A Microarray, Validation, and Gene-Enrichment Approach for Assessing Differentially Expressed Circulating miRNAs in Obese and Lean Heart Failure Patients: A Case–Control Study
Abstract
1. Introduction
2. Results
2.1. Discovery Cohort
2.1.1. Baseline Characteristics
2.1.2. Microarray Results
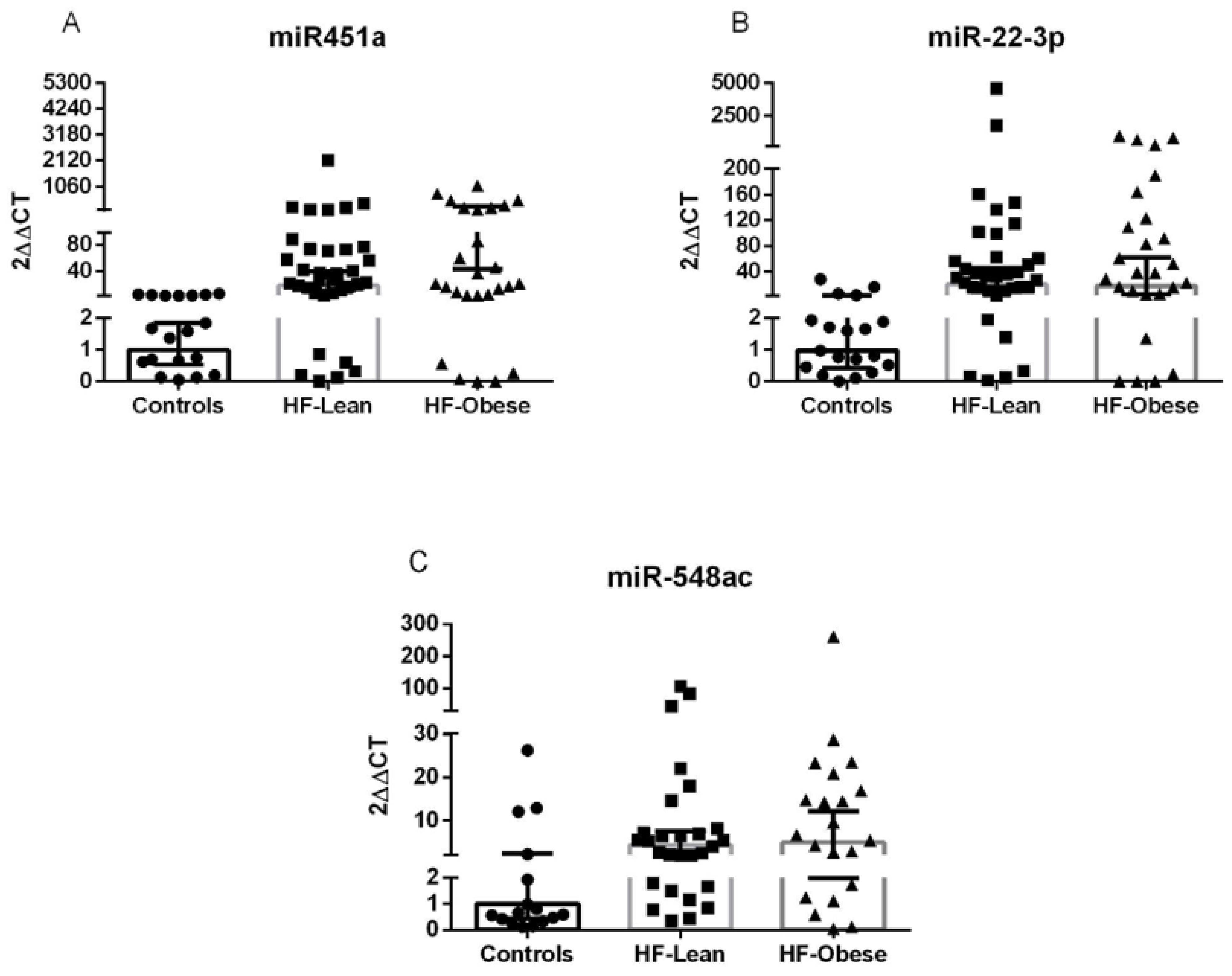
2.2. Validation Cohort
2.2.1. Baseline Characteristics
2.2.2. Validation Results
2.2.3. Construction of HF-miR–Gene Network
3. Discussion
4. Materials and Methods
4.1. Patients and Controls
4.2. Data Collection
4.3. Sample Preparation
4.4. Microarray Analysis
4.5. RT-qPCR
4.6. Target and Pathway Analysis of Validated miRNAs
4.7. Ethical Considerations
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prospective Studies Collaboration; Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-Mass Index and Cause-Specific Mortality in 900,000 Adults: Collaborative Analyses of 57 Prospective Studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Després, J.-P.; Davis, N.; Grossniklaus, D.A.; Klein, S.; Lamarche, B.; Lopez-Jimenez, F.; Rao, G.; St-Onge, M.-P.; Towfighi, A.; et al. Assessing Adiposity: A Scientific Statement from the American Heart Association. Circulation 2011, 124, 1996–2019. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Sesso, H.D.; Gaziano, J.M. Body Mass Index and Vigorous Physical Activity and the Risk of Heart Failure among Men. Circulation 2009, 119, 44–52. [Google Scholar] [CrossRef]
- Oguntade, A.S.; Islam, N.; Malouf, R.; Taylor, H.; Jin, D.; Lewington, S.; Lacey, B. Body Composition and Risk of Incident Heart Failure in 1 Million Adults: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2023, 12, e029062. [Google Scholar] [CrossRef]
- Joyce, E.; Lala, A.; Stevens, S.R.; Cooper, L.B.; AbouEzzeddine, O.F.; Groarke, J.D.; Grodin, J.L.; Braunwald, E.; Anstrom, K.J.; Redfield, M.M.; et al. Prevalence, Profile, and Prognosis of Severe Obesity in Contemporary Hospitalized Heart Failure Trial Populations. JACC Heart Fail. 2016, 4, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Oktay, A.A.; Lavie, C.J.; Kokkinos, P.F.; Parto, P.; Pandey, A.; Ventura, H.O. The Interaction of Cardiorespiratory Fitness with Obesity and the Obesity Paradox in Cardiovascular Disease. Prog. Cardiovasc. Dis. 2017, 60, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Padwal, R.; McAlister, F.A.; McMurray, J.J.V.; Cowie, M.R.; Rich, M.; Pocock, S.; Swedberg, K.; Maggioni, A.; Gamble, G.; Ariti, C.; et al. The Obesity Paradox in Heart Failure Patients with Preserved versus Reduced Ejection Fraction: A Meta-Analysis of Individual Patient Data. Int. J. Obes. 2014, 38, 1110–1114. [Google Scholar]
