Navigating the Central Nervous System (CNS): A Pharmacokinetic Approach to the Treatment of CNS Tumors, Glioblastoma Multiforme (GBM), in Particular
Abstract
1. Introduction
2. Glioblastoma Multiforme (GBM)
2.1. Drug Efficacy
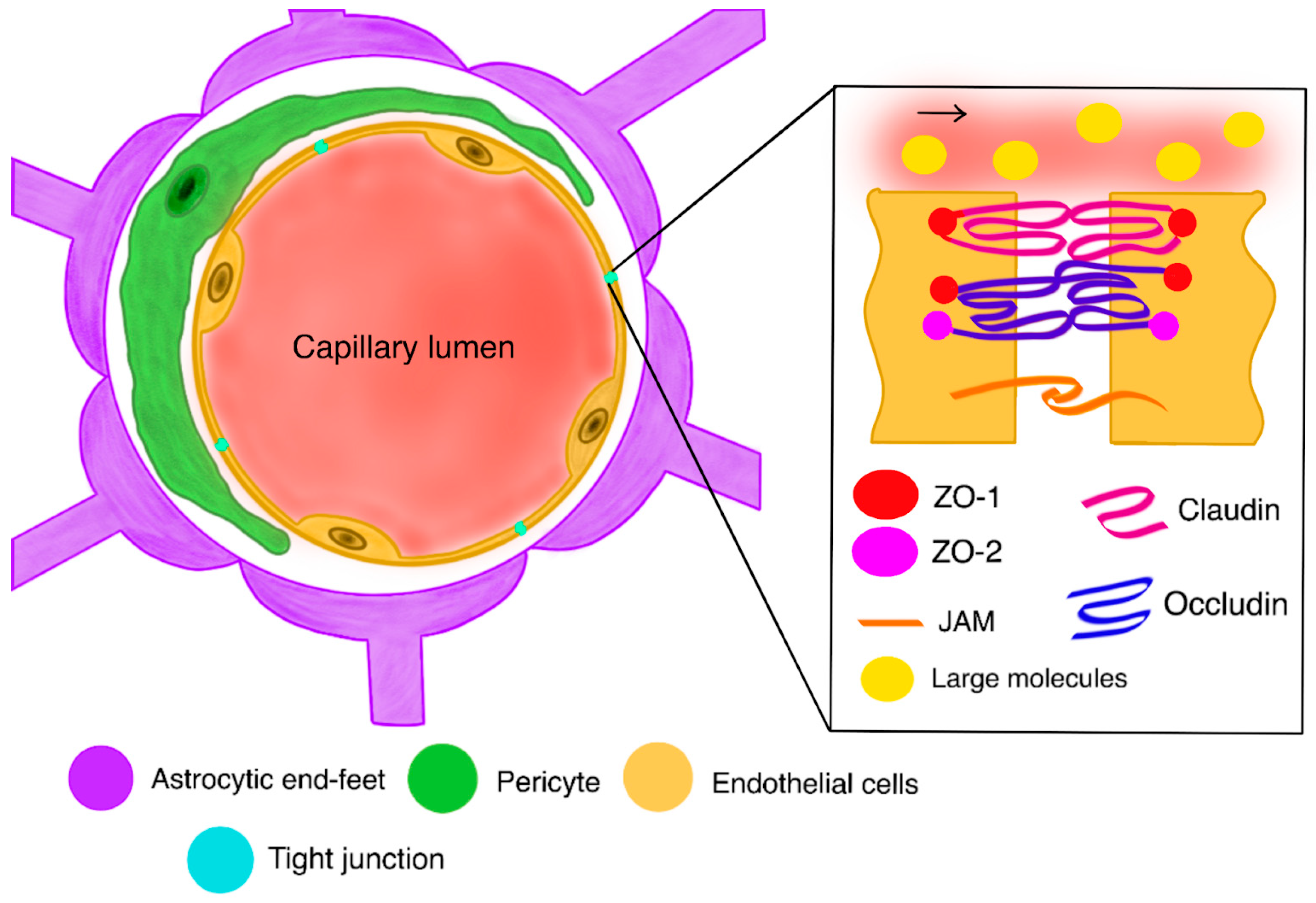
2.2. Blood–Brain Barrier (BBB)
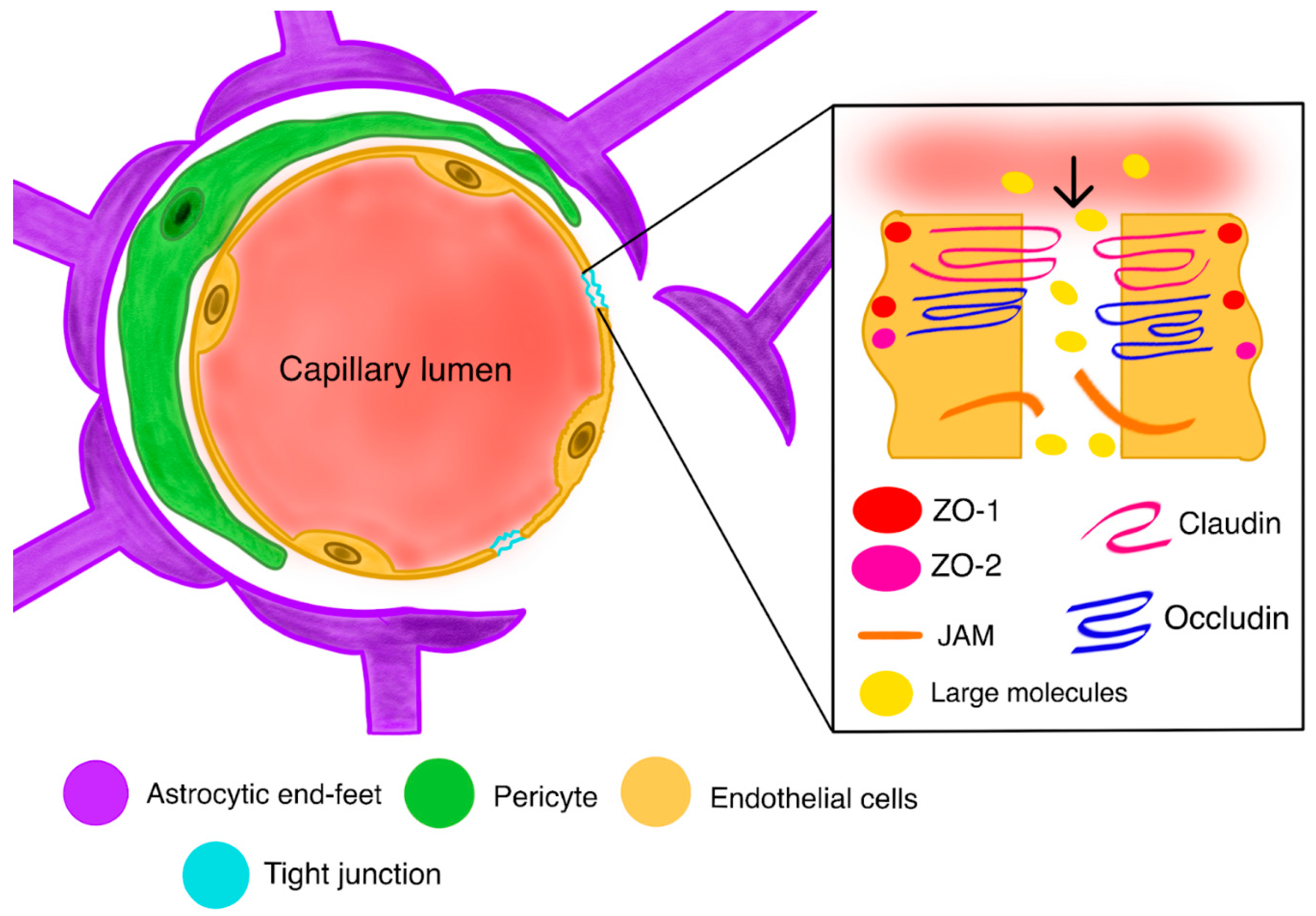
2.3. Blood–Tumor Barrier (BTB)
2.4. A Further Drug Route in the CNS
2.5. Bypassing the Barriers
2.6. High-Dose Systemic Therapy
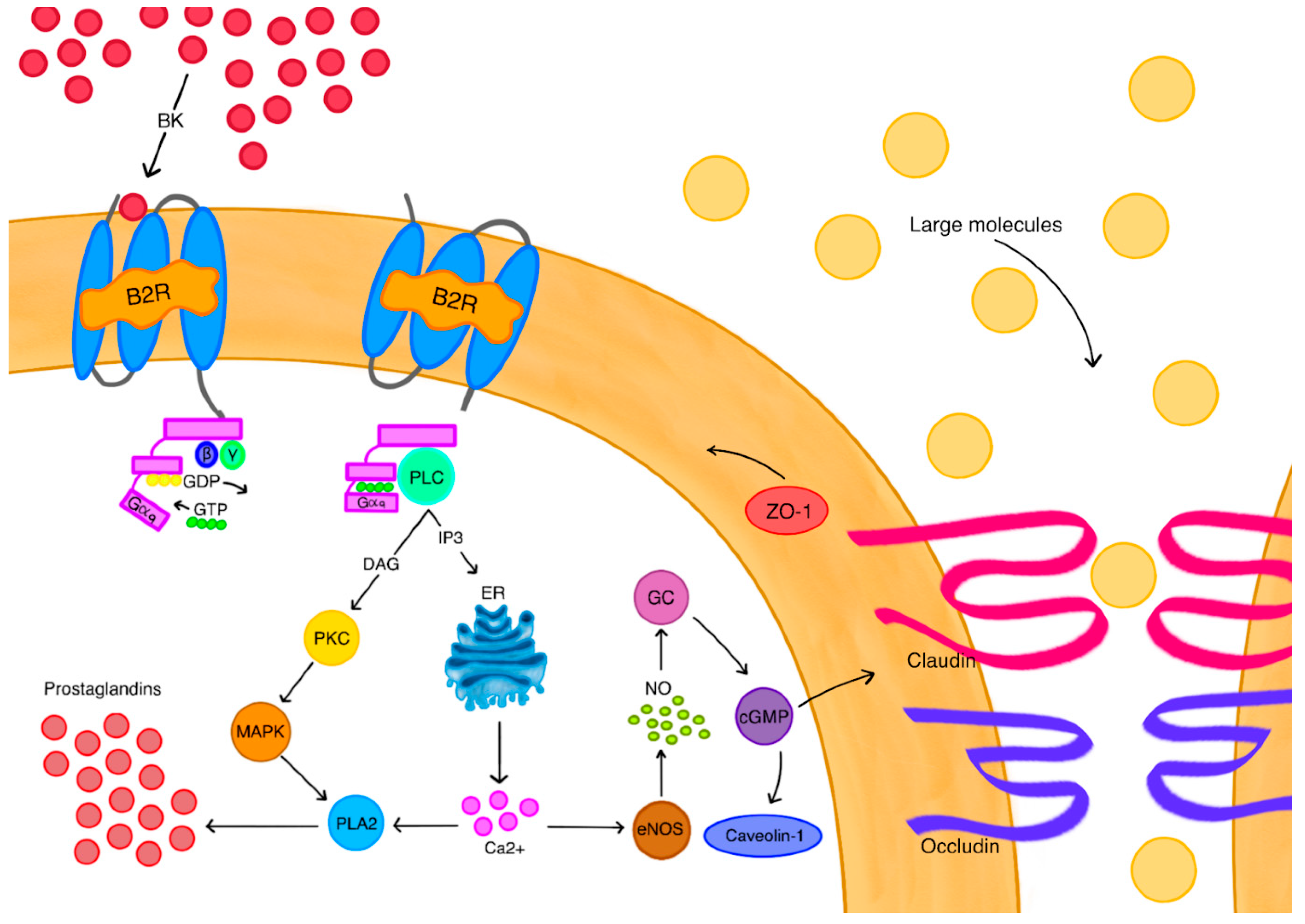
2.7. BBB Disruption
3. Discussion
3.1. Inhibition of Efflux Transporters
3.2. Regional Therapy
4. Conclusions and Future Direction
Author Contributions
Funding
Conflicts of Interest
References
- Lu-Emerson, C.; Chowdhary, S.; Kotecha, R.; Sharma, A.; Odia, Y.; Vaillant, B.; Redfern, C.; Mammoser, A.; Shih, K.; Kesari, S.; et al. A pilot survey into the landscape of neuro-oncology care in the community. Oncologist 2025, 30, oyaf047. [Google Scholar] [CrossRef]
- Smith, E.J.; Naik, A.; Goel, M.; Wen, P.Y.; Lim, M.; Chang, S.M.; Germano, I.M. Adult neuro-oncology trials in the United States over 5 decades: Analysis of trials completion rate to guide the path forward. Neuro-Oncol. Adv. 2024, 6, vdad169. [Google Scholar] [CrossRef]
- Thau, L.; Reddy, V.; Singh, P. Anatomy, central nervous system. In StatPearls—NCBI Bookshelf; StatPearls: Treasure Island, FL, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542179 (accessed on 15 March 2025).
