Microfluidic-Based Technologies for Crossing the Blood–Brain Barrier Against Alzheimer’s Disease: Novel Strategies and Challenges
Abstract
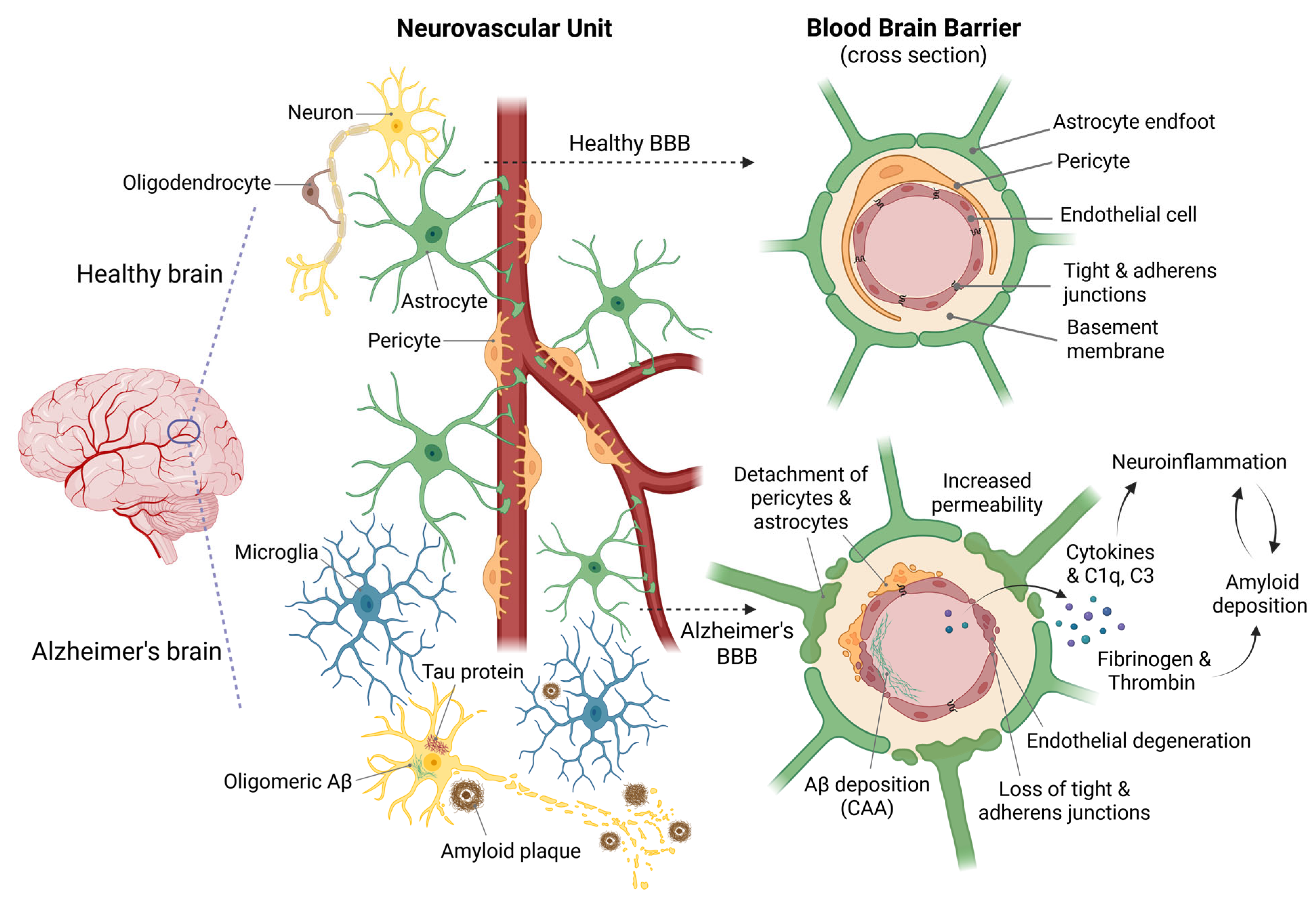
1. Introduction
2. Models for Studying the BBB
2.1. Current In Vitro Models of the BBB: Advantages and Limitations
| BBB Model | Advantages | Limitations |
|---|---|---|
| Transwell systems [40,41,52,53] |
|
|
| Co-Culture Models [47,48,49] |
|
|
| 3D Culture Models (Organoids) [55,56,57,58,59,60,61,62,63,64,65] |
|
|
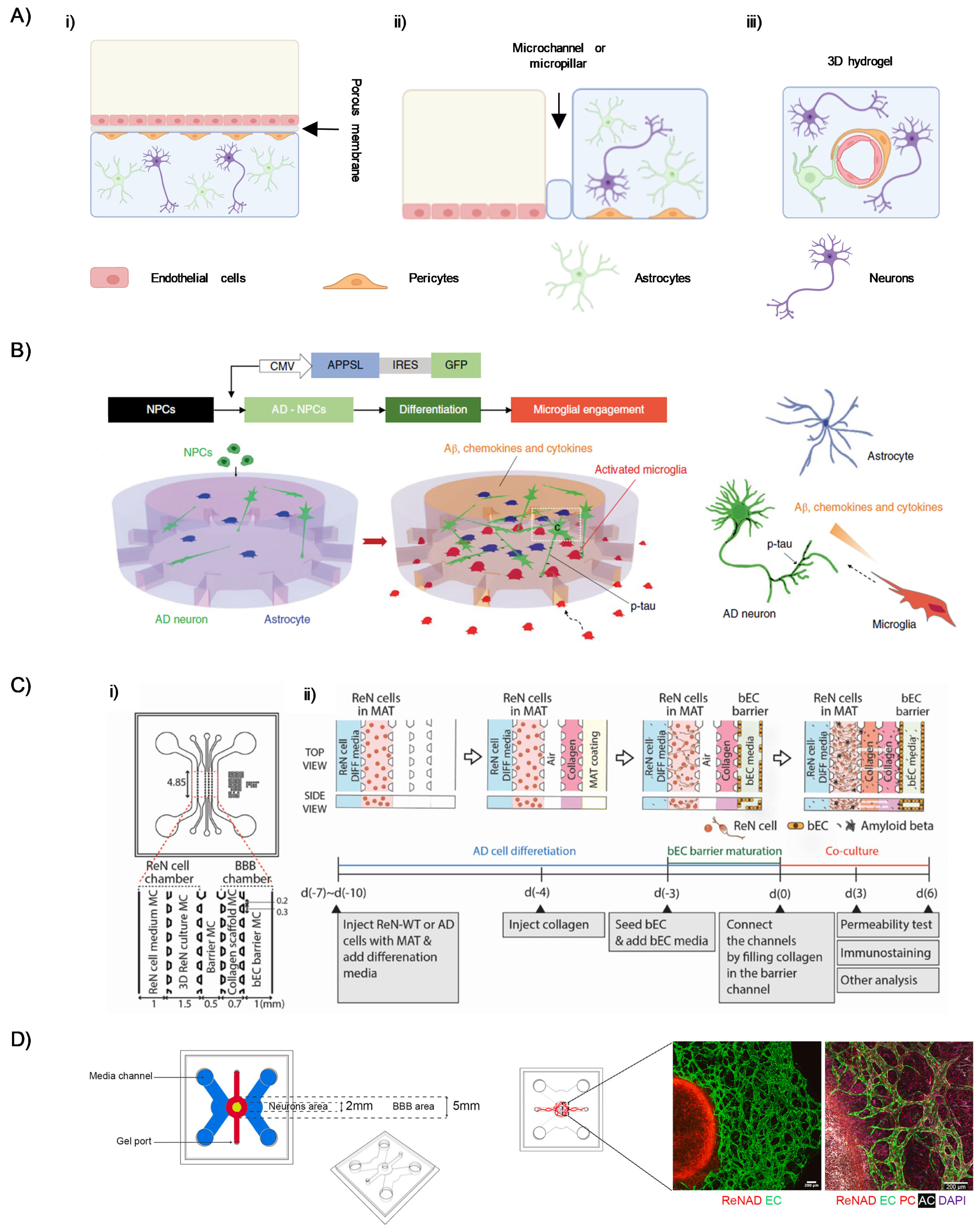
2.2. Microfluidic Models of the BBB in the Setting of AD
2.3. Regulatory and Ethical Issues of OoC
3. Microfluidic Synthesis of Nanocarriers for AD Therapy
Routes of Drug Administration
4. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamatham, P.T.; Shukla, R.; Khatri, D.K.; Vora, L.K. Pathogenesis, Diagnostics, and Therapeutics for Alzheimer’s Disease: Breaking the Memory Barrier. Ageing Res. Rev. 2024, 101, 102481. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-Targeting Therapies for Alzheimer Disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Cavallucci, V.; D’Amelio, M.; Cecconi, F. Aβ Toxicity in Alzheimer’s Disease. Mol. Neurobiol. 2012, 45, 366–378. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, M.; Rossini, P.M. Brain Excitability and Connectivity of Neuronal Assemblies in Alzheimer’s Disease: From Animal Models to Human Findings. Prog. Neurobiol. 2012, 99, 42–60. [Google Scholar] [CrossRef]
- Zenaro, E.; Piacentino, G.; Constantin, G. The Blood-Brain Barrier in Alzheimer’s Disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef]
- Do, T.M.; Dodacki, A.; Alata, W.; Calon, F.; Nicolic, S.; Scherrmann, J.-M.; Farinotti, R.; Bourasset, F. Age-Dependent Regulation of the Blood-Brain Barrier Influx/Efflux Equilibrium of Amyloid-β Peptide in a Mouse Model of Alzheimer’s Disease (3xTg-AD). J. Alzheimer’s Dis. 2015, 49, 287–300. [Google Scholar] [CrossRef]
- Viswanathan, A.; Greenberg, S.M. Cerebral Amyloid Angiopathy in the Elderly. Ann. Neurol. 2011, 70, 871–880. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral Amyloid Angiopathy and Alzheimer Disease—One Peptide, Two Pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef]
- Cai, Z.; Qiao, P.-F.; Wan, C.-Q.; Cai, M.; Zhou, N.-K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 1223–1234. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes Regulate the Blood–Brain Barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Brown, L.S.; Foster, C.G.; Courtney, J.-M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front. Cell. Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the Neurovascular Unit: Key Functions and Signaling Pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef]
- Kugler, E.C.; Greenwood, J.; MacDonald, R.B. The “Neuro-Glial-Vascular” Unit: The Role of Glia in Neurovascular Unit Formation and Dysfunction. Front. Cell Dev. Biol. 2021, 9, 732820. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Zhang, G.; Tian, Y.; Huang, J.; Tao, R.; Liao, M.; Lu, Y.; Ye, W.; Wang, R.; Fukunaga, K.; Lou, Y.; et al. The γ-Secretase Blocker DAPT Reduces the Permeability of the Blood–Brain Barrier by Decreasing the Ubiquitination and Degradation of Occludin During Permanent Brain Ischemia. CNS Neurosci. Ther. 2013, 19, 53–60. [Google Scholar] [CrossRef]
- Cai, Z.; Hussain, M.D.; Yan, L.-J. Microglia, Neuroinflammation, and Beta-Amyloid Protein in Alzheimer’s Disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef]
- Kanekiyo, T.; Bu, G. The Low-Density Lipoprotein Receptor-Related Protein 1 and amyloid-β Clearance in Alzheimer’s Disease. Front. Aging Neurosci. 2014, 6, 93. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Kanekiyo, T. Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1965. [Google Scholar] [CrossRef]
- Wan, W.; Chen, H.; Li, Y. The Potential Mechanisms of Aβ-Receptor for Advanced Glycation End-Products Interaction Disrupting Tight Junctions of the Blood-Brain Barrier in Alzheimer’s Disease. Int. J. Neurosci. 2014, 124, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kantawala, B.; Shariff, S.; Ramadan, N.; Fawaz, V.; Hassan, Y.; Mugisha, N.; Yenkoyan, K.; Nazir, A.; Uwishema, O. Revolutionizing Neurotherapeutics: Blood-Brain Barrier-on-a-Chip Technologies for Precise Drug Delivery. Ann. Med. Surg. 2024, 86, 2794–2804. [Google Scholar] [CrossRef] [PubMed]
- Khawli, L.A.; Prabhu, S. Drug Delivery across the Blood–Brain Barrier. Mol. Pharm. 2013, 10, 1471–1472. [Google Scholar] [CrossRef]
