In Vitro Evaluation of the Regenerative Potential of Autologous Platelet-Rich Fibrin (PRF) on Human Primary Periodontal Ligament Cells
Abstract
1. Introduction
2. Results
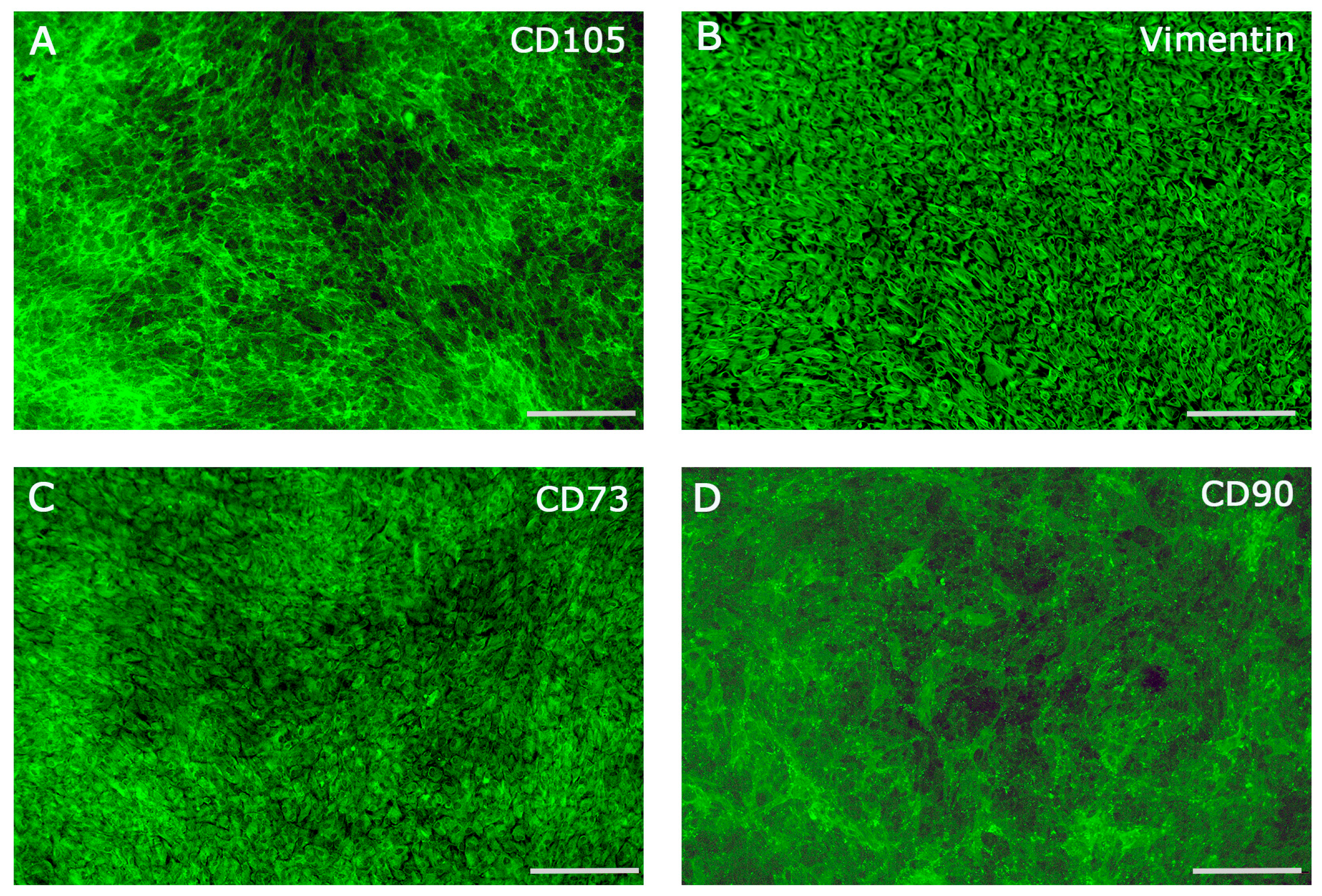
2.1. Characterization of Periodontal Ligament Cells
2.2. Effect of Different PRF Treatment Methods on hPDLCs
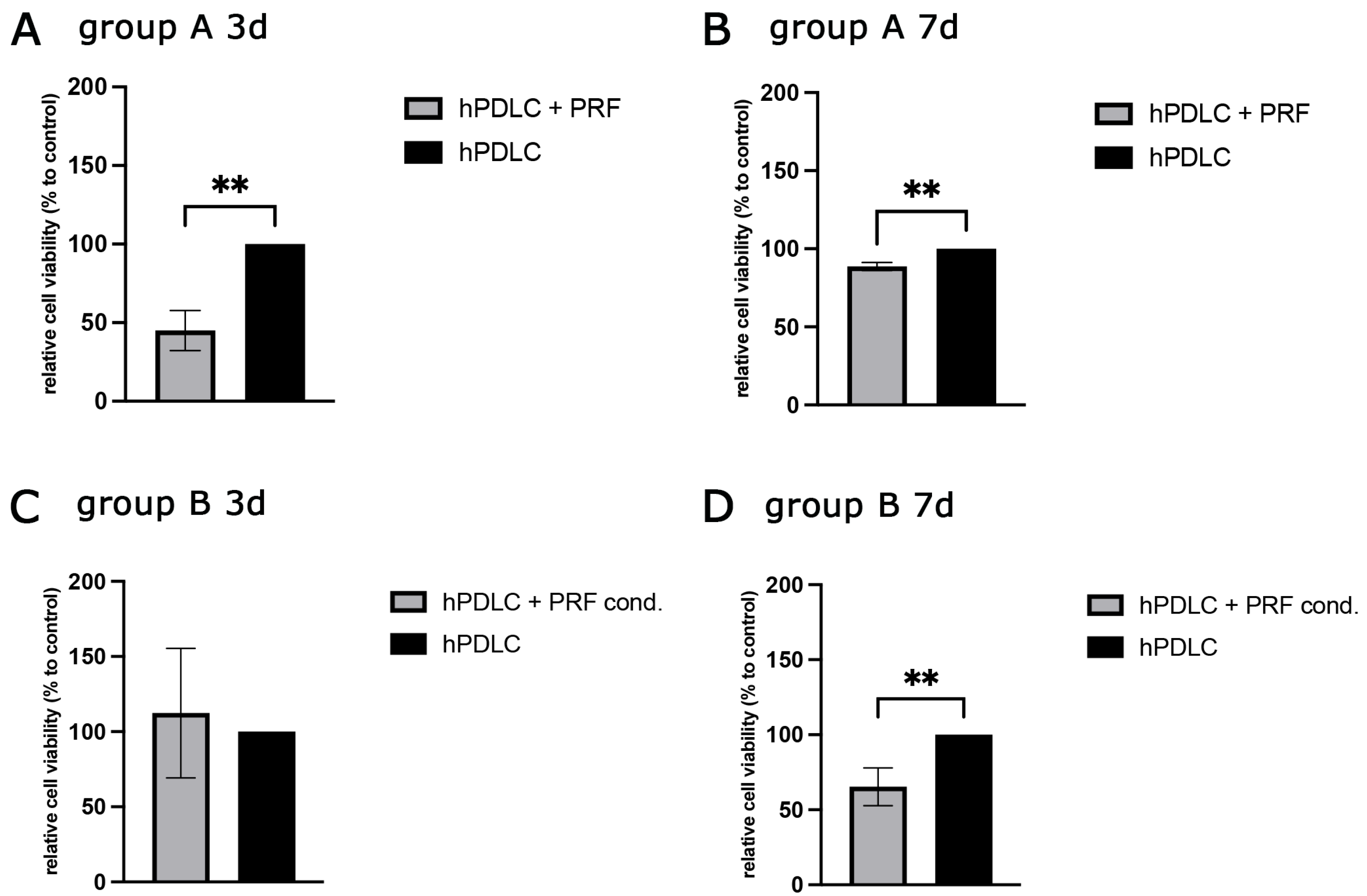
2.2.1. PRF-Mediated Effect on hPDLCs Cell Viability
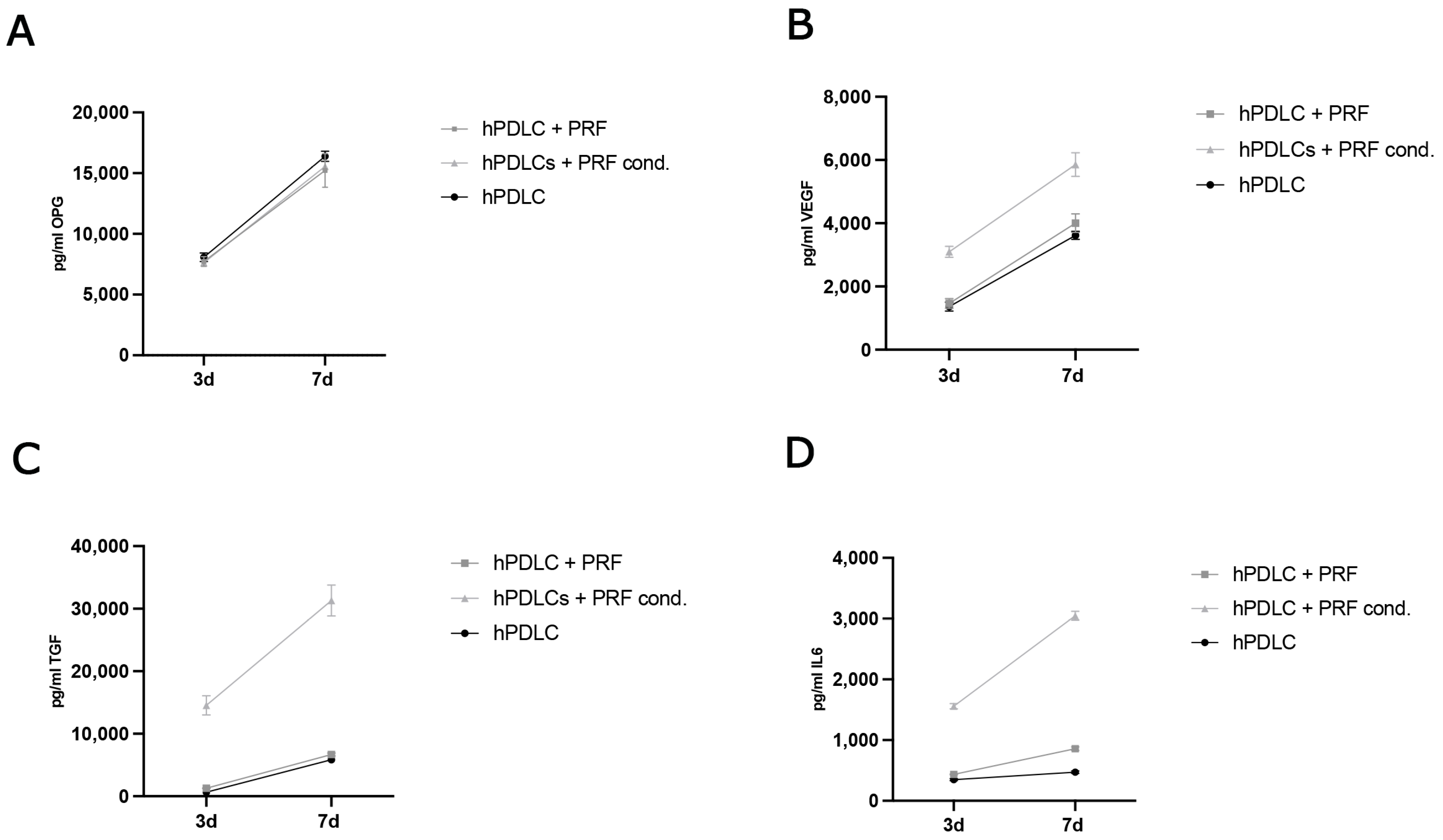
2.2.2. Evaluation of Protein Concentration in Cell Culture Supernatants
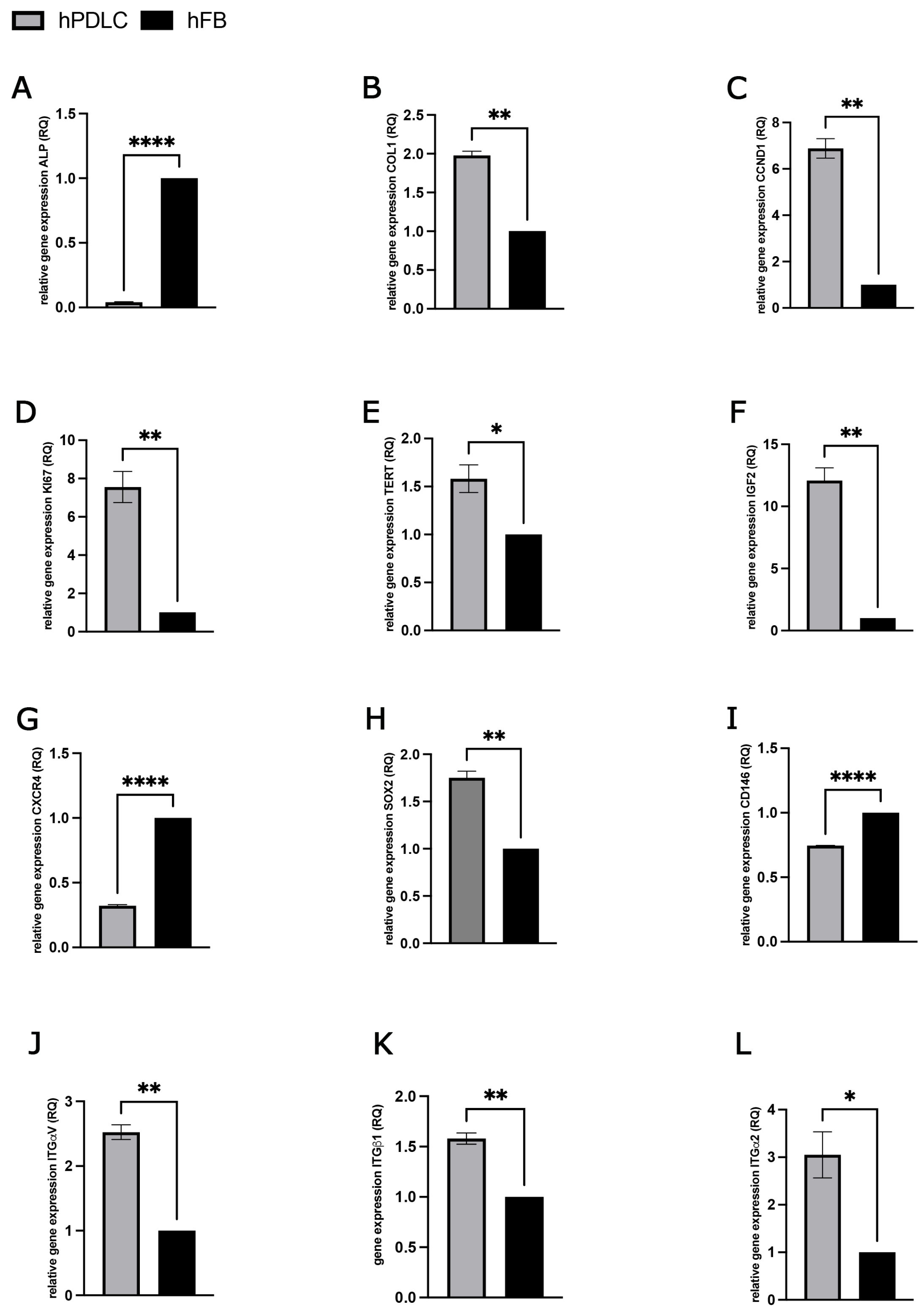
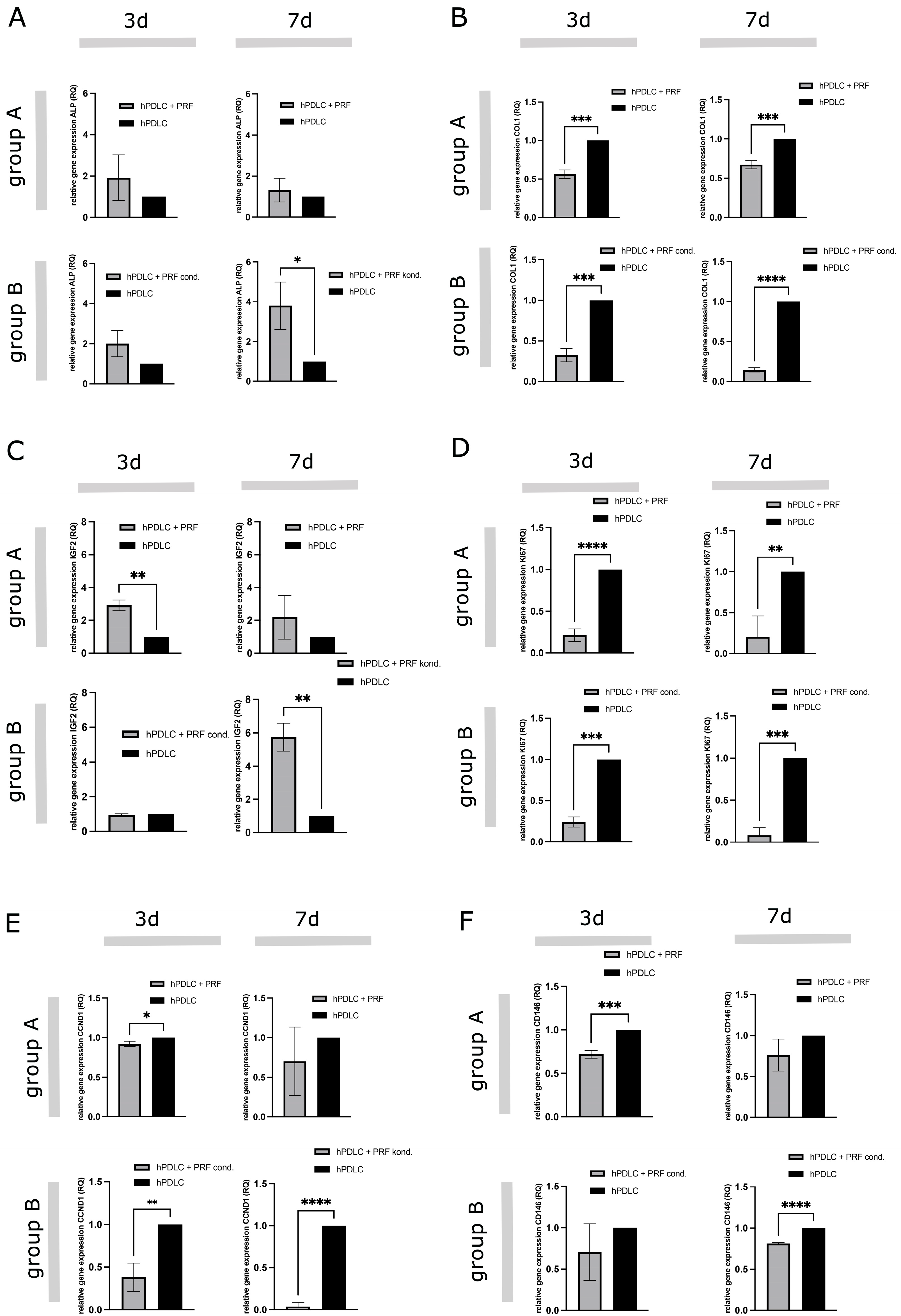
2.2.3. PRF-Mediated Effect on Gene Expression of Differentiation, Proliferation and Stem Cell Associated Markers in hPDLCs
2.2.4. Alkaline Phosphatase Activity in PRF Treated hPDLCs
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Primary Cells
4.3. Preparation of PRF
4.4. Indirect and Direct PRF Treatment of hPDLCs
4.5. Cell Viability Assay
4.6. Immunofluorescence Staining
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. RNA Isolation and Gene Expression Analyses
4.9. ALP-Activity Assay
4.10. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Tooth Loss: A Systematic Review and Meta-analysis. J. Dent. Res. 2014, 93 (Suppl. S7), 20S–28S. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Nyman, S.; Gottlow, J.; Karring, T.; Lindhe, J. The regenerative potential of the periodontal ligament: An experimental study in the monkey. J. Clin. Periodontol. 1982, 9, 257–265. [Google Scholar] [CrossRef]
