Enantioselective Complexation of Xylopinine: A Cyclodextrin-Assisted CE and NMR Study
Abstract
1. Introduction
2. Results and Discussion
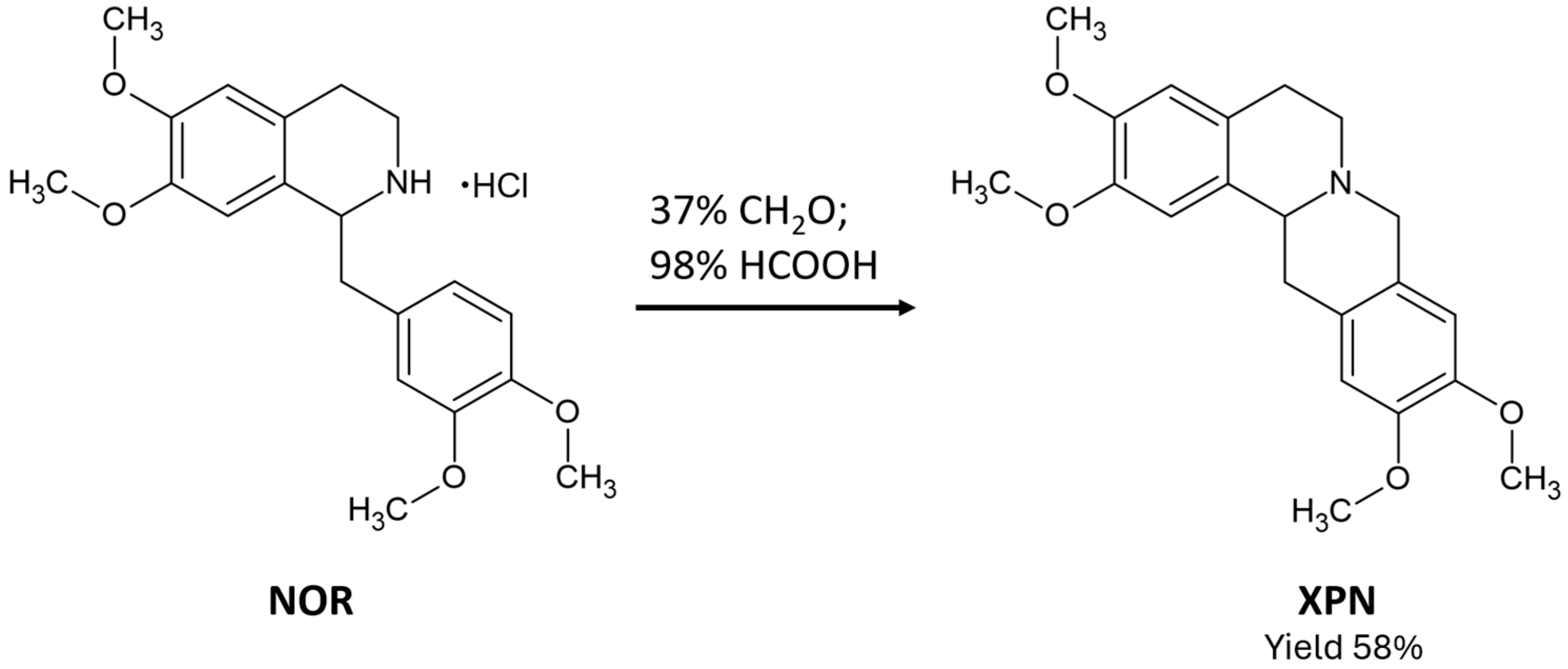
2.1. Synthetic Procedures
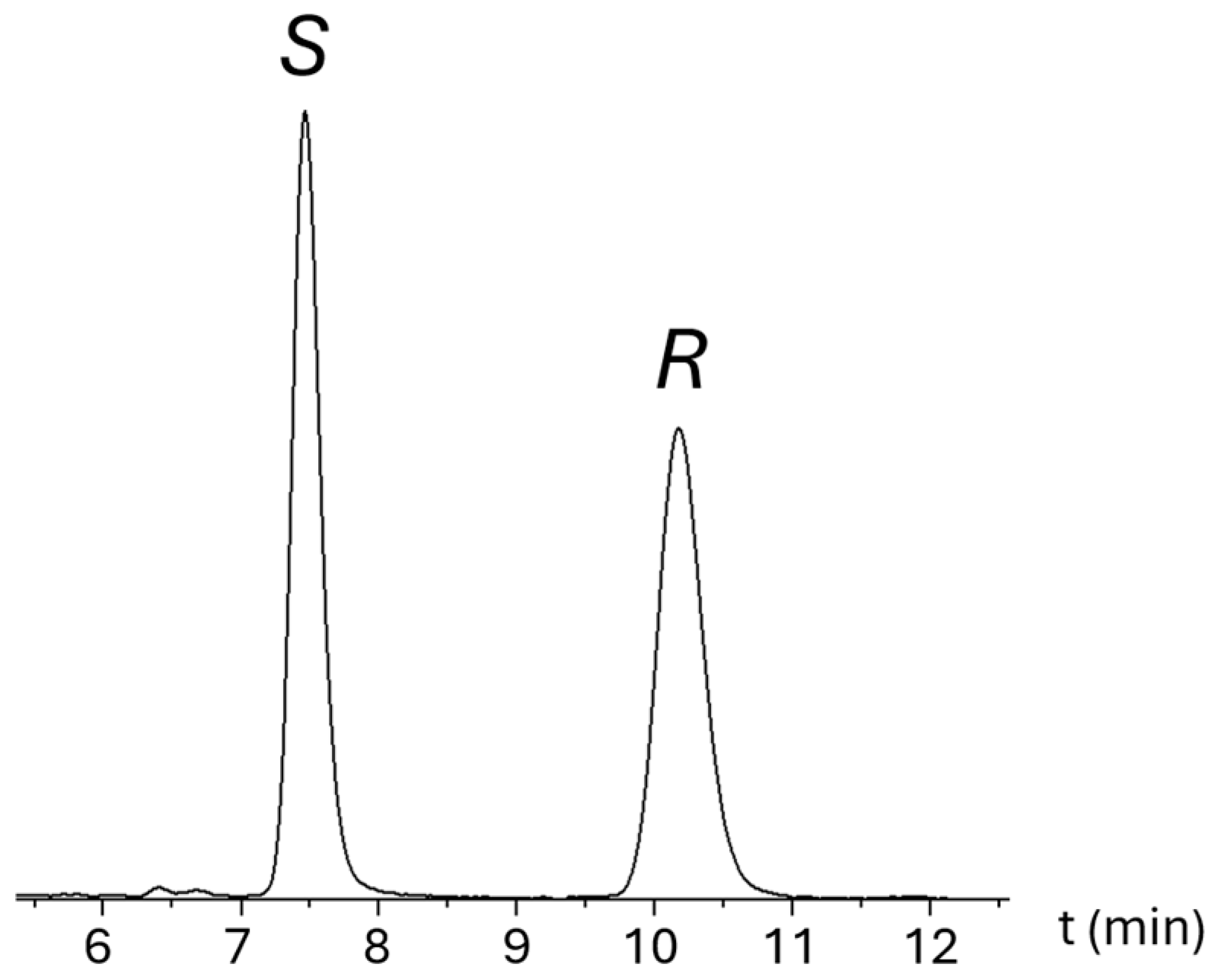
2.2. Semi-Preparative Isolation of XPN Enantiomers
2.3. CyD-Mediated Capillary Electrophoretic Study of XPN
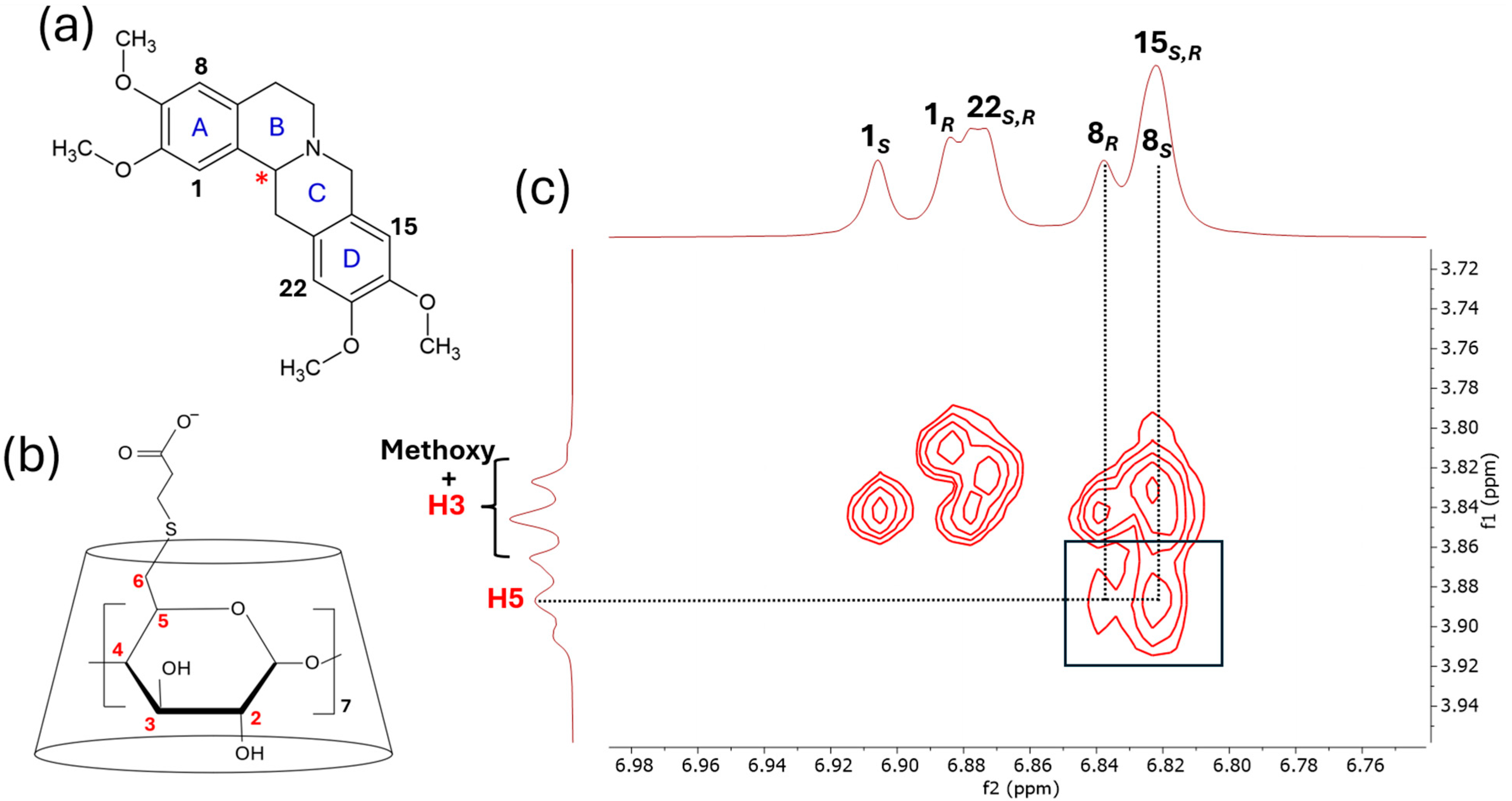
2.4. Characterization of the CyD Complexes by NMR Spectroscopy
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Racemic XPN
3.3. Chiral HPLC
3.4. Circular Dichroism Spectroscopy
3.5. Capillary Electrophoresis
3.6. NMR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kato, N.; Dubouzet, E.; Kokabu, Y.; Yoshida, S.; Taniguchi, Y.; Dubouzet, J.G.; Yazaki, K.; Sato, F. Identification of a WRKY Protein as a Transcriptional Regulator of Benzylisoquinoline Alkaloid Biosynthesis in Coptis Japonica. Plant Cell Physiol. 2007, 48, 8–18. [Google Scholar] [CrossRef]
- O’Connor, S.E. ChemInform Abstract: Alkaloid Biosynthesis. ChemInform 2010, 41. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Cicero, A.F.G. Berberine on Metabolic and Cardiovascular Risk Factors: An Analysis from Preclinical Evidences to Clinical Trials. Expert Opin. Biol. Ther. 2012, 12, 1113–1124. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine and Barberry (Berberis vulgaris): A Clinical Review. Phyther. Res. 2019, 33, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Grycová, L.; Dostál, J.; Marek, R. Quaternary Protoberberine Alkaloids. Phytochemistry 2007, 68, 150–175. [Google Scholar] [CrossRef]
- Menezes, L.R.A.; D’Sousa Costa, C.O.; Rodrigues, A.C.B.D.C.; Santo, F.R.D.E.; Nepel, A.; Dutra, L.M.; Silva, F.M.A.; Soares, M.B.P.; Barison, A.; Costa, E.V.; et al. Cytotoxic Alkaloids from the Stem of Xylopia laevigata. Molecules 2016, 21, 890. [Google Scholar] [CrossRef]