- Cancer Research UK. Brain Tumour Symptoms. Available online: https://www.cancerresearchuk.org/about-cancer/brain-tumours/symptoms (accessed on 15 March 2025).
- Shan, F.Y.; Zhong, D.; Hu, W.; Patel, N.; Fonkem, E.; Feng, D.; Zhang, Y.; Huang, J.H.; Rao, A. Neoplasms of Central Nervous System: A Diagnostic approach. In Neoplasm; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R.; et al. Glioma. Nat. Rev. Dis. Primers 2015, 1, 15017. [Google Scholar] [CrossRef]
- Maher, E.A.; Furnari, F.B.; Bachoo, R.M.; Rowitch, D.H.; Louis, D.N.; Cavenee, W.K.; DePinho, R.A. Malignant glioma: Genetics and biology of a grave matter. Genes Dev. 2001, 15, 1311–1333. [Google Scholar] [CrossRef]
- Bian, W.; Khayal, I.S.; Lupo, J.M.; McGue, C.; Vandenberg, S.; Lamborn, K.R.; Chang, S.M.; Cha, S.; Nelson, S.J. Multiparametric characterization of Grade 2 glioma subtypes using magnetic resonance spectroscopic, perfusion, and diffusion imaging. Transl. Oncol. 2009, 2, 271–280. [Google Scholar] [CrossRef]
- Jo, J.; Schiff, D. Current considerations in the treatment of Grade 3 Gliomas. Curr. Treat. Options Oncol. 2022, 23, 1219–1232. [Google Scholar] [CrossRef]
- Mair, M.J.; Geurts, M.; Van Den Bent, M.J.; Berghoff, A.S. A basic review on systemic treatment options in WHO grade II–III gliomas. Cancer Treat. Rev. 2020, 92, 102124. [Google Scholar] [CrossRef]
- Gallego, O. Nonsurgical treatment of recurrent glioblastoma. Curr. Oncol. 2015, 22, 273–281. [Google Scholar] [CrossRef]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, M.A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of resistance and current treatment options for glioblastoma multiforme (GBM). Cancers 2023, 15, 2116. [Google Scholar] [CrossRef]
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in Glioblastoma therapy: An update on current approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro-Oncology 2018, 20 (Suppl. S4), iv1–iv86. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Tran, B.; Rosenthal, M.A. Survival comparison between glioblastoma multiforme and other incurable cancers. J. Clin. Neurosci. 2010, 17, 417–421. [Google Scholar] [CrossRef]
- Mehta, M.; Wen, P.; Nishikawa, R.; Reardon, D.; Peters, K. Critical review of the addition of treating fields (TTFields) to the existing standard of care for newly diagnosed glioblastoma patients. Crit. Rev. Oncol. Hematol. 2017, 111, 60–65. [Google Scholar] [CrossRef]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Nelson, J.S.; Burchfiel, C.M.; Fekedulegn, D.; Andrew, M.E. Potential risk factors for incident glioblastoma multiforme: The Honolulu Heart Program and Honolulu-Asia Aging Study. J. Neurooncol. 2012, 109, 315–321. [Google Scholar] [CrossRef]
- Grunert, M.; Kassubek, R.; Danz, B.; Klemenz, B.; Hasslacher, S.; Stroh, S.; Schneele, L.; Langhans, J.; Ströbele, S.; Barry, S.E.; et al. Radiation and Brain Tumors: An Overview. Crit. Rev. Oncog. 2018, 23, 119–138. [Google Scholar] [CrossRef]
- Tamimi, A.F.; Juweid, M. Epidemiology and outcome of glioblastoma. In Codon Publications eBooks; Exon Publications: Brisbane, Australia, 2017; pp. 143–153. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Singh, S.; Dey, D.; Barik, D.; Mohapatra, I.; Kim, S.; Sharma, M.; Prasad, S.; Wang, P.; Singh, A.; Singh, G. Glioblastoma at the crossroads: Current understanding and future therapeutic horizons. Signal Transduct. Target. Ther. 2025, 10, 213. [Google Scholar] [CrossRef]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2009, 37, 13–25. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood–brain barrier: An overview. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Savidge, T.C.; Newman, P.; Pothoulakis, C.; Ruhl, A.; Neunlist, M.; Bourreille, A.; Hurst, R.; Sofroniew, M.V. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 2007, 132, 1344–1358. [Google Scholar] [CrossRef]
- Araya, R.; Kudo, M.; Kawano, M.; Ishii, K.; Hashikawa, T.; Iwasato, T.; Itohara, S.; Terasaki, T.; Oohira, A.; Mishina, Y.; et al. BMP signaling through BMPRIA in astrocytes is essential for proper cerebral angiogenesis and formation of the blood–brain-barrier. Mol. Cell. Neurosci. 2008, 38, 417–430. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2005, 7, 41–53. [Google Scholar] [CrossRef]
- Armulik, A.; Mäe, M.; Betsholtz, C. Pericytes and the blood–brain barrier: Recent Advances and Implications for the Delivery of CNS Therapy. Ther. Deliv. 2011, 2, 419–422. [Google Scholar] [CrossRef]
- Brown, L.S.; Foster, C.G.; Courtney, J.-M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and neurovascular function in the healthy and diseased brain. Front. Cell. Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef]
- Liao, K.; Niu, F.; Hu, G.; Buch, S. Morphine-mediated release of astrocyte-derived extracellular vesicle miR-23a induces loss of pericyte coverage at the blood-brain barrier: Implications for neuroinflammation. Front. Cell Dev. Biol. 2022, 10, 984375. [Google Scholar] [CrossRef]
- Jeske, R.; Albo, J.; Marzano, M.; Bejoy, J.; Li, Y. Engineering Brain-Specific Pericytes from Human Pluripotent Stem Cells. Tissue Eng. Part. B Rev. 2020, 26, 367–382. [Google Scholar] [CrossRef]
