Monoclonal Antibodies as Therapeutic Agents in Autoimmune and Neurodegenerative Diseases of the Central Nervous System: Current Evidence on Molecular Mechanisms and Future Directions
Abstract
1. Introduction
2. Autoimmune Demyelinating Diseases of the CNS
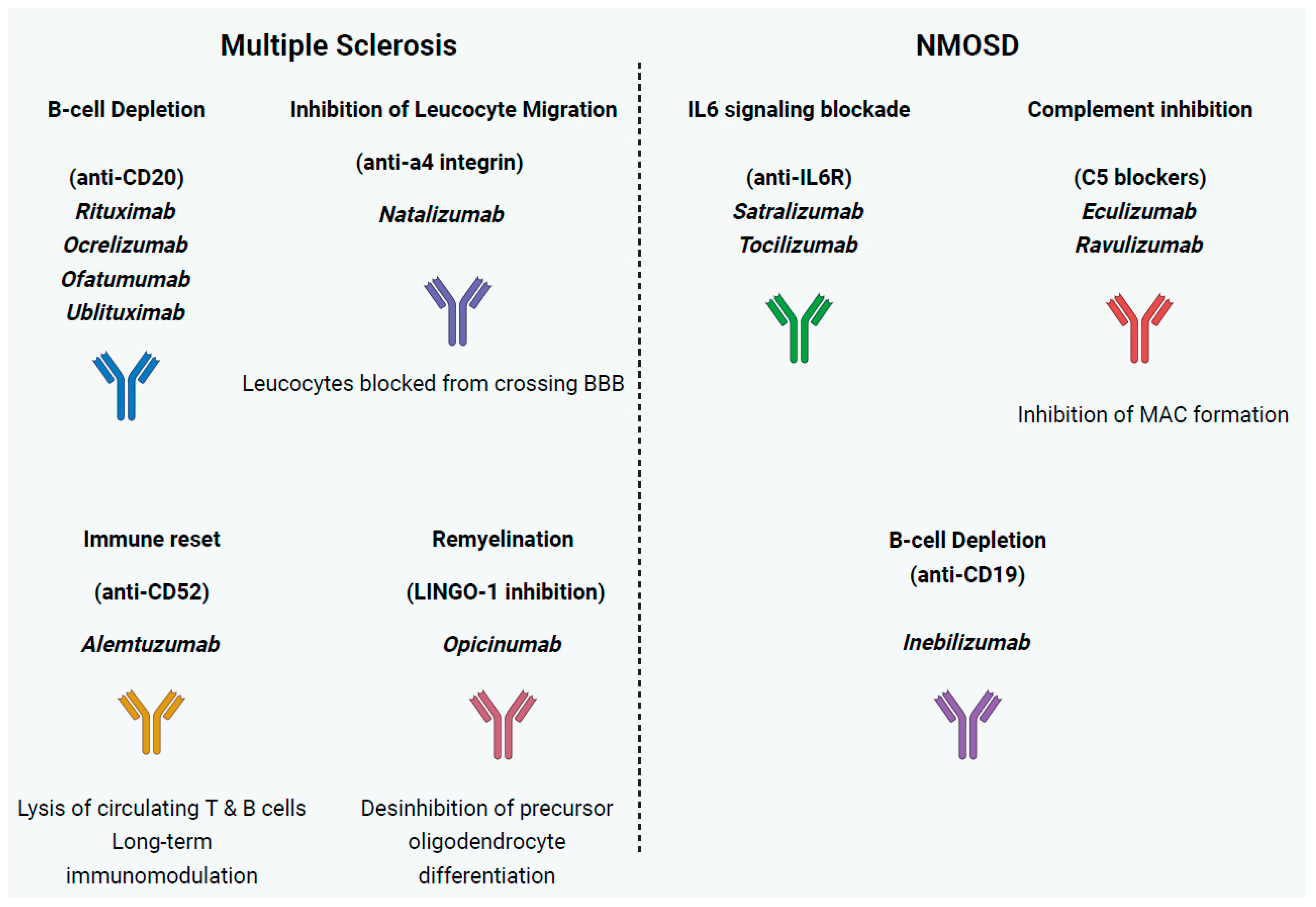
2.1. Multiple Sclerosis
2.1.1. mAbs Inhibiting Leukocyte Migration into the CNS
2.1.2. B Cell Depletion mAb Therapies
2.1.3. mAb Immune Reset
2.1.4. mAbs Promoting Remyelination
2.1.5. Other mAbs in MS
2.2. Neuromyelitis Optica Spectrum Disorder
2.2.1. mAbs Targeting IL-6
2.2.2. Complement Inhibition
2.2.3. Other B Cell Depletion Therapies
3. Neurodegenerative Diseases of the CNS
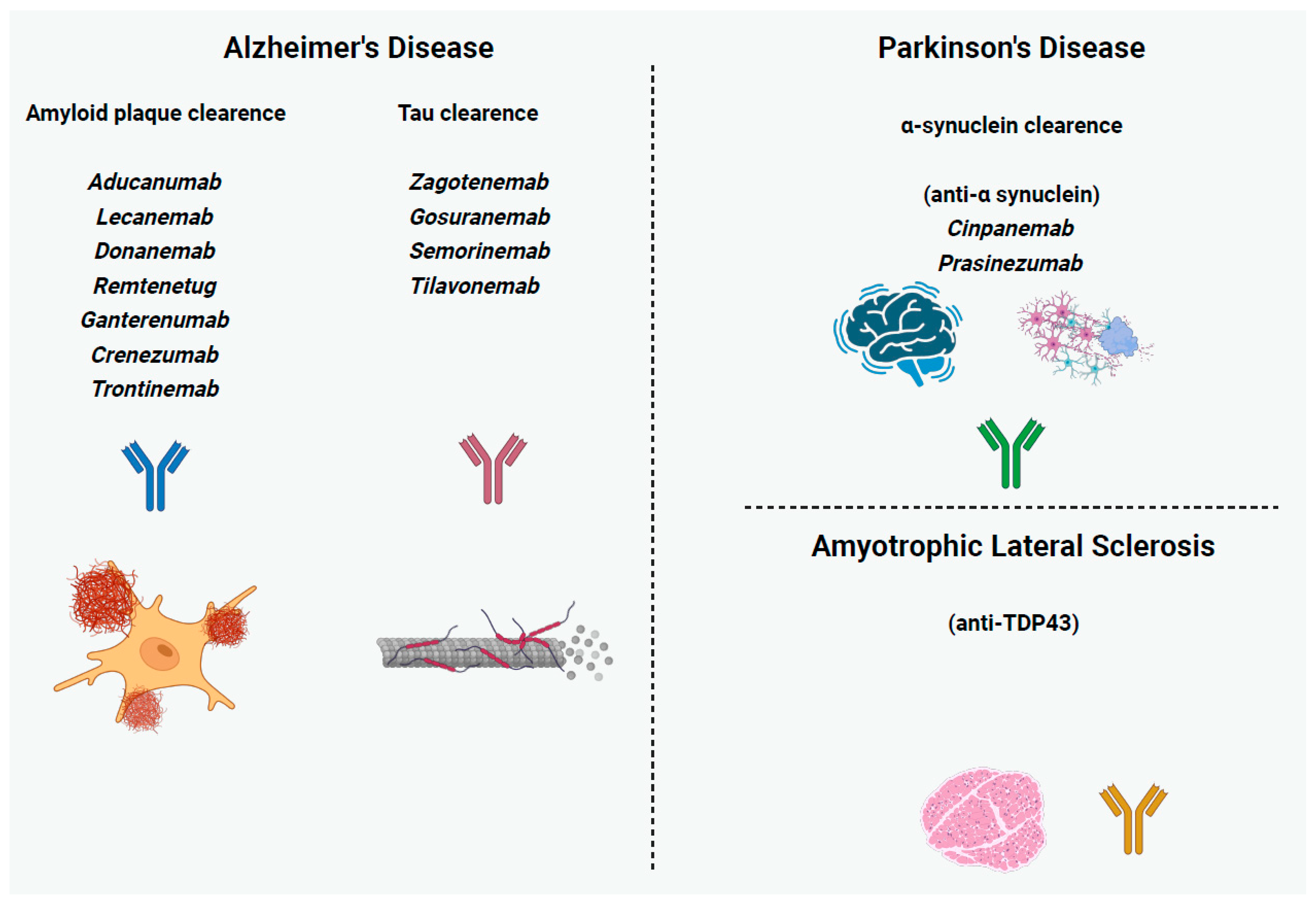
3.1. Alzheimer’s Disease
3.1.1. Anti-Amyloid mAbs
3.1.2. Anti-Tau mAbs
3.2. Parkinson’s Disease
Anti-a-Synuclein mAbs
4. Neurodegenerative Diseases of the CNS and PNS
4.1. Amyotrophic Lateral Sclerosis
Anti-TAR DNA-Binding Protein 43 (TDP43) mAbs
5. Conclusions
Funding
Conflicts of Interest
References
- Smith, S.L. Ten Years of Orthoclone OKT3 (Muromonab-CD3): A Review. J. Transpl. Coord. 1996, 6, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef] [PubMed]
- Skarlis, C.; Kotsari, M.; Anagnostouli, M. Advancing Treatment in Pediatric Multiple Sclerosis: The Promise of B-Cell-Targeting Therapies. Int. J. Mol. Sci. 2025, 26, 5989. [Google Scholar] [CrossRef] [PubMed]
- Skarlis, C.; Argyriou, E.; Mavragani, C.P. Lymphoma in Sjögren’s Syndrome: Predictors and Therapeutic Options. Curr. Treat. Options Rheum. 2020, 6, 1–17. [Google Scholar] [CrossRef]
- Skarlis, C.; Marketos, N.; Mavragani, C.P. Biologics in Sjögren’s Syndrome. Pharmacol. Res. 2019, 147, 104389. [Google Scholar] [CrossRef]
- Papadopoulos, V.E.; Skarlis, C.; Evangelopoulos, M.-E.; Mavragani, C.P. Type I Interferon Detection in Autoimmune Diseases: Challenges and Clinical Applications. Expert. Rev. Clin. Immunol. 2021, 17, 883–903. [Google Scholar] [CrossRef]
- Elices, M.J. Natalizumab. Elan/Biogen. Curr. Opin. Investig. Drugs 2003, 4, 1354–1362. [Google Scholar]
- Kharel, S.; Ojha, R. Future of Monoclonal Antibody Therapy in Parkinson’s Disease. Ann. Neurosci. 2023, 30, 8–10. [Google Scholar] [CrossRef]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the Clinical Course of Multiple Sclerosis. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Waubant, E.; Lucas, R.; Mowry, E.; Graves, J.; Olsson, T.; Alfredsson, L.; Langer-Gould, A. Environmental and Genetic Risk Factors for MS: An Integrated Review. Ann. Clin. Transl. Neurol. 2019, 6, 1905–1922. [Google Scholar] [CrossRef] [PubMed]
- Skarlis, C.; Anagnostouli, M. The Role of Melatonin in Multiple Sclerosis. Neurol. Sci. 2020, 41, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Manna, I.; De Benedittis, S.; Porro, D. A Comprehensive Examination of the Role of Epigenetic Factors in Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 8921. [Google Scholar] [CrossRef]
- Munger, K.L.; Chitnis, T.; Ascherio, A. Body Size and Risk of MS in Two Cohorts of US Women. Neurology 2009, 73, 1543–1550. [Google Scholar] [CrossRef]
- Mikaeloff, Y.; Caridade, G.; Tardieu, M.; Suissa, S.; KIDSEP Study Group. Parental Smoking at Home and the Risk of Childhood-Onset Multiple Sclerosis in Children. Brain 2007, 130, 2589–2595. [Google Scholar] [CrossRef]
- Banwell, B.; Krupp, L.; Kennedy, J.; Tellier, R.; Tenembaum, S.; Ness, J.; Belman, A.; Boiko, A.; Bykova, O.; Waubant, E.; et al. Clinical Features and Viral Serologies in Children with Multiple Sclerosis: A Multinational Observational Study. Lancet Neurol. 2007, 6, 773–781. [Google Scholar] [CrossRef]
- Hedström, A.K.; Sundqvist, E.; Bäärnhielm, M.; Nordin, N.; Hillert, J.; Kockum, I.; Olsson, T.; Alfredsson, L. Smoking and Two Human Leukocyte Antigen Genes Interact to Increase the Risk for Multiple Sclerosis. Brain 2011, 134, 653–664. [Google Scholar] [CrossRef]
- Bjornevik, K.; Münz, C.; Cohen, J.I.; Ascherio, A. Epstein–Barr Virus as a Leading Cause of Multiple Sclerosis: Mechanisms and Implications. Nat. Rev. Neurol. 2023, 19, 160–171. [Google Scholar] [CrossRef]
- Anagnostouli, M.; Anagnostoulis, G.; Katsavos, S.; Panagiotou, M.; Kararizou, E.; Davaki, P. HLA-DRB1 15:01 and Epstein-Barr Virus in a Multiple Sclerosis Patient with Psoriasis, Nasopharyngeal and Breast Cancers. Lessons for Possible Hidden Links for Autoimmunity and Cancer. J. Neurol. Sci. 2014, 339, 26–31. [Google Scholar] [CrossRef]
- International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2; Sawcer, S.; Hellenthal, G.; Pirinen, M.; Spencer, C.C.A.; Patsopoulos, N.A.; Moutsianas, L.; Dilthey, A.; Su, Z.; et al. Genetic Risk and a Primary Role for Cell-Mediated Immune Mechanisms in Multiple Sclerosis. Nature 2011, 476, 214–219. [Google Scholar] [CrossRef]
