Impaired Formation of Primary Cilia in Olfactory Neuronal Precursors Is Associated with Decreased Proliferation and Maturation in Individuals with Hyposmia
Abstract
1. Introduction
2. Results
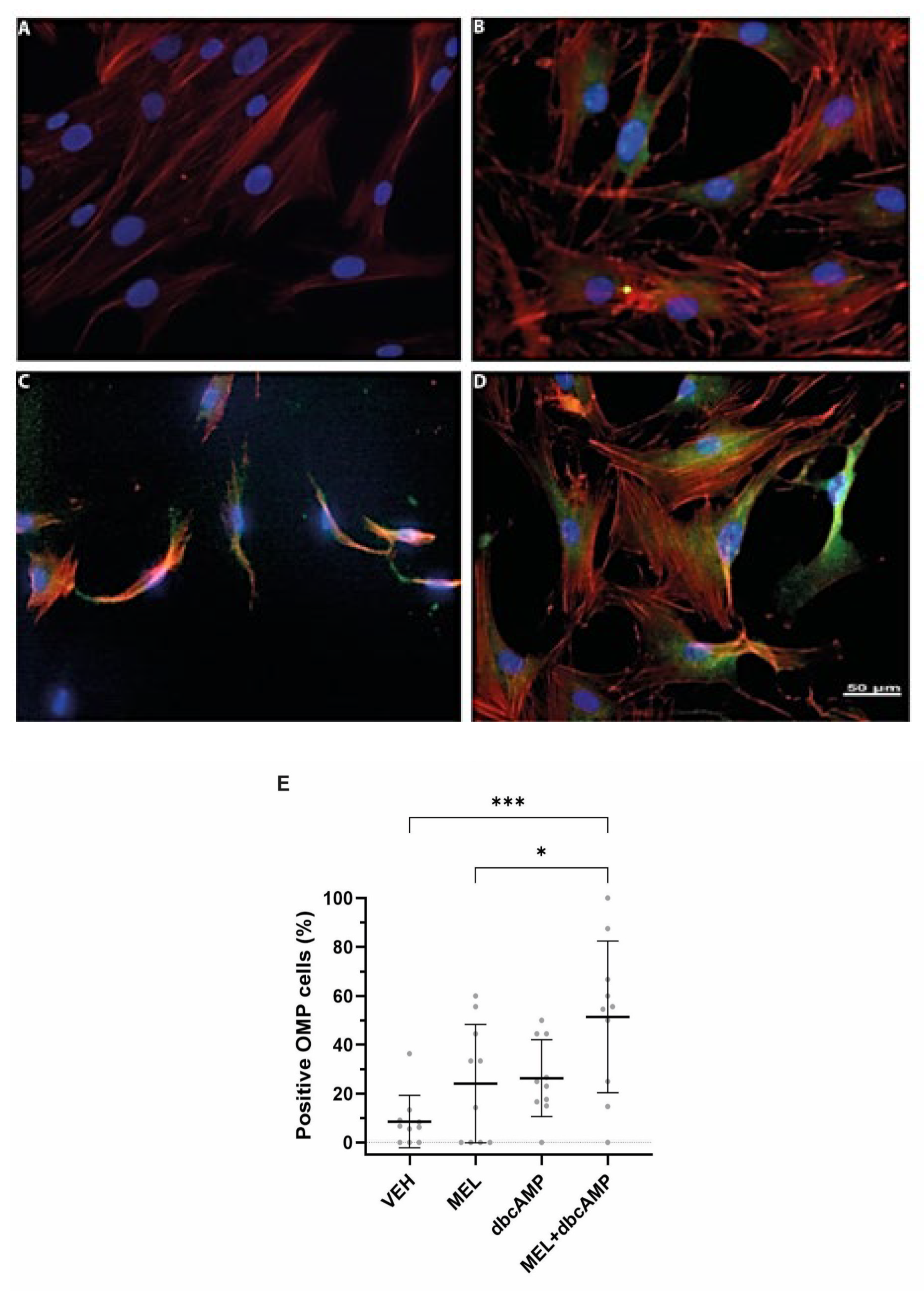
2.1. The Combination of Melatonin and Dibutyryl Cyclic AMP Stimulates Maturation and Cilia Biogenesis in Olfactory Neuronal Precursors
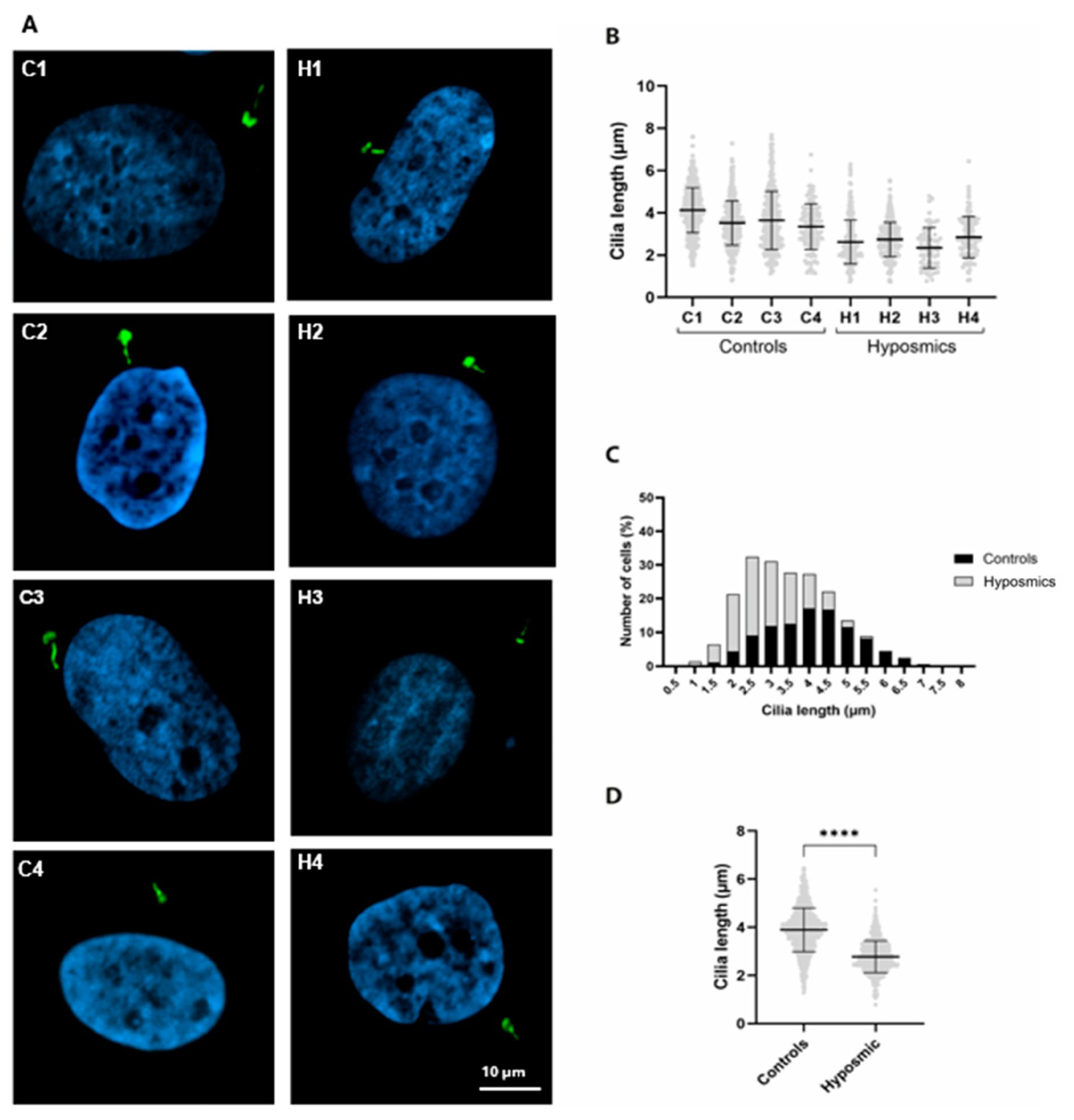
2.2. Cilia Axonemes Are Longer in Olfactory Neuronal Precursors Cultured with Melatonin and Dibutyryl Cyclic AMP
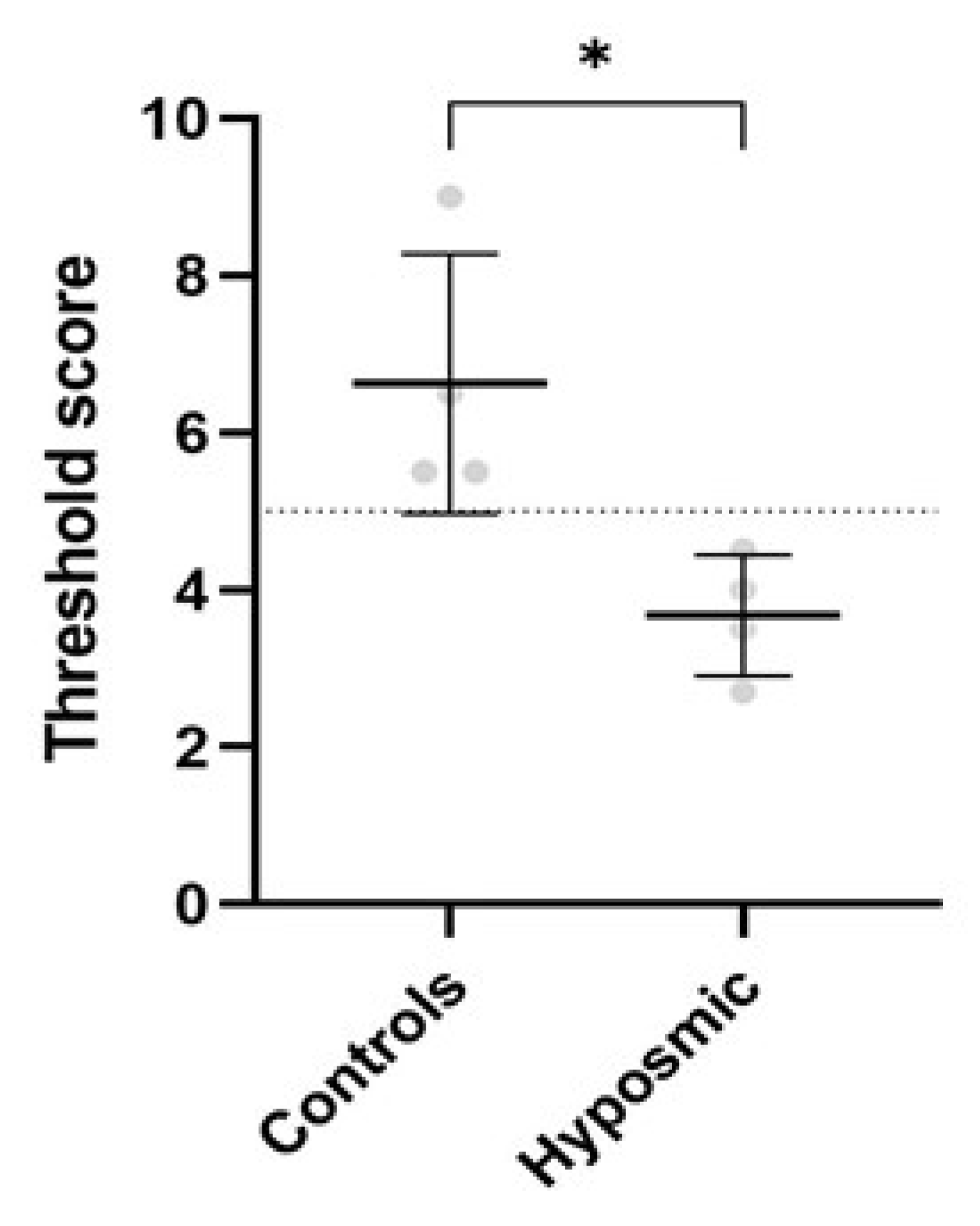
2.3. Olfactory Test Results in Control and Hyposmic Subjects
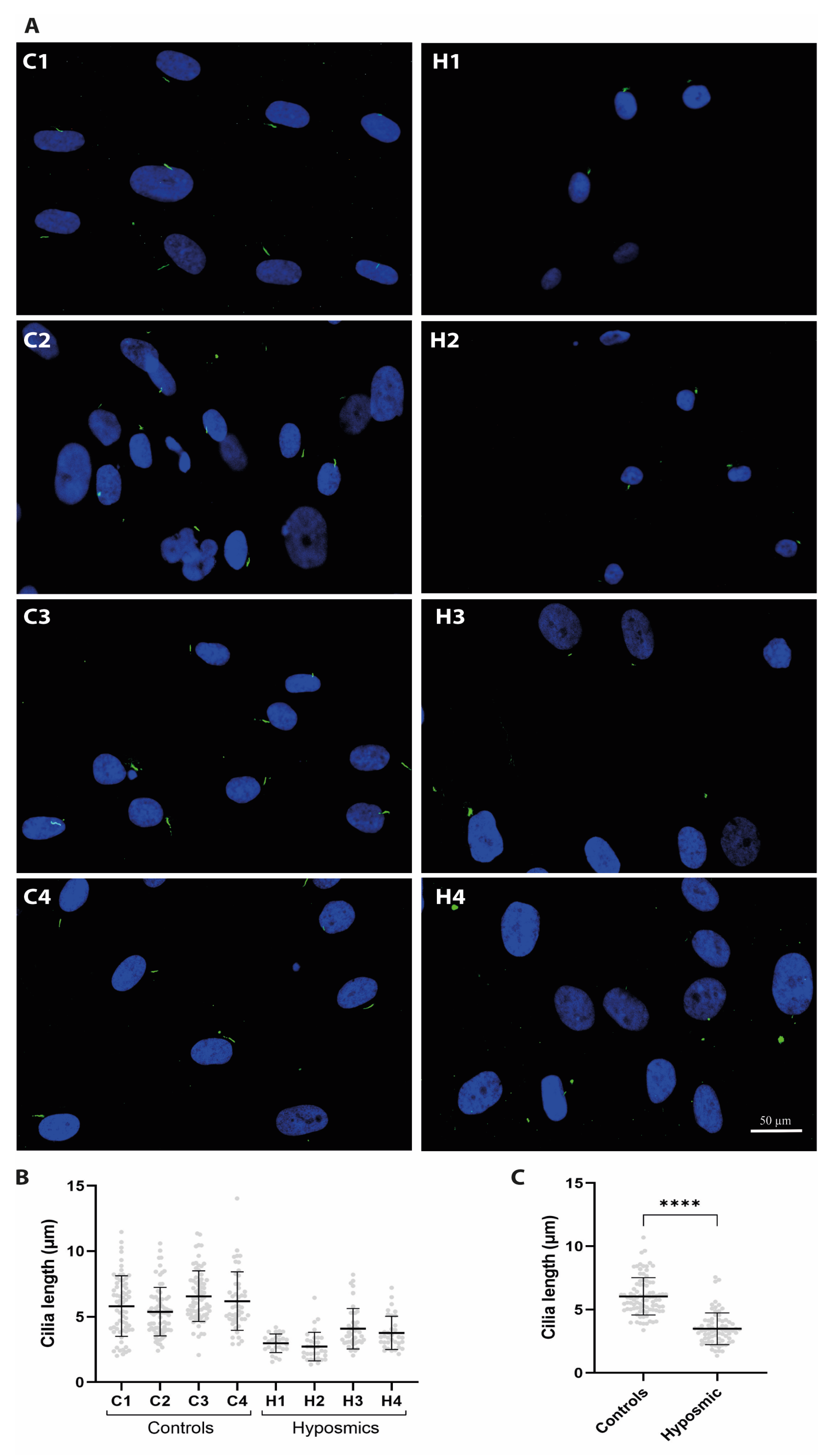
2.4. Cilia Are Shorter in Individuals with Hyposmia
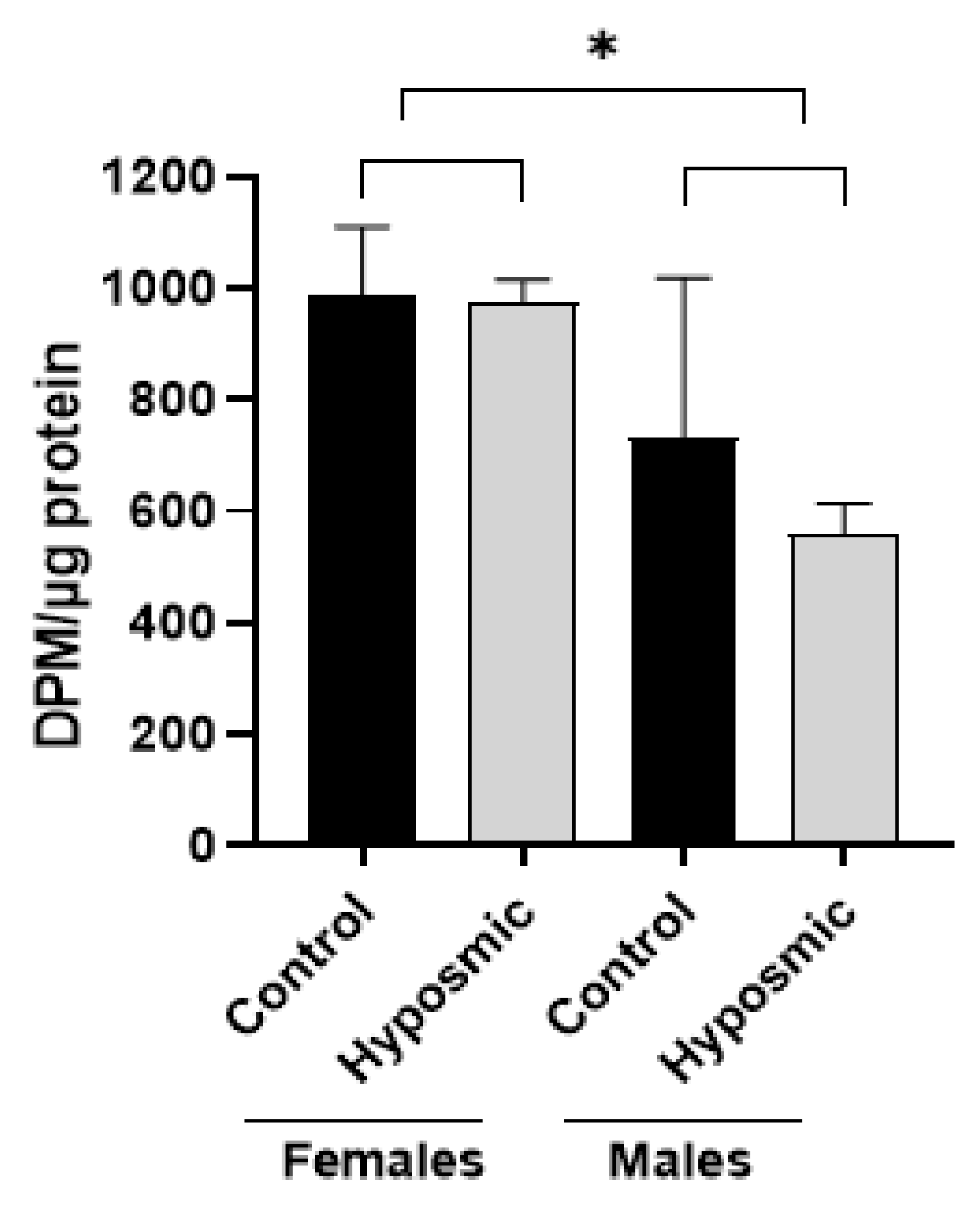
2.5. Prtoein Acetylatransferase Activity Hyposmic and Control Participants
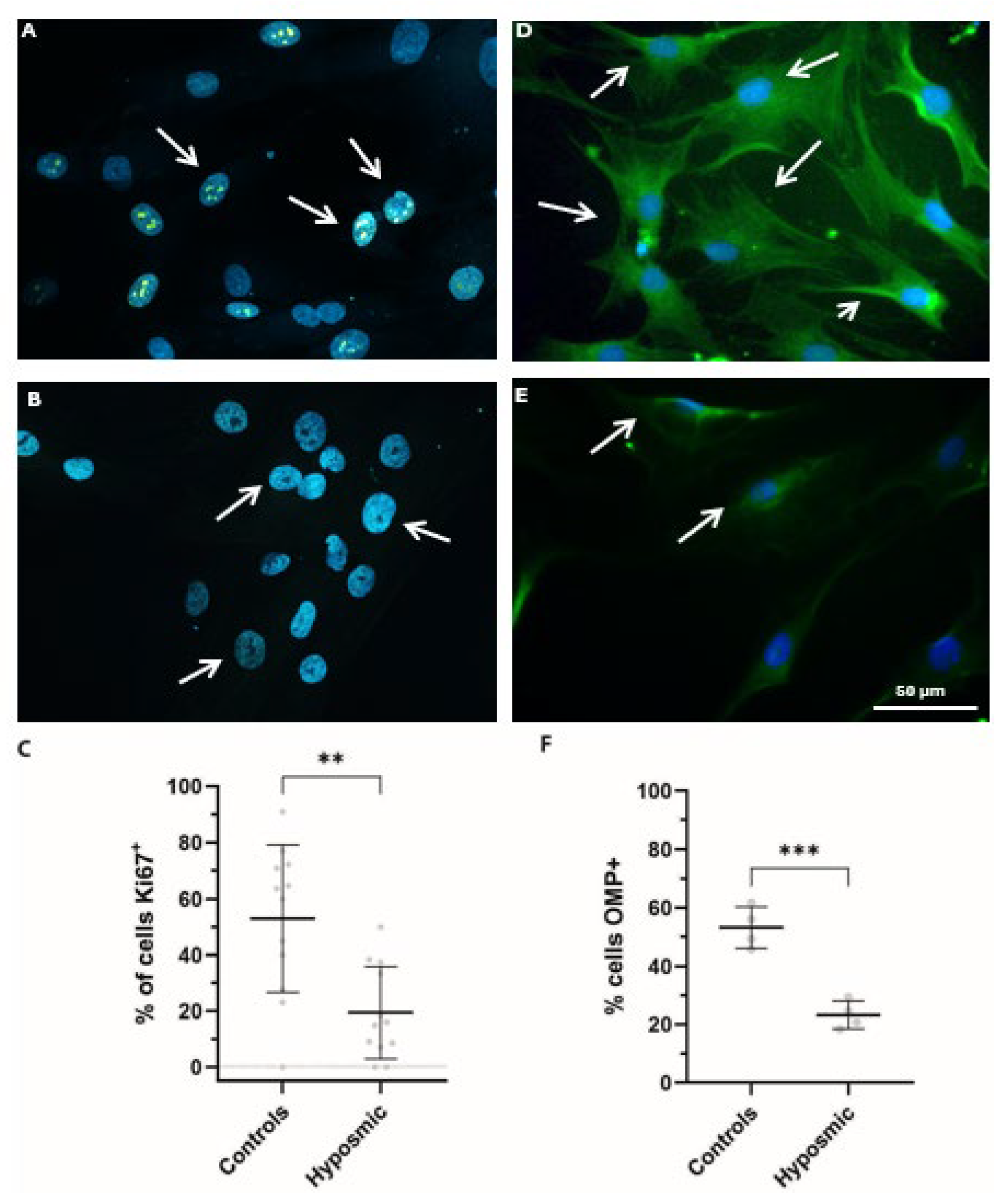
2.6. Proliferation and Differentiation of Olfactory Neuronal Precursors Are Impaired in Subjects with Hyposmia
3. Discussion
4. Materials and Methods
4.1. Participants
Exclusion Criteria
4.2. Olfactory Test
4.3. Obtaining Olfactory Neuronal Precursors and Cell Culture
4.4. Cilia Formation
4.5. Immunofluorescence Staining
4.6. Determination of Primary Cilia Length and Frequencies for Cell Proliferation and Differentiation
4.7. Protein Acetyltransferase Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ANOVA | Two-way analysis of variance |
| C | Control |
| DAPI | 4′,6-DiAmino-2-PhenylIndole |
| dbcAMP | dibutyryl cyclic AMP |
| DMEM | Dulbecco‘s-Modified Eagle Medium |
| DPM | Disintegrations Per Minute |
| EC | Entorhinal Cortex |
| F | Female |
| GPCRs | G Protein-Coupled Receptors |
| H | hyposmic |
| Ki67 | Nuclear Protein used as a Marker of Proliferating Cells |
| M | Male |
| MEL | Melatonin |
| OMP | Olfactory Marker Protein ONPs |
| ONPs | Olfactory Neuron Precursors |
| OSNs | Olfactory Sensory Neurons |
| OR | Olfactory Receptor |
| PCM-1 | Pericentriolar Material |
| PD | Parkinson’s Disease |
| SD | Standard Deviation |
| VEH | Vehicle |
