Dermatophyte-Selective Imidazole-Thiosemicarbazides: Potent In Vitro Activity Against Trichophyton and Microsporum with No Anti-Candida Effect
Abstract
1. Introduction
2. Results and Discussion
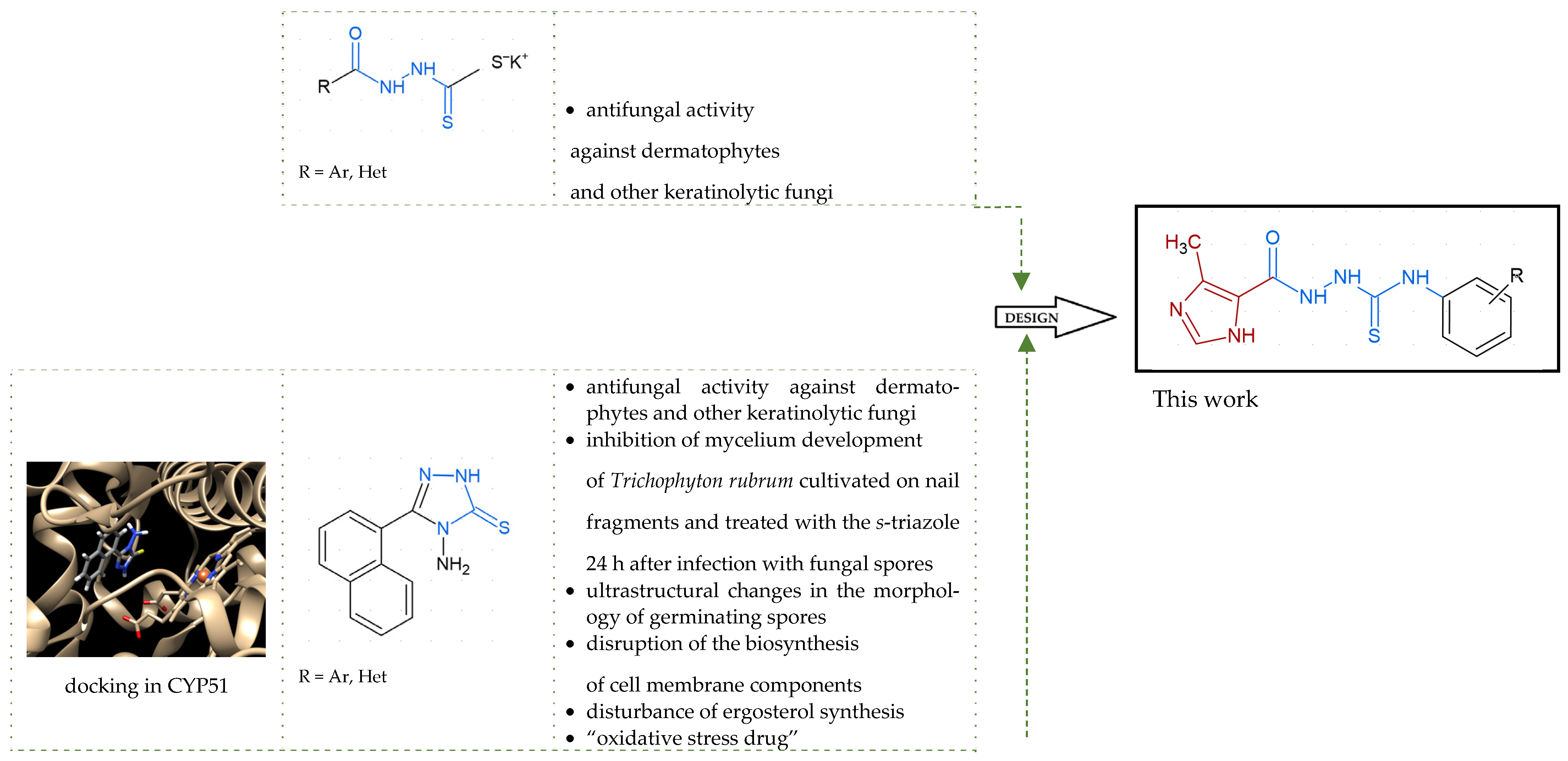
2.1. Rationale
2.2. In Vitro Antifungal Activity
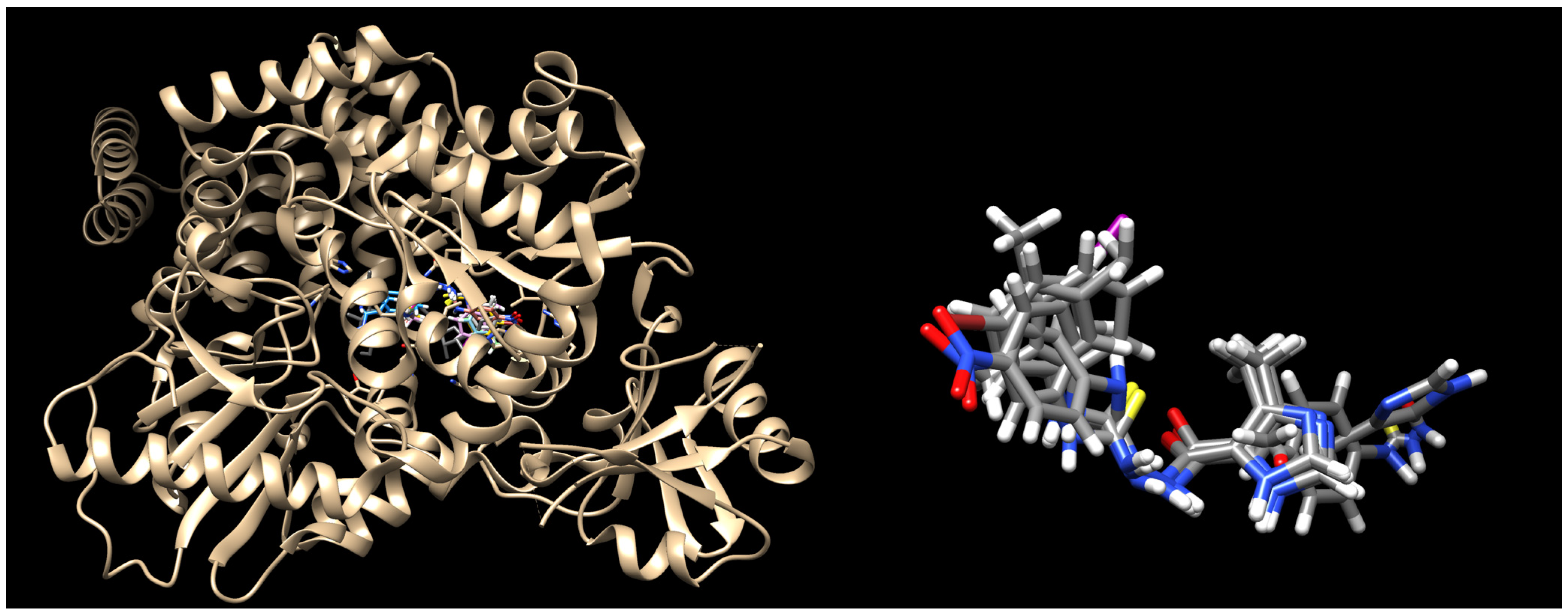
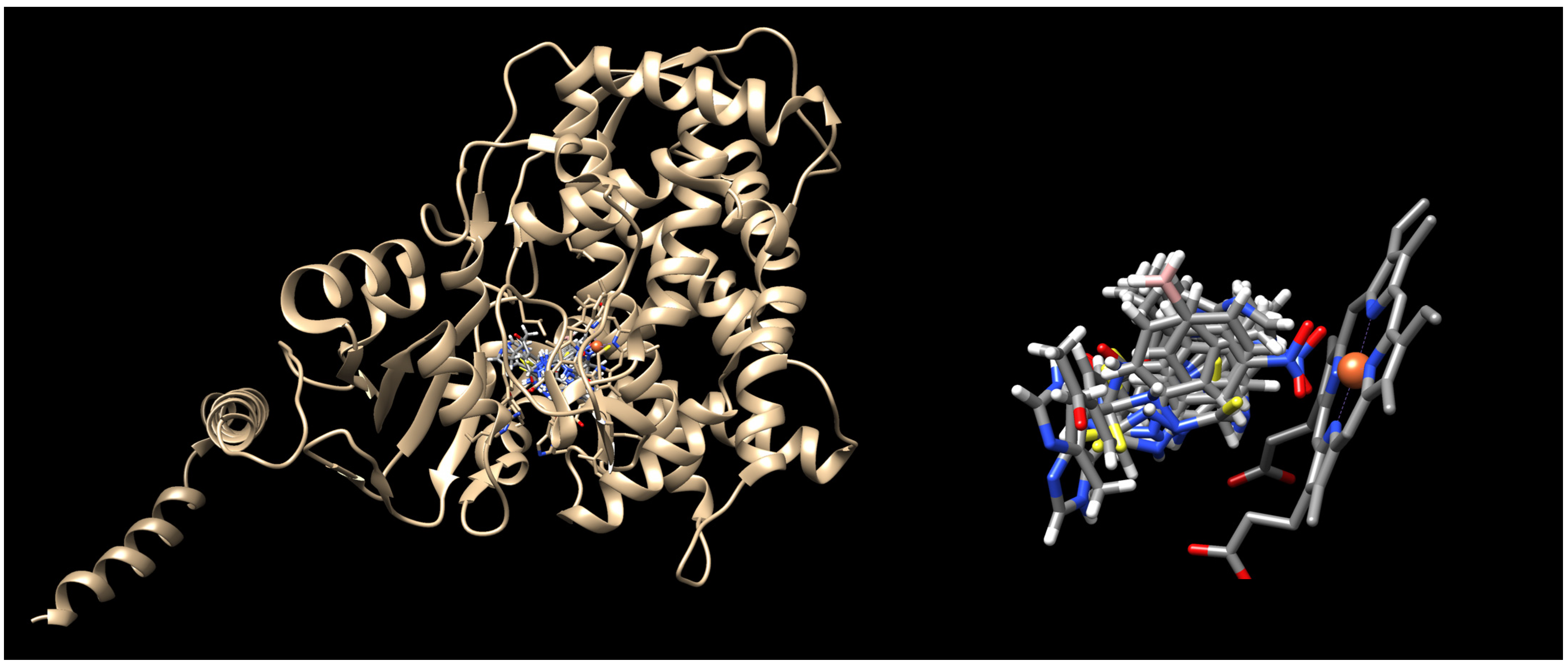
2.3. Molecular Docking
3. Materials and Methods
3.1. Chemistry
3.2. Fungal Strains Used in This Study
3.3. Antifungal Activity Assays
3.4. Resazurin Microtiter Assay
3.5. Anti-Candida Activity Assay
3.6. Docking Methodology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weitzman, I.; Summerbell, R.C. The dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar] [CrossRef]
- White, T.C.; Findley, K.; Dawson, T.L., Jr.; Scheynius, A.; Boekhout, T.; Cuomo, C.A.; Xu, J.; Saunders, C.W. Fungi on the Skin: Dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 2014, 4, a019802. [Google Scholar] [CrossRef] [PubMed]
- Kruithoff, C.; Gamal, A.; McCormick, T.S.; Ghannoum, M.A. Dermatophyte Infections Worldwide: Increase in Incidence and Associated Antifungal Resistance. Life 2024, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Ringworm (Tinea): Fact Sheet. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/ringworm-(tinea) (accessed on 1 July 2025).
- Urban, K.; Chu, S.; Scheufele, C.; Giesey, R.L.; Mehrmal, S.; Uppal, P.; Delost, G.R. The Global, Regional, and National Burden of Fungal Skin Diseases in 195 Countries and Territories: A Cross-Sectional Analysis from the Global Burden of Disease Study 2017. JAAD Int. 2020, 2, 22–27. [Google Scholar] [CrossRef]
- Arya, K.; Chaturvedi, S.; Usmani, S.A.; Chandra, S.; Bhardwaj, N.; Kumar, M.; Kumari, S.; Prasad, R.; Singh, A. Dermatophytosis in Context of Trichophyton rubrum: Host Defence Mechanisms, Virulence Factors, and Treatment Innovations. Mol. Biol. Rep. 2025, 52, 634. [Google Scholar] [CrossRef]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The Global Burden of Skin Disease in 2010: An Analysis of the Prevalence and Impact of Skin Conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef]