- Sharma, A.; Lavie, C.J.; Borer, J.S.; Vallakati, A.; Goel, S.; Lopez-Jimenez, F.; Arbab-Zadeh, A.; Mukherjee, D.; Lazar, J.M. Meta-Analysis of the Relation of Body Mass Index to All-Cause and Cardiovascular Mortality and Hospitalization in Patients with Chronic Heart Failure. Am. J. Cardiol. 2015, 115, 1428–1434. [Google Scholar]
- Alebna, P.L.; Mehta, A.; Yehya, A.; daSilva-deAbreu, A.; Lavie, C.J.; Carbone, S. Update on Obesity, the Obesity Paradox, and Obesity Management in Heart Failure. Prog. Cardiovasc. Dis. 2024, 82, 34–42. [Google Scholar] [CrossRef]
- Thaker, V.V. Genetic and Epigenetic Causes of Obesity. Adolesc. Med. State Art Rev. 2017, 28, 379–405. [Google Scholar]
- Sahu, P.; Bestepe, F.; Vehbi, S.; Ghanem, G.F.; Blanton, R.M.; Icli, B. Obesity and Heart Failure: Mechanistic Insights and the Regulatory Role of MicroRNAs. Genes 2025, 16, 647. [Google Scholar] [CrossRef]
- Kim, S.Y.; Morales, C.R.; Gillette, T.G.; Hill, J.A. Epigenetic Regulation in Heart Failure. Curr. Opin. Cardiol. 2016, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Thomé, J.G.; Mendoza, M.R.; Cheuiche, A.V.; La Porta, V.L.; Silvello, D.; Dos Santos, K.G.; Andrades, M.E.; Clausell, N.; Rohde, L.E.; Biolo, A. Circulating microRNAs in Obese and Lean Heart Failure Patients: A Case-Control Study with Computational Target Prediction Analysis. Gene 2015, 574, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sonsöz, M.R.; Yilmaz, M.; Cevik, E.; Orta, H.; Bilge, A.K.; Elitok, A.; Onur, I.; Komurcu-Bayrak, E. Circulating Levels of MicroRNAs in Hypertrophic Cardiomyopathy: The Relationship with Left Ventricular Hypertrophy, Left Atrial Dilatation and Ventricular Depolarisation-Repolarisation Parameters. Heart Lung Circ. 2022, 31, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Scrimgeour, N.R.; Wrobel, A.; Pinho, M.J.; Høydal, M.A. microRNA-451a Prevents Activation of Matrix Metalloproteinases 2 and 9 in Human Cardiomyocytes during Pathological Stress Stimulation. Am. J. Physiol. Cell Physiol. 2020, 318, C94–C102. [Google Scholar] [CrossRef]
- Deng, H.-Y.; He, Z.-Y.; Dong, Z.-C.; Zhang, Y.-L.; Han, X.; Li, H.-H. MicroRNA-451a Attenuates Angiotensin II-Induced Cardiac Fibrosis and Inflammation by Directly Targeting T-box1. J. Physiol. Biochem. 2022, 78, 257–269. [Google Scholar] [CrossRef]
- Lage, R.; Cebro-Márquez, M.; Vilar-Sánchez, M.E.; González-Melchor, L.; García-Seara, J.; Martínez-Sande, J.L.; Fernández-López, X.A.; Aragón-Herrera, A.; Martínez-Monzonís, M.A.; González-Juanatey, J.R.; et al. Circulating miR-451a Expression May Predict Recurrence in Atrial Fibrillation Patients after Catheter Pulmonary Vein Ablation. Cells 2023, 12, 638. [Google Scholar] [CrossRef]
