Defective IgG Class Switching in the Spleen of TRAF5-Deficient Mice Reveals a Role for TRAF5 in CD40-Mediated B Cell Responses During Obesity-Associated Inflammation
Abstract
1. Introduction
2. Result
2.1. Elavated Splenic Expression of the Pro-Inflammatory Cytokine mRNAs Tnf (TNF-α) and Cd40lg (CD40L) Is Observed in Traf5−/− Mice Fed a High-Fat Diet
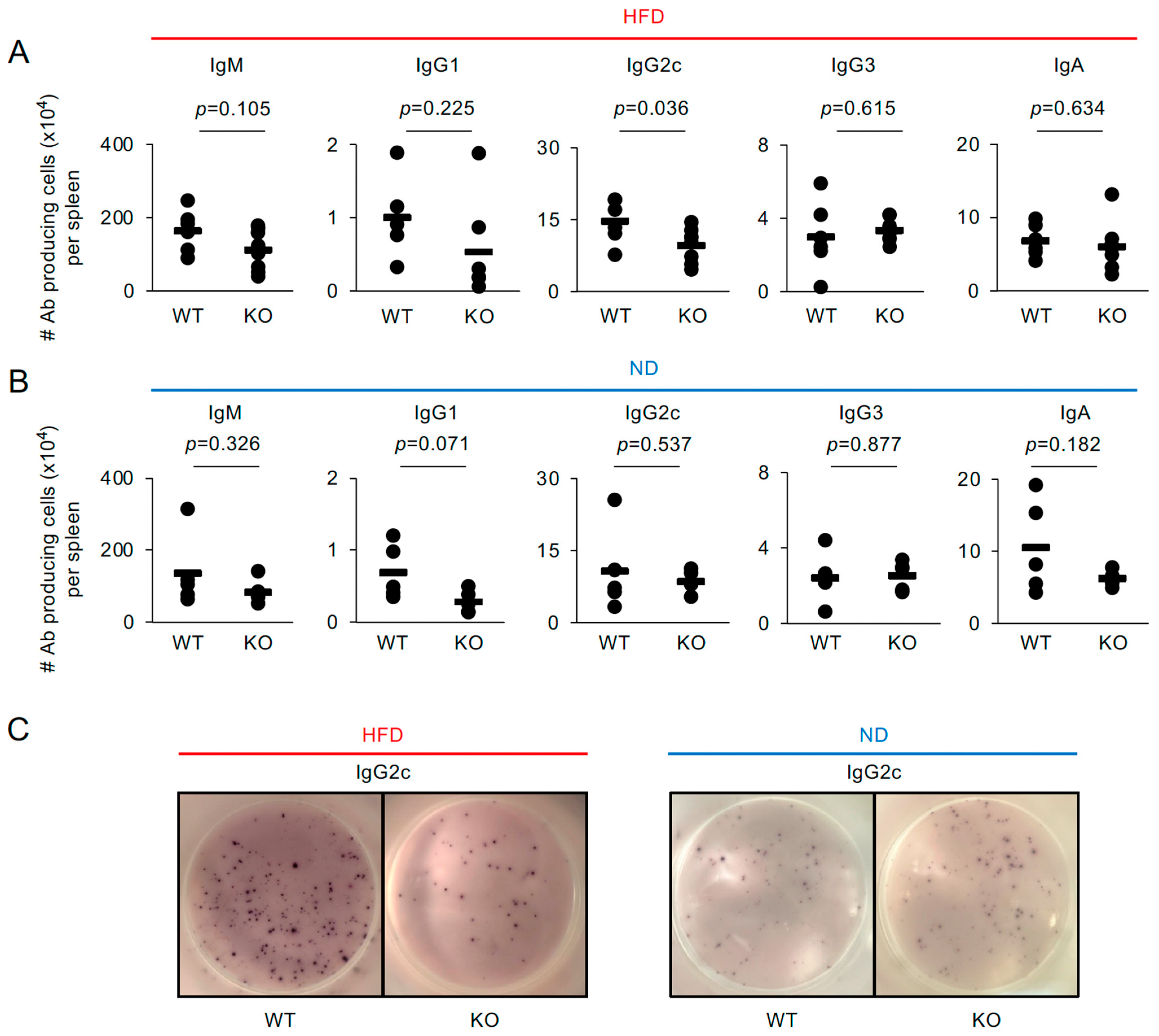
2.2. IgG2c-Producing Cells Are Decreased in the Spleens of Traf5−/− Mice Fed a High-Fat Diet
2.3. Splenic T Cell Phenotype in High-Fat Diet-Fed Traf5+/+ and Traf5−/− Mice
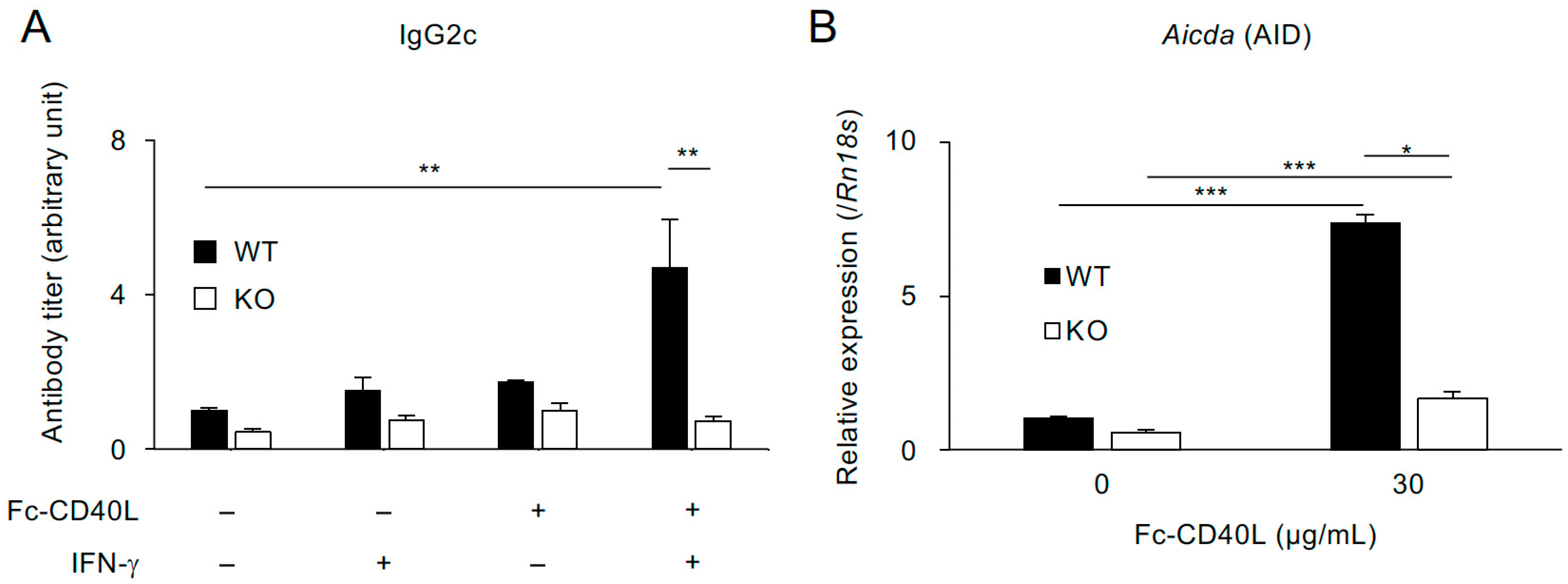
2.4. TRAF5 Promotes Aicda (AID) Expression and Class-Switch Recombination to IgG2c Mediated by Soluble CD40L and IFN-γ in B Cells from Normal-Diet-Fed Mice
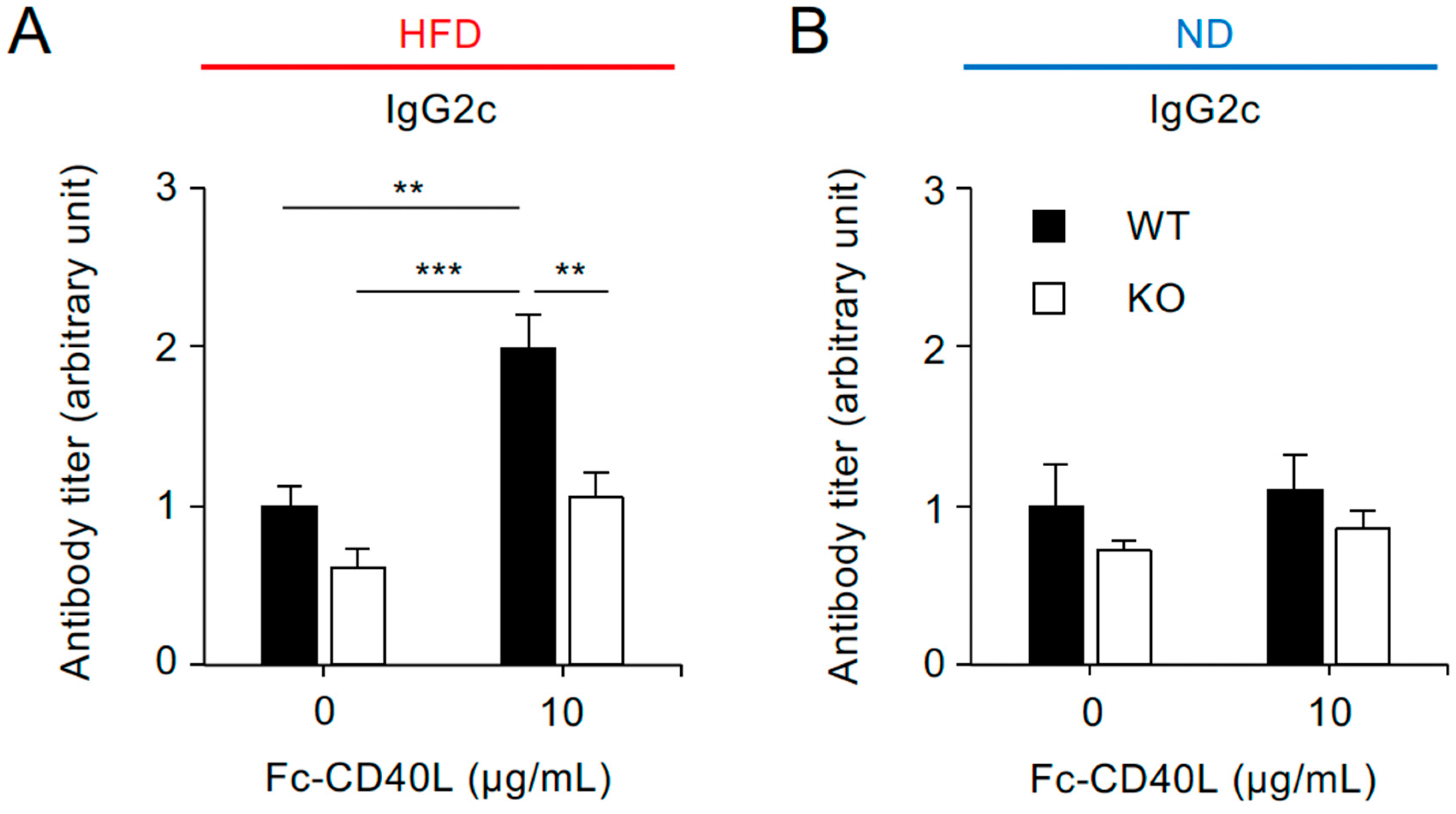
2.5. CD40-Mediated IgG2c Production Is Reduced in Splenocytes from Traf5−/− Mice Fed a High-Fat Diet
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Antibodies
4.3. ELISPOT Assay
4.4. Detection of IgG2c
4.5. Real-Time RT-PCR
4.6. Flow Cytometry
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AID (Aicda) | activation-induced cytidine deaminase |
| CD40L (Cd40lg) | CD40 ligand |
| CSR | class-switch recombination |
| ELISA | enzyme-linked immunosorbent assay |
| ELISPOT | enzyme-linked immunospot |
| HFD | high-fat diet |
| IFN-γ | interferon-gamma |
| IgA | immunoglobulin A |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| IL | interleukin |
| KO | knock-out |
| ND | normal diet |
| Th17 | T helper 17 |
| TNF-α (Tnf) | tumor necrosis factor-alpha |
| TNFR | tumor necrosis factor receptor |
| TRAF5 (Traf5) | Tumor necrosis factor-associated factor 5 |
| WT | wild-type |
References
- So, T. The immunological significance of tumor necrosis factor receptor-associated factors (TRAFs). Int. Immunol. 2022, 34, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka Kuniishi, M.; Ishii, N.; So, T. Role of tumor necrosis factor receptor-associated factor 5 in B- and T-lymphocytes. Explor. Immunol. 2023, 3, 40–55. [Google Scholar] [CrossRef]
- Nagashima, H.; Ishii, N.; So, T. Regulation of Interleukin-6 Receptor Signaling by TNF Receptor-Associated Factor 2 and 5 During Differentiation of Inflammatory CD4+ T Cells. Front. Immunol. 2018, 9, 1986. [Google Scholar] [CrossRef]
- Hildebrand, J.M.; Yi, Z.; Buchta, C.M.; Poovassery, J.; Stunz, L.L.; Bishop, G.A. Roles of tumor necrosis factor receptor associated factor 3 (TRAF3) and TRAF5 in immune cell functions. Immunol. Rev. 2011, 244, 55–74. [Google Scholar] [CrossRef]
