New Concepts of Regeneration and Renewal of Adrenal Chromaffin Cells
Abstract
1. Introduction
2. The Origin of the Chromaffin Cells
3. Regulation of Chromaffin Cell Embryonic Development
3.1. Glucocorticoid Signaling
3.2. Transcriptional Regulation
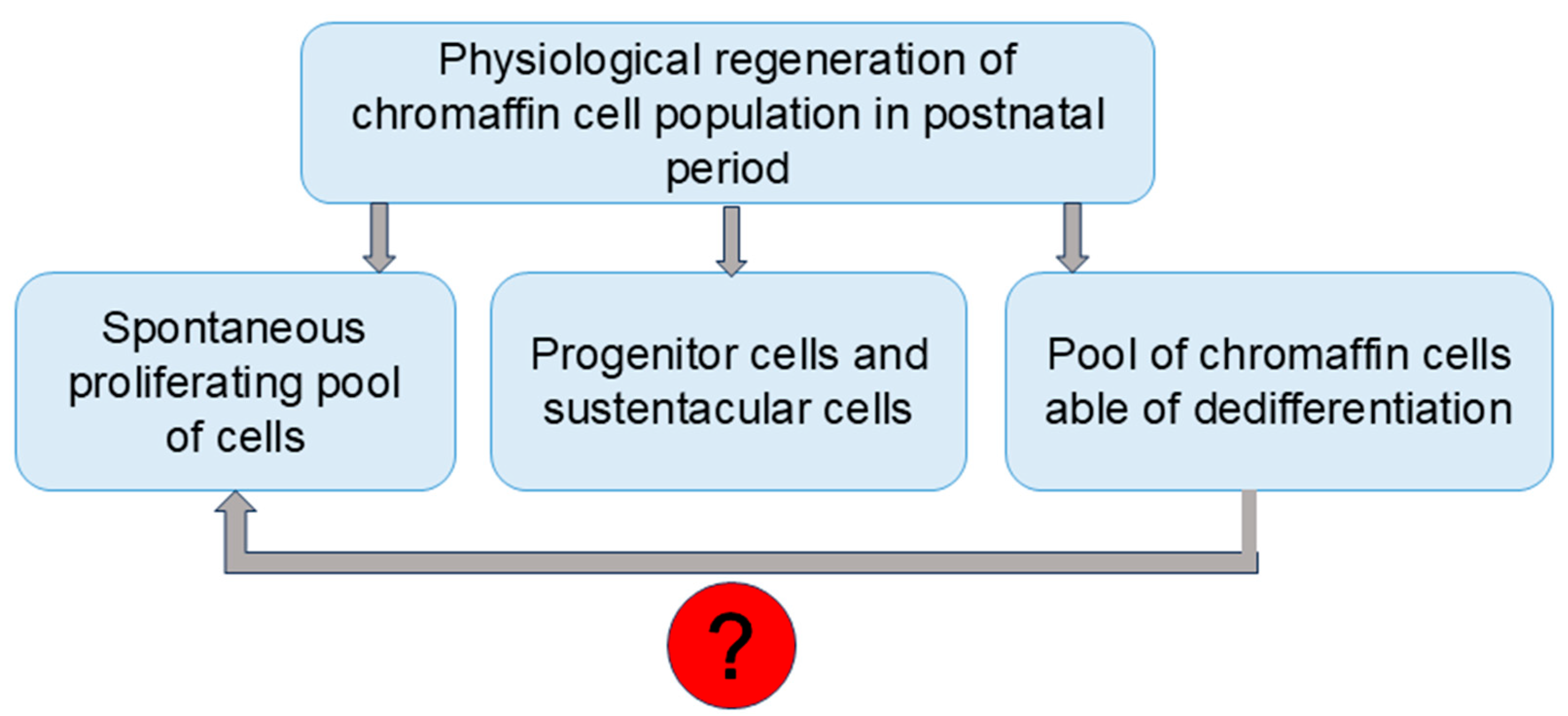
4. Sources of Chromaffin Cell Postnatal Maintenance and Reparation
4.1. Proliferating Cells in the Adrenal Medulla
| Method Used | Rate of Cell Division | Animal, Strain | Age | Reference |
|---|---|---|---|---|
| Mitotic count | 3 mitoses in 1 section No mitoses | Albino rat | 56 days 340 days | Jackson C.M., 1919 [62] |
| Mitotic count following colchicine | Average of 3 mitoses per section No mitoses | Albino rats of both sexes | From birth to 63 days | Mitchell R. et al., 1948 [60] |
| [3H]thymidine | 0.5% medullary cells labeled | Sherman albino rat | 200 g | Messier B. et al., 1960 [65] |
| Mitotic count | Average of 20 dividing cells per adrenal (0.004%) | female Sprague–Dawley rats | 22–36 weeks | Malvaldi G. et al., 1968 [63] |
| Light and electron microscopic autoradiography | 9.4% in 2-week-old and less than 1% in adult | Mus musculus | From 2 weeks to adulthood | Jureska W. et al., 1978 [67] |
| Mitotic count following colcemid | 0.3% of the cells divide | Long–Evans and Sprague–Dawley rats | 9 months | Tischler A. et al., 1988 [68] |
| [3H]thymidine autoradiographia | Fluctuating mitotic rate from 1 to 70% | CS1 mice | Newborn within 24 h | Monkhouse W. et al., 1988 [59] |
| Mitotic count following Colcemid BrdU 6 h before Killing | 1 cell or less per section Up to 7 labeled cells per section | Long–Evans and Sprague–Dawley rats | 9 months | Tischler A. et al., 1989 [69] |
| Percentage of metaphases | 0.5–0.8% | Wistar rats | 300–350 gg (12–15 weeks) | Plecas B. et al., 1990 [64] |
| BrdU 50 μg/kg i.p. BrdU 1 μg/kg/h i.p. | 0.92 + 0.09% after 192 h 41.96 + 1.43 after 1752 h | Sprague–Dawley rats | 22–36 weeks | Verhofstad A., 1993 [66] |
| Ki-67 immunohistochemical evaluation | 2.11 ± 0.15% in 6-week-old 1.65 ± 0.10 in 10-week-old | Wister rat | 6–10 weeks old | Yaglova N.V. et al., 2018 [61] |
4.2. Stem/Progenitor Cells in the Adrenal Medulla
5. New Findings in Chromaffin Population Renewal
6. Discussion
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Madrazo, I.; Drucker-Colín, R.; Díaz, V.; Martínez-Mata, J.; Torres, C.; Becerril, J.J. Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson’s disease. N. Engl. J. Med. 1987, 316, 831–834. [Google Scholar] [CrossRef]
- Feeney, D.M.; Weisend, M.P.; Kline, A.E. Noradrenergic pharmacotherapy, intracerebral infusion, and adrenal transplantation promote functional recovery aft.er cortical damage. J. Neural Transplant. Plast. 1993, 4, 199–213. [Google Scholar] [CrossRef]
- Sagen, J. Chromaffin cell transplants for alleviation of chronic pain. ASAIO J. 1992, 38, 24–28. [Google Scholar] [CrossRef]
- Eaton, M.J.; Berrocal, Y.; Wolfe, S.Q.; Widerström-Noga, E. Review of the history and current status of cell-transplant approaches for the management of neuropathic pain. Pain Res. Treat. 2012, 2012, 263972. [Google Scholar] [CrossRef]
- Quinn, N.P. The clinical application of cell grafting techniques in patients with Parkinson’s disease. Prog. Brain Res. 1990, 82, 619–625. [Google Scholar] [CrossRef]
- Bés, J.C.; Tkaczuk, J.; Czech, K.A.; Tafani, M.; Bastide, R.; Caratero, C.; Pappas, G.D.; Lazorthes, Y. One-year chromaffin cell allograft survival in cancer patients with chronic pain: Morphological and functional evidence. Cell Transplant. 1998, 7, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Espejo, E.; Armengol, J.A.; Flores, J.A.; Galan-Rodriguez, B.; Ramiro, S. Cells of the sympathoadrenal lineage: Biological properties as donor tissue for cell-replacement therapies for Parkinson’s disease. Brain Res. Brain Res. Rev. 2005, 49, 343–354. [Google Scholar] [CrossRef]
