DNA Methylation Mediates the Association Between Prenatal Maternal Stress and the Broad Autism Phenotype in Human Adolescents: Project Ice Storm
Abstract
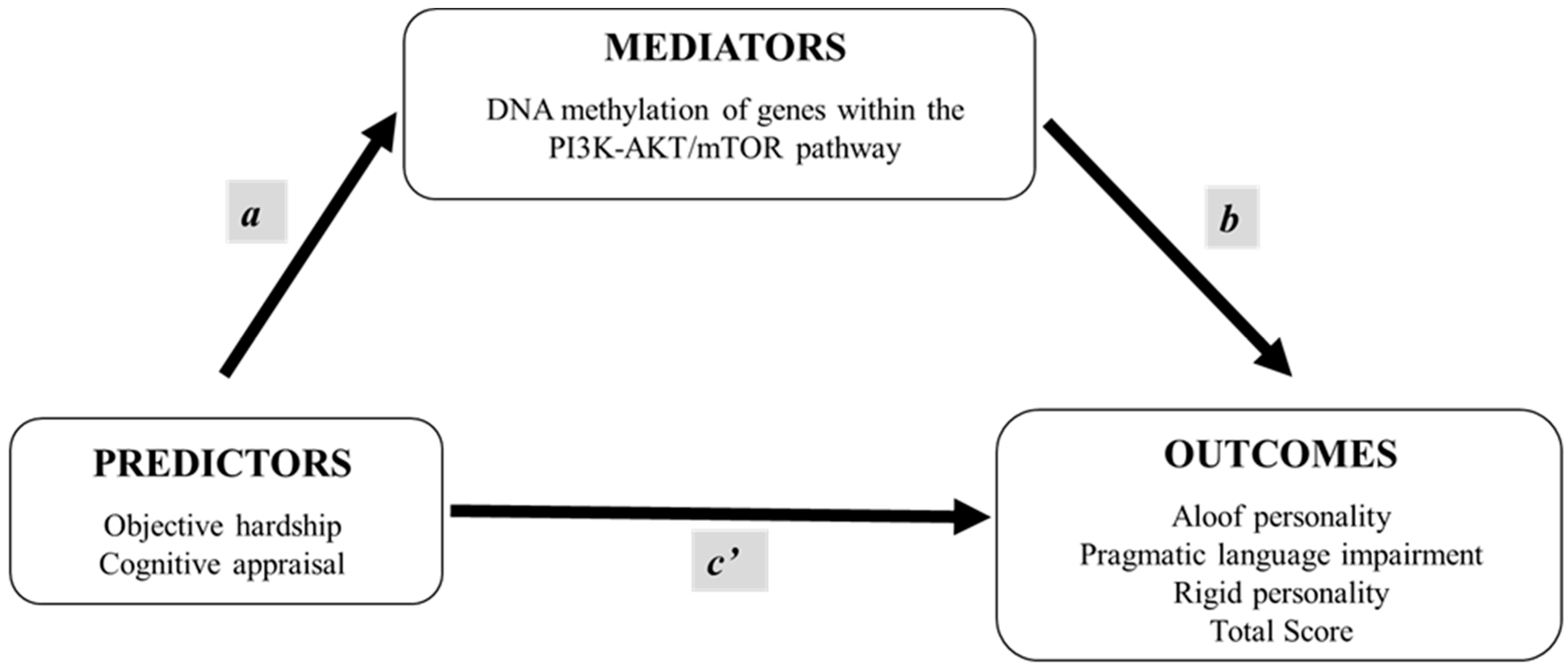
1. Introduction
2. Results
2.1. Descriptive Statistics
2.2. Correlation Analyses
2.3. Mediation Analyses
2.3.1. Methylation Levels of CpGs Mediate the Association Between Maternal Objective Hardship and Components of BAP (Table 3)
2.3.2. Methylation Levels of CpGs Mediate the Association Between Maternal Cognitive Appraisal and Components of BAP (Table 3)
2.4. Summary
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Questionnaires
4.3. Blood Collection and DNA Extraction
4.4. Infinium Human Methylation 450K BeadChip Array and Data Analysis
4.5. Selection of CpGs Within Genes of the PI3K-AKT/mTOR Pathway
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | AKT Serine/Threonine Kinase |
| ASD | Autism Spectrum Disorder |
| BCL2L1 | BCL2 Like |
| DNA | Deoxyribonucleic Acid |
| FNBP1 | Formin Binding Protein 1 |
| IES-R | Impact of Event Scale—Revised |
| mTOR | mechanistic target of rapamycin |
| NFKBIA | NFKB Inhibitor Alpha |
| P13K | phosphatidylinositol 3-kinase |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PIK3CD | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Delta |
| PNMS | Prenatal Maternal Stress |
| PPP2R5C | Protein Phosphatase 2 Regulatory Subunit B’Gamma |
| PPP2R5E | Protein Phosphatase 2 Regulatory Subunit B’Epsilon |
| PRKCH | Protein Kinase C Eta |
| PRRSL | Proline Rich 5 Like |
| PTSD | Post-Traumatic Stress Disorder |
| RPTOR | Regulatory Associated Protein of mTOR Complex 1 |
References
- Grzadzinski, R.; Huerta, M.; Lord, C. DSM-5 and autism spectrum disorders (ASDs): An opportunity for identifying ASD subtypes. Mol. Autism 2013, 4, 12. [Google Scholar] [CrossRef]
- Zaky, E. Autism spectrum disorder (asd); The Past, The Present, and The Future. J. Child Adolesc. Behav. 2017, 5, e116. [Google Scholar] [CrossRef]
- Genovese, A.; Butler, M.G. Clinical assessment, genetics, and treatment approaches in autism spectrum disorder (ASD). Int. J. Mol. Sci. 2020, 21, 4726. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association, D.; American Psychiatric Association, D. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013; Volume 5, pp. 36–37. [Google Scholar]
- Piven, J. The broad autism phenotype: A complementary strategy for molecular genetic studies of autism. Am. J. Med. Genet. 2001, 105, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.S.; Losh, M.; Parlier, M.; Reznick, J.S.; Piven, J. The broad autism phenotype questionnaire. J. Autism Dev. Disord. 2007, 37, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Sasson, N.J.; Lam, K.S.; Childress, D.; Parlier, M.; Daniels, J.L.; Piven, J. The B road A utism P henotype Q uestionnaire: Prevalence and Diagnostic Classification. Autism Res. 2013, 6, 134–143. [Google Scholar] [CrossRef]
- Gerdts, J.; Bernier, R. The broader autism phenotype and its implications on the etiology and treatment of autism spectrum disorders. Autism Res. Treat. 2011, 2011, 545901. [Google Scholar] [CrossRef]
