The Interplay Between CB2 and NMDA Receptors in Parkinson’s Disease
Abstract
1. Introduction
2. Results
2.1. α-Syn Fibrils Alter the Conformation of CB2R-NMDAR Complexes
2.2. α-Syn Fibrils Do Not Affect CB2R Affinity for Its Ligands
2.3. CB2R Signaling Is Decreased upon Treatment with α-Syn Fibrils
2.4. Signaling of CB2R-NMDAR Complexes Is Decreased upon α-Syn Fibrils Treatment in a Heterologous System
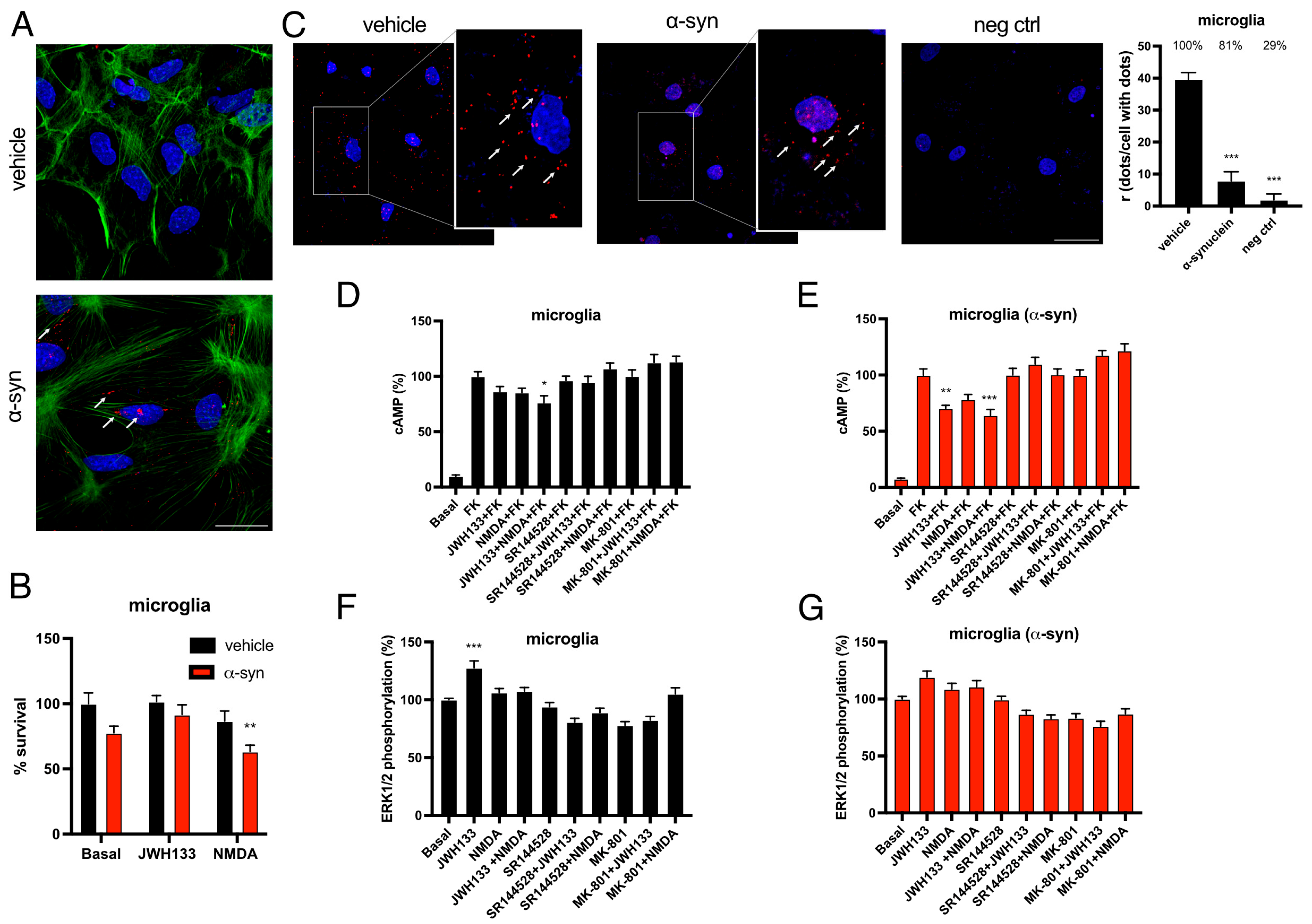
2.5. The Expression and Function of CB2R-NMDAR Complexes Are Altered by α-Syn Fibrils in Rat Microglia
2.6. CB2R Activation Counteracts the Detrimental Phenotype of Microglia Activation Induced by α-Syn Fibrils
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Cell Culture and Transient Transfection
4.3. Fusion Proteins and Expression Vectors
4.4. α-Synuclein Fibrils Treatment
4.5. Immunofluorescence Studies
4.6. Homogeneous Competition Binding Assays
4.7. Bioluminescence Resonance Energy Transfer (BRET) Assays
4.8. cAMP Level Determination
4.9. Extracellular Signal-Regulated Kinase Phosphorylation Assays
4.10. Detection of Cytoplasmic Calcium Levels
4.11. β-Arrestin II Recruitment
4.12. Cell Viability
4.13. In Situ and In Vitro Proximity Ligation Assay (PLA)
4.14. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| Arg-1 | Arginase-1 |
| BBB | Blood–brain barrier |
| BRET | Bioluminiscence Resonance Energy Transfer |
| CB2R | Cannabinoid receptor 2 |
| GHSR1A | Ghrelin receptor 1A |
| GPCRs | G protein-coupled receptors |
| HEK-293T | Human Embryonic Kidney 293T |
| HTRF | Homogeneous Time-Resolved FRET |
| Iba-1 | Ionized calcium-binding adaptor molecule 1 |
| iNOS | Inducible Nitric Oxide Synthase |
| L-DOPA | Levodopa |
| LPS | Lipopolysaccharide |
| mBU | Milli BRET units |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NMDA | N-methyl-D-aspartate |
| NMDAR | NMDA receptor |
| PD | Parkinson’s disease |
| PLA | Proximity Ligation Assay |
| Rluc | Renilla luciferase |
| YFP | Yellow fluorescent protein |
| α-syn | Alpha synuclein |
References
- de Rijk, M.C.; Rocca, W.A.; Anderson, D.W.; Melcon, M.O.; Breteler, M.M.B.; Maraganore, D.M. A Population Perspective on Diagnostic Criteria for Parkinson’s Disease. Neurology 1997, 48, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H.V. Parkinson Disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K. Incidence of Parkinson’s Disease: Variation by Age, Gender, and Race/Ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The Epidemiology of Parkinson’s Disease: Risk Factors and Prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-Synuclein in Parkinson’s Disease and Other Synucleinopathies: From Overt Neurodegeneration Back to Early Synaptic Dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Yoo, G.; Shin, Y.-K.; Lee, N.K. The Role of α-Synuclein in SNARE-Mediated Synaptic Vesicle Fusion. J. Mol. Biol. 2023, 435, 167775. [Google Scholar] [CrossRef]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef]
- Simón-Sánchez, J.; Schulte, C.; Bras, J.M.; Sharma, M.; Gibbs, J.R.; Berg, D.; Paisan-Ruiz, C.; Lichtner, P.; Scholz, S.W.; Hernandez, D.G.; et al. Genome-Wide Association Study Reveals Genetic Risk Underlying Parkinson’s Disease. Nat. Genet. 2009, 41, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy Bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Ahlskog, J.E.; Muenter, M.D. Frequency of Levodopa-related Dyskinesias and Motor Fluctuations as Estimated from the Cumulative Literature. Mov. Disord. 