- International Multiple Sclerosis Genetics Consortium. Multiple Sclerosis Genomic Map Implicates Peripheral Immune Cells and Microglia in Susceptibility. Science 2019, 365, eaav7188. [Google Scholar] [CrossRef]
- Anagnostouli, M.; Artemiadis, A.; Gontika, M.; Skarlis, C.; Markoglou, N.; Katsavos, S.; Kilindireas, K.; Doxiadis, I.; Stefanis, L. HLA-DPB1*03 as Risk Allele and HLA-DPB1*04 as Protective Allele for Both Early- and Adult-Onset Multiple Sclerosis in a Hellenic Cohort. Brain Sci. 2020, 10, 374. [Google Scholar] [CrossRef]
- Patsopoulos, N.A.; Barcellos, L.F.; Hintzen, R.Q.; Schaefer, C.; van Duijn, C.M.; Noble, J.A.; Raj, T.; IMSGC; ANZgene; Gourraud, P.-A.; et al. Fine-Mapping the Genetic Association of the Major Histocompatibility Complex in Multiple Sclerosis: HLA and Non-HLA Effects. PLoS Genet. 2013, 9, e1003926. [Google Scholar] [CrossRef]
- Patsopoulos, N.A. Genetics of Multiple Sclerosis: An Overview and New Directions. Cold Spring Harb. Perspect. Med. 2018, 8, a028951. [Google Scholar] [CrossRef]
- Skarlis, C.; Markoglou, N.; Gontika, M.; Artemiadis, A.; Pons, M.-R.; Stefanis, L.; Dalakas, M.; Chrousos, G.; Anagnostouli, M. The Impact of HLA-DRB1 Alleles in a Hellenic, Pediatric-Onset Multiple Sclerosis Cohort: Implications on Clinical and Neuroimaging Profile. Neurol. Sci. 2024, 45, 5405–5411. [Google Scholar] [CrossRef] [PubMed]
- Moutsianas, L.; Jostins, L.; Beecham, A.H.; Dilthey, A.T.; Xifara, D.K.; Ban, M.; Shah, T.S.; Patsopoulos, N.A.; Alfredsson, L.; Anderson, C.A.; et al. Class II HLA Interactions Modulate Genetic Risk for Multiple Sclerosis. Nat. Genet. 2015, 47, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, L.F.; Sawcer, S.; Ramsay, P.P.; Baranzini, S.E.; Thomson, G.; Briggs, F.; Cree, B.C.A.; Begovich, A.B.; Villoslada, P.; Montalban, X.; et al. Heterogeneity at the HLA-DRB1 Locus and Risk for Multiple Sclerosis. Hum. Mol. Genet. 2006, 15, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Sawcer, S.; Franklin, R.J.M.; Ban, M. Multiple Sclerosis Genetics. Lancet Neurol. 2014, 13, 700–709. [Google Scholar] [CrossRef]
- Skarlis, C.; Papadopoulos, V.; Raftopoulou, S.; Mavragani, C.P.; Evangelopoulos, M.-E. B-Cell Activating Factor Gene Variants in Multiple Sclerosis: Possible Associations with Disease Susceptibility among Females. Clin. Immunol. 2023, 257, 109847. [Google Scholar] [CrossRef]
- Ntellas, P.; Dardiotis, E.; Sevdali, E.; Siokas, V.; Aloizou, A.-M.; Tsinti, G.; Germenis, A.E.; Hadjigeorgiou, G.M.; Eibel, H.; Speletas, M. TNFRSF13C/BAFFR P21R and H159Y Polymorphisms in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2020, 37, 101422. [Google Scholar] [CrossRef] [PubMed]
- Steri, M.; Orrù, V.; Idda, M.L.; Pitzalis, M.; Pala, M.; Zara, I.; Sidore, C.; Faà, V.; Floris, M.; Deiana, M.; et al. Overexpression of the Cytokine BAFF and Autoimmunity Risk. N. Engl. J. Med. 2017, 376, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B.M. Impact of B Cells to the Pathophysiology of Multiple Sclerosis. J. Neuroinflammation 2019, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple Sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Artemiadis, A.K.; Anagnostouli, M.C. Apoptosis of Oligodendrocytes and Post-Translational Modifications of Myelin Basic Protein in Multiple Sclerosis: Possible Role for the Early Stages of Multiple Sclerosis. Eur. Neurol. 2010, 63, 65–72. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Iezzi, E.; Centonze, D. Multiple Sclerosis: Inflammation, Autoimmunity and Plasticity. Handb. Clin. Neurol. 2022, 184, 457–470. [Google Scholar] [CrossRef]
- Mazzeo, A.C.; Calabresi, L.; Damato, V.; Spagni, G.; Massacesi, L.; Mariottini, A. CD20+ T Cells in Multiple Sclerosis: From Pathogenesis to Treatment-Induced Depletion. Int. J. Mol. Sci. 2025, 26, 6655. [Google Scholar] [CrossRef]
- Margoni, M.; Preziosa, P.; Filippi, M.; Rocca, M.A. Anti-CD20 Therapies for Multiple Sclerosis: Current Status and Future Perspectives. J. Neurol. 2022, 269, 1316–1334. [Google Scholar] [CrossRef]
- Salzer, J.; Svenningsson, R.; Alping, P.; Novakova, L.; Björck, A.; Fink, K.; Islam-Jakobsson, P.; Malmeström, C.; Axelsson, M.; Vågberg, M.; et al. Rituximab in Multiple Sclerosis: A Retrospective Observational Study on Safety and Efficacy. Neurology 2016, 87, 2074–2081. [Google Scholar] [CrossRef]
- Alping, P.; Frisell, T.; Novakova, L.; Islam-Jakobsson, P.; Salzer, J.; Björck, A.; Axelsson, M.; Malmeström, C.; Fink, K.; Lycke, J.; et al. Rituximab versus Fingolimod after Natalizumab in Multiple Sclerosis Patients. Ann. Neurol. 2016, 79, 950–958. [Google Scholar] [CrossRef]
- Granqvist, M.; Boremalm, M.; Poorghobad, A.; Svenningsson, A.; Salzer, J.; Frisell, T.; Piehl, F. Comparative Effectiveness of Rituximab and Other Initial Treatment Choices for Multiple Sclerosis. JAMA Neurol. 2018, 75, 320–327. [Google Scholar] [CrossRef]