- Wang, T.; Wu, F.; Yu, D. miR-144/451 in Hematopoiesis and beyond. ExRNA 2019, 1, 16. [Google Scholar] [CrossRef]
- van Boven, N.; Akkerhuis, K.M.; Anroedh, S.S.; Rizopoulos, D.; Pinto, Y.; Battes, L.C.; Hillege, H.L.; Caliskan, K.C.; Germans, T.; Manintveld, O.C.; et al. Serially Measured Circulating miR-22-3p Is a Biomarker for Adverse Clinical Outcome in Patients with Chronic Heart Failure: The Bio-SHiFT Study. Int. J. Cardiol. 2017, 235, 124–132. [Google Scholar] [CrossRef]
- Zhao, X.-S.; Ren, Y.; Wu, Y.; Ren, H.-K.; Chen, H. MiR-30b-5p and miR-22-3p Restrain the Fibrogenesis of Post-Myocardial Infarction in Mice via Targeting PTAFR. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3993–4004. [Google Scholar]
- Guo, S.; Jin, Y.; Zhou, J.; Zhu, Q.; Jiang, T.; Bian, Y.; Zhang, R.; Chang, C.; Xu, L.; Shen, J.; et al. MicroRNA Variants and HLA-miRNA Interactions Are Novel Rheumatoid Arthritis Susceptibility Factors. Front. Genet. 2021, 12, 747274. [Google Scholar] [CrossRef]
- Song, F.; Yang, Y.; Liu, J. MicroRNA-548ac Induces Apoptosis in Laryngeal Squamous Cell Carcinoma Cells by Targeting Transmembrane Protein 158. Oncol. Lett. 2020, 20, 69. [Google Scholar] [CrossRef]
- Lyu, P.; Hao, Z.; Zhang, H.; Li, J. Identifying Pancreatic Cancer-Associated miRNAs Using Weighted Gene Co-Expression Network Analysis. Oncol. Lett. 2022, 24, 297. [Google Scholar] [CrossRef] [PubMed]
- Barquet-Muñoz, S.A.; Pedroza-Torres, A.; Perez-Plasencia, C.; Montaño, S.; Gallardo-Alvarado, L.; Pérez-Montiel, D.; Herrera-Montalvo, L.A.; Cantú-de León, D. MicroRNA Profile Associated with Positive Lymph Node Metastasis in Early-Stage Cervical Cancer. Curr. Oncol. 2022, 29, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Schiattarella, G.G.; Lindberg, F.; Anker, M.S.; Bayes-Genis, A.; Bäck, M.; Braunschweig, F.; Bucciarelli-Ducci, C.; Butler, J.; Cannata, A.; et al. Heart Failure and Obesity: Translational Approaches and Therapeutic Perspectives. A Scientific Statement of the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2025, 27, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Alpert, M.A.; Arena, R.; Mehra, M.R.; Milani, R.V.; Ventura, H.O. Impact of Obesity and the Obesity Paradox on Prevalence and Prognosis in Heart Failure. JACC Heart Fail. 2013, 1, 93–102. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X. Obesity: Criteria and Classification. Proc. Nutr. Soc. 2000, 59, 505–509. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The Sva Package for Removing Batch Effects and Other Unwanted Variation in High-Throughput Experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Lin, Y.-C.-D.; Li, J.; Huang, K.-Y.; Shrestha, S.; Hong, H.-C.; Tang, Y.; Chen, Y.-G.; Jin, C.-N.; Yu, Y.; et al. miRTarBase 2020: Updates to the Experimentally Validated microRNA-Target Interaction Database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G.; et al. DIANA-TarBase v8: A Decade-Long Collection of Experimentally Supported miRNA–gene Interactions. Nucleic Acids Res. 2018, 46, D239–D245. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape Plug-in to Decipher Functionally Grouped Gene Ontology and Pathway Annotation Networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape Plugin: Pathway Insights Using Integrated Experimental and in Silico Data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Nakaya, A. The KEGG Databases at GenomeNet. Nucleic Acids Res. 2002, 30, 42–46. [Google Scholar] [CrossRef]



| Characteristics | Controls (n = 10) | HF–Lean (n = 10) | HF–Obese (n = 10) | p-Value |
|---|---|---|---|---|
| Age (years) | 54.09 ± 10.70 | 53.11 ± 10.87 | 54.56 ± 15.00 | 0.965 |
| Male sex (%) | 7 (70) | 7 (70) | 5 (50) | 0.563 |
| White ethnicity (%) | 9 (90) | 6 (60) | 7 (70) | 0.535 |
| Body weight (kg) | 69.80 ± 11.86 | 60.00 ± 11.44 | 106.23 ± 24.41 ** | 0.000004 |
| Body mass index (kg/m2) | 23.77 ± 2.10 | 21.62 ± 2.07 | 38.09 ± 5.91 ** | <0.000001 |
| Body fat (%) | 15.91 ± 6.98 | 14.28 ± 5.13 | 34.92 ± 5.44 ** | <0.000001 |
| Waist circumference (cm) | 82.82 ± 9.19 | 119.34 ± 13.23 ** | 0.000001 | |
| Heart rate (beats/min) | 77.44 ± 11.95 | 76.22 ± 12.02 | 0.831 | |
| Echocardiography indices | ||||
| LVEF (%) | 29.30 ± 7.09 | 29.20 ± 10.15 | 0.980 | |
| LVEDD (mm) | 64.70 ± 11.62 | 66.30 ± 4.52 | 0.690 | |
| LVESD (mm) | 55.80 ± 11.65 | 56.10 ± 5.90 | 0.943 | |
| NYHA class (%) | ||||
| I or II | 9 (90) | 8 (80) | 0.531 | |
| III | 1 (10) | 2 (20) | 0.531 | |
| HF etiology (%) | ||||
| Ischemic | 5 (50) | 6 (60) | 0.653 | |
| Non-ischemic | 5 (50) | 4 (40) | 0.653 | |
| Medical history (%) | ||||
| Hypertension | 4 (40) | 6 (60) | 0.371 | |
| Diabetes | 3 (30) | 3 (30) | 1.0 | |
| Atrial fibrillation or flutter | 2 (20) | 2 (20) | 1.0 | |
| Myocardial Infarction | 4 (40) | 5 (50) | 0.653 | |
| Medication (%) | ||||
| ACE inhibitor or ARB | 9 (90) | 10 (100) | 0.305 | |
| Beta-blocker | 6(60) | 8 (80) | 0.329 | |
| Diuretic | 8 (80) | 9 (90) | 0.531 | |
| Statins | 4 (40) | 7 (70) | 0.178 | |
| Calcium channel blocker | 0 (0%) | 3 (30) | 0.06 | |
| Vasodilators | 3 (30) | 2 (20) | 0.606 | |