- Tillie, R.J.H.A.; Theelen, T.L.; Van Kuijk, K.; Temmerman, L.; De Bruijn, J.; Gijbels, M.; Betsholtz, C.; Biessen, E.A.L.; Sluimer, J.C.A. A Switch from Cell-Associated to Soluble PDGF-B Protects against Atherosclerosis, despite Driving Extramedullary Hematopoiesis. Cells 2021, 10, 1746. [Google Scholar] [CrossRef]
- Sengillo, J.D.; Winkler, E.A.; Walker, C.T.; Sullivan, J.S.; Johnson, M.; Zlokovic, B.V. Deficiency in Mural Vascular Cells Coincides with Blood–Brain Barrier Disruption in Alzheimer’s Disease. Brain Pathol. 2012, 23, 303–310. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Breaking through the tight junction barrier. J. Cell Biol. 1993, 123, 1631–1633. [Google Scholar] [CrossRef]
- Gumbiner, B. Structure, biochemistry, and assembly of epithelial tight junctions. AJP Cell Physiol. 1987, 253, C749–C758. [Google Scholar] [CrossRef]
- Luissint, A.-C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.-O. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef]
- Zhao, Y.; Gan, L.; Ren, L.; Lin, Y.; Ma, C.; Lin, X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022, 1788, 147937. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Warren, K.E. Beyond the Blood:Brain Barrier: The Importance of Central Nervous System (CNS) Pharmacokinetics for the Treatment of CNS Tumors, Including Diffuse Intrinsic Pontine Glioma. Front. Oncol. 2018, 8, 239. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Patching, S.G. Glucose transporters at the Blood-Brain Barrier: Function, regulation and gateways for drug delivery. Mol. Neurobiol. 2016, 54, 1046–1077. [Google Scholar] [CrossRef]
- Erdő, F.; Denes, L.; De Lange, E. Age-associated physiological and pathological changes at the blood–brain barrier: A review. J. Cereb. Blood Flow. Metab. 2016, 37, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Hottinger, A.F.; Stupp, R.; Homicsko, K. Standards of care and novel approaches in the management of glioblastoma multiforme. Chin. J. Cancer 2014, 33, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Barani, I.J.; Larson, D.A. Radiation Therapy of Glioblastoma; Cancer Treatment and Research; Springer: Cham, Switzerland, 2014; pp. 49–73. [Google Scholar] [CrossRef]
- Schneider, S.W.; Ludwig, T.; Tatenhorst, L.; Braune, S.; Oberleithner, H.; Senner, V.; Paulus, W. Glioblastoma cells release factors that disrupt blood-brain barrier features. Acta Neuropathol. 2004, 107, 272–276. [Google Scholar] [CrossRef]
- Ishihara, H.; Kubota, H.; Lindberg, R.L.P.; Leppert, D.; Gloor, S.M.; Errede, M.; Virgintino, D.; Fontana, A.; Yonekawa, Y.; Frei, K. Endothelial Cell Barrier Impairment Induced by Glioblastomas and Transforming Growth Factor β2Involves Matrix Metalloproteinases and Tight Junction Proteins. J. Neuropathol. Exp. Neurol. 2008, 67, 435–448. [Google Scholar] [CrossRef]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; De Sampaio Spohr, T.C.L.; Porto-Carreiro, I.; Pereira, C.M.; Balça-Silva, J.; Kahn, S.A.; DosSantos, M.F.; et al. Gliomas and the vascular fragility of the blood brain barrier. Front. Cell. Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology 2017, 20, 184–191. [Google Scholar] [CrossRef]
- Dandy, W.E. Removal of right cerebral hemisphere for certain tumors with hemiplegia. J. Am. Med. Assoc. 1928, 90, 823. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, C.-L.; Liu, C.-M. Drug delivery strategies to enhance the permeability of the blood–brain barrier for treatment of glioma. Drug Des. Dev. Ther. 2015, 9, 2089. [Google Scholar] [CrossRef]
- Lemée, J.-M.; Clavreul, A.; Menei, P. Intratumoral heterogeneity in glioblastoma: Don’t forget the peritumoral brain zone. Neuro-Oncology 2015, 17, 1322–1332. [Google Scholar] [CrossRef]
- Steeg, P.S. The blood–tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 696–714. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2019, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous blood–tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef]
- Banks, W.A. From blood–brain barrier to blood–brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Soria, F.N.; Miguelez, C.; Peñagarikano, O.; Tønnesen, J. Current techniques for investigating the brain extracellular space. Front. Neurosci. 2020, 14, 570750. [Google Scholar] [CrossRef]
- Nicholson, C.; Hrabětová, S. Brain extracellular space: The final frontier of neuroscience. Biophys. J. 2017, 113, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Syková, E.; Nicholson, C. Diffusion in brain extracellular space. Physiol. Rev. 2008, 88, 1277–1340. [Google Scholar] [CrossRef] [PubMed]
- Morrison, P.F.; Dedrick, R.L. Transport of cisplatin in rat brain following microinfusion: An analysis. J. Pharm. Sci. 1986, 75, 120–128. [Google Scholar] [CrossRef]
- Emerich, D.F.; Dean, R.L.; Osborn, C.; Bartus, R.T. The development of the bradykinin agonist labradimil as a means to increase the permeability of the blood-brain barrier. Clin. Pharmacokinet. 2001, 40, 105–123. [Google Scholar] [CrossRef]
- Central Nervous System Tumors Treatment (PDQ®). Cancer.gov. Available online: https://www.cancer.gov/types/brain/hp/adult-brain-treatment-pdq (accessed on 15 March 2025).
