1. Introduction
Clostridium perfringens is an anaerobic zoonotic pathogen that is widely distributed in natural environments and the digestive tracts of animals [
1].
C. perfringens is the primary agent responsible for necrotic enteritis (NE) in poultry, leading to intestinal bleeding, mucosal necrosis, growth retardation, and even high mortality rates, resulting in significant economic losses [
2]. Historically, antibiotics were frequently employed as efficacious substances incorporated into feed for the treatment of NE. In recent years, the overuse of antibiotics has led to increased antibiotic resistance in
C. perfringens, posing a serious threat to the promotion of antibiotic-free farming [
3]. This limitation has spurred the investigation of various antibiotic alternatives for preventing
C. perfringens infections, such as acidifiers [
4], polysaccharides [
5], probiotics [
6], herbal extracts [
7], and fatty acids [
8].
Among these alternatives, probiotics have demonstrated considerable benefits for improving growth, immune function, and gut health of livestock and poultry [
9,
10,
11]. Probiotics currently used in animal production primarily include
Bacillus,
Bifidobacterium, and
Lactobacillus, with
Bacillus considered the most promising owing to its widespread availability, strong stress tolerance, and capacity to produce various digestive enzymes and antibacterial compounds [
12,
13]. Predominant
Bacillus spp. studied as monogastric probiotics include
B. subtilis,
B. licheniformis,
B. coagulans,
B. amyloliquefaciens,
B. velezensis, and
B. cereus, typically isolated from soil or animal gastrointestinal tracts [
14]. Supplementation with
Bacillus probiotics has been shown to enhance the growth performance, meat quality, antioxidant capacity, and immune function of broiler chickens, as well as reduce intestinal damage and inflammatory responses triggered by
C. perfringens infection [
15,
16,
17,
18]. Although numerous
Bacillus probiotics have been reported, there are few studies on specific
Bacillus strains that can effectively inhibit pathogenic bacteria, and the sources of these strains are limited. There remains a need to identify novel
Bacillus strains with enhanced antibacterial efficacy and broader functional features for use in modern livestock production.
Therefore, this study aimed to isolate and evaluate novel Bacillus strains with potent activity against C. perfringens. In this study, we isolated Bacillus strains from the intestinal contents of diverse animal species by leveraging their heat-resistant and spore-forming properties. Through in vitro screening, five Bacillus strains demonstrating effective inhibition of C. perfringens growth were selected. We then evaluated the probiotic properties of these antimicrobial Bacillus strains, including acid tolerance, bile salt resistance, antibiotic susceptibility, and enzyme production capabilities. Broiler trials were conducted to evaluate the impact of these five strains on growth performance and gut health, and to further explore their in vivo probiotic functions.
3. Discussion
Enterocolitis induced by
C. perfringens in poultry has become a major public health concern [
19]. In light of antibiotic overuse and escalating resistance, the development of effective agents against
C. perfringens was imperative for the treatment of associated diseases in the future.
Bacillus is a well-known beneficial bacterium that is extensively used in animal feed additives due to its broad-spectrum antibacterial properties and relatively high activity [
20,
21]. The present study screened five strains of
Bacillus exhibiting strong
C. perfringens inhibition and proceeded to analyze their probiotic properties. These five strains were then incorporated into broiler diets to investigate their impact on the chickens’ growth performance and gut health.
Bacillus bacteria can form spores to withstand adverse environmental conditions, enabling survival in high temperatures, acidic, alkaline, and saline environments [
22]. This study took advantage of their characteristics by using high-temperature screening, which greatly improved screening efficiency. The Oxford Cup experiment was typically employed to assess the antimicrobial activity of various materials against pathogens [
23]. In vitro antibacterial testing revealed that five
Bacillus strains exhibit excellent antibacterial activity against
C. perfringens, with antibacterial zone diameters exceeding 15 mm. The antimicrobial activity of
Bacillus spp. may derive from bioactive metabolites secreted by viable bacteria. Previous studies have demonstrated that
Bacillus culture supernatants exert potent inhibitory effects against
C. perfringens by disrupting cell wall integrity, inducing cytoplasmic condensation and vacuolization [
16,
24].
Probiotics added to feed must pass through the gastrointestinal tract and proliferate within it. The acidic and alkaline environments in the gastrointestinal tract generally inhibit bacterial growth and activity, thereby affecting the efficacy of probiotics [
25]. After 2 h of treatment with hydrochloric acid and bile salts, the survival rate of the five
Bacillus strains remained above 90%, indicating good tolerance to the gastrointestinal environment. Additionally, the ability to produce enzymes is also one of the criteria for evaluating probiotics. Proteases and amylases are enzymes that break down proteins and carbohydrates into amino acids, aldehydes and amines [
26]. All five
Bacillus strains in this experiment can produce amylase and protease. Although broilers naturally produce digestive enzymes, the production of additional enzymes by gut probiotics further aids carbohydrate and protein digestion, thereby enhancing nutrient absorption [
27].