| Nitrates | 4 (40) | 4 (40) | 1.0 | |
| Antiarrhythmics | 1 (10) | 0 (0) | 0.305 | |
| Digitalis | 9 (90) | 9 (90) | 1.0 | |
| Antiplatelet agents | 6 (60) | 5 (50) | 0.653 | |
| Oral anticoagulants | 3 (30) | 0 (0) | 0.06 | |
| Hypoglycemic | 2 (20) | 0 (0) | 0.136 | |
| Blood pressure (mmHg) | ||||
| Systolic | 125.00 ± 22.77 | 118.80 ± 20.81 | 0.533 | |
| Diastolic | 73.40 ± 12.58 | 77.10 ± 14.41 | 0.548 | |
| Laboratory | ||||
| Creatinine (mg/dL) | 1.26 ± 0.48 | 1.14 ± 0.27 | 0.492 | |
| Hemoglobin (g/dL) | 13.40 ± 1.42 | 13.65 ± 1.73 | 0.761 | |
| Total cholesterol (mg/dL) | 150.14 ± 30.71 | 170.14 ± 27.40 | 0.223 | |
| LDL cholesterol (mg/dL) | 67.15± 35.21 | 77.83 ± 41.08 | 0.597 | |
| HDL cholesterol (mg/dL) | 42.29 ± 16.05 | 38.86 ± 6.82 | 0.612 | |
| Triglycerides (mg/dL) | 155.57 ± 95.34 | 164.33 ± 41.87 | 0.839 | |
| Albumin (g/dL) | 4.38 ± 0.24 | 4.43 ± 0.16 | 0.671 |
| Characteristics | Controls (n = 19) | HF–Lean (n = 35) | HF–Obese (n = 26) | p-Value |
|---|---|---|---|---|
| Age (years) | 49.3 ± 12.43 | 56.22 ± 10.54 | 56.50 ± 13.44 | 0.089 |
| Male sex (%) | 12 (63.2) | 22 (62.9) | 17 (65.4) | 0.978 |
| White ethnicity (%) | 17 (89.5) | 24 (68.6) | 17 (68.0) | 0.258 |
| Body weight (kg) | 69.29 ± 10.98 | 60.32 ± 9.09 | 100.32 ± 18.65 * | <0.001 |
| Body mass index (kg/m2) | 24.40 ± 2.51 | 21.74 ± 2.06 * | 36.98 ± 4.96 * | <0.001 |
| Body fat (%) | 15.88 ± 5.68 | 16.94 ± 6.35 | 32.94 ± 5.19 * | <0.001 |
| Waist circumference (cm) | 84.57 ± 7.84 | 116.73 ± 11.04 | <0.001 | |
| Heart rate (beats/min) | 73.15 ± 14.21 | 80.08 ± 13.95 | 0.069 | |
| Echocardiography indices | ||||
| LVEF (%) | 31.85 ± 10.25 | 30.04 ± 8.85 | 0.474 | |
| LVEDD (mm) | 63.68 ± 9.44 | 65.50 ± 6.77 | 0.408 | |
| LVESD (mm) | 51.48 ± 13.68 | 55.62 ± 7.43 | 0.171 | |
| NYHA class (%) | ||||
| I or II | 30 (85.7) | 21 (80.8) | 0.606 | |
| III | 5 (14.3) | 5 (19.2) | 0.606 | |
| HF etiology (%) | ||||
| Ischemic | 12 (34.3) | 8 (30.8) | 0.772 | |
| Non-ischemic | 23 (65.7) | 18 (69.2) | 0.772 | |
| Medical history (%) | ||||
| Hypertension | 13 (37.1) | 7 (26.9) | 0.05 | |
| Diabetes | 9 (25.7) | 9 (34.6) | 0.451 | |
| Atrial fibrillation or flutter | 8 (22.9) | 10 (38.5) | 0.186 | |
| Myocardial Infarction | 9 (25.7) | 7 (26.9) | 0.915 | |
| Medication (%) | ||||
| ACE inhibitor or ARB | 34 (97.1) | 25 (96.2) | 0.830 | |
| Beta-blocker | 27 (77.1) | 20 (76.9) | 0.984 | |
| Diuretic | 28 (80) | 25 (96.2) | 0.065 | |
| Statins | 14 (40) | 13 (50) | 0.437 | |
| Calcium channel blocker | 0 (0) | 4 (15.4) | 0.016 | |
| Vasodilators | 6 (17.1) | 8 (30.8) | 0.211 | |