- Teraiya, M.; Perreault, H.; Chen, V.C. An overview of glioblastoma multiforme and temozolomide resistance: Can LC-MS-based proteomics reveal the fundamental mechanism of temozolomide resistance? Front. Oncol. 2023, 13, 1166207. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma—A comprehensive review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Li, H.; Wu, Y.; Chen, Y.; Lv, J.; Qu, C.; Mei, T.; Zheng, Y.; Ye, C.; Li, F.; Ge, S.; et al. Overcoming temozolomide resistance in glioma: Recent advances and mechanistic insights. Acta Neuropathol. Commun. 2025, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Strik, H.M.; Buhk, J.H.; Bock, C.; Hoffmann, A.L.; Wrede, A.; Marosi, C.; Kaiser, U.; Christmann, M.; Kaina, B.; Bähr, M. Tegwondo: Development of a novel near-continuous dose-dense temozolomide regimen for the treatment of recurrent brain tumors. J. Clin. Oncol. 2008, 26 (Suppl. S15), 13016. [Google Scholar] [CrossRef]
- Wick, W.; Steinbach, J.P.; Küker, W.M.; Dichgans, J.; Bamberg, M.; Weller, M. One week on/one week off: A novel active regimen of temozolomide for recurrent glioblastoma. Neurology 2004, 62, 2113–2115. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.; Felsberg, J.; Steinbach, J.P.; Herrlinger, U.; Platten, M.; Blaschke, B.; Meyermann, R.; Reifenberger, G.; Weller, M.; Wick, W. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J. Clin. Oncol. 2007, 25, 3357–3361. [Google Scholar] [CrossRef]
- Boockvar, J.A.; Tsiouris, A.J.; Hofstetter, C.P.; Kovanlikaya, I.; Fralin, S.; Kesavabhotla, K.; Seedial, S.M.; Pannullo, S.C.; Schwartz, T.H.; Stieg, P.; et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. J. Neurosurg. 2010, 114, 624–632. [Google Scholar] [CrossRef]
- Zünkeler, B.; Carson, R.E.; Olson, J.; Blasberg, R.G.; Devroom, H.; Lutz, R.J.; Saris, S.C.; Wright, D.C.; Kammerer, W.; Patronas, N.J.; et al. Quantification and pharmacokinetics of blood-brain barrier disruption in humans. J. Neurosurg. 1996, 85, 1056–1065. [Google Scholar] [CrossRef]
- Giantini-Larsen, A.M.; Pandey, A.; Garton, A.L.A.; Rampichini, M.; Winston, G.; Goldberg, J.L.; Magge, R.; Stieg, P.E.; Souweidane, M.M.; Ramakrishna, R. Therapeutic manipulation and bypass of the blood brain barrier: Powerful tools in glioma treatment. Neuro-Oncol. Adv. 2025, 7, vdae201. [Google Scholar] [CrossRef]
- Ferreira, C.; Ferreira, M.Y.; Singh, F.; Wong, T.; Bokil, S.; Massimo, S.; Cavallaro, J.; Albers, O.; D’Amico, R.; Langer, D.; et al. Superselective intra-arterial cerebral infusion of chemotherapeutics after osmotic blood–brain barrier disruption in newly diagnosed or recurrent glioblastoma: Technical insights and clinical outcomes from a single-center experience. J. NeuroInterventional Surg. 2024, 16, 3382. [Google Scholar] [CrossRef] [PubMed]
- Ting, W.-H.; Chen, H.-H.; Wei, M.-C.; Sun, H.-D.; Hsiao, S.-M. Intraperitoneal Chemotherapy without Bevacizumab versus Intravenous Chemotherapy with Bevacizumab as the Frontline Adjuvant Therapy in Advanced Ovarian Cancer. Cancers 2024, 16, 3382. [Google Scholar] [CrossRef] [PubMed]
- Kazazi-Hyseni, F.; Beijnen, J.H.; Schellens, J.H. Bevacizumab. Oncologist 2010, 15, 819–825. [Google Scholar] [CrossRef]
- Ningaraj, N.S.; Rao, M.K.; Black, K.L. Adenosine 5′-triphosphate-sensitive potassium channel-mediated Blood-Brain tumor barrier permeability increase in a rat brain tumor model. Cancer Res. 2003, 63, 8899–8911. [Google Scholar] [PubMed]
- Gu, Y.-T.; Xue, Y.-X.; Zhang, H.; Li, Y.; Liang, X.-Y. Adenosine 5′-Triphosphate-Sensitive potassium channel activator induces the Up-Regulation of caveolin-1 expression in a rat brain tumor model. Cell. Mol. Neurobiol. 2011, 31, 629–634. [Google Scholar] [CrossRef]
- Gu, Y.-T.; Xue, Y.-X.; Wang, Y.-F.; Wang, J.-H.; Chen, X.; ShangGuan, Q.-R.; Lian, Y.; Zhong, L.; Meng, Y.-N. Minoxidil sulfate induced the increase in blood–brain tumor barrier permeability through ROS/RhoA/PI3K/PKB signaling pathway. Neuropharmacology 2013, 75, 407–415. [Google Scholar] [CrossRef]
- Ningaraj, N.S.; Rao, M.; Hashizume, K.; Asotra, K.; Black, K.L. Regulation of Blood-Brain tumor barrier permeability by Calcium-Activated potassium channels. J. Pharmacol. Exp. Ther. 2002, 301, 838–851. [Google Scholar] [CrossRef]
- Ningaraj, N.S.; Rao, M.; Black, K.L. Calcium-dependent potassium channels as a target protein for modulation of the blood-brain tumor barrier. Drug News Perspect. 2003, 16, 291. [Google Scholar] [CrossRef]
- Hu, J.; Yuan, X.; Ko, M.K.; Yin, D.; Sacapano, M.R.; Wang, X.; Konda, B.M.; Espinoza, A.; Prosolovich, K.; Ong, J.M.; et al. Calcium-activated potassium channels mediated blood-brain tumor barrier opening in a rat metastatic brain tumor model. Mol. Cancer 2007, 6, 22. [Google Scholar] [CrossRef]
