Application of Induced Pluripotent Stem Cells (iPSCs) in Hereditary and Viral Diseases of the Liver: Modeling and Treatment
Abstract
1. Introduction
2. Classification and Statistics
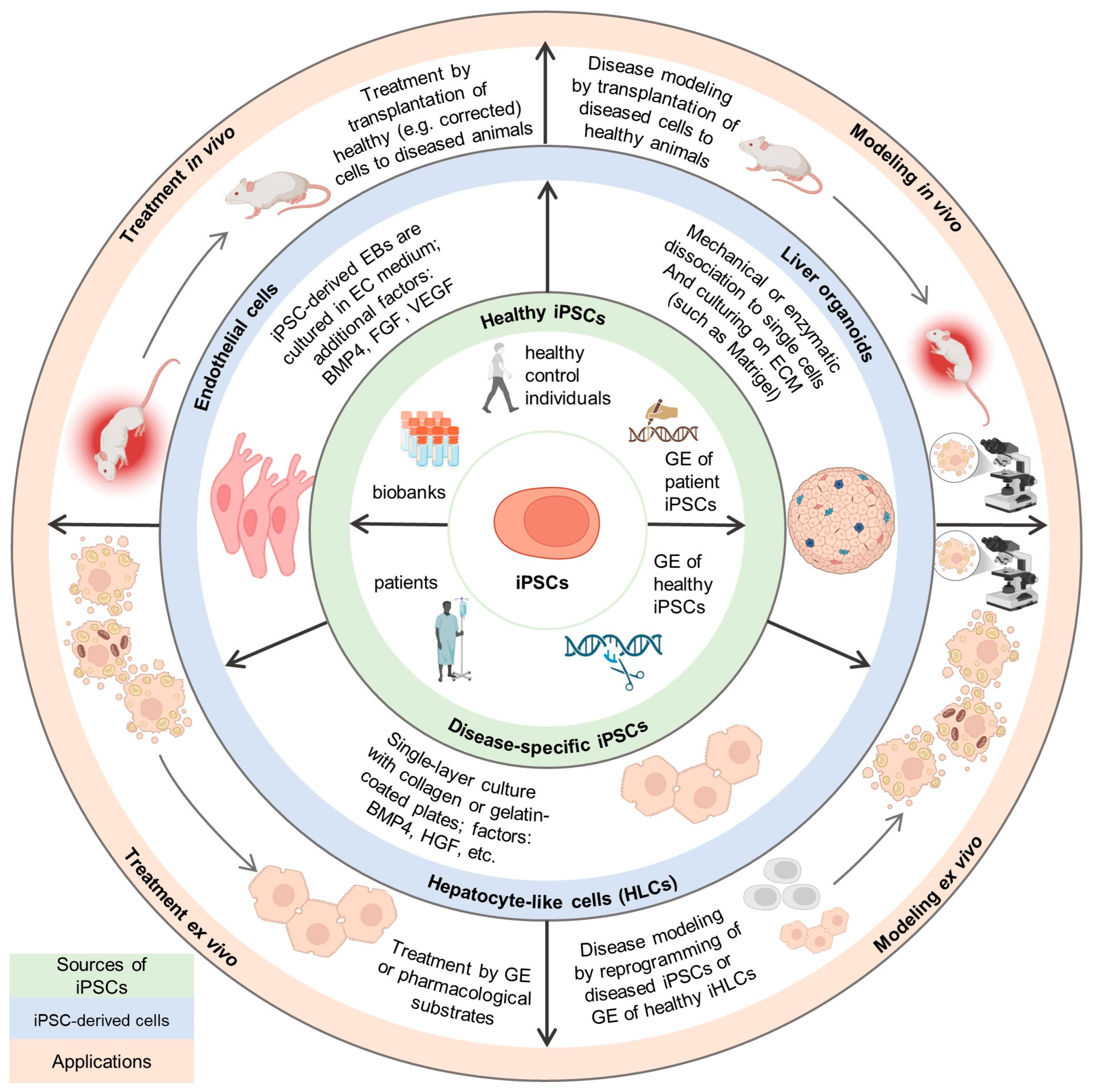
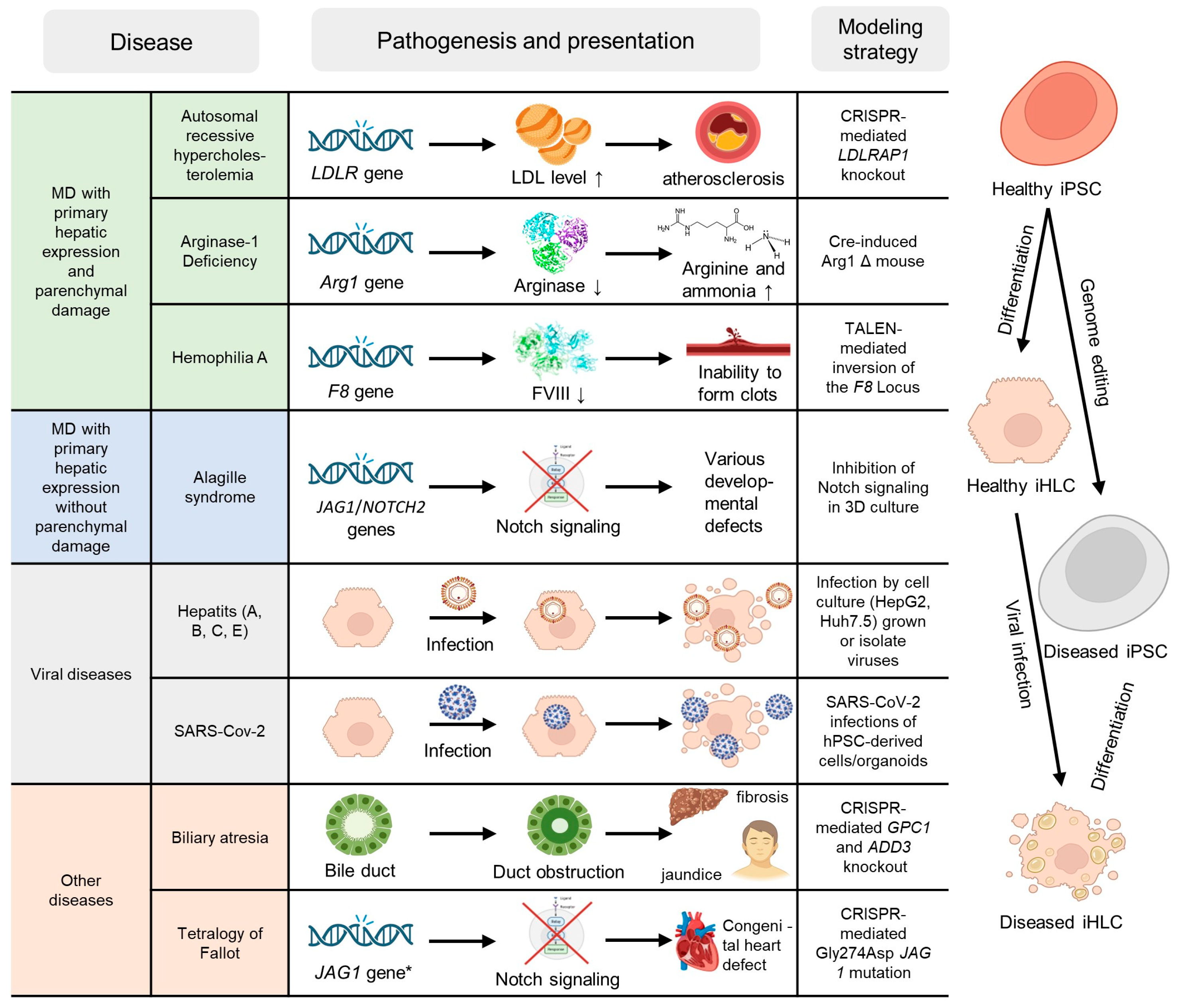
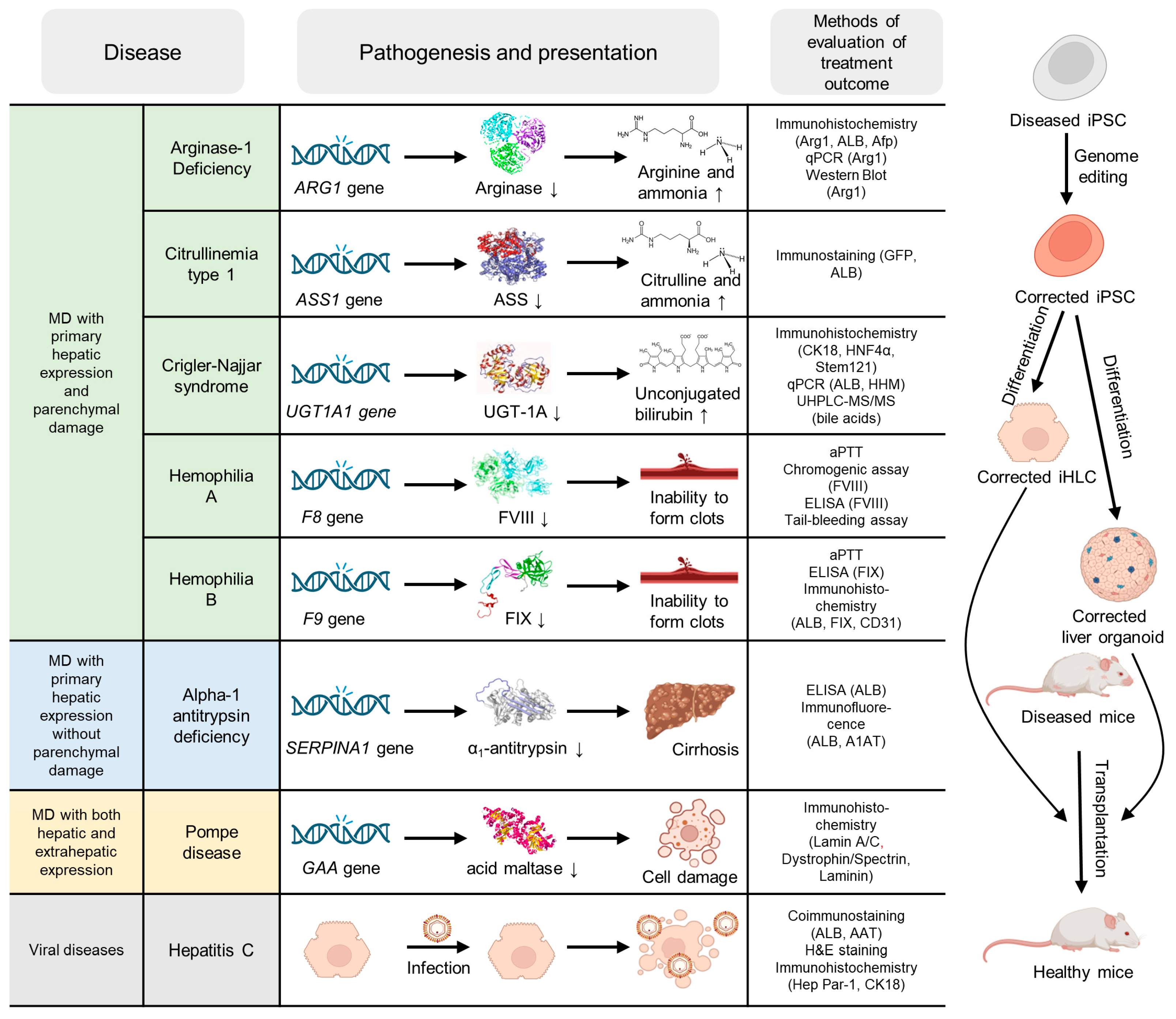
3. Ex Vivo Studies
3.1. Monogenic Diseases with Primary Hepatic Expression Without Significant Parenchymal Damage
3.2. Monogenic Diseases with Primary Hepatic Expression and Parenchymal Damage
3.3. Monogenic Diseases with Both Hepatic and Extrahepatic Expression
3.4. Viral and Other Liver Diseases
4. In Vivo Studies
| Disease | Mouse Model | Cell Type | Cell Number, mln | Injection Site | Assessment Type, Duration, Result | Reference |
|---|---|---|---|---|---|---|
| HA | FVIII −/− ID mice | iECs | 1 | IV injection in tail vein | Factor VIII activity was measured using a chromogenic assay and tail clip challenge. A single transplant of edited iECs resulted in amelioration of the hemophilia phenotype for more than 3 months | [187] |
| FVIII −/− IC mice | iECs, 3D liver organoids | 2–4 | SC injection | Good cell engraftment (more than 3 months) and reversed bleeding phenotypes against lethal wounding challenges | [190] | |
| B6 FVIII −/− mice | CM-Dil labeled iMSCs | 2 | IV injection | Performed a tail-bleeding assay, collected plasma 1, 2, 3, and 4 weeks after transplantation, and determined aPTT. Long-term engraftment of corrected iMSC with restoration of FVIII function and phenotypic rescue was observed in HA mice transplanted with cells | [186] | |
| Adult and neonatal B6 FVIII −/− mice | iECs | 10; 2–3 | SC injection | The titer of coagulation factor VIII remained high (11.2–369.2% of the standard—healthy human plasma) at 2 weeks post-transplantation, and treated mice showed reduced blood loss in a tail-clip bleeding test compared with non-transplanted HA mice | [189] | |
| FVIII −/− NOD/SCID mice | iECs | 2 | IV injection in the peritoneal cavity | Mice showed a stable increase in factor VIII activity, which remained stable up to 12 weeks after transplantation (aPTT test). Recovery of factor VIII activity was also demonstrated after intraperitoneal injection of iECs combined with microcarrier beads supporting their viability | [191] | |
| B6 FVIII −/− mice | iECs | N/A | SC injection | Three of the nine mice that received edited cells survived the tail-clip challenge (endpoint—2 days); the remaining six also showed increased survival (on average 111 min) compared to control mice that did not receive any cells or received cells derived from non-corrected patient iPSCs | [188] | |
| FVIII −/− NOD/SCID mice | iECs | N/A | N/A | HA-corrected iECs were detected in the liver up to 1 m post-transplantation | [192] | |
| HB | B6 FIX −/− mice | iHLCs | 0.1 | Transcutaneous injection into the liver | ELISA and chromogenic assay were performed on plasma of mice sacrificed 2 weeks after transplantation. As a result, restoration of FIX activity in vivo was demonstrated | [111] |
| FIX −/− ID (RGFKO) mice | iHLCs | N/A | IS injection | A chromogenic test was used to show the bleeding phenotype being rescued; the clotting FIX concentration was 0.7 μg/mL (nearly equal to a non-HB mouse) | [184] | |
| B6 FIX −/− mice | iHLCs | 2 | Injection under the kidney capsule | The clotting activities of transplanted mice were approximately 5% of the wild-type values (2 weeks post transplantation), and the bleeding time was shorter than that of non-transplanted mice. | [193] | |
| NOD/SCID mice | iHLCs | 42 per kg | IS injection | Cells not only successfully engraft but also secrete hFIX. Only two weeks after transplantation the hFIX antigen was detected, and after four weeks or more, it was no longer detectable in any group | [181] | |
| FIX −/− mice | iHLCs | 0.5 | IS injection | Two sequential IS injections with a 1-week interval of 5 × 105 of healthy murine iHLCs to HB mice. The recipient mice were sacrificed at 1 to 4 weeks after transplantation. Hemostatic function was assayed by thromboelastography using the citrated kaolin mode. The authors studied a set of parameters: the time to initial fibrin formation, the speed to reach a specific level of clot strength, and others. iHLCs transplantation improved hemostasis measures, thrombus formation, and coagulation factor IX activity. The transferred cells were located in the liver of recipient HB mice | [183] | |
| HCV | ID MUP-uPA/SCID/Bg mice | iHLCs | 4 | IS injection | The authors infected mice with HCV after IS injection of healthy iHLCs, creating an in vivo model for studying hepatitis, and measured HCV RNA titers and HCV core antigen concentrations in the sera of mice and showed that it is human iHLCs that carry the virus, since mice cannot be infected with HCV. | [194] |
| CTLN1 | ID (NSG) mice | Hepatic organoids | 2 | SC injection | The authors demonstrated successful engraftment using immunostaining of GFP and human-specific albumin antibodies separately | [93] |
| ASA | NOD/SCID/IL2Rγ− mice | iECs | 0.4 | SC injection | As expected, ASLD iECs showed decreased ability to form blood capillaries and arterioles in vivo | [180] |
| GSDII | ID (NSG) mice | iMPs | 0.5 | IM injection | Myogenic progenitors engrafted into murine muscle formed human myofibers | [195] |
| Crigler-Najjar syndrome | Gunn rats with mutated Ugt1a1 | iMSCs | 4 | IS injection | The iMSCs engrafted and survived in the liver for up to 2 months. The expression of several human-specific hepatocyte markers, including albumin, demonstrated that the transplanted iMSCs differentiated into functional hepatocytes | [196] |
| AD | Arg1Δ mice | Murine iHLCs | 2 | IS injection | Even though iPSCs with Arg1Δ alleles in their genome were successfully repaired, mice that received iHLC showed an insignificant recovery in urea cycle function when compared to control mice, and some mice’ survival in this lethal model was prolonged by only up to a week | [197] |
| AATD | ID (Alb-uPA+/+; Rag2−/−; Il2rg−/−) mice | iHLCs | 0.5 | IS injection | Successful engraftment was demonstrated by human albumin being detected in the serum of transplanted animals for at least 5 weeks. Authors demonstrated the potential of combining human iPSCs with genetic correction to generate clinically relevant cells for autologous cell-based therapies | [198] |
| - | ID (NSG) mice | IH-ICC organoids | 0.5 per 1 scaffold | IH injection | The organoids formed vascularized tissue following intrahepatic injection into ID NSG mice | [199] |
5. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAT | alpha-1-antitrypsin |
| AATD | alpha-1-antitrypsin deficiency |
| ABL | abetalipoproteinemia |
| AD | arginase deficiency |
| AGT | glyoxylate aminotransferase |
| AHS | Alpers-Huttenlocher syndrome |
| ALGS | Alagille syndrome |
| aPTT | activated partial thromboplastin time |
| ARH | autosomal recessive hypercholesterolemia |
| ASL, ASA | Argininosuccinate Lyase Deficiency |
| ATTR | transthyretin amyloidosis |
| ATZ | mutant alpha-1 antitrypsin Z |
| BA | biliary atresia |
| BSEP | bile salt export pump |
| cccDNA | covalently closed circular DNA |
| CF | cystic fibrosis |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| CK18 | cytokeratin 18 |
| CLCs | cholangiocyte-like cells |
| CPS I deficiency | carbamoyl phosphate synthetase 1 deficiency |
| CTLN1 | citrullinemia type 1 |
| CYP | cytochrome P450 |
| DE | definitive endoderm |
| DGUOK | deoxyribonucleoside kinase |
| ECs | endothelial cells |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| ELISA | enzyme-linked immunosorbent assay |
| ESCs | embryonic stem cells |
| FAP | familial amyloid polyneuropathy |
| FGF | fibroblast growth factor |
| FH | familial hypercholesterolemia |
| FIX | coagulation factor IX |
| FVIII | coagulation factor VIII |
| GAA | acid alpha-glucosidase |
| GE | gene editing |
| GSDIb | glycogen storage disease type Ib |
| HA | hemophilia a |
| HB | hemophilia b |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| HDR | homology-directed repair |
| HEV | hepatitis E viral |
| HGF | hepatocyte growth factor |
| HO | hepatic organoid |
| HPBCD | hydroxypropyl-β-cyclodextrin |
| HPC | hepatic progenitor cells |
| HPGCD | 2-hydroxypropyl-γ-cyclodextrin |
| iECs | induced pluripotent stem cell-derived endothelial cells |
| iHPCs | induced pluripotent stem cell-derived hepatic progenitor cells |
| iMSC | induced pluripotent stem cell-derived mesenchymal stem cells |
| iPSCs | induced pluripotent stem cells |
| LAL | lysosomal acid lipase |
| LDL-C | low-density lipid-cholesterol |
| LDLR | low-density lipoprotein receptor |
| MSC | mesenchymal stem cells |
| MTTP | microsomal triglyceride transfer protein |
| NOD SCID | non-obese diabetic severe combined immunodeficient |
| NP-C | Niemann–Pick type C |
| NSG | NOD scid gamma |
| NTCP | sodium taurocholate co-transporting polypeptide |
| OTC | ornithine transcarbamylase |
| OTCD | ornithine transcarbamylase deficiency |
| PFIC | progressive familial intrahepatic cholestasis |
| PGC1α | proliferator-activated receptor gamma coactivator 1-alpha |
| PH1 | primary hyperoxaluria type 1 |
| PHH | primary hepatocyte |
| PLD | polycystic liver disease |
| rER | rough endoplasmic reticulum |
| SARS-CoV-2 | severe acute respiratory syndrome-related coronavirus 2 |
| sgRNA | subgenomic RNA |
| shRNAs | short hairpin RNA |
| siRNA | small interfering RNA |
| TALEN | transcription activator-like effector nucleases |
| TCA | tricarboxylic acid |
| TOF | tetralogy of Fallot |
| TTR | transthyretin |
| UCD | urea cycle disorders |
| VPA | valproic acid |
| WD | Wilson’s disease |
| WTTA | wild-type transthyretin amyloid |
References
- Hepatitis. Available online: https://www.who.int/health-topics/hepatitis (accessed on 6 August 2025).
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global Burden of Liver Disease: 2023 Update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef]
- Blivet-Van Eggelpoël, M.-J.; Chettouh, H.; Fartoux, L.; Aoudjehane, L.; Barbu, V.; Rey, C.; Priam, S.; Housset, C.; Rosmorduc, O.; Desbois-Mouthon, C. Epidermal Growth Factor Receptor and HER-3 Restrict Cell Response to Sorafenib in Hepatocellular Carcinoma Cells. J. Hepatol. 2012, 57, 108–115. [Google Scholar] [CrossRef]