- Au, P.Y.; Yeh, W.C. Physiological roles and mechanisms of signaling by TRAF2 and TRAF5. Adv. Exp. Med. Biol. 2007, 597, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Oshima, H.; Chung, W.; Williams-Abbott, L.; Ware, C.F.; Yagita, H.; Okumura, K. TRAF5, an activator of NF-kappaB and putative signal transducer for the lymphotoxin-beta receptor. J. Biol. Chem. 1996, 271, 14661–14664. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.K.; Tojo, T.; Aoki, T.; Kobayashi, N.; Ohishi, T.; Watanabe, T.; Yamamoto, T.; Inoue, J. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc. Natl. Acad. Sci. USA 1996, 93, 9437–9442. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, S.; Nakano, H.; Ishida, T.; Horie, R.; Nagai, M.; Ito, K.; Yagita, H.; Okumura, K.; Inoue, J.; Watanabe, T. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NFkappaB activation. J. Biol. Chem. 1997, 272, 2042–2045. [Google Scholar] [CrossRef]
- Marsters, S.A.; Ayres, T.M.; Skubatch, M.; Gray, C.L.; Rothe, M.; Ashkenazi, A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J. Biol. Chem. 1997, 272, 14029–14032. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Solovyev, I.; Colombero, A.; Elliott, R.; Kelley, M.; Boyle, W.J. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. J. Biol. Chem. 1997, 272, 13471–13474. [Google Scholar] [CrossRef]
- Kawamata, S.; Hori, T.; Imura, A.; Takaori-Kondo, A.; Uchiyama, T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J. Biol. Chem. 1998, 273, 5808–5814. [Google Scholar] [CrossRef]
- Akiba, H.; Nakano, H.; Nishinaka, S.; Shindo, M.; Kobata, T.; Atsuta, M.; Morimoto, C.; Ware, C.F.; Malinin, N.L.; Wallach, D.; et al. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. J. Biol. Chem. 1998, 273, 13353–13358. [Google Scholar] [CrossRef]
- Darnay, B.G.; Haridas, V.; Ni, J.; Moore, P.A.; Aggarwal, B.B. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J. Biol. Chem. 1998, 273, 20551–20555. [Google Scholar] [CrossRef]
- Nakano, H.; Sakon, S.; Koseki, H.; Takemori, T.; Tada, K.; Matsumoto, M.; Munechika, E.; Sakai, T.; Shirasawa, T.; Akiba, H.; et al. Targeted disruption of Traf5 gene causes defects in CD40- and CD27-mediated lymphocyte activation. Proc. Natl. Acad. Sci. USA 1999, 96, 9803–9808. [Google Scholar] [CrossRef]
- Xia, X.Z.; Treanor, J.; Senaldi, G.; Khare, S.D.; Boone, T.; Kelley, M.; Theill, L.E.; Colombero, A.; Solovyev, I.; Lee, F.; et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J. Exp. Med. 2000, 192, 137–143. [Google Scholar] [CrossRef]
