Hepatocellular EVs Regulate Lipid Metabolism via SIRT1/SREBP−1c/PGC−1α Signaling in Primary Calf Hepatocytes
Abstract
1. Introduction
2. Results
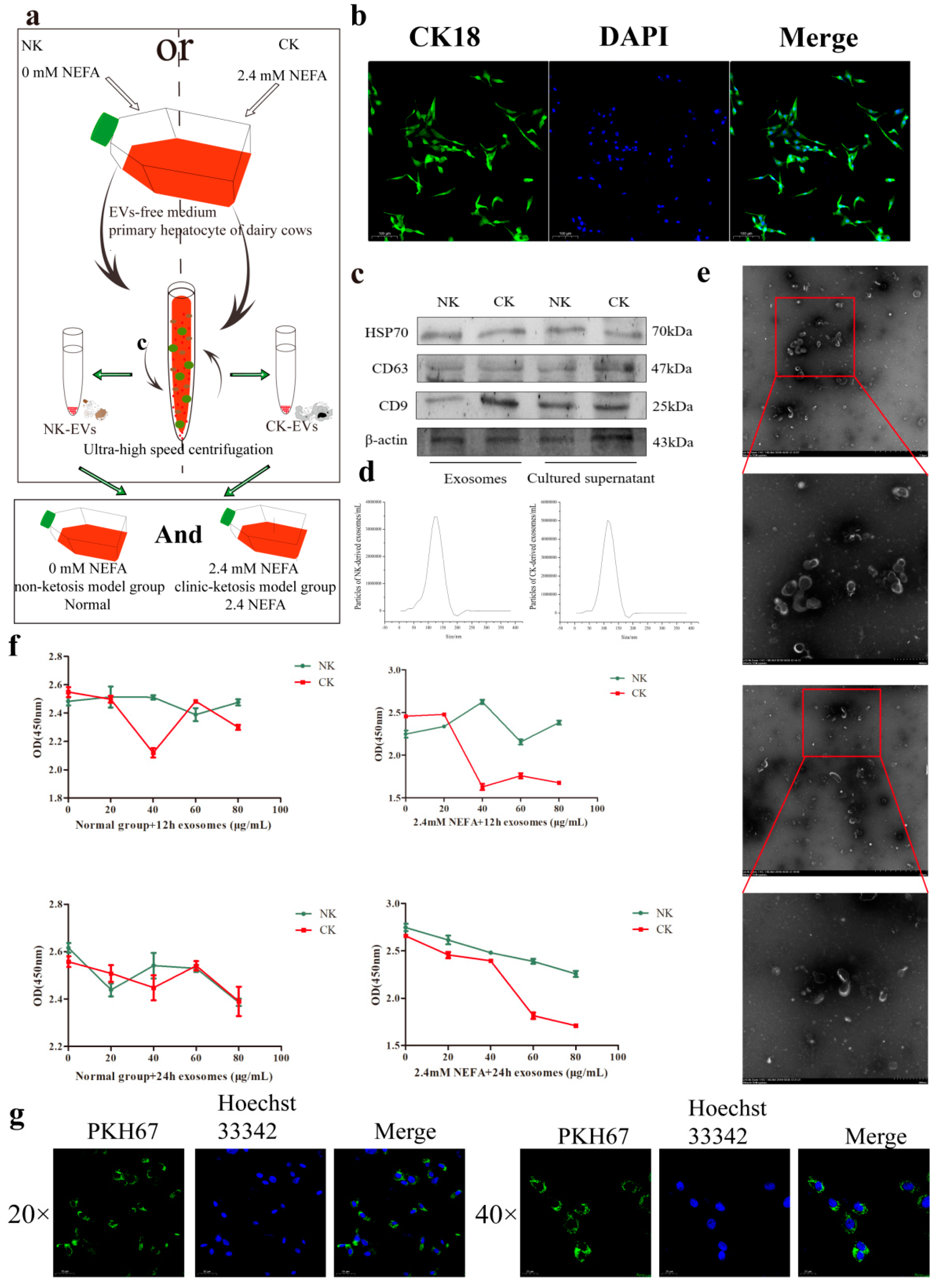
2.1. Characterization of Hepatic Cells and EVs
2.2. Determination of the Optimum Concentration and Treatment Time of EVs
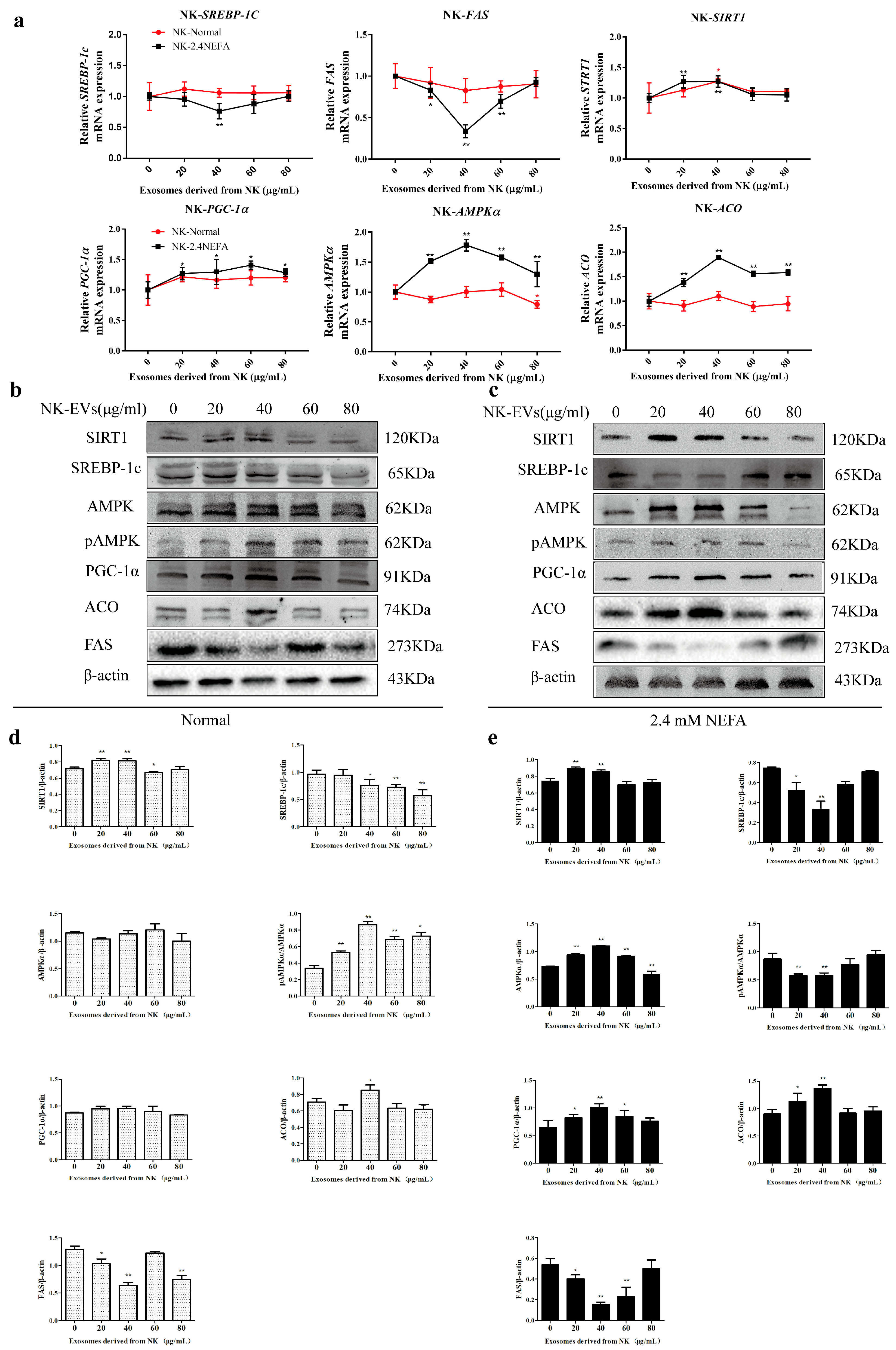
2.3. Effects of NK−Derived EVs on SIRT1/SREBP−1c/PGC−1α Pathway-Related Gene and Protein Expression in Normal Calf Hepatocytes and Those Treated with NEFAs at 2.4 mM
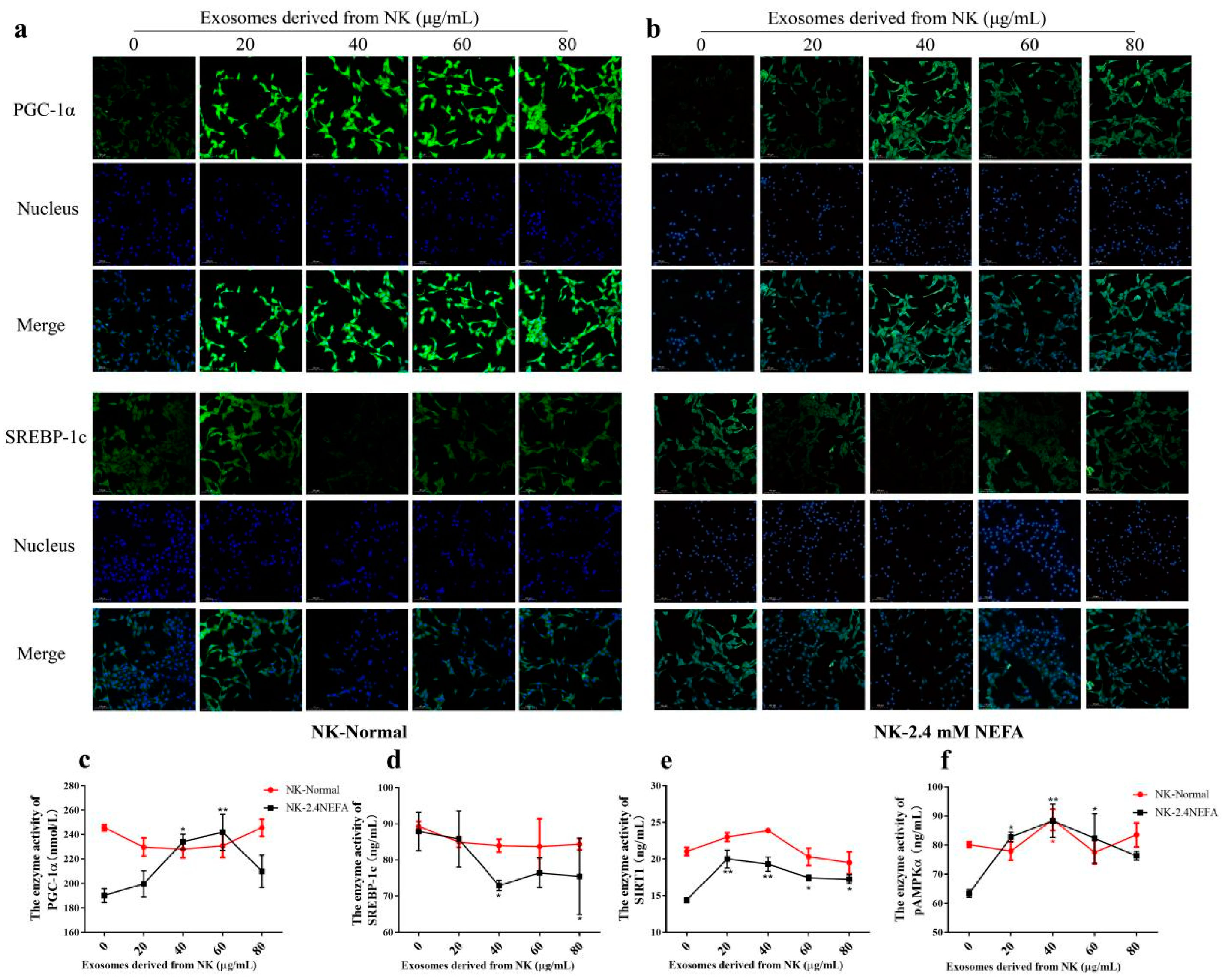
2.4. Effects of NK EVs on SREBP−1c and PGC−1α Expression and SIRT1/SREGBP−1c/PGC−1α Pathway-Related Enzyme Activity in Normal and 2.4 mM NEFA-Treated Dairy Cow Hepatocytes
2.5. Effects of CK EVs on the Relative mRNA and Protein Expression Levels of Genes Associated with the SIRT1/SREBP−1c/PGC−1α Pathway in Normal and 2.4 mM NEFA−Treated Dairy Cow Hepatocytes
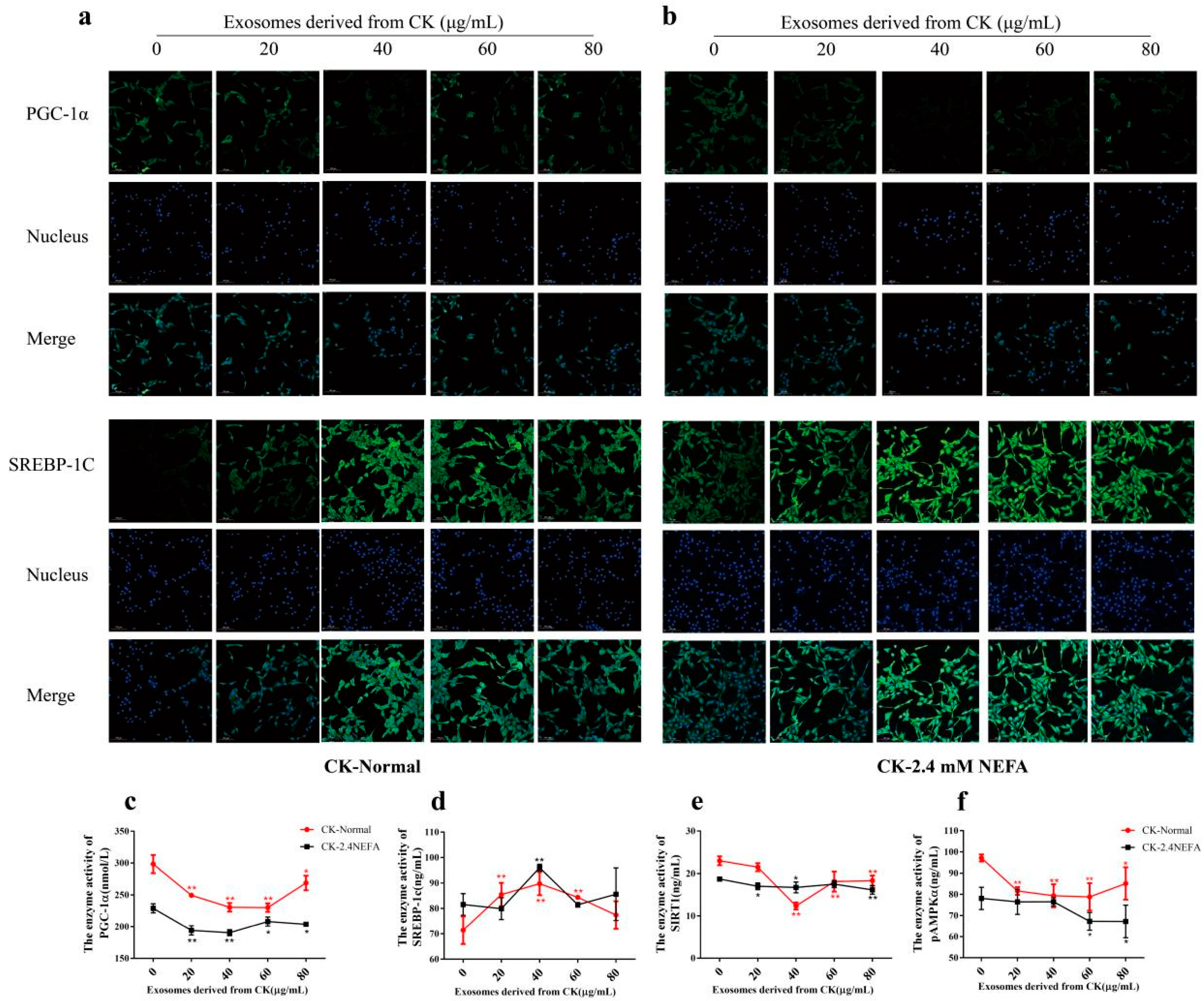
2.6. Effects of CK EVs on SREBP−1c and PGC−1α Expression and SIRT1/SREGBP−1c/PGC−1α Pathway−Related Enzyme Activity in Normal and 2.4 mM NEFA-Treated Dairy Cow Hepatocytes
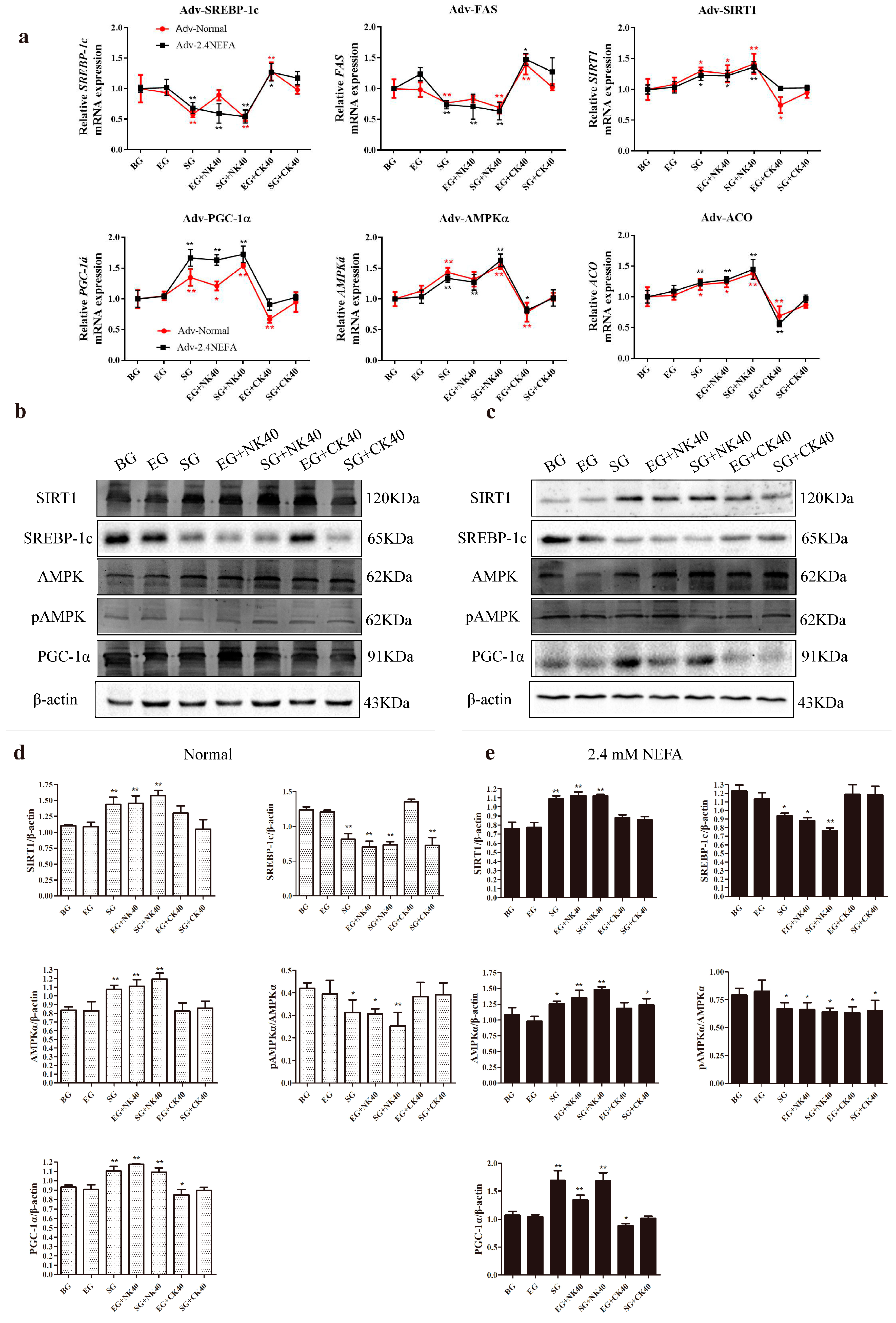
2.7. Effects of SIRT1 Overexpression and Calf Hepatocyte EVs on SIRT1/SREBP−1c/PGC−1α Pathway mRNA and Protein Expression in Normal Hepatocytes and Those Treated with NEFAs at 2.4 mM
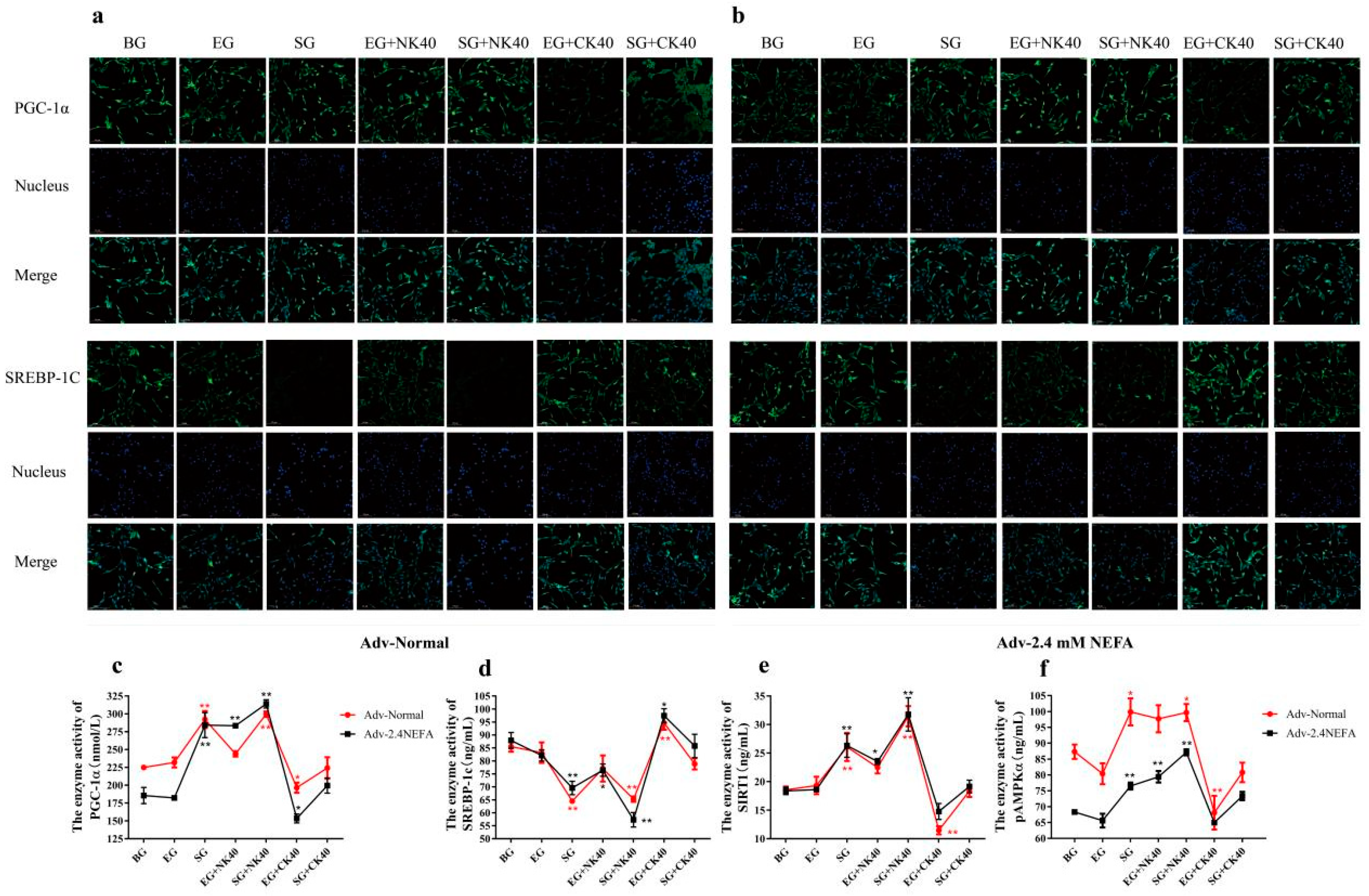
2.8. Effects of SIRT1 Overexpression and Calf Hepatocyte EVs on SREBP−1c and PGC−1α Immunofluorescence and the Activities of Enzymes Related to the SIRT1/SREBP−1c/PGC−1α Pathway in Normal Hepatocytes and Those Treated with NEFAs at 2.4 mM
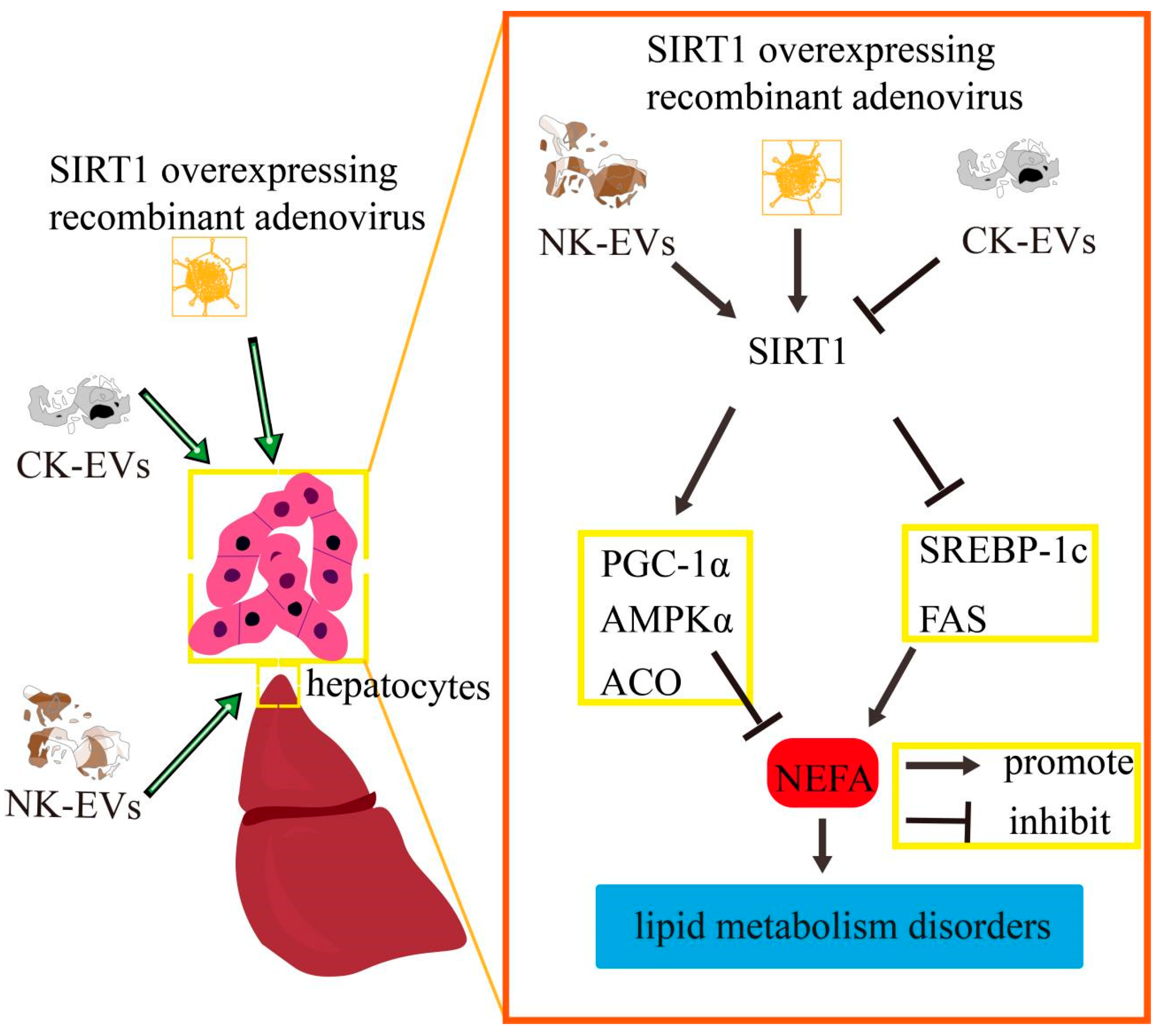
3. Discussion
4. Materials and Methods
4.1. Cells Culture
4.2. EV Isolation and Characterization
4.3. Nanoparticle Tracking Analysis (NTA)
4.4. Transmission Electron Microscopy (TEM)
4.5. Fusion of EVs and Calf Hepatocytes
4.6. Cell Viability Assay
4.7. Construction of the SIRT1-Overexpressing Adenovirus