- Major, E.O.; Yousry, T.A.; Clifford, D.B. Pathogenesis of Progressive Multifocal Leukoencephalopathy and Risks Associated with Treatments for Multiple Sclerosis: A Decade of Lessons Learned. Lancet Neurol. 2018, 17, 467–480, Correction in Lancet Neurol. 2018, 17, 862. [Google Scholar] [CrossRef]
- Hawker, K.; O’Connor, P.; Freedman, M.S.; Calabresi, P.A.; Antel, J.; Simon, J.; Hauser, S.; Waubant, E.; Vollmer, T.; Panitch, H.; et al. Rituximab in Patients with Primary Progressive Multiple Sclerosis: Results of a Randomized Double-Blind Placebo-Controlled Multicenter Trial. Ann. Neurol. 2009, 66, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; de Seze, J.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef]
- Rindi, L.V.; Zaçe, D.; Braccialarghe, N.; Massa, B.; Barchi, V.; Iannazzo, R.; Fato, I.; De Maria, F.; Kontogiannis, D.; Malagnino, V.; et al. Drug-Induced Progressive Multifocal Leukoencephalopathy (PML): A Systematic Review and Meta-Analysis. Drug Saf. 2024, 47, 333–354. [Google Scholar] [CrossRef]
- Steinman, L. The Discovery of Natalizumab, a Potent Therapeutic for Multiple Sclerosis. J. Cell Biol. 2012, 199, 413–416. [Google Scholar] [CrossRef]
- Rudick, R.A.; Stuart, W.H.; Calabresi, P.A.; Confavreux, C.; Galetta, S.L.; Radue, E.-W.; Lublin, F.D.; Weinstock-Guttman, B.; Wynn, D.R.; Lynn, F.; et al. Natalizumab plus Interferon Beta-1a for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2006, 354, 911–923. [Google Scholar] [CrossRef]
- Polman, C.H.; O’Connor, P.W.; Havrdova, E.; Hutchinson, M.; Kappos, L.; Miller, D.H.; Phillips, J.T.; Lublin, F.D.; Giovannoni, G.; Wajgt, A.; et al. A Randomized, Placebo-Controlled Trial of Natalizumab for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2006, 354, 899–910. [Google Scholar] [CrossRef]
- Ghezzi, A.; Moiola, L.; Pozzilli, C.; Brescia-Morra, V.; Gallo, P.; Grimaldi, L.M.E.; Filippi, M.; Comi G., G.; MS Study Group-Italian Society of Neurology. Natalizumab in the Pediatric MS Population: Results of the Italian Registry. BMC Neurol. 2015, 15, 174. [Google Scholar] [CrossRef]
- Margoni, M.; Rinaldi, F.; Riccardi, A.; Franciotta, S.; Perini, P.; Gallo, P. No Evidence of Disease Activity Including Cognition (NEDA-3 plus) in Naïve Pediatric Multiple Sclerosis Patients Treated with Natalizumab. J. Neurol. 2020, 267, 100–105. [Google Scholar] [CrossRef]
- Gontika, M.; Skarlis, C.; Markoglou, N.; Tzanetakos, D.; Vakrakou, A.; Toulas, P.; Koutsis, G.; Evangelopoulos, M.-E.; Pons, R.; Dardiotis, E.; et al. Natalizumab Therapy in Patients with Pediatric-Onset Multiple Sclerosis in Greece: Clinical and Immunological Insights of Time-Long Administration and Future Directions-a Single-Center Retrospective Observational Study. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 933–943. [Google Scholar] [CrossRef]
- Sirbu, C.A.; Ghinescu, M.C.; Axelerad, A.D.; Sirbu, A.M.; Ionita-Radu, F. A New Era for Monoclonal Antibodies with Applications in Neurology (Review). Exp. Ther. Med. 2021, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.; Steinman, L.; Fox, E.J.; Hartung, H.-P.; Qian, P.; Wray, S.; Robertson, D.; Selmaj, K.; Wynn, D.; Mok, K.; et al. Improvements in No Evidence of Disease Activity with Ublituximab vs. Teriflunomide in the ULTIMATE Phase 3 Studies in Relapsing Multiple Sclerosis. Front. Neurol. 2024, 15, 1473284. [Google Scholar] [CrossRef] [PubMed]
- Smets, I.; Giovannoni, G. Derisking CD20-Therapies for Long-Term Use. Mult. Scler. Relat. Disord. 2022, 57, 103418. [Google Scholar] [CrossRef] [PubMed]
- DEMİR, S.; ATMACA, M.M.; TOGROL, R.E. The First Cure Experience of A Clinic: Approach to The Patient to Start Ocrelizumab. Noro Psikiyatr. Ars. 2019, 58, 52–56. [Google Scholar] [CrossRef]
- Wang, M.; Otto, C.; Fernández Zapata, C.; Dehlinger, A.; Gallaccio, G.; Diekmann, L.-M.; Niederschweiberer, M.; Schindler, P.; Körtvélyessy, P.; Kunkel, D.; et al. Comprehensive Analysis of B Cell Repopulation in Ocrelizumab-Treated Patients with Multiple Sclerosis by Mass Cytometry and Proteomics. iScience 2025, 28, 112383. [Google Scholar] [CrossRef]
- Coles, A.J.; Twyman, C.L.; Arnold, D.L.; Cohen, J.A.; Confavreux, C.; Fox, E.J.; Hartung, H.-P.; Havrdova, E.; Selmaj, K.W.; Weiner, H.L.; et al. Alemtuzumab for Patients with Relapsing Multiple Sclerosis after Disease-Modifying Therapy: A Randomised Controlled Phase 3 Trial. Lancet 2012, 380, 1829–1839. [Google Scholar] [CrossRef]
- Holmøy, T.; von der Lippe, H.; Leegaard, T.M. Listeria Monocytogenes Infection Associated with Alemtuzumab—A Case for Better Preventive Strategies. BMC Neurol. 2017, 17, 65. [Google Scholar] [CrossRef]
- Croteau, D.; Flowers, C.; Kulick, C.G.; Brinker, A.; Kortepeter, C.M. Acute Acalculous Cholecystitis: A New Safety Risk for Patients with MS Treated with Alemtuzumab. Neurology 2018, 90, e1548–e1552. [Google Scholar] [CrossRef]