- Tada, K.; Okazaki, T.; Sakon, S.; Kobarai, T.; Kurosawa, K.; Yamaoka, S.; Hashimoto, H.; Mak, T.W.; Yagita, H.; Okumura, K.; et al. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-kappa B activation and protection from cell death. J. Biol. Chem. 2001, 276, 36530–36534. [Google Scholar] [CrossRef] [PubMed]
- Esparza, E.M.; Lindsten, T.; Stockhausen, J.M.; Arch, R.H. Tumor necrosis factor receptor (TNFR)-associated factor 5 is a critical intermediate of costimulatory signaling pathways triggered by glucocorticoid-induced TNFR in T cells. J. Biol. Chem. 2006, 281, 8559–8564. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Santamaria, R.; Xu, W.; Cols, M.; Chen, K.; Puga, I.; Shan, M.; Xiong, H.; Bussel, J.B.; Chiu, A.; et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat. Immunol. 2010, 11, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Phung, H.T.; Nagashima, H.; Kobayashi, S.; Asano, N.; Machiyama, T.; Sakurai, T.; Tayama, S.; Asao, A.; Imatani, A.; Kawabe, T.; et al. TRAF5 Deficiency Ameliorates the Severity of Dextran Sulfate Sodium Colitis by Decreasing TRAF2 Expression in Nonhematopoietic Cells. Immunohorizons 2020, 4, 129–139. [Google Scholar] [CrossRef]
- Kawahara, E.; Azuma, M.; Nagashima, H.; Omori, K.; Akiyama, S.; Fujimori, Y.; Oishi, M.; Shibui, N.; Kawaguchi, K.; Morita, M.; et al. TNF Receptor-Associated Factor 5 Limits IL-27 Receptor Signaling in CD4+ T Lymphocytes. J. Immunol. 2022, 208, 642–650. [Google Scholar] [CrossRef]
- Hikosaka-Kuniishi, M.; Iwata, C.; Ozawa, Y.; Ogawara, S.; Wakaizumi, T.; Itaya, R.; Sunakawa, R.; Sato, A.; Nagai, H.; Morita, M.; et al. The Role of TNF Receptor-Associated Factor 5 in the Formation of Germinal Centers by B Cells During the Primary Phase of the Immune Response in Mice. Int. J. Mol. Sci. 2024, 25, 12331. [Google Scholar] [CrossRef] [PubMed]
- So, T.; Salek-Ardakani, S.; Nakano, H.; Ware, C.F.; Croft, M. TNF receptor-associated factor 5 limits the induction of Th2 immune responses. J. Immunol. 2004, 172, 4292–4297. [Google Scholar] [CrossRef] [PubMed]
- Missiou, A.; Rudolf, P.; Stachon, P.; Wolf, D.; Varo, N.; Aichele, P.; Colberg, C.; Hoppe, N.; Ernst, S.; Munkel, C.; et al. TRAF5 deficiency accelerates atherogenesis in mice by increasing inflammatory cell recruitment and foam cell formation. Circ. Res. 2010, 107, 757–766. [Google Scholar] [CrossRef]
- Nagashima, H.; Okuyama, Y.; Asao, A.; Kawabe, T.; Yamaki, S.; Nakano, H.; Croft, M.; Ishii, N.; So, T. The adaptor TRAF5 limits the differentiation of inflammatory CD4+ T cells by antagonizing signaling via the receptor for IL-6. Nat. Immunol. 2014, 15, 449–456. [Google Scholar] [CrossRef]
