The Photodynamic Antibacterial Potential of New Tetracationic Zinc(II) Phthalocyanines Bearing 4-((Diethylmethylammonium)methyl)phenoxy Substituents
Abstract
1. Introduction
2. Results and Discussion
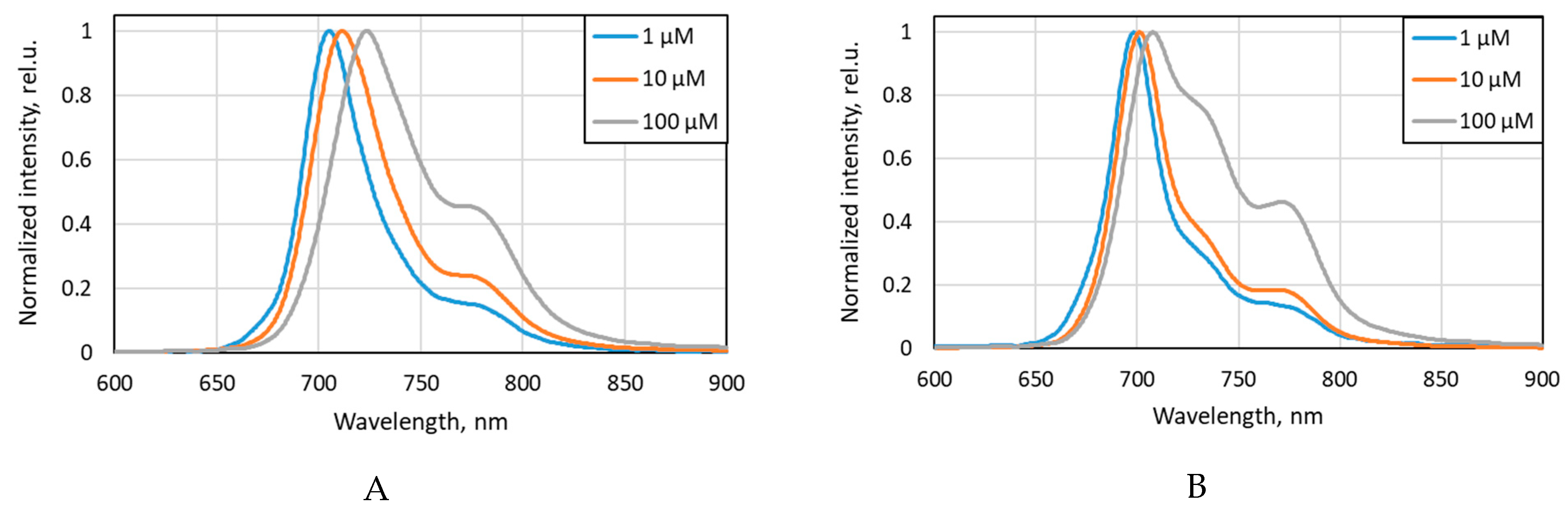
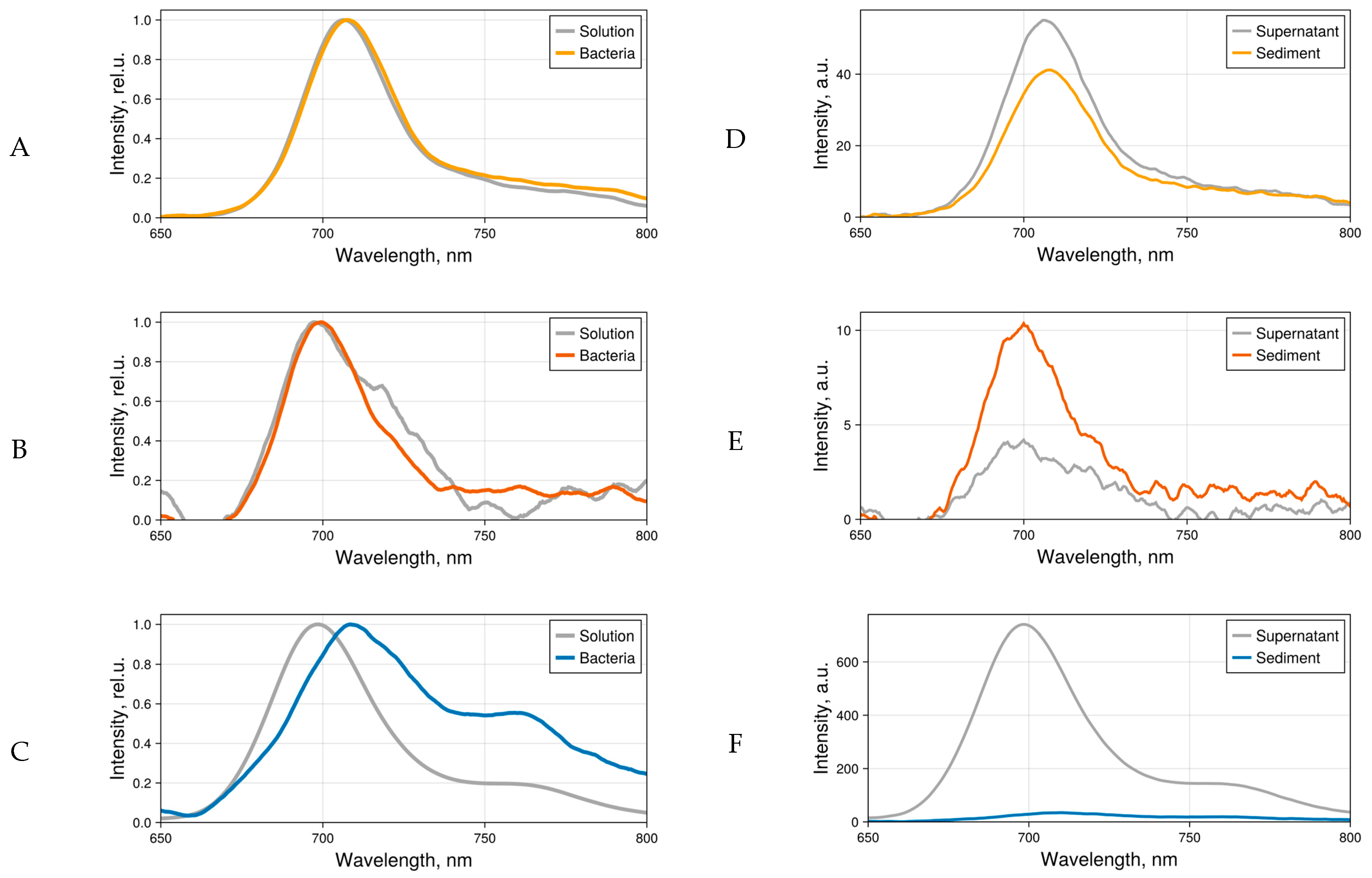
2.1. Dye Fluorescence
2.2. Fluorescence Microscopy Images
2.3. Influence of Polycationic Phthalocyanines on Zeta Potential of Bacterial Cells
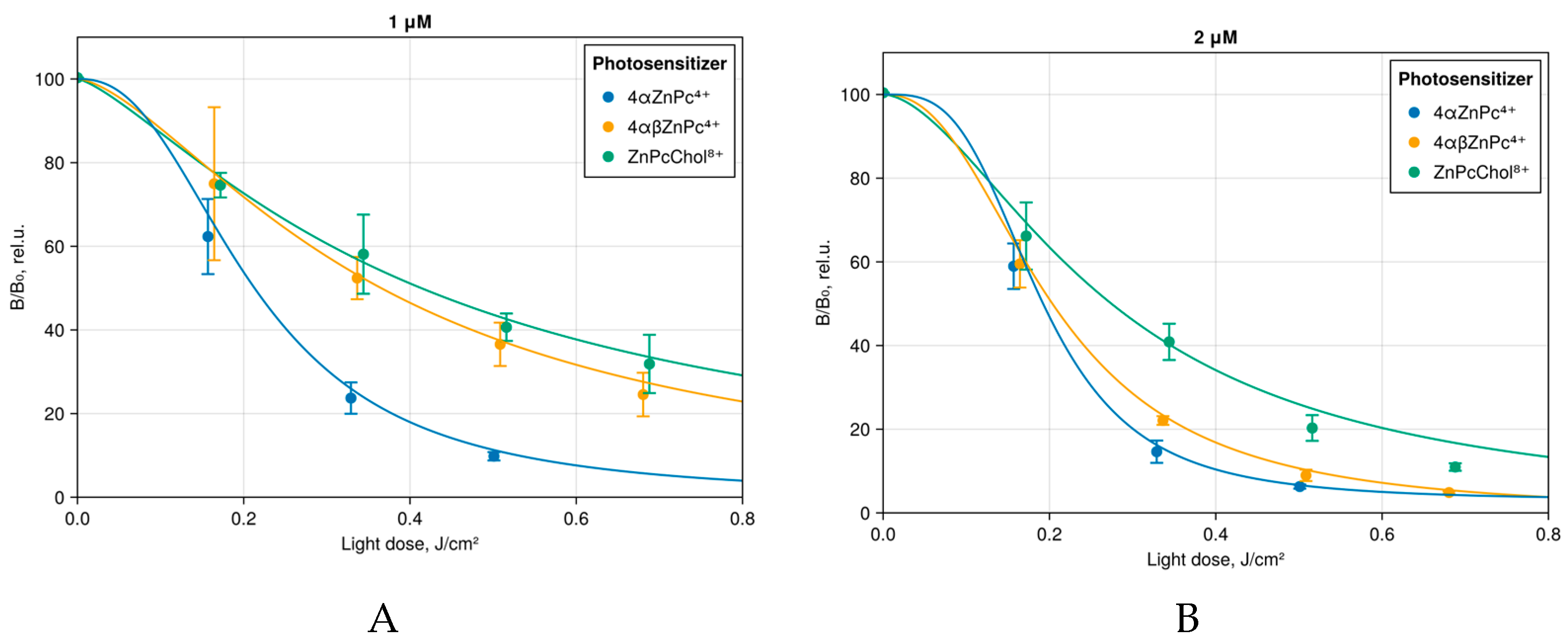
2.4. Testing the Antibacterial Photodynamic Activity of Phthalocyanine Derivatives
3. Materials and Methods
3.1. Phthalocyanine Derivatives
3.2. Fluorescence
3.3. Biosensor Escherichia coli
3.4. Fluorescence Microscopy of Biosensor E. coli with the Studied Photosensitizers
3.5. Zeta Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Niño-Vega, G.A.; Ortiz-Ramírez, J.A.; López-Romero, E. Novel Antibacterial Approaches and Therapeutic Strategies. Antibiotics 2025, 14, 404. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 2023. [Google Scholar] [CrossRef]
- MacDermott-Opeskin, H.I.; Gupta, V.; O’Mara, M.L. Lipid-Mediated Antimicrobial Resistance: A Phantom Menace or a New Hope? Biophys. Rev. 2022, 14, 145–162. [Google Scholar] [CrossRef]
- Sun, J.; Rutherford, S.T.; Silhavy, T.J.; Huang, K.C. Physical Properties of the Bacterial Outer Membrane. Nat. Rev. Microbiol. 2022, 20, 236–248. [Google Scholar] [CrossRef]
- Silipo, A. Lipid A Structure. In Bacterial Lipopolysaccharides; Springer: Vienna, Austria, 2011; pp. 1–20. [Google Scholar]
- Brüssow, H. The Antibiotic Resistance Crisis and the Development of New Antibiotics. Microb. Biotechnol. 2024, 17, e14510. [Google Scholar] [CrossRef]
- Lang, M.; Carvalho, A.; Baharoglu, Z.; Mazel, D. Aminoglycoside uptake, stress, and potentiation in Gram-negative bacteria: New therapies with old molecules. Microbiol. Mol. Biol. Rev. 2023, 87, e0003622. [Google Scholar] [CrossRef]