- Cadavid, D.; Mellion, M.; Hupperts, R.; Edwards, K.R.; Calabresi, P.A.; Drulović, J.; Giovannoni, G.; Hartung, H.-P.; Arnold, D.L.; Fisher, E.; et al. Safety and Efficacy of Opicinumab in Patients with Relapsing Multiple Sclerosis (SYNERGY): A Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Neurol. 2019, 18, 845–856. [Google Scholar] [CrossRef]
- Eisen, A.; Greenberg, B.M.; Bowen, J.D.; Arnold, D.L.; Caggiano, A.O. A Double-Blind, Placebo-Controlled, Single Ascending-Dose Study of Remyelinating Antibody rHIgM22 in People with Multiple Sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2017, 3, 2055217317743097. [Google Scholar] [CrossRef]
- Giovannoni, G.; Gold, R.; Selmaj, K.; Havrdova, E.; Montalban, X.; Radue, E.-W.; Stefoski, D.; McNeill, M.; Amaravadi, L.; Sweetser, M.; et al. Daclizumab High-Yield Process in Relapsing-Remitting Multiple Sclerosis (SELECTION): A Multicentre, Randomised, Double-Blind Extension Trial. Lancet Neurol. 2014, 13, 472–481. [Google Scholar] [CrossRef]
- Fox, R.J.; Bar-Or, A.; Traboulsee, A.; Oreja-Guevara, C.; Giovannoni, G.; Vermersch, P.; Syed, S.; Li, Y.; Vargas, W.S.; Turner, T.J.; et al. Tolebrutinib in Nonrelapsing Secondary Progressive Multiple Sclerosis. N. Engl. J. Med. 2025, 392, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Arnold, D.L.; Cree, B.A.C.; Ionete, C.; Kim, H.J.; Sormani, M.P.; Syed, S.; Chen, Y.; Maxwell, C.R.; Benoit, P.; et al. Tolebrutinib versus Teriflunomide in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2025, 392, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Dufek, M.; Budincevic, H.; Drulovic, J.; Habek, M.; Hua, L.H.; Weber, M.S.; Thomas, P.; Napieralski, J.; Mitzner, M.C.; et al. Safety and Efficacy of Fenebrutinib in Relapsing Multiple Sclerosis (FENopta): A Multicentre, Double-Blind, Randomised, Placebo-Controlled, Phase 2 Trial and Open-Label Extension Study. Lancet Neurol. 2025, 24, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Hatchwell, E.; Smith, E.B.; Jalilzadeh, S.; Bruno, C.D.; Taoufik, Y.; Hendel-Chavez, H.; Liblau, R.; Brassat, D.; Martin-Blondel, G.; Wiendl, H.; et al. Progressive Multifocal Leukoencephalopathy Genetic Risk Variants for Pharmacovigilance of Immunosuppressant Therapies. Front. Neurol. 2022, 13, 1016377. [Google Scholar] [CrossRef]
- Skarlis, C.; Papadopoulos, V.; Raftopoulou, S.; Mavragani, C.P.; Evangelopoulos, M.-E. Association of B-Cell Activating Factor Gene Variants with Serum Anti-JCV Antibody Positivity in Male Patients with Multiple Sclerosis under Natalizumab Treatment: Implications for Progressive Multifocal Leukoencephalopathy Risk Stratification. J. Neurol. Sci. 2024, 461, 123046. [Google Scholar] [CrossRef]
- de la Hera, B.; Urcelay, E.; Brassat, D.; Chan, A.; Vidal-Jordana, A.; Salmen, A.; Villar, L.M.; Álvarez-Cermeño, J.C.; Izquierdo, G.; Fernández, O.; et al. Natalizumab-Related Anaphylactoid Reactions in MS Patients Are Associated with HLA Class II Alleles. Neurol. Neuroimmunol. Neuroinflamm. 2014, 1, e47. [Google Scholar] [CrossRef]
- Chu, M.M.; Li, V.; Kalincik, T.; Lui, E.; Seet, M.-R.; Jackson, S.; Kilpatrick, T. A Single Nucleotide Polymorphism in the MerTK Gene Is Associated with Increased Radiological Disease Activity in Patients with Multiple Sclerosis on Natalizumab Therapy. Mult. Scler. Relat. Disord. 2025, 102, 106635. [Google Scholar] [CrossRef]
- Carlson, A.K.; Amin, M.; Cohen, J.A. Drugs Targeting CD20 in Multiple Sclerosis: Pharmacology, Efficacy, Safety, and Tolerability. Drugs 2024, 84, 285–304. [Google Scholar] [CrossRef]
- Bar-Or, A.; Grove, R.A.; Austin, D.J.; Tolson, J.M.; VanMeter, S.A.; Lewis, E.W.; Derosier, F.J.; Lopez, M.C.; Kavanagh, S.T.; Miller, A.E.; et al. Subcutaneous Ofatumumab in Patients with Relapsing-Remitting Multiple Sclerosis: The MIRROR Study. Neurology 2018, 90, e1805–e1814, Correction in Neurology 2018, 91, 538. [Google Scholar] [CrossRef] [PubMed]
- Protopapa, M.; Schraad, M.; Pape, K.; Steffen, F.; Steenken, L.; Zipp, F.; Fleischer, V.; Bittner, S. Recurrent Late-Onset Neutropenia Following Treatment with Different B Cell-Depleting Strategies in Multiple Sclerosis. Med 2025, 6, 100529. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L.; Fox, E.; Hartung, H.-P.; Alvarez, E.; Qian, P.; Wray, S.; Robertson, D.; Huang, D.; Selmaj, K.; Wynn, D.; et al. Ublituximab versus Teriflunomide in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2022, 387, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Coles, A.J.; Cohen, J.A.; Fox, E.J.; Giovannoni, G.; Hartung, H.-P.; Havrdova, E.; Schippling, S.; Selmaj, K.W.; Traboulsee, A.; Compston, D.A.S.; et al. Alemtuzumab CARE-MS II 5-Year Follow-up: Efficacy and Safety Findings. Neurology 2017, 89, 1117–1126. [Google Scholar] [CrossRef]
- Havrdova, E.; Arnold, D.L.; Cohen, J.A.; Hartung, H.-P.; Fox, E.J.; Giovannoni, G.; Schippling, S.; Selmaj, K.W.; Traboulsee, A.; Compston, D.A.S.; et al. Alemtuzumab CARE-MS I 5-Year Follow-up: Durable Efficacy in the Absence of Continuous MS Therapy. Neurology 2017, 89, 1107–1116, Correction in Neurology 2018, 90, 755. [Google Scholar] [CrossRef]