- Robinton, D.A.; Daley, G.Q. The Promise of Induced Pluripotent Stem Cells in Research and Therapy. Nature 2012, 481, 295–305. [Google Scholar] [CrossRef]
- Kato, Y.; Inaba, T.; Shinke, K.; Hiramatsu, N.; Horie, T.; Sakamoto, T.; Hata, Y.; Sugihara, E.; Takimoto, T.; Nagai, N.; et al. Comprehensive Search for Genes Involved in Thalidomide Teratogenicity Using Early Differentiation Models of Human Induced Pluripotent Stem Cells: Potential Applications in Reproductive and Developmental Toxicity Testing. Cells 2025, 14, 215. [Google Scholar] [CrossRef]
- Dawoody Nejad, L.; Pioro, E.P. Modeling ALS with Patient-Derived iPSCs: Recent Advances and Future Potentials. Brain Sci. 2025, 15, 134. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, N.; Kalsan, M.; Saini, A.; Chandra, R. Mechanism of Induction: Induced Pluripotent Stem Cells (iPSCs). J. Stem Cells 2015, 10, 43–62. [Google Scholar]
- Flahou, C.; Morishima, T.; Takizawa, H.; Sugimoto, N. Fit-For-All iPSC-Derived Cell Therapies and Their Evaluation in Humanized Mice with NK Cell Immunity. Front. Immunol. 2021, 12, 662360. [Google Scholar] [CrossRef]
- Mah, N.; Kurtz, A.; Fuhr, A.; Seltmann, S.; Chen, Y.; Bultjer, N.; Dewender, J.; Lual, A.; Steeg, R.; Mueller, S.C. The Management of Data for the Banking, Qualification, and Distribution of Induced Pluripotent Stem Cells: Lessons Learned from the European Bank for Induced Pluripotent Stem Cells. Cells 2023, 12, 2756. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Liu, C.-L.; Ting, C.-Y.; Chiu, Y.-T.; Cheng, Y.-C.; Nicholson, M.W.; Hsieh, P.C.H. Human iPSC Banking: Barriers and Opportunities. J. Biomed. Sci. 2019, 26, 87. [Google Scholar] [CrossRef] [PubMed]
- Kobold, S.; Bultjer, N.; Stacey, G.; Mueller, S.C.; Kurtz, A.; Mah, N. History and Current Status of Clinical Studies Using Human Pluripotent Stem Cells. Stem Cell Rep. 2023, 18, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.K.; Yamanaka, S. iPS Cell Therapy 2.0: Preparing for next-Generation Regenerative Medicine. Bioessays 2024, 46, e2400072. [Google Scholar] [CrossRef] [PubMed]
- Nikasa, P.; Tricot, T.; Mahdieh, N.; Baharvand, H.; Totonchi, M.; Hejazi, M.S.; Verfaillie, C.M. Patient-Specific Induced Pluripotent Stem Cell-Derived Hepatocyte-Like Cells as a Model to Study Autosomal Recessive Hypercholesterolemia. Stem Cells Dev. 2021, 30, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-Y.; Kim, J.; Kweon, J.; Son, J.S.; Lee, J.S.; Yoo, J.-E.; Cho, S.-R.; Kim, J.-H.; Kim, J.-S.; Kim, D.-W. Targeted Inversion and Reversion of the Blood Coagulation Factor 8 Gene in Human iPS Cells Using TALENs. Proc. Natl. Acad. Sci. USA 2014, 111, 9253–9258. [Google Scholar] [CrossRef]
- Sampaziotis, F.; de Brito, M.C.; Madrigal, P.; Bertero, A.; Saeb-Parsy, K.; Soares, F.A.C.; Schrumpf, E.; Melum, E.; Karlsen, T.H.; Bradley, J.A.; et al. Cholangiocytes Derived from Human Induced Pluripotent Stem Cells for Disease Modeling and Drug Validation. Nat. Biotechnol. 2015, 33, 845–852. [Google Scholar] [CrossRef]
- Sakurai, F.; Mitani, S.; Yamamoto, T.; Takayama, K.; Tachibana, M.; Watashi, K.; Wakita, T.; Iijima, S.; Tanaka, Y.; Mizuguchi, H. Human Induced-Pluripotent Stem Cell-Derived Hepatocyte-like Cells as an in Vitro Model of Human Hepatitis B Virus Infection. Sci. Rep. 2017, 7, 45698. [Google Scholar] [CrossRef]
- Schöbel, A.; Rösch, K.; Herker, E. Functional Innate Immunity Restricts Hepatitis C Virus Infection in Induced Pluripotent Stem Cell–Derived Hepatocytes. Sci. Rep. 2018, 8, 3893. [Google Scholar] [CrossRef]
- Walsh, C.; Jin, S. Induced Pluripotent Stem Cells and CRISPR-Cas9 Innovations for Treating Alpha-1 Antitrypsin Deficiency and Glycogen Storage Diseases. Cells 2024, 13, 1052. [Google Scholar] [CrossRef]
- Sun, X.-C.; Kong, D.; Zhao, J.; Faber, K.N.; Xia, Q.; He, K. Liver Organoids: Established Tools for Disease Modeling and Drug Development. Hepatol. Commun. 2023, 7, e0105. [Google Scholar] [CrossRef]
- Ouchi, R.; Koike, H. Modeling Human Liver Organ Development and Diseases with Pluripotent Stem Cell-Derived Organoids. Front. Cell Dev. Biol. 2023, 11, 1133534. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, N.; Que, H.; Mai, E.; Hu, Y.; Tan, R.; Gu, J.; Gong, P. Pluripotent Stem Cell-Derived Hepatocyte-like Cells: Induction Methods and Applications. Int. J. Mol. Sci. 2023, 24, 11592. [Google Scholar] [CrossRef]
- Duff, C.; Baruteau, J. Modelling Urea Cycle Disorders Using iPSCs. NPJ Regen. Med. 2022, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Kaserman, J.E.; Wilson, A.A. Patient-Derived Induced Pluripotent Stem Cells for Alpha-1 Antitrypsin Deficiency Disease Modeling and Therapeutic Discovery. Chronic Obstr. Pulm. Dis. 2018, 5, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yu, Y.; Nyberg, S.L. Induced Pluripotent Stem Cells for the Treatment of Liver Diseases: Novel Concepts. Cells Tissues Organs 2022, 211, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pu, K.; Liu, X.; Bae, S.D.W.; Nguyen, R.; Bai, S.; Li, Y.; Qiao, L. The Application of Induced Pluripotent Stem Cells Against Liver Diseases: An Update and a Review. Front. Med. 2021, 8, 644594. [Google Scholar] [CrossRef]
- Tafaleng, E.N.; Malizio, M.R.; Fox, I.J.; Soto-Gutierrez, A. Synthetic Human Livers for Modeling Metabolic Diseases. Curr. Opin. Gastroenterol. 2021, 37, 224–230. [Google Scholar] [CrossRef]
- Blaszkiewicz, J.; Duncan, S.A. Advancements in Disease Modeling and Drug Discovery Using iPSC-Derived Hepatocyte-like Cells. Genes 2022, 13, 573. [Google Scholar] [CrossRef]
- Fagiuoli, S.; Daina, E.; D’Antiga, L.; Colledan, M.; Remuzzi, G. Monogenic Diseases That Can Be Cured by Liver Transplantation. J. Hepatol. 2013, 59, 595–612. [Google Scholar] [CrossRef]
- Salen, P.; Babiker, H.M. Hemophilia A. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Alshaikhli, A.; Killeen, R.B.; Rokkam, V.R. Hemophilia B. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Summar, M.L.; Koelker, S.; Freedenberg, D.; Le Mons, C.; Haberle, J.; Lee, H.-S.; Kirmse, B. The Incidence of Urea Cycle Disorders. Mol. Genet. Metab. 2013, 110, 179–180. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Asuka, E.; Jialal, I. Hypercholesterolemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Fargue, S.; Acquaviva Bourdain, C. Primary Hyperoxaluria Type 1: Pathophysiology and Genetics. Clin. Kidney J. 2022, 15, i4–i8. [Google Scholar] [CrossRef]
- Tanskanen, M.; Peuralinna, T.; Polvikoski, T.; Notkola, I.-L.; Sulkava, R.; Hardy, J.; Singleton, A.; Kiuru-Enari, S.; Paetau, A.; Tienari, P.J.; et al. Senile Systemic Amyloidosis Affects 25% of the Very Aged and Associates with Genetic Variation in Alpha2-Macroglobulin and Tau: A Population-Based Autopsy Study. Ann. Med. 2008, 40, 232–239. [Google Scholar] [CrossRef]
- Schmidt, H.H.; Waddington-Cruz, M.; Botteman, M.F.; Carter, J.A.; Chopra, A.S.; Hopps, M.; Stewart, M.; Fallet, S.; Amass, L. Estimating the Global Prevalence of Transthyretin Familial Amyloid Polyneuropathy. Muscle Nerve 2018, 57, 829–837. [Google Scholar] [CrossRef]
- Collaud, F.; Bortolussi, G.; Guianvarc’h, L.; Aronson, S.J.; Bordet, T.; Veron, P.; Charles, S.; Vidal, P.; Sola, M.S.; Rundwasser, S.; et al. Preclinical Development of an AAV8-hUGT1A1 Vector for the Treatment of Crigler-Najjar Syndrome. Mol. Ther. Methods Clin. Dev. 2018, 12, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Nana, M.; Anastasopoulou, C.; Parikh, N.S.; Ahlawat, R. Glycogen Storage Disease Type I. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Meseeha, M.; Sankari, A.; Attia, M. Alpha-1 Antitrypsin Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Leonard, L.D.; Chao, G.; Baker, A.; Loomes, K.; Spinner, N.B. Clinical Utility Gene Card for: Alagille Syndrome (ALGS). Eur. J. Hum. Genet. 2014, 22, 435. [Google Scholar] [CrossRef] [PubMed]
- A Siddiqi, I.; Tadi, P. Progressive Familial Intrahepatic Cholestasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sreenath Nagamani, S.C.; Erez, A.; Lee, B. Argininosuccinate Lyase Deficiency. Genet. Med. 2012, 14, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Immergluck, J.; Grant, L.M.; Anilkumar, A.C. Wilson Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sniderman King, L.; Trahms, C.; Scott, C.R. Tyrosinemia Type I. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Mitchell, G.A.; Grompe, M.; Lambert, M.; Tanguay, R.M. Hypertyrosinemia. In The Online Metabolic and Molecular Bases of Inherited Disease; McGraw Hill Medical: New York, NY, USA, 2019; Available online: https://ommbid.mhmedical.com/content.aspx?bookid=2709§ionid=225082825 (accessed on 7 August 2025).
- Schaefer, A.M.; Taylor, R.W.; Turnbull, D.M.; Chinnery, P.F. The Epidemiology of Mitochondrial Disorders–Past, Present and Future. Biochim. Biophys. Acta 2004, 1659, 115–120. [Google Scholar] [CrossRef]
- Patterson, M. Niemann-Pick Disease Type C. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Pericleous, M.; Kelly, C.; Wang, T.; Livingstone, C.; Ala, A. Wolman’s Disease and Cholesteryl Ester Storage Disorder: The Phenotypic Spectrum of Lysosomal Acid Lipase Deficiency. Lancet Gastroenterol. Hepatol. 2017, 2, 670–679. [Google Scholar] [CrossRef]
- Colburn, R.; Lapidus, D. An Analysis of Pompe Newborn Screening Data: A New Prevalence at Birth, Insight and Discussion. Front. Pediatr. 2023, 11, 1221140. [Google Scholar] [CrossRef]
- Scotet, V.; L’Hostis, C.; Férec, C. The Changing Epidemiology of Cystic Fibrosis: Incidence, Survival and Impact of the CFTR Gene Discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef]
- Junaid, S.Z.S.; Patel, K. Abetalipoproteinemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 7 August 2025).
- Hepatitis C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 7 August 2025).
- Hepatitis E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 7 August 2025).
- Jimenez-Rivera, C.; Jolin-Dahel, K.S.; Fortinsky, K.J.; Gozdyra, P.; Benchimol, E.I. International Incidence and Outcomes of Biliary Atresia. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 344–354. [Google Scholar] [CrossRef]