4.8. Quantitative Real Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.9. Enzyme Linked Immunosorbent Assay (ELISA)
4.10. Western Blotting Assays
4.11. Immunofluorescence
4.12. Statistical Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACO | Acyl-coa oxidase |
| AMPKα | Adenosine 5′-monophosphate-activated protein kinase α |
| BG | Blank group |
| CCK-8 | Cell counting kit 8 |
| CK | Clinical ketosis |
| CK40 | 40 μg/mlck−derived evs |
| DAPI | 4′,6-diamidino-2-phenylindole |
| EG | Empty carrier group |
| EVs | Extracellular vesicles |
| FAS | Fatty acid synthase |
| FBS | Fetal bovine serum |
| FITC | Fluorescein isothiocyanate |
| hep-EVs | hepatocyte−derived extracellular vesicles |
| HSP70 | Heat shock protein 70 |
| NEB | Negative energy balance |
| NEFA | Nonesterified fatty acid |
| NF-κB | Nuclear transcription factor-κb |
| NK | Non-ketosis |
| NK40 | 40 μg/mlnk−derived evs |
| NTA | Nanoparticle tracking analysis |
| PPAR | Peroxisome proliferator-activated receptor |
| qRT-PCR | Quantitative real-time reverse transcription polymerase chain reaction |
| ROS | Reactive oxygen species |
| SG | Sirt1-overexpression group |
| SIRT1 | Silent information regulator 1 |
| SREBP−1c | Sterol regulatory element-binding protein 1c |
| TEM | Transmission electron microscopy |
| TG | Triglyceride |
References
- Rasmussen, P.; Barkema, H.W.; Osei, P.P.; Taylor, J.; Shaw, A.P.; Conrady, B.; Chaters, G.; Muñoz, V.; Hall, D.C.; Apenteng, O.O.; et al. Global losses due to dairy cattle diseases: A comorbidity-adjusted economic analysis. J. Dairy Sci. 2024, 107, 6945–6970. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.H.; Mauck, J.; Loor, J.J.; Wei, B.; Shen, B.Y.; Wang, Y.Z.; Zhao, C.X.; Zhu, X.Y.; Wang, J.G. Plasma and milk metabolomics profiles in dairy cows with subclinical and clinical ketosis. J. Dairy Sci. 2024, 107, 6340–6357. [Google Scholar] [CrossRef]