- La Barbera, L.; Mauri, E.; D’Amelio, M.; Gori, M. Functionalization Strategies of Polymeric Nanoparticles for Drug Delivery in Alzheimer’s Disease: Current Trends and Future Perspectives. Front. Neurosci. 2022, 16, 939855. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Santhosh, M.; Choi, J.-W. In Vitro Blood–Brain Barrier-Integrated Neurological Disorder Models Using a Microfluidic Device. Micromachines 2019, 11, 21. [Google Scholar] [CrossRef]
- Ahn, S.I.; Sei, Y.J.; Park, H.-J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.-J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered Human Blood–Brain Barrier Platform for Understanding Nanoparticle Transport Mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef]
- Lei, T.; Yang, Z.; Li, H.; Qin, M.; Gao, H. Interactions between Nanoparticles and Pathological Changes of Vascular in Alzheimer’s Disease. Adv. Drug Deliv. Rev. 2024, 207, 115219. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, J.; Shah, Z.; Awasthi, A.; Mahajan, A.; Kim, Y. Advanced Human BBB-on-a-Chip: A New Platform for Alzheimer’s Disease Studies. Adv. Healthc. Mater. 2021, 10, 2002285. [Google Scholar] [CrossRef]
- Mehraji, S.; DeVoe, D.L. Microfluidic Synthesis of Lipid-Based Nanoparticles for Drug Delivery: Recent Advances and Opportunities. Lab Chip 2024, 24, 1154–1174. [Google Scholar] [CrossRef]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The Effect of Nanoparticle Size on the Ability to Cross the Blood–Brain Barrier: An In Vivo Study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Qosa, H.; Abuasal, B.S.; Romero, I.A.; Weksler, B.; Couraud, P.-O.; Keller, J.N.; Kaddoumi, A. Differences in Amyloid-β Clearance across Mouse and Human Blood–Brain Barrier Models: Kinetic Analysis and Mechanistic Modeling. Neuropharmacology 2014, 79, 668–678. [Google Scholar] [CrossRef]
- Hoshi, Y.; Uchida, Y.; Tachikawa, M.; Inoue, T.; Ohtsuki, S.; Terasaki, T. Quantitative Atlas of Blood-Brain Barrier Transporters, Receptors, and Tight Junction Proteins in Rats and Common Marmoset. J. Pharm. Sci. 2013, 102, 3343–3355. [Google Scholar] [CrossRef] [PubMed]
- Soliman, Y.; Al-khodor, J.; Yildirim Köken, G.; Mustafaoglu, N. A Guide for Blood–Brain Barrier Models. FEBS Lett. 2025, 599, 599–644. [Google Scholar] [CrossRef] [PubMed]
- Grobstein, C. Morphogenetic Interaction between Embryonic Mouse Tissues Separated by a Membrane Filter. Nature 1953, 172, 869–870. [Google Scholar] [CrossRef] [PubMed]
- DeBault, L.E.; Kahn, L.E.; Frommes, S.P.; Cancilla, P.A. Cerebral Microvessels and Derived Cells in Tissue Culture: Isolation and Preliminary Characterization. In Vitro 1979, 15, 473–487. [Google Scholar] [CrossRef]
- Bowman, P.D.; Betz, A.L.; Ar, D.; Wolinsky, J.S.; Penney, J.B.; Shivers, R.R.; Goldstein, G.W. Primary Culture of Capillary Endothelium from Rat Brain. In Vitro 1981, 17, 353–362. [Google Scholar] [CrossRef]
- Bolden, C.T.; Skibber, M.A.; Olson, S.D.; Zamorano Rojas, M.; Milewicz, S.; Gill, B.S.; Cox, C.S. Validation and Characterization of a Novel Blood–Brain Barrier Platform for Investigating Traumatic Brain Injury. Sci. Rep. 2023, 13, 16150. [Google Scholar] [CrossRef]
- Berezowski, V.; Miecz, D.; Marszałek, M.; Bröer, A.; Bröer, S.; Cecchelli, R.; Nałecz, K.A. Involvement of OCTN2 and B0,+ in the Transport of Carnitine through an in Vitro Model of the Blood-Brain Barrier. J. Neurochem. 2004, 91, 860–872. [Google Scholar] [CrossRef]
- Demeuse, P.; Kerkhofs, A.; Struys-Ponsar, C.; Knoops, B.; Remacle, C.; van den Bosch de Aguilar, P. Compartmentalized Coculture of Rat Brain Endothelial Cells and Astrocytes: A Syngenic Model to Study the Blood–Brain Barrier. J. Neurosci. Methods 2002, 121, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Nomura, M.; Yamagishi, S.-I.; Harada, S.-I.; Yamashita, J.; Yamamoto, H. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia 1997, 19, 13–26. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakao, S.; Nakaoke, R.; Nakagawa, S.; Kitagawa, N.; Niwa, M. Effects of Hypoxia on Endothelial/Pericytic Co-Culture Model of the Blood–Brain Barrier. Regul. Pept. 2004, 123, 77–83. [Google Scholar] [CrossRef]
- Zozulya, A.; Weidenfeller, C.; Galla, H.-J. Pericyte-Endothelial Cell Interaction Increases MMP-9 Secretion at the Blood-Brain Barrier in Vitro. Brain Res. 2008, 1189, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vandenhaute, E.; Culot, M.; Gosselet, F.; Dehouck, L.; Godfraind, C.; Franck, M.; Plouët, J.; Cecchelli, R.; Dehouck, M.-P.; Ruchoux, M.-M. Brain Pericytes from Stress-Susceptible Pigs Increase Blood-Brain Barrier Permeability in Vitro. Fluids Barriers CNS 2012, 9, 11. [Google Scholar] [CrossRef]
- Nakagawa, S.; Deli, M.A.; Kawaguchi, H.; Shimizudani, T.; Shimono, T.; Kittel, Á.; Tanaka, K.; Niwa, M. A New Blood–Brain Barrier Model Using Primary Rat Brain Endothelial Cells, Pericytes and Astrocytes. Neurochem. Int. 2009, 54, 253–263. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Burkhart, A.; Moos, T. A Triple Culture Model of the Blood-Brain Barrier Using Porcine Brain Endothelial Cells, Astrocytes and Pericytes. PLoS ONE 2015, 10, e0134765. [Google Scholar] [CrossRef]