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New attachment following surgical treatment of human periodontal disease. J. Clin. Periodontol. 1982, 9, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Mark Bartold, P.; Batouli, S.; Brahim, J.; Young, M.; Gehron Robey, P.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Ivanovski, S.; Gronthos, S.; Shi, S.; Bartold, P. Stem cells in the periodontal ligament. Oral Dis. 2006, 12, 358–363. [Google Scholar] [CrossRef]
- Fujii, S.; Maeda, H.; Tomokiyo, A.; Monnouchi, S.; Hori, K.; Wada, N.; Akamine, A. Effects of TGF-β1 on the proliferation and differentiation of human periodontal ligament cells and a human periodontal ligament stem/progenitor cell line. Cell Tissue Res. 2010, 342, 233–242. [Google Scholar] [CrossRef]
- Hyun, S.-Y.; Lee, J.-H.; Kang, K.-J.; Jang, Y.-J. Effect of FGF-2, TGF-β-1, and BMPs on Teno/Ligamentogenesis and Osteo/Cementogenesis of Human Periodontal Ligament Stem Cells. Mol. Cells 2017, 40, 550–557. [Google Scholar] [CrossRef]
- Silvério, K.G.; Rodrigues, T.L.; Coletta, R.D.; Benevides, L.; Da Silva, J.S.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H. Mesenchymal Stem Cell Properties of Periodontal Ligament Cells From Deciduous and Permanent Teeth. J. Periodontol. 2010, 81, 1207–1215. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endodontology 2006, 101, e51–e55. [Google Scholar] [CrossRef]
- Ghanaati, S.; Herrera-Vizcaino, C.; Al-Maawi, S.; Lorenz, J.; Miron, R.J.; Nelson, K.; Schwarz, F.; Choukroun, J.; Sader, R. Fifteen Years of Platelet Rich Fibrin in Dentistry and Oromaxillofacial Surgery: How High is the Level of Scientific Evidence? J. Oral. Implantol. 