- Fan, X.; Xu, J.; Hu, Y.; Wang, K.; Zhao, Y.; Cai, J.; Zhang, X.; Pan, B.; Xu, A.; Chen, Y.; et al. Effect of high NEFA concentration on lipid metabolism disorders in hepatocytes based on lipidomics. Front. Pharmacol. 2024, 15, 1372296. [Google Scholar] [CrossRef] [PubMed]
- Mir, B.; Goettsch, C. Extracellular Vesicles as Delivery Vehicles of Specific Cellular Cargo. Cells 2020, 9, 1601. [Google Scholar] [CrossRef]
- Pérez-Boza, J.; Pegtel, D.M. Exosomes take (germinal) center stage. EMBO Rep. 2020, 21, e50190. [Google Scholar] [CrossRef]
- Eguchi, A.; Iwasa, M.; Nakagawa, H. Extracellular vesicles in fatty liver disease and steatohepatitis: Role as biomarkers and therapeutic targets. Liver Int. 2023, 43, 292–298. [Google Scholar] [CrossRef]
- Muñoz-Hernández, R.; Rojas, Á.; Gato, S.; Gallego, J.; Gil-Gómez, A.; Castro, M.J.; Ampuero, J.; Romero-Gómez, M. Extracellular Vesicles as Biomarkers in Liver Disease. Int. J. Mol. Sci. 2022, 23, 16217. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, X.; Qiu, T.; An, Y.; Zhang, G.; Li, Q.; Jiang, L.; Yang, G.; Cao, J.; Sun, X.; et al. Exosomal miR−181a-2-3p derived from citreoviridin-treated hepatocytes activates hepatic stellate cells trough inducing mitochondrial calcium overload. Chem.-Biol. Interact. 2022, 358, 109899. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cheng, F.; Zhang, Z.; Xu, R.; Shi, H.; Yan, Y. The role of hepatocyte-derived extracellular vesicles in liver and extrahepatic diseases. Biomed. Pharmacother. 2024, 180, 117502. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, T.; Chen, T.; Sun, J.; Luo, J.; He, J.; Wei, L.; Zeng, B.; Zhang, H.; Li, W.; et al. Hepatic exosome-derived miR−130a-3p attenuates glucose intolerance via suppressing PHLPP2 gene in adipocyte. Metabolism 2020, 103, 154006. [Google Scholar] [CrossRef]
- Li, Y.; Luan, Y.; Li, J.; Song, H.; Li, Y.; Qi, H.; Sun, B.; Zhang, P.; Wu, X.; Liu, X.; et al. Exosomal miR−199a-5p promotes hepatic lipid accumulation by modulating MST1 expression and fatty acid metabolism. Hepatol. Int. 2020, 14, 1057–1074. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, H.; Bai, Y.; Zhi, F. Gut dysbiosis-derived exosomes trigger hepatic steatosis by transiting HMGB1 from intestinal to liver in mice. Biochem. Biophys. Res. Commun. 2019, 509, 767–772. [Google Scholar] [CrossRef]
