Cardioprotective Effects of Simvastatin in Doxorubicin-Induced Acute Cardiomyocyte Injury
Abstract
1. Introduction
2. Results
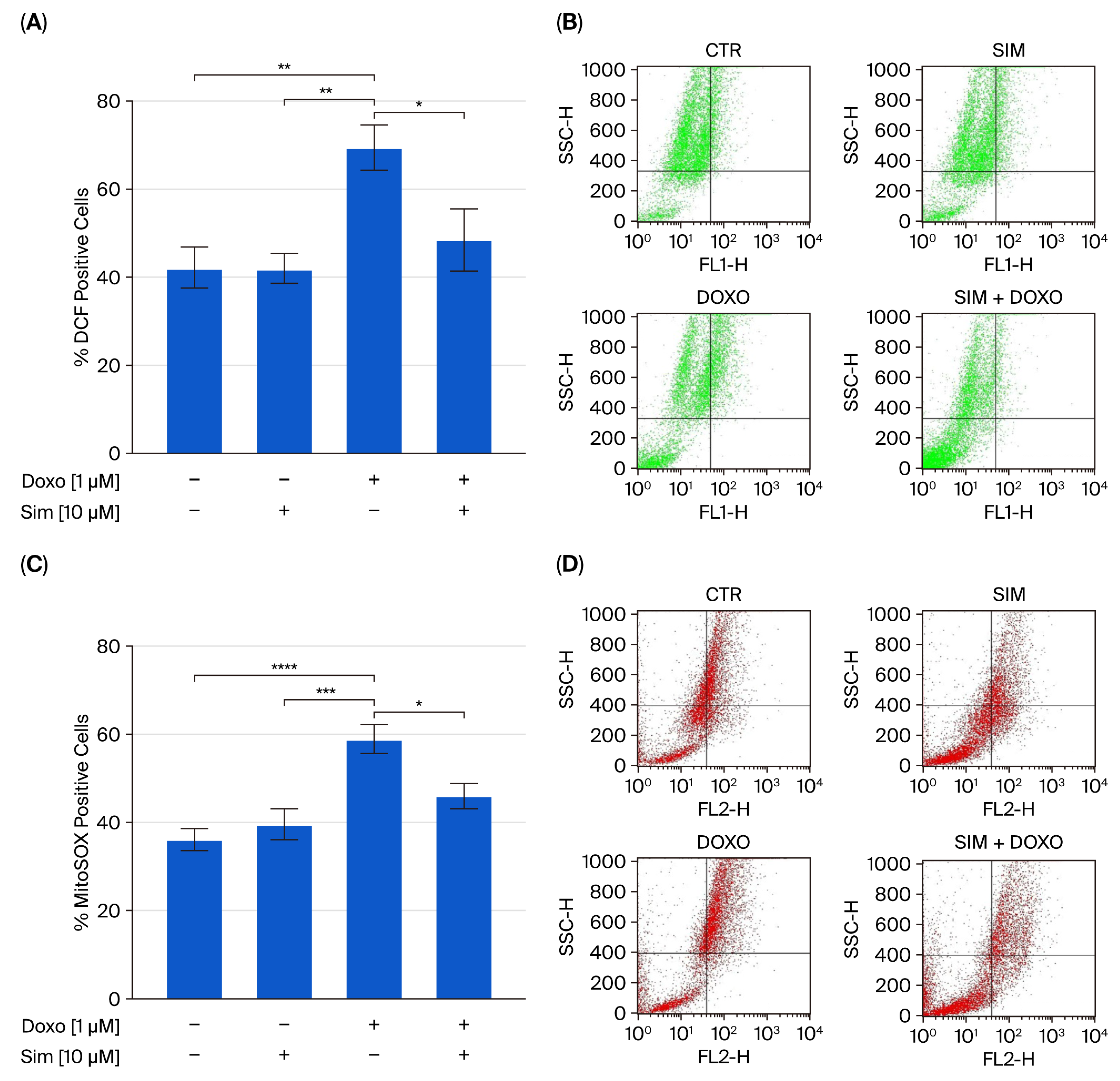
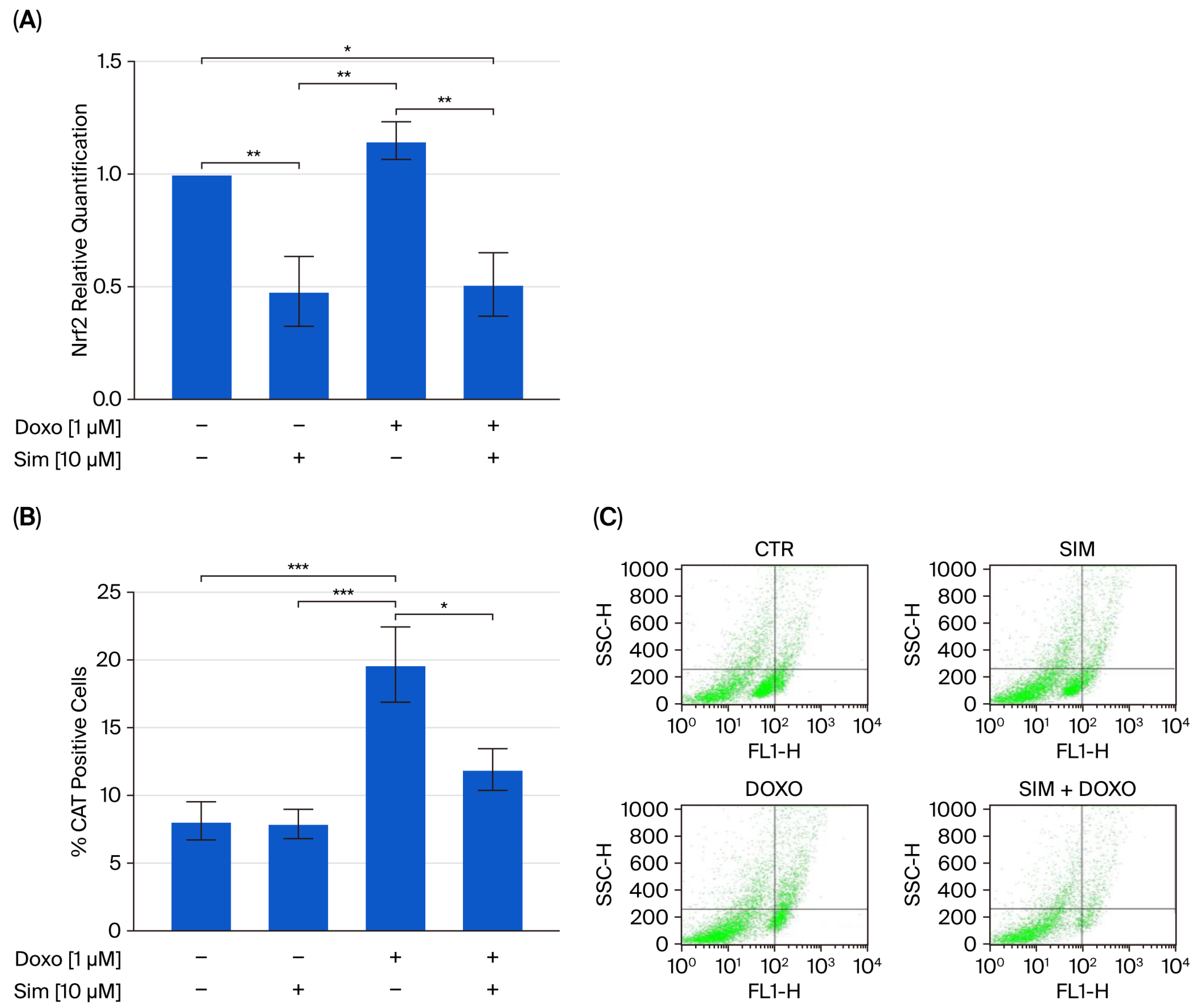
2.1. Effect of Simvastatin Co-Treatment on Doxo-Induced Oxidative Stress
2.2. Effect of Simvastatin Co-Treatment on Intracellular Calcium Signaling and Mitochondrial Membrane Potential (MMP)
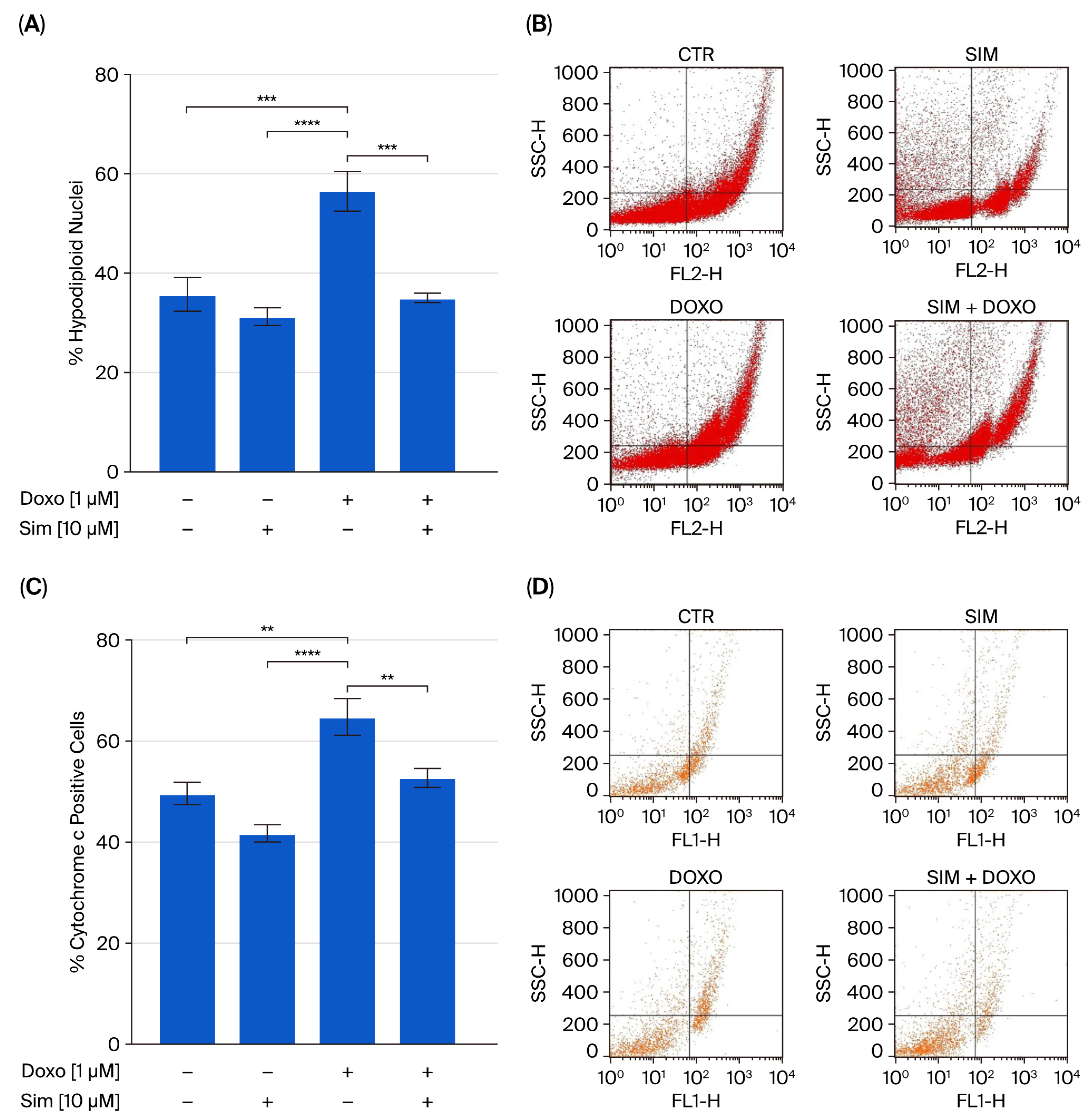
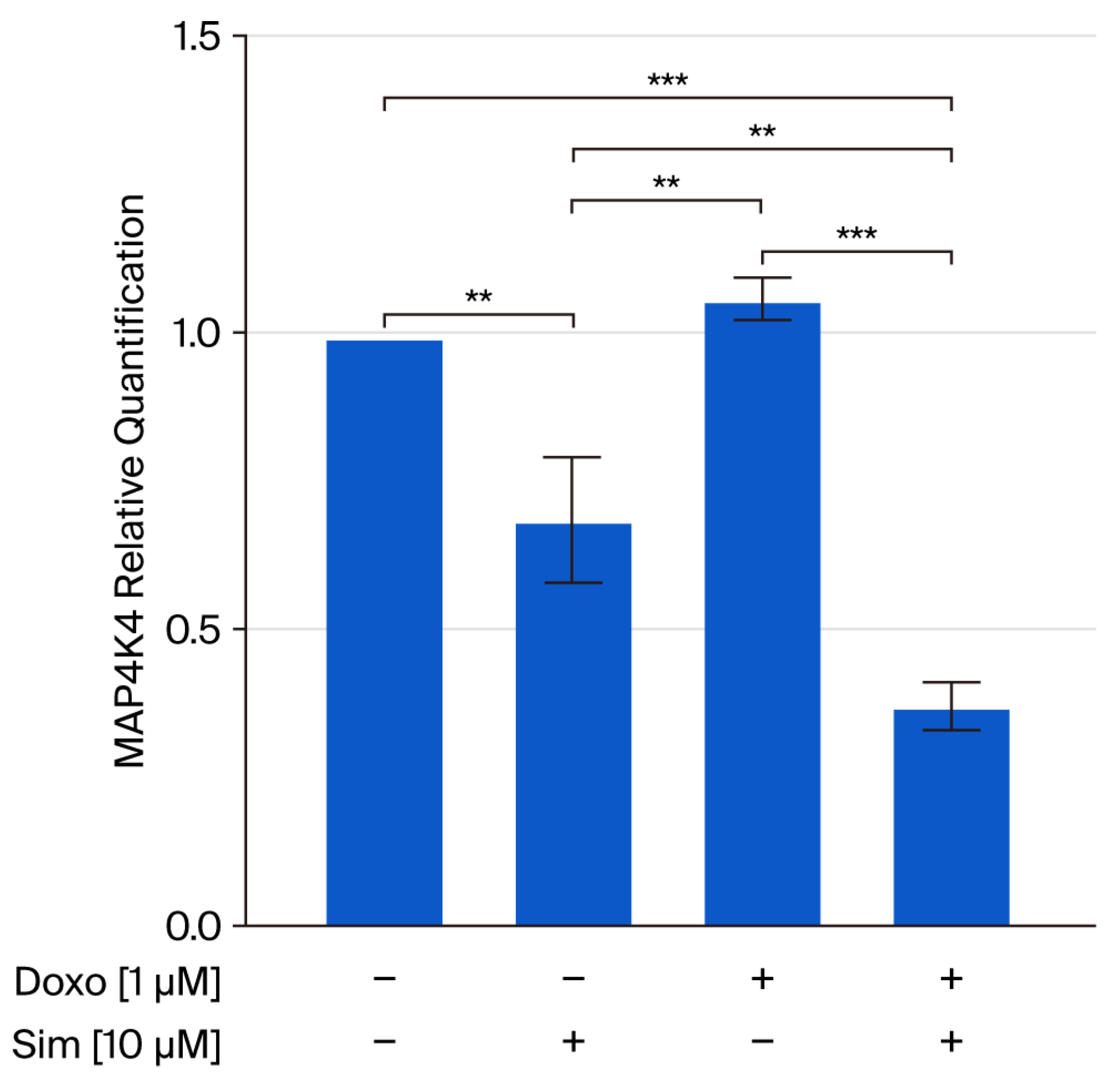
2.3. Effect of Simvastatin Co-Treatment on Doxorubicin-Induced Apoptosis
3. Discussion
4. Methods
4.1. Cell Line and Treatment
4.2. Measurement of Intracellular Reactive Oxygen Species (ROS)
4.3. Detection of Mitochondrial Superoxide Formation
4.4. Analysis of Apoptosis
4.5. Flow Cytometry Analysis
4.6. Evaluation of Intracellular Calcium Signaling
4.7. Measurement of Mitochondrial Membrane Depolarization
4.8. RNA Extraction and Quantitative Real Time RT-PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, J.; Ells, Z.; Dolle, B.; Rouzina, I.F.; Williams, M.C.; Paramanathan, T. Optical tweezers reveal nanomolar DNA binding affinity of doxorubicin. Biophys. J. 2024, 123, 225a. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell. Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Matusik, K.; Kamińska, K.; Sobiborowicz-Sadowska, A.; Borzuta, H.; Buczma, K.; Cudnoch-Jędrzejewska, A. The significance of the apelinergic system in doxorubicin-induced cardiotoxicity. Heart Fail. Rev. 2024, 29, 969–988. [Google Scholar] [CrossRef]