References
- Soudry, Y.; Lemogne, C.; Malinvaud, D.; Consoli, S.-M.; Bonfils, P. Olfactory System and Emotion: Common Substrates. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Hoskison, E.E. Olfaction, Pheromones and Life. J. Laryngol. Otol. 2013, 127, 1156–1159. [Google Scholar] [CrossRef]
- Bathini, P.; Brai, E.; Auber, L.A. Olfactory Dysfunction in the Pathophysiological Continuum of Dementia. Ageing Res. Rev. 2019, 55, 100956. [Google Scholar] [CrossRef]
- Kamath, V.; Paksarian, D.; Cui, L.; Moberg, P.J.; Turetsky, B.I.; Merikangas, K.R. Olfactory Processing in Bipolar Disorder, Major Depression, and Anxiety. Bipolar Disord. 2018, 20, 547–555. [Google Scholar] [CrossRef]
- Pabel, L.D.; Hummel, T.; Weidner, K.; Croy, I. The Impact of Severity, Course and Duration of Depression on Olfactory Function. J. Affect. Disord. 2018, 238, 194–203. [Google Scholar] [CrossRef]
- Turetsky, B.I.; Hahn, C.-G.; Arnold, S.E.; Moberg, P.J. Olfactory Receptor Neuron Dysfunction in Schizophrenia. Neuropsychopharmacology 2009, 34, 767–774. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Ma, M.; Sawa, A.; Lane, A.P.; Kamiya, A. Olfactory Impairment in Psychiatric Disorders: Does Nasal Inflammation Impact Disease Psychophysiology? Transl. Psychiatry 2022, 12, 314. [Google Scholar] [CrossRef]
- KOVACS, T. Mechanisms of Olfactory Dysfunction in Aging and Neurodegenerative Disorders. Ageing Res. Rev. 2004, 3, 215–232. [Google Scholar] [CrossRef]
- Hua, K.; Ferland, R.J. Primary Cilia Proteins: Ciliary and Extraciliary Sites and Functions. Cell. Mol. Life Sci. 2018, 75, 1521–1540. [Google Scholar] [CrossRef]
- Chinen, T.; Yamazaki, K.; Hashimoto, K.; Fujii, K.; Watanabe, K.; Takeda, Y.; Yamamoto, S.; Nozaki, Y.; Tsuchiya, Y.; Takao, D.; et al. Centriole and PCM Cooperatively Recruit CEP192 to Spindle Poles to Promote Bipolar Spindle Assembly. J. Cell Biol. 2021, 220, e202006085. [Google Scholar] [CrossRef]