- Bailliard, F.; Anderson, R.H. Tetralogy of Fallot. Orphanet J. Rare Dis. 2009, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Kothadia, J.P.; Kreitman, K.; Shah, J.M. Polycystic Liver Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Loewa, A.; Feng, J.J.; Hedtrich, S. Human Disease Models in Drug Development. Nat. Rev. Bioeng. 2023, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kidambi, S.; Ortega-Ribera, M.; Thuy, L.T.T.; Nieto, N.; Cogger, V.C.; Xie, W.-F.; Tacke, F.; Gracia-Sancho, J. In Vitro Models for the Study of Liver Biology and Diseases: Advances and Limitations. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Nam, Y.; Rim, Y.A.; Ju, J.H. Advanced Animal Replacement Testing Strategies Using Stem Cell and Organoids. Int. J. Stem Cells 2025, 18, 107–125. [Google Scholar] [CrossRef]
- Moravcová, A.; Červinková, Z.; Kučera, O.; Mezera, V.; Rychtrmoc, D.; Lotková, H. The Effect of Oleic and Palmitic Acid on Induction of Steatosis and Cytotoxicity on Rat Hepatocytes in Primary Culture. Physiol. Res. 2015, 64, S627–S636. [Google Scholar] [CrossRef]
- Alkhatatbeh, M.J.; Lincz, L.F.; Thorne, R.F. Low Simvastatin Concentrations Reduce Oleic Acid-Induced Steatosis in HepG2 Cells: An in Vitro Model of Non-Alcoholic Fatty Liver Disease. Exp. Ther. Med. 2016, 11, 1487–1492. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, Z.; Li, Z.; Pang, J.; Feng, M.; Hu, X.; Wang, X.; Lin-Peng, S.; Liu, B.; Chen, F.; et al. In Situ Genetic Correction of F8 Intron 22 Inversion in Hemophilia A Patient-Specific iPSCs. Sci. Rep. 2016, 6, 18865. [Google Scholar] [CrossRef]
- Soldatow, V.Y.; Lecluyse, E.L.; Griffith, L.G.; Rusyn, I. In Vitro Models for Liver Toxicity Testing. Toxicol. Res. 2013, 2, 23–39. [Google Scholar] [CrossRef]
- Gupta, R.; Schrooders, Y.; Hauser, D.; van Herwijnen, M.; Albrecht, W.; Ter Braak, B.; Brecklinghaus, T.; Castell, J.V.; Elenschneider, L.; Escher, S.; et al. Comparing in Vitro Human Liver Models to in Vivo Human Liver Using RNA-Seq. Arch. Toxicol. 2021, 95, 573–589. [Google Scholar] [CrossRef]
- Binda, D.; Lasserre-Bigot, D.; Bonet, A.; Thomassin, M.; Come, M.P.; Guinchard, C.; Bars, R.; Jacqueson, A.; Richert, L. Time Course of Cytochromes P450 Decline during Rat Hepatocyte Isolation and Culture: Effect of l-NAME. Toxicol. Vitr. 2003, 17, 59–67. [Google Scholar] [CrossRef]
- Kaur, I.; Vasudevan, A.; Rawal, P.; Tripathi, D.M.; Ramakrishna, S.; Kaur, S.; Sarin, S.K. Primary Hepatocyte Isolation and Cultures: Technical Aspects, Challenges and Advancements. Bioengineering 2023, 10, 131. [Google Scholar] [CrossRef]
- Vinken, M. Primary Hepatocyte Cultures for Liver Disease Modeling. Curr. Opin. Toxicol. 2021, 25, 1–5. [Google Scholar] [CrossRef]
- Lauschke, V.M.; Vorrink, S.U.; Moro, S.M.L.; Rezayee, F.; Nordling, Å.; Hendriks, D.F.G.; Bell, C.C.; Sison-Young, R.; Park, B.K.; Goldring, C.E.; et al. Massive Rearrangements of Cellular MicroRNA Signatures Are Key Drivers of Hepatocyte Dedifferentiation. Hepatology 2016, 64, 1743–1756. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, J.; Bolleyn, J.; Vanhaecke, T.; Rogiers, V.; Vinken, M. Primary Hepatocyte Cultures for Pharmaco-Toxicological Studies: At the Busy Crossroad of Various Anti-Dedifferentiation Strategies. Arch. Toxicol. 2013, 87, 577–610. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol. Biol. 2015, 1250, 77–93. [Google Scholar] [CrossRef]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef]
- Pramfalk, C.; Larsson, L.; Härdfeldt, J.; Eriksson, M.; Parini, P. Culturing of HepG2 Cells with Human Serum Improve Their Functionality and Suitability in Studies of Lipid Metabolism. Biochim. et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 51–59. [Google Scholar] [CrossRef]
- Štampar, M.; Breznik, B.; Filipič, M.; Žegura, B. Characterization of In Vitro 3D Cell Model Developed from Human Hepatocellular Carcinoma (HepG2) Cell Line. Cells 2020, 9, 2557. [Google Scholar] [CrossRef]
- Westerink, W.M.A.; Schoonen, W.G.E.J. Cytochrome P450 Enzyme Levels in HepG2 Cells and Cryopreserved Primary Human Hepatocytes and Their Induction in HepG2 Cells. Toxicol. Vitr. 2007, 21, 1581–1591. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Fleming, H.E.; Khetani, S.R.; Bhatia, S.N. Pluripotent Stem Cell-Derived Hepatocyte-like Cells. Biotechnol. Adv. 2014, 32, 504–513. [Google Scholar] [CrossRef]
- Gandhi, N.; Wills, L.; Akers, K.; Su, Y.; Niccum, P.; Murali, T.M.; Rajagopalan, P. Comparative Transcriptomic and Phenotypic Analysis of Induced Pluripotent Stem Cell Hepatocyte-like Cells and Primary Human Hepatocytes. Cell Tissue Res. 2024, 396, 119–139. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Bram, Y.; Frankel, A. Pluripotent Stem Cell-Derived Hepatocyte-like Cells: A Tool to Study Infectious Disease. Curr. Pathobiol. Rep. 2016, 4, 147–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guan, Y.; Enejder, A.; Wang, M.; Fang, Z.; Cui, L.; Chen, S.-Y.; Wang, J.; Tan, Y.; Wu, M.; Chen, X.; et al. A Human Multi-Lineage Hepatic Organoid Model for Liver Fibrosis. Nat. Commun. 2021, 12, 6138. [Google Scholar] [CrossRef] [PubMed]
- Choucha Snouber, L.; Bunescu, A.; Naudot, M.; Legallais, C.; Brochot, C.; Dumas, M.E.; Elena-Herrmann, B.; Leclerc, E. Metabolomics-on-a-Chip of Hepatotoxicity Induced by Anticancer Drug Flutamide and Its Active Metabolite Hydroxyflutamide Using HepG2/C3a Microfluidic Biochips. Toxicol. Sci. 2013, 132, 8–20. [Google Scholar] [CrossRef]
- Westra, I.M.; Mutsaers, H.A.M.; Luangmonkong, T.; Hadi, M.; Oosterhuis, D.; de Jong, K.P.; Groothuis, G.M.M.; Olinga, P. Human Precision-Cut Liver Slices as a Model to Test Antifibrotic Drugs in the Early Onset of Liver Fibrosis. Toxicol. Vitr. 2016, 35, 77–85. [Google Scholar] [CrossRef]
- Li, S.; Huang, S.-Q.; Zhao, Y.-X.; Ding, Y.-J.; Ma, D.-J.; Ding, Q.-R. Derivation and Applications of Human Hepatocyte-like Cells. World J. Stem Cells 2019, 11, 535–547. [Google Scholar] [CrossRef]
- Qiu, S.; Li, Y.; Imakura, Y.; Mima, S.; Hashita, T.; Iwao, T.; Matsunaga, T. An Efficient Method for the Differentiation of Human iPSC-Derived Endoderm toward Enterocytes and Hepatocytes. Cells 2021, 10, 812. [Google Scholar] [CrossRef]
- Fiorotto, R.; Amenduni, M.; Mariotti, V.; Fabris, L.; Spirli, C.; Strazzabosco, M. Liver Diseases in the Dish: iPSC and Organoids as a New Approach to Modeling Liver Diseases. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 920–928. [Google Scholar] [CrossRef]
- Graffmann, N.; Scherer, B.; Adjaye, J. In Vitro Differentiation of Pluripotent Stem Cells into Hepatocyte like Cells-Basic Principles and Current Progress. Stem Cell Res. 2022, 61, 102763. [Google Scholar] [CrossRef]
- Huch, M.; Knoblich, J.A.; Lutolf, M.P.; Martinez-Arias, A. The Hope and the Hype of Organoid Research. Development 2017, 144, 938–941. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Weng, Y.; Han, S.; Sekyi, M.T.; Su, T.; Mattis, A.N.; Chang, T.T. Self-Assembled Matrigel-Free iPSC-Derived Liver Organoids Demonstrate Wide-Ranging Highly Differentiated Liver Functions. Stem Cells 2023, 41, 126–139. [Google Scholar] [CrossRef]
- Olgasi, C.; Cucci, A.; Follenzi, A. iPSC-Derived Liver Organoids: A Journey from Drug Screening, to Disease Modeling, Arriving to Regenerative Medicine. Int. J. Mol. Sci. 2020, 21, 6215. [Google Scholar] [CrossRef]
- Harrison, S.P.; Baumgarten, S.F.; Verma, R.; Lunov, O.; Dejneka, A.; Sullivan, G.J. Liver Organoids: Recent Developments, Limitations and Potential. Front. Med. 2021, 8, 574047. [Google Scholar] [CrossRef]
- Althali, N.J.; Hentges, K.E. Genetic Insights into Non-Syndromic Tetralogy of Fallot. Front. Physiol. 2022, 13, 1012665. [Google Scholar] [CrossRef]
- Yoshitoshi-Uebayashi, E.Y.; Toyoda, T.; Yasuda, K.; Kotaka, M.; Nomoto, K.; Okita, K.; Yasuchika, K.; Okamoto, S.; Takubo, N.; Nishikubo, T.; et al. Modelling Urea-Cycle Disorder Citrullinemia Type 1 with Disease-Specific iPSCs. Biochem. Biophys. Res. Commun. 2017, 486, 613–619. [Google Scholar] [CrossRef]
- Akbari, S.; Sevinç, G.G.; Ersoy, N.; Basak, O.; Kaplan, K.; Sevinç, K.; Ozel, E.; Sengun, B.; Enustun, E.; Ozcimen, B.; et al. Robust, Long-Term Culture of Endoderm-Derived Hepatic Organoids for Disease Modeling. Stem Cell Rep. 2019, 13, 627–641. [Google Scholar] [CrossRef]
- Lee, P.C.; Truong, B.; Vega-Crespo, A.; Gilmore, W.B.; Hermann, K.; Angarita, S.A.; Tang, J.K.; Chang, K.M.; Wininger, A.E.; Lam, A.K.; et al. Restoring Ureagenesis in Hepatocytes by CRISPR/Cas9-Mediated Genomic Addition to Arginase-Deficient Induced Pluripotent Stem Cells. Mol. Ther. Nucleic Acids 2016, 5, e394. [Google Scholar] [CrossRef]
- Sin, Y.Y.; Price, P.R.; Ballantyne, L.L.; Funk, C.D. Proof-of-Concept Gene Editing for the Murine Model of Inducible Arginase-1 Deficiency. Sci. Rep. 2017, 7, 2585. [Google Scholar] [CrossRef]
- Yang, X.; Yan, B.; Zhang, H.; Ma, Y.; Zhou, Q.; Li, Y.; Guan, J.; Wang, D.; Liu, Y.; Gai, Z. Generation of an Induced Pluripotent Stem Cell Line (SDQLCHi009-A) from a Patient with 47,XXY and Ornithine Transcarbamylase Deficiency Carrying a Hemizygote Mutation in OTC. Stem Cell Res. 2020, 43, 101704. [Google Scholar] [CrossRef]
- Guan, J.; Yan, B.; Zhang, H.; Liu, C.; Li, Y.; Yang, X.; Li, Z.; Gai, Z.; Liu, Y. Generation of a Human Induced Pluripotent Stem Cell Line (SDQLCHi036-A) from a Patient with Ornithine Transcarbamylase Deficiency Carrying a Deletion Involving 3–9 Exons of OTC Gene. Stem Cell Res. 2021, 52, 102220. [Google Scholar] [CrossRef] [PubMed]
- Zabulica, M.; Jakobsson, T.; Ravaioli, F.; Vosough, M.; Gramignoli, R.; Ellis, E.; Rooyackers, O.; Strom, S.C. Gene Editing Correction of a Urea Cycle Defect in Organoid Stem Cell Derived Hepatocyte-like Cells. Int. J. Mol. Sci. 2021, 22, 1217. [Google Scholar] [CrossRef] [PubMed]
- Laemmle, A.; Poms, M.; Hsu, B.; Borsuk, M.; Rüfenacht, V.; Robinson, J.; Sadowski, M.C.; Nuoffer, J.; Häberle, J.; Willenbring, H. Aquaporin 9 Induction in Human iPSC-derived Hepatocytes Facilitates Modeling of Ornithine Transcarbamylase Deficiency. Hepatology 2022, 76, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.J. Familial Hypercholesterolemia: A Review. Ann. Pediatr. Cardiol. 2014, 7, 107–117. [Google Scholar] [CrossRef]