- Pisula, E.; Ziegart-Sadowska, K. Broader autism phenotype in siblings of children with ASD—A review. Int. J. Mol. Sci. 2015, 16, 13217–13258. [Google Scholar] [CrossRef]
- Rubenstein, E.; Chawla, D. Broader autism phenotype in parents of children with autism: A systematic review of percentage estimates. J. Child Fam. Stud. 2018, 27, 1705–1720. [Google Scholar] [CrossRef]
- Barker, D.J. In utero programming of chronic disease. Clin. Sci. 1998, 95, 115–128. [Google Scholar] [CrossRef]
- Barouki, R.; Gluckman, P.D.; Grandjean, P.; Hanson, M.; Heindel, J.J. Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health 2012, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Cummings, E.M.; Davies, P.T. Maternal depression and child development. J. Child Psychol. Psychiatry 1994, 35, 73–112. [Google Scholar] [CrossRef] [PubMed]
- Deave, T.; Heron, J.; Evans, J.; Emond, A. The impact of maternal depression in pregnancy on early child development. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1043–1051. [Google Scholar] [CrossRef]
- Urizar, G.G., Jr.; Muñoz, R.F. Role of maternal depression on child development: A prospective analysis from pregnancy to early childhood. Child Psychiatry Hum. Dev. 2022, 53, 502–514. [Google Scholar] [CrossRef]
- Graignic-Philippe, R.; Dayan, J.; Chokron, S.; Jacquet, A.; Tordjman, S. Effects of prenatal stress on fetal and child development: A critical literature review. Neurosci. Biobehav. Rev. 2014, 43, 137–162. [Google Scholar] [CrossRef]
- Pianta, R.C.; Egeland, B.; Sroufe, L.A. Maternal stress and children’s development: Prediction of school outcomes and identification of protective factors. Risk Prot. Factors Dev. Psychopathol. 1990, 215–235. [Google Scholar]
- Wazana, A.; Székely, E.; Oberlander, T.F. Prenatal Stress and Child Development; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Lei, G.; Xi, Q.Q.; Jun, W.; Yu, H.; Wei, D.; Su, Y.Y.; Zhang, X. Association between prenatal environmental factors and child autism: A case control study in Tianjin, China. Biomed. Environ. Sci. 2015, 28, 642–650. [Google Scholar]
- Say, G.N.; Karabekiroğlu, K.; Babadağı, Z.; Yüce, M. Maternal stress and perinatal features in autism and attention deficit/hyperactivity disorder. Pediatr. Int. 2016, 58, 265–269. [Google Scholar] [CrossRef]
- Lafortune, S.; Laplante, D.P.; Elgbeili, G.; Li, X.; Lebel, S.; Dagenais, C.; King, S. Effect of Natural Disaster-Related Prenatal Maternal Stress on Child Development and Health: A Meta-Analytic Review. Int. J. Environ. Res. Public Health 2021, 18, 8332. [Google Scholar] [CrossRef]
- Kinney, D.K.; Miller, A.M.; Crowley, D.J.; Huang, E.; Gerber, E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J. Autism Dev. Disord. 2008, 38, 481–488. [Google Scholar] [CrossRef]
- Nomura, Y.; Zhang, W.; Hurd, Y.L. Stress in pregnancy: Clinical and adaptive behavior of offspring following Superstorm Sandy. Dev. Psychopathol. 2022, 34, 1249–1259. [Google Scholar] [CrossRef]