- Karimkhani, C.; Dellavalle, R.P.; Coffeng, L.E.; Flohr, C.; Hay, R.J.; Langan, S.M.; Nsoesie, E.O.; Ferrari, A.J.; Erskine, H.E.; Silverberg, J.I.; et al. Global Skin Disease Morbidity and Mortality: An Update From the Global Burden of Disease Study 2013. JAMA Dermatol. 2017, 153, 406–412. [Google Scholar] [CrossRef]
- Brasch, J. Current Knowledge of Host Response in Human Tinea. Mycoses 2009, 52, 304–312. [Google Scholar] [CrossRef]
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clin. Infect. Dis. 2019, 68, 1791–1797. [Google Scholar] [CrossRef]
- Benedict, K.; Whitham, H.K.; Jackson, B.R. The burden of fungal diseases in the United States: Estimation and economic analysis. Open Forum Infect. Dis. 2022, 9, ofac097. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Tinea Pedis: An Updated Review. Drugs Context 2023, 12, 2023-5-1. [Google Scholar] [CrossRef]
- Legge, B.S.; Grady, J.F.; Lacey, A.M. The Iincidence of Tinea Pedis in Diabetic Versus Nondiabetic Patients with Interdigital Macerations. J. Am. Podiatr. Med. Assoc. 2008, 98, 353–356. [Google Scholar] [CrossRef]
- Rand, S. Overview: The Treatment of Dermatophytosis. J. Am. Acad. Dermatol. 2000, 43, S104–S112. [Google Scholar] [CrossRef]
- Al-Khikani, F.H.O.; Ayit, A.S. Major Challenges in Dermatophytosis Treatment: Current Options and Future Visions. Egypt. J. Dermatol. Venereol. 2020, 41, 1–9. [Google Scholar] [CrossRef]
- Lauharanta, J. Comparative Efficacy And Safety of Amorolfine Nail Lacquer 2% Versus 5% Once Weekly. Clin. Exp. Dermatol. 1992, 17, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Tabara, K.; Szewczyk, A.E.; Bienias, W.; Wojciechowska, A.; Pastuszka, M.; Oszukowska, M.; Kaszuba, A. Amorolfine vs. Ciclopirox—Lacquers for the Treatment of Onychomycosis. Postep. Dermatol. Alergol. 2015, 32, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E.; Tavakkol, A. Safety and Tolerability of Oral Antifungal Agents in the Treatment of Fungal Nail Disease: A Proven Reality. Ther. Clin. Risk Manag. 2005, 1, 299–306. [Google Scholar]
- Sacheli, R.; Hayette, M.-P. Antifungal Resistance in Dermatophytes: Genetic Considerations, Clinical Presentations and Alternative Therapies. J. Fungi 2021, 7, 983. [Google Scholar] [CrossRef]