- Bory, S.; Bun, S.S.; Baghdikian, B.; Dumètre, A.; Hutter, S.; Mabrouki, F.; Bun, H.; Elias, R.; Azas, N.; Ollivier, E. HPLC Analysis of Stephania rotunda Extracts and Correlation with Antiplasmodial Activity. Phyther. Res. 2013, 27, 278–284. [Google Scholar] [CrossRef]
- Desgrouas, C.; Taudon, N.; Bun, S.S.; Baghdikian, B.; Bory, S.; Parzy, D.; Ollivier, E. Ethnobotany, Phytochemistry and Pharmacology of Stephania rotunda Lour. J. Ethnopharmacol. 2014, 154, 537–563. [Google Scholar] [CrossRef] [PubMed]
- Chea, A.; Hout, S.; Bun, S.S.; Tabatadze, N.; Gasquet, M.; Azas, N.; Elias, R.; Balansard, G. Antimalarial Activity of Alkaloids Isolated from Stephania rotunda. J. Ethnopharmacol. 2007, 112, 132–137. [Google Scholar] [CrossRef]
- Montenegro, H.; Gutiérez, M.; Romero, L.I.; Ortega-Barría, E.; Capson, T.L.; Rios, L.C. Aporphine Alkaloids from Guatteria spp. with Leishmanicidal Activity. Planta Med. 2003, 69, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Baghdikian, B.; Mahiou-Leddet, V.; Bory, S.; Bun, S.S.; Dumetre, A.; Mabrouki, F.; Hutter, S.; Azas, N.; Ollivier, E. New Antiplasmodial Alkaloids from Stephania rotunda. J. Ethnopharmacol. 2013, 145, 381–385. [Google Scholar] [CrossRef]
- Nakanishi, H.; Okegawa, T.; Shimamoto, K. Comparison of the Optical Isomers of Xylopinine. Jpn. J. Pharmacol. 1966, 16, 10–24. [Google Scholar] [CrossRef]
- Ge, H.; Bian, Y.; He, X.; Xie, X.Q.; Wang, J. Significantly Different Effects of Tetrahydroberberrubine Enantiomers on Dopamine D1/D2 Receptors Revealed by Experimental Study and Integrated in Silico Simulation. J. Comput. Aided. Mol. Des. 2019, 33, 447–459. [Google Scholar] [CrossRef]
- Hong, Z.Y.; Fan, G.R.; Chai, Y.F.; Yin, X.P.; Wen, J.; Wu, Y.T. Chiral Liquid Chromatography Resolution and Stereoselective Pharmacokinetic Study of Tetrahydropalmatine Enantiomers in Dogs. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 826, 108–113. [Google Scholar] [CrossRef]