2001, 16, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.F.; Collier, T.J.; Patterson, J.R.; Kemp, C.J.; Luk, K.C.; Tansey, M.G.; Paumier, K.L.; Kanaan, N.M.; Fischer, D.L.; Polinski, N.K.; et al. Lewy Body-like Alpha-Synuclein Inclusions Trigger Reactive Microgliosis Prior to Nigral Degeneration. J. Neuroinflamm. 2018, 15, 129. [Google Scholar] [CrossRef]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-Synuclein: Pathology, Mitochondrial Dysfunction and Neuroinflammation in Parkinson’s Disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Imamura, K.; Hishikawa, N.; Sawada, M.; Nagatsu, T.; Yoshida, M.; Hashizume, Y. Distribution of Major Histocompatibility Complex Class II-Positive Microglia and Cytokine Profile of Parkinson’s Disease Brains. Acta Neuropathol. 2003, 106, 518–526. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive Microglia Are Positive for HLA-DR in the Substantia Nigra of Parkinson’s and Alzheimer’s Disease Brains. Neurology 1988, 38, 1285. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Zhou, T. Alpha-Synuclein Induced Immune Cells Activation and Associated Therapy in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 769506. [Google Scholar] [CrossRef]
- Pérez-Olives, C.; Rivas-Santisteban, R.; Lillo, J.; Navarro, G.; Franco, R. Recent Advances in the Potential of Cannabinoids for Neuroprotection in Alzheimer’s, Parkinson’s, and Huntington’s Diseases. In Cannabinoids and Neuropsychiatric Disorders; Springer: Cham, Switzerland, 2021; pp. 81–92. [Google Scholar]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Cannabinoid Signaling and Neuroinflammatory Diseases: A Melting Pot for the Regulation of Brain Immune Responses. J. Neuroimmune Pharmacol. 2015, 10, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Navarro, G.; Borroto-Escuela, D.; Angelats, E.; Etayo, Í.; Reyes-Resina, I.; Pulido-Salgado, M.; Rodríguez-Pérez, A.I.; Canela, E.I.; Saura, J.; Lanciego, J.L.; et al. Receptor-Heteromer Mediated Regulation of Endocannabinoid Signaling in Activated Microglia. Role of CB1 and CB2 Receptors and Relevance for Alzheimer’s Disease and Levodopa-Induced Dyskinesia. Brain. Behav. Immun. 2018, 67, 139–151. [Google Scholar] [CrossRef]
- Chen, D.; Gao, M.; Gao, F.; Su, Q.; Wu, J. Brain Cannabinoid Receptor 2: Expression, Function and Modulation. Acta Pharmacol. Sin. 2017, 38, 312–316. [Google Scholar] [CrossRef]
- Gómez-Gálvez, Y.; Palomo-Garo, C.; Fernández-Ruiz, J.; García, C. Potential of the Cannabinoid CB2 Receptor as a Pharmacological Target against Inflammation in Parkinson’s Disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 200–208. [Google Scholar] [CrossRef]
- Price, D.A.; Martinez, A.A.; Seillier, A.; Koek, W.; Acosta, Y.; Fernandez, E.; Strong, R.; Lutz, B.; Marsicano, G.; Roberts, J.L.; et al. WIN55,212-2, a Cannabinoid Receptor Agonist, Protects against Nigrostriatal Cell Loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine Mouse Model of Parkinson’s Disease. Eur. J. Neurosci. 2009, 29, 2177–2186. [Google Scholar] [CrossRef]
- Concannon, R.M.; Okine, B.N.; Finn, D.P.; Dowd, E. Differential Upregulation of the Cannabinoid CB2 Receptor in Neurotoxic and Inflammation-Driven Rat Models of Parkinson’s Disease. Exp. Neurol. 2015, 269, 133–141. [Google Scholar] [CrossRef]