- Unsicker, K. The chromaffin cell: Paradigm in cell, developmental and growth factor biology. J. Anat. 1993, 183 Pt 2, 207–221. [Google Scholar] [PubMed] [PubMed Central]
- Landis, S.C.; Patterson, P.H. Neural crest cell lineages. Trends Neurosci. 1981, 4, 172–175. [Google Scholar] [CrossRef]
- Smitten, N.A. Sympathoadrenal System in Phylogenesis and Ontogenesis of Vertebrates; Science: Moscow, Russia, 1972; 348p. (In Russian) [Google Scholar]
- Doupe, A.J.; Landis, S.C.; Patterson, P.H. Environmental influences in the development of neural crest derivatives: Glucocorticoids, growth factors, and chromaffin cell plasticity. J. Neurosci. 1985, 5, 2119–2142. [Google Scholar] [CrossRef][Green Version]
- Anderson, D.J.; Axel, R. A bipotential neuroendocrine precursor whose choice of cell fate is determined by NGF and glucocorticoids. Cell 1986, 47, 1079–1090. [Google Scholar] [CrossRef]
- Anderson, D.J.; Carnahan, J.F.; Michelsohn, A.; Patterson, P.H. Antibody markers identify a common progenitor to sympathetic neurons and chromaffin cells in vivo and reveal the timing of commitment to neuronal differentiation in the sympathoadrenal lineage. J. Neurosci. 1991, 11, 3507–3519. [Google Scholar] [CrossRef]
- Furlan, A.; Dyachuk, V.; Kastriti, M.E.; Calvo-Enrique, L.; Abdo, H.; Hadjab, S.; Chontorotzea, T.; Akkuratova, N.; Usoskin, D.; Kamenev, D.; et al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 2017, 357, eaal3753. [Google Scholar] [CrossRef]
- Lousado, L.; Prazeres, P.H.D.M.; Andreotti, J.P.; Paiva, A.E.; Azevedo, P.O.; Santos, G.S.P.; Filev, R.; Mintz, A.; Birbrair, A. Schwann cell precursors as a source for adrenal gland chromaffin cells. Cell Death Dis. 2017, 8, e3072. [Google Scholar] [CrossRef]
- Lumb, R.; Tata, M.; Xu, X.; Joyce, A.; Marchant, C.; Harvey, N.; Ruhrberg, C.; Schwarz, Q. Neuropilins guide preganglionic sympathetic axons and chromaffin cell precursors to establish the adrenal medulla. Development 2018, 145, dev162552. [Google Scholar] [CrossRef] [PubMed]
- Kastriti, M.E.; Kameneva, P.; Kamenev, D.; Dyachuk, V.; Furlan, A.; Hampl, M.; Memic, F.; Marklund, U.; Lallemend, F.; Hadjab, S.; et al. Schwann Cell Precursors Generate the Majority of Chromaffin Cells in Zuckerkandl Organ and Some Sympathetic Neurons in Paraganglia. Front. Mol. Neurosci. 2019, 12, 6. [Google Scholar] [CrossRef]
- Huber, K.; Kalcheim, C.; Unsicker, K. The development of the chromaffin cell lineage from the neural crest. Auton. Neurosci. 2009, 151, 10–16. [Google Scholar] [CrossRef]
- Kameda, Y. Signaling molecules and transcription factors involved in the development of the sympathetic nervous system, with special emphasis on the superior cervical ganglion. Cell Tissue Res. 2014, 357, 527–548. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.; Brühl, B.; Guillemot, F.; Olson, E.N.; Ernsberger, U.; Unsicker, K. Development of chromaffin cells depends on MASH1 function. Development 2002, 129, 4729–4738. [Google Scholar] [CrossRef] [PubMed]
- Axelson, H. The Notch signaling cascade in neuroblastoma: Role of the basic helix-loop-helix proteins HASH-1 and HES-1. Cancer Lett. 2004, 204, 171–178. [Google Scholar] [CrossRef]
- Aminot, A.; Roffi, J. Perinatal evolution and hormonal control of adrenal tyrosine hydroxylase activity in the rat. Enzyme 1979, 24, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Godefroy, D.; Rostène, W.; Anouar, Y.; Goazigo, A.R. Tyrosine-hydroxylase immunoreactivity in the mouse transparent brain and adrenal glands. J. Neural Transm. 2019, 126, 367–375, Erratum in J. Neural Transm. 2019, 126, 695. [Google Scholar] [CrossRef] [PubMed]
- Theiler, K. The House Mouse: Atlas of Embryonic Development; Springer: New York, NY, USA, 1989. [Google Scholar]
- Ehrhart-Bornstein, M.; Hinson, J.P.; Bornstein, S.R.; Scherbaum, W.A.; Vinson, G.P. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr. Rev. 1998, 19, 101–143. [Google Scholar] [CrossRef]