- Unsel-Bolat, G.; Yildirim, S.; Kilicaslan, F.; Caparros-Gonzalez, R.A. Natural Disasters as a Maternal Prenatal Stressor and Children’s Neurodevelopment: A Systematic Review. Behav. Sci. 2024, 14, 1054. [Google Scholar] [CrossRef]
- Dieckmann, L.; Czamara, D. Epigenetics of prenatal stress in humans: The current research landscape. Clin. Epigenetics 2024, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Mandy, M.; Nyirenda, M. Developmental Origins of Health and Disease: The relevance to developing nations. Int. Health 2018, 10, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Alberts, I.; Li, X. Dysregulation of the IGF-I/PI3K/AKT/mTOR signaling pathway in autism spectrum disorders. Int. J. Dev. Neurosci. 2014, 35, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.X.; Zhang, Q.L. PI3K/AKT/mTOR-mediated autophagy in the development of autism spectrum disorder. Brain Res. Bull. 2016, 125, 152–158. [Google Scholar] [CrossRef]
- Yeung, K.S.; Tso, W.W.Y.; Ip, J.J.K.; Mak, C.C.Y.; Leung, G.K.C.; Tsang, M.H.Y.; Ying, D.; Pei, S.L.C.; Lee, S.L.; Yang, W. Identification of mutations in the PI3K-AKT-mTOR signalling pathway in patients with macrocephaly and developmental delay and/or autism. Mol. Autism 2017, 8, 66. [Google Scholar] [CrossRef]
- Thomas, S.D.; Jha, N.K.; Ojha, S.; Sadek, B. mTOR signaling disruption and its association with the development of autism spectrum disorder. Molecules 2023, 28, 1889. [Google Scholar] [CrossRef]
- Onore, C.; Yang, H.; Van de Water, J.; Ashwood, P. Dynamic Akt/mTOR signaling in children with autism spectrum disorder. Front. Pediatr. 2017, 5, 43. [Google Scholar] [CrossRef]
- Sharma, A.; Mehan, S. Targeting PI3K-AKT/mTOR signaling in the prevention of autism. Neurochem. Int. 2021, 147, 105067. [Google Scholar] [CrossRef]
- Jahrling, J.B.; Laberge, R.M. Age-Related Neurodegeneration Prevention Through mTOR Inhibition: Potential Mechanisms and Remaining Questions. Curr. Top. Med. Chem. 2015, 15, 2139–2151. [Google Scholar] [CrossRef]
- Enriquez-Barreto, L.; Morales, M. The PI3K signaling pathway as a pharmacological target in Autism related disorders and Schizophrenia. Mol. Cell Ther. 2016, 4, 2. [Google Scholar] [CrossRef]
- Chang, S.E.; McDaniels, T.L.; Mikawoz, J.; Peterson, K. Infrastructure failure interdependencies in extreme events: Power outage consequences in the 1998 Ice Storm. Nat. Hazards 2007, 41, 337–358. [Google Scholar] [CrossRef]
- Lecomte, E.L.; Pang, A.W.; Russell, J.W. Ice storm’98; Institute for Catastrophic Loss Reduction: Toronto, ON, Canada, 1998. [Google Scholar]
- Kerry, M.; Kelk, G.; Etkin, D.; Burton, I.; Kalhok, S. Glazed over: Canada copes with the ice storm of 1998. Environ. Sci. Policy Sustain. Dev. 1999, 41, 6–11. [Google Scholar] [CrossRef]
- King, S.; Dancause, K.; Turcotte-Tremblay, A.M.; Veru, F.; Laplante, D.P. Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Res. C Embryo. Today 2012, 96, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Walder, D.J.; Laplante, D.P.; Sousa-Pires, A.; Veru, F.; Brunet, A.; King, S. Prenatal maternal stress predicts autism traits in 6½ year-old children: Project Ice Storm. Psychiatry Res. 2014, 219, 353–360. [Google Scholar] [CrossRef]