- Williams-Medina, A.; Deblock, M.; Janigro, D. In Vitro Models of the Blood–Brain Barrier: Tools in Translational Medicine. Front. Med. Technol. 2021, 2, 623950. [Google Scholar] [CrossRef]
- Cucullo, L.; Hossain, M.; Puvenna, V.; Marchi, N.; Janigro, D. The Role of Shear Stress in Blood-Brain Barrier Endothelial Physiology. BMC Neurosci. 2011, 12, 40. [Google Scholar] [CrossRef]
- Choublier, N.; Taghi, M.; Menet, M.-C.; Le Gall, M.; Bruce, J.; Chafey, P.; Guillonneau, F.; Moreau, A.; Denizot, C.; Parmentier, Y.; et al. Exposure of Human Cerebral Microvascular Endothelial Cells hCMEC/D3 to Laminar Shear Stress Induces Vascular Protective Responses. Fluids Barriers CNS 2022, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Cucullo, L.; Hossain, M.; Tierney, W.; Janigro, D. A New Dynamic In Vitro Modular Capillaries-Venules Modular System: Cerebrovascular Physiology in a Box. BMC Neurosci. 2013, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Santaguida, S.; Janigro, D.; Hossain, M.; Oby, E.; Rapp, E.; Cucullo, L. Side by Side Comparison between Dynamic versus Static Models of Blood–Brain Barrier in Vitro: A Permeability Study. Brain Res. 2006, 1109, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chaulagain, B.; Gothwal, A.; Lamptey, R.N.L.; Trivedi, R.; Mahanta, A.K.; Layek, B.; Singh, J. Experimental Models of In Vitro Blood–Brain Barrier for CNS Drug Delivery: An Evolutionary Perspective. Int. J. Mol. Sci. 2023, 24, 2710. [Google Scholar] [CrossRef]
- Workman, M.J.; Svendsen, C.N. Recent Advances in Human iPSC-Derived Models of the Blood–Brain Barrier. Fluids Barriers CNS 2020, 17, 30. [Google Scholar] [CrossRef]
- Marton, R.M.; Pașca, S.P. Organoid and Assembloid Technologies for Investigating Cellular Crosstalk in Human Brain Development and Disease. Trends Cell Biol. 2020, 30, 133–143. [Google Scholar] [CrossRef]
- Logan, S.; Arzua, T.; Canfield, S.G.; Seminary, E.R.; Sison, S.L.; Ebert, A.D.; Bai, X. Studying Human Neurological Disorders Using Induced Pluripotent Stem Cells: From 2D Monolayer to 3D Organoid and Blood Brain Barrier Models. In Comprehensive Physiology; Prakash, Y.S., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 565–611. ISBN 978-0-470-65071-4. [Google Scholar]
- Yan, L.; Moriarty, R.A.; Stroka, K.M. Recent Progress and New Challenges in Modeling of Human Pluripotent Stem Cell-Derived Blood-Brain Barrier. Theranostics 2021, 11, 10148–10170. [Google Scholar] [CrossRef]
- Bhalerao, A.; Sivandzade, F.; Archie, S.R.; Chowdhury, E.A.; Noorani, B.; Cucullo, L. In Vitro Modeling of the Neurovascular Unit: Advances in the Field. Fluids Barriers CNS 2020, 17, 22. [Google Scholar] [CrossRef]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 2019, 24, 995–1005.e6. [Google Scholar] [CrossRef]
- Nzou, G.; Wicks, R.T.; VanOstrand, N.R.; Mekky, G.A.; Seale, S.A.; EL-Taibany, A.; Wicks, E.E.; Nechtman, C.M.; Marrotte, E.J.; Makani, V.S.; et al. Multicellular 3D Neurovascular Unit Model for Assessing Hypoxia and Neuroinflammation Induced Blood-Brain Barrier Dysfunction. Sci. Rep. 2020, 10, 9766. [Google Scholar] [CrossRef]
- Kistemaker, L.; Van Bodegraven, E.J.; De Vries, H.E.; Hol, E.M. Vascularized Human Brain Organoids: Current Possibilities and Prospects. Trends Biotechnol. 2025, 43, 1275–1285. [Google Scholar] [CrossRef]
- Sharma, A.; Fernandes, D.C.; Reis, R.L.; Gołubczyk, D.; Neumann, S.; Lukomska, B.; Janowski, M.; Kortylewski, M.; Walczak, P.; Oliveira, J.M.; et al. Cutting-Edge Advances in Modeling the Blood–Brain Barrier and Tools for Its Reversible Permeabilization for Enhanced Drug Delivery into the Brain. Cell Biosci. 2023, 13, 137. [Google Scholar] [CrossRef]
- Simöes Da Gama, C.; Morin-Brureau, M. Study of BBB Dysregulation in Neuropathogenicity Using Integrative Human Model of Blood–Brain Barrier. Front. Cell. Neurosci. 2022, 16, 863836. [Google Scholar] [CrossRef] [PubMed]
- Urrestizala-Arenaza, N.; Cerchio, S.; Cavaliere, F.; Magliaro, C. Limitations of Human Brain Organoids to Study Neurodegenerative Diseases: A Manual to Survive. Front. Cell. Neurosci. 2024, 18, 1419526. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-Brain Barrier Breakdown in Alzheimer Disease and Other Neurodegenerative Disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Levy, N. The Use of Animal as Models: Ethical Considerations. Int. J. Stroke 2012, 7, 440–442. [Google Scholar] [CrossRef]
- Andersen, M.L.; Winter, L.M.F. Animal Models in Biological and Biomedical Research—Experimental and Ethical Concerns. An. Acad. Bras. Ciênc. 2017, 91, e20170238. [Google Scholar] [CrossRef]