- Finotto, S.; Krieglstein, K.; Schober, A.; Deimling, F.; Lindner, K.; Brühl, B.; Beier, K.; Metz, J.; Garcia-Arraras, J.E.; Roig-Lopez, J.L.; et al. Analysis of mice carrying targeted mutations of the glucocorticoid receptor gene argues against an essential role of glucocorticoid signalling for generating adrenal chromaffin cells. Development 1999, 126, 2935–2944. [Google Scholar] [CrossRef]
- Michelsohn, A.M.; Anderson, D.J. Changes in competence determine the timing of two sequential glucocorticoid effects on sympathoadrenal progenitors. Neuron 1992, 8, 589–604. [Google Scholar] [CrossRef]
- Unsicker, K.; Huber, K.; Schober, A.; Kalcheim, C. Resolved and open issues in chromaffin cell development. Mech. Dev. 2013, 130, 324–329. [Google Scholar] [CrossRef]
- Huber, K. The sympathoadrenal cell lineage: Specification, diversification, and new perspectives. Dev. Biol. 2006, 298, 335–343. [Google Scholar] [CrossRef]
- Gut, P.; Huber, K.; Lohr, J.; Brühl, B.; Oberle, S.; Treier, M.; Ernsberger, U.; Kalcheim, C.; Unsicker, K. Lack of an adrenal cortex in Sf1 mutant mice is compatible with the generation and differentiation of chromaffin cells. Development 2005, 132, 4611–4619. [Google Scholar] [CrossRef]
- Parlato, R.; Otto, C.; Tuckermann, J.; Stotz, S.; Kaden, S.; Gröne, H.J.; Unsicker, K.; Schütz, G. Conditional inactivation of glucocorticoid receptor gene in dopamine-beta-hydroxylase cells impairs chromaffin cell survival. Endocrinology 2009, 150, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Kastriti, M.E.; Kameneva, P.; Adameyko, I. Stem cells, evolutionary aspects and pathology of the adrenal medulla: A new developmental paradigm. Mol. Cell. Endocrinol. 2020, 518, 110998. [Google Scholar] [CrossRef]
- Huber, K.; Karch, N.; Ernsberger, U.; Goridis, C.; Unsicker, K. The role of Phox2B in chromaffin cell development. Dev. Biol. 2005, 279, 501–508. [Google Scholar] [CrossRef]
- Pattyn, A.; Guillemot, F.; Brunet, J.F. Delays in neuronal differentiation in Mash1/Ascl1 mutants. Dev. Biol. 2006, 295, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.R.; Tiveron, M.C.; Guillemot, F.; Brunet, J.F.; Goridis, C. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development 1998, 125, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Wildner, H.; Gierl, M.S.; Strehle, M.; Pla, P.; Birchmeier, C. Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development 2008, 135, 473–481. [Google Scholar] [CrossRef]
- Hendershot, T.J.; Liu, H.; Clouthier, D.E.; Shepherd, I.T.; Coppola, E.; Studer, M.; Firulli, A.B.; Pittman, D.L.; Howard, M.J. Conditional deletion of Hand2 reveals critical functions in neurogenesis and cell type-specific gene expression for development of neural crest-derived noradrenergic sympathetic ganglion neurons. Dev. Biol. 2008, 319, 179–191. [Google Scholar] [CrossRef]
- Morikawa, Y.; D’Autréaux, F.; Gershon, M.D.; Cserjesi, P. Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev. Biol. 2007, 307, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Tsarovina, K.; Reiff, T.; Stubbusch, J.; Kurek, D.; Grosveld, F.G.; Parlato, R.; Schütz, G.; Rohrer, H. The Gata3 transcription factor is required for the survival of embryonic and adult sympathetic neurons. J. Neurosci. 2010, 30, 10833–10843. [Google Scholar] [CrossRef]
- Moriguchi, T.; Takako, N.; Hamada, M.; Maeda, A.; Fujioka, Y.; Kuroha, T.; Huber, R.E.; Hasegawa, S.L.; Rao, A.; Yamamoto, M.; et al. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development 2006, 133, 3871–3881. [Google Scholar] [CrossRef]
- Schmidt, M.; Huber, L.; Majdazari, A.; Schütz, G.; Williams, T.; Rohrer, H. The transcription factors AP-2β and AP-2α are required for survival of sympathetic progenitors and differentiated sympathetic neurons. Dev. Biol. 2011, 355, 89–100. [Google Scholar] [CrossRef]
- Huber, K.; Narasimhan, P.; Shtukmaster, S.; Pfeifer, D.; Evans, S.M.; Sun, Y. The LIM-Homeodomain transcription factor Islet-1 is required for the development of sympathetic neurons and adrenal chromaffin cells. Dev. Biol. 2013, 380, 286–298. [Google Scholar] [CrossRef]