- Bian, Z.; Dai, J.; Hiroyasu, N.; Guan, H.; Yuan, Y.; Gan, L.; Zhou, H.; Zong, J.; Zhang, Y.; Li, F.; et al. Disruption of tumor necrosis factor receptor associated factor 5 exacerbates pressure overload cardiac hypertrophy and fibrosis. J. Cell Biochem. 2014, 115, 349–358. [Google Scholar] [CrossRef]
- Gao, L.; Wang, P.X.; Zhang, Y.; Yu, C.J.; Ji, Y.; Wang, X.; Zhang, P.; Jiang, X.; Jin, H.; Huang, Z.; et al. Tumor necrosis factor receptor-associated factor 5 (Traf5) acts as an essential negative regulator of hepatic steatosis. J. Hepatol. 2016, 65, 125–136. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, L.; Ma, S.; Zhang, Y.; Cai, Z.; Zhang, K.; Jin, D. TRAF5 protects against myocardial ischemia reperfusion injury via AKT signaling. Eur. J. Pharmacol. 2020, 878, 173092. [Google Scholar] [CrossRef]
- Gissler, M.C.; Anto-Michel, N.; Pennig, J.; Scherrer, P.; Li, X.; Marchini, T.; Pfeiffer, K.; Hardtner, C.; Abogunloko, T.; Mwinyella, T.; et al. Genetic Deficiency of TRAF5 Promotes Adipose Tissue Inflammation and Aggravates Diet-Induced Obesity in Mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2563–2574. [Google Scholar] [CrossRef]
- Potter, C.; Eyre, S.; Cope, A.; Worthington, J.; Barton, A. Investigation of association between the TRAF family genes and RA susceptibility. Ann. Rheum. Dis. 2007, 66, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Chen, L.; Hou, S.; Fang, J.; Zhou, Y.; Bai, L.; Liu, Y.; Kijlstra, A.; Yang, P. TRAF5 and TRAF3IP2 gene polymorphisms are associated with Behcet’s disease and Vogt-Koyanagi-Harada syndrome: A case-control study. PLoS ONE 2014, 9, e84214. [Google Scholar] [CrossRef]
- Emami, H.; Singh, P.; MacNabb, M.; Vucic, E.; Lavender, Z.; Rudd, J.H.; Fayad, Z.A.; Lehrer-Graiwer, J.; Korsgren, M.; Figueroa, A.L.; et al. Splenic metabolic activity predicts risk of future cardiovascular events: Demonstration of a cardiosplenic axis in humans. JACC Cardiovasc. Imaging 2015, 8, 121–130. [Google Scholar] [CrossRef]
- Lori, A.; Perrotta, M.; Lembo, G.; Carnevale, D. The Spleen: A Hub Connecting Nervous and Immune Systems in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2017, 18, 1216. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Park, J.Y.; Yu, R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res. Clin. Pract. 2005, 69, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Folco, E.J.; Sukhova, G.; Shimizu, K.; Gotsman, I.; Vernon, A.H.; Libby, P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: A role for adaptive immunity in obesity. Circ. Res. 2008, 103, 467–476. [Google Scholar] [CrossRef]