- Tranchita, E.; Murri, A.; Grazioli, E.; Cerulli, C.; Emerenziani, G.P.; Ceci, R.; Caporossi, D.; Dimauro, I.; Parisi, A. The Beneficial Role of Physical Exercise on Anthracyclines Induced Cardiotoxicity in Breast Cancer Patients. Cancers 2022, 14, 2288. [Google Scholar] [CrossRef]
- Sangweni, N.F.; van Vuuren, D.; Mabasa, L.; Gabuza, K.; Huisamen, B.; Naidoo, S.; Barry, R.; Johnson, R. Prevention of Anthracycline-Induced Cardiotoxicity: The Good and Bad of Current and Alternative Therapies. Front. Cardiovasc. Med. 2022, 9, 907266. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Pecoraro, M.; Marzocco, S.; Belvedere, R.; Petrella, A.; Franceschelli, S.; Popolo, A. Simvastatin Reduces Doxorubicin-Induced Cardiotoxicity: Effects beyond Its Antioxidant Activity. Int. J. Mol. Sci. 2023, 24, 7573. [Google Scholar] [CrossRef]
- Avagimyan, A.; Pogosova, N.; Kakturskiy, L.; Sheibani, M.; Challa, A.; Kogan, E.; Fogacci, F.; Mikhaleva, L.; Vandysheva, R.; Yakubovskaya, M.; et al. Doxorubicin-related cardiotoxicity: Review of fundamental pathways of cardiovascular system injury. Cardiovasc. Pathol. 2024, 73, 107683. [Google Scholar] [CrossRef]
- Cappetta, D.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative stress and cellular response to doxorubicin: A common factor in the complex milieu of anthracycline cardiotoxicity. Oxid. Med. Cell Longev. 2017, 2017, 1521020. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Wang, G.; Ren, J. Molecular Mechanisms and Therapeutic Targeting of Ferroptosis in Doxorubicin-Induced Cardiotoxicity. JACC Basic Transl. Sci. 2024, 9, 811–826. [Google Scholar] [CrossRef]