- Dauger, S.; Pattyn, A.; Lofaso, F.; Gaultier, C.; Goridis, C.; Gallego, J.; Brunet, J.F. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development 2003, 130, 6635–6642. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, A.; Morin, X.; Cremer, H.; Goridis, C.; Brunet, J.-F. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 1999, 399, 366–370. [Google Scholar] [CrossRef]
- Parras, C.M.; Schuurmans, C.; Scardigli, R.; Kim, J.; Anderson, D.J.; Guillemot, F. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002, 16, 324–338. [Google Scholar] [CrossRef]
- Peng, E.; Hu, C.; Feng, J.; He, R. MASH1 induces neuron transdifferentiation of adrenal medulla chromaffin cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban = J. Cent. South Univ. Med. Sci. 2023, 48, 526–537. [Google Scholar] [CrossRef]
- Maleki, Z.; Nadella, A.; Nadella, M.; Patel, G.; Patel, S.; Kholová, I. INSM1, a Novel Biomarker for Detection of Neuroendocrine Neoplasms: Cytopathologists’ View. Diagnostics 2021, 11, 2172. [Google Scholar] [CrossRef]
- Lan, M.S.; Breslin, M.B. Structure, expression, and biological function of INSM1 transcription factor in neuroendocrine differentiation. FASEB J. 2009, 23, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lan, M.S. Interplay: The Essential Role between INSM1 and N-Myc in Aggressive Neuroblastoma. Biology 2022, 11, 1376. [Google Scholar] [CrossRef]
- Abou Nader, N.; Zamberlam, G.; Boyer, A. Transgenic Mouse Models to Study the Development and Maintenance of the Adrenal Cortex. Int. J. Mol. Sci. 2022, 23, 14388. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C.; Okumura, L.M.; Page, D.C. Gata4 is required for formation of the genital ridge in mice. PLoS Genet. 2013, 9, e1003629. [Google Scholar] [CrossRef]
- Hong, S.J.; Huh, Y.; Chae, H.; Hong, S.; Lardaro, T.; Kim, K.S. GATA-3 regulates the transcriptional activity of tyrosine hydroxylase by interacting with CREB. J. Neurochem. 2006, 98, 773–781. [Google Scholar] [CrossRef]
- Liang, X.; Song, M.R.; Xu, Z.; Lanuza, G.M.; Liu, Y.; Zhuang, T.; Chen, Y.; Pfaff, S.L.; Evans, S.M.; Sun, Y. Isl1 is required for multiple, aspects of motor neuron development. Mol. Cell. Neurosci. 2011, 47, 215–222. [Google Scholar] [CrossRef]
- Elshatory, Y.; Everhart, D.; Deng, M.; Xie, X.; Barlow, R.B.; Gan, L. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J. Neurosci. 2007, 27, 12707–12720. [Google Scholar] [CrossRef]
- Cai, C.L.; Liang, X.; Shi, Y.; Chu, P.H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell. 2003, 5, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Huh, Y.H.; Leung, A.; Choi, H.J.; Ding, Y.; Kang, U.J.; Yoo, S.H.; Buettner, R.; Kim, K.S. Transcription factor AP-2β regulates the neurotransmitter phenotype and maturation of chromaffin cells. Mol. Cell. Neurosci. 2011, 46, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Reiprich, S.; Stolt, C.C.; Schreiner, S.; Parlato, R.; Wegner, M. SoxE proteins are differentially required in mouse adrenal gland development. Mol. Biol. Cell. 2008, 19, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Potzner, M.R.; Tsarovina, K.; Binder, E.; Penzo-Méndez, A.; Lefebvre, V.; Rohrer, H.; Wegner, M.; Sock, E. Sequential requirement of Sox4 and Sox11 during development of the sympathetic nervous system. Development 2010, 137, 775–784. [Google Scholar] [CrossRef]
- Monkhouse, W.S.; Fussell, I. A fraction of labelled mitoses study on adrenal chromaffin tissue in the newborn mouse and the effect of hydrocortisone. J. Anat. 1988, 157, 105–109. [Google Scholar] [PubMed] [PubMed Central]
- Mitchell, R.M. Histological changes and mitotic activity in the rat adrenal during postnatal development. Anat. Rec. 1948, 101, 161–185. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Obernikhin, S.S.; Tsomartova, D.A.; Nazimova, S.V.; Yaglov, V.V. Expression of Transcription Factor PRH/Hhex in Adrenal Chromaffin Cells in the Postnatal Development and Its Role in the Regulation of Proliferative Processes. Bull. Exp. Biol. Med. 2018, 165, 508–511. [Google Scholar] [CrossRef]