- Serwas, D.; Su, T.Y.; Roessler, M.; Wang, S.; Dammermann, A. Centrioles Initiate Cilia Assembly but Are Dispensable for Maturation and Maintenance in C. elegans. J. Cell Biol. 2017, 216, 1659–1671. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, E.; Greenan, G.; Lange, K.I.; Srayko, M.; Müller-Reichert, T. The Role of γ-Tubulin in Centrosomal Microtubule Organization. PLoS ONE 2012, 7, e29795. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.M.; Constable, S.; Nguyen, T.M.; White, K.; Lee, W.-C.A.; Lippincott-Schwartz, J.; Mukhopadhyay, S. Permanent Deconstruction of Intracellular Primary Cilia in Differentiating Granule Cell Neurons. J. Cell Biol. 2024, 223, e202404038. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, F.; Benzing, T.; Katsanis, N. Ciliopathies. N. Engl. J. Med. 2011, 364, 1533–1543. [Google Scholar] [CrossRef]
- Reiter, J.F.; Leroux, M.R. Genes and Molecular Pathways Underpinning Ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef]
- Braun, J.-J.; Noblet, V.; Durand, M.; Scheidecker, S.; Zinetti-Bertschy, A.; Foucher, J.; Marion, V.; Muller, J.; Riehm, S.; Dollfus, H.; et al. Olfaction Evaluation and Correlation with Brain Atrophy in Bardet-Biedl Syndrome. Clin. Genet. 2014, 86, 521–529. [Google Scholar] [CrossRef]
- Muñoz-Estrada, J.; Lora-Castellanos, A.; Meza, I.; Alarcón Elizalde, S.; Benítez-King, G. Primary Cilia Formation Is Diminished in Schizophrenia and Bipolar Disorder: A Possible Marker for These Psychiatric Diseases. Schizophr. Res. 2018, 195, 412–420. [Google Scholar] [CrossRef]
- Benítez-King, G.; Riquelme, A.; Ortíz-López, L.; Berlanga, C.; Rodríguez-Verdugo, M.S.; Romo, F.; Calixto, E.; Solís-Chagoyán, H.; Jímenez, M.; Montaño, L.M.; et al. A Non-Invasive Method to Isolate the Neuronal Linage from the Nasal Epithelium from Schizophrenic and Bipolar Diseases. J. Neurosci. Methods 2011, 201, 35–45. [Google Scholar] [CrossRef]
- Miranda-Riestra, A.; Estrada-Reyes, R.; Torres-Sanchez, E.D.; Carreño-García, S.; Ortiz, G.G.; Benítez-King, G. Melatonin: A Neurotrophic Factor? Molecules 2022, 27, 7742. [Google Scholar] [CrossRef]