- Cayo, M.A.; Cai, J.; DeLaForest, A.; Noto, F.K.; Nagaoka, M.; Clark, B.S.; Collery, R.F.; Si-Tayeb, K.; Duncan, S.A. ‘JD’ iPS Cell–Derived Hepatocytes Faithfully Recapitulate the Pathophysiology of Familial Hypercholesterolemia. Hepatology 2012, 56, 2163–2171. [Google Scholar] [CrossRef]
- Cayo, M.A.; Mallanna, S.K.; Di Furio, F.; Jing, R.; Tolliver, L.B.; Bures, M.; Urick, A.; Noto, F.K.; Pashos, E.E.; Greseth, M.D.; et al. A Drug Screen Using Human iPSC-Derived Hepatocyte-like Cells Identifies Cardiac Glycosides as a Potential Treatment for Hypercholesterolemia. Cell Stem Cell 2017, 20, 478–489.e5. [Google Scholar] [CrossRef]
- Omer, L.; Hudson, E.A.; Zheng, S.; Hoying, J.B.; Shan, Y.; Boyd, N.L. CRISPR Correction of a Homozygous Low-density Lipoprotein Receptor Mutation in Familial Hypercholesterolemia Induced Pluripotent Stem Cells. Hepatol. Commun. 2017, 1, 886–898. [Google Scholar] [CrossRef]
- Fattahi, F.; Asgari, S.; Pournasr, B.; Seifinejad, A.; Totonchi, M.; Taei, A.; Aghdami, N.; Salekdeh, G.H.; Baharvand, H. Disease-Corrected Hepatocyte-like Cells from Familial Hypercholesterolemia-Induced Pluripotent Stem Cells. Mol. Biotechnol. 2013, 54, 863–873. [Google Scholar] [CrossRef]
- Konkle, B.A.; Nakaya Fletcher, S. Hemophilia A.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Goodeve, A.C. Hemophilia B: Molecular Pathogenesis and Mutation Analysis. J. Thromb. Haemost. 2015, 13, 1184–1195. [Google Scholar] [CrossRef]
- Jia, B.; Chen, S.; Zhao, Z.; Liu, P.; Cai, J.; Qin, D.; Du, J.; Wu, C.; Chen, Q.; Cai, X.; et al. Modeling of Hemophilia A Using Patient-Specific Induced Pluripotent Stem Cells Derived from Urine Cells. Life Sci. 2014, 108, 22–29. [Google Scholar] [CrossRef]
- Martorell, L.; Luce, E.; Vazquez, J.L.; Richaud-Patin, Y.; Jimenez-Delgado, S.; Corrales, I.; Borras, N.; Casacuberta-Serra, S.; Weber, A.; Parra, R.; et al. Advanced Cell-Based Modeling of the Royal Disease: Characterization of the Mutated F9 mRNA. J. Thromb. Haemost. 2017, 15, 2188–2197. [Google Scholar] [CrossRef]
- Sung, J.J.; Park, S.; Choi, S.-H.; Kim, J.; Cho, M.S.; Kim, D.-W. Generation of a Gene Edited Hemophilia A Patient-Derived iPSC Cell Line, YCMi001-B-1, by Targeted Insertion of Coagulation Factor FVIII Using CRISPR/Cas9. Stem Cell Res. 2020, 48, 101948. [Google Scholar] [CrossRef]
- Park, C.-Y.; Sung, J.J.; Cho, S.-R.; Kim, J.; Kim, D.-W. Universal Correction of Blood Coagulation Factor VIII in Patient-Derived Induced Pluripotent Stem Cells Using CRISPR/Cas9. Stem Cell Rep. 2019, 12, 1242–1249. [Google Scholar] [CrossRef]
- Luce, E.; Steichen, C.; Allouche, M.; Messina, A.; Heslan, J.-M.; Lambert, T.; Weber, A.; Nguyen, T.H.; Christophe, O.; Dubart-Kupperschmitt, A. In Vitro Recovery of FIX Clotting Activity as a Marker of Highly Functional Hepatocytes in a Hemophilia B iPSC Model. Hepatology 2022, 75, 866–880. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, Z.; Zhao, J.; Zhou, T.; Tang, S.; Wang, P.; Xiao, R.; Chen, Y.; Wu, L.; Zhou, M.; et al. CRISPR-Mediated In Situ Introduction or Integration of F9-Padua in Human iPSCs for Gene Therapy of Hemophilia B. Int. J. Mol. Sci. 2023, 24, 9013. [Google Scholar] [CrossRef]
- Hiramoto, T.; Kashiwakura, Y.; Hayakawa, M.; Baatartsogt, N.; Kamoshita, N.; Abe, T.; Inaba, H.; Nishimasu, H.; Uosaki, H.; Hanazono, Y.; et al. PAM-Flexible Cas9-Mediated Base Editing of a Hemophilia B Mutation in Induced Pluripotent Stem Cells. Commun. Med. 2023, 3, 56. [Google Scholar] [CrossRef]
- Bayarsaikhan, D.; Bayarsaikhan, G.; Lee, J.; Okano, T.; Kim, K.; Lee, B. Development of iPSC-Derived FIX-Secreting Hepatocyte Sheet as a Novel Treatment Tool for Hemophilia B Treatment. Stem Cell Res. Ther. 2025, 16, 88. [Google Scholar] [CrossRef]
- Niemietz, C.; Fleischhauer, L.; Sandfort, V.; Guttmann, S.; Zibert, A.; Schmidt, H.H.-J. Hepatocyte-like Cells Reveal Novel Role of SERPINA1 in Transthyretin Amyloidosis. J. Cell Sci. 2018, 131, jcs219824. [Google Scholar] [CrossRef]
- Niemietz, C.J.; Sauer, V.; Stella, J.; Fleischhauer, L.; Chandhok, G.; Guttmann, S.; Avsar, Y.; Guo, S.; Ackermann, E.J.; Gollob, J.; et al. Evaluation of Therapeutic Oligonucleotides for Familial Amyloid Polyneuropathy in Patient-Derived Hepatocyte-Like Cells. PLoS ONE 2016, 11, e0161455. [Google Scholar] [CrossRef]
- Choi, S.M.; Liu, H.; Chaudhari, P.; Kim, Y.; Cheng, L.; Feng, J.; Sharkis, S.; Ye, Z.; Jang, Y.-Y. Reprogramming of EBV-Immortalized B-Lymphocyte Cell Lines into Induced Pluripotent Stem Cells. Blood 2011, 118, 1801–1805. [Google Scholar] [CrossRef]
- Tafaleng, E.N.; Chakraborty, S.; Han, B.; Hale, P.; Wu, W.; Soto-Gutierrez, A.; Feghali-Bostwick, C.A.; Wilson, A.A.; Kotton, D.N.; Nagaya, M.; et al. Induced Pluripotent Stem Cells Model Personalized Variations in Liver Disease Due to A1-Antitrypsin Deficiency. Hepatology 2015, 62, 147–157. [Google Scholar] [CrossRef]
- Segeritz, C.-P.; Rashid, S.T.; de Brito, M.C.; Serra, M.P.; Ordonez, A.; Morell, C.M.; Kaserman, J.E.; Madrigal, P.; Hannan, N.R.F.; Gatto, L.; et al. hiPSC Hepatocyte Model Demonstrates the Role of Unfolded Protein Response and Inflammatory Networks in A1-Antitrypsin Deficiency. J. Hepatol. 2018, 69, 851–860. [Google Scholar] [CrossRef]
- Pastore, N.; Attanasio, S.; Granese, B.; Castello, R.; Teckman, J.; Wilson, A.A.; Ballabio, A.; Brunetti-Pierri, N. Activation of the C-Jun N-Terminal Kinase Pathway Aggravates Proteotoxicity of Hepatic Mutant Z Alpha1-Antitrypsin. Hepatology 2017, 65, 1865–1874. [Google Scholar] [CrossRef]
- Wilson, A.A.; Ying, L.; Liesa, M.; Segeritz, C.-P.; Mills, J.A.; Shen, S.S.; Jean, J.; Lonza, G.C.; Liberti, D.C.; Lang, A.H.; et al. Emergence of a Stage-Dependent Human Liver Disease Signature with Directed Differentiation of Alpha-1 Antitrypsin-Deficient iPS Cells. Stem Cell Rep. 2015, 4, 873–885. [Google Scholar] [CrossRef]
- Kaserman, J.E.; Hurley, K.; Dodge, M.; Villacorta-Martin, C.; Vedaie, M.; Jean, J.-C.; Liberti, D.C.; James, M.F.; Higgins, M.I.; Lee, N.J.; et al. A Highly Phenotyped Open Access Repository of Alpha-1 Antitrypsin Deficiency Pluripotent Stem Cells. Stem Cell Rep. 2020, 15, 242–255. [Google Scholar] [CrossRef]
- Smith, C.; Abalde-Atristain, L.; He, C.; Brodsky, B.R.; Braunstein, E.M.; Chaudhari, P.; Jang, Y.-Y.; Cheng, L.; Ye, Z. Efficient and Allele-Specific Genome Editing of Disease Loci in Human iPSCs. Mol. Ther. 2015, 23, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Eggenschwiler, R.; Loya, K.; Wu, G.; Sharma, A.D.; Sgodda, M.; Zychlinski, D.; Herr, C.; Steinemann, D.; Teckman, J.; Bals, R.; et al. Sustained Knockdown of a Disease-Causing Gene in Patient-Specific Induced Pluripotent Stem Cells Using Lentiviral Vector-Based Gene Therapy. Stem Cells Transl. Med. 2013, 2, 641–654. [Google Scholar] [CrossRef]
- Choi, S.M.; Kim, Y.; Shim, J.S.; Park, J.T.; Wang, R.-H.; Leach, S.D.; Liu, J.O.; Deng, C.-X.; Ye, Z.; Jang, Y.-Y. Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology 2013, 57, 2458–2468. [Google Scholar] [CrossRef]
- Werder, R.B.; Kaserman, J.E.; Packer, M.S.; Lindstrom-Vautrin, J.; Villacorta-Martin, C.; Young, L.E.; Aratyn-Schaus, Y.; Gregoire, F.; Wilson, A.A. Adenine Base Editing Reduces Misfolded Protein Accumulation and Toxicity in Alpha-1 Antitrypsin Deficient Patient iPSC-Hepatocytes. Mol. Ther. 2021, 29, 3219–3229. [Google Scholar] [CrossRef]
- Brooks, B.M.; Pradhan, M.; Cheng, Y.-S.; Gorshkov, K.; Farkhondeh, A.; Chen, C.Z.; Beers, J.; Liu, C.; Baumgaertel, K.; Rodems, S.; et al. Generation of an Induced Pluripotent Stem Cell Line (TRNDi031-A) from a Patient with Alagille Syndrome Type 1 Carrying a Heterozygous p. C312X (c. 936 T > A) Mutation in JAGGED-1. Stem Cell Res. 2021, 54, 102447. [Google Scholar] [CrossRef]
- Guan, Y.; Xu, D.; Garfin, P.M.; Ehmer, U.; Hurwitz, M.; Enns, G.; Michie, S.; Wu, M.; Zheng, M.; Nishimura, T.; et al. Human Hepatic Organoids for the Analysis of Human Genetic Diseases. JCI Insight 2017, 2, e94954. [Google Scholar] [CrossRef]
- Imagawa, K.; Takayama, K.; Isoyama, S.; Tanikawa, K.; Shinkai, M.; Harada, K.; Tachibana, M.; Sakurai, F.; Noguchi, E.; Hirata, K.; et al. Generation of a Bile Salt Export Pump Deficiency Model Using Patient-Specific Induced Pluripotent Stem Cell-Derived Hepatocyte-like Cells. Sci. Rep. 2017, 7, 41806. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Osaka, S.; Sakabe, K.; Fukami, A.; Kishimoto, E.; Aihara, E.; Sabu, Y.; Mizutani, A.; Kusuhara, H.; Naritaka, N.; et al. Modeling Human Bile Acid Transport and Synthesis in Stem Cell-Derived Hepatocytes with a Patient-Specific Mutation. Stem Cell Rep. 2021, 16, 309–323. [Google Scholar] [CrossRef]
- Yi, F.; Qu, J.; Li, M.; Suzuki, K.; Kim, N.Y.; Liu, G.-H.; Belmonte, J.C.I. Establishment of Hepatic and Neural Differentiation Platforms of Wilson’s Disease Specific Induced Pluripotent Stem Cells. Protein Cell 2012, 3, 855–863. [Google Scholar] [CrossRef]
- Parisi, S.; Polishchuk, E.V.; Allocca, S.; Ciano, M.; Musto, A.; Gallo, M.; Perone, L.; Ranucci, G.; Iorio, R.; Polishchuk, R.S.; et al. Characterization of the Most Frequent ATP7B Mutation Causing Wilson Disease in Hepatocytes from Patient Induced Pluripotent Stem Cells. Sci. Rep. 2018, 8, 6247. [Google Scholar] [CrossRef]
- Overeem, A.W.; Klappe, K.; Parisi, S.; Klöters-Planchy, P.; Mataković, L.; du Teil Espina, M.; Drouin, C.A.; Weiss, K.H.; van IJzendoorn, S.C.D. Pluripotent Stem Cell-Derived Bile Canaliculi-Forming Hepatocytes to Study Genetic Liver Diseases Involving Hepatocyte Polarity. J. Hepatol. 2019, 71, 344–356. [Google Scholar] [CrossRef]
- Groba, S.R.; Guttmann, S.; Niemietz, C.; Bernick, F.; Sauer, V.; Hachmöller, O.; Karst, U.; Zischka, H.; Zibert, A.; Schmidt, H.H. Downregulation of Hepatic Multi-Drug Resistance Protein 1 (MDR1) after Copper Exposure. Metallomics 2017, 9, 1279–1287. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Li, W.; Guo, X.; Zhao, P.; Xu, J.; Chen, Y.; Pan, Q.; Liu, X.; Zychlinski, D.; et al. Rescue of ATP7B Function in Hepatocyte-like Cells from Wilson’s Disease Induced Pluripotent Stem Cells Using Gene Therapy or the Chaperone Drug Curcumin. Hum. Mol. Genet. 2011, 20, 3176–3187. [Google Scholar] [CrossRef]