- Pippin, J.J.; Cavanaugh, S.E.; Pistollato, F. Animal Research for Alzheimer Disease: Failures of Science and Ethics. In Animal Experimentation: Working Towards a Paradigm Change; BRILL: Leiden, The Netherlands, 2019; pp. 480–516. ISBN 978-90-04-35618-4. [Google Scholar]
- LaFerla, F.M.; Green, K.N. Animal Models of Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006320. [Google Scholar] [CrossRef]
- Woodruff-Pak, D.S. Animal Models of Alzheimer’s Disease: Therapeutic Implications. J. Alzheimer’s Dis. 2008, 15, 507–521. [Google Scholar] [CrossRef]
- Blanchard, J.W.; Victor, M.B.; Tsai, L.-H. Dissecting the Complexities of Alzheimer Disease with In Vitro Models of the Human Brain. Nat. Rev. Neurol. 2022, 18, 25–39. [Google Scholar] [CrossRef]
- Battat, S.; Weitz, D.A.; Whitesides, G.M. An Outlook on Microfluidics: The Promise and the Challenge. Lab Chip 2022, 22, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.I.; Kim, Y. Human Blood–Brain Barrier on a Chip: Featuring Unique Multicellular Cooperation in Pathophysiology. Trends Biotechnol. 2021, 39, 749–752. [Google Scholar] [CrossRef]
- van der Helm, M.W.; van der Meer, A.D.; Eijkel, J.C.T.; van den Berg, A.; Segerink, L.I. Microfluidic Organ-on-Chip Technology for Blood-Brain Barrier Research. Tissue Barriers 2016, 4, e1142493. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, M.; Huang, R.; Wang, K.; Zeng, Z.; Xiao, L.; Lin, Y.; Liu, D. Blood–Brain Barrier Microfluidic Chips and Their Applications. Organs-on-a-Chip 2023, 5, 100027. [Google Scholar] [CrossRef]
- Peng, B.; Hao, S.; Tong, Z.; Bai, H.; Pan, S.; Lim, K.-L.; Li, L.; Voelcker, N.H.; Huang, W. Blood-Brain Barrier (BBB)-on-a-Chip: A Promising Breakthrough in Brain Disease Research. Lab Chip 2022, 22, 3579–3602. [Google Scholar] [CrossRef]
- Park, J.; Wetzel, I.; Marriott, I.; Dréau, D.; D’Avanzo, C.; Kim, D.Y.; Tanzi, R.E.; Cho, H. A 3D Human Triculture System Modeling Neurodegeneration and Neuroinflammation in Alzheimer’s Disease. Nat. Neurosci. 2018, 21, 941–951. [Google Scholar] [CrossRef]
- Shin, Y.; Choi, S.H.; Kim, E.; Bylykbashi, E.; Kim, J.A.; Chung, S.; Kim, D.Y.; Kamm, R.D.; Tanzi, R.E. Blood-Brain Barrier Dysfunction in a 3D In Vitro Model of Alzheimer’s Disease. Adv. Sci. 2019, 6, 1900962. [Google Scholar] [CrossRef]
- Pavlou, G.; Spitz, S.; Pramotton, F.M.; Tsai, A.; Li, B.M.; Wang, X.; Barr, O.M.; Ko, E.C.; Zhang, S.; Ashley, S.J.; et al. Engineered 3D Human Neurovascular Model of Alzheimer’s Disease to Study Vascular Dysfunction. Biomaterials 2025, 314, 122864. [Google Scholar] [CrossRef]
- Blanchard, J.W.; Bula, M.; Davila-Velderrain, J.; Akay, L.A.; Zhu, L.; Frank, A.; Victor, M.B.; Bonner, J.M.; Mathys, H.; Lin, Y.-T.; et al. Reconstruction of the Human Blood–Brain Barrier In Vitro Reveals a Pathogenic Mechanism of APOE4 in Pericytes. Nat. Med. 2020, 26, 952–963. [Google Scholar] [CrossRef]
- Jang, M.; Choi, N.; Kim, H.N. Hyperglycemic Neurovasculature-On-A-Chip to Study the Effect of SIRT1-Targeted Therapy for the Type 3 Diabetes “Alzheimer’s Disease. ” Adv. Sci. 2022, 9, e2201882. [Google Scholar] [CrossRef]
- Gu, L.; Mao, X.; Tian, C.; Yang, Y.; Yang, K.; Canfield, S.G.; Zhu, D.; Gu, M.; Guo, F. Engineering Blood-Brain Barrier Microphysiological Systems to Model Alzheimer’s Disease Monocyte Penetration and Infiltration. Biomater. Sci. 2025, 13, 3650–3661. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-J.; Otieno, M.A.; Ronxhi, J.; Lim, H.-K.; Ewart, L.; Kodella, K.R.; Petropolis, D.B.; Kulkarni, G.; Rubins, J.E.; Conegliano, D.; et al. Reproducing Human and Cross-Species Drug Toxicities Using a Liver-Chip. Sci. Transl. Med. 2019, 11, eaax5516. [Google Scholar] [CrossRef]
- Ewart, L.; Apostolou, A.; Briggs, S.A.; Carman, C.V.; Chaff, J.T.; Heng, A.R.; Jadalannagari, S.; Janardhanan, J.; Jang, K.J.; Joshipura, S.R.; et al. Performance Assessment and Economic Analysis of a Human Liver-Chip for Predictive Toxicology. Commun. Med. 2022, 2, 154, Corrcetion in Commun. Med. 2023, 3, 16. [Google Scholar] [CrossRef]
- Nahak, B.K.; Mishra, A.; Preetam, S.; Tiwari, A. Advances in Organ-on-a-Chip Materials and Devices. ACS Appl. Bio Mater. 2022, 5, 3576–3607. [Google Scholar] [CrossRef]
- Han, J.J. FDA Modernization Act 2.0 Allows for Alternatives to Animal Testing. Artif. Organs 2023, 47, 449–450. [Google Scholar] [CrossRef]
- FDA’s Center for Drug Evaluation and Research (CDER). FDA’s ISTAND Pilot Program Accepts a Submission of First Organ-on-a-Chip Technology Designed to Predict Human Drug-Induced Liver Injury (DILI); FDA: Silver Spring, MD, USA, 2024. [Google Scholar]