- Li, X.; Laplante, D.P.; Elgbeili, G.; King, S. Preconception and prenatal maternal stress are associated with broad autism phenotype in young adults: Project Ice Storm. J. Dev. Orig. Health Dis. 2023, 14, 481–489. [Google Scholar] [CrossRef]
- Cao-Lei, L.; de Rooij, S.R.; King, S.; Matthews, S.G.; Metz, G.A.S.; Roseboom, T.J.; Szyf, M. Prenatal stress and epigenetics. Neurosci. Biobehav. Rev. 2020, 117, 198–210. [Google Scholar] [CrossRef]
- Bateson, P.; Gluckman, P.; Hanson, M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J. Physiol. 2014, 592, 2357–2368. [Google Scholar] [CrossRef]
- Cao-Lei, L.; Dancause, K.N.; Elgbeili, G.; Massart, R.; Szyf, M.; Liu, A.; Laplante, D.P.; King, S. DNA methylation mediates the impact of exposure to prenatal maternal stress on BMI and central adiposity in children at age 13½ years: Project Ice Storm. Epigenetics 2015, 10, 749–761. [Google Scholar] [CrossRef]
- Cao-Lei, L.; van den Heuvel, M.I.; Huse, K.; Platzer, M.; Elgbeili, G.; Braeken, M.; Otte, R.A.; Witte, O.W.; Schwab, M.; Van den Bergh, B.R.H. Epigenetic Modifications Associated with Maternal Anxiety during Pregnancy and Children’s Behavioral Measures. Cells 2021, 10, 2421. [Google Scholar] [CrossRef]
- Lussier, A.A.; Smith, B.J.; Fisher, J.; Luo, M.; Cerutti, J.; Schneper, L.; Smith, T.; Cecil, C.A.; Felix, J.F.; Mitchell, C. DNA methylation mediates the link between adversity and depressive symptoms. Nat. Ment. Health 2024, 2, 1476–1485. [Google Scholar] [CrossRef]
- Schuurmans, I.K.; Dunn, E.C.; Lussier, A.A. DNA methylation as a possible mechanism linking childhood adversity and health: Results from a 2-sample mendelian randomization study. Am. J. Epidemiol. 2024, 193, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Pei, L.; Xiao, X.; Wei, Q.; Chen, J.K.; Ding, H.F.; Huang, S.; Fan, G.; Shi, H.; Dong, Z. DNA methylation protects against cisplatin-induced kidney injury by regulating specific genes, including interferon regulatory factor 8. Kidney Int. 2017, 92, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Varley, K.E.; Gertz, J.; Bowling, K.M.; Parker, S.L.; Reddy, T.E.; Pauli-Behn, F.; Cross, M.K.; Williams, B.A.; Stamatoyannopoulos, J.A.; Crawford, G.E.; et al. Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res. 2013, 23, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Caradonna, S.G.; Einhorn, N.R.; Saudagar, V.; Khalil, H.; Petty, G.H.; Lihagen, A.; LeFloch, C.; Lee, F.S.; Akil, H.; Guidotti, A. Corticosterone induces discrete epigenetic signatures in the dorsal and ventral hippocampus that depend upon sex and genotype: Focus on methylated Nr3c1 gene. Transl. Psychiatry 2022, 12, 109. [Google Scholar] [CrossRef]
- Öztürk, K.H.; Ünal, G.Ö.; Doğuç, D.K.; Toğay, V.A.; Koşar, P.A.; Sezik, M. Hypothalamic NR3C1 DNA methylation in rats exposed to prenatal stress. Mol. Biol. Rep. 2022, 49, 7921–7928. [Google Scholar] [CrossRef]