- Lichtmannegger, J.; Leitzinger, C.; Wimmer, R.; Schmitt, S.; Schulz, S.; Kabiri, Y.; Eberhagen, C.; Rieder, T.; Janik, D.; Neff, F.; et al. Methanobactin Reverses Acute Liver Failure in a Rat Model of Wilson Disease. J. Clin. Investig. 2016, 126, 2721–2735. [Google Scholar] [CrossRef]
- Satoh, D.; Maeda, T.; Ito, T.; Nakajima, Y.; Ohte, M.; Ukai, A.; Nakamura, K.; Enosawa, S.; Toyota, M.; Miyagawa, Y.; et al. Establishment and Directed Differentiation of Induced Pluripotent Stem Cells from Glycogen Storage Disease Type Ib Patient. Genes Cells 2013, 18, 1053–1069. [Google Scholar] [CrossRef]
- Soga, M.; Ishitsuka, Y.; Hamasaki, M.; Yoneda, K.; Furuya, H.; Matsuo, M.; Ihn, H.; Fusaki, N.; Nakamura, K.; Nakagata, N.; et al. HPGCD Outperforms HPBCD as a Potential Treatment for Niemann-Pick Disease Type C during Disease Modeling with iPS Cells. Stem Cells 2015, 33, 1075–1088. [Google Scholar] [CrossRef]
- Völkner, C.; Pantoom, S.; Liedtke, M.; Lukas, J.; Hermann, A.; Frech, M.J. Assessment of FDA-Approved Drugs as a Therapeutic Approach for Niemann-Pick Disease Type C1 Using Patient-Specific iPSC-Based Model Systems. Cells 2022, 11, 319. [Google Scholar] [CrossRef]
- Maetzel, D.; Sarkar, S.; Wang, H.; Abi-Mosleh, L.; Xu, P.; Cheng, A.W.; Gao, Q.; Mitalipova, M.; Jaenisch, R. Genetic and Chemical Correction of Cholesterol Accumulation and Impaired Autophagy in Hepatic and Neural Cells Derived from Niemann-Pick Type C Patient-Specific iPS Cells. Stem Cell Rep. 2014, 2, 866–880. [Google Scholar] [CrossRef]
- Guo, J.; Duan, L.; He, X.; Li, S.; Wu, Y.; Xiang, G.; Bao, F.; Yang, L.; Shi, H.; Gao, M.; et al. A Combined Model of Human iPSC-Derived Liver Organoids and Hepatocytes Reveals Ferroptosis in DGUOK Mutant mtDNA Depletion Syndrome. Adv. Sci. 2021, 8, 2004680. [Google Scholar] [CrossRef]
- Jing, R.; Corbett, J.L.; Cai, J.; Beeson, G.C.; Beeson, C.C.; Chan, S.S.; Dimmock, D.P.; Lazcares, L.; Geurts, A.M.; Lemasters, J.J.; et al. A Screen Using iPSC-Derived Hepatocytes Reveals NAD+ as a Potential Treatment for mtDNA Depletion Syndrome. Cell Rep. 2018, 25, 1469–1484.e5. [Google Scholar] [CrossRef]
- Li, S.; Guo, J.; Ying, Z.; Chen, S.; Yang, L.; Chen, K.; Long, Q.; Qin, D.; Pei, D.; Liu, X. Valproic Acid-Induced Hepatotoxicity in Alpers Syndrome Is Associated with Mitochondrial Permeability Transition Pore Opening-Dependent Apoptotic Sensitivity in an Induced Pluripotent Stem Cell Model. Hepatology 2015, 61, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Ogawa, S.; Bear, C.E.; Ahmadi, S.; Chin, S.; Li, B.; Grompe, M.; Keller, G.; Kamath, B.M.; Ghanekar, A. Directed Differentiation of Cholangiocytes from Human Pluripotent Stem Cells. Nat. Biotechnol. 2015, 33, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Fiorotto, R.; Amenduni, M.; Mariotti, V.; Fabris, L.; Spirli, C.; Strazzabosco, M. Src Kinase Inhibition Reduces Inflammatory and Cytoskeletal Changes in ΔF508 Human Cholangiocytes and Improves CFTR Correctors Efficacy. Hepatology 2018, 67, 972–988. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-S.; Li, R.; Baskfield, A.; Beers, J.; Zou, J.; Liu, C.; Zheng, W. A Human Induced Pluripotent Stem Cell Line (TRNDi007-B) from an Infantile Onset Pompe Patient Carrying p.R854X Mutation in the GAA Gene. Stem Cell Res. 2019, 37, 101435. [Google Scholar] [CrossRef]
- Ouchi, R.; Togo, S.; Kimura, M.; Shinozawa, T.; Koido, M.; Koike, H.; Thompson, W.; Karns, R.; Mayhew, C.; McGrath, P.S.; et al. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019, 30, 374–384.e6. [Google Scholar] [CrossRef]
- Liu, Y.; Conlon, D.M.; Bi, X.; Slovik, K.J.; Shi, J.; Edelstein, H.I.; Millar, J.S.; Javaheri, A.; Cuchel, M.; Pashos, E.E.; et al. Lack of MTTP Protein in Pluripotent Stem Cell-Derived Hepatocytes/Cardiomyocytes Abolishes apoB Secretion and Increases Cell Stress. Cell Rep. 2017, 19, 1456–1466. [Google Scholar] [CrossRef]
- Estève, J.; Blouin, J.-M.; Lalanne, M.; Azzi-Martin, L.; Dubus, P.; Bidet, A.; Harambat, J.; Llanas, B.; Moranvillier, I.; Bedel, A.; et al. Generation of Induced Pluripotent Stem Cells-Derived Hepatocyte-like Cells for Ex Vivo Gene Therapy of Primary Hyperoxaluria Type 1. Stem Cell Res. 2019, 38, 101467. [Google Scholar] [CrossRef] [PubMed]
- Shlomai, A.; Schwartz, R.E.; Ramanan, V.; Bhatta, A.; de Jong, Y.P.; Bhatia, S.N.; Rice, C.M. Modeling Host Interactions with Hepatitis B Virus Using Primary and Induced Pluripotent Stem Cell-Derived Hepatocellular Systems. Proc. Natl. Acad. Sci. USA 2014, 111, 12193–12198. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-W.; Himeno, M.; Koui, Y.; Sugiyama, M.; Nishitsuji, H.; Mizokami, M.; Shimotohno, K.; Miyajima, A.; Kido, T. Modulation of Hepatitis B Virus Infection by Epidermal Growth Factor Secreted from Liver Sinusoidal Endothelial Cells. Sci. Rep. 2020, 10, 14349. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Carpentier, A.; Cheng, X.; Daniel Block, P.; Zhao, Y.; Zhang, Z.; Protzer, U.; Liang, T.J. Human Stem Cell-Derived Hepatocytes as a Model for Hepatitis B Virus Infection, Spreading and Virus-Host Interactions. J. Hepatol. 2017, 66, 494–503. [Google Scholar] [CrossRef]
- Nie, Y.-Z.; Zheng, Y.-W.; Miyakawa, K.; Murata, S.; Zhang, R.-R.; Sekine, K.; Ueno, Y.; Takebe, T.; Wakita, T.; Ryo, A.; et al. Recapitulation of Hepatitis B Virus–Host Interactions in Liver Organoids from Human Induced Pluripotent Stem Cells. eBioMedicine 2018, 35, 114–123. [Google Scholar] [CrossRef]
- Kaneko, S.; Kakinuma, S.; Asahina, Y.; Kamiya, A.; Miyoshi, M.; Tsunoda, T.; Nitta, S.; Asano, Y.; Nagata, H.; Otani, S.; et al. Human Induced Pluripotent Stem Cell-Derived Hepatic Cell Lines as a New Model for Host Interaction with Hepatitis B Virus. Sci. Rep. 2016, 6, 29358. [Google Scholar] [CrossRef]
- Wu, X.; Robotham, J.M.; Lee, E.; Dalton, S.; Kneteman, N.M.; Gilbert, D.M.; Tang, H. Productive Hepatitis C Virus Infection of Stem Cell-Derived Hepatocytes Reveals a Critical Transition to Viral Permissiveness during Differentiation. PLoS Pathog. 2012, 8, e1002617. [Google Scholar] [CrossRef]
- Sa-ngiamsuntorn, K.; Wongkajornsilp, A.; Phanthong, P.; Borwornpinyo, S.; Kitiyanant, N.; Chantratita, W.; Hongeng, S. A Robust Model of Natural Hepatitis C Infection Using Hepatocyte-like Cells Derived from Human Induced Pluripotent Stem Cells as a Long-Term Host. Virol. J. 2016, 13, 59. [Google Scholar] [CrossRef]
- Sakurai, F.; Kunito, T.; Takayama, K.; Hashimoto, R.; Tachibana, M.; Sakamoto, N.; Wakita, T.; Mizuguchi, H. Hepatitis C Virus-Induced Innate Immune Responses in Human iPS Cell-Derived Hepatocyte-like Cells. Virus Res. 2017, 242, 7–15. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Trehan, K.; Andrus, L.; Sheahan, T.P.; Ploss, A.; Duncan, S.A.; Rice, C.M.; Bhatia, S.N. Modeling Hepatitis C Virus Infection Using Human Induced Pluripotent Stem Cells. Proc. Natl. Acad. Sci. USA 2012, 109, 2544–2548. [Google Scholar] [CrossRef]
- Yoshida, T.; Takayama, K.; Kondoh, M.; Sakurai, F.; Tani, H.; Sakamoto, N.; Matsuura, Y.; Mizuguchi, H.; Yagi, K. Use of Human Hepatocyte-like Cells Derived from Induced Pluripotent Stem Cells as a Model for Hepatocytes in Hepatitis C Virus Infection. Biochem. Biophys. Res. Commun. 2011, 416, 119–124. [Google Scholar] [CrossRef]
- Helsen, N.; Debing, Y.; Paeshuyse, J.; Dallmeier, K.; Boon, R.; Coll, M.; Sancho-Bru, P.; Claes, C.; Neyts, J.; Verfaillie, C.M. Stem Cell-Derived Hepatocytes: A Novel Model for Hepatitis E Virus Replication. J. Hepatol. 2016, 64, 565–573. [Google Scholar] [CrossRef]
- Dao Thi, V.L.; Debing, Y.; Wu, X.; Rice, C.M.; Neyts, J.; Moradpour, D.; Gouttenoire, J. Sofosbuvir Inhibits Hepatitis E Virus Replication In Vitro and Results in an Additive Effect When Combined with Ribavirin. Gastroenterology 2016, 150, 82–85.e4. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Thi, V.L.D.; Liu, P.; Takacs, C.N.; Xiang, K.; Andrus, L.; Gouttenoire, J.; Moradpour, D.; Rice, C.M. Pan-Genotype Hepatitis E Virus Replication in Stem Cell-Derived Hepatocellular Systems. Gastroenterology 2018, 154, 663–674.e7. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, Y.; Nilsson-Payant, B.E.; Gupta, V.; Wang, P.; Duan, X.; Tang, X.; Zhu, J.; Zhao, Z.; Jaffré, F.; et al. A Human Pluripotent Stem Cell-Based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell 2020, 27, 125–136.e7. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Ye, Z.; Kafka, K.; Stewart, D.; Anders, R.; Schwarz, K.B.; Jang, Y.-Y. Biliary Atresia Relevant Human Induced Pluripotent Stem Cells Recapitulate Key Disease Features in a Dish. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 56–63. [Google Scholar] [CrossRef]
- Goessling, W.; Sadler, K.C. Zebrafish: An Important Tool for Liver Disease Research. Gastroenterology 2015, 149, 1361–1377. [Google Scholar] [CrossRef]
- Elgilani, F.; Mao, S.A.; Glorioso, J.M.; Yin, M.; Iankov, I.D.; Singh, A.; Amiot, B.; Rinaldo, P.; Marler, R.J.; Ehman, R.L.; et al. Chronic Phenotype Characterization of a Large-Animal Model of Hereditary Tyrosinemia Type 1. Am. J. Pathol. 2017, 187, 33–41. [Google Scholar] [CrossRef]
- Wang, H.; Tan, T.; Wang, J.; Niu, Y.; Yan, Y.; Guo, X.; Kang, Y.; Duan, Y.; Chang, S.; Liao, J.; et al. Rhesus Monkey Model of Liver Disease Reflecting Clinical Disease Progression and Hepatic Gene Expression Analysis. Sci. Rep. 2015, 5, 15019. [Google Scholar] [CrossRef]
- McLean, E.K.; McLean, A.E.; Sutton, P.M. Instant Cirrhosis. An Improved Method for Producing Cirrhosis of the Liver in Rats by Simultaneous Administration of Carbon Tetrachloride and Phenobarbitone. Br. J. Exp. Pathol. 1969, 50, 502–506. [Google Scholar]
- Newell, P.; Villanueva, A.; Friedman, S.L.; Koike, K.; Llovet, J.M. Experimental Models of Hepatocellular Carcinoma. J. Hepatol. 2008, 48, 858–879. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Wei, C.; Chen, L.; Huang, J.; Yang, S.; Diehl, A.M. Oxidative DNA Damage and DNA Repair Enzyme Expression Are Inversely Related in Murine Models of Fatty Liver Disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1070–G1077. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Lee, Y.A.; Fujiwara, N.; Ybanez, M.; Allen, B.; Martins, S.; Fiel, M.I.; Goossens, N.; Chou, H.-I.; Hoshida, Y.; et al. A Simple Diet-and Chemical-Induced Murine NASH Model with Rapid Progression of Steatohepatitis, Fibrosis and Liver Cancer. J. Hepatol. 2018, 69, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Popov, Y.; Sverdlov, D.Y.; Bhaskar, K.R.; Sharma, A.K.; Millonig, G.; Patsenker, E.; Krahenbuhl, S.; Krahenbuhl, L.; Schuppan, D. Macrophage-Mediated Phagocytosis of Apoptotic Cholangiocytes Contributes to Reversal of Experimental Biliary Fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G323–G334. [Google Scholar] [CrossRef]