- Concannon, R.M.; Okine, B.N.; Finn, D.P.; Dowd, E. Upregulation of the Cannabinoid CB2 Receptor in Environmental and Viral Inflammation-Driven Rat Models of Parkinson’s Disease. Exp. Neurol. 2016, 283, 204–212. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S.K. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease. Front. Neurosci. 2016, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Abellanas, M.A.; Aymerich, M.S. Cannabinoid Receptor Type 2 as a Therapeutic Target for Parkinson’s Disease. In Diagnosis and Management in Parkinson’s Disease; Elsevier: Amsterdam, The Netherlands, 2020; pp. 557–573. [Google Scholar]
- Borroto-Escuela, D.O.; Carlsson, J.; Ambrogini, P.; Narváez, M.; Wydra, K.; Tarakanov, A.O.; Li, X.; Millón, C.; Ferraro, L.; Cuppini, R.; et al. Understanding the Role of GPCR Heteroreceptor Complexes in Modulating the Brain Networks in Health and Disease. Front. Cell. Neurosci. 2017, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Santisteban, R.; Lillo, A.; Lillo, J.; Rebassa, J.-B.; Contestí, J.S.; Saura, C.A.; Franco, R.; Navarro, G. N-Methyl-D-Aspartate (NMDA) and Cannabinoid CB2 Receptors Form Functional Complexes in Cells of the Central Nervous System: Insights into the Therapeutic Potential of Neuronal and Microglial NMDA Receptors. Alzheimer’s Res. Ther. 2021, 13, 184. [Google Scholar] [CrossRef]
- Reyes-Resina, I.; Lillo, J.; Raïch, I.; Rebassa, J.B.; Navarro, G. The Expression and Functionality of CB1R-NMDAR Complexes Are Decreased in A Parkinson’s Disease Model. Int. J. Mol. Sci. 2024, 25, 3021. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA Receptor Subunit Diversity: Impact on Receptor Properties, Synaptic Plasticity and Disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Synaptic versus Extrasynaptic NMDA Receptor Signalling: Implications for Neurodegenerative Disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef]
- Bading, H. Therapeutic Targeting of the Pathological Triad of Extrasynaptic NMDA Receptor Signaling in Neurodegenerations. J. Exp. Med. 2017, 214, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA Receptor Involvement in Central Nervous System Disorders. Neuron 2014, 82, 279–293. [Google Scholar] [CrossRef]
- Dunah, A.W.; Wang, Y.; Yasuda, R.P.; Kameyama, K.; Huganir, R.L.; Wolfe, B.B.; Standaert, D.G. Alterations in Subunit Expression, Composition, and Phosphorylation of Striatal N-Methyl-D-Aspartate Glutamate Receptors in a Rat 6-Hydroxydopamine Model of Parkinson’s Disease. Mol. Pharmacol. 2000, 57, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.D.; Russell, D.; Vaughan, C.L.; Chase, T.N. Enhanced Tyrosine Phosphorylation of Striatal NMDA Receptor Subunits: Effect of Dopaminergic Denervation and l-DOPA Administration. Brain Res. 1998, 813, 150–159. [Google Scholar] [CrossRef]
- Mellone, M.; Stanic, J.; Hernandez, L.F.; Iglesias, E.; Zianni, E.; Longhi, A.; Prigent, A.; Picconi, B.; Calabresi, P.; Hirsch, E.C.; et al. NMDA Receptor GluN2A/GluN2B Subunit Ratio as Synaptic Trait of Levodopa-Induced Dyskinesias: From Experimental Models to Patients. Front. Cell. Neurosci. 2015, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Qi, C.; Mao, L.-M.; Liu, Z. Changes in Surface Expression of N-Methyl-D-Aspartate Receptors in the Striatum in a Rat Model of Parkinson’s Disease. Drug Des. Devel. Ther. 2014, 165, 165–173. [Google Scholar] [CrossRef]
- Melo-Thomas, L.; Gil-Martínez, A.L.; Cuenca, L.; Estrada, C.; Gonzalez-Cuello, A.; Schwarting, R.K.; Herrero, M.T. Electrical Stimulation or MK-801 in the Inferior Colliculus Improve Motor Deficits in MPTP-Treated Mice. Neurotoxicology 2018, 65, 38–43. [Google Scholar] [CrossRef]