- Martinez-Rossi, N.M.; Bitencourt, T.A.; Peres, N.T.A.; Lang, E.A.S.; Gomes, E.V.; Quaresemin, N.R.; Martins, M.P.; Lopes, L.; Rossi, A. Dermatophyte Resistance to Antifungal Drugs: Mechanisms and Prospectus. Front. Microbiol. 2018, 9, 1108. [Google Scholar] [CrossRef]
- Lo Re, V., 3rd; Carbonari, D.M.; Lewis, J.D.; Forde, K.A.; Goldberg, D.S.; Reddy, K.R.; Haynes, K.; Roy, J.A.; Sha, D.; Marks, A.R.; et al. Oral Azole Antifungal Medications and Risk of Acute Liver Injury, Overall and by Chronic Liver Disease Status. Am. J. Med. 2016, 129, 283–291.e5. [Google Scholar] [CrossRef]
- Zapata-Garrido, A.J.; Romo, A.C.; Padilla, F.B. Terbinafine Hepatotoxicity. A Case Report and Review of Literature. Ann. Hepatol. 2003, 2, 45–51. [Google Scholar] [CrossRef]
- Spernovasilis, N.; Kofteridis, D.P. Pre-Existing Liver Disease and Toxicity of Antifungals. J. Fungi 2018, 4, 133. [Google Scholar] [CrossRef]
- Maskan Bermudez, N.; Rodríguez-Tamez, G.; Perez, S.; Tosti, A. Onychomycosis: Old and New. J. Fungi 2023, 9, 559. [Google Scholar] [CrossRef]
- Ciesielska, A.; Kowalczyk, A.; Paneth, A.; Stączek, P. Evaluation of the Antidermatophytic Activity of Potassium Salts of N-acylhydrazinecarbodithioates and Their Aminotriazole-thione Derivatives. Sci. Rep. 2024, 14, 3521. [Google Scholar] [CrossRef]
- Moriello, K.A.; Coyner, K.; Paterson, S.; Mignon, B. Diagnosis and Treatment of Dermatophytosis in Dogs and Cats. Vet. Dermatol. 2017, 28, 266–268. [Google Scholar] [CrossRef]
- Graser, Y.; El Fari, M.; Presber, W.; Kuijpers, A.F.A.; De Hoog, G.S. Molecular and Conventional Taxonomy of the Microsporum canis Complex. Med. Mycol. 2000, 38, 143–153. [Google Scholar] [CrossRef]
- Paneth, A.; Węglińska, L.; Bekier, A.; Stefaniszyn, E.; Wujec, M.; Trotsko, N.; Hawrył, A.; Hawrył, M.; Dzitko, K. Discovery of Potent and Selective Halogen-Substituted Imidazole-Thiosemicarbazides for Inhibition of Toxoplasma gondii Growth In Vitro via Structure-Based Design. Molecules 2019, 24, 1618. [Google Scholar] [CrossRef]