- Polgar, Z.; Li, Y.; Wang, X.L.; Guha, C.; Roy-Chowdhury, N.; Roy-Chowdhury, J. Gunn Rats as a Surrogate Model for Evaluation of Hepatocyte Transplantation-Based Therapies of Crigler-Najjar Syndrome Type 1. Methods Mol. Biol. 2017, 1506, 131–147. [Google Scholar] [CrossRef]
- Morrey, J.D.; Korba, B.E.; Sidwell, R.W. Transgenic Mice as a Chemotherapeutic Model for Hepatitis B Virus Infection. Antivir. Ther. 1998, 3, 59–68. [Google Scholar]
- Takehara, T.; Tatsumi, T.; Suzuki, T.; Rucker, E.B.; Hennighausen, L.; Jinushi, M.; Miyagi, T.; Kanazawa, Y.; Hayashi, N. Hepatocyte-Specific Disruption of Bcl-xL Leads to Continuous Hepatocyte Apoptosis and Liver Fibrotic Responses. Gastroenterology 2004, 127, 1189–1197. [Google Scholar] [CrossRef]
- Ilan, E.; Arazi, J.; Nussbaum, O.; Zauberman, A.; Eren, R.; Lubin, I.; Neville, L.; Ben-Moshe, O.; Kischitzky, A.; Litchi, A.; et al. The Hepatitis C Virus (HCV)-Trimera Mouse: A Model for Evaluation of Agents against HCV. J. Infect. Dis. 2002, 185, 153–161. [Google Scholar] [CrossRef]
- Kabbani, M.; Michailidis, E.; Steensels, S.; Fulmer, C.G.; Luna, J.M.; Le Pen, J.; Tardelli, M.; Razooky, B.; Ricardo-Lax, I.; Zou, C.; et al. Human Hepatocyte PNPLA3-148M Exacerbates Rapid Non-Alcoholic Fatty Liver Disease Development in Chimeric Mice. Cell Rep. 2022, 40, 111321. [Google Scholar] [CrossRef]
- Sundaram, S.S.; Whitington, P.F.; Green, R.M. Steatohepatitis Develops Rapidly in Transgenic Mice Overexpressing Abcb11 and Fed a Methionine-Choline-Deficient Diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1321–G1327. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Denayer, T.; Stöhr, T.; Van Roy, M. Animal Models in Translational Medicine: Validation and Prediction. New Horiz. Transl. Med. 2014, 2, 5–11. [Google Scholar] [CrossRef]
- Kho, J.; Tian, X.; Wong, W.-T.; Bertin, T.; Jiang, M.-M.; Chen, S.; Jin, Z.; Shchelochkov, O.A.; Burrage, L.C.; Reddy, A.K.; et al. Argininosuccinate Lyase Deficiency Causes an Endothelial-Dependent Form of Hypertension. Am. J. Hum. Genet. 2018, 103, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Shen, J.; Wang, R.; Gu, H.; Zhang, J.; Xue, F.; Liu, X.; Liu, W.; Fu, R.; Zhang, L.; et al. Targeted Genome Engineering in Human Induced Pluripotent Stem Cells from Patients with Hemophilia B Using the CRISPR-Cas9 System. Stem Cell Res. Ther. 2018, 9, 92. [Google Scholar] [CrossRef]
- Liu, H.; Kim, Y.; Sharkis, S.; Marchionni, L.; Jang, Y.-Y. In Vivo Liver Regeneration Potential of Human Induced Pluripotent Stem Cells from Diverse Origins. Sci. Transl. Med. 2011, 3, 82ra39. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Huang, Y.-J.; Chen, P.; Hsu, Y.-C.; Lin, S.-W.; Lai, H.-S.; Lee, H.-S. Hepatocyte-Like Cells Derived from Mouse Induced Pluripotent Stem Cells Produce Functional Coagulation Factor IX in a Hemophilia B Mouse Model. Cell Transplant. 2016, 25, 1237–1246. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Tonnu, N.; Menon, T.; Lewis, B.M.; Green, K.T.; Wampler, D.; Monahan, P.E.; Verma, I.M. Autologous and Heterologous Cell Therapy for Hemophilia B toward Functional Restoration of Factor IX. Cell Rep. 2018, 23, 1565–1580. [Google Scholar] [CrossRef]
- Zanolini, D.; Merlin, S.; Feola, M.; Ranaldo, G.; Amoruso, A.; Gaidano, G.; Zaffaroni, M.; Ferrero, A.; Brunelleschi, S.; Valente, G.; et al. Extrahepatic Sources of Factor VIII Potentially Contribute to the Coagulation Cascade Correcting the Bleeding Phenotype of Mice with Hemophilia A. Haematologica 2015, 100, 881–892. [Google Scholar] [CrossRef]
- Qiu, L.; Xie, M.; Zhou, M.; Liu, X.; Hu, Z.; Wu, L. Restoration of FVIII Function and Phenotypic Rescue in Hemophilia A Mice by Transplantation of MSCs Derived from F8-Modified iPSCs. Front. Cell Dev. Biol. 2021, 9, 630353. [Google Scholar] [CrossRef]
- Kim, D.-H.; Choi, S.-H.; Sung, J.J.; Kim, S.; Yi, H.; Park, S.; Park, C.W.; Oh, Y.W.; Lee, J.; Kim, D.-S.; et al. Long-Term Correction of Hemophilia A via Integration of a Functionally Enhanced FVIII Gene into the AAVS1 Locus by Nickase in Patient-Derived iPSCs. Exp. Mol. Med. 2025, 57, 184–192. [Google Scholar] [CrossRef]
- Park, C.-Y.; Kim, D.H.; Son, J.S.; Sung, J.J.; Lee, J.; Bae, S.; Kim, J.-H.; Kim, D.-W.; Kim, J.-S. Functional Correction of Large Factor VIII Gene Chromosomal Inversions in Hemophilia A Patient-Derived iPSCs Using CRISPR-Cas9. Cell Stem Cell 2015, 17, 213–220. [Google Scholar] [CrossRef]
- Rose, M.; Gao, K.; Cortez-Toledo, E.; Agu, E.; Hyllen, A.A.; Conroy, K.; Pan, G.; Nolta, J.A.; Wang, A.; Zhou, P. Endothelial Cells Derived from Patients’ Induced Pluripotent Stem Cells for Sustained Factor VIII Delivery and the Treatment of Hemophilia A. Stem Cells Transl. Med. 2020, 9, 686–696. [Google Scholar] [CrossRef]
- Son, J.S.; Park, C.-Y.; Lee, G.; Park, J.Y.; Kim, H.J.; Kim, G.; Chi, K.Y.; Woo, D.-H.; Han, C.; Kim, S.K.; et al. Therapeutic Correction of Hemophilia A Using 2D Endothelial Cells and Multicellular 3D Organoids Derived from CRISPR/Cas9-Engineered Patient iPSCs. Biomaterials 2022, 283, 121429. [Google Scholar] [CrossRef] [PubMed]
- Olgasi, C.; Talmon, M.; Merlin, S.; Cucci, A.; Richaud-Patin, Y.; Ranaldo, G.; Colangelo, D.; Di Scipio, F.; Berta, G.N.; Borsotti, C.; et al. Patient-Specific iPSC-Derived Endothelial Cells Provide Long-Term Phenotypic Correction of Hemophilia A. Stem Cell Rep. 2018, 11, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Talmon, M.; Ranaldo, G.; Grosso, C.; Lombardo, A.; Merlin, S.; Cannizzo, S.E.; Raya, A.; Naldini, L.; Schinco, P.; Follenzi, A. 469. iPSC-Based Strategy To Correct the Bleeding Phenotype in Hemophilia A Targeting FVIII Expression to Endothelial Cells. Mol. Ther. 2013, 21, S180. [Google Scholar] [CrossRef][Green Version]
- Okamoto, R.; Takayama, K.; Akita, N.; Nagamoto, Y.; Hosokawa, D.; Iizuka, S.; Sakurai, F.; Suemizu, H.; Ohashi, K.; Mizuguchi, H. Human iPS Cell-Based Liver-like Tissue Engineering at Extrahepatic Sites in Mice as a New Cell Therapy for Hemophilia B. Cell Transplant. 2018, 27, 299–309. [Google Scholar] [CrossRef]
- Carpentier, A.; Tesfaye, A.; Chu, V.; Nimgaonkar, I.; Zhang, F.; Lee, S.B.; Thorgeirsson, S.S.; Feinstone, S.M.; Liang, T.J. Engrafted Human Stem Cell-Derived Hepatocytes Establish an Infectious HCV Murine Model. J. Clin. Investig. 2014, 124, 4953–4964. [Google Scholar] [CrossRef]
- van der Wal, E.; Herrero-Hernandez, P.; Wan, R.; Broeders, M.; in ’t Groen, S.L.M.; van Gestel, T.J.M.; van IJcken, W.F.J.; Cheung, T.H.; van der Ploeg, A.T.; Schaaf, G.J.; et al. Large-Scale Expansion of Human iPSC-Derived Skeletal Muscle Cells for Disease Modeling and Cell-Based Therapeutic Strategies. Stem Cell Rep. 2018, 10, 1975–1990. [Google Scholar] [CrossRef]
- Spitzhorn, L.-S.; Kordes, C.; Megges, M.; Sawitza, I.; Götze, S.; Reichert, D.; Schulze-Matz, P.; Graffmann, N.; Bohndorf, M.; Wruck, W.; et al. Transplanted Human Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Support Liver Regeneration in Gunn Rats. Stem Cells Dev. 2018, 27, 1702–1714. [Google Scholar] [CrossRef]
- Sin, Y.Y.; Ballantyne, L.L.; Richmond, C.R.; Funk, C.D. Transplantation of Gene-Edited Hepatocyte-like Cells Modestly Improves Survival of Arginase-1-Deficient Mice. Mol. Ther. Nucleic Acids 2017, 10, 122–130. [Google Scholar] [CrossRef]
- Yusa, K.; Rashid, S.T.; Strick-Marchand, H.; Varela, I.; Liu, P.-Q.; Paschon, D.E.; Miranda, E.; Ordóñez, A.; Hannan, N.; Rouhani, F.J.; et al. Targeted Gene Correction of A1-Antitrypsin Deficiency in Induced Pluripotent Stem Cells. Nature 2011, 478, 391–394. [Google Scholar] [CrossRef]
- Ng, S.S.; Saeb-Parsy, K.; Blackford, S.J.I.; Segal, J.M.; Serra, M.P.; Horcas-Lopez, M.; No, D.Y.; Mastoridis, S.; Jassem, W.; Frank, C.W.; et al. Human iPS Derived Progenitors Bioengineered into Liver Organoids Using an Inverted Colloidal Crystal Poly (Ethylene Glycol) Scaffold. Biomaterials 2018, 182, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Panferov, E.; Dodina, M.; Reshetnikov, V.; Ryapolova, A.; Ivanov, R.; Karabelsky, A.; Minskaia, E. Induced Pluripotent (iPSC) and Mesenchymal (MSC) Stem Cells for In Vitro Disease Modeling and Regenerative Medicine. Int. J. Mol. Sci. 2025, 26, 5617. [Google Scholar] [CrossRef] [PubMed]
- Dao Thi, V.L.; Wu, X.; Belote, R.L.; Andreo, U.; Takacs, C.N.; Fernandez, J.P.; Vale-Silva, L.A.; Prallet, S.; Decker, C.C.; Fu, R.M.; et al. Stem Cell-Derived Polarized Hepatocytes. Nat. Commun. 2020, 11, 1677. [Google Scholar] [CrossRef] [PubMed]
- Scesa, G.; Adami, R.; Bottai, D. iPSC Preparation and Epigenetic Memory: Does the Tissue Origin Matter? Cells 2021, 10, 1470. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lehle, J.D.; McCarrey, J.R. Source Cell-Type Epigenetic Memory Persists in Induced Pluripotent Cells but Is Lost in Subsequently Derived Germline Cells. Front. Cell Dev. Biol. 2024, 12, 1306530. [Google Scholar] [CrossRef]
- Zhao, M.-T.; Chen, H.; Liu, Q.; Shao, N.-Y.; Sayed, N.; Wo, H.-T.; Zhang, J.Z.; Ong, S.-G.; Liu, C.; Kim, Y.; et al. Molecular and Functional Resemblance of Differentiated Cells Derived from Isogenic Human iPSCs and SCNT-Derived ESCs. Proc. Natl. Acad. Sci. USA 2017, 114, E11111–E11120. [Google Scholar] [CrossRef]
- Sun, H.; Shi, C.; Ye, Z.; Yao, B.; Li, C.; Wang, X.; Qian, Q. The Role of Mesenchymal Stem Cells in Liver Injury. Cell. Biol. Int. 2022, 46, 501–511. [Google Scholar] [CrossRef]
- Oswald, J.; Boxberger, S.; Jørgensen, B.; Feldmann, S.; Ehninger, G.; Bornhäuser, M.; Werner, C. Mesenchymal Stem Cells Can Be Differentiated into Endothelial Cells in Vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef]
- Pareja, E.; Gómez-Lechón, M.J.; Tolosa, L. Induced Pluripotent Stem Cells for the Treatment of Liver Diseases: Challenges and Perspectives from a Clinical Viewpoint. Ann. Transl. Med. 2020, 8, 566. [Google Scholar] [CrossRef]