- Leite, S.B.; Beken, S.; Brendler-Schwaab, S.; Corvi, R.; Daskalopoulos, E.-P.; Delrue, N.; Fitzpatrick, S.; Piergiovanni, M.; Tarazona, J.; van Engelen, J.; et al. Resources for Organ-on-Chip Validation and Qualification—EUROoCS RAB; European Commission, Joint Research Centre (JRC): Brussels, Belgium, 2021; Available online: http://data.europa.eu/89h/7bcb1db5-5c7e-460b-b79e-ca5f642514a4 (accessed on 3 September 2025).
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine—Challenge and Perspectives. Angew. Chem. Int. Ed. 2009, 48, 872–897. [Google Scholar] [CrossRef]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Eltaib, L. Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication. Polymers 2025, 17, 833. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Khan, S.; Sharma, A.; Jain, V. An Overview of Nanostructured Lipid Carriers and Its Application in Drug Delivery through Different Routes. Adv. Pharm. Bull. 2023, 13, 446–460. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Ghiuță, I.; Cristea, D. 15—Silver Nanoparticles for Delivery Purposes. In Nanoengineered Biomaterials for Advanced Drug Delivery; Mozafari, M., Ed.; Woodhead Publishing Series in Biomaterials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 347–371. ISBN 978-0-08-102985-5. [Google Scholar]
- Croitoru, G.A.; Pîrvulescu, D.C.; Niculescu, A.G.; Grumezescu, A.M.; Antohi, A.M.; Nicolae, C.L. Metallic Nanomaterials—Targeted Drug Delivery Approaches for Improved Bioavailability, Reduced Side Toxicity, and Enhanced Patient Outcomes. Rom. J. Morphol. Embryol. 2024, 65, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, E.; Devnarain, N.; Mohammed, M.; Govender, T.; Omolo, C.A. Engineering Hybrid Nanosystems for Efficient and Targeted Delivery against Bacterial Infections. J. Control. Release 2022, 351, 598–622. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Martin, B.; Fernandez-Barbero, A. Inorganic/Polymer Hybrid Nanoparticles for Sensing Applications. Adv. Colloid Interface Sci. 2016, 233, 25–37. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming Blood–Brain Barrier Transport: Advances in Nanoparticle-Based Drug Delivery Strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef]
- Yao, F.; Zhu, P.; Chen, J.; Li, S.; Sun, B.; Li, Y.; Zou, M.; Qi, X.; Liang, P.; Chen, Q. Synthesis of Nanoparticles via Microfluidic Devices and Integrated Applications. Microchim. Acta 2023, 190, 256. [Google Scholar] [CrossRef]
- Moragues, T.; Arguijo, D.; Beneyton, T.; Modavi, C.; Simutis, K.; Abate, A.R.; Baret, J.-C.; deMello, A.J.; Densmore, D.; Griffiths, A.D. Droplet-Based Microfluidics. Nat. Rev. Methods Primers 2023, 3, 32. [Google Scholar] [CrossRef]
- Nunziata, G.; Borroni, A.; Rossi, F. Advanced Microfluidic Strategies for Core-Shell Nanoparticles: The next-Generation of Polymeric and Lipid-Based Drug Nanocarriers. Chem. Eng. J. Adv. 2025, 22, 100759. [Google Scholar] [CrossRef]
- Hamdallah, S.I.; Zoqlam, R.; Erfle, P.; Blyth, M.; Alkilany, A.M.; Dietzel, A.; Qi, S. Microfluidics for Pharmaceutical Nanoparticle Fabrication: The Truth and the Myth. Int. J. Pharm. 2020, 584, 119408. [Google Scholar] [CrossRef]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of Polymeric Nanoparticles for Blood-Brain Barrier Transfer-Strategies and Challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef]
- Dong, N.; Ali-Khiavi, P.; Ghavamikia, N.; Pakmehr, S.; Sotoudegan, F.; Hjazi, A.; Gargari, M.K.; Gargari, H.K.; Behnamrad, P.; Rajabi, M.; et al. Nanomedicine in the Treatment of Alzheimer’s Disease: Bypassing the Blood-Brain Barrier with Cutting-Edge Nanotechnology. Neurol. Sci. 2025, 46, 1489–1507. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef] [PubMed]
- Asimakidou, E.; Tan, J.K.S.; Zeng, J.; Lo, C.H. Blood–Brain Barrier-Targeting Nanoparticles: Biomaterial Properties and Biomedical Applications in Translational Neuroscience. Pharmaceuticals 2024, 17, 612. [Google Scholar] [CrossRef] [PubMed]
- Floryanzia, S.D.; Nance, E. Applications and Considerations for Microfluidic Systems to Model the Blood–Brain Barrier. ACS Appl. Bio Mater. 2023, 6, 3617–3632. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Liu, H.; Li, H.; Li, H.; Wong, K.L.; All, A.H. Functionalized Nanomaterials Capable of Crossing the Blood–Brain Barrier. ACS Nano 2024, 18, 1820–1845. [Google Scholar] [CrossRef]
- Perxés Perich, M.; Palma-Florez, S.; Solé, C.; Goberna-Ferrón, S.; Samitier, J.; Gómez-Romero, P.; Mir, M.; Lagunas, A. Polyoxometalate-Decorated Gold Nanoparticles Inhibit β-Amyloid Aggregation and Cross the Blood–Brain Barrier in a Μphysiological Model. Nanomaterials 2023, 13, 2697. [Google Scholar] [CrossRef]