- Michel, A.; Nicolas, J.-M.; Rose, S.; Jackson, M.; Colman, P.; Briône, W.; Sciberras, D.; Muglia, P.; Scheller, D.K.; Citron, M.; et al. Antiparkinsonian Effects of the “Radiprodil and Tozadenant” Combination in MPTP-Treated Marmosets. PLoS ONE 2017, 12, e0182887. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease. JAMA 2020, 323, 548. [Google Scholar] [CrossRef]
- Dale, N.C.; Johnstone, E.K.M.; Pfleger, K.D.G. GPCR Heteromers: An Overview of Their Classification, Function and Physiological Relevance. Front. Endocrinol. 2022, 13, 931573. [Google Scholar] [CrossRef]
- Navarro, G.; Raïch, I.; Rebassa, J.B.; Lillo, J.; Pérez-Olives, C.; Capó, T.; Cubeles, E.; Saura, C.A.; Cordomí, A.; Sotelo, E.; et al. Cannabinoid CB1 Receptor Activation Mitigates N-Methyl-d-Aspartate Receptor-Mediated Neurotoxicity. ACS Pharmacol. Transl. Sci. 2025, 8, 3019–3032. [Google Scholar] [CrossRef]
- Ferré, S.; Navarro, G.; Casadó, V.; Cortés, A.; Mallol, J.; Canela, E.I.; Lluís, C.; Franco, R. G Protein-Coupled Receptor Heteromers as New Targets for Drug Development. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2010; pp. 41–52. [Google Scholar]
- Chen, T.-W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive Fluorescent Proteins for Imaging Neuronal Activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Di Marzo, V.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; van der Stelt, M. Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958, Erratum in Pharmacol. Rev. 2023, 76, 194. [Google Scholar] [CrossRef]
- Pisani, A.; Fezza, F.; Galati, S.; Battista, N.; Napolitano, S.; Finazzi-Agrò, A.; Bernardi, G.; Brusa, L.; Pierantozzi, M.; Stanzione, P.; et al. High Endogenous Cannabinoid Levels in the Cerebrospinal Fluid of Untreated Parkinson’s Disease Patients. Ann. Neurol. 2005, 57, 777–779. [Google Scholar] [CrossRef]
- Pisani, V.; Moschella, V.; Bari, M.; Fezza, F.; Galati, S.; Bernardi, G.; Stanzione, P.; Pisani, A.; Maccarrone, M. Dynamic Changes of Anandamide in the Cerebrospinal Fluid of Parkinson’s Disease Patients. Mov. Disord. 2010, 25, 920–924. [Google Scholar] [CrossRef]
- Kelly, R.; Bemelmans, A.-P.; Joséphine, C.; Brouillet, E.; McKernan, D.P.; Dowd, E. Time-Course of Alterations in the Endocannabinoid System after Viral-Mediated Overexpression of α-Synuclein in the Rat Brain. Molecules 2022, 27, 507. [Google Scholar] [CrossRef]
- Soti, M.; Ranjbar, H.; Kohlmeier, K.A.; Shabani, M. Parkinson’s Disease Related Alterations in Cannabinoid Transmission. Brain Res. Bull. 2022, 178, 82–96. [Google Scholar] [CrossRef]
- García, C.; Palomo-Garo, C.; García-Arencibia, M.; Ramos, J.; Pertwee, R.; Fernández-Ruiz, J. Symptom-relieving and Neuroprotective Effects of the Phytocannabinoid Δ 9 -THCV in Animal Models of Parkinson’s Disease. Br. J. Pharmacol. 2011, 163, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- García, M.C.; Cinquina, V.; Palomo-Garo, C.; Rábano, A.; Fernández-Ruiz, J. Identification of CB2 Receptors in Human Nigral Neurons That Degenerate in Parkinson’s Disease. Neurosci. Lett. 2015, 587, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, G.; Bucci, G.; Emanuele, M.; Leo, D.; Sotnikova, T.D.; Mus, L.V.; Soubrane, C.H.; Dallas, M.L.; Thalhammer, A.; Cingolani, L.A.; et al. Exogenous -Synuclein Decreases Raft Partitioning of Cav2.2 Channels Inducing Dopamine Release. J. Neurosci. 2014, 34, 10603–10615. [Google Scholar] [CrossRef]