- Sadowska, A.; Osiński, P.; Roztocka, A.; Kaczmarz-Chojnacka, K.; Zapora, E.; Sawicka, D.; Car, H. Statins—From Fungi to Pharmacy. Int. J. Mol. Sci. 2024, 25, 466. [Google Scholar] [CrossRef]
- Ma, Y.; Yuan, Q.; Aizeze, A. Statins to prevent anthracyclines-induced cardiotoxicity. Austin Cardio 2023, 8, 1037. [Google Scholar] [CrossRef]
- TamehriZadeh, S.S.; Khalaji, M.; Tajdari, M.; Mavaddat, H.; Szmit, S.; Lashgari, N.A.; Roudsari, N.M.; Abbasi-Kashkoli, H.; Banach, M.; Abdolghaffari, A.H. Statins: Novel Approaches for the Management of Doxorubicin-Induced Cardiotoxicity-A Literature Review. Cardiovasc. Toxicol. 2025, 25, 1429–1452. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.L.; El-Abd, A.A.; Mohamed, H.G.; Noufal, A.M.; Hennawy, B.S. Role of Statin Therapy in Prevention of Anthracycline-Induced Cardiotoxicity: A Three Dimentional Echocardiography Study. Curr. Probl. Cardiol. 2024, 49, 102130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lou, L.; Shi, W.; Chen, Y.; Fu, Z.; Liu, S.; Sok, T.; Li, Z.; Zhang, X.; Yang, J. Statins in Mitigating Anticancer Treatment-Related Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 10177. [Google Scholar] [CrossRef] [PubMed]
- Vitale, R.; Marzocco, S.; Popolo, A. Role of Oxidative Stress and Inflammation in Doxorubicin-Induced Cardiotoxicity: A Brief Account. Int. J. Mol. Sci. 2024, 25, 7477. [Google Scholar] [CrossRef]
- Songbo, M.; Lang, H.; Xinyong, C.; Bin, X.; Ping, Z.; Liang, S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef]