- Bockmühl, Y.; Patchev, A.V.; Madejska, A.; Hoffmann, A.; Sousa, J.C.; Sousa, N.; Holsboer, F.; Almeida, O.F.; Spengler, D. Methylation at the CpG island shore region upregulates Nr3c1 promoter activity after early-life stress. Epigenetics 2015, 10, 247–257. [Google Scholar] [CrossRef]
- Brouwers, E.P.; van Baar, A.L.; Pop, V.J. Maternal anxiety during pregnancy and subsequent infant development. Infant Behav. Dev. 2001, 24, 95–106. [Google Scholar] [CrossRef]
- Szyf, M.; Weaver, I.; Meaney, M. Maternal care, the epigenome and phenotypic differences in behavior. Reprod. Toxicol. 2007, 24, 9–19. [Google Scholar] [CrossRef]
- Meaney, M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001, 24, 1161–1192. [Google Scholar] [CrossRef] [PubMed]
- Kappeler, L.; Meaney, M.J. Epigenetics and parental effects. Bioessays 2010, 32, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Low, F.M.; Gluckman, P.D.; Hanson, M.A. Developmental plasticity, epigenetics and human health. Evol. Biol. 2012, 39, 650–665. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Lillycrop, K.A.; Burdge, G.C.; Gluckman, P.D.; Hanson, M.A. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr. Res. 2007, 61, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef]
- Li, X.; Qureshi, M.N.I.; Laplante, D.P.; Elgbeili, G.; Jones, S.L.; Long, X.; Paquin, V.; Bezgin, G.; Lussier, F.; King, S.; et al. Atypical brain structure and function in young adults exposed to disaster-related prenatal maternal stress: Project Ice Storm. J. Neurosci. Res. 2023, 101, 1849–1863. [Google Scholar] [CrossRef]
- Li, X.; Qureshi, M.N.I.; Laplante, D.P.; Elgbeili, G.; Jones, S.L.; King, S.; Rosa-Neto, P. Amygdala and Hippocampal Contributions to Broad Autism Phenotype: Project Ice Storm. Preprints 2025. [Google Scholar] [CrossRef]
- Ram, S.; Howland, M.A.; Sandman, C.A.; Davis, E.P.; Glynn, L.M. Prenatal risk for ASD: Fetal cortisol exposure predicts child autism-spectrum disorder symptoms. Clin. Psychol. Sci. A J. Assoc. Psychol. Sci. 2018, 7, 349. [Google Scholar]
- Beversdorf, D.Q.; Stevens, H.E.; Jones, K.L. Prenatal Stress, Maternal Immune Dysregulation, and Their Association With Autism Spectrum Disorders. Curr. Psychiatry Rep. 2018, 20, 76. [Google Scholar] [CrossRef]
- Nishitani, S.; Isozaki, M.; Yao, A.; Higashino, Y.; Yamauchi, T.; Kidoguchi, M.; Kawajiri, S.; Tsunetoshi, K.; Neish, H.; Imoto, H. Cross-tissue correlations of genome-wide DNA methylation in Japanese live human brain and blood, saliva, and buccal epithelial tissues. Transl. Psychiatry 2023, 13, 72. [Google Scholar] [CrossRef]
- Mendonça, V.; Soares-Lima, S.C.; Moreira, M.A.M. Exploring cross-tissue DNA methylation patterns: Blood–brain CpGs as potential neurodegenerative disease biomarkers. Commun. Biol. 2024, 7, 904. [Google Scholar] [CrossRef]