2018, 44, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.-L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral. Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endodontology 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Dohle, E.; El Bagdadi, K.; Sader, R.; Choukroun, J.; James Kirkpatrick, C.; Ghanaati, S. Platelet-rich fibrin-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, 598–610. [Google Scholar] [CrossRef]
- Herrera-Vizcaíno, C.; Dohle, E.; Al-Maawi, S.; Booms, P.; Sader, R.; Kirkpatrick, C.J.; Choukroun, J.; Ghanaati, S. Platelet-rich fibrin secretome induces three dimensional angiogenic activation in vitro. Eur. Cell Mater. 2019, 37, 250–264. [Google Scholar] [CrossRef]
- Wend, S.; Kubesch, A.; Orlowska, A.; Al-Maawi, S.; Zender, N.; Dias, A.; Miron, R.J.; Sader, R.; Booms, P.; Kirkpatrick, C.J.; et al. Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J. Mater. Sci. Mater. Med. 2017, 28, 188. [Google Scholar] [CrossRef]
- Choukroun, J.; Ghanaati, S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: The first introduction to the low speed centrifugation concept. Eur. J. Trauma. Emerg. Surg. 2018, 44, 87–95. [Google Scholar] [CrossRef]
- Ajwani, H.; Shetty, S.; Gopalakrishnan, D.; Kathariya, R.; Kulloli, A.; Dolas, R.S.; Pradeep, A.R. Comparative evaluation of platelet-rich fibrin biomaterial and open flap debridement in the treatment of two and three wall intrabony defects. J. Int. Oral. Health 2015, 7, 32–37. [Google Scholar]