Previous research confirms that dietary supplementation with
Bacillus strains as probiotics in broiler feed improves feed utilization efficiency and enhances growth performance [
28,
29]. However, this study found that, relative to CON, both the ANT group and the five
Bacillus groups significantly improved the FCR of 42-day-old broilers, but did not influence their average body weight and BWG. The addition of
Bacillus or antibiotics may induce changes in the cecal microorganisms, causing amino acids to be prioritized for microbial protein synthesis rather than muscle deposition [
30]. Furthermore, studies have shown that dietary supplementation with
B. amyloliquefaciens did not significantly improve growth performance at 9 weeks of age, but enhanced growth parameters in red-feathered native chickens by 11 weeks of age [
31]. The 1–42 day observation period selected in this study may not have captured the stage-specific characteristics of body weight gain in broilers. The breast muscle index serves as a critical measure of broilers’ growth performance. Dietary supplementation with C271 significantly increased breast muscle yield and improved slaughter performance in broilers. Meanwhile, dietary inclusion of C66, Y7, and L15 showed a tendency to enhance 42-day breast muscle yield, demonstrating comparable effects to antibiotic treatment. These findings corroborate prior studies showing that dietary supplementation with
Bacillus subtilis–enzyme complex increased breast muscle percentage in broilers [
32].
The thymus, spleen, and bursa of Fabricius constitute pivotal immune structures in broilers, and their immune organ indices serve as reliable indicators of immunological responsiveness in broilers. Notably, the bursa exhibits greater sensitivity to dietary interventions compared to other organs in broilers [
33]. This study demonstrated that ANT and C271 treatments significantly reduced the bursa of Fabricius index in 42-day-old broilers, while C377 had no significant effect. Zou et al. [
34] reported that broilers fed multi-strain probiotics showed significantly increased spleen index, while thymus and bursa of Fabricius indices remained unchanged. Other studies have demonstrated that
Bacillus supplementation had no effect on thymus and spleen weights but increased the bursa of Fabricius index [
32]. These differential effects may result from variations in bacterial strains, dosages, and host status, which can lead to either immunostimulatory or immunosuppressive outcomes.
Serum immunoglobulin reflects the humoral immune status of broilers. The main mechanisms of probiotics’ immune-modulating effects include: increasing the production of antimicrobial peptides [
35], neutralizing dysbiosis [
36], stimulating mucosal immunity [
37], and increasing the production of specific antibodies [
38]. The study found that supplementation with C271 tended to increase serum IgA concentration in 21-day-old broilers. This finding aligns with a previous study demonstrating that supplementation with
B. subtilis enhanced IgA and IgY levels in chickens [
39]. Notably, dietary supplementation with C377 significantly reduced serum IgY levels in 42-day-old broilers. The decreased IgY concentration might be attributed to the probiotic’s elimination of pathogens that would otherwise stimulate natural antibody production [
40]. The immune system-derived inflammatory cytokines serve crucial functions in protecting against bacterial and viral infections while balancing immunological homeostasis [
41]. The current results revealed that dietary
Bacillus supplementation had no significant effect on inflammatory cytokine expression in 42-day-old broilers, consistent with the observed reduction in the bursa of Fabricius index. Due to the addition of exogenous
Bacillus and antibiotics, the microbial diversity in the intestines of broilers decreases, and the immune system’s mobilization requirements are reduced.
Broiler growth rate is strongly correlated with intestinal morphogenesis, where increased VH and VH/CD ratio reflect greater absorptive surface area and improved nutrient uptake efficiency [
42]. Existing research indicates that the dietary addition of
B. subtilis significantly enhanced jejunal VH and VH/CD ratio, concurrently decreasing CD in 21-day-old broilers [
43]. In this study, C271 supplementation significantly improved jejunal VH and VH/CD ratio in broilers at 42 days of age (
p < 0.05). Meanwhile, C377 reduced jejunal CD and increased VH/CD ratio in 42-day-old broilers (
p < 0.05).
Bacillus strains could enhance jejunal structure in broilers through intestinal tissue repair and beneficial microbiota colonization [
44].