- Ningaraj, N.S.; Sankpal, U.T.; Khaitan, D.; Meister, E.A.; Vats, T.S. Modulation of KCa channels increases anticancer drug delivery to brain tumors and prolongs survival in xenograft model. Cancer Biol. Ther. 2009, 8, 1924–1933. [Google Scholar] [CrossRef][Green Version]
- Cai, R.-P.; Xue, Y.-X.; Huang, J.; Wang, J.-H.; Wang, J.-H.; Zhao, S.-Y.; Guan, T.-T.; Zhang, Z.; Gu, Y.-T. NS1619 regulates the expression of caveolin-1 protein in a time-dependent manner via ROS/PI3K/PKB/FoxO1 signaling pathway in brain tumor microvascular endothelial cells. J. Neurol. Sci. 2016, 369, 109–118. [Google Scholar] [CrossRef]
- Perry, V.L.; Lenz, F.A. Ablative therapy for movement disorders. Thalamotomy for Parkinson’s Disease. Neurosurg. Clin. N. Am. 1998, 9, 313–327. [Google Scholar] [CrossRef]
- Fidler, I.J.; Liotta, L.A. The blood-brain barrier and cancer metastasis to the brain. Cancer Treat. Res. 2002, 114, 129–148. [Google Scholar]
- Inamura, T.; Black, K.L. Bradykinin selectively opens Blood-Tumor barrier in experimental brain tumors. J. Cereb. Blood Flow. Metab. 1994, 14, 862–870. [Google Scholar] [CrossRef]
- Raymond, J.J.; Robertson, D.M.; Dinsdale, H.B. Pharmacological modification of bradykinin induced breakdown of the blood-brain barrier. Can. J. Neurol. Sci. 1986, 13, 214–220. [Google Scholar] [CrossRef]
- Su, B.; Wang, R.; Xie, Z.; Ruan, H.; Li, J.; Xie, C.; Lu, W.; Wang, J.; Wang, D.; Liu, M. Effect of Retro-Inverso isomer of bradykinin on Size-Dependent penetration of Blood–Brain tumor barrier. Small 2018, 14, 1702331. [Google Scholar] [CrossRef]
- Bartus, R.T.; Elliott, P.; Hayward, N.; Dean, R.; McEwen, E.L.; Fisher, S.K. Permeability of the blood brain barrier by the bradykinin agonist, RMP-7: Evidence for a sensitive, auto-regulated, receptor-mediated system. Immunopharmacology 1996, 33, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Chen, Z.; Liu, Y.; Black, K.L. Overexpression of bradykinin type 2 receptors on glioma cells enhances bradykinin-mediated blood–brain tumor barrier permeability increase. Neurol. Res. 2002, 24, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hashizume, K.; Chen, Z.; Samoto, K.; Ningaraj, N.; Asotra, K.; Black, K.L. Correlation between bradykinin-induced blood–tumor barrier permeability and B2 receptor expression in experimental brain tumors. Neurol. Res. 2001, 23, 379–387. [Google Scholar] [CrossRef]
- Zhao, Y.; Xue, Y.; Liu, Y.; Fu, W.; Jiang, N.; An, P.; Wang, P.; Yang, Z.; Wang, Y. Study of correlation between expression of bradykinin B2 receptor and pathological grade in human gliomas. Br. J. Neurosurg. 2005, 19, 322–326. [Google Scholar] [CrossRef]
- Côté, J.; Savard, M.; Bovenzi, V.; Dubuc, C.; Tremblay, L.; Tsanaclis, A.M.; Fortin, D.; Lepage, M.; Gobeil, F. Selective tumor blood–brain barrier opening with the kinin B2 receptor agonist [Phe8ψ(CH2NH)Arg9]-BK in a F98 glioma rat model: An MRI study. Neuropeptides 2010, 44, 177–185. [Google Scholar] [CrossRef]
- Liu, L.; Xue, Y.; Liu, Y.; Wang, Y. Bradykinin increases blood–tumor barrier permeability by down-regulating the expression levels of ZO-1, occludin, and claudin-5 and rearranging actin cytoskeleton. J. Neurosci. Res. 2008, 86, 1153–1168. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Hsu, J.-W.; Lin, H.-Y.; Lai, S.-W.; Huang, B.-R.; Tsai, C.-F.; Lu, D.-Y. Bradykinin B1 receptor contributes to interleukin-8 production and glioblastoma migration through interaction of STAT3 and SP-1. Neuropharmacology 2018, 144, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Arvanitis, C.D.; Alexander, P.M.; McDannold, N. Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev. 2014, 72, 94–109. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.; Hynynen, K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol. 2004, 30, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Maroufi, S.F.; Fallahi, M.S.; Maroufi, S.P.; Sheehan, J.P. Focused ultrasound blood-brain barrier disruption in high-grade gliomas: Scoping review of clinical studies. J. Clin. Neurosci. 2024, 128, 110786. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef]
- Ter Linden, E.; Abels, E.R.; Van Solinge, T.S.; Neefjes, J.; Broekman, M.L.D. Overcoming barriers in Glioblastoma—Advances in drug delivery strategies. Cells 2024, 13, 998. [Google Scholar] [CrossRef]
- Borst, P.; Schinkel, A.H. P-glycoprotein ABCB1: A major player in drug handling by mammals. J. Clin. Investig. 2013, 123, 4131–4133. [Google Scholar] [CrossRef]
- Engle, K.; Kumar, G. Cancer multidrug-resistance reversal by ABCB1 inhibition: A recent update. Eur. J. Med. Chem. 2022, 239, 114542. [Google Scholar] [CrossRef]
- Crowley, E.; McDevitt, C.A.; Callaghan, R. Generating inhibitors of P-Glycoprotein: Where to, now? In Multi-Drug Resistance in Cancer; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; pp. 405–432. [Google Scholar] [CrossRef]