- Pullishery, F.; Hussein Alattas, M.; Roshdy Abdelrasoul, M.; Fouad Hassan, A.; Abdelhamid Ahmed Derbala, D.; Hashir, S. Effectiveness of i-PRF in periodontal regeneration—A systematic review and meta-analysis. Saudi Dent. J. 2024, 36, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, K.; Komaki, M.; Sekiya, I.; Sakaguchi, Y.; Noguchi, K.; Oda, S.; Muneta, T.; Ishikawa, I. Stem cell properties of human periodontal ligament cells. J. Periodontal Res. 2006, 41, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-T.; Liu, C.-L.; Chen, T.-H.; Lee, O.K. Optimization of Culture Conditions for Stem Cells Derived from Human Anterior Cruciate Ligament and Bone Marrow. Cell Transpl. 2014, 23, 791–803. [Google Scholar] [CrossRef]
- Gronthos, S.; Mrozik, K.; Shi, S.; Bartold, P.M. Ovine Periodontal Ligament Stem Cells: Isolation, Characterization, and Differentiation Potential. Calcif. Tissue Int. 2006, 79, 310–317. [Google Scholar] [CrossRef]
- Franke, W.W.; Grund, C.; Kuhn, C.; Jackson, B.W.; Illmensee, K. Formation of Cytoskeletal Elements During Mouse Embryogenesis. Differentiation 1982, 23, 43–59. [Google Scholar] [CrossRef]
- Duff, S.E.; Li, C.; Garland, J.M.; Kumar, S. CD105 is important for angiogenesis: Evidence and potential applications. FASEB J. 2003, 17, 984–992. [Google Scholar] [CrossRef]
- Gay, I.; Chen, S.; MacDougall, M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod. Craniofacial Res. 2007, 10, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, B.J.; Bhat, K.G.; Kukreja, P.; Kumber, V.M.; Balakrishnan, R.; Govila, V. Isolation and immunohistochemical characterization of periodontal ligament stem cells: A preliminary study. J. Indian. Soc. Periodontol. 2021, 25, 295–299. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Kawase, T.; Sato, S.; Miake, K.; Saito, S. Alkaline Phosphatase of Human Periodontal Ligament Fibroblast-Like Cells. Adv. Dent. Res. 1988, 2, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Somerman, M.J.; Archer, S.Y.; Imm, G.R.; Foster, R.A. A Comparative Study of Human Periodontal Ligament Cells and Gingival Fibroblasts in vitro. J. Dent. Res. 1988, 67, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Tomokiyo, A.; Wada, N.; Maeda, H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev. 2019, 28, 974–985. [Google Scholar] [CrossRef]
- Calabrese, E.J. Human periodontal ligament stem cells and hormesis: Enhancing cell renewal and cell differentiation. Pharmacol. Res. 2021, 173, 105914. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wei, X.; Huang, R.; Ling, J.; Wu, L.; Xiao, Y. Effect of Bone Morphogenetic Protein-4 on the Expression of Sox2, Oct-4, and c-Myc in Human Periodontal Ligament Cells During Long-Term Culture. Stem Cells Dev. 2013, 22, 1670–1677. [Google Scholar] [CrossRef]
- Hosiriluck, N.; Kashio, H.; Takada, A.; Mizuguchi, I.; Arakawa, T. The profiling and analysis of gene expression in human periodontal ligament tissue and fibroblasts. Clin. Exp. Dent. Res. 2022, 8, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Varga, F.; Fischer, M.B.; Watzek, G. Platelets stimulate proliferation of bone cells: Involvement of platelet-derived growth factor, microparticles and membranes. Clin. Oral. Implant. Res. 2002, 13, 529–535. [Google Scholar] [CrossRef]