- Paneth, A.; Węglińska, L.; Bekier, A.; Stefaniszyn, E.; Wujec, M.; Trotsko, N.; Dzitko, K. Systematic Identification of Thiosemicarbazides for Inhibition of Toxoplasma gondii Growth In Vitro. Molecules 2019, 24, 614. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, C.; Zhang, Y.; Li, R. Invasive Dermatophyte Infection: A Systematic Review. Mycoses 2021, 64, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Rouzaud, C.; Hay, R.; Chosidow, O.; Dupin, N.; Puel, A.; Lortholary, O.; Lanternier, F. Severe Dermatophytosis and Acquired or Innate Immunodeficiency: A Review. J. Fungi 2016, 2, 4. [Google Scholar] [CrossRef]
- Dubljanin, E.; Zunic, J.; Vujcic, I.; Colovic Calovski, I.; Sipetic Grujicic, S.; Mijatovic, S.; Dzamic, A. Host-Pathogen Interaction and Resistance Mechanisms in Dermatophytes. Pathogens 2024, 13, 657. [Google Scholar] [CrossRef] [PubMed]
- Celestrino, G.A.; Veasey, J.V.; Benard, G.; Sousa, M.G. Host Immune Responses in Dermatophytes Infection. Mycoses 2012, 64, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Athouf, D.A. Opportunistic Dermatophyte Infections in Immunocompromised Patients with Rheumatoid Arthritis: A Review Article. Indones. J. Health Sci. 2025, 2, 1–34. [Google Scholar]
- Sooriya, S.; Jayapalan, S.; Mini, G.; Manjusree, S.; Nandakumar, L. Chronic Dermatophytosis: Clinico-Mycological Determinants and Antifungal Susceptibility Pattern. Indian J. Dermatol. 2021, 66, 329. [Google Scholar] [CrossRef]
- Machnikowski, N.; Barańska-Rybak, W.; Wilkowska, A.; Nowicki, R. Diagnosis of Dermatophytoses Still Problematic for General Practitioners—10 Case Studies and Review of Literature. Forum Dermat. 2017, 3, 157–165. [Google Scholar]
- Prabhu, N.; Rajeshwari, K.A.; Sangolli, P.M. Quality of Life in Patients with Chronic Dermatophytosis: A Cross-Sectional Study. Our Dermatol. Online 2024, 15, 349–352. [Google Scholar] [CrossRef]
- Hassan, N.H.; El-Hawary, S.S.; Emam, M.; Rabeh, M.A.; Abdelmohsen, U.R.; Selim, N.M. Potential Inhibitors of CYP51 Enzyme in Dermatophytes by Red Sea Soft Coral Nephthea sp.: In Silico and Molecular Networking Studies. ACS Omega 2022, 7, 13808–13817. [Google Scholar] [CrossRef]
- Ni, T.; Xie, F.; Hao, Y.; Li, L.; Zhu, S.; Wu, H.; Chi, X.; Yan, L.; Jiang, Y.; Zhang, D. Discovery of Novel Orally Bioavailable Triazoles with Potent and Broad-Spectrum Antifungal Activity In Vitro and In Vivo. J. Med. Chem. 2022, 65, 16665–16678. [Google Scholar] [CrossRef]