- Thomas, H.; Coley, H.M. Overcoming multidrug resistance in cancer: An update on the clinical strategy of inhibiting P-Glycoprotein. Cancer Control. 2003, 10, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Planting, A.S.T.; Sonneveld, P.; Van Der Gaast, A.; Sparreboom, A.; Van Der Burg, M.E.L.; Luyten, G.P.M.; De Leeuw, K.; De Boer-Dennert, M.; Wissel, P.S.; Jewell, R.C.; et al. A phase I and pharmacologic study of the MDR converter GF120918 in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2004, 55, 91–99. [Google Scholar] [CrossRef]
- Loya, J.; Zhang, C.; Cox, E.; Achrol, A.S.; Kesari, S. Biological intratumoral therapy for the high-grade glioma part I: Intratumoral delivery and immunotoxins. CNS Oncol. 2019, 8, CNS38. [Google Scholar] [CrossRef]
- Garfield, J.; Dayan, A.D. Postoperative intracavitary chemotherapy of malignant gliomas. J. Neurosurg. 1973, 39, 315–322. [Google Scholar] [CrossRef]
- Sendelbeck, S.L.; Urquhart, J. Spatial distribution of dopamine, methotrexate and antipyrine during continuous intracerebral microperfusion. Brain Res. 1985, 328, 251–258. [Google Scholar] [CrossRef]
- Zhou, Z.; Singh, R.; Souweidane, M.M. Convection-Enhanced delivery for diffuse intrinsic pontine glioma treatment. Curr. Neuropharmacol. 2016, 15, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.M.; Laske, D.W.; Morrison, P.F.; Bankiewicz, K.S.; Oldfield, E.H. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J. Neurosurg. 1995, 82, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Akabani, G.; Friedman, A.H.; Bigner, D.; Kunwar, S.; Berger, M.S.; Bankiewicz, K.S. Comparison of intratumoral bolus injection and convection-enhanced delivery of radiolabeled antitenascin monoclonal antibodies. Neurosurg. Focus 2006, 20, E14. [Google Scholar] [CrossRef]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro-Oncol. 2010, 12, 871–881. [Google Scholar] [CrossRef]
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-enhanced delivery in glioblastoma: A review of preclinical and clinical studies. J. Neurosurg. 2016, 126, 191–200. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brewer, C.; Barnett, G.H.; Mohammadi, A.M. First-in-human investigation of skull implanted ultrasound for blood-brain barrier opening in glioblastoma patients. J. Neurooncol. 2022, 159, 101–112. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem. Biophys. Res. Commun. 2006, 340, 1085–1090. [Google Scholar] [CrossRef]
- Ningaraj, N.S.; Rao, M.K.; Black, K.L. Adenosine 2A receptor agonist enhances delivery of chemotherapeutic agent to rat brain tumors via blood-brain tumor barrier modulation. Clin. Cancer Res. 2003, 9, 5448–5454. [Google Scholar]
- St-Coeur, P.D.; Poitras, J.J.; Cuperlovic-Culf, M.; Touaibia, M.; Morin, P., Jr. Investigating a signature of temozolomide resistance in GBM cell lines using metabolomics. J. Neurooncol. 2015, 125, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, K.; Jażdżewska, A.; Kozłowski, J.; Zacny, A.; Lorenc, T.; Olejarz, W. Revolutionizing Glioblastoma Treatment: A Comprehensive Overview of Modern Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 5774. [Google Scholar] [CrossRef]
- Ntafoulis, I.; Koolen, S.L.W.; Leenstra, S.; Lamfers, M.L.M. Drug Repurposing, a Fast-Track Approach to Develop Effective Treatments for Glioblastoma. Cancers 2022, 14, 3705. [Google Scholar] [CrossRef]
- Pouyan, A.; Ghorbanlo, M.; Eslami, M.; Jahanshahi, M.; Ziaei, E.; Salami, A.; Mokhtari, K.; Shahpasand, K.; Farahani, N.; Meybodi, T.E.; et al. Glioblastoma multiforme: Insights into pathogenesis, key signaling pathways, and therapeutic strategies. Mol. Cancer 2025, 24, 58. [Google Scholar] [CrossRef]
- Vaz-Salgado, M.A.; Villamayor, M.; Albarrán, V.; Alía, V.; Sotoca, P.; Chamorro, J.; Rosero, D.; Barrill, A.M.; Martín, M.; Fernandez, E.; et al. Recurrent Glioblastoma: A Review of the Treatment Options. Cancers 2023, 15, 4279. [Google Scholar] [CrossRef]
- Dey, S.; Mathur, P.; Mukherjee, S.; Chowdhury, R.; Majumder, S.; Roy, A.; Chowdhury, S. Repurposing of CNS accumulating drugs Gemfibrozil and Doxylamine for enhanced sensitization of glioblastoma cells through modulation of autophagy. Sci. Rep. 2025, 15, 20560. [Google Scholar] [CrossRef]
- Meng, J.; Agrahari, V.; Youm, I. Advances in Targeted Drug Delivery Approaches for the Central Nervous System Tumors: The Inspiration of Nanobiotechnology. J. Neuroimmune Pharmacol. 2017, 12, 84–98. [Google Scholar] [CrossRef]
- Beylerli, O.; Gareev, I.; Musaev, E.; Roumiantsev, S.; Chekhonin, V.; Ahmad, A.; Chao, Y.; Yang, G. New approaches to targeted drug therapy of intracranial tumors. Cell Death Discov. 2025, 11, 111. [Google Scholar] [CrossRef]
- Khasraw, M.; Fujita, Y.; Lee-Chang, C.; Balyasnikova, I.V.; Najem, H.; Heimberger, A.B. New Approaches to Glioblastoma. Annu. Rev. Med. 2022, 73, 279–292. [Google Scholar] [CrossRef]