- Braun, P.R.; Han, S.; Hing, B.; Nagahama, Y.; Gaul, L.N.; Heinzman, J.T.; Grossbach, A.J.; Close, L.; Dlouhy, B.J.; Howard, M.A., III; et al. Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Transl. Psychiatry 2019, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lei, L.; Massart, R.; Suderman, M.J.; Machnes, Z.; Elgbeili, G.; Laplante, D.P.; Szyf, M.; King, S. DNA methylation signatures triggered by prenatal maternal stress exposure to a natural disaster: Project Ice Storm. PLoS ONE 2014, 9, e107653. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; St-Hilaire, A.; Jehel, L.; King, S. Validation of a French version of the impact of event scale-revised. Can. J. Psychiatry 2003, 48, 56–61. [Google Scholar] [CrossRef]
- Cao-Lei, L.; Elgbeili, G.; Massart, R.; Laplante, D.P.; Szyf, M.; King, S. Pregnant women’s cognitive appraisal of a natural disaster affects DNA methylation in their children 13 years later: Project Ice Storm. Transl. Psychiatry 2015, 5, e515. [Google Scholar] [CrossRef]
- Preacher, K.J.; Hayes, A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. Comput. 2004, 36, 717–731. [Google Scholar] [CrossRef]
- Shrout, P.E.; Bolger, N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol. Methods 2002, 7, 422–445. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R. An Introduction to the Bootstrap, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 1993. [Google Scholar]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis; The Guilford Press: New York, NY, USA, 2013. [Google Scholar]
| Age 15 Years (n = 27) | Age 16 Years (n = 22) | Age 19 Years (n = 13) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (or n) | SD | Min. | Max. | Mean (or n) | SD | Min. | Max. | Mean (or n) | SD | Min. | Max. | |
| Sex of child | ||||||||||||
| Male | 15 | 13 | 4 | |||||||||
| Female | 12 | 9 | 9 | |||||||||
| Objective Hardship | 10.9615 | 3.96465 | 5.00 | 21.00 | 11.2857 | 4.38341 | 5.00 | 21.00 | 10.1538 | 3.02341 | 6.00 | 15.00 |
| Cognitive Appraisal | ||||||||||||
| Very Positive | 1 | 1 | 0 | |||||||||
| Neutral/Positive | 18 | 14 | 8 | |||||||||
| Negative | 8 | 7 | 5 | |||||||||
| Very Negative | 0 | 0 | 0 | |||||||||
| BAPQ_Aloof | 2.27333 | 0.600103 | 1.170 | 3.180 | 2.63273 | 0.902395 | 1.080 | 4.750 | 2.63538 | 0.933601 | 1.330 | 4.420 |
| BAPQ_PragLan | 2.37926 | 0.451407 | 1.330 | 3.170 | 2.42364 | 0.553186 | 1.080 | 3.580 | 2.15385 | 0.607447 | 1.000 | 2.750 |
| BAPQ_Rigid | 2.56741 | 0.564314 | 1.000 | 3.420 | 2.88591 | 0.712558 | 1.750 | 4.670 | 2.85846 | 0.687300 | 1.920 | 3.830 |
| BAPQ_Total | 2.40667 | 0.430724 | 1.470 | 3.030 | 2.64773 | 0.603221 | 1.610 | 3.670 | 2.55000 | 0.652712 | 1.530 | 3.310 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Objective Hardship | 1 | |||||||||||||
| 2. Cognitive Appraisal | 0.276 | 1 | ||||||||||||
| 3. K15_BAPQ_Aloof | 0.186 | 0.112 | 1 | |||||||||||
| 4. K15_BAPQ_PragLan | 0.083 | −0.069 | 0.485 * | 1 | ||||||||||
| 5. K15_BAPQ_Rigid | 0.312 | 0.140 | 0.481 * | 0.407 * | 1 | |||||||||
| 6. K15_BAPQ_ToT | 0.249 | 0.088 | 0.842 ** | 0.751 ** | 0.800 ** | 1 | ||||||||
| 7. K16_BAPQ_Aloof | 0.028 | −0.191 | 0.534 * | 0.324 | 0.347 | 0.548 * | 1 | |||||||
| 8. K16_BAPQ_PragLan | 0.083 | −0.174 | 0.517 * | 0.774 ** | 0.378 | 0.719 ** | 0.435 * | 1 | ||||||