- Palma-Florez, S.; López-Canosa, A.; Moralez-Zavala, F.; Castaño, O.; Kogan, M.J.; Samitier, J.; Lagunas, A.; Mir, M. BBB-on-a-Chip with Integrated Micro-TEER for Permeability Evaluation of Multi-Functionalized Gold Nanorods against Alzheimer’s Disease. J. Nanobiotechnol. 2023, 21, 115. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, C.; Deng, N.; Gao, Z.; Jiang, Z.; Li, X.; Zhou, Y.; Pei, H.; Li, L.; Tang, B. Understanding Drug Nanocarrier and Blood–Brain Barrier Interaction Based on a Microfluidic Microphysiological Model. Lab Chip 2023, 23, 1935–1944. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, C.; Xia, X.; Wang, Y.; Yan, C.; Wang, X.; Lei, T.; Yang, X.; Yang, W.; Cheng, G.; et al. Pathological BBB Crossing Melanin-Like Nanoparticles as Metal-Ion Chelators and Neuroinflammation Regulators against Alzheimer’s Disease. Research 2023, 6, 0180. [Google Scholar] [CrossRef]
- Hassan, N.; Cordero, M.L.; Sierpe, R.; Almada, M.; Juárez, J.; Valdez, M.; Riveros, A.; Vargas, E.; Abou-Hassan, A.; Ruso, J.M.; et al. Peptide Functionalized Magneto-Plasmonic Nanoparticles Obtained by Microfluidics for Inhibition of β-Amyloid Aggregation. J. Mater. Chem. B 2018, 6, 5091–5099. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ye, X.; Du, Y.; Yang, W.; Tong, F.; Li, W.; Huang, Q.; Chen, Y.; Li, H.; Gao, H.; et al. Nose-to-Brain Delivery of Targeted Lipid Nanoparticles as Two-Pronged β-Amyloid Nanoscavenger for Alzheimer’s Disease Therapy. Acta. Pharm. Sin. B 2025, 15, 2884–2899. [Google Scholar] [CrossRef] [PubMed]
- Mohebichamkhorami, F.; Faizi, M.; Mahmoudifard, M.; Hajikarim-Hamedani, A.; Mohseni, S.S.; Heidari, A.; Ghane, Y.; Khoramjouy, M.; Khayati, M.; Ghasemi, R.; et al. Microfluidic Synthesis of Ultrasmall Chitosan/Graphene Quantum Dots Particles for Intranasal Delivery in Alzheimer’s Disease Treatment. Small 2023, 19, e2207626. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, L.; Luo, Z.; Han, D.; Luo, W.; Wan, R.; Li, Y.; Ge, Y.; Lin, W.W.; Xie, Y.; et al. Pantothenate-Encapsulated Liposomes Combined with Exercise for Effective Inhibition of CRM1-Mediated PKM2 Translocation in Alzheimer’s Therapy. J. Control. Release 2024, 373, 336–357. [Google Scholar] [CrossRef]
- Yao, W.; Che, J.; Zhao, C.; Zhang, X.; Zhou, H.; Bai, F. Treatment of Alzheimer’s Disease by Microcapsule Regulates Neurotransmitter Release via Microfluidic Technology. Eng. Regen. 2023, 4, 183–192. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, W.; Zhou, H.; Wang, H.; Kong, B.; Bai, F. Ginkgo Biloba Extract-Loaded PLGA Microcapsules Generated from Microfluidics for Alzheimer’s Disease Treatment. Mater. Des. 2024, 238, 112735. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, W.; Shan, Q.; Zhang, X.; Zhang, D.; Che, J.; Bai, F. Hierarchical Adhesive Hydrogel Microparticles with Galantamine Hydrobromide Encapsulation for the Treatment of Alzheimer’s Disease. Mater. Des. 2024, 246, 113322. [Google Scholar] [CrossRef]
- Sarode, A.; Fan, Y.; Byrnes, A.E.; Hammel, M.; Hura, G.L.; Fu, Y.; Kou, P.; Hu, C.; Hinz, F.I.; Roberts, J.; et al. Predictive High-Throughput Screening of PEGylated Lipids in Oligonucleotide-Loaded Lipid Nanoparticles for Neuronal Gene Silencing. Nanoscale Adv. 2022, 4, 2107–2123. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, L.; Xiao, F.; Wang, L.; Liu, X.; Zhu, L.; Lu, Y.; Zheng, W.; Jiang, X. Dual Targeting Nanoparticles for Epilepsy Therapy. Chem. Sci. 2022, 13, 12913–12920. [Google Scholar] [CrossRef]
- Hou, Q.; Zhu, L.; Wang, L.; Liu, X.; Xiao, F.; Xie, Y.; Zheng, W.; Jiang, X. Screening On-Chip Fabricated Nanoparticles for Penetrating the Blood–Brain Barrier. Nanoscale 2022, 14, 3234–3241, Corrcetion in Nanoscale 2022, 14, 3971–3971. [Google Scholar] [CrossRef]
- Mendanha, D.; Gimondi, S.; Costa, B.M.; Ferreira, H.; Neves, N.M. Microfluidic-Derived Docosahexaenoic Acid Liposomes for Glioblastoma Therapy. Nanomed. Nanotechnol. Biol. Med. 2023, 53, 102704. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.; Wu, Z.; Cui, B.; Zou, C.; Zhang, P.; Yao, S. Microfluidics-Prepared Ultra-Small Biomimetic Nanovesicles for Brain Tumor Targeting. Adv. Healthc. Mater. 2024, 13, 2302302. [Google Scholar] [CrossRef] [PubMed]
- Sommonte, F.; Arduino, I.; Iacobazzi, R.M.; Laera, L.; Silvestri, T.; Lopedota, A.A.; Castegna, A.; Denora, N. Microfluidic Development of Brain-Derived Neurotrophic Factor Loaded Solid Lipid Nanoparticles: An in. Vitro. Evaluation in the Post-Traumatic Brain Injury Neuroinflammation Model. J. Drug Deliv. Sci. Technol. 2024, 96, 105699. [Google Scholar] [CrossRef]
- Gao, M.; Li, Y.; Ho, W.; Chen, C.; Chen, Q.; Li, F.; Tang, M.; Fan, Q.; Wan, J.; Yu, W.; et al. Targeted mRNA Nanoparticles Ameliorate Blood-Brain Barrier Disruption Postischemic Stroke by Modulating Microglia Polarization. ACS Nano 2024, 18, 3260–3275. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Che, X.; Zheng, G.; Liu, Z.; Xie, D.; Wang, L. Microfluidic Preparation and Evaluation of Multivesicular Liposomes Containing Gastrodin for Oral Delivery across the Blood–Brain Barrier. Mol. Pharm. 2024, 21, 5607–5618. [Google Scholar] [CrossRef]