- Armentero, M.-T.; Fancellu, R.; Nappi, G.; Bramanti, P.; Blandini, F. Prolonged Blockade of NMDA or MGluR5 Glutamate Receptors Reduces Nigrostriatal Degeneration While Inducing Selective Metabolic Changes in the Basal Ganglia Circuitry in a Rodent Model of Parkinson’s Disease. Neurobiol. Dis. 2006, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, J.; Mague, S.; Pijanowski, R.; Harris, R.; Kleckner, N.; Matthews, R. NMDA Receptor Antagonists Ameliorate the Stepping Deficits Produced by Unilateral Medial Forebrain Bundle Injections of 6-OHDA in Rats. Psychopharmacology 2004, 175, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, J.; Ossowska, K.; Schulze, G.; Coper, H.; Wolfarth, S. L-701,324, a Selective Antagonist at the Glycine Site of the NMDA Receptor, Counteracts Haloperidol-Induced Muscle Rigidity in Rats. Psychopharmacology 1999, 143, 235–243. [Google Scholar] [CrossRef]
- Egunlusi, A.O.; Joubert, J. NMDA Receptor Antagonists: Emerging Insights into Molecular Mechanisms and Clinical Applications in Neurological Disorders. Pharmaceuticals 2024, 17, 639. [Google Scholar] [CrossRef]
- Bortolanza, M.; Bariotto-dos-Santos, K.D.; Dos-Santos-Pereira, M.; Da-Silva, C.A.; Del-Bel, E. Antidyskinetic Effect of 7-Nitroindazole and Sodium Nitroprusside Associated with Amantadine in a Rat Model of Parkinson’s Disease. Neurotox. Res. 2016, 30, 88–100. [Google Scholar] [CrossRef]
- Flores, A.J.; Bartlett, M.J.; Root, B.K.; Parent, K.L.; Heien, M.L.; Porreca, F.; Polt, R.; Sherman, S.J.; Falk, T. The Combination of the Opioid Glycopeptide MMP-2200 and a NMDA Receptor Antagonist Reduced l-DOPA-Induced Dyskinesia and MMP-2200 by Itself Reduced Dopamine Receptor 2-like Agonist-Induced Dyskinesia. Neuropharmacology 2018, 141, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Steece-Collier, K.; Chambers, L.K.; Jaw-Tsai, S.S.; Menniti, F.S.; Greenamyre, J.T. Antiparkinsonian Actions of CP-101,606, an Antagonist of NR2B Subunit-Containing N-Methyl-d-Aspartate Receptors. Exp. Neurol. 2000, 163, 239–243. [Google Scholar] [CrossRef]
- Igarashi, M.; Habata, T.; Akita, H.; Noda, K.; Ogata, M.; Saji, M. The NR2B Antagonist, Ifenprodil, Corrects the l-DOPA-Induced Deficit of Bilateral Movement and Reduces c-Fos Expression in the Subthalamic Nucleus of Hemiparkinsonian Rats. Neurosci. Res. 2015, 96, 45–53. [Google Scholar] [CrossRef]
- Michel, A.; Downey, P.; Van Damme, X.; De Wolf, C.; Schwarting, R.; Scheller, D. Behavioural Assessment of the A2a/NR2B Combination in the Unilateral 6-OHDA-Lesioned Rat Model: A New Method to Examine the Therapeutic Potential of Non-Dopaminergic Drugs. PLoS ONE 2015, 10, e0135949. [Google Scholar] [CrossRef]
- Gerhard, A.; Pavese, N.; Hotton, G.; Turkheimer, F.; Es, M.; Hammers, A.; Eggert, K.; Oertel, W.; Banati, R.B.; Brooks, D.J. In Vivo Imaging of Microglial Activation with [11C](R)-PK11195 PET in Idiopathic Parkinson’s Disease. Neurobiol. Dis. 2006, 21, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.-S.; et al. Aggregated Alpha-Synuclein Activates Microglia: A Process Leading to Disease Progression in Parkinson’s Disease. FASEB J. 2005, 19, 533–542. [Google Scholar] [CrossRef]
- Hoffmann, A.; Ettle, B.; Bruno, A.; Kulinich, A.; Hoffmann, A.-C.; von Wittgenstein, J.; Winkler, J.; Xiang, W.; Schlachetzki, J.C.M. Alpha-Synuclein Activates BV2 Microglia Dependent on Its Aggregation State. Biochem. Biophys. Res. Commun. 2016, 479, 881–886. [Google Scholar] [CrossRef]
- Hoenen, C.; Gustin, A.; Birck, C.; Kirchmeyer, M.; Beaume, N.; Felten, P.; Grandbarbe, L.; Heuschling, P.; Heurtaux, T. Alpha-Synuclein Proteins Promote Pro-Inflammatory Cascades in Microglia: Stronger Effects of the A53T Mutant. PLoS ONE 2016, 11, e0162717. [Google Scholar] [CrossRef]