- Li, X.; Huang, W.; Gu, J.; Du, X.; Lei, L.; Yuan, X.; Sun, G.; Wang, Z.; Li, X.; Liu, G. SREBP−1c overactivates ROS-mediated hepatic NF-κB inflammatory pathway in dairy cows with fatty liver. Cell. Signal. 2015, 27, 2099–2109. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Wang, Z.; Yao, J.; Zhao, B.; Liu, G. Non-esterified fatty acids promote expression and secretion of angiopoietin-like protein 4 in calf hepatocytes cultured in vitro. Mol. Cell Biochem. 2015, 401, 141–146. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Guan, Y.; Li, X.; Lei, L.; Liu, J.; Yin, L.; Liu, G.; Wang, Z. Acetic acid activates the AMP-activated protein kinase signaling pathway to regulate lipid metabolism in bovine hepatocytes. PLoS ONE 2013, 8, e67880. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, Y.; Zhang, Y.; Long, M.; Guo, Y.; Wang, Z.; Li, X.; Zhang, C.; Li, X.; He, J.; et al. Effect of non-esterified fatty acids on fatty acid metabolism-related genes in calf hepatocytes cultured in vitro. Cell. Physiol. Biochem. 2013, 32, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, X.; Zhao, B. PSXIII-41 Fibroblast growth factor 21 promotes lipid oxidation and transportation while inhibits lipogenesis via AMP-activated protein kinase signaling pathway in bovine hepatocytes. J. Anim. Sci. 2019, 97 (Suppl. 3), 368. [Google Scholar] [CrossRef]
- Loiklung, C.; Sukon, P.; Thamrongyoswittayakul, C. Global prevalence of subclinical ketosis in dairy cows: A systematic review and meta-analysis. Res. Vet. Sci. 2022, 144, 66–76. [Google Scholar] [CrossRef]
- Ha, S.; Kang, S.; Han, M.; Lee, J.; Chung, H.; Oh, S.-I.; Kim, S.; Park, J. Predicting ketosis during the transition period in Holstein Friesian cows using hematological and serum biochemical parameters on the calving date. Sci. Rep. 2022, 12, 853. [Google Scholar] [CrossRef]