- Ude, J.; Tripathi, V.; Buyck, J.M.; Söderholm, S.; Cunrath, O.; Fanous, J.; Claudi, B.; Egli, A.; Schleberger, C.; Hiller, S.; et al. Outer Membrane Permeability: Antimicrobials and Diverse Nutrients Bypass Porins in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2021, 118, e2107644118. [Google Scholar] [CrossRef] [PubMed]
- Kholina, E.G.; Kovalenko, I.B.; Strakhovskaya, M.G. Investigation of Porin-Dependent Translocation of Methylene Blue and Gentamicin through the Outer Membrane of Gram-Negative Bacteria Using Molecular Dynamics Methods. Mathematical Biol. Bioinform. 2025, 20, 71–82. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are We Afraid of the Light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as Medicinal Photosensitizers: Developments in the Last Five Years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Kollar, J.; Machacek, M.; Halaskova, M.; Lenco, J.; Kucera, R.; Demuth, J.; Rohlickova, M.; Hasonova, K.; Miletin, M.; Novakova, V.; et al. Cationic Versus Anionic Phthalocyanines for Photodynamic Therapy: What a Difference the Charge Makes. J. Med. Chem. 2020, 63, 7616–7632. [Google Scholar] [CrossRef]
- Mantareva, V. Phthalocyanine Photosensitizers Towards Drug-Resistant Pathogens; BP International: Kowloon, Hong Kong, 2025; ISBN 978-93-499-7020-5. [Google Scholar]
- Pereira, G.F.M.; Tasso, T.T. From cuvette to cells: How the central metal ion modulates the properties of phthalocyanines and porphyrazines as photosensitizers. Inorg. Chim. Acta 2021, 519, 120271. [Google Scholar] [CrossRef]
- Gorman, A.; Killoran, J.; O’Shea, C.; Kenna, T.; Gallagher, W.M.; O’Shea, D.F. In Vitro Demonstration of the Heavy-Atom Effect for Photodynamic Therapy. J. Am. Chem. Soc. 2004, 126, 10619–10631. [Google Scholar] [CrossRef] [PubMed]