- Grozdanov, V.; Bousset, L.; Hoffmeister, M.; Bliederhaeuser, C.; Meier, C.; Madiona, K.; Pieri, L.; Kiechle, M.; McLean, P.J.; Kassubek, J.; et al. Increased Immune Activation by Pathologic A-Synuclein in Parkinson’s Disease. Ann. Neurol. 2019, 86, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Young, A.P.; Denovan-Wright, E.M. The Dynamic Role of Microglia and the Endocannabinoid System in Neuroinflammation. Front. Pharmacol. 2022, 12, 806417. [Google Scholar] [CrossRef]
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the Cannabinoid CB 2 Receptor in Microglial Cells in Response to Inflammatory Stimuli. J. Neurochem. 2005, 95, 437–445. [Google Scholar] [CrossRef]
- Medina-Vera, D.; Zhao, H.; Bereczki, E.; Rosell-Valle, C.; Shimozawa, M.; Chen, G.; de Fonseca, F.R.; Nilsson, P.; Tambaro, S. The Expression of the Endocannabinoid Receptors CB2 and GPR55 Is Highly Increased during the Progression of Alzheimer’s Disease in AppNL-G-F Knock-In Mice. Biology 2023, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Solas, M.; Francis, P.T.; Franco, R.; Ramirez, M.J. CB2 Receptor and Amyloid Pathology in Frontal Cortex of Alzheimer’s Disease Patients. Neurobiol. Aging 2013, 34, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Codolo, G.; Plotegher, N.; Pozzobon, T.; Brucale, M.; Tessari, I.; Bubacco, L.; de Bernard, M. Triggering of Inflammasome by Aggregated α–Synuclein, an Inflammatory Response in Synucleinopathies. PLoS ONE 2013, 8, e55375. [Google Scholar] [CrossRef]
- Fellner, L.; Irschick, R.; Schanda, K.; Reindl, M.; Klimaschewski, L.; Poewe, W.; Wenning, G.K.; Stefanova, N. Toll-like Receptor 4 Is Required for α-Synuclein Dependent Activation of Microglia and Astroglia. Glia 2013, 61, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Ardah, M.T.; Merghani, M.M.; Haque, M.E. Thymoquinone Prevents Neurodegeneration against MPTP in Vivo and Modulates α-Synuclein Aggregation in Vitro. Neurochem. Int. 2019, 128, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Dammer, E.B.; Malovic, E.; Olsen, A.L.; Raza, S.A.; Gao, T.; Xiao, H.; Oliver, D.L.; Duong, D.; Joers, V.; et al. Molecular Signatures of Neuroinflammation Induced by ASynuclein Aggregates in Microglial Cells. Front. Immunol. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.D.; Glanzer, J.G.; Kadiu, I.; Ricardo-Dukelow, M.; Chaudhuri, A.; Ciborowski, P.; Cerny, R.; Gelman, B.; Thomas, M.P.; Mosley, R.L.; et al. Nitrated Alpha-Synuclein-Activated Microglial Profiling for Parkinson’s Disease. J. Neurochem. 2008, 104, 1504–1525. [Google Scholar] [CrossRef]
- Kim, C.; Ho, D.-H.; Suk, J.-E.; You, S.; Michael, S.; Kang, J.; Joong Lee, S.; Masliah, E.; Hwang, D.; Lee, H.-J.; et al. Neuron-Released Oligomeric α-Synuclein Is an Endogenous Agonist of TLR2 for Paracrine Activation of Microglia. Nat. Commun. 2013, 4, 1562. [Google Scholar] [CrossRef]
- Rojanathammanee, L.; Murphy, E.J.; Combs, C.K. Expression of Mutant Alpha-Synuclein Modulates Microglial Phenotype in Vitro. J. Neuroinflamm. 2011, 8, 44. [Google Scholar] [CrossRef]