- Ruppert, P.M.M.; Kersten, S. Mechanisms of hepatic fatty acid oxidation and ketogenesis during fasting. Trends Endocrinol. Metab. 2024, 35, 107–124. [Google Scholar] [CrossRef]
- Li, Y.; Ding, H.; Liu, L.; Song, Y.; Du, X.; Feng, S.; Wang, X.; Li, X.; Wang, Z.; Li, X.; et al. Non-esterified Fatty Acid Induce Dairy Cow Hepatocytes Apoptosis via the Mitochondria-Mediated ROS-JNK/ERK Signaling Pathway. Front. Cell Dev. Biol. 2020, 8, 245, Erratum in Front. Cell Dev. Biol. 2020, 8, 462. [Google Scholar]
- Zhao, T.; Yang, X.; Duan, G.; Chen, J.; He, K.; Chen, Y.X.; Luo, S.Z. Phosphorylation-regulated phase separation of syndecan-4 and syntenin promotes the biogenesis of exosomes. Cell Prolif. 2024, 57, e13645. [Google Scholar] [CrossRef]
- Povero, D.; Yamashita, H.; Ren, W.; Subramanian, M.G.; Myers, R.P.; Eguchi, A.; Simonetto, D.A.; Goodman, Z.D.; Harrison, S.A.; Sanyal, A.J.; et al. Characterization and Proteome of Circulating Extracellular Vesicles as Potential Biomarkers for NASH. Hepatol. Commun. 2020, 4, 1263–1278. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Seo, W.; Kim, D.K.; Moon, P.G.; Kim, S.H.; Lee, B.H.; Song, B.J.; Baek, M.C. Exogenous exosomes from mice with acetaminophen-induced liver injury promote toxicity in the recipient hepatocytes and mice. Sci. Rep. 2018, 8, 16070. [Google Scholar] [CrossRef]
- Yan, C.; Tian, X.; Li, J.; Liu, D.; Ye, D.; Xie, Z.; Han, Y.; Zou, M.H. A High-Fat Diet Attenuates AMPK α1 in Adipocytes to Induce Exosome Shedding and Nonalcoholic Fatty Liver Development In Vivo. Diabetes 2021, 70, 577–588, Erratum in Diabetes 2023, 72, 1035. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Y.; Kim, I.-m.; Liu, Y.; Cai, J.; Berman, A.E.; Nilsson, K.R.; Weintraub, N.L.; Tang, Y. Electrical Stimulation Increases the Secretion of Cardioprotective Extracellular Vesicles from Cardiac Mesenchymal Stem Cells. Cells 2023, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Berumen, S.G.; Bunn, K.E.; Pua, H.H.; Rafat, M. Extracellular vesicles: Mediators of intercellular communication in tissue injury and disease. Cell Commun. Signal. 2021, 19, 104. [Google Scholar] [CrossRef]