- Amemori, S.; Sasaki, Y.; Yanai, N.; Kimizuka, N. Near-infrared-to-visible photon upconversion sensitized by a metal complex with spin-forbidden yet strong S0–T1 absorption. J. Am. Chem. Soc. 2016, 138, 8702–8705. [Google Scholar] [CrossRef] [PubMed]
- Strakhovskaya, M.G.; Antonenko, Y.N.; Pashkovskaya, A.A.; Kotova, E.A.; Kireev, V.; Zhukhovitsky, V.G.; Kuznetsova, N.A.; Yuzhakova, O.A.; Negrimovsky, V.M.; Rubin, A.B. Electrostatic binding of substituted metal phthalocyanines to enterobacterial cells: Its role in photodynamic inactivation. Biochem. Mosc. 2009, 74, 1305–1314. [Google Scholar] [CrossRef]
- Muehler, D.; Brandl, E.; Hiller, K.-A.; Cieplik, F.; Maisch, T. Membrane Damage as Mechanism of Photodynamic Inactivation Using Methylene Blue and TMPyP in Escherichia Coli and Staphylococcus Aureus. Photochem. Photobiol. Sci. 2022, 21, 209–220. [Google Scholar] [CrossRef]
- Anandan, A.; Vrielink, A. Structure and function of lipid A-modifying enzymes. Ann. N. Y. Acad. Sci. 2020, 1459, 19–37. [Google Scholar] [CrossRef]
- Kelbauskas, L.; Dietel, W. Internalization of Aggregated Photosensitizers by Tumor Cells: Subcellular Time-Resolved Fluorescence Spectroscopy on Derivatives of Pyropheophorbide-a Ethers and Chlorin E6 under Femtosecond One- and Two-Photon Excitation. Photochem. Photobiol. 2002, 76, 686. [Google Scholar] [CrossRef]
- Halaskova, M.; Kostelansky, F.; Demuth, J.; Hlbocanova, I.; Miletin, M.; Zimcik, P.; Machacek, M.; Novakova, V. Amphiphilic Cationic Phthalocyanines for Photodynamic Therapy of Cancer. ChemPlusChem 2022, 87, e202200133. [Google Scholar] [CrossRef] [PubMed]