- Mahreen Ameen, M.D. Epidemiology of Superficial Fungal Infections. Clin. Dermatol. 2010, 28, 197–201. [Google Scholar] [CrossRef]
- El-Saghier, A.M.; Abdou, A.; Mohamed, M.A.A.; AbdEl-Lateef, H.M.; Kadry, A.M. Novel 2-Acetamido-2-ylidene-4-imidazole Derivatives (El-Saghier Reaction): Green Synthesis, Biological Assessment, and Molecular Docking. ACS Omega 2023, 8, 30519–30531. [Google Scholar] [CrossRef]
- Siwach, A.; Verma, P.K. Synthesis and Therapeutic Potential of Imidazole Containing Compounds. BMC Chem. 2021, 15, 12. [Google Scholar] [CrossRef]
- Bekier, A.; Gatkowska, J.; Chyb, M.; Sokołowska, J.; Chwatko, G.; Głowacki, R.; Paneth, A.; Dzitko, K. 4-Arylthiosemicarbazide Derivatives—Pharmacokinetics, Toxicity and Anti-Toxoplasma gondii Activity In Vivo. Eur. J. Med. Chem. 2022, 244, 114812. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Kahlmeter, G.; Guinea, J.; Meletiadis, J. Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). How to: Perform Antifungal Susceptibility Testing of Microconidia-Forming Dermatophytes Following the New Reference EUCAST Method E.Def 11.0, Exemplified by Trichophyton. Clin. Microbiol. Infect. 2021, 27, 55–60. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard—Second Edition; CLSI document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Arendrup, M.C.; Jørgensen, K.M.; Guinea, J.; Lagrou, K.; Chryssanthou, E.; Hayette, M.-P.; Barchiesi, F.; Lass-Flörl, C.; Hamal, P.; Dannaoui, E.; et al. Multicentre validation of a EUCAST method for the antifungal susceptibility testing of microconidia-forming dermatophytes. J. Antimicrob. Chemother. 2020, 75, 1807–1819. [Google Scholar] [CrossRef]
- Gupta, A.K.; Summerbell, R.C. Tinea capitis. Med. Mycol. 2000, 38, 255–287. [Google Scholar] [CrossRef]
- ATCC 102231. Available online: https://www.atcc.org/products/10231 (accessed on 22 September 2025).
- ATCC 66032. Available online: https://www.atcc.org/products/66032 (accessed on 22 September 2025).
- CDC B11903. Available online: https://www.microbiologics.com/01256P (accessed on 22 September 2025).
- ATCC 14243. Available online: https://www.atcc.org/products/14243 (accessed on 22 September 2025).
- ATCC 22019. Available online: https://www.atcc.org/products/22019 (accessed on 22 September 2025).