- Veshkini, A.; Gnott, M.; Vogel, L.; Kröger-Koch, C.; Tuchscherer, A.; Tröscher, A.; Bernabucci, U.; Trevisi, E.; Starke, A.; Mielenz, M.; et al. Abomasal infusion of essential fatty acids and conjugated linoleic acid during late pregnancy and early lactation affects immunohematological and oxidative stress markers in dairy cows. J. Dairy Sci. 2023, 106, 5096–5114. [Google Scholar] [CrossRef]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwivedi, U.N.; Kakkar, P. AMP-activated protein kinase: An energy sensor and survival mechanism in the reinstatement of metabolic homeostasis. Exp. Cell. Res. 2023, 428, 113614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ding, H.; Liu, C.; Huang, Y.; Tai, W.; Feng, S.; Wang, X.; Zhao, C.; Li, Y. Circulating exosome-mediated AMPKalpha-SIRT1 pathway regulates lipid metabolism disorders in calf hepatocytes. Res. Vet. Sci. 2024, 169, 105177. [Google Scholar] [CrossRef]
- Pang, J.; Yin, L.; Jiang, W.; Wang, H.; Cheng, Q.; Jiang, Z.; Cao, Y.; Zhu, X.; Li, B.; Qian, S.; et al. Sirt1-mediated deacetylation of PGC−1alpha alleviated hepatic steatosis in type 2 diabetes mellitus via improving mitochondrial fatty acid oxidation. Cell. Signal. 2024, 124, 111478. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Hardie, D.G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 2023, 24, 255–272. [Google Scholar] [CrossRef]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome proliferator-activated receptor gamma coactivator−1 (PGC−1) family in physiological and pathophysiological process and diseases. Signal Transduct. Target. Ther. 2024, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, C.; Chen, X.; Fu, E.; Zhang, K.; Tao, H.; Han, Z.; Han, Z.C.; Li, Z. Phosphatidylserine-mediated uptake of extracellular vesicles by hepatocytes ameliorates