- Makarov, D.A.; Yuzhakova, O.A.; Slivka, L.K.; Kuznetsova, N.A.; Negrimovsky, V.M.; Kaliya, O.L.; Lukyanets, E.A. Cationic Zn and Al Phthalocyanines: Synthesis, Spectroscopy and Photosensitizing Properties. J. Porphyr. Phthaloc 2007, 11, 586–595. [Google Scholar] [CrossRef]
- Bunin, D.A.; Akasov, R.A.; Martynov, A.G.; Stepanova, M.P.; Monich, S.V.; Tsivadze, A.Y.u.; Gorbunova, Y.G. Pivotal Role of the Intracellular Microenvironment in the High Photodynamic Activity of Cationic Phthalocyanines. J. Med. Chem. 2025, 68, 658–673. [Google Scholar] [CrossRef]
- Bunin, D.A.; Martynov, A.G.; Safonova, E.A.; Tsivadze, A.Y.u.; Gorbunova, Y.G. Robust Route toward Cationic Phthalocyanines through Reductive Amination. Dye. Pigment. 2022, 207, 110768. [Google Scholar] [CrossRef]
- Bunin, D.A.; Martynov, A.G.; Gvozdev, D.A.; Gorbunova, Y.G. Phthalocyanine aggregates in the photodynamic therapy: Dogmas, controversies, and future prospects. Biophys. Rev. 2023, 15, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Villagrán, M.; Paulus, L.; Leger, D.Y.; Therrien, B.; Liagre, B. Dimethyl Sulfoxide: A Bio-Friendly or Bio-Hazard Chemical? The Effect of DMSO in Human Fibroblast-like Synoviocytes. Molecules 2022, 27, 4472. [Google Scholar] [CrossRef] [PubMed]
- Meerovich, G.A.; Romanishkin, I.D.; Akhlyustina, E.V.; Loschenov, V.B. On the Dependence of Fluorescence Intensity on the Concentration of Photosensitizer Solutions. Biomed. Photonics 2025, 14, 21–26. [Google Scholar] [CrossRef]
- Ekineker, G.; Göksel, M. Synthesis of both peripheral and non-peripheral substituted metal-free phthalocyanines and characterization. Tetrahedron 2020, 76, 130878. [Google Scholar] [CrossRef]
- Liu, D.; Li, L.; Chen, J.; Chen, Z.; Jiang, L.; Yuan, C.; Huang, M. Dissociation of zinc phthalocyanine aggregation on bacterial surface is key for photodynamic antimicrobial effect. J. Porphyr. Phthalocyanines 2018, 22, 925–934. [Google Scholar] [CrossRef]