| 9. K16_BAPQ_Rigid | 0.394 | −0.021 | 0.410 | 0.394 | 0.688 ** | 0.667 ** | 0.601 ** | 0.555 ** | 1 | |||||
| 10. K16_BAPQ_ToT | 0.204 | −0.156 | 0.589 ** | 0.555 * | 0.541 * | 0.750 ** | 0.868 ** | 0.741 ** | 0.864 ** | 1 | ||||
| 11. K19_BAPQ_Aloof | 0.370 | 0.015 | 0.708 ** | 0.225 | 0.471 | 0.596 * | 0.538 | 0.364 | 0.626 | 0.582 | 1 | |||
| 12. K19_BAPQ_PragLan | 0.051 | −0.116 | 0.659 * | 0.754 ** | 0.620 * | 0.827 ** | 0.518 | 0.849 ** | 0.474 | 0.677 | 0.650 * | 1 | ||
| 13. K19_BAPQ_Rigid | 0.396 | 0.426 | 0.202 | −0.072 | 0.644 * | 0.350 | 0.218 | −0.044 | 0.548 | 0.268 | 0.709 ** | 0.555 * | 1 | |
| 14. K19_BAPQ_ToT | 0.329 | 0.119 | 0.623 * | 0.308 | 0.659 * | 0.672 * | 0.501 | 0.405 | 0.657 | 0.586 | 0.929 ** | 0.817 ** | 0.861 ** | 1 |
| Significant Mediation Effects | Significant Effect Sizes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CpGs (Genes) | Range of Significant Mediating Effects | Range of Significant Effect Sizes (R2) | ||||||||
| PNMS | BAPQ | Age | Total Tested | Significant Mediating | Minimum (Location) | Maximum (Location) | Mean Mediation | Minimum (Location) | Maximum (Location) | Mean R2 |
| Objective Hardship | Aloof | 15 | 27 (18) | 20 (15) | −0.0200 (cg08792630 in FOXO3) | −0.0361 (cg00689225 in NFKBIA) | −0.0284 | 0.1791 (cg20171453 in RHOH) | 0.3119 (cg00689225 in NFKBIA) | 0.2260 |
| 16 | 18 (13) | −0.0262 (cg17904575 in PPP2R5C) | −0.0547 (cg00689225 in NFKBIA) | −0.0343 | 0.0941 (cg01320698 in PIK3CD) | 0.3823 (cg00689225 in NFKBIA) | 0.1967 | |||
| 19 | 7 (7) | −0.0945 (cg00689225 located in NFKBIA) | −0.1161 (cg26360197 located in RPTOR) | −0.1028 | 0.3378 (cg00689225 located in NFKBIA) | 0.4512 (cg11833768 located in PPP2R5E) | 0.4064 | |||
| Pragmatic language impairment | 15 | 17 (13) | −0.0129 (cg16518861 located in NFKBIA) | −0.0251 (cg06491415 located in RPS6KA2) | −0.0179 | 0.1007 (cg08223235 located in BCL2) | 0.2519 (cg05651511 located in RPTOR) | 0.1531 | ||
| 16 | 11 (8) | −0.0203 (cg07499142 in PIK3CD) | −0.0506 (cg01320698 in PIK3CD) | −0.0287 | 0.1544 (cg18758433 in RPTOR) | 0.3609 (cg01320698 in PIK3CD) | 0.2339 | |||
| 19 | 11 (10) | −0.0500 (cg18758433 in RPTOR) | −0.0929 (cg26360197 in RPTOR) | −0.0777 | 0.1834 (cg18758433 in RPTOR) | 0.4273 (cg23575275 in CDKN1A) | 0.3277 | |||
| Rigid personality | 15 | |||||||||
| 16 | 1 (1) | −0.0283 (cg26601310 located in PRR5L) | 0.2867 (cg26601310 in PRR5L) | |||||||
| 19 | ||||||||||
| Total BAP score | 15 | 18 (14) | −0.0138 (cg07499142 located in PIK3CD) | −0.0237 (cg06491415 located in RPS6KA2) | −0.0189 | 0.1512 (cg07499142 located in PIK3CD) | 0.3432 (cg05651511 located in RPTOR) | 0.2256 | ||
| 16 | 15 (11) | −0.0196 (cg17306848 in PRKCH) | −0.0381 (cg01320698 in PIK3CD) | −0.0258 | 0.1777 (cg13989999 in BCL2L1) | 0.3834 (cg00689225 in NFKBIA) | 0.2492 | |||
| 19 | 4 (4) | −0.0640 (cg23575275 in CDKN1A) | −0.0785 (cg26360197 in RPTOR) | −0.0686 | 0.0578 (cg23575275 in DKN1A) | 0.3849 (cg11833768 in PPP2R5E) | 0.2823 | |||