- Adediran, E.; Vijayanand, S.; Kale, A.; Gulani, M.; Wong, J.C.; Escayg, A.; Murnane, K.S.; D’Souza, M.J. Microfluidics-Assisted Formulation of Polymeric Oxytocin Nanoparticles for Targeted Brain Delivery. Pharmaceutics 2025, 17, 452. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Preda, M.D.; Niculescu, A.-G.; Vladâcenco, O.; Radu, C.I.; Grumezescu, A.M.; Teleanu, D.M. Current Strategies to Enhance Delivery of Drugs across the Blood–Brain Barrier. Pharmaceutics 2022, 14, 987. [Google Scholar] [CrossRef]
- Qu, Z.; Luo, J.; Li, Z.; Yang, R.; Zhao, J.; Chen, X.; Yu, S.; Shu, H. Advancements in Strategies for Overcoming the Blood–Brain Barrier to Deliver Brain-Targeted Drugs. Front. Aging. Neurosci. 2024, 16, 1353003. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Lu, Y.; Quan, H.; Wang, Y.; Song, S.; Guo, H. Advanced Oral Drug Delivery Systems for Gastrointestinal Targeted Delivery: The Design Principles and Foundations. J. Nanobiotechnol. 2025, 23, 400. [Google Scholar] [CrossRef]
- Baryakova, T.H.; Pogostin, B.H.; Langer, R.; McHugh, K.J. Overcoming Barriers to Patient Adherence: The Case for Developing Innovative Drug Delivery Systems. Nat. Rev. Drug Discov. 2023, 22, 387–409. [Google Scholar] [CrossRef] [PubMed]
- St Clair-Jones, A.; Prignano, F.; Goncalves, J.; Paul, M.; Sewerin, P. Understanding and Minimising Injection-Site Pain Following Subcutaneous Administration of Biologics: A Narrative Review. Rheumatol. Ther. 2020, 7, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.; Rayner, S.L.; Chung, R.; Shi, B.Y.; Liang, X.J. Advances in Nanotechnology-Based Strategies for the Treatments of Amyotrophic Lateral Sclerosis. Mater. Today Bio 2020, 6, 100055. [Google Scholar] [CrossRef]
- Dighe, S.; Jog, S.; Momin, M.; Sawarkar, S.; Omri, A. Intranasal Drug Delivery by Nanotechnology: Advances in and Challenges for Alzheimer’s Disease Management. Pharmaceutics 2024, 16, 58. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of Intranasal Drug Delivery Directly to the Brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Nguyen, L.T.-T.; Duong, V.-A. Nose-to-Brain Drug Delivery. Encyclopedia 2025, 5, 91. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Limiti, E.; Mozetic, P.; Giannitelli, S.M.; Pinelli, F.; Han, X.; Del Rio, D.; Abbruzzese, F.; Basoli, F.; Rosanò, L.; Scialla, S.; et al. Hyaluronic Acid–Polyethyleneimine Nanogels for Controlled Drug Delivery in Cancer Treatment. ACS Appl. Nano Mater. 2022, 5, 5544–5557. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Limiti, E.; Mozetic, P.; Pinelli, F.; Han, X.; Abbruzzese, F.; Basoli, F.; Rio, D.D.; Scialla, S.; Rossi, F.; et al. Droplet-Based Microfluidic Synthesis of Nanogels for Controlled Drug Delivery: Tailoring Nanomaterial Properties via Pneumatically Actuated Flow-Focusing Junction. Nanoscale 2022, 14, 11415–11428. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s Disease: Experimental Models and Reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical Considerations Regarding Animal Experimentation. J. Prev. Med. Hyg. 2022, 63, E255. [Google Scholar] [CrossRef]
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-Enhanced Blood-Brain Barrier Chip Recapitulates Human Barrier Function and Shuttling of Drugs and Antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef]
- Robert, J.; Button, E.B.; Yuen, B.; Gilmour, M.; Kang, K.; Bahrabadi, A.; Stukas, S.; Zhao, W.; Kulic, I.; Wellington, C.L. Clearance of Beta-Amyloid Is Facilitated by Apolipoprotein E and Circulating High-Density Lipoproteins in Bioengineered Human Vessels. eLife 2017, 6, e29595. [Google Scholar] [CrossRef]
| Device Configuration | Application | Complexity | REF | |
|---|---|---|---|---|
| Technological | Biological | |||
| Parallel (Cylindrical) | Microglia recruitment, neuroinflammation, Aβ accumulation, and p-tau aggregation | • | • | [78] |
| Parallel | BBB disfunction and Aβ deposition | •• | •• | [79] |
| Full-contact | High-resolution tracking of Alzheimer’s-related changes in BBB function | ••• | ••• | [80] |
| Full-contact | Pathogenic mechanism of APOE4 expression in pericytes | • | ••• | [81] |
| Full-contact | Diabetes mellitus contribution to Alzheimer’s disease | •• | •• | [82] |
| Vertical design | Immune-brain interaction in Alzheimer’s disease | • | •• | [83] |
| Material Class | Composition | Target | Mechanism of Action | Biological Validation | REF | |||
|---|---|---|---|---|---|---|---|---|
| In Vitro | In Vivo | |||||||