- Meerovich, G.A.; Akhlyustina, E.V.; Romanishkin, I.D.; Makarova, E.A.; Tiganova, I.G.; Zhukhovitsky, V.G.; Kholina, E.G.; Kovalenko, I.B.; Romanova, Y.M.; Loschenov, V.B.; et al. Photodynamic Inactivation of Bacteria: Why It Is Not Enough to Excite a Photosensitizer. Photodiagnosis Photodyn. Ther. 2023, 44, 103853. [Google Scholar] [CrossRef]
- Sakalauskienė, G.V.; Malcienė, L.; Stankevičius, E.; Radzevičienė, A. Unseen Enemy: Mechanisms of Multidrug Antimicrobial Resistance in Gram-Negative ESKAPE Pathogens. Antibiotics 2025, 14, 63. [Google Scholar] [CrossRef]
- Strakhovskaya, M.G.; Zarubina, A.; Zhukhovitskii, V.G.; Mironov, A.F.; Rubin, A.B. Bioluminescent genetically transformed bacteria as a new effective tool for testing photosensitization activity. Dokl. Biochem. Biophys. 2004, 396, 177–180. [Google Scholar] [CrossRef]
- Strakhovskaya, M.G.; Parkhomenko, I.M.; Rumbal’, Y.V.; Zarubina, A.P.; Danilov, V.S.; Stranadko, E.F. Photoquenching of the Bioluminescence of the Genetically Engineered Escherichia Coli TG1 (pXen7) Strain in the Presence of Photodithazine. Microbiology 2002, 71, 294–297. [Google Scholar] [CrossRef]
- Rapacka-Zdończyk, A.; Woźniak, A.; Michalska, K.; Pierański, M.; Ogonowska, P.; Grinholc, M.; Nakonieczna, J. Factors Determining the Susceptibility of Bacteria to Antibacterial Photodynamic Inactivation. Front. Med. 2021, 8, 64260. [Google Scholar] [CrossRef] [PubMed]



| N | 4αZnPc4+ | 4αβZnPc4+ | ZnPcChol8+ |
|---|---|---|---|
| 1 | 4.38 | 7.23 | 5.08 |
| 2 | 3.12 | 6.01 | 1.63 |
| 3 | 5.05 | 6.42 | 2.51 |
| 4 | 3.57 | 13.06 | 5.20 |
| 5 | 1.90 | 7.25 | 8.14 |
| Phthalocyanines (Pcs) | Pcs Concentration, 1 µM | Pcs Concentration, 2 µM |
|---|---|---|
| 4αZnPc4+ | −28.6 (±6.8) | −12.7 (±5.4) |