- Jackson, C.M. The postnatal development of the suprarenal gland and the effect of inanition upon its growth and structure in the albino rat. Am. J. Anat. 1919, 25, 221–289. [Google Scholar] [CrossRef]
- Malvaldi, G.; Mencacci, P.; Viola-Magni, M.P. Mitoses in the adrenal medullary cells. Experientia 1968, 24, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Plećas, B.; Hristić, M.; Popović, A.; Jovović, D. Mitotic stimulation of adrenal medullary chromaffin cells by oxytocin. Horm. Metab. Res. 1990, 22, 402–403. [Google Scholar] [CrossRef] [PubMed]
- Messier, B.; Leblond, C.P. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am. J. Anat. 1960, 106, 247–285. [Google Scholar] [CrossRef]
- Verhofstad, A.A. Kinetics of adrenal medullary cells. J. Anat. 1993, 183 Pt 2, 315–326. [Google Scholar] [PubMed] [PubMed Central]
- Jurecka, W.; Lassmann, H.; Hörandner, H. The proliferation of adrenal medullary cells in newborn and adult mice. A light and electron microscopic autoradiographic study. Cell Tissue Res. 1978, 189, 305–312. [Google Scholar] [CrossRef]
- Tischler, A.S.; DeLellis, R.A.; Nunnemacher, G.; Wolfe, H.J. Acute stimulation of chromaffin cell proliferation in the adult rat adrenal medulla. Lab. Investig. 1988, 58, 733–735. [Google Scholar] [PubMed]
- Tischler, A.S.; Ruzicka, L.A.; Donahue, S.R.; DeLellis, R.A. Chromaffin cell proliferation in the adult rat adrenal medulla. Int. J. Dev. Neurosci. 1989, 7, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Tischler, A.S.; McClain, R.M.; Childers, H.; Downing, J. Neurogenic signals regulate chromaffin cell proliferation and mediate the mitogenic effect of reserpine in the adult rat adrenal medulla. Lab. Investig. 1991, 65, 374–376. [Google Scholar] [PubMed]
- Huff, K.; End, D.; Guroff, G. Nerve growth factor-induced alteration in the response of PC12 pheochromocytoma cells to epidermal growth factor. J. Cell Biol. 1981, 88, 189–198. [Google Scholar] [CrossRef]
- Chabot, J.G.; Walker, P.; Pelletier, G. Distribution of epidermal growth factor binding sites in the adult rat adrenal gland by light microscope autoradiography. Acta Endocrinol. 1986, 113, 391–395. [Google Scholar] [CrossRef]
- Bamberger, A.M.; Schulte, H.M.; Wullbrand, A.; Jung, R.; Beil, F.U.; Bamberger, C.M. Expression of leukemia inhibitory factor (LIF) and LIF receptor (LIF-R) in the human adrenal cortex: Implications for steroidogenesis. Mol. Cell. Endocrinol. 2000, 162, 145–149. [Google Scholar] [CrossRef]
- Ware, C.B.; Kariagina, A.; Zonis, S.; Alon, D.; Chesnokova, V. Leukemia inhibitory factor signaling is implicated in embrionic development of the HPA axis. FEBS Lett. 2005, 579, 4465–4469. [Google Scholar] [CrossRef][Green Version]
- Oberle, S.; Schober, A.; Meyer, V.; Holtmann, B.; Henderson, C.; Sendtner, M.; Unsicker, K. Loss of leukemia inhibitory factor receptor beta or cardiotrophin-1 causes similar deficits in preganglionic sympathetic neurons and adrenal medulla. J. Neurosci. 2006, 26, 1823–1832. [Google Scholar] [CrossRef]
- Combs, S.E.; Krieglstein, K.; Unsicker, K. Reduction of endogenous TGF-beta increases proliferation of developing adrenal chromaffin cells in vivo. J. Neurosci. Res. 2000, 59, 379–383. [Google Scholar] [CrossRef]
- Sicard, F.; Krug, A.W.; Ziegler, C.G.; Sperber, S.; Ehrhart-Bornstein, M.; Bornstein, S.R. Role of DHEA and growth factors in chromaffin cell proliferation. Ann. N. Y. Acad. Sci. 2006, 1073, 312–326. [Google Scholar] [CrossRef]