- Ho, E.K.; Tsai, A.E.; Stearns, T. Transient Primary Cilia Mediate Robust Hedgehog Pathway-Dependent Cell Cycle Control. Curr. Biol. 2020, 30, 2829–2835.e5. [Google Scholar] [CrossRef]
- Boward, B.; Wu, T.; Dalton, S. Concise Review: Control of Cell Fate Through Cell Cycle and Pluripotency Networks. Stem Cells 2016, 34, 1427–1436. [Google Scholar] [CrossRef]
- Tucker, E.S.; Lehtinen, M.K.; Maynard, T.; Zirlinger, M.; Dulac, C.; Rawson, N.; Pevny, L.; LaMantia, A.-S. Proliferative and Transcriptional Identity of Distinct Classes of Neural Precursors in the Mammalian Olfactory Epithelium. Development 2010, 137, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Kiprilov, E.N.; Awan, A.; Desprat, R.; Velho, M.; Clement, C.A.; Byskov, A.G.; Andersen, C.Y.; Satir, P.; Bouhassira, E.E.; Christensen, S.T.; et al. Human Embryonic Stem Cells in Culture Possess Primary Cilia with Hedgehog Signaling Machinery. J. Cell. Biol. 2008, 180, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Habif, J.C.; Xie, C.; de Celis, C.; Ukhanov, K.; Green, W.W.; Moretta, J.C.; Zhang, L.; Campbell, R.J.; Martens, J.R. The Role of a Ciliary GTPase in the Regulation of Neuronal Maturation of Olfactory Sensory Neurons. Development 2023, 150, dev201116. [Google Scholar] [CrossRef] [PubMed]
- Shida, T.; Cueva, J.G.; Xu, Z.; Goodman, M.B.; Nachury, M.V. The Major α-Tubulin K40 Acetyltransferase ATAT1 Promotes Rapid Ciliogenesis and Efficient Mechanosensation. Proc. Natl. Acad. Sci. USA 2010, 107, 21517–21522. [Google Scholar] [CrossRef]
- Woodruff, J.B.; Wueseke, O.; Hyman, A.A. Pericentriolar Material Structure and Dynamics. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130459. [Google Scholar] [CrossRef]
- Nakakura, T.; Asano-Hoshino, A.; Suzuki, T.; Arisawa, K.; Tanaka, H.; Sekino, Y.; Kiuchi, Y.; Kawai, K.; Hagiwara, H. The Elongation of Primary Cilia via the Acetylation of α-Tubulin by the Treatment with Lithium Chloride in Human Fibroblast KD Cells. Med. Mol. Morphol. 2015, 48, 44–53. [Google Scholar] [CrossRef]