- Sevastre, A.S.; Costachi, A.; Tataranu, L.G.; Brandusa, C.; Artene, S.A.; Stovicek, O.; Alexandru, O.; Danoiu, S.; Sfredel, V.; Dricu, A. Glioblastoma pharmacotherapy: A multifaceted perspective of conventional and emerging treatments (Review). Exp. Ther. Med. 2021, 22, 1408. [Google Scholar] [CrossRef]
- Majc, B.; Novak, M.; Kopitar-Jerala, N.; Jewett, A.; Breznik, B. Immunotherapy of Glioblastoma: Current Strategies and Challenges in Tumor Model Development. Cells 2021, 10, 265. [Google Scholar] [CrossRef]
- Strika, Z.; Petković, K.; Likić, R. Effectiveness and Safety of mRNA Vaccines in the Therapy of Glioblastoma. J. Pers. Med. 2024, 14, 993. [Google Scholar] [CrossRef]
- Kiel, K.; Piranlioglu, R.; Godlewski, J.; Bronisz, A. Harnessing immunotherapy: Cancer vaccines as novel therapeutic strategies for brain tumor. Front. Immunol. 2025, 16, 1588081. [Google Scholar] [CrossRef] [PubMed]
- Kardani, K.; Sanchez Gil, J.; Rabkin, S.D. Oncolytic herpes simplex viruses for the treatment of glioma and targeting glioblastoma stem-like cells. Front. Cell Infect. Microbiol. 2023, 13, 1206111. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Peláez, M.A.; Maradei Anaya, S.J.; Bedoya-Rodríguez, I.J.; González-Ipuz, K.G.; Vera-Palacios, D.; Buitrago, I.V.; Castellanos, J.E.; Velandia-Romero, M.L. Zika Virus: A Neurotropic Warrior against High-Grade Gliomas-Unveiling Its Potential for Oncolytic Virotherapy. Viruses 2024, 16, 561. [Google Scholar] [CrossRef] [PubMed]
- Francipane, M.G.; Douradinha, B.; Chinnici, C.M.; Russelli, G.; Conaldi, P.G.; Iannolo, G. Zika Virus: A New Therapeutic Candidate for Glioblastoma Treatment. Int. J. Mol. Sci. 2021, 22, 10996. [Google Scholar] [CrossRef]
| No | Key Challenges in CNS Drug Delivery | Description | References |
|---|---|---|---|
| 1 | Blood–Brain Barrier (BBB) | The BBB, a protective barrier, restricts the entry of many systemic drugs into the brain, significantly limiting therapeutic options. | [118] |
| 2 | Tumor Infiltration | GBM infiltrates the brain extensively, making complete surgical removal difficult and requiring systemic treatments to reach microscopic tumor cells. | [119] |
| 3 | Tumor Heterogeneity | GBM is highly heterogeneous, with different cell populations having varying responses to treatments, leading to drug resistance. | [120] |
| 4 | Drug Resistance | Mechanisms like the DNA repair enzyme O6-methylguanine methyltransferase (MGMT) can counteract the effects of chemotherapy, reducing drug efficacy. | [121] |
| 5 | Targeted Drug Delivery | Developing methods to increase drug concentrations at the tumor site while minimizing systemic side effects is key. | [122] |
| 6 | Modifying Drug Properties | Researchers are investigating drugs with better lipophilicity and lower molecular weight to enhance BBB penetration. | [123] |
| 7 | Drug Delivery Systems | Utilizing Nanotechnology, such as nanoparticles, can encapsulate drugs, protecting them from degradation and aiding in crossing the BBB. | [124] |
| 8 | Disrupting the BBB | Methods for temporarily disrupting the BBB, like using focused ultrasound, can increase drug delivery to the tumor. | [125] |
| 9 | Targeting Tumor-Specific Transporters | Some tumors in the brain have upregulated transporter proteins that can be exploited for targeted drug delivery. | [126] |
| 10 | Current and Emerging Treatments | Temozolomide (TMZ) Tumor-Treating Fields (TTFields) Molecularly Targeted Therapies Immunotherapy Drug Repurposing | [127,128,129,130,131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Tylutki, J.; Tylutki, M.; Leś, D.; Aebisher, D. Navigating the Central Nervous System (CNS): A Pharmacokinetic Approach to the Treatment of CNS Tumors, Glioblastoma Multiforme (GBM), in Particular. Int. J. Mol. Sci. 2025, 26, 9418. https://doi.org/10.3390/ijms26199418
Bartusik-Aebisher D, Tylutki J, Tylutki M, Leś D, Aebisher D. Navigating the Central Nervous System (CNS): A Pharmacokinetic Approach to the Treatment of CNS Tumors, Glioblastoma Multiforme (GBM), in Particular. International Journal of Molecular Sciences. 2025; 26(19):9418. https://doi.org/10.3390/ijms26199418
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Jakub Tylutki, Michał Tylutki, Dominika Leś, and David Aebisher. 2025. "Navigating the Central Nervous System (CNS): A Pharmacokinetic Approach to the Treatment of CNS Tumors, Glioblastoma Multiforme (GBM), in Particular" International Journal of Molecular Sciences 26, no. 19: 9418. https://doi.org/10.3390/ijms26199418
APA StyleBartusik-Aebisher, D., Tylutki, J., Tylutki, M., Leś, D., & Aebisher, D. (2025). Navigating the Central Nervous System (CNS): A Pharmacokinetic Approach to the Treatment of CNS Tumors, Glioblastoma Multiforme (GBM), in Particular. International Journal of Molecular Sciences, 26(19), 9418. https://doi.org/10.3390/ijms26199418