- Sicard, F.; Ehrhart-Bornstein, M.; Corbeil, D.; Sperber, S.; Krug, A.W.; Ziegler, C.G.; Rettori, V.; McCann, S.M.; Bornstein, S.R. Age-dependent regulation of chromaffin cell proliferation by growth factors, dehydroepiandrosterone (DHEA), and DHEA sulfate. Proc. Natl. Acad. Sci. USA 2007, 104, 2007–2012. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Tsomartova, D.A.; Obernikhin, S.S.; Nazimova, S.V.; Yaglov, V.V. Regulation of Proliferative Processes in Rat Adrenal Cortex by Transcriptional Factor PRH under Conditions of Developmental Exposure to Endocrine Disruptor DDT. Bull. Exp. Biol. Med. 2019, 167, 404–407. [Google Scholar] [CrossRef]
- Gaston, K.; Tsitsilianos, M.A.; Wadey, K.; Jayaraman, P.S. Misregulation of the proline rich homeodomain (PRH/HHEX) protein in cancer cells and its consequences for tumour growth and invasion. Cell Biosci. 2016, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Noy, P.; Williams, H.; Sawasdichai, A.; Gaston, K.; Jayaraman, P.S. PRH/Hhex controls cell survival through coordinate transcriptional regulation of vascular endothelial growth factor signaling. Mol. Cell. Biol. 2010, 30, 2120–2134. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.T.; Nutt, S.L.; McCormack, M.P. The Haematopoietically-expressed homeobox transcription factor: Roles in development, physiology and disease. Front. Immunol. 2023, 14, 1197490. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Tsomartova, D.A.; Obernikhin, S.S.; Nazimova, S.V.; Ivanova, M.Y.; Chereshneva, E.V.; Yaglov, V.V.; Lomanovskaya, T.A. Transcription factors β-catenin and Hex in postnatal development of the rat adrenal cortex: Implication in proliferation control. Heliyon 2021, 7, e05932. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Nazimova, S.V.; Obernikhin, S.S.; Tsomartova, D.A.; Yaglov, V.V.; Timokhina, E.P.; Tsomartova, E.S.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Developmental Exposure to DDT Disrupts Transcriptional Regulation of Postnatal Growth and Cell Renewal of Adrenal Medulla. Int. J. Mol. Sci. 2023, 24, 2774. [Google Scholar] [CrossRef]
- Łukowicz, K.; Grygier, B.; Basta-Kaim, A. Emerging role of neural stem/progenitor cell secretome in brain inflammatory response modulation. Pharmacol. Rep. 2025, 77, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Mariniello, K.; Ruiz-Babot, G.; McGaugh, E.C.; Nicholson, J.G.; Gualtieri, A.; Gaston-Massuet, C.; Nostro, M.C.; Guasti, L. Stem Cells, Self-Renewal, and Lineage Commitment in the Endocrine System. Front. Endocrinol. 2019, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Mitani, F.; Mukai, K.; Miyamoto, H.; Suematsu, M.; Ishimura, Y. Development of functional zonation in the rat adrenal cortex. Endocrinology 1999, 140, 3342–3353. [Google Scholar] [CrossRef]
- Pihlajoki, M.; Dörner, J.; Cochran, R.S.; Heikinheimo, M.; Wilson, D.B. Adrenocortical zonation, renewal, and remodeling. Front. Endocrinol. 2015, 6, 27. [Google Scholar] [CrossRef]
- Wood, M.A.; Acharya, A.; Finco, I.; Swonger, J.M.; Elston, M.J.; Tallquist, M.D.; Hammer, G.D. Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development 2013, 140, 4522–4532. [Google Scholar] [CrossRef]
- Chung, K.-F.; Sicard, F.; Vukicevic, V.; Hermann, A.; Storch, A.; Huttner, W.B.; Bornstein, S.R.; Ehrhard-Bornstein, M. Isolation of neural crest derived chromaffin progenitors from adult adrenal medulla. Stem Cells 2009, 27, 2602–2613. [Google Scholar] [CrossRef]
- Santana, M.M.; Chung, K.-F.; Vukicevic, V.; Rosmaninho-Salgado, J.; Kanczkowski, W.; Cortez, V.; Hackmann, K.; Bastos, C.A.; Mota, A.; Schrock, E.; et al. Isolation, characterization, and differentiation of progenitor cells from human adult adrenal medulla. Stem Cells Transl. Med. 2012, 1, 783–791. [Google Scholar] [CrossRef]
- Chen-Pan, C.; Pan, I.J.; Yamamoto, Y.; Sakogawa, T.; Yamada, J.; Hayashi, Y. Prompt recovery of damaged adrenal medullae induced by salinomycin. Toxicol. Pathol. 1999, 27, 563–572. [Google Scholar] [CrossRef]
- Chen-Pan, C.; Pan, I.J.; Yamamoto, Y.; Chen, H.H.; Hayashi, Y. Recovery of injured adrenal medulla by differentiation of pre-existing undifferentiated chromaffin cells. Toxicol. Pathol. 2002, 30, 165–172. [Google Scholar] [CrossRef]