| Nitrates | 9 (25.7) | 7 (26.9) | 0.915 | |
| Antiarrhythmics | 3 (8.6) | 1 (3.8) | 0.461 | |
| Digitalis | 30 (85.7) | 22 (42.3) | 0.905 | |
| Antiplatelet agents | 16 (45.7) | 10 (38.5) | 0.571 | |
| Oral anticoagulants | 9 (25.7) | 4 (30.8) | 0.330 | |
| Hypoglycemic | 4 (11.4) | 4 (15.4) | 0.651 | |
| Blood pressure (mmHg) | ||||
| Systolic | 116.69 ± 23.95 | 136.96 ± 31.20 | 0.006 | |
| Diastolic | 70.46 ± 14.58 | 84.69 ± 14.14 | <0.001 | |
| Laboratory | ||||
| Creatinine (mg/dL) | 1.18 ± 0.41 | 1.12 ± 0.36 | 0.609 | |
| Hemoglobin (g/dL) | 13.65 ± 1.57 | 56.27 ± 205.51 | 0.252 | |
| Total cholesterol (mg/dL) | 172.81 ± 47.09 | 180.42 ± 92.89 | 0.717 | |
| LDL cholesterol (mg/dL) | 93.07 ± 40.18 | 82.34 ± 8.03 | 0.361 | |
| HDL cholesterol (mg/dL) | 42.70 ± 11.94 | 39.11 ± 10.61 | 0.302 | |
| Triglycerides (mg/dL) | 210.56 ± 316.25 | 375.22 ± 954.58 | 0.409 | |
| Albumin (g/dL) | 4.34 ± 0.26 | 4.44 ± 0.21 | 0.279 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, D.d.S.; Lopes, A.; Recamonde-Mendoza, M.; Bueno, R.H.; Calloni, R.; Clausell, N.; Tobar Leitão, S.A.; Biolo, A. A Microarray, Validation, and Gene-Enrichment Approach for Assessing Differentially Expressed Circulating miRNAs in Obese and Lean Heart Failure Patients: A Case–Control Study. Int. J. Mol. Sci. 2025, 26, 9475. https://doi.org/10.3390/ijms26199475
Soares DdS, Lopes A, Recamonde-Mendoza M, Bueno RH, Calloni R, Clausell N, Tobar Leitão SA, Biolo A. A Microarray, Validation, and Gene-Enrichment Approach for Assessing Differentially Expressed Circulating miRNAs in Obese and Lean Heart Failure Patients: A Case–Control Study. International Journal of Molecular Sciences. 2025; 26(19):9475. https://doi.org/10.3390/ijms26199475
Chicago/Turabian StyleSoares, Douglas dos Santos, Amanda Lopes, Mariana Recamonde-Mendoza, Rodrigo Haas Bueno, Raquel Calloni, Nadine Clausell, Santiago Alonso Tobar Leitão, and Andreia Biolo. 2025. "A Microarray, Validation, and Gene-Enrichment Approach for Assessing Differentially Expressed Circulating miRNAs in Obese and Lean Heart Failure Patients: A Case–Control Study" International Journal of Molecular Sciences 26, no. 19: 9475. https://doi.org/10.3390/ijms26199475
APA StyleSoares, D. d. S., Lopes, A., Recamonde-Mendoza, M., Bueno, R. H., Calloni, R., Clausell, N., Tobar Leitão, S. A., & Biolo, A. (2025). A Microarray, Validation, and Gene-Enrichment Approach for Assessing Differentially Expressed Circulating miRNAs in Obese and Lean Heart Failure Patients: A Case–Control Study. International Journal of Molecular Sciences, 26(19), 9475. https://doi.org/10.3390/ijms26199475