- Berger, T.; Elovaara, I.; Fredrikson, S.; McGuigan, C.; Moiola, L.; Myhr, K.-M.; Oreja-Guevara, C.; Stoliarov, I.; Zettl, U.K. Alemtuzumab Use in Clinical Practice: Recommendations from European Multiple Sclerosis Experts. CNS Drugs 2017, 31, 33–50. [Google Scholar] [CrossRef]
- Kazakou, P.; Tzanetakos, D.; Vakrakou, A.G.; Tzartos, J.S.; Evangelopoulos, Μ.-E.; Anagnostouli, M.; Stathopoulos, P.; Kassi, G.N.; Stefanis, L.; Kilidireas, C.; et al. Thyroid Autoimmunity Following Alemtuzumab Treatment in Multiple Sclerosis Patients: A Prospective Study. Clin. Exp. Med. 2023, 23, 2885–2894. [Google Scholar] [CrossRef]
- Saarela, M.; Senthil, K.; Jones, J.; Tienari, P.J.; Soilu-Hänninen, M.; Airas, L.; Coles, A.; Saarinen, J.T. Hemophagocytic Lymphohistiocytosis in 2 Patients with Multiple Sclerosis Treated with Alemtuzumab. Neurology 2018, 90, 849–851. [Google Scholar] [CrossRef]
- Ferraro, D.; Camera, V.; Vitetta, F.; Zennaro, M.; Ciolli, L.; Nichelli, P.F.; Sola, P. Acute Coronary Syndrome Associated with Alemtuzumab Infusion in Multiple Sclerosis. Neurology 2018, 90, 852–854. [Google Scholar] [CrossRef]
- Cadavid, D.; Balcer, L.; Galetta, S.; Aktas, O.; Ziemssen, T.; Vanopdenbosch, L.; Frederiksen, J.; Skeen, M.; Jaffe, G.J.; Butzkueven, H.; et al. Safety and Efficacy of Opicinumab in Acute Optic Neuritis (RENEW): A Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Neurol. 2017, 16, 189–199. [Google Scholar] [CrossRef]
- Krämer, J.; Wiendl, H. What Have Failed, Interrupted, and Withdrawn Antibody Therapies in Multiple Sclerosis Taught Us? Neurotherapeutics 2022, 19, 785–807. [Google Scholar] [CrossRef]
- Vermersch, P.; Granziera, C.; Mao-Draayer, Y.; Cutter, G.; Kalbus, O.; Staikov, I.; Dufek, M.; Saubadu, S.; Bejuit, R.; Truffinet, P.; et al. Inhibition of CD40L with Frexalimab in Multiple Sclerosis. N. Engl. J. Med. 2024, 390, 589–600. [Google Scholar] [CrossRef]
- Fatima, T.; Mirza, A.; Fatima, F.; Karamat, R.I.; Ahmad, B.; Naeem, S.; Shahid, I.; Akilimali, A. Frexalimab (SAR441344) as a Potential Multiautoimmune Disorder Tackling mAB Targeting the CD40-CD40L Pathway Undergoing Clinical Trials: A Review. Ann. Med. Surg. 2024, 86, 7305–7313. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Singhal, T.; Zurawski, J.; Saraceno, T.J.; Gopalakrishnan, N.; Cain, L.; LaBarre, B.; King, D.; Bergmark, R.W.; Maxfield, A.Z.; et al. Nasal Foralumab Treatment of PIRA Induces Regulatory Immunity, Dampens Microglial Activation and Stabilizes Clinical Progression in Non-Active Secondary Progressive MS. medRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Siriratnam, P.; Huda, S.; Butzkueven, H.; van der Walt, A.; Jokubaitis, V.; Monif, M. A Comprehensive Review of the Advances in Neuromyelitis Optica Spectrum Disorder. Autoimmun. Rev. 2023, 22, 103465. [Google Scholar] [CrossRef] [PubMed]
- Carnero Contentti, E.; Correale, J. Neuromyelitis Optica Spectrum Disorders: From Pathophysiology to Therapeutic Strategies. J. Neuroinflammation 2021, 18, 208. [Google Scholar] [CrossRef]
- Huang, T.-L.; Wang, J.-K.; Chang, P.-Y.; Hsu, Y.-R.; Lin, C.-H.; Lin, K.-H.; Tsai, R.-K. Neuromyelitis Optica Spectrum Disorder: From Basic Research to Clinical Perspectives. Int. J. Mol. Sci. 2022, 23, 7908. [Google Scholar] [CrossRef]
- Shen, X. Research Progress on Pathogenesis and Clinical Treatment of Neuromyelitis Optica Spectrum Disorders (NMOSDs). Clin. Neurol. Neurosurg. 2023, 231, 107850. [Google Scholar] [CrossRef]
- Giglhuber, K.; Berthele, A. Adverse Events in NMOSD Therapy. Int. J. Mol. Sci. 2022, 23, 4154. [Google Scholar] [CrossRef]
- Rigal, J.; Pugnet, G.; Ciron, J.; Lépine, Z.; Biotti, D. Off-Label Use of Tocilizumab in Neuromyelitis Optica Spectrum Disorders and MOG-Antibody-Associated Diseases: A Case-Series. Mult. Scler. Relat. Disord. 2020, 46, 102483. [Google Scholar] [CrossRef]
- Ramirez, A.D.; Dresser, L.; Abuaf, A.; Javed, A. Treatment Options for NMOSD in Children: Effectiveness and Safety of Subcutaneous Tocilizumab (P12-8.009). Neurology 2025, 104, 5335. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Yao, M.; Yang, Z.; Li, B.; Yin, L.; Geng, X. Tocilizumab Treatment in Neuromyelitis Optica Spectrum Disorders: Updated Meta-Analysis of Efficacy and Safety. Mult. Scler. Relat. Disord. 2023, 80, 105062. [Google Scholar] [CrossRef] [PubMed]
- FDA. Approves Alexion’s Eculizumab for Neuromyelitis Optica Spectrum Disorder. Available online: https://www.centerforbiosimilars.com/view/fda-approves-alexions-eculizumab-for-neuromyelitis-optica-spectrum-disorder- (accessed on 10 September 2025).
- Food and Drug Administration. ULTOMIRIS® (ravulizumab-cwvz) Injection, for Intravenous Use. 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761108s023lbl.pdf (accessed on 10 July 2024).