liver ischemia-reperfusion injury. Apoptosis 2024, 30, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jin, S.; Huang, W.; Li, Y.; Wang, J.; Ling, X.; Huang, Y.; Hu, Y.; Li, C.; Meng, Y.; et al. LPS-induced macrophage HMGB1-loaded extracellular vesicles trigger hepatocyte pyroptosis by activating the NLRP3 inflammasome. Cell Death Discov. 2021, 7, 337. [Google Scholar] [CrossRef]
- Rakshe, P.S.; Dutta, B.J.; Chib, S.; Maurya, N.; Singh, S. Unveiling the interplay of AMPK/SIRT1/PGC−1α axis in brain health: Promising targets against aging and NDDs. Ageing Res. Rev. 2024, 96, 102255. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Zhu, N.; Li, M.; Wang, Z.; Lin, X. CTRP6-mediated cardiac protection in heart failure via the AMPK/SIRT1/PGC−1alpha signalling pathway. Exp. Physiol. 2024, 109, 2031–2045. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Zhou, W.; Lu, C.; Ji, T.; Yang, W.; Jin, Z.; Tian, Y.; Lei, W.; Wu, S.; et al. SIRT1/PGC−1α signaling activation by mangiferin attenuates cerebral hypoxia/reoxygenation injury in neuroblastoma cells. Eur. J. Pharmacol. 2021, 907, 174236. [Google Scholar] [CrossRef]
- Liu, S.; Cui, F.; Ning, K.; Wang, Z.; Fu, P.; Wang, D.; Xu, H. Role of irisin in physiology and pathology. Front. Endocrinol. 2022, 13, 962968. [Google Scholar] [CrossRef]
- Li, Y.; Ding, H.; Wang, X.; Liu, L.; Huang, D.; Zhang, R.; Guo, L.; Wang, Z.; Li, X.; Liu, G.; et al. High levels of acetoacetate and glucose increase expression of cytokines in bovine hepatocytes, through activation of the NF-kappaB signalling pathway. J. Dairy Res. 2016, 83, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.A.; Majumdar, N.; Yang, P.; Subbaiah, P.V.; Kineman, R.D.; Cordoba-Chacon, J. Hepatocyte-specific, PPARγ-regulated mechanisms to promote steatosis in adult mice. J. Endocrinol. 2017, 232, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Nasukawa, T.; Uchiyama, J.; Taharaguchi, S.; Ota, S.; Ujihara, T.; Matsuzaki, S.; Murakami, H.; Mizukami, K.; Sakaguchi, M. Virus purification by CsCl density gradient using general centrifugation. Arch. Virol. 2017, 162, 3523–3528. [Google Scholar] [CrossRef] [PubMed]