- Itakura, G.; Kobayashi, Y.; Nishimura, S.; Iwai, H.; Takano, M.; Iwanami, A.; Toyama, Y.; Okano, H.; Nakamura, M. Controlling Immune Rejection Is a Fail-Safe System against Potential Tumorigenicity after Human iPSC-Derived Neural Stem Cell Transplantation. PLoS ONE 2015, 10, e0116413. [Google Scholar] [CrossRef]
- Wang, L.; Cao, J.; Wang, Y.; Lan, T.; Liu, L.; Wang, W.; Jin, N.; Gong, J.; Zhang, C.; Teng, F.; et al. Immunogenicity and Functional Evaluation of iPSC-Derived Organs for Transplantation. Cell Discov. 2015, 1, 15015. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Z.-N.; Rong, Z.; Xu, Y. Immunogenicity of Induced Pluripotent Stem Cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a Clinical Hurdle for Pluripotent Stem Cell Therapies. Nat. Med. 2013, 19, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Hotta, A.; Ellis, J. Retroviral Vector Silencing during iPS Cell Induction: An Epigenetic Beacon That Signals Distinct Pluripotent States. J. Cell. Biochem. 2008, 105, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Shinozaki, F.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Ishige, N. Hepatocyte Selection Medium Eliminating Induced Pluripotent Stem Cells among Primary Human Hepatocytes. World J. Methodol. 2015, 5, 108–114. [Google Scholar] [CrossRef]
- Li, H.-Y.; Chien, Y.; Chen, Y.-J.; Chen, S.-F.; Chang, Y.-L.; Chiang, C.-H.; Jeng, S.-Y.; Chang, C.-M.; Wang, M.-L.; Chen, L.-K.; et al. Reprogramming Induced Pluripotent Stem Cells in the Absence of C-Myc for Differentiation into Hepatocyte-like Cells. Biomaterials 2011, 32, 5994–6005. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Fu, X.; Xu, Y. The Immunogenicity and Immune Tolerance of Pluripotent Stem Cell Derivatives. Front. Immunol. 2017, 8, 645. [Google Scholar] [CrossRef]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Wahl, A.; Garcia, J.V.; Reichenspurner, H.; et al. Hypoimmunogenic Derivatives of Induced Pluripotent Stem Cells Evade Immune Rejection in Fully Immunocompetent Allogeneic Recipients. Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef]
- Hannan, N.R.F.; Segeritz, C.-P.; Touboul, T.; Vallier, L. Production of Hepatocyte-like Cells from Human Pluripotent Stem Cells. Nat. Protoc. 2013, 8, 430–437. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Tseng, C.-Y.; Wang, H.-W.; Kuo, H.-C.; Yang, V.W.; Lee, O.K. Rapid Generation of Mature Hepatocyte-like Cells from Human Induced Pluripotent Stem Cells by an Efficient Three-Step Protocol. Hepatology 2012, 55, 1193–1203. [Google Scholar] [CrossRef]
- Si-Tayeb, K.; Noto, F.K.; Nagaoka, M.; Li, J.; Battle, M.A.; Duris, C.; North, P.E.; Dalton, S.; Duncan, S.A. Highly Efficient Generation of Human Hepatocyte-like Cells from Induced Pluripotent Stem Cells. Hepatology 2010, 51, 297–305. [Google Scholar] [CrossRef]
- Nitzahn, M.; Truong, B.; Khoja, S.; Vega-Crespo, A.; Le, C.; Eliav, A.; Makris, G.; Pyle, A.D.; Häberle, J.; Lipshutz, G.S. CRISPR-Mediated Genomic Addition to CPS1 Deficient iPSCs Is Insufficient to Restore Nitrogen Homeostasis. Yale J. Biol. Med. 2021, 94, 545–557. [Google Scholar] [PubMed]
- Baxter, M.; Withey, S.; Harrison, S.; Segeritz, C.-P.; Zhang, F.; Atkinson-Dell, R.; Rowe, C.; Gerrard, D.T.; Sison-Young, R.; Jenkins, R.; et al. Phenotypic and Functional Analyses Show Stem Cell-Derived Hepatocyte-like Cells Better Mimic Fetal Rather than Adult Hepatocytes. J. Hepatol. 2015, 62, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, H.; Ikeda, Y.; Amiot, B.P.; Rinaldo, P.; Duncan, S.A.; Nyberg, S.L. Hepatocyte-like Cells Differentiated from Human Induced Pluripotent Stem Cells: Relevance to Cellular Therapies. Stem Cell Res. 2012, 9, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Rao, J.; Tan, Z.; Xun, T.; Zhao, J.; Yang, X. Inflammatory Signaling on Cytochrome P450-Mediated Drug Metabolism in Hepatocytes. Front. Pharmacol. 2022, 13, 1043836. [Google Scholar] [CrossRef]
- Lercher, A.; Bhattacharya, A.; Popa, A.M.; Caldera, M.; Schlapansky, M.F.; Baazim, H.; Agerer, B.; Gürtl, B.; Kosack, L.; Májek, P.; et al. Type I Interferon Signaling Disrupts the Hepatic Urea Cycle and Alters Systemic Metabolism to Suppress T Cell Function. Immunity 2019, 51, 1074–1087.e9. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Koh, K.H.; Jeong, H. Isoform-Specific Regulation of Cytochromes P450 Expression by Estradiol and Progesterone. Drug Metab. Dispos. 2013, 41, 263–269. [Google Scholar] [CrossRef]
- Manganelli, M.; Mazzoldi, E.L.; Ferraro, R.M.; Pinelli, M.; Parigi, M.; Aghel, S.A.M.; Bugatti, M.; Collo, G.; Stocco, G.; Vermi, W.; et al. Progesterone Receptor Is Constitutively Expressed in Induced Pluripotent Stem Cells (iPSCs). Stem Cell Rev. Rep. 2024, 20, 2303–2317. [Google Scholar] [CrossRef]
- Kajiwara, M.; Aoi, T.; Okita, K.; Takahashi, R.; Inoue, H.; Takayama, N.; Endo, H.; Eto, K.; Toguchida, J.; Uemoto, S.; et al. Donor-Dependent Variations in Hepatic Differentiation from Human-Induced Pluripotent Stem Cells. Proc. Natl. Acad. Sci. USA 2012, 109, 12538–12543. [Google Scholar] [CrossRef]
- LaLone, V.; Aizenshtadt, A.; Goertz, J.; Skottvoll, F.S.; Mota, M.B.; You, J.; Zhao, X.; Berg, H.E.; Stokowiec, J.; Yu, M.; et al. Quantitative Chemometric Phenotyping of Three-Dimensional Liver Organoids by Raman Spectral Imaging. Cell Rep. Methods 2023, 3, 100440. [Google Scholar] [CrossRef]
- Yang, A.S.P.; Dutta, D.; Kretzschmar, K.; Hendriks, D.; Puschhof, J.; Hu, H.; Boonekamp, K.E.; van Waardenburg, Y.; Chuva de Sousa Lopes, S.M.; van Gemert, G.-J.; et al. Development of Plasmodium Falciparum Liver-Stages in Hepatocytes Derived from Human Fetal Liver Organoid Cultures. Nat. Commun. 2023, 14, 4631. [Google Scholar] [CrossRef]
- Shrestha, S.; Lekkala, V.K.R.; Acharya, P.; Kang, S.-Y.; Vanga, M.G.; Lee, M.-Y. Reproducible Generation of Human Liver Organoids (HLOs) on a Pillar Plate Platform via Microarray 3D Bioprinting. Lab A Chip 2024, 24, 2747–2761. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.N.; Zarrinpar, A. Hepatocyte Transplantation: Past Efforts, Current Technology, and Future Expansion of Therapeutic Potential. J. Surg. Res. 2018, 226, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Murata, S.; Hasegawa, S.; Mikami, S.; Enosawa, S.; Hsu, H.-C.; Fukuda, A.; Okamoto, S.; Mori, A.; Matsuo, M.; et al. Investigation of Clinical Safety of Human iPS Cell-Derived Liver Organoid Transplantation to Infantile Patients in Porcine Model. Cell Transplant. 2020, 29, 963689720964384. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Seed, T.M. How Necessary Are Animal Models for Modern Drug Discovery? Expert Opin. Drug Discov. 2021, 16, 1391–1397. [Google Scholar] [CrossRef]
- Teufel, A.; Itzel, T.; Erhart, W.; Brosch, M.; Wang, X.Y.; Kim, Y.O.; von Schönfels, W.; Herrmann, A.; Brückner, S.; Stickel, F.; et al. Comparison of Gene Expression Patterns Between Mouse Models of Nonalcoholic Fatty Liver Disease and Liver Tissues from Patients. Gastroenterology 2016, 151, 513–525.e0. [Google Scholar] [CrossRef]
- Hammer, H.; Schmidt, F.; Marx-Stoelting, P.; Pötz, O.; Braeuning, A. Cross-Species Analysis of Hepatic Cytochrome P450 and Transport Protein Expression. Arch. Toxicol. 2021, 95, 117–133. [Google Scholar] [CrossRef]
- Albadry, M.; Küttner, J.; Grzegorzewski, J.; Dirsch, O.; Kindler, E.; Klopfleisch, R.; Liska, V.; Moulisova, V.; Nickel, S.; Palek, R.; et al. Cross-Species Variability in Lobular Geometry and Cytochrome P450 Hepatic Zonation: Insights into CYP1A2, CYP2D6, CYP2E1 and CYP3A4. Front. Pharmacol. 2024, 15, 1404938. [Google Scholar] [CrossRef]
- Cunningham, R.P.; Porat-Shliom, N. Liver Zonation-Revisiting Old Questions with New Technologies. Front. Physiol. 2021, 12, 732929. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Seki, E. In Vivo and In Vitro Models to Study Liver Fibrosis: Mechanisms and Limitations. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 355–367. [Google Scholar] [CrossRef]
- Shao, W.; Xu, H.; Zeng, K.; Ye, M.; Pei, R.; Wang, K. Advances in Liver Organoids: Replicating Hepatic Complexity for Toxicity Assessment and Disease Modeling. Stem Cell Res. Ther. 2025, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Hartlage, A.S.; Kapoor, A. Hepatitis C Virus Vaccine Research: Time to Put Up or Shut Up. Viruses 2021, 13, 1596. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Broering, R.; Li, X.; Zhang, X.; Liu, J.; Yang, D.; Lu, M. In Vivo Mouse Models for Hepatitis B Virus Infection and Their Application. Front. Immunol. 2021, 12, 766534. [Google Scholar] [CrossRef] [PubMed]
- Scheer, N.; Wilson, I.D. A Comparison between Genetically Humanized and Chimeric Liver Humanized Mouse Models for Studies in Drug Metabolism and Toxicity. Drug Discov. Today 2016, 21, 250–263. [Google Scholar] [CrossRef]
- Washburn, M.L.; Bility, M.T.; Zhang, L.; Kovalev, G.I.; Buntzman, A.; Frelinger, J.A.; Barry, W.; Ploss, A.; Rice, C.M.; Su, L. A Humanized Mouse Model to Study Hepatitis C Virus Infection, Immune Response, and Liver Disease. Gastroenterology 2011, 140, 1334–1344. [Google Scholar] [CrossRef]
- Taymour, R.; Chicaiza-Cabezas, N.A.; Gelinsky, M.; Lode, A. Core-Shell Bioprinting of Vascularizedin Vitroliver Sinusoid Models. Biofabrication 2022, 14, 045019. [Google Scholar] [CrossRef]
- Skardal, A.; Devarasetty, M.; Kang, H.-W.; Seol, Y.-J.; Forsythe, S.D.; Bishop, C.; Shupe, T.; Soker, S.; Atala, A. Bioprinting Cellularized Constructs Using a Tissue-Specific Hydrogel Bioink. J. Vis. Exp. 2016, e53606. [Google Scholar] [CrossRef]
- Wang, B.; Jakus, A.E.; Baptista, P.M.; Soker, S.; Soto-Gutierrez, A.; Abecassis, M.M.; Shah, R.N.; Wertheim, J.A. Functional Maturation of Induced Pluripotent Stem Cell Hepatocytes in Extracellular Matrix-A Comparative Analysis of Bioartificial Liver Microenvironments. Stem Cells Transl. Med. 2016, 5, 1257–1267. [Google Scholar] [CrossRef]
- Kasturi, M.; Mathur, V.; Gadre, M.; Srinivasan, V.; Vasanthan, K.S. Three Dimensional Bioprinting for Hepatic Tissue Engineering: From In Vitro Models to Clinical Applications. Tissue Eng. Regen. Med. 2024, 21, 21–52. [Google Scholar] [CrossRef]