| Cognitive Appraisal | Aloof | 15 | 61 (41) | 39 (33) | −0.0876 (cg14001486 in PRKCH) | −0.2037 (cg04610450 in PIK3R2) | −0.1458 | 0.1121 (cg22666015 in INPP5D) | 0.3008 (cg12873919 in BCL2L1) | 0.1891 |
| 16 | 31 (27) | −0.1370 (cg13916261 in FNBP1) | −0.3626 (cg18758433 in RPTOR) | −0.2278 | 0.1422 (cg00278517 in PS6KA2) | 0.3870 (cg18758433 in RPTOR) | 0.2166 | |||
| 19 | 4 (3) | −0.3259 (cg02481000 in PRKCZ) | −0.3947 (cg22666015 in INPP5D) | −0.3633 | 0.2544 (cg00300046 in PRKCZ) | 0.4497 (cg23575275 in CDKN1A) | 0.3273 | |||
| Pragmatic language impairment | 15 | 29 (24) | −0.0673 (cg25342409 in PIK3R5) | −0.1180 (cg08557970 in RPS6KA2) | −0.0897 | 0.0817 (cg25342409 in PIK3R5) | 0.1867 (cg16994041 in PRKAG2) | 0.1350 | ||
| 1 Positive Mediation | 0.0531 cg08257293 in BCL2L1 | 0.0966 cg08257293 in BCL2L1 | ||||||||
| 16 | 14 (13) | −0.0940 (cg00319686 in MAP2K1) | −0.1519 (cg17904575 in PPP2R5C) | −0.1259 | 0.1533 (cg26601310 in PRR5L) | 0.2632 (cg02015053 in EIF3J) | 0.1975 | |||
| 19 | 22 (18) | −0.1339 (cg24585377 in RPS6KA1) | −0.3377 (cg02481000 in PRKCZ) | −0.2165 | 0.2089 (cg24585377 in PS6KA1) | 0.7253 (cg02481000 in PRKCZ) | 0.3304 | |||
| Rigid personality | 15 | |||||||||
| 16 | 1 (1) | −0.1577 cg13916261 in FNBP1 | 0.2440 cg13916261 in FNBP1 | |||||||
| 19 | ||||||||||
| Total BAP score | 15 | 31 (28) | −0.1242 (cg04610450 in PIK3R2) | −0.0570 (cg23174662 in HIF1A) | −0.0925 | 0.0889 (cg25342409 in PIK3R5) | 0.2234 (cg12873919 in BCL2L1) | 0.1454 | ||
| 16 | 10 (9) | −0.1123 (cg00319686 in MAP2K1) | −0.1897 (cg18758433 in RPTOR) | −0.1468 | 0.1772 (cg05581469 in RKAG1) | 0.2390 (cg18758433 in RPTOR) | 0.2071 | |||
| 19 | 4 (3) | −0.1966 (cg23575275 in CDKN1A) | −0.3006 (cg22666015 in INPP5D) | −0.2571 | 0.2741 (cg23575275 in DKN1A) | 0.4031 (cg02481000 in PRKCZ) | 0.3406 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao-Lei, L.; Elgbeili, G.; Laplante, D.P.; Szyf, M.; King, S. DNA Methylation Mediates the Association Between Prenatal Maternal Stress and the Broad Autism Phenotype in Human Adolescents: Project Ice Storm. Int. J. Mol. Sci. 2025, 26, 9468. https://doi.org/10.3390/ijms26199468
Cao-Lei L, Elgbeili G, Laplante DP, Szyf M, King S. DNA Methylation Mediates the Association Between Prenatal Maternal Stress and the Broad Autism Phenotype in Human Adolescents: Project Ice Storm. International Journal of Molecular Sciences. 2025; 26(19):9468. https://doi.org/10.3390/ijms26199468
Chicago/Turabian StyleCao-Lei, Lei, Guillaume Elgbeili, David P. Laplante, Moshe Szyf, and Suzanne King. 2025. "DNA Methylation Mediates the Association Between Prenatal Maternal Stress and the Broad Autism Phenotype in Human Adolescents: Project Ice Storm" International Journal of Molecular Sciences 26, no. 19: 9468. https://doi.org/10.3390/ijms26199468
APA StyleCao-Lei, L., Elgbeili, G., Laplante, D. P., Szyf, M., & King, S. (2025). DNA Methylation Mediates the Association Between Prenatal Maternal Stress and the Broad Autism Phenotype in Human Adolescents: Project Ice Storm. International Journal of Molecular Sciences, 26(19), 9468. https://doi.org/10.3390/ijms26199468