- O’Rourke, R.W.; White, A.E.; Metcalf, M.D.; Winters, B.R.; Diggs, B.S.; Zhu, X.; Marks, D.L. Systemic inflammation and insulin sensitivity in obese IFN-gamma knockout mice. Metabolism 2012, 61, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Dib, M.; Hausding, M.; Kashani, F.; Oelze, M.; Kroller-Schon, S.; Hanf, A.; Daub, S.; Roohani, S.; Gramlich, Y.; et al. CD40L controls obesity-associated vascular inflammation, oxidative stress, and endothelial dysfunction in high fat diet-treated and db/db mice. Cardiovasc. Res. 2018, 114, 312–323. [Google Scholar] [CrossRef]
- Bae, H.R.; Choi, M.S.; Kim, S.; Young, H.A.; Gershwin, M.E.; Jeon, S.M.; Kwon, E.Y. IFNgamma is a Key Link between Obesity and Th1-Mediated AutoImmune Diseases. Int. J. Mol. Sci. 2020, 22, 208. [Google Scholar] [CrossRef]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- de Baat, A.; Trinh, B.; Ellingsgaard, H.; Donath, M.Y. Physiological role of cytokines in the regulation of mammalian metabolism. Trends Immunol. 2023, 44, 613–627. [Google Scholar] [CrossRef]
- Winer, S.; Paltser, G.; Chan, Y.; Tsui, H.; Engleman, E.; Winer, D.; Dosch, H.M. Obesity predisposes to Th17 bias. Eur. J. Immunol. 2009, 39, 2629–2635. [Google Scholar] [CrossRef]
- Endo, Y.; Asou, H.K.; Matsugae, N.; Hirahara, K.; Shinoda, K.; Tumes, D.J.; Tokuyama, H.; Yokote, K.; Nakayama, T. Obesity Drives Th17 Cell Differentiation by Inducing the Lipid Metabolic Kinase, ACC1. Cell Rep. 2015, 12, 1042–1055. [Google Scholar] [CrossRef]
- Jiang, Z.; Tabuchi, C.; Gayer, S.G.; Bapat, S.P. Immune Dysregulation in Obesity. Annu. Rev. Pathol. 2025, 20, 483–509. [Google Scholar] [CrossRef]
- Winer, D.A.; Winer, S.; Shen, L.; Wadia, P.P.; Yantha, J.; Paltser, G.; Tsui, H.; Wu, P.; Davidson, M.G.; Alonso, M.N.; et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011, 17, 610–617. [Google Scholar] [CrossRef]
- Conway, B.; Rene, A. Obesity as a disease: No lightweight matter. Obes. Rev. 2004, 5, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best. Pract. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef]
- Shiozawa, M.; Kaneko, H.; Itoh, H.; Morita, K.; Okada, A.; Matsuoka, S.; Kiriyama, H.; Kamon, T.; Fujiu, K.; Michihata, N.; et al. Association of Body Mass Index with Ischemic and Hemorrhagic Stroke. Nutrients 2021, 13, 2343. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef]
- Ralston, J.C.; Lyons, C.L.; Kennedy, E.B.; Kirwan, A.M.; Roche, H.M. Fatty Acids and NLRP3 Inflammasome-Mediated Inflammation in Metabolic Tissues. Annu. Rev. Nutr. 2017, 37, 77–102. [Google Scholar] [CrossRef]
- Grant, R.W.; Dixit, V.D. Adipose tissue as an immunological organ. Obesity 2015, 23, 512–518. [Google Scholar] [CrossRef]