- Hong, Z.; Fan, G.; Chai, Y.; Yin, X.; Wu, Y. Stereoselective Pharmacokinetics of Tetrahydropalmatine after Oral Administration of (-)-Enantiomer and the Racemate. Chirality 2005, 17, 293–296. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, K.; Wen, J.; Fan, G.; Chai, Y.; Hong, Z. Chiral HPLC Determination and Stereoselective Pharmacokinetics of Tetrahydroberberine Enantiomers in Rats. Chirality 2011, 43, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Sauvageot-Witzku, K.; Geisslinger, F.; Urban, N.; Schaefer, M.; Bartel, K.; Bracher, F. The Ethoxycarbonyl Group as Both Activating and Protective Group in N-Acyl-Pictet-Spengler Reactions Using Methoxystyrenes. A Short Approach to Racemic 1-Benzyltetrahydroisoquinoline Alkaloids. Beilstein J. Org. Chem. 2021, 17, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.D.; Shi, Y.P.; Wu, X.M.; Luo, X.P. Chiral High-Performance Liquid Chromatographic Separation of the Enantiomers of Tetrahydropalmatine and Tetrahydroberberine, Isolated from Corydalis Yanhusuo. Anal. Bioanal. Chem. 2006, 384, 939–945. [Google Scholar] [CrossRef]
- Várnai, B.; Zsila, F.; Szakács, Z.; Garádi, Z.; Malanga, M.; Béni, S. Sulfobutylation of Beta-Cyclodextrin Enhances the Complex Formation with Mitragynine: An NMR and Chiroptical Study. Int. J. Mol. Sci. 2022, 23, 3844. [Google Scholar] [CrossRef] [PubMed]
- Dohárszky, A.; Vági, E.M.; Könczöl, Á.; Simon, A.; Várnagy, E.; Muratov, M.; Steiger, K.I.; Várnai, B.; Béni, S.; Riethmüller, E.; et al. Kratom Alkaloids: A Blood–Brain Barrier Specific Membrane Permeability Assay-Guided Isolation and Cyclodextrin Complexation Study. Molecules 2024, 29, 5302. [Google Scholar] [CrossRef]
- Fejős, I.; Kalydi, E.; Malanga, M.; Benkovics, G.; Béni, S. Single Isomer Cyclodextrins as Chiral Selectors in Capillary Electrophoresis. J. Chromatogr. A 2020, 1627, 461375. [Google Scholar] [CrossRef]
- Koyama, J.; Morita, I.; Kino, A.; Iwasa, K.; Tagahara, K. Enantiomeric Separation by Cyclodextrin Modified Capillary Zone Electrophoresis (CD-CZE) of Quaternary Tetrahydroprotoberberine Alkaloids. Chem. Pharm. Bull. 2000, 57, 364–370. [Google Scholar] [CrossRef]
