Y. Effects of Different Environmental Factors on the Growth, Development and Physiological Responses of Spinach. Master’s Thesis, Shanghai Normal University, Shanghai, China, 2020. (In Chinese). [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Khalid, E.; Mao, H.; Tong, Y.; Xue, X.; Tang, Y.; Cai, L.; Ming, R. Gibberellin Disrupts Hormonal Homeostasis and Anther Integrity to Trigger Sex Reversal in Spinach. Int. J. Mol. Sci. 2025, 26, 9505. https://doi.org/10.3390/ijms26199505
Wang T, Khalid E, Mao H, Tong Y, Xue X, Tang Y, Cai L, Ming R. Gibberellin Disrupts Hormonal Homeostasis and Anther Integrity to Trigger Sex Reversal in Spinach. International Journal of Molecular Sciences. 2025; 26(19):9505. https://doi.org/10.3390/ijms26199505
Chicago/Turabian StyleWang, Tengqi, Ehsan Khalid, Haoming Mao, Yihan Tong, Xinyu Xue, Yuru Tang, Lingmin Cai, and Ray Ming. 2025. "Gibberellin Disrupts Hormonal Homeostasis and Anther Integrity to Trigger Sex Reversal in Spinach" International Journal of Molecular Sciences 26, no. 19: 9505. https://doi.org/10.3390/ijms26199505
APA StyleWang, T., Khalid, E., Mao, H., Tong, Y., Xue, X., Tang, Y., Cai, L., & Ming, R. (2025). Gibberellin Disrupts Hormonal Homeostasis and Anther Integrity to Trigger Sex Reversal in Spinach. International Journal of Molecular Sciences, 26(19), 9505. https://doi.org/10.3390/ijms26199505