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Jacks, R.D.; Lumeng, C.N. Macrophage and T cell networks in adipose tissue. Nat. Rev. Endocrinol. 2024, 20, 50–61. [Google Scholar] [CrossRef]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta 2013, 417, 80–84. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef]
- Brandes, R.P. Endothelial dysfunction and hypertension. Hypertension 2014, 64, 924–928. [Google Scholar] [CrossRef]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar] [CrossRef]
- Gutierrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar] [CrossRef]
- Sandireddy, R.; Sakthivel, S.; Gupta, P.; Behari, J.; Tripathi, M.; Singh, B.K. Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases. Front. Cell Dev. Biol. 2024, 12, 1433857. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Sechi, L.A.; Navarese, E.P.; Casu, G.; Vidili, G. Metabolic dysfunction-associated steatotic liver disease and cardiovascular risk: A comprehensive review. Cardiovasc. Diabetol. 2024, 23, 346. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021, 23 (Suppl. 1), 3–16. [Google Scholar] [CrossRef]
- Lopez-Jimenez, F.; Almahmeed, W.; Bays, H.; Cuevas, A.; Di Angelantonio, E.; le Roux, C.W.; Sattar, N.; Sun, M.C.; Wittert, G.; Pinto, F.J.; et al. Obesity and cardiovascular disease: Mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. Eur. J. Prev. Cardiol. 2022, 29, 2218–2237. [Google Scholar] [CrossRef]
- Conrad, N.; Verbeke, G.; Molenberghs, G.; Goetschalckx, L.; Callender, T.; Cambridge, G.; Mason, J.C.; Rahimi, K.; McMurray, J.J.V.; Verbakel, J.Y. Autoimmune diseases and cardiovascular risk: A population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet 2022, 400, 733–743. [Google Scholar] [CrossRef]
- Zirlik, A.; Bavendiek, U.; Libby, P.; MacFarlane, L.; Gerdes, N.; Jagielska, J.; Ernst, S.; Aikawa, M.; Nakano, H.; Tsitsikov, E.; et al. TRAF-1, -2, -3, -5, and -6 are induced in atherosclerotic plaques and differentially mediate proinflammatory functions of CD40L in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Strohm, L.; Ubbens, H.; Munzel, T.; Daiber, A.; Daub, S. Role of CD40(L)-TRAF signaling in inflammation and resolution-a double-edged sword. Front. Pharmacol. 2022, 13, 995061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gang, X.; Yang, S.; Cui, M.; Sun, L.; Li, Z.; Wang, G. The Alterations in and the Role of the Th17/Treg Balance in Metabolic Diseases. Front. Immunol. 2021, 12, 678355. [Google Scholar] [CrossRef]