- Calof, A.L.; Mumm, J.S.; Rim, P.C.; Shou, J. The Neuronal Stem Cell of the Olfactory Epithelium. J. Neurobiol. 1998, 36, 190–205. [Google Scholar] [CrossRef]
- Doyle, K.L.; Khan, M.; Cunningham, A.M. Expression of the Intermediate Filament Protein Nestin by Sustentacular Cells in Mature Olfactory Neuroepithelium. J. Comp. Neurol. 2001, 437, 186–195. [Google Scholar] [CrossRef]
- Deems, D.A.; Doty, R.L.; Settle, R.G.; Moore-Gillon, V.; Shaman, P.; Mester, A.F.; Kimmelman, C.P.; Brightman, V.J.; Snow, J.B. Smell and Taste Disorders, A Study of 750 Patients from the University of Pennsylvania Smell and Taste Center. Arch. Otolaryngol. Head Neck Surg. 1991, 117, 519–528. [Google Scholar] [CrossRef]
- Romano, I.R.; D’Angeli, F.; Gili, E.; Fruciano, M.; Lombardo, G.A.G.; Mannino, G.; Vicario, N.; Russo, C.; Parenti, R.; Vancheri, C.; et al. Melatonin Enhances Neural Differentiation of Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 4891. [Google Scholar] [CrossRef]
- Goldstein, N.I.; Quinn, M.R.; Teeter, J.H. Differentiation of a Cell Line from Olfactory Epithelium Is Induced by Dibutyryladenosine 3′, 5′-Cyclic Monophosphate And12-O-Tetradecanoylphorbol-13-Acetate. Brain Res. 1986, 370, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, K.; Kasahara, K.; Miyazaki, I.; Asanuma, M. Lithium Treatment Elongates Primary Cilia in the Mouse Brain and in Cultured Cells. Biochem. Biophys. Res. Commun. 2009, 388, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Sakrajda, K.; Rybakowski, J.K. The Mechanisms of Lithium Action: The Old and New Findings. Pharmaceuticals 2025, 18, 467. [Google Scholar] [CrossRef] [PubMed]
- Galván-Arrieta, T.; Trueta, C.; Cercós, M.G.; Valdés-Tovar, M.; Alarcón, S.; Oikawa, J.; Zamudio-Meza, H.; Benítez-King, G. The Role of Melatonin in the Neurodevelopmental Etiology of Schizophrenia: A Study in Human Olfactory Neuronal Precursors. J. Pineal Res. 2017, 63, e12421. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, W.; Liang, X.; Rao, Y. Both the Establishment and the Maintenance of Neuronal Polarity Require Active Mechanisms. Cell 2005, 120, 123–135. [Google Scholar] [CrossRef]