- Kamenev, D.; Sunadome, K.; Shirokov, M.; Chagin, A.S.; Singh, A.; Irion, U.; Adameyko, I.; Fried, K.; Dyachuk, V. Schwann cell precursors generate sympathoadrenal system during zebrafish development. J. Neurosci. Res. 2021, 99, 2540–2557. [Google Scholar] [CrossRef]
- Magro, G.; Grasso, S. Immunohistochemical identification and comparison of glial cell lineage in foetal, neonatal, adult and neoplastic human adrenal medulla. Histochem. J. 1997, 29, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M. Immunohistochemical study of sustentacular cells in adrenal medulla of neonatal and adult rats using an antibody against S-100 protein. Folia Morphol. 2017, 76, 246–251. [Google Scholar] [CrossRef]
- Gallol, L.E.; Mohamed, F.H. Immunomorphometric variations of sustentacular cells of the male viscacha adrenal medulla during the annual reproductive cycle. Effects of androgens and melatonin. Acta Histochem. 2018, 120, 363–372. [Google Scholar] [CrossRef]
- Kameda, Y. Immunoelectron microscopic localization of vimentin in sustentacular cells of the carotid body and the adrenal medulla of guinea pigs. J. Histochem. Cytochem. 1996, 44, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.; Filippa, V.; Mohamed, F.; Dominguez, S.; Scardapane, L. Interaction between chromaffin and sustentacular cells in adrenal medulla of viscacha (Lagostomus maximus maximus). Anat. Histol. Embryol. 2007, 36, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Rubin de Celis, M.F.; Garcia-Martin, R.; Wittig, D.; Valencia, G.D.; Enikolopov, G.; Funk, R.H.; Chavakis, T.; Bornstein, S.R.; Androutsellis-Theotokis, A.; Ehrhart-Bornstein, M. Multipotent glia-like stem cells mediate stress adaptation. Stem Cells 2015, 33, 2037–2051. [Google Scholar] [CrossRef]
- Santambrogio, A.; Kemkem, Y.; Willis, T.L.; Berger, I.; Kastriti, M.E.; Faure, L.; Russell, J.P.; Lodge, E.J.; Yianni, V.; Kövér, B.; et al. SOX2+ sustentacular cells are stem cells of the postnatal adrenal medulla. Nat. Commun. 2025, 16, 16. [Google Scholar] [CrossRef]
- Pignatelli, D.; Xiao, F.; Gouveia, A.M.; Ferreira, J.G.; Vinson, G.P. Adrenarche in the rat. J. Endocrinol. 2006, 191, 301–308. [Google Scholar] [CrossRef]
- Obernikhin, S.S.; Yaglova, N.V.; Timokhina, E.P.; Nazimova, S.V.; Yaglov, V.V. Regulation of Morphogenetic Processes during Postnatal Development and Physiological Regeneration of the Adrenal Medulla. Bull. Exp. Biol. Med. 2023, 175, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, K.A.; Cheyette, B.N. Wnt signaling in vertebrate neural development and function. J. Neuroimmune Pharmacol. 2012, 7, 774–787. [Google Scholar] [CrossRef]
- Wilson, N.H.; Stoeckli, E.T. Sonic hedgehog regulates Wnt activity during neural circuit formation. Vitam. Horm. 2012, 88, 173–209. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.S.; Scott, M.P. Sonic hedgehog in the nervous system: Functions, modifications and mechanisms. Curr. Opin. Neurobiol. 2002, 12, 57–63. [Google Scholar] [CrossRef]
- Finco, I.; Lerario, A.M.; Hammer, G.D. Sonic hedgehog and WNT signaling promote adrenal gland regeneration in male mice. Endocrinology 2018, 159, 579–596. [Google Scholar] [CrossRef]
- Ji, H.; Miao, J.; Zhang, X.; Du, Y.; Liu, H.; Li, S.; Li, L. Inhibition of sonic hedgehog signaling aggravates brain damage associated with the down-regulation of Gli 1, Ptch 1 and SOD1 expression in acute ischemic stroke. Neurosci. Lett. 2012, 506, 1–6. [Google Scholar] [CrossRef]
- Sims, J.R.; Lee, S.W.; Topalkara, K.; Qiu, J.; Xu, J.; Zhou, Z.; Moskowitz, M.A. Sonic hedgehog regulates ischemia/hypoxia-induced neural progenitor proliferation. Stroke 2009, 40, 3618–3626. [Google Scholar] [CrossRef]
- Esch, D.; Vahokoski, J.; Groves, M.R.; Pogenberg, V.; Cojocaru, V.; Vom Bruch, H.; Han, D.; Drexler, H.C.A.; Araúzo-Bravo, M.J.; Ng, C.K.L.; et al. A unique Oct4 interface is crucial for reprogramming to pluripotency. Nat. Cell Biol. 2013, 15, 295–301. [Google Scholar] [CrossRef]