- Yang, H.B.; Lu, Z.Y.; Yuan, W.; Li, W.D.; Mao, S. Selenium Attenuates Doxorubicin-Induced Cardiotoxicity Through Nrf2-NLRP3 Pathway. Biol. Trace Elem. Res. 2022, 200, 2848–2856. [Google Scholar] [CrossRef]
- Bkaily, G.; Danielle, J. Calcium Homeostasis, Transporters, and Blockers in Health and Diseases of the Cardiovascular System. Int. J. Mol. Sci. 2023, 24, 8803. [Google Scholar] [CrossRef]
- Pecoraro, M.; Sorrentino, R.; Franceschelli, S.; Del Pizzo, M.; Pinto, A.; Popolo, A. Doxorubicin-Mediated Cardiotoxicity: Role of Mitochondrial Connexin 43. Cardiovasc. Toxicol. 2015, 15, 366–376. [Google Scholar] [CrossRef]
- Angsutararux, P.; Luanpitpong, S.; Issaragrisil, S. Chemotherapy-Induced Cardiotoxicity: Overview of the Roles of Oxidative Stress. Oxid. Med. Cell Longev. 2015, 2015, 795602. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, J.; Yang, P. The mechanism and therapeutic strategies in doxorubicin induced cardiotoxicity: Role of programmed cell death. Cell Stress Chaperones 2024, 29, 666–680, Erratum in Cell Stress Chaperones 2024, 29, 720. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, L.R.; Chapman, K.; Xie, M.; Maifoshie, E.; Jenkins, M.; Golforoush, P.A.; Bellahcene, M.; Noseda, M.; Faust, D.; Jarvis, A.; et al. MAP4K4 Inhibition Promotes Survival of Human Stem Cell-Derived Cardiomyocytes and Reduces Infarct Size In Vivo. Cell Stem Cell. 2019, 24, 579–591, Correction in: Cell Stem Cell. 2020, 26, 458. [Google Scholar] [CrossRef] [PubMed]
- Golforoush, P.A.; Narasimhan, P.; Chaves-Guerrero, P.P.; Lawrence, E.; Newton, G.; Yan, R.; Harding, S.E.; Perrior, T.; Chapman, K.L.; Schneider, M.D. Selective protection of human cardiomyocytes from anthracycline cardiotoxicity by small molecule inhibitors of MAP4K4. Sci. Rep. 2020, 10, 12060. [Google Scholar] [CrossRef]