- Paolocci, E.; Zaccolo, M. Compartmentalised CAMP Signalling in the Primary Cilium. Front. Physiol. 2023, 14, 1187134. [Google Scholar] [CrossRef]
- Canonico, B.; Carloni, S.; Montanari, M.; Ambrogini, P.; Papa, S.; Alonso-Alconada, D.; Balduini, W. Melatonin Modulates Cell Cycle Dynamics and Promotes Hippocampal Cell Proliferation After Ischemic Injury in Neonatal Rats. Mol. Neurobiol. 2024, 61, 6910–6919. [Google Scholar] [CrossRef]
- Macarelli, V.; Leventea, E.; Merkle, F.T. Regulation of the Length of Neuronal Primary Cilia and Its Potential Effects on Signalling. Trends Cell Biol. 2023, 33, 979–990. [Google Scholar] [CrossRef]
- Palazzo, A.; Ackerman, B.; Gundersen, G.G. Tubulin Acetylation and Cell Motility. Nature 2003, 421, 230. [Google Scholar] [CrossRef]
- Coelho, D.H.; Costanzo, R.M. Posttraumatic Olfactory Dysfunction. Auris Nasus Larynx 2016, 43, 137–143. [Google Scholar] [CrossRef]
- Kurtenbach, S.; Goss, G.M.; Goncalves, S.; Choi, R.; Hare, J.M.; Chaudhari, N.; Goldstein, B.J. Cell-Based Therapy Restores Olfactory Function in an Inducible Model of Hyposmia. Stem Cell Rep. 2019, 12, 1354–1365. [Google Scholar] [CrossRef]
- Yuan, Z.-Q.; Peng, X.-C.; Liu, L.; Yang, F.-Y.; Qian, F. Olfactory Receptors and Human Diseases. Cell Tissue Res. 2025, 401, 1–14. [Google Scholar] [CrossRef]
- Hummel, T.; Whitcroft, K.L.; Andrews, P.; Altundag, A.; Cinghi, C.; Costanzo, R.M.; Damm, M.; Frasnelli, J.; Gudziol, H.; Gupta, N.; et al. Position Paper on Olfactory Dysfunction. Rhinol. J. 2017, 54, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Rumeau, C.; Nguyen, D.T.; Jankowski, R. How to Assess Olfactory Performance with the Sniffin’ Sticks Test®. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Hummel, C.; Zucco, G.M.; Iannilli, E.; Maboshe, W.; Landis, B.N.; Hummel, T. OLAF: Standardization of International Olfactory Tests. Eur. Arch. Oto-Rhino-Laryngol. 