| 4αβZnPc4+ | −25.7 (±4.9) | −19.8 (±7.8) |
| ZnPcChol8+ | −24.8 (±5.7) | −15.7 (±6.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meerovich, G.; Bunin, D.; Akhlyustina, E.; Romanishkin, I.; Levkin, V.; Kharnas, S.; Stepanova, M.; Martynov, A.; Loschenov, V.; Gorbunova, Y.; et al. The Photodynamic Antibacterial Potential of New Tetracationic Zinc(II) Phthalocyanines Bearing 4-((Diethylmethylammonium)methyl)phenoxy Substituents. Int. J. Mol. Sci. 2025, 26, 9414. https://doi.org/10.3390/ijms26199414
Meerovich G, Bunin D, Akhlyustina E, Romanishkin I, Levkin V, Kharnas S, Stepanova M, Martynov A, Loschenov V, Gorbunova Y, et al. The Photodynamic Antibacterial Potential of New Tetracationic Zinc(II) Phthalocyanines Bearing 4-((Diethylmethylammonium)methyl)phenoxy Substituents. International Journal of Molecular Sciences. 2025; 26(19):9414. https://doi.org/10.3390/ijms26199414
Chicago/Turabian StyleMeerovich, Gennady, Dmitry Bunin, Ekaterina Akhlyustina, Igor Romanishkin, Vladimir Levkin, Sergey Kharnas, Maria Stepanova, Alexander Martynov, Victor Loschenov, Yulia Gorbunova, and et al. 2025. "The Photodynamic Antibacterial Potential of New Tetracationic Zinc(II) Phthalocyanines Bearing 4-((Diethylmethylammonium)methyl)phenoxy Substituents" International Journal of Molecular Sciences 26, no. 19: 9414. https://doi.org/10.3390/ijms26199414
APA StyleMeerovich, G., Bunin, D., Akhlyustina, E., Romanishkin, I., Levkin, V., Kharnas, S., Stepanova, M., Martynov, A., Loschenov, V., Gorbunova, Y., & Strakhovskaya, M. (2025). The Photodynamic Antibacterial Potential of New Tetracationic Zinc(II) Phthalocyanines Bearing 4-((Diethylmethylammonium)methyl)phenoxy Substituents. International Journal of Molecular Sciences, 26(19), 9414. https://doi.org/10.3390/ijms26199414