- Yan, B.; Huang, Z.A.; Yahaya, N.; Chen, D.D.Y. Enantioselective Analysis in Complex Matrices Using Capillary Electrophoresis-Mass Spectrometry: A Case Study of the Botanical Drug Corydalis Rhizoma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1152, 122216. [Google Scholar] [CrossRef] [PubMed]
- Benmekhbi, L.; Louafi, F.; Roisnel, T.; Hurvois, J.P. Synthesis of Tetrahydroisoquinoline Alkaloids and Related Compounds through the Alkylation of Anodically Prepared α-Amino Nitriles. J. Org. Chem. 2016, 81, 6721–6739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, X. Asymmetric Synthesis of (S)-(-)-Xylopinine and (S)-(+)-Laudanosine. Chinese J. Org. Chem. 2023, 43, 3297. [Google Scholar] [CrossRef]
- Mujahidin, D.; Doye, S. Enantioselective Synthesis of (+)-(S)-Laudanosine and (–)-(S)-Xylopinine. Eur. J. Org. Chem. 2005, 2005, 2689–2693. [Google Scholar] [CrossRef]
- Elavarasan, S.; Preety, J.; Abinaya, R.; Saravanan, T.; Balasubramanian, K.K.; Venkatramaiah, N.; Baskar, B. Visible Light Driven Metal-Free Photoredox Catalyzed α-Benzylation and α-Oxygenation of N-Substituted Tetrahydroisoquinolines: Applications to Synthesis of Natural Products. Chem. An Asian J. 2022, 17, e202200878. [Google Scholar] [CrossRef]
- Várnagy, E.; Tóth, G.; Hosztafi, S.; Dobó, M.; Fejős, I.; Béni, S. Chiral Recognition Mechanism of Benzyltetrahydroisoquinoline Alkaloids: Cyclodextrin-Mediated Capillary Electrophoresis, Chiral HPLC, and NMR Spectroscopy Study. Molecules 2025, 30, 1125. [Google Scholar] [CrossRef]
- Craig, L.E.; Tarbell, D.S. Curariform Activity and Chemical Structure. II. Synthesis in the Benzyltetrahydroisoquinoline Series1. J. Am. Chem. Soc. 1948, 70, 2783–2785. [Google Scholar] [CrossRef]
- Kametani, T.; Terui, T.; Agui, H.; Fukumoto, K. A Modified Synthesis of Codamine under Eschweiler-Clarke Conditions. J. Heterocycl. Chem. 1968, 5, 753–755. [Google Scholar] [CrossRef]
- Szabó, Z.I.; Foroughbakhshfasaei, M.; Noszál, B.; Tóth, G. Enantioseparation of Racecadotril Using Polysaccharide-Type Chiral Stationary Phases in Polar Organic Mode. Chirality 2018, 30, 95–105. [Google Scholar] [CrossRef]