- Deng, J.; Wei, W.; Chen, Z.; Lin, B.; Zhao, W.; Luo, Y.; Zhang, X. Engineered Liver-on-a-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines 2019, 10, 676. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Wang, J.-R.; Wu, J.-L.; Meng, X.; Hu, P.; Mu, X.; Liang, Q.-L.; Luo, G. Design and Fabrication of a Liver-on-a-Chip Platform for Convenient, High-Efficient, and Safe in Situ Perfusion Culture of 3D Hepatic Spheroids. Lab A Chip 2018, 18, 2547–2562. [Google Scholar] [CrossRef]
- Weltin, A.; Hammer, S.; Noor, F.; Kaminski, Y.; Kieninger, J.; Urban, G.A. Accessing 3D Microtissue Metabolism: Lactate and Oxygen Monitoring in Hepatocyte Spheroids. Biosens. Bioelectron. 2017, 87, 941–948. [Google Scholar] [CrossRef]
- Ahmed, H.M.M.; Salerno, S.; Morelli, S.; Giorno, L.; De Bartolo, L. 3D Liver Membrane System by Co-Culturing Human Hepatocytes, Sinusoidal Endothelial and Stellate Cells. Biofabrication 2017, 9, 025022. [Google Scholar] [CrossRef]
- Du, Y.; Li, N.; Yang, H.; Luo, C.; Gong, Y.; Tong, C.; Gao, Y.; Lu, S.; Long, M. Mimicking Liver Sinusoidal Structures and Functions Using a 3D-Configured Microfluidic Chip. Lab A Chip 2017, 17, 782–794. [Google Scholar] [CrossRef]
- Kogler, S.; Aizenshtadt, A.; Harrison, S.; Skottvoll, F.S.; Berg, H.E.; Abadpour, S.; Scholz, H.; Sullivan, G.; Thiede, B.; Lundanes, E.; et al. “Organ-in-a-Column” Coupled On-Line with Liquid Chromatography-Mass Spectrometry. Anal. Chem. 2022, 94, 17677–17684. [Google Scholar] [CrossRef] [PubMed]
- Tutty, M.A.; Movia, D.; Prina-Mello, A. Three-Dimensional (3D) Liver Cell Models—A Tool for Bridging the Gap between Animal Studies and Clinical Trials When Screening Liver Accumulation and Toxicity of Nanobiomaterials. Drug Deliv. Transl. Res. 2022, 12, 2048–2074. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, A.; Duche, D.; Ouedraogo, G.; Nahmias, Y. Challenges and Opportunities in the Design of Liver-on-Chip Microdevices. Annu. Rev. Biomed. Eng. 2019, 21, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Chliara, M.A.; Elezoglou, S.; Zergioti, I. Bioprinting on Organ-on-Chip: Development and Applications. Biosensors 2022, 12, 1135. [Google Scholar] [CrossRef]
| Disease Group | Disease | Disease Prevalence | Phenotype | Studies Discussed in This Review | ||
|---|---|---|---|---|---|---|
| Ex Vivo | In Vivo | |||||
| Modeling | Treatment | |||||
| Monogenic diseases with primary hepatic expression without significant parenchymal damage | Hemophilia A | 1:5000 (mostly males) [30] | Deficiency of coagulation factor VIII leads to impaired blood clotting and increased bleeding | + | + | + |
| Hemophilia B | 1:20,000 (mostly males) [31] | Factor IX deficiency causes prolonged bleeding after injuries, surgery, or even spontaneously | + | N/A | + | |
| UCDs | 1:35,000 including ASL deficiency) [32] | Mutations lead to deficiencies of the various enzymes and transporters involved in the urea cycle, causing hyperammonemia or the buildup of cycle intermediates | + | + | + | |
| Hypercholesterolemia | 1:250 (mostly familial heterozygous) [33] | High levels of blood cholesterol as a result of autosomal recessive and familial hypercholesterolemia | + | + | N/A | |
| PH1 | 1–3:1,000,000 [34] | Increased secretion of oxalate caused by mutations in the AGXT gene leads to kidney stones and potential kidney damage | N/A | + | N/A | |
| WTTA | 1:4 (very aged patients) [35] | Misfolded TTR deposits in various tissues mostly affect the heart and tendons of the elderly adults | + | N/A | N/A | |
| FAP | 10,186 patients (extrapolated globally) [36] | Also known as TTR-related amyloidosis. Buildup of abnormal amyloid deposits in the nervous system and other organs causes pain and muscular weakness and may affect the kidneys and the heart | N/A | + | N/A | |
| Crigler-Najjar syndrome | 1:1,000,000 [37] | Improper processing of bilirubin leads to an increase in bilirubin in the blood, causing potential brain damage and severe jaundice | + | N/A | + | |
| GSDIa | 1:125,000 [38] | Lack of release of glucose during fasting and accumulation of excess glycogen and fat in the liver and kidney provoke severe hypoglycemia and other metabolic pathologies | + | N/A | N/A | |
| Monogenic diseases with primary hepatic expression and parenchymal damage | AATD | 2–5:10,000 [39] | Deficiency in alpha-1 antitrypsin results in lung and liver diseases such as COPD and cirrhosis | + | + | + |
| ALGS | 20–33:1,000,000 [40] | Lack or complete absence of bile ducts is accompanied by accumulation of bile in the liver, which leads to severe heart and/or liver disease | + | + | N/A | |
| PFIC | 1–2:100,000 [41] | Impaired bile acid transport and secretion from the liver associated with inadequate bile accumulation and liver disease | + | + | N/A | |
| ASA | 1:70,000 [42] | Urea cycle disorder caused by deficiency or absence of the enzyme ASL may provoke progressive liver damage | N/A | + | + | |
| WD | 1:30,000 [43] | Copper is accumulated due to its defective transport to bile and excretion in waste products, causing symptoms related to the brain and liver | + | + | N/A | |
| GSDIb | 1:500,000 [38] | A deficiency in the G6PT results in disrupted glucose homeostasis and immunological impairment, particularly characterized by neutropenia and neutrophil dysfunction | + | N/A | N/A | |
| TYR1 | 5–6:600,000 [44,45] | Inability to effectively break down the amino acid tyrosine. Accumulation of tyrosine and its byproducts leads to severe health consequences, including liver and renal disorders | + | N/A | N/A | |
| Monogenic diseases with both hepatic and extrahepatic expression | MDDS, AHS | 1–2:10,000 [46] | Mutations in the POLG gene, crucial for mitochondrial DNA replication and repair, lead to progressive developmental regression, uncontrollable seizures, and liver degradation, typically occurring in infants and young children. | N/A | + | N/A |
| NP-C | 4–6:600,000 [47] | The accumulation of cholesterol and glycolipids caused by mutations in NPC1 and NPC2 impairs the body’s ability to process and transport them. This buildup mostly impacts the brain, liver, and spleen, resulting in a variety of systemic and neurological symptoms | N/A | + | N/A | |
| Wolman disease | 1:500,000 [48] | Deficiency in LAL provokes the accumulation of cholesteryl esters and triglycerides in various tissues and organs. Gastrointestinal symptoms and liver and spleen enlargement are present | N/A | + | N/A | |
| Pompe disease | 1:18,711 [49] | Also known as GSDII. Deficiency in the acid GAA causes a buildup of glycogen in cells, leading to muscle and nerve cell damage in the body | + | N/A | + | |
| CF | 1–2:6000 [50] | Mutations in both alleles of the gene encoding the CFTR protein lead to the impaired mucus clearance from the lungs and the colonization of the lungs by bacteria. The pancreas, liver, kidneys, and intestine are also affected | N/A | + | N/A | |
| Abetalipoproteinemia | 1:1,000,000 [51] | Mutations in the microsomal MTTP are associated with the impaired absorption of fats and fat-soluble vitamins, causing low or absent levels of plasma cholesterol, LDLs, and VLDLs. The symptoms affect the gastrointestinal system, nervous system, and eyes. | N/A | + | N/A | |
| Viral hepatitis | Hepatitis B | 254 million people worldwide (2022) [52] | Viral infections that cause liver inflammation. Hepatitis viruses B and C cause chronic disease, whereas hepatitis E is usually self-limiting and resolves within 2–6 weeks | N/A | + | N/A |
| Hepatitis C | 50 million people globally [53] | + | + | + | ||
| Hepatitis E | 19.47 million cases of acute hepatitis E (AHE) globally in 2021 [54] | N/A | + | N/A | ||
| Other disorders | BA | 5–10:100,000 [55] | The bile ducts of a child are narrowed, blocked, or absent, causing liver failure or cirrhosis | + | N/A | N/A |
| TOF | 3:10,000 [56] | Congenital heart anomaly with four specific cardiac defects | N/A | + | N/A | |
| PLD | 1–10:1,000,000 [57] | Numerous cysts are present in liver tissue, which may cause sudden pain, inflammation, and other symptoms | N/A | + | N/A | |
| Cell Type | Relevance | Ease of Acquisition and Use | Throughput | Loss of Viability in Long-Term Culture |
|---|---|---|---|---|
| Primary hepatocytes [66,67,68,69,70] | High, but cells dedifferentiate rapidly in a matter of days | Difficult to acquire and handle | Very low | Occurs quickly |
| Immortalized hepatocytes [71,72,73,74,75] | Comparatively low, especially under 2D conditions, cells are highly variable | Inexpensive, easy to acquire and handle | Very high | With adequate technique, culture lifespan is unlimited |
| iPSC-derived hepatocyte-like cells [76,77,78] | Adequate; cells are somewhat variable and tend to exhibit a fetal phenotype | Expensive and moderately difficult to acquire and handle | Medium-low | Occurs, but is comparatively slower |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andriianov, V.; Malyutina, A.; Panferov, E.; Karabelsky, A.; Ivanov, R.; Minskaia, E.; Reshetnikov, V. Application of Induced Pluripotent Stem Cells (iPSCs) in Hereditary and Viral Diseases of the Liver: Modeling and Treatment. Int. J. Mol. Sci. 2025, 26, 9432. https://doi.org/10.3390/ijms26199432
Andriianov V, Malyutina A, Panferov E, Karabelsky A, Ivanov R, Minskaia E, Reshetnikov V. Application of Induced Pluripotent Stem Cells (iPSCs) in Hereditary and Viral Diseases of the Liver: Modeling and Treatment. International Journal of Molecular Sciences. 2025; 26(19):9432. https://doi.org/10.3390/ijms26199432
Chicago/Turabian StyleAndriianov, Vladimir, Alina Malyutina, Egor Panferov, Alexander Karabelsky, Roman Ivanov, Ekaterina Minskaia, and Vasiliy Reshetnikov. 2025. "Application of Induced Pluripotent Stem Cells (iPSCs) in Hereditary and Viral Diseases of the Liver: Modeling and Treatment" International Journal of Molecular Sciences 26, no. 19: 9432. https://doi.org/10.3390/ijms26199432
APA StyleAndriianov, V., Malyutina, A., Panferov, E., Karabelsky, A., Ivanov, R., Minskaia, E., & Reshetnikov, V. (2025). Application of Induced Pluripotent Stem Cells (iPSCs) in Hereditary and Viral Diseases of the Liver: Modeling and Treatment. International Journal of Molecular Sciences, 26(19), 9432. https://doi.org/10.3390/ijms26199432