- Koga, H.; Nanjoh, Y.; Makimura, K.; Tsuboi, R. In Vitro Antifungal Activities of Luliconazole, a New Topical Imidazole. Med. Mycol. 2009, 47, 640–647. [Google Scholar] [CrossRef]
- Nazia, H.; Manvi, S.; Sufiyanu, S.; Pooja, J.; Kalicharan, S.; Shyamasree, N.; Mridu, D.; Asgar, A.; Zeenat, I. Molecular Docking-Guided Ungual Drug-Delivery Design For Amelioration of Onychomycosis. ACS Omega 2019, 4, 9583–9592. [Google Scholar]
- Pathak, S.; Alonso, J.; Schimpl, M.; Rafie, K.; Blair, D.E.; Borodkin, V.S.; Schuttelkopf, A.W.; Albarbarawi, O.; van Aalten, D.M. The Active Site of O-GlcNAc Transferase Imposes Constraints on Substrate Sequence. Nat. Struct. Mol. Biol. 2015, 22, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Sagatova, A.A.; Keniya, M.V.; Wilson, R.K.; Sabherwal, M.; Tyndall, J.D.; Monk, B.C. Triazole Resistance Mediated By Mutations of a Conserved Active Site Tyrosine in Fungal Lanosterol 14 Alpha-Demethylase. Sci. Rep. 2016, 6, 26213. [Google Scholar] [CrossRef] [PubMed]
- Bekier, A.; Węglińska, L.; Paneth, A.; Paneth, P.; Dzitko, K. 4-Arylthiosemicarbazide Derivatives as a New Class of Tyrosinase Inhibitors and Anti-Toxoplasma gondii Agents. J. Enzym. Inhib. Med. Chem. 2021, 36, 1145–1164. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Tudela, J.L.; Chryssanthou, E.; Petrikkou, E.; Mosquera, J.; Denning, D.W.; Cuenca-Estrella, M. Interlaboratory Evaluation of Hematocytometer Method of Inoculum Preparation For Testing Antifungal Susceptibilities of Filamentous Fungi. J. Clin. Microbiol. 2003, 41, 5236–5237. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Howard, S.; Lass-Fl€orl, C.; Mouton, J.W.; Meletiadis, J.; Cuenca-Estrella, M. EUCAST Testing of Isavuconazole Susceptibility in Aspergillus: Comparison of Results for Inoculum Standardization Using Conidium Counting Versus Optical Density. Antimicrob. Agents Chemother. 2014, 58, 6432–6436. [Google Scholar] [CrossRef] [PubMed]
- Markantonatou, A.M.; Samaras, K.; Zachrou, E.; Vyzantiadis, T.A. Comparison of Four Methods for the in vitro Susceptibility Testing of Dermatophytes. Front. Microbiol. 2020, 14, 1593. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.; Pearson, J. Susceptibility Testing: Accurate and Reproducible Minimum Inhibitory Concentration (MIC) and Non-Inhibitory Concentration (NIC) values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef] [PubMed]
- ATCC 90030. Available online: https://www.atcc.org/products/90030 (accessed on 22 September 2025).
- Berman, H.M.; Henrick, K.; Nakamura, H. Announcing the Worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef]

| MIC (μg/mL; [μM]) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | T. ton. | T. rub. | T. men. | M. can. | A. ker. | Ch. que. | A. pan. | Ch. tro. | |
| 1 | 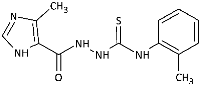 | 2.93 [10.14] | >289.36 [>1000] | >289.36 [>1000] | >289.36 [>1000] | >289.36 [>1000] | >289.36 [>1000] | >289.36 [>1000] | 127.19 [>439.56] |
| 2 |  | 1.43 [4.94] | >289.36 [>1000] | >289.36 [>1000] | >289.36 [>1000] | >289.36 [>1000] | 70.75 [244.51] | 177.27 [612.65] | 162.81 [562.67] |
| 3 |  | <0.29 [<1] | >289.36 [>1000] | >289.36 [>1000] | >289.36 [>1000] | >289.36 [>1000] | 153.45 [530.32] | 133.58 [461.65] | 136.75 [472.61] |
| 4 | 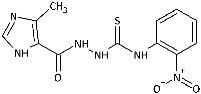 | 98.81 [308.46] | 78.00 [243.50] | 149.35 [466.23] | 147.45 [460.30] | >320.33 [>1000] | 42.28 [132.00] | 41.64 [130.00] | 146.28 [456.66] |