- Kraus, Z.J.; Haring, J.S.; Bishop, G.A. TNF receptor-associated factor 5 is required for optimal T cell expansion and survival in response to infection. J. Immunol. 2008, 181, 7800–7809. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Kinoshita, K.; Fagarasan, S.; Yamada, S.; Shinkai, Y.; Honjo, T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000, 102, 553–563. [Google Scholar] [CrossRef]
- Lee, M.S.J.; Natsume-Kitatani, Y.; Temizoz, B.; Fujita, Y.; Konishi, A.; Matsuda, K.; Igari, Y.; Tsukui, T.; Kobiyama, K.; Kuroda, E.; et al. B cell-intrinsic MyD88 signaling controls IFN-gamma-mediated early IgG2c class switching in mice in response to a particulate adjuvant. Eur. J. Immunol. 2019, 49, 1433–1440. [Google Scholar] [CrossRef]
- Kobayashi, S.; Sakurai, T.; So, T.; Shiota, Y.; Asao, A.; Phung, H.T.; Tanaka, R.; Kawabe, T.; Maruyama, T.; Kanno, E.; et al. TNF Receptor-Associated Factor 5 Limits Function of Plasmacytoid Dendritic Cells by Controlling IFN Regulatory Factor 5 Expression. J. Immunol. 2019, 203, 1447–1456. [Google Scholar] [CrossRef]
- Buchta, C.M.; Bishop, G.A. TRAF5 negatively regulates TLR signaling in B lymphocytes. J. Immunol. 2014, 192, 145–150. [Google Scholar] [CrossRef]
- Levitan, I.; Volkov, S.; Subbaiah, P.V. Oxidized LDL: Diversity, patterns of recognition, and pathophysiology. Antioxid. Redox Signal 2010, 13, 39–75. [Google Scholar] [CrossRef]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, T.; Naka, T.; Yoshida, K.; Tanaka, T.; Fujiwara, H.; Suematsu, S.; Yoshida, N.; Kishimoto, T.; Kikutani, H. The immune responses in CD40-deficient mice: Impaired immunoglobulin class switching and germinal center formation. Immunity 1994, 1, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Foy, T.M.; Laman, J.D.; Elliott, E.A.; Dunn, J.J.; Waldschmidt, T.J.; Elsemore, J.; Noelle, R.J.; Flavell, R.A. Mice deficient for the CD40 ligand. Immunity 1994, 1, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, B.R.; Fanslow, W.C., 3rd; Armitage, R.J.; Campbell, K.A.; Liggitt, D.; Wright, B.; Davison, B.L.; Maliszewski, C.R. Humoral immune responses in CD40 ligand-deficient mice. J. Exp. Med. 1994, 180, 1889–1900. [Google Scholar] [CrossRef]
- Foy, T.M.; Aruffo, A.; Bajorath, J.; Buhlmann, J.E.; Noelle, R.J. Immune regulation by CD40 and its ligand GP39. Annu. Rev. Immunol. 1996, 14, 591–617. [Google Scholar] [CrossRef]
- Woolaver, R.A.; Wang, X.; Dollin, Y.; Xie, P.; Wang, J.H.; Chen, Z. TRAF2 Deficiency in B Cells Impairs CD40-Induced Isotype Switching That Can Be Rescued by Restoring NF-kappaB1 Activation. J. Immunol. 2018, 201, 3421–3430. [Google Scholar] [CrossRef]