- Balaban, D.T.; Levy, M.; Borrow, R.; Anderson, M.R. An Evaluation of Ravulizumab for the Treatment of Neuromyelitis Optica Spectrum Disorder. Expert. Opin. Biol. Ther. 2024, 24, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Kim, H.J.; Weinshenker, B.G.; Pittock, S.J.; Wingerchuk, D.M.; Fujihara, K.; Paul, F.; Cutter, G.R.; Marignier, R.; Green, A.J.; et al. Safety and Efficacy of Inebilizumab for the Treatment of Neuromyelitis Optica Spectrum Disorder: End-of-Study Results from the Open-Label Period of the N-MOmentum Trial. Lancet Neurol. 2024, 23, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Bennett, J.L.; Kim, H.J.; Weinshenker, B.G.; Pittock, S.J.; Wingerchuk, D.M.; Fujihara, K.; Paul, F.; Cutter, G.R.; Marignier, R.; et al. Inebilizumab for the Treatment of Neuromyelitis Optica Spectrum Disorder (N-MOmentum): A Double-Blind, Randomised Placebo-Controlled Phase 2/3 Trial. Lancet 2019, 394, 1352–1363. [Google Scholar] [CrossRef]
- Ali, F.; Sharma, K.; Anjum, V.; Ali, A. Inebilizumab-Cdon: USFDA Approved for the Treatment of NMOSD (Neuromyelitis Optica Spectrum Disorder). Curr. Drug Discov. Technol. 2022, 19, e140122193419. [Google Scholar] [CrossRef]
- Stone, J.H.; Khosroshahi, A.; Zhang, W.; Della Torre, E.; Okazaki, K.; Tanaka, Y.; Löhr, J.M.; Schleinitz, N.; Dong, L.; Umehara, H.; et al. Inebilizumab for Treatment of IgG4-Related Disease. N. Engl. J. Med. 2025, 392, 1168–1177. [Google Scholar] [CrossRef]
- Pittock, S.J.; Lennon, V.A.; McKeon, A.; Mandrekar, J.; Weinshenker, B.G.; Lucchinetti, C.F.; O’Toole, O.; Wingerchuk, D.M. Eculizumab in AQP4-IgG-Positive Relapsing Neuromyelitis Optica Spectrum Disorders: An Open-Label Pilot Study. Lancet Neurol. 2013, 12, 554–562. [Google Scholar] [CrossRef]
- Clardy, S.L.; Pittock, S.J.; Aktas, O.; Nakahara, J.; Isobe, N.; Centonze, D.; Fam, S.; Kielhorn, A.; Yu, J.C.; Jansen, J.; et al. Network Meta-Analysis of Ravulizumab and Alternative Interventions for the Treatment of Neuromyelitis Optica Spectrum Disorder. Neurol. Ther. 2024, 13, 535–549, Correction in Neurol. Ther. 2024, 13, 1313–1314. [Google Scholar] [CrossRef]
- Bennett, J.L.; Aktas, O.; Rees, W.A.; Smith, M.A.; Gunsior, M.; Yan, L.; She, D.; Cimbora, D.; Pittock, S.J.; Weinshenker, B.G.; et al. Association between B-Cell Depletion and Attack Risk in Neuromyelitis Optica Spectrum Disorder: An Exploratory Analysis from N-MOmentum, a Double-Blind, Randomised, Placebo-Controlled, Multicentre Phase 2/3 Trial. EBioMedicine 2022, 86, 104321. [Google Scholar] [CrossRef] [PubMed]
- Uplizna® (Inebilizumab-Cdon) Is Now the First and Only Fda-Approved Treatment for Igg4-Related Disease. Available online: https://www.amgen.com/newsroom/press-releases/2025/04/uplizna-inebilizumabcdon-is-now-the-first-and-only-fdaapproved-treatment-for-igg4related-disease (accessed on 4 August 2025).
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Alzheimer’s Disease: From Immunotherapy to Immunoprevention. Cell 2023, 186, 4260–4270. [Google Scholar] [CrossRef] [PubMed]
- Beshir, S.A.; Hussain, N.; Menon, V.B.; Al Haddad, A.H.I.; Al Zeer, R.A.h.; Elnour, A.A. Advancements and Challenges in Antiamyloid Therapy for Alzheimer’s Disease: A Comprehensive Review. Int. J. Alzheimers Dis. 2024, 2024, 2052142. [Google Scholar] [CrossRef]
- Liu, A.; Wang, T.; Yang, L.; Zhou, Y. The APOE-Microglia Axis in Alzheimer’s Disease: Functional Divergence and Therapeutic Perspectives-A Narrative Review. Brain Sci. 2025, 15, 675. [Google Scholar] [CrossRef]
- Topalis, V.; Voros, C.; Ziaka, M. Targeting Inflammation in Alzheimer’s Disease: Insights into Pathophysiology and Therapeutic Avenues—A Comprehensive Review. J. Geriatr. Psychiatry Neurol. 2025, 23, 8919887251361578. [Google Scholar] [CrossRef]
- Haddad, H.W.; Malone, G.W.; Comardelle, N.J.; Degueure, A.E.; Kaye, A.M.; Kaye, A.D. Aducanumab, a Novel Anti-Amyloid Monoclonal Antibody, for the Treatment of Alzheimer’s Disease: A Comprehensive Review. Health Psychol. Res. 2022, 10, 31925. [Google Scholar] [CrossRef]
- Brockmann, R.; Nixon, J.; Love, B.L.; Yunusa, I. Impacts of FDA Approval and Medicare Restriction on Antiamyloid Therapies for Alzheimer’s Disease: Patient Outcomes, Healthcare Costs, and Drug Development. Lancet Reg. Health Am. 2023, 20, 100467. [Google Scholar] [CrossRef]
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443, Correction in Drugs 2021, 81, 1701. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Hoy, S.M. Lecanemab: First Approval. Drugs 2023, 83, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Babar, Z.; Babar, A. FDA’s New Approval; Kisunla (Donanemab-Azbt): A Major Breakthrough in Alzheimer’s Care. J. Pak. Med. Assoc. 2025, 75, 1156. [Google Scholar] [CrossRef] [PubMed]
- Siebrand, C.J.; Bergo, N.J.; Lee, S.; Andersen, J.K.; Walton, C.C. Chimeric Antigen Receptors Discriminate between Tau and Distinct Amyloid-Beta Species. J. Transl. Med. 2025, 23, 605. [Google Scholar] [CrossRef]
- Walsh, S.; Howard, R.; Richard, E.; Milne, R.; Brayne, C. Interpreting the Evidence on Gantenerumab for Dominantly Inherited Alzheimer’s Disease. Lancet Neurol. 2025, 24, 634. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.; Chen, C.D.; Gordon, B.A.; Joseph-Mathurin, N.; Jack, C.R.; Koeppe, R.; Hornbeck, R.; Koudelis, D.; McKay, N.S.; Hobbs, D.A.; et al. Regional Effects of Gantenerumab on Neuroimaging Biomarkers in the DIAN-TU-001 Trial. Alzheimers Dement. 2025, 21, e70347. [Google Scholar] [CrossRef]
- Ostrowitzki, S.; Bittner, T.; Sink, K.M.; Mackey, H.; Rabe, C.; Honig, L.S.; Cassetta, E.; Woodward, M.; Boada, M.; van Dyck, C.H.; et al. Evaluating the Safety and Efficacy of Crenezumab vs Placebo in Adults with Early Alzheimer Disease: Two Phase 3 Randomized Placebo-Controlled Trials. JAMA Neurol. 2022, 79, 1113–1121. [Google Scholar] [CrossRef]
- Grimm, H.P.; Schumacher, V.; Schäfer, M.; Imhof-Jung, S.; Freskgård, P.-O.; Brady, K.; Hofmann, C.; Rüger, P.; Schlothauer, T.; Göpfert, U.; et al. Delivery of the BrainshuttleTM Amyloid-Beta Antibody Fusion Trontinemab to Non-Human Primate Brain and Projected Efficacious Dose Regimens in Humans. Mabs 2023, 15, 2261509. [Google Scholar] [CrossRef]
- Muliaditan, M.; van Steeg, T.J.; Avery, L.B.; Sun, W.; Hammond, T.R.; Hijdra, D.; Choi, S.-L.; Pillai, N.; Leksa, N.C.; Mavroudis, P.D. Translational Minimal Physiologically Based Pharmacokinetic Model for Transferrin Receptor-Mediated Brain Delivery of Antibodies. MAbs 2025, 17, 2515414. [Google Scholar] [CrossRef]