| Genes | Primer Sequences | Accession Number | Product Size/bp |
|---|---|---|---|
| SIRT1 | F: GCTTACAGGGCCTATCCAG R: CATGCGAGGCTCTATCATCT | NM_001192980.2 | 186 |
| AMPKα | F: ACCAAGGTGTAAGGAAAGCA R: ACGGGTTTACAACCTTCCAT | NM_001109802.2 | 126 |
| FAS | F: TTCTTAGACAAGCCCCTCTC R: TAGGTAGTTCGGAGCATCTG | NM_174662.2 | 150 |
| ACO | F: AGACCACTATTACAAGGCCG R: AATACGTGCATGTGTGGTTG | NM_001205495.1 | 127 |
| PGC1A | F: GCCCCAGGTGGTGGA R: GTTACTTTCCAGAGGAGGCA | NM_177945.3 | 109 |
| SREBP1C | F: GCTGACCGACATAGAAGACAT R: CCAGGAAGCCTTCAAGTGAG | NM_001113302.1 | 188 |
| ACTB | F: GCCCTGAGGCTCTCTTCCA R: GCGGATGTCGACGTCACA | NM_173979.3 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Tang, J.; Liu, L.; Zhao, C.; Feng, S.; Wang, X.; Ding, H.; Li, Y. Hepatocellular EVs Regulate Lipid Metabolism via SIRT1/SREBP−1c/PGC−1α Signaling in Primary Calf Hepatocytes. Int. J. Mol. Sci. 2025, 26, 9392. https://doi.org/10.3390/ijms26199392
Zhang D, Tang J, Liu L, Zhao C, Feng S, Wang X, Ding H, Li Y. Hepatocellular EVs Regulate Lipid Metabolism via SIRT1/SREBP−1c/PGC−1α Signaling in Primary Calf Hepatocytes. International Journal of Molecular Sciences. 2025; 26(19):9392. https://doi.org/10.3390/ijms26199392
Chicago/Turabian StyleZhang, Daoliang, Jishun Tang, Leihong Liu, Chang Zhao, Shibin Feng, Xichun Wang, Hongyan Ding, and Yu Li. 2025. "Hepatocellular EVs Regulate Lipid Metabolism via SIRT1/SREBP−1c/PGC−1α Signaling in Primary Calf Hepatocytes" International Journal of Molecular Sciences 26, no. 19: 9392. https://doi.org/10.3390/ijms26199392
APA StyleZhang, D., Tang, J., Liu, L., Zhao, C., Feng, S., Wang, X., Ding, H., & Li, Y. (2025). Hepatocellular EVs Regulate Lipid Metabolism via SIRT1/SREBP−1c/PGC−1α Signaling in Primary Calf Hepatocytes. International Journal of Molecular Sciences, 26(19), 9392. https://doi.org/10.3390/ijms26199392