| Alzheimer’s disease | Nanoparticles | Inorganic NPs | Chitosan coated Gold nanorods & Spion NPs functionalized with peptide D1 | Reduced Aβ deposition and plaque formation | Binding of Aβ through Ab-D1 interaction | SH-SY5Y neuroblastoma cells in a 2D model | - | [116] |
| Organic NPs | Lipid nanoparticles co-encapsulating a-mangostin (a-M) and b-site APP cleaving enzyme 1 (BACE1) siRNA | Neuroprotection & gene silencing to reduce Aβ synthesis | Delivery of a-M and BACE1 SiRNAs | BV-2, PC-12, and Calu-3 cell lines in 2D and transwell model | Male APP/PS1 mice and Male C57BL/6 mice | [117] | ||
| Hybrid NPs | Chitosan & graphene quantum dots | Neuroprotection and neuroinflammation | anti-inflammatory effect and regulation of the brain tissue microenvironment | C6 glioma cells (2D model) & bEnd.3, astrocytes and BV-2 glial cells in a transwell model | Sprague-Dawley Male rats | [118] | ||
| Organic NPs | Pantothenate encapsulated in transferrin modified liposomes (Pan@TRF@Liposome NPs) | Neuroinflammation, metabolic disfunction, neuronal death | modulation of CRM1-mediated PKM2 nuclear translocation | BV-2 glial cells in a 2D model | Male APP/PS1 mice and C57BL/6J mice | [119] | ||
| Microparticles | Hydrogel microparticles | alginate-dopamine core-shell microcapsules | Reduced Aβ deposition, and acetylcholinesterase (AChE) activity & neuroprotection | Delivery of Donepezil | N2A neuroblastoma cells and 3T3 cells in 2D models | APP/PS1 mice and C57BL/6 mice | [120] | |
| Hydrogel microparticles | PLGA-GelMa core-shell microcapsules | Reduced Aβ deposition & neuroprotection | Delivery of Ginkgo biloba extract | N2A neuroblastoma cells and 3T3 cells in 2D models | APP/PS1 mice and C57BL/6J mice | [121] | ||
| Hydrogel microparticles & inorganic NPs | Mesoporous silica nanocarriers encapsulated into alginate-dopamine core-shell microcapsules | Reduced Aβ deposition and AChE activity | Delivery of Galantamine hydrobromide | N2A, Caco-2 and 3T3 cells in a 2D model | APP/PS1 mice and C57BL/6 mice | [122] | ||
| Material class | Composition | Application | REF | |||||
| Other CNS disorders | Organic NPs | PEGylated lipid nanoparticles loaded with antisense oligonucleotides | Neuronal gene silencing | [123] | ||||
| Hybrid NPs | PLGA core with lamotrigine, lipid shell functionalized with D-T7 and Tet1 peptides | Epilepsy therapy via dual BBB and neuron targeting | [124] | |||||
| Hybrid NPs | PLGA core with lamotrigine, lipid shell modified with peptides (T7, D-T7, GSH, TGN, CGN, TAT) | BBB penetration and epilepsy therapy | [125] | |||||
| Organic NPs | Docosahexaenoic acid liposomes | Glioblastoma therapy | [126] | |||||
| Organic NPs | Macrophage cell membrane-derived nanovesicles loaded with indocyanine green | Targeted brain tumor theranostics | [127] | |||||
| Organic NPs | Solid lipid nanoparticles loaded with brain-derived neurotrophic factor | Brain delivery for post-traumatic brain injury neuroinflammation | [128] | |||||
| Organic NPs | Lipid nanoparticles delivering mRNA encoding IL-10 | Stroke therapy, BBB protection, neuroinflammation modulation | [129] | |||||
| Organic NPs | Multivesicular liposomes containing gastrodin | Oral delivery across BBB for CNS diseases | [130] | |||||
| Organic NPs | Polymeric nanoparticles (cross-linked BSA) conjugated with RVG peptide | Targeted brain delivery for seizure control and CNS disorders | [131] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, I.; Limiti, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A.; D’Amelio, M.; La Barbera, L.; Gori, M. Microfluidic-Based Technologies for Crossing the Blood–Brain Barrier Against Alzheimer’s Disease: Novel Strategies and Challenges. Int. J. Mol. Sci. 2025, 26, 9478. https://doi.org/10.3390/ijms26199478
Ferrari I, Limiti E, Giannitelli SM, Trombetta M, Rainer A, D’Amelio M, La Barbera L, Gori M. Microfluidic-Based Technologies for Crossing the Blood–Brain Barrier Against Alzheimer’s Disease: Novel Strategies and Challenges. International Journal of Molecular Sciences. 2025; 26(19):9478. https://doi.org/10.3390/ijms26199478
Chicago/Turabian StyleFerrari, Irene, Emanuele Limiti, Sara Maria Giannitelli, Marcella Trombetta, Alberto Rainer, Marcello D’Amelio, Livia La Barbera, and Manuele Gori. 2025. "Microfluidic-Based Technologies for Crossing the Blood–Brain Barrier Against Alzheimer’s Disease: Novel Strategies and Challenges" International Journal of Molecular Sciences 26, no. 19: 9478. https://doi.org/10.3390/ijms26199478
APA StyleFerrari, I., Limiti, E., Giannitelli, S. M., Trombetta, M., Rainer, A., D’Amelio, M., La Barbera, L., & Gori, M. (2025). Microfluidic-Based Technologies for Crossing the Blood–Brain Barrier Against Alzheimer’s Disease: Novel Strategies and Challenges. International Journal of Molecular Sciences, 26(19), 9478. https://doi.org/10.3390/ijms26199478