- Al Khafaji, A.T.; Barakat, A.M.; Shayyal, A.J.; Taan, A.A.; Aboqader Al-Aouadi, R.F. Managing Doxorubicin Cardiotoxicity: Insights Into Molecular Mechanisms and Protective Strategies. J. Biochem. Mol. Toxicol. 2025, 39, e70155. [Google Scholar] [CrossRef]
- Kundnani, N.R.; Passini, V.; Stefania Carlogea, I.; Dumitrescu, P.; Meche, V.; Buzas, R.; Duda-Seiman, D.M. Overview of Oncology: Drug-Induced Cardiac Toxicity. Medicina 2025, 61, 709. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Yi, Z.; Dong, H. Simvastatin protects cardiomyocytes from doxorubicin cardiotoxicity by suppressing endoplasmic reticulum stress and activating Akt signaling. Int. J. Clin. Exp. Med. 2016, 9, 2193–2201. [Google Scholar]
- Vitale, R.; Marzocco, S.; Popolo, A. Simvastatin Enhances the Cytotoxic Effects of Doxorubicin in a Mammary Adenocarcinoma Cell Model by Involving Connexin 43. J. Biochem. Mol. Toxicol. 2025, 39, e70214. [Google Scholar] [CrossRef]
- Quagliariello, V.; Berretta, M.; Bisceglia, I.; Giacobbe, I.; Iovine, M.; Giordano, V.; Arianna, R.; Barbato, M.; Izzo, F.; Maurea, C.; et al. The sGCa Vericiguat Exhibit Cardioprotective and Anti-Sarcopenic Effects through NLRP-3 Pathways: Potential Benefits for Anthracycline-Treated Cancer Patients. Cancers 2024, 16, 1487. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Fard, J.K.; Ghafoor, D.; Eid, A.H.; Sahebkar, A. Paradoxical effects of statins on endothelial and cancer cells: The impact of concentrations. Cancer Cell Int. 2023, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, V.; Vakilpour, A.; Scherrer-Crosbie, M. Statins for the Primary Prevention of Anthracycline Cardiotoxicity: A Comprehensive Review. Curr. Oncol. Rep. 2024, 26, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, F.; Quiñones-Galvan, A.; Regoli, F.; Ferrannini, E.; Galetta, F. A comparative study of the in vitro antioxidant activity of statins. Int. J. Cardiol. 2003, 90, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, R.; Castillo, R.L.; Gormaz, J.G.; Carrillo, M.; Thavendiranathan, P. Role of Oxidative Stress in the Mechanisms of Anthracycline-Induced Cardiotoxicity: Effects of Preventive Strategies. Oxid. Med. Cell Longev. 2021, 2021, 8863789. [Google Scholar] [CrossRef]
- Reis-Mendes, A.; Ferreira, M.; Padrão, A.I.; Duarte, J.A.; Duarte-Araújo, M.; Remião, F.; Carvalho, F.; Sousa, E.; Bastos, M.L.; Costa, V.M. The Role of Nrf2 and Inflammation on the Dissimilar Cardiotoxicity of Doxorubicin in Two-Time Points: A Cardio-Oncology In Vivo Study Through Time. Inflammation 2024, 47, 264–284. [Google Scholar] [CrossRef]
- Shi, S.; Chen, Y.; Luo, Z.; Nie, G.; Dai, Y. Role of oxidative stress and inflammation-related signaling pathways in doxorubicin-induced cardiomyopathy. Cell Commun. Signal. 2023, 21, 61. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Maghraby, N.; El-Baz, M.A.H.; Hassan, A.M.A.; Abd-Elghaffar, S.K.; Ahmed, A.S.; Sabra, M.S. Metformin Alleviates Doxorubicin-Induced Cardiotoxicity via Preserving Mitochondrial Dynamics Balance and Calcium Homeostasis. Appl. Biochem. Biotechnol. 2025, 197, 2713–2733. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 2015, 460, 72–81. [Google Scholar] [CrossRef]