| 5 |  | 1.59 [4.96] | >320.33 [>1000] | >320.33 [>1000] | >320.33 [>1000] | >320.33 [>1000] | 38.86 [121.31] | 36.48 [113.87] | 131.01 [409.00] |
| 6 | 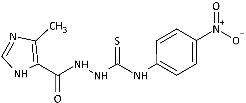 | 3.65 [11.40] | 73.38 [229.07] | >320.33 [>1000] | >320.33 [>1000] | n.a. | 39.41 [123.02] | 49.82 [155.54] | 52.69 [164.48] |
| 7 |  | 73.46 [207.39] | >354.23 [>1000] | 271.58 [766.70] | 7.38 [20.83] | >354.23 [>1000] | 21.86 [61.71] | 43.69 [123.34] | 169.62 [478.86] |
| 8 | 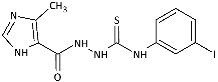 | 15.15 [37.77] | >401.23 [>1000] | 165.51 [412.51] | 3.87 [9.64] | >401.23 [>1000] | 24.38 [60.76] | 46.81 [116.67] | 86.35 [215.21] |
| 9 | 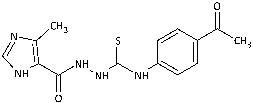 | 13.65 [43.02] | 144.82 [456.31] | >317.37 [>1000] | 136.05 [428.69] | n.a. | 39.56 [124.64] | 76.84 [242.11] | 67.71 [213.35] |
| 10 |  | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| 11 | 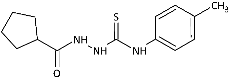 | 122.77 [442.61] | 197.98 [713.75] | >277.39 [>1000] | 152.26 [548.91] | 141.44 [509.91] | 143.72 [518.12] | 170.93 [616.21] | 3.68 [13.25] |
| 12 | 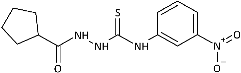 | 226.14 [733.38] | >308.36 [>1000] | >308.36 [>1000] | >308.36 [>1000] | >308.36 [>1000] | >308.36 [>1000] | >308.36 [>1000] | 3.98 [12.91] |
| 13 | 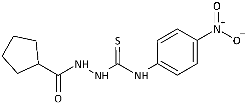 | 68.66 [222.66] | 152.46 [494.44] | 278.36 [902.71] | 163.24 [529.40] | 108.05 [350.41] | 149.46 [484.71] | 170.25 [552.11] | 1.61 [5.23] |
| 14 | 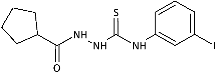 | 225.41 [579.09] | 283.05 [727.15] | >389.26 [>1000] | >389.26 [>1000] | 457.02 [1174.09] | 355.12 [912.31] | >389.26 [>1000] | 6.08 [15.61] |
| AMB | 4.00 [3.70] | 4.00 [3.70] | 4.00 [3.70] | 8.00 [7.39] | 4.00 [3.70] | nt | nt | nt | |
| KCZ | 0.50 [0.27] | 2.00 [1.06] | 1.00 [0.53] | 1.00 [0.53] | 1.00 [0.53] | nt | nt | nt | |
| IC50 [μM]; SI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | T. ton. | T. rub. | T. men. | M. can. | A. ker. | Ch. que. | A. pan. | Ch. tro. |
| 1 | [<1] SI > 996 | [580.09] SI = 1.7 | [55.14] SI = 18.1 | [491.58] SI = 2.0 | [>1000] SI < 1 | [>1000] SI < 1 | [460.47] SI = 2.2 | [52.54] SI = 19.0 |
| 2 | [10.14] SI = 17.1 | [>1000] SI < 1 | [>1000] SI < 1 | [>1000] SI < 1 | [>1000] SI < 1 | [>1000] SI < 1 | [>1000] SI < 1 | [>439.56] SI < 1 |
| 3 | [<1] SI > 361 | [>1000] SI < 1 | [32.07] SI = 11.3 | [361.91] SI < 1 | [>1000] SI < 1 | [224.91] SI = 1.6 | [305.70] SI = 1.2 | [44.77] SI = 8.1 |
| 4 | [2.05] SI = 276.5 | [185.69] SI = 3.1 | [415.33] SI = 1.4 | [339.15] SI = 1.7 | [>1000] SI < 1 | [90.55] SI = 6.3 | [92.77] SI = 6.1 | [39.72] SI = 14.3 |
| 5 | [<1] SI > 440 | [>1000] SI < 1 | [344.69] SI = 1.3 | [269.64] SI = 1.6 | [>1000] SI < 1 | [78.52] SI = 5.6 | [74.23] SI = 5.9 | [76.05] SI = 5.8 |
| 6 | [<1] SI > 682 | [149.69] SI = 4.6 | [>1000] SI < 1 | [625.89] SI = 1.1 | n.a. | [83.06] SI = 8.2 | [100.39] SI = 6.8 | [44.77] SI = 15.3 |
| 7 | [<1] SI > 846 | [>1000] SI < 1 | [127.41] SI = 6.6 | [11.06] SI = 76.6 | [>1000] SI < 1 | [38.17] SI = 22.2 | [68.34] SI = 12.4 | [111.99] SI = 7.6 |
| 8 | [<1] SI > 213 | [489.32] SI < 1 | [66.49] SI = 3.2 | [7.21] SI = 29.6 | [619.86] SI < 1 | [35.39] SI = 6.0 | [46.24] SI = 4.6 | [82.51] SI = 2.6 |