- Yeh, W.C.; Shahinian, A.; Speiser, D.; Kraunus, J.; Billia, F.; Wakeham, A.; de la Pompa, J.L.; Ferrick, D.; Hum, B.; Iscove, N.; et al. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 1997, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Duncan, G.S.; Mirtsos, C.; Ng, M.; Speiser, D.E.; Shahinian, A.; Marino, M.W.; Mak, T.W.; Ohashi, P.S.; Yeh, W.C. TRAF2 deficiency results in hyperactivity of certain TNFR1 signals and impairment of CD40-mediated responses. Immunity 1999, 11, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Y.; Chiu, C.J.; Hsing, C.H.; Hsu, Y.H. Interferon Family Cytokines in Obesity and Insulin Sensitivity. Cells 2022, 11, 4041. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005, 310, 1510–1512. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Hikosaka, M.; Murata, A.; Yoshino, M.; Hayashi, S.I. Correlation between cell aggregation and antibody production in IgE-producing plasma cells. Biochem. Biophys. Rep. 2017, 10, 224–231. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakaizumi, T.; Hikosaka-Kuniishi, M.; Ozawa, Y.; Sato, A.; Iwata, C.; Wada, T.; Sasaoka, T.; Morita, M.; So, T. Defective IgG Class Switching in the Spleen of TRAF5-Deficient Mice Reveals a Role for TRAF5 in CD40-Mediated B Cell Responses During Obesity-Associated Inflammation. Int. J. Mol. Sci. 2025, 26, 9494. https://doi.org/10.3390/ijms26199494
Wakaizumi T, Hikosaka-Kuniishi M, Ozawa Y, Sato A, Iwata C, Wada T, Sasaoka T, Morita M, So T. Defective IgG Class Switching in the Spleen of TRAF5-Deficient Mice Reveals a Role for TRAF5 in CD40-Mediated B Cell Responses During Obesity-Associated Inflammation. International Journal of Molecular Sciences. 2025; 26(19):9494. https://doi.org/10.3390/ijms26199494
Chicago/Turabian StyleWakaizumi, Tomomi, Mari Hikosaka-Kuniishi, Yusuke Ozawa, Ayaka Sato, Chieri Iwata, Tsutomu Wada, Toshiyasu Sasaoka, Masashi Morita, and Takanori So. 2025. "Defective IgG Class Switching in the Spleen of TRAF5-Deficient Mice Reveals a Role for TRAF5 in CD40-Mediated B Cell Responses During Obesity-Associated Inflammation" International Journal of Molecular Sciences 26, no. 19: 9494. https://doi.org/10.3390/ijms26199494
APA StyleWakaizumi, T., Hikosaka-Kuniishi, M., Ozawa, Y., Sato, A., Iwata, C., Wada, T., Sasaoka, T., Morita, M., & So, T. (2025). Defective IgG Class Switching in the Spleen of TRAF5-Deficient Mice Reveals a Role for TRAF5 in CD40-Mediated B Cell Responses During Obesity-Associated Inflammation. International Journal of Molecular Sciences, 26(19), 9494. https://doi.org/10.3390/ijms26199494