2012, 269, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Benítez-King, G.; Valdés-Tovar, M.; Trueta, C.; Galván-Arrieta, T.; Argueta, J.; Alarcón, S.; Lora-Castellanos, A.; Solís-Chagoyán, H. The Microtubular Cytoskeleton of Olfactory Neurons Derived from Patients with Schizophrenia or with Bipolar Disorder: Implications for Biomarker Characterization, Neuronal Physiology and Pharmacological Screening. Mol. Cell. Neurosci. 2016, 73, 84–95. [Google Scholar] [CrossRef]
- Larkins, C.E.; Aviles, G.D.G.; East, M.P.; Kahn, R.A.; Caspary, T. Arl13b Regulates Ciliogenesis and the Dynamic Localization of Shh Signaling Proteins. Mol. Biol. Cell 2011, 22, 4694–4703. [Google Scholar] [CrossRef]
- Delaval, B.; Doxsey, S.J. Pericentrin in Cellular Function and Disease. J. Cell Biol. 2010, 188, 181–190. [Google Scholar] [CrossRef]
- Ueha, R.; Shichino, S.; Ueha, S.; Kondo, K.; Kikuta, S.; Nishijima, H.; Matsushima, K.; Yamasoba, T. Reduction of Proliferating Olfactory Cells and Low Expression of Extracellular Matrix Genes Are Hallmarks of the Aged Olfactory Mucosa. Front. Aging Neurosci. 2018, 10, 86. [Google Scholar] [CrossRef]

| Participant | Diagnosis | Drug | Diagnosis [Year] | Sample Obtaining | Sex | Age | Olfactory Threshold | Smoke | Concomitant Diseases |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C1 | None | None | 2015 | F | 32 | 6.5 | + | AH |
| 2 | C2 | None | None | 2017 | F | 37 | 9.0 | - | AH |
| 3 | C3 | LT | None | 2020 | M | 28 | 5.5 | - | HD |
| 4 | C4 | None | None | 2016 | M | 73 | 5.5 | AH | |
| n = 4 | |||||||||
| 1 | H1 | None | 2015 | 2015 | F | 32 | 4.5 | - | AH |
| 2 | H2 | None | 2015 | 2015 | F | 39 | 3.5 | + | AH |
| 3 | H3 | None | 2015 | 2015 | M | 30 | 4.9 | - | AH |
| 4 | H4 | None | 2016 | 2016 | M | 88 | 2.75 | + | AH |
| n = 4 |
| Groups | Age | DPM/µg Protein (Mean) |
|---|---|---|
| Female control | 32 | 1007.0 |
| 950.6 | ||
| 37 | 1142.4 | |
| 845.9 | ||
| Male control | 28 | 1038.8 |
| 73 | 907.4 | |
| 551.0 | ||
| 430.2 | ||
| Female hyposmic | 28 | 995.8 |
| 920.2 | ||
| 39 | 1011.8 | |
| 975.1 | ||
| Male hyposmic | 30 | 639.3 |
| 88 | 542.5 | |
| 539.7 | ||