- Fejős, I.; Tóth, G.; Várnai, B.; Szabó, Z.I.; Köteles, I.; Malanga, M.; Béni, S. Enantioseparation of Solriamfetol and Its Major Impurity Phenylalaninol by Capillary Electrophoresis Using Sulfated Gamma Cyclodextrin. Electrophoresis 2021, 42, 1818–1825. [Google Scholar] [CrossRef]
- Hazra, S.; Hossain, M.; Kumar, G.S. Studies on α-, β-, and γ-Cyclodextrin Inclusion Complexes of Isoquinoline Alkaloids Berberine, Palmatine and Coralyne. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 311–323. [Google Scholar] [CrossRef]
- Kamigauchi, M.; Kanbara, N.; Sugiura, M.; Iwasa, K.; Ohishi, H.; Ishida, T. Berberine/γ-Cyclodextrin Inclusion Structure Studied by 1H-NMR Spectroscopy and Molecular-Dynamics Calculations. Helv. Chim. Acta 2004, 87, 264–271. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, S.; Qin, Z.; Ai, G.; Li, M.; Gong, S.; Liu, Y.; Zeng, H.; Chen, J.; Su, Z.; et al. Supersaturated Drug Delivery System of Oxyberberine Based on Cyclodextrin Nanoaggregates: Preparation, Characterization, and In Vivo Application. Int. J. Nanomedicine 2024, 19, 5297–5316. [Google Scholar] [CrossRef]
- Miskolczy, Z.; Megyesi, M.; Biczók, L. Entropy-Driven Inclusion of Natural Protoberberine Alkaloids in Sulfobutylether-β-Cyclodextrin. Molecules 2022, 27, 7514. [Google Scholar] [CrossRef] [PubMed]
- Dubský, P.; Ördögová, M.; Malý, M.; Riesová, M. CEval: All-in-one software for data processing and statistical evalua-tions in affinity capillary electrophoresis. J. Chromatogr. A 2016, 1445, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, J.; Jensen, H.; Holm, R. Affinity capillary electrophoresis method for investigation of bile salts complexation with sulfobutyl ether-β-cyclodextrin. J. Sep. Sci. 2012, 35, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Beneš, M.; Zusková, I.; Svobodová, J.; Gaš, B. Determination of stability constants of complexes of neutral analytes with charged cyclodextrins by affinity capillary electrophoresis. Electrophoresis 2012, 33, 1032–1039. [Google Scholar] [CrossRef] [PubMed]