- Sripusanapan, A.; Piriyakulthorn, C.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. Ivabradine ameliorates doxorubicin-induced cardiotoxicity through improving mitochondrial function and cardiac calcium homeostasis. Biochem. Pharmacol. 2025, 236, 116881. [Google Scholar] [CrossRef]
- Parihar, A.; Parihar, M.; Zenebe, W.; Ghafourifar, P. Statins lower calcium-induced oxidative stress in isolated mitochondria. Hum. Exp. Toxicol. 2011, 31, 355–363. [Google Scholar] [CrossRef]
- Gao, X.; Gao, C.; Liu, G.; Hu, J. MAP4K4: An emerging therapeutic target in cancer. Cell Biosci. 2016, 6, 56. [Google Scholar] [CrossRef]
- Hosseini, F.S.; Ahmadi, A.; Kesharwani, P.; Hosseini, H.; Sahebkar, A. Regulatory effects of statins on Akt signaling for prevention of cancers. Cell Signal. 2024, 120, 111213. [Google Scholar] [CrossRef]
- Salim, E.I.; Elsebakhy, S.; Hessien, M. Repurposing of atorvastatin and metformin denotes their individual and combined antiproliferative effects in non-small cell lung cancer. Fundam. Clin. Pharmacol. 2024, 38, 550–560. [Google Scholar] [CrossRef]
- Popolo, A.; Piccinelli, A.L.; Morello, S.; Sorrentino, R.; Osmany, C.R.; Rastrelli, L.; Pinto, A. Cytotoxic activity of nemorosone in human MCF-7 breast cancer cells. Can. J. Physiol. Pharmacol. 2011, 89, 50–57. [Google Scholar] [CrossRef]