- Fleisher, A.S.; Munsie, L.M.; Perahia, D.G.S.; Andersen, S.W.; Higgins, I.A.; Hauck, P.M.; Lo, A.C.; Sims, J.R.; Brys, M.; Mintun, M.; et al. Assessment of Efficacy and Safety of Zagotenemab: Results From PERISCOPE-ALZ, a Phase 2 Study in Early Symptomatic Alzheimer Disease. Neurology 2024, 102, e208061, Correction in Neurology 2025, 104, 9. [Google Scholar] [CrossRef]
- Dam, T.; Boxer, A.L.; Golbe, L.I.; Höglinger, G.U.; Morris, H.R.; Litvan, I.; Lang, A.E.; Corvol, J.-C.; Aiba, I.; Grundman, M.; et al. Safety and Efficacy of Anti-Tau Monoclonal Antibody Gosuranemab in Progressive Supranuclear Palsy: A Phase 2, Randomized, Placebo-Controlled Trial. Nat. Med. 2021, 27, 1451–1457. [Google Scholar] [CrossRef]
- Teng, E.; Manser, P.T.; Pickthorn, K.; Brunstein, F.; Blendstrup, M.; Sanabria Bohorquez, S.; Wildsmith, K.R.; Toth, B.; Dolton, M.; Ramakrishnan, V.; et al. Safety and Efficacy of Semorinemab in Individuals with Prodromal to Mild Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Toth, B.; Brunstein, F.; Bobbala, A.; Datta, S.; Ceniceros, R.; Sanabria Bohorquez, S.M.; Anania, V.G.; Wildsmith, K.R.; Schauer, S.P.; et al. Randomized Phase II Study of the Safety and Efficacy of Semorinemab in Participants with Mild-to-Moderate Alzheimer Disease: Lauriet. Neurology 2023, 101, e1391–e1401. [Google Scholar] [CrossRef] [PubMed]
- Florian, H.; Wang, D.; Arnold, S.E.; Boada, M.; Guo, Q.; Jin, Z.; Zheng, H.; Fisseha, N.; Kalluri, H.V.; Rendenbach-Mueller, B.; et al. Tilavonemab in Early Alzheimer’s Disease: Results from a Phase 2, Randomized, Double-Blind Study. Brain 2023, 146, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.; Rasool, A.; Shaheryar, M.; Sarfraz, A.; Sarfraz, Z.; Robles-Velasco, K.; Cherrez-Ojeda, I. Donanemab for Alzheimer’s Disease: A Systematic Review of Clinical Trials. Healthcare 2022, 11, 32. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Mital, S.; Knopman, D.S.; Alexander, G.C. Cost-Effectiveness of Lecanemab for Individuals with Early-Stage Alzheimer Disease. Neurology 2024, 102, e209218. [Google Scholar] [CrossRef]
- Willis, B.A.; Lo, A.C.; Dage, J.L.; Shcherbinin, S.; Chinchen, L.; Andersen, S.W.; LaBell, E.S.; Perahia, D.G.S.; Hauck, P.M.; Lowe, S.L. Safety, Tolerability, and Pharmacokinetics of Zagotenemab in Participants with Symptomatic Alzheimer’s Disease: A Phase I Clinical Trial. J. Alzheimers Dis. Rep. 2023, 7, 1015–1024. [Google Scholar] [CrossRef]
- Shulman, M.; Kong, J.; O’Gorman, J.; Ratti, E.; Rajagovindan, R.; Viollet, L.; Huang, E.; Sharma, S.; Racine, A.M.; Czerkowicz, J.; et al. TANGO: A Placebo-Controlled Randomized Phase 2 Study of Efficacy and Safety of the Anti-Tau Monoclonal Antibody Gosuranemab in Early Alzheimer’s Disease. Nat. Aging 2023, 3, 1591–1601. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The Epidemiology of Parkinson’s Disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The Pathogenesis of Parkinson’s Disease. Lancet 2024, 403, 293–304. [Google Scholar] [CrossRef]
- MacMahon Copas, A.N.; McComish, S.F.; Fletcher, J.M.; Caldwell, M.A. The Pathogenesis of Parkinson’s Disease: A Complex Interplay Between Astrocytes, Microglia, and T Lymphocytes? Front. Neurol. 2021, 12, 666737. [Google Scholar] [CrossRef]
- Funayama, M.; Nishioka, K.; Li, Y.; Hattori, N. Molecular Genetics of Parkinson’s Disease: Contributions and Global Trends. J. Hum. Genet. 2023, 68, 125–130. [Google Scholar] [CrossRef]
- Lang, A.E.; Siderowf, A.D.; Macklin, E.A.; Poewe, W.; Brooks, D.J.; Fernandez, H.H.; Rascol, O.; Giladi, N.; Stocchi, F.; Tanner, C.M.; et al. Trial of Cinpanemab in Early Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 408–420. [Google Scholar] [CrossRef]
- Hutchison, R.M.; Fraser, K.; Yang, M.; Fox, T.; Hirschhorn, E.; Njingti, E.; Scott, D.; Bedell, B.J.; Kistner, K.M.; Cedarbaum, J.M.; et al. Cinpanemab in Early Parkinson Disease: Evaluation of Biomarker Results from the Phase 2 SPARK Clinical Trial. Neurology 2024, 102, e209137. [Google Scholar] [CrossRef]
- Games, D.; Valera, E.; Spencer, B.; Rockenstein, E.; Mante, M.; Adame, A.; Patrick, C.; Ubhi, K.; Nuber, S.; Sacayon, P.; et al. Reducing C-Terminal-Truncated Alpha-Synuclein by Immunotherapy Attenuates Neurodegeneration and Propagation in Parkinson’s Disease-like Models. J. Neurosci. 2014, 34, 9441–9454. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Taylor, K.I.; Anzures-Cabrera, J.; Marchesi, M.; Simuni, T.; Marek, K.; Postuma, R.B.; Pavese, N.; Stocchi, F.; Azulay, J.-P.; et al. Trial of Prasinezumab in Early-Stage Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, M.; He, Q.; Chen, S.; Simayi, D.; Ma, X.; Zhao, J.; Sun, X.; Yang, P.; Mao, Q.; et al. Production and Characterization of Novel Monoclonal Antibodies against Pathological Human TDP-43 Proteins. J. Neuropathol. Exp. Neurol. 2024, 83, 655–669. [Google Scholar] [CrossRef]
- van den Bos, M.A.J.; Geevasinga, N.; Higashihara, M.; Menon, P.; Vucic, S. Pathophysiology and Diagnosis of ALS: Insights from Advances in Neurophysiological Techniques. Int. J. Mol. Sci. 2019, 20, 2818. [Google Scholar] [CrossRef]
- Longinetti, E.; Regodón Wallin, A.; Samuelsson, K.; Press, R.; Zachau, A.; Ronnevi, L.-O.; Kierkegaard, M.; Andersen, P.M.; Hillert, J.; Fang, F.; et al. The Swedish Motor Neuron Disease Quality Registry. Amyotroph. Lateral Scler. Front. Degener. 2018, 19, 528–537. [Google Scholar] [CrossRef]
- Jun, K.Y.; Park, J.; Oh, K.-W.; Kim, E.M.; Bae, J.S.; Kim, I.; Kim, S.H. Epidemiology of ALS in Korea Using Nationwide Big Data. J. Neurol. Neurosurg. Psychiatry 2019, 90, 395–403. [Google Scholar] [CrossRef]
- Leighton, D.J.; Newton, J.; Stephenson, L.J.; Colville, S.; Davenport, R.; Gorrie, G.; Morrison, I.; Swingler, R.; Chandran, S.; Pal, S.; et al. Changing Epidemiology of Motor Neurone Disease in Scotland. J. Neurol. 2019, 266, 817–825. [Google Scholar] [CrossRef]
- Eisen, A.; Vucic, S.; Mitsumoto, H. History of ALS and the Competing Theories on Pathogenesis: IFCN Handbook Chapter. Clin. Neurophysiol. Pract. 2024, 9, 1–12. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, R. Current Insights in the Molecular Genetic Pathogenesis of Amyotrophic Lateral Sclerosis. Front. Neurosci. 2023, 17, 1189470. [Google Scholar] [CrossRef]
- Kiani, L. ALS Pathogenesis Linked to Actin Barrier Collapse. Nat. Rev. Neurol. 2024, 20, 1. [Google Scholar] [CrossRef]
- McGuigan, A.; Blair, H.A. Tofersen: A Review in Amyotrophic Lateral Sclerosis Associated with SOD1 Mutations. CNS Drugs 2025, 39, 903–912. [Google Scholar] [CrossRef]
- Castellanos Otero, P.; Todd, T.W.; Shao, W.; Jones, C.J.; Huang, K.; Daughrity, L.M.; Yue, M.; Sheth, U.; Gendron, T.F.; Prudencio, M.; et al. Generation and Characterization of Monoclonal Antibodies against Pathologically Phosphorylated TDP-43. PLoS ONE 2024, 19, e0298080. [Google Scholar] [CrossRef]