- Wang, K.; Ma, C.; Feng, J.Q.; Jing, Y. The Emerging Role of Cell Transdifferentiation in Skeletal Development and Diseases. Int. J. Mol. Sci. 2022, 23, 5974. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Zhang, M.; Liu, N.-X.; Lv, X.; Zhang, J.; Chen, F.-M.; Chen, Y.-J. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials 2013, 34, 5506–5520. [Google Scholar] [CrossRef]
- Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Russell, R.P.; Apostolakos, J.; Ramos, D.M.; Kumbar, S.G.; Imhoff, A.B.; Arciero, R.A.; Mazzocca, A.D. Properties of Biologic Scaffolds and Their Response to Mesenchymal Stem Cells. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Bucur, M.; Constantin, C.; Neagu, M.; Zurac, S.; Dinca, O.; Vladan, C.; Cioplea, M.; Popp, C.; Nichita, L.; Ionescu, E. Alveolar blood clots and platelet-rich fibrin induce in vitro fibroblast proliferation and migration. Exp. Ther. Med. 2018, 17, 982–989. [Google Scholar] [CrossRef]
- Saeed, M.A.; El-Rahman, M.A.; Helal, M.E.; Zaher, A.R.; Grawish, M.E. Efficacy of Human Platelet Rich Fibrin Exudate vs Fetal Bovine Serum on Proliferation and Differentiation of Dental Pulp Stem Cells. Int. J. Sport Commun. 2017, 10, 38–47. [Google Scholar] [CrossRef]
- Silva, I.; Branco, J.C. Rank/Rankl/opg: Literature review. Acta Reum. Port. 2011, 36, 209–218. [Google Scholar]
- Chang, I.C.; Tsai, C.H.; Chang, Y.C. Platelet-rich fibrin modulates the expression of extracellular signal-regulated protein kinase and osteoprotegerin in human osteoblasts. J. Biomed. Mater. Res. 2010, 95A, 327–332. [Google Scholar] [CrossRef]