| 9 | [<1] SI > 650 | [419.46] SI = 1.6 | [>1000] SI < 1 | [404.29] SI = 1.6 | n.a. | [84.81] SI = 7.7 | [150.86] SI = 4.3 | [42.07] SI = 15.5 |
| 11 | [65.87] SI = 7.0 | [510.74] SI < 1 | [225.16] SI = 2.1 | [390.66] SI = 1.2 | [208.88] SI = 2.2 | [380.97] SI = 1.2 | [393.09] SI = 1.2 | [<1] SI > 463 |
| 12 | [216.97] SI = 2.8 | [>1000] SI < 1 | [563.64] SI = 1.1 | [>1000] SI < 1 | [398.01] SI = 1.5 | [>1000] SI < 1 | [>1000] SI < 1 | [2.51] SI = 245.6 |
| 13 | [36.58] SI = 10.3 | [447.71] SI < 1 | [66.50] SI = 5.7 | [280.86] SI = 1.3 | [120.08] SI = 3.2 | [329.15] SI = 1.1 | [283.14] SI = 1.1 | [<1] SI > 378 |
| 14 | [206.11] SI < 1 | [538.26] SI < 1 | [86.77] SI = 1.4 | [>1000] SI < 1 | [420.92] SI < 1 | [306.34] SI < 1 | [>1000] SI < 1 | [1.16] SI = 101.4 |
| C. albicans | C. glabrata | C. auris | C. krusei | C. parapsilosis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| 1 | >1000 | >1000 | >1000 | >1000 | 500 | 1000 | >1000 | >1000 | 500 | >1000 |
| 2 | 1000 | 1000 | 1000 | >1000 | 250 | 500 | 1000 | 1000 | 500 | 1000 |
| 3 | 1000 | 1000 | 1000 | >1000 | 250 | 500 | 1000 | >1000 | 250 | 1000 |
| 4 | >1000 | >1000 | >1000 | >1000 | 250 | 250 | 1000 | >1000 | 250 | 1000 |
| 5 | 500 | >1000 | 1000 | 1000 | 250 | 500 | 1000 | 1000 | 250 | 1000 |
| 6 | 500 | 500 | 1000 | >1000 | 62.5 | 125 | 500 | 500 | 250 | 500 |
| 7 | 1000 | >1000 | 1000 | >1000 | 500 | 1000 | 1000 | >1000 | 500 | 1000 |
| 8 | 500 | >1000 | >1000 | >1000 | 500 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 9 | 1000 | 1000 | 1000 | >1000 | 250 | 250 | 1000 | 1000 | 500 | 1000 |
| Nystatin | 0.24 | 0.48 | 0.48 | 0.48 | 0.48 | 0.48 | 0.24 | 0.24 | 0.24 | 0.48 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | NI * | |
|---|---|---|---|---|---|---|---|---|---|---|
| α-keratin | −36.4 | −39.2 | −37.8 | −43.3 | −49.7 | −38.7 | −39.2 | −38.5 | −38.7 | −58.9 |
| lanosterol−14-α demethylase | −32.1 | −33.5 | −28.7 | −33.8 | −33.8 | −38.2 | −30.2 | −29.0 | −33.0 | −27.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paneth, A.; Dzitko, K.; Bekier, A.; Trotsko, N.; Suśniak, K.; Ciesielska, A.; Paneth, P. Dermatophyte-Selective Imidazole-Thiosemicarbazides: Potent In Vitro Activity Against Trichophyton and Microsporum with No Anti-Candida Effect. Int. J. Mol. Sci. 2025, 26, 9437. https://doi.org/10.3390/ijms26199437
Paneth A, Dzitko K, Bekier A, Trotsko N, Suśniak K, Ciesielska A, Paneth P. Dermatophyte-Selective Imidazole-Thiosemicarbazides: Potent In Vitro Activity Against Trichophyton and Microsporum with No Anti-Candida Effect. International Journal of Molecular Sciences. 2025; 26(19):9437. https://doi.org/10.3390/ijms26199437
Chicago/Turabian StylePaneth, Agata, Katarzyna Dzitko, Adrian Bekier, Nazar Trotsko, Katarzyna Suśniak, Anita Ciesielska, and Piotr Paneth. 2025. "Dermatophyte-Selective Imidazole-Thiosemicarbazides: Potent In Vitro Activity Against Trichophyton and Microsporum with No Anti-Candida Effect" International Journal of Molecular Sciences 26, no. 19: 9437. https://doi.org/10.3390/ijms26199437
APA StylePaneth, A., Dzitko, K., Bekier, A., Trotsko, N., Suśniak, K., Ciesielska, A., & Paneth, P. (2025). Dermatophyte-Selective Imidazole-Thiosemicarbazides: Potent In Vitro Activity Against Trichophyton and Microsporum with No Anti-Candida Effect. International Journal of Molecular Sciences, 26(19), 9437. https://doi.org/10.3390/ijms26199437