| Column | Mobile Phase | t1 | Rs |
|---|---|---|---|
| Chiral CyD-Ph | ACN | 5.97 | - |
| MeOH | 11.42 | 1.9 | |
| Cyclobond I | ACN | 6.11 | - |
| MeOH | 4.93 | - | |
| Nucleodex β-CyD | ACN | 2.70 | - |
| MeOH | 2.97 | - | |
| Chiralcel OD | ACN | 6.58 | 0.60 |
| MeOH | 9.10 | 0.60 | |
| Chiralpak AD | ACN | 5.74 | - |
| MeOH | 7.46 | 5.50 | |
| Chiralpak IA | ACN | 3.84 | - |
| MeOH | 5.64 | 2.90 |
| CyDs | Concentration | pH 4.5 | pH 7.4 | ||
|---|---|---|---|---|---|
| (mM) | Rs | EMO | Rs | EMO | |
| β-CyD | 10 | - | - | 1.4 | R,S |
| S-β-CyD | 10 | 3.2 | R,S | <1 | R,S |
| SBE-β-CyD | 10 | 4.6 | S,R | <1 | S,R |
| CM-β-CyD | 10 | - | - | <0.5 | - |
| SBX | 2.5 | n.d. | n.d. | 9.3 | R,S |
| SGX | 7.5 | n.d. | n.d. | 1.4 | R,S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Várnagy, E.; Tóth, G.; Hosztafi, S.; Malanga, M.; Fejős, I.; Béni, S. Enantioselective Complexation of Xylopinine: A Cyclodextrin-Assisted CE and NMR Study. Int. J. Mol. Sci. 2025, 26, 9405. https://doi.org/10.3390/ijms26199405
Várnagy E, Tóth G, Hosztafi S, Malanga M, Fejős I, Béni S. Enantioselective Complexation of Xylopinine: A Cyclodextrin-Assisted CE and NMR Study. International Journal of Molecular Sciences. 2025; 26(19):9405. https://doi.org/10.3390/ijms26199405
Chicago/Turabian StyleVárnagy, Erzsébet, Gergő Tóth, Sándor Hosztafi, Milo Malanga, Ida Fejős, and Szabolcs Béni. 2025. "Enantioselective Complexation of Xylopinine: A Cyclodextrin-Assisted CE and NMR Study" International Journal of Molecular Sciences 26, no. 19: 9405. https://doi.org/10.3390/ijms26199405
APA StyleVárnagy, E., Tóth, G., Hosztafi, S., Malanga, M., Fejős, I., & Béni, S. (2025). Enantioselective Complexation of Xylopinine: A Cyclodextrin-Assisted CE and NMR Study. International Journal of Molecular Sciences, 26(19), 9405. https://doi.org/10.3390/ijms26199405