- Masui, S.; Nakatake, Y.; Toyooka, Y.; Shimosato, D.; Yagi, R.; Takahashi, K.; Okochi, H.; Okuda, A.; Matoba, R.; Sharov, A.A.; et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 2007, 9, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Obernikhin, S.S.; Nazimova, S.V.; Timokhina, E.P.; Yaglov, V.V. Changes in the Expression of Transcription Factor Oct4 during Postnatal Development of Adrenal Medulla. Bull. Exp. Biol. Med. 2022, 173, 783–786. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 18, 45–57. [Google Scholar] [CrossRef]
- Mansouri, A.; Cregut, M.; Abbes, C.; Durand, M.-J.; Landoulsi, A.; Thouand, G. The environmental issues of DDT pollution and bioremediation: A multidisciplinary review. Appl. Biochem. Biotechnol. 2017, 181, 309–339. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Timokhina, E.P.; Yaglov, V.V.; Obernikhin, S.S.; Nazimova, S.V.; Tsomartova, D.A. Changes in Histophysiology of the Adrenal Medulla in Rats after Prenatal and Postnatal Exposure to Endocrine Disruptor DDT. Bull. Exp. Biol. Med. 2020, 169, 398–400. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Obernikhin, S.S.; Nazimova, S.V.; Tsomartova, D.A.; Timokhina, E.P.; Yaglov, V.V.; Tsomartova, E.S.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Postnatal Exposure to the Endocrine Disruptor Dichlorodiphenyltrichloroethane Affects Adrenomedullary Chromaffin Cell Physiology and Alters the Balance of Mechanisms Underlying Cell Renewal. Int. J. Mol. Sci. 2024, 25, 1494. [Google Scholar] [CrossRef] [PubMed]
- Espejo, E.F.; Gonzalez-Albo, M.C.; Moraes, J.P.; El Banoua, F.; Flores, J.A.; Caraballo, I. Functional regeneration in a rat Parkinson’s model after intrastriatal grafts of glial cell line-derived neurotrophic factor and transforming growth factor beta1-expressing extra-adrenal chromaffin cells of the Zuckerkandl’s organ. J. Neurosci. 2001, 21, 9888–9895. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart-Bornstein, M.; Vukicevic, V.; Chung, K.F.; Ahmad, M.; Bornstein, S.R. Chromaffin progenitor cells from the adrenal medulla. Cell. Mol. Neurobiol. 2010, 30, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Moattari, M.; Moattari, F.; Kaka, G.; Kouchesfahani, H.M.; Sadraie, S.H.; Naghdi, M. Comparison of neuroregeneration in central nervous system and peripheral nervous system. Otorhinolaryngol. Head Neck Surg. 2018, 3. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaglova, N.V.; Obernikhin, S.S.; Nazimova, S.V.; Yaglov, V.V.; Timokhina, E.P.; Tsomartova, E.S.; Ivanova, M.Y.; Chereshneva, E.V.; Lomanovskaya, T.A.; Tsomartova, D.A. New Concepts of Regeneration and Renewal of Adrenal Chromaffin Cells. Int. J. Mol. Sci. 2025, 26, 9369. https://doi.org/10.3390/ijms26199369
Yaglova NV, Obernikhin SS, Nazimova SV, Yaglov VV, Timokhina EP, Tsomartova ES, Ivanova MY, Chereshneva EV, Lomanovskaya TA, Tsomartova DA. New Concepts of Regeneration and Renewal of Adrenal Chromaffin Cells. International Journal of Molecular Sciences. 2025; 26(19):9369. https://doi.org/10.3390/ijms26199369
Chicago/Turabian StyleYaglova, Nataliya V., Sergey S. Obernikhin, Svetlana V. Nazimova, Valentin V. Yaglov, Ekaterina P. Timokhina, Elina S. Tsomartova, Marina Y. Ivanova, Elizaveta V. Chereshneva, Tatiana A. Lomanovskaya, and Dibakhan A. Tsomartova. 2025. "New Concepts of Regeneration and Renewal of Adrenal Chromaffin Cells" International Journal of Molecular Sciences 26, no. 19: 9369. https://doi.org/10.3390/ijms26199369
APA StyleYaglova, N. V., Obernikhin, S. S., Nazimova, S. V., Yaglov, V. V., Timokhina, E. P., Tsomartova, E. S., Ivanova, M. Y., Chereshneva, E. V., Lomanovskaya, T. A., & Tsomartova, D. A. (2025). New Concepts of Regeneration and Renewal of Adrenal Chromaffin Cells. International Journal of Molecular Sciences, 26(19), 9369. https://doi.org/10.3390/ijms26199369