- Boyce, B.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Dohle, E.; Lim, J.; Weigl, P.; Teoh, S.H.; Sader, R.; Ghanaati, S. Biologization of Pcl-Mesh Using Platelet Rich Fibrin (Prf) Enhances Its Regenerative Potential In Vitro. Int. J. Mol. Sci. 2021, 22, 2159. [Google Scholar] [CrossRef]
- Sonnenschein, S.K.; Meyle, J. Local inflammatory reactions in patients with diabetes and periodontitis. Periodontol. 2000 2015, 69, 221–254. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part. B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Zhao, J.H. Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust. Dent. J. 2011, 56, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Stucki, U.; Schmid, J.; Hämmerle, C.F.; Lang, N.P. Temporal and local appearance of alkaline phosphatase activity in early stages of guided bone regeneration. A descriptive histochemical study in humans. Clin. Oral. Implant. Res. 2001, 12, 121–127. [Google Scholar] [CrossRef]
- Demirel, E.; Yildiz, K.; Çadirci, K.; Aygün, H.; Şenocak, E.; Gündoğdu, B. Effect of platelet-rich fibrin on epidural fibrosis and comparison to ADCON® Gel and hyaluronic acid. Acta Orthop. Traumatol. Turc. 2018, 52, 469–474. [Google Scholar] [CrossRef]
- O’Dell, S.D.; Day, I.N.M. Molecules in focus Insulin-like growth factor II (IGF-II). Int. J. Biochem. Cell Biol. 1998, 30, 767–771. [Google Scholar] [CrossRef]
- Temmerman, A.; Vandessel, J.; Castro, A.; Jacobs, R.; Teughels, W.; Pinto, N.; Quirynen, M. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: A split-mouth, randomized, controlled clinical trial. J. Clin. Periodontol. 