- Liberatore, G.T.; Jackson-Lewis, V.; Vukosavic, S.; Mandir, A.S.; Vila, M.; McAuliffe, W.G.; Dawson, V.L.; Dawson, T.M.; Przedborski, S. Inducible Nitric Oxide Synthase Stimulates Dopaminergic Neurodegeneration in the MPTP Model of Parkinson Disease. Nat. Med. 1999, 5, 1403–1409. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Glial Reactions in Parkinson’s Disease. Mov. Disord. 2008, 23, 474–483. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Qin, L.; Gao, H.-M.; Wilson, B.; Ali, S.F.; Zhang, W.; Hong, J.-S.; Liu, B. Neuroprotective Effect of Dextromethorphan in the MPTP Parkinson’s Disease Model: Role of NADPH Oxidase. FASEB J. 2004, 18, 589–591. [Google Scholar] [CrossRef]
- Wu, D.-C.; Teismann, P.; Tieu, K.; Vila, M.; Jackson-Lewis, V.; Ischiropoulos, H.; Przedborski, S. NADPH Oxidase Mediates Oxidative Stress in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Model of Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2003, 100, 6145–6150. [Google Scholar] [CrossRef]
- Karpenko, M.N.; Vasilishina, A.A.; Gromova, E.A.; Muruzheva, Z.M.; Bernadotte, A. Interleukin-1β, Interleukin-1 Receptor Antagonist, Interleukin-6, Interleukin-10, and Tumor Necrosis Factor-α Levels in CSF and Serum in Relation to the Clinical Diversity of Parkinson’s Disease. Cell. Immunol. 2018, 327, 77–82. [Google Scholar] [CrossRef]
- George, S.; Rey, N.L.; Tyson, T.; Esquibel, C.; Meyerdirk, L.; Schulz, E.; Pierce, S.; Burmeister, A.R.; Madaj, Z.; Steiner, J.A.; et al. Microglia Affect α-Synuclein Cell-to-Cell Transfer in a Mouse Model of Parkinson’s Disease. Mol. Neurodegener. 2019, 14, 34. [Google Scholar] [CrossRef]
- Choi, I.; Zhang, Y.; Seegobin, S.P.; Pruvost, M.; Wang, Q.; Purtell, K.; Zhang, B.; Yue, Z. Microglia Clear Neuron-Released α-Synuclein via Selective Autophagy and Prevent Neurodegeneration. Nat. Commun. 2020, 11, 1386. [Google Scholar] [CrossRef]
- Park, H.J.; Oh, S.H.; Kim, H.N.; Jung, Y.J.; Lee, P.H. Mesenchymal Stem Cells Enhance α-Synuclein Clearance via M2 Microglia Polarization in Experimental and Human Parkinsonian Disorder. Acta Neuropathol. 2016, 132, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Feliú, A.; Carrillo-Salinas, F.J.; Rueda-Zubiaurre, A.; Ortega-Gutiérrez, S.; de Sola, R.G.; Guaza, C. Endocannabinoids Drive the Acquisition of an Alternative Phenotype in Microglia. Brain. Behav. Immun. 2015, 49, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Jia, J.; Liu, X.; Bai, F.; Wang, Q.; Xiong, L. Activation of Murine Microglial N9 Cells Is Attenuated through Cannabinoid Receptor CB2 Signaling. Biochem. Biophys. Res. Commun. 2015, 458, 92–97. [Google Scholar] [CrossRef]
- Correa, F.; Hernangómez, M.; Mestre, L.; Loría, F.; Spagnolo, A.; Docagne, F.; Di Marzo, V.; Guaza, C. Anandamide Enhances IL-10 Production in Activated Microglia by Targeting CB 2 Receptors: Roles of ERK1/2, JNK, and NF-κB. Glia 2010, 58, 135–147. [Google Scholar] [CrossRef]
- Malek, N.; Popiolek-Barczyk, K.; Mika, J.; Przewlocka, B.; Starowicz, K. Anandamide, Acting via CB2 Receptors, Alleviates LPS-Induced Neuroinflammation in Rat Primary Microglial Cultures. Neural Plast. 2015, 2015, 130639. [Google Scholar] [CrossRef]
- Chung, Y.C.; Shin, W.-H.; Baek, J.Y.; Cho, E.J.; Baik, H.H.; Kim, S.R.; Won, S.-Y.; Jin, B.K. CB2 Receptor Activation Prevents Glial-Derived Neurotoxic Mediator Production, BBB Leakage and Peripheral Immune Cell Infiltration and Rescues Dopamine Neurons in the MPTP Model of Parkinson’s Disease. Exp. Mol. Med. 2016, 48, e205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xiao, F.; Xiao, Y.; Guo, Y.; Shan, X.; Zhang, Z.; Zhang, L.; Guo, H. Targeting CB2R in Astrocytes for Parkinson’s Disease Therapy: Unraveling the Foxg1-Mediated Neuroprotective Mechanism through Autophagy-Mediated NLRP3 Degradation. J. Neuroinflamm. 2023, 20, 304. [Google Scholar] [CrossRef]