| 512.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarcón-Elizalde, S.; Lora-Castellanos, A.; Santillán-Morales, V.; Reséndiz-Gachús, M.A.; Estrada-Reyes, R.; Oikawa-Sala, J.; Muñoz-Estrada, J.; Mayagoitia-Novales, L.; Constantino-Jonapa, L.A.; Martín-Higueras, C.; et al. Impaired Formation of Primary Cilia in Olfactory Neuronal Precursors Is Associated with Decreased Proliferation and Maturation in Individuals with Hyposmia. Int. J. Mol. Sci. 2025, 26, 9435. https://doi.org/10.3390/ijms26199435
Alarcón-Elizalde S, Lora-Castellanos A, Santillán-Morales V, Reséndiz-Gachús MA, Estrada-Reyes R, Oikawa-Sala J, Muñoz-Estrada J, Mayagoitia-Novales L, Constantino-Jonapa LA, Martín-Higueras C, et al. Impaired Formation of Primary Cilia in Olfactory Neuronal Precursors Is Associated with Decreased Proliferation and Maturation in Individuals with Hyposmia. International Journal of Molecular Sciences. 2025; 26(19):9435. https://doi.org/10.3390/ijms26199435
Chicago/Turabian StyleAlarcón-Elizalde, Salvador, Alejandra Lora-Castellanos, Valeria Santillán-Morales, Miguel A. Reséndiz-Gachús, Rosa Estrada-Reyes, Julián Oikawa-Sala, Jesús Muñoz-Estrada, Lilian Mayagoitia-Novales, Luis A. Constantino-Jonapa, Cristina Martín-Higueras, and et al. 2025. "Impaired Formation of Primary Cilia in Olfactory Neuronal Precursors Is Associated with Decreased Proliferation and Maturation in Individuals with Hyposmia" International Journal of Molecular Sciences 26, no. 19: 9435. https://doi.org/10.3390/ijms26199435
APA StyleAlarcón-Elizalde, S., Lora-Castellanos, A., Santillán-Morales, V., Reséndiz-Gachús, M. A., Estrada-Reyes, R., Oikawa-Sala, J., Muñoz-Estrada, J., Mayagoitia-Novales, L., Constantino-Jonapa, L. A., Martín-Higueras, C., Acebes, Á., & Benítez-King, G. (2025). Impaired Formation of Primary Cilia in Olfactory Neuronal Precursors Is Associated with Decreased Proliferation and Maturation in Individuals with Hyposmia. International Journal of Molecular Sciences, 26(19), 9435. https://doi.org/10.3390/ijms26199435