- Pecoraro, M.; Marzocco, S.; Franceschelli, S.; Popolo, A. Trastuzumab and Doxorubicin Sequential Administration Increases Oxidative Stress and Phosphorylation of Connexin 43 on Ser368. Int. J. Mol. Sci. 2022, 23, 6375. [Google Scholar] [CrossRef]


| Genes | Forward Primer Sequence (5′–3′) | Reverse Primer Sequence (5′–3′) |
|---|---|---|
| GAPDH | CAACTTTGGTATCGTGGAAGGAC | ACAGTCTTCTGGGTGGCAGTG |
| NRF2 | CCTGGGATTTATAGCAGCAGAC | TGACACCAACCAGAGCTGAG |
| MAP4K4 | GTTAAAACGGGTCAGTTGGC | CCCCACAGAACTCCATAACAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, R.; Mazzone, M.; Di Marcantonio, M.C.; Marzocco, S.; Mincione, G.; Popolo, A. Cardioprotective Effects of Simvastatin in Doxorubicin-Induced Acute Cardiomyocyte Injury. Int. J. Mol. Sci. 2025, 26, 9440. https://doi.org/10.3390/ijms26199440
Vitale R, Mazzone M, Di Marcantonio MC, Marzocco S, Mincione G, Popolo A. Cardioprotective Effects of Simvastatin in Doxorubicin-Induced Acute Cardiomyocyte Injury. International Journal of Molecular Sciences. 2025; 26(19):9440. https://doi.org/10.3390/ijms26199440
Chicago/Turabian StyleVitale, Roberta, Mariangela Mazzone, Maria Carmela Di Marcantonio, Stefania Marzocco, Gabriella Mincione, and Ada Popolo. 2025. "Cardioprotective Effects of Simvastatin in Doxorubicin-Induced Acute Cardiomyocyte Injury" International Journal of Molecular Sciences 26, no. 19: 9440. https://doi.org/10.3390/ijms26199440
APA StyleVitale, R., Mazzone, M., Di Marcantonio, M. C., Marzocco, S., Mincione, G., & Popolo, A. (2025). Cardioprotective Effects of Simvastatin in Doxorubicin-Induced Acute Cardiomyocyte Injury. International Journal of Molecular Sciences, 26(19), 9440. https://doi.org/10.3390/ijms26199440