- Joers, V.; Murray, B.C.; McLaughlin, C.; Oliver, D.; Staley, H.; Coronado, J.; Achat-Mendes, C.; Golshani, S.; Kelly, S.D.; Goodson, M.; et al. Modulation of Cannabinoid Receptor 2 Alters Neuroinflammation and Reduces Formation of Alpha-Synuclein Aggregates in a Rat Model of Nigral Synucleinopathy. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Lo, H.; You, H.; Wu, W.; Cheng, X.; Xin, J.; Ye, Z.; Chen, X.; Pan, X. Loss of Cannabinoid Receptor 2 Promotes α-Synuclein-Induced Microglial Synaptic Pruning in Nucleus Accumbens by Modulating the PCREB-c-Fos Signaling Pathway and Complement System. Exp. Neurol. 2023, 359, 114230. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E.; Brotchie, J.M.; Gross, C.E. Pathophysiology of Levodopa-Induced Dyskinesia: Potential for New Therapies. Nat. Rev. Neurosci. 2001, 2, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, P.; Stayte, S.; Egan, T.; Clark, I.; Vissel, B. Targeting the Cannabinoid Receptor CB2 in a Mouse Model of L-Dopa Induced Dyskinesia. Neurobiol. Dis. 2020, 134, 104646. [Google Scholar] [CrossRef]
- Raïch, I.; Lillo, J.; Rebassa, J.B.; Griñán-Ferré, C.; Bellver-Sanchis, A.; Reyes-Resina, I.; Franco, R.; Pallàs, M.; Navarro, G. Cannabidiol as a Multifaceted Therapeutic Agent: Mitigating Alzheimer’s Disease Pathology and Enhancing Cognitive Function. Alzheimer’s Res. Ther. 2025, 17, 109. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Rabal, O.; Reyes-Resina, I.; Zamarbide, M.; Navarro, G.; Sánchez-Arias, J.A.; De Miguel, I.; Lanciego, J.L.; Oyarzabal, J.; Franco, R. Two Affinity Sites of the Cannabinoid Subtype 2 Receptor Identified by a Novel Homogeneous Binding Assay. J. Pharmacol. Exp. Ther. 2016, 358, 580–587. [Google Scholar] [CrossRef]
- Franco, R.; Aguinaga, D.; Reyes, I.; Canela, E.I.; Lillo, J.; Tarutani, A.; Hasegawa, M.; del Ser-Badia, A.; del Rio, J.A.; Kreutz, M.R.; et al. N-Methyl-D-Aspartate Receptor Link to the MAP Kinase Pathway in Cortical and Hippocampal Neurons and Microglia Is Dependent on Calcium Sensors and Is Blocked by α-Synuclein, Tau, and Phospho-Tau in Non-Transgenic and Transgenic APPSw,Ind Mice. Front. Mol. Neurosci. 2018, 11, 273. [Google Scholar] [CrossRef]
- Masuda-Suzukake, M.; Nonaka, T.; Hosokawa, M.; Kubo, M.; Shimozawa, A.; Akiyama, H.; Hasegawa, M. Pathological Alpha-Synuclein Propagates through Neural Networks. Acta Neuropathol. Commun. 2014, 2, 88. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Resina, I.; Lillo, J.; Raïch, I.; Rebassa, J.B.; Capó, T.; Badia, P.; Navarro, G. The Interplay Between CB2 and NMDA Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2025, 26, 9419. https://doi.org/10.3390/ijms26199419
Reyes-Resina I, Lillo J, Raïch I, Rebassa JB, Capó T, Badia P, Navarro G. The Interplay Between CB2 and NMDA Receptors in Parkinson’s Disease. International Journal of Molecular Sciences. 2025; 26(19):9419. https://doi.org/10.3390/ijms26199419
Chicago/Turabian StyleReyes-Resina, Irene, Jaume Lillo, Iu Raïch, Joan Biel Rebassa, Toni Capó, Pau Badia, and Gemma Navarro. 2025. "The Interplay Between CB2 and NMDA Receptors in Parkinson’s Disease" International Journal of Molecular Sciences 26, no. 19: 9419. https://doi.org/10.3390/ijms26199419
APA StyleReyes-Resina, I., Lillo, J., Raïch, I., Rebassa, J. B., Capó, T., Badia, P., & Navarro, G. (2025). The Interplay Between CB2 and NMDA Receptors in Parkinson’s Disease. International Journal of Molecular Sciences, 26(19), 9419. https://doi.org/10.3390/ijms26199419