| Mechanism of Action | Medication | Disease | Clinical Trial Registration Number | Type of Study | Status | References |
|---|---|---|---|---|---|---|
| B cell depletion (anti-CD20) | Rituximab | MS (RRMS, SPMS, PPMS) | NCT00087529, NCT02932304 | Phase II/III, randomized, double-blind | Completed | [38,39,40,41] |
| Ocrelizumab | RRMS, PPMS | NCT01247324, NCT01194570 | Phase III, randomized, double-blind | Approved (FDA/EMA) | [41,42,43] | |
| Ofatumumab | RRMS | NCT02077361 | Phase III, randomized, double-blind | Approved (FDA/EMA) | [44,45] | |
| Ublituximab | RRMS | NCT03277261, NCT03277248 | Phase III, randomized, double-blind | Approved (FDA) | [44,46] | |
| Inhibition of leukocyte migration (anti-α4 integrin) | Natalizumab | RRMS, POMS | NCT00027300 | Phase III, randomized, double-blind | Approved (FDA/EMA) | [42,47,48,49,50,51,52,53] |
| Immune reset (anti-CD52) | Alemtuzumab | RRMS | NCT00548405, NCT00530348 | Phase III, randomized, open-label | Approved (restricted use EMA) | [52,54,55,56,57,58] |
| Remyelination (LINGO-1 inhibition) | Opicinumab | MS (RRMS, acute optic neuritis) | NCT01721161, NCT01894684 | Phase II, randomized, double-blind | Completed-Discontinued | [59,60] |
| T-B cell interaction blockade (anti-CD40L) | Frexalimab | MS | NCT04879628 | Phase II, randomized, double-blind | Active, not recruiting | [61,62] |
| Anti-CD3 mAb | Foralumab | SPMS | NCT04594085 | Open-label | Completed | [63] |
| BTKi | Tolebrutinib | RRMS | NCT04410991 | Phase III, randomized, double-blind | Completed | [64,65] |
| PPMS | NCT04458051 | |||||
| SPMS | NCT04411641 | |||||
| Fenebrutinib | RRMS | NCT05119569 | Phase II, randomized, double-blind | Active, not recruiting | [66] |
| Mechanism of Action | Medication | Disease | Clinical Trial Registration Number | Type of Study | Status | References |
|---|---|---|---|---|---|---|
| IL-6 receptor blockade | Satralizumab | NMOSD (AQP4-IgG+) | NCT02073279, NCT02028884 | Phase III, randomized, double-blind | Approved (FDA/EMA) | [90] |
| Tocilizumab | NMOSD (AQP4-IgG+) | NCT02028884 | Phase II, open-label, extension | Off-label | [91,92,93] | |
| Complement inhibition (C5 blockade) | Eculizumab | NMOSD (AQP4-IgG+) | NCT01892345 | Phase III, randomized, double-blind | Approved (FDA/EMA) | [94] |
| Ravulizumab | NMOSD (AQP4-IgG+) | NCT03330418 | Phase III, randomized, double-blind | Approved (FDA/EMA) | [95,96] | |
| B cell depletion (anti-CD19) | Inebilizumab | NMOSD (AQP4-IgG+) | NCT02200770 (NMOmentum) | Phase II/III, randomized, double-blind | Approved (FDA) | [97,98,99,100] |
| Mechanism of Action | Medication | Disease | Clinical Trial Registration Number | Type of Study | Status | References |
|---|---|---|---|---|---|---|
| Anti-amyloid (Aβ fibrils/plaques) | Aducanumab | AD (MCI, mild dementia) | NCT02477800NCT02484547 | Phase III, randomized, double-blind | FDA accelerated approval (2021) | [110,111,112] |
| Anti-amyloid (Aβ protofibrils) | Lecanemab | AD (early AD) | NCT01767311NCT03887455 | Phase II/III, randomized, double-blind | FDA full approval (2023), EMA/Japan approved | [113,114] |
| Anti-amyloid (Aβ pyroglutamate) | Donanemab | AD (early AD, ApoE ε4 non-carriers/heterozygotes) | NCT04437511 | Phase III, randomized, double-blind | FDA full approval (2024), EMA positive opinion (2025) | [115] |
| Anti-amyloid (N3pG-Aβ) | Remternetug | AD (early AD) | NCT05869306NCT05869319 | Phase III, randomized, double-blind | Ongoing | [116] |
| Anti-amyloid (Aβ plaques) | Gantenerumab | AD (early AD) | NCT03443973NCT03444870 | Phase III, randomized, double-blind | Terminated | [117,118] |
| Anti-amyloid (Aβ oligomers) | Crenezumab | AD (prodromal to mild) | NCT02670083NCT03114657 | Phase III, randomized, double-blind | Terminated | [119] |
| Anti-amyloid with brain shuttle | Trontinemab | AD (early AD) | NCT04639050 | Phase II, randomized, double-blind | Active, not recruiting | [120,121] |
| Anti-tau | Zagotenemab | AD (early symptomatic) | NCT03019536 | Phase II, randomized, double-blind | Completed | [122] |
| Anti-tau | Gosuranemab | AD | NCT03352557NCT03068468 | Phase II, randomized, double-blind | Terminated | [123] |
| Anti-tau | Semorinemab | AD (mild to moderate) | NCT03828747NCT03352557 | Phase II, randomized, double-blind | Completed | [124,125] |
| Anti-tau | Tilavonemab | AD | NCT02880956 | Phase II, randomized, double-blind | Completed | [126] |
| Mechanism of Action | Medication | Disease | Clinical Trial Registration Number | Type of Study | Status | References |
|---|---|---|---|---|---|---|
| Anti-α-synuclein | Cinpanemab | PD (early PD) | NCT03318523 (SPARK) | Phase II, randomized, double-blind | Terminated | [135,136] |
| Anti-α-synuclein | Prasinezumab | PD (early PD) | NCT03100149 (PASADENA) | Phase II, randomized, double-blind | Active, not recruiting | [137,138] |
| Anti-TDP-43 (pS409/410) | Experimental mAbs | ALS/FTD | - | Preclinical/exploratory | Ongoing (preclinical) | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skarlis, C.; Angelopoulou, E.; Rentzos, M.; Papageorgiou, S.G.; Anagnostouli, M. Monoclonal Antibodies as Therapeutic Agents in Autoimmune and Neurodegenerative Diseases of the Central Nervous System: Current Evidence on Molecular Mechanisms and Future Directions. Int. J. Mol. Sci. 2025, 26, 9398. https://doi.org/10.3390/ijms26199398
Skarlis C, Angelopoulou E, Rentzos M, Papageorgiou SG, Anagnostouli M. Monoclonal Antibodies as Therapeutic Agents in Autoimmune and Neurodegenerative Diseases of the Central Nervous System: Current Evidence on Molecular Mechanisms and Future Directions. International Journal of Molecular Sciences. 2025; 26(19):9398. https://doi.org/10.3390/ijms26199398
Chicago/Turabian StyleSkarlis, Charalampos, Efthalia Angelopoulou, Michail Rentzos, Sokratis G. Papageorgiou, and Maria Anagnostouli. 2025. "Monoclonal Antibodies as Therapeutic Agents in Autoimmune and Neurodegenerative Diseases of the Central Nervous System: Current Evidence on Molecular Mechanisms and Future Directions" International Journal of Molecular Sciences 26, no. 19: 9398. https://doi.org/10.3390/ijms26199398
APA StyleSkarlis, C., Angelopoulou, E., Rentzos, M., Papageorgiou, S. G., & Anagnostouli, M. (2025). Monoclonal Antibodies as Therapeutic Agents in Autoimmune and Neurodegenerative Diseases of the Central Nervous System: Current Evidence on Molecular Mechanisms and Future Directions. International Journal of Molecular Sciences, 26(19), 9398. https://doi.org/10.3390/ijms26199398