2016, 43, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhang, X.; Zhao, Q.; Chai, J.; Zhang, Y. Liquid platelet-rich fibrin promotes the regenerative potential of human periodontal ligament cells. Oral. Dis. 2020, 26, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Guo, X.; Cai, X. SDF-1/CXCR4 axis promotes osteogenic differentiation of BMSCs through the JAK2/STAT3 pathway. Folia Histochem. Cytobiol. 2021, 59, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013, 330, 150–162. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dohle, E.; Quernheim, M.; Sader, R.; Ghanaati, S. In Vitro Evaluation of the Regenerative Potential of Autologous Platelet-Rich Fibrin (PRF) on Human Primary Periodontal Ligament Cells. Int. J. Mol. Sci. 2025, 26, 9459. https://doi.org/10.3390/ijms26199459
Dohle E, Quernheim M, Sader R, Ghanaati S. In Vitro Evaluation of the Regenerative Potential of Autologous Platelet-Rich Fibrin (PRF) on Human Primary Periodontal Ligament Cells. International Journal of Molecular Sciences. 2025; 26(19):9459. https://doi.org/10.3390/ijms26199459
Chicago/Turabian StyleDohle, Eva, Marlene Quernheim, Robert Sader, and Shahram Ghanaati. 2025. "In Vitro Evaluation of the Regenerative Potential of Autologous Platelet-Rich Fibrin (PRF) on Human Primary Periodontal Ligament Cells" International Journal of Molecular Sciences 26, no. 19: 9459. https://doi.org/10.3390/ijms26199459
APA StyleDohle, E., Quernheim, M., Sader, R., & Ghanaati, S. (2025). In Vitro Evaluation of the Regenerative Potential of Autologous Platelet-Rich Fibrin (PRF) on Human Primary Periodontal Ligament Cells. International Journal of Molecular Sciences, 26(19), 9459. https://doi.org/10.3390/ijms26199459