Gut microorganisms perform critical host functions: digesting nutrients, preventing pathogen colonization, strengthening intestinal barrier properties, and stimulating immune system development [
45]. Existing research indicates that
B. licheniformis fermentation products reduced fecal bacterial diversity in broilers [
46], while
B. subtilis-fermented feed decreased cecal microbial richness [
47]. These findings are partially consistent with our results, where supplementation with C271 and C377 reduced bacterial richness and diversity in the cecum. This may be related to the antibacterial activity of the added
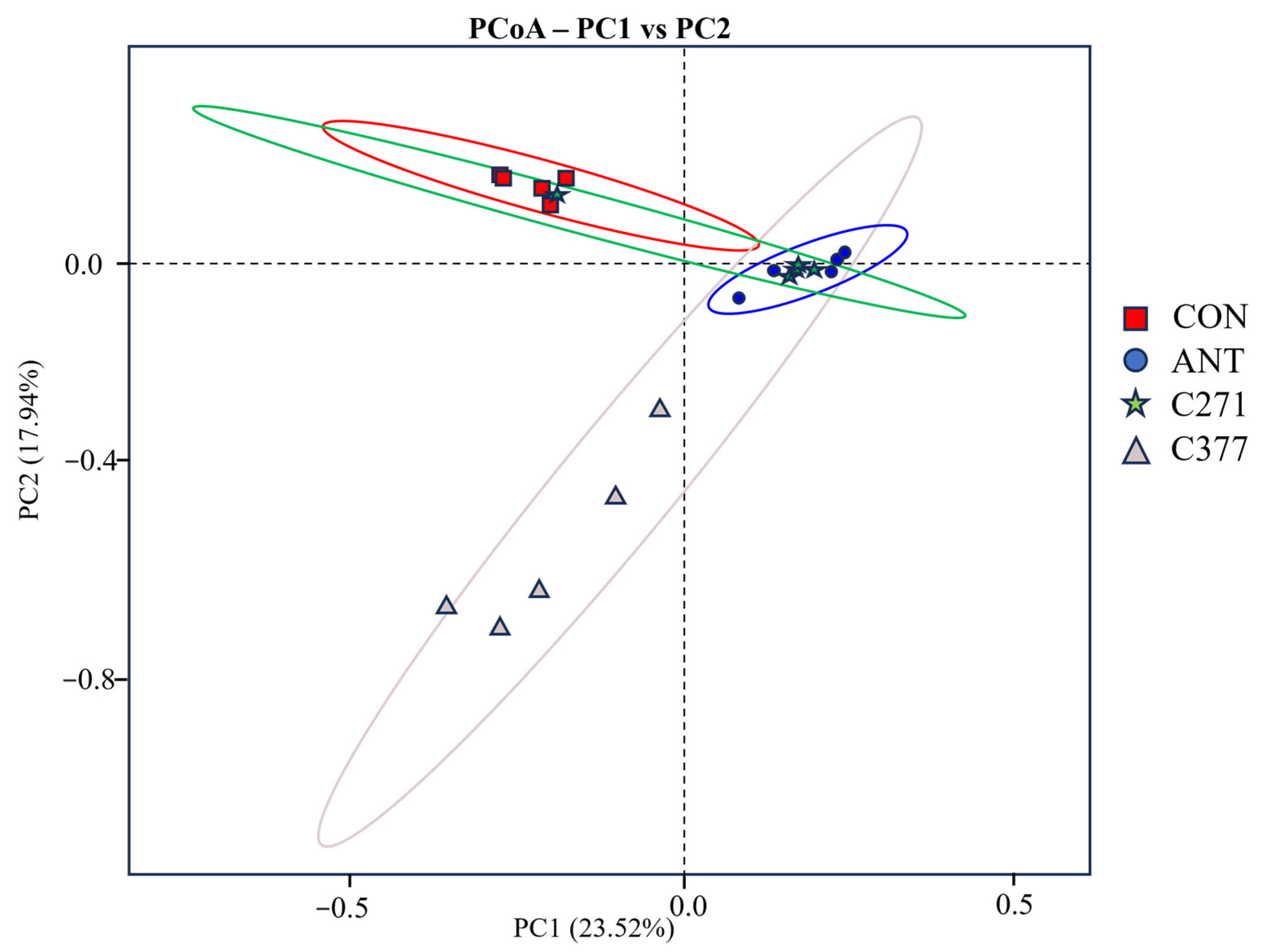
Bacillus strains. PCoA demonstrated two primary clusters: one comprising control and C271 with conserved microbiota, and another containing antibiotic and C377 groups, indicating C377’s antibiotic-mimicking effects on microbial composition, possibly due to antimicrobial action.
Firmicutes and Bacteroidetes constituted the predominant phyla in broiler cecal communities. Similar findings were also reported in cecal samples from probiotic-supplemented broilers [
48,
49]. Supplementation with C271 and C377 increased the relative abundance of Bacteroidetes. Multiple species of this phylum contribute to intestinal nutrient metabolism and are capable of fermenting carbohydrates to produce short-chain fatty acids [
50]. It is worth noting that supplementation with C271 and C377 can increase the relative abundance of Verrucomicrobiota. Studies have shown that Verrucomicrobiota can improve diverse intestinal indicators, including VH, VH/CD, and intestinal length [
51,
52]. Aligning with these results, our study found a significant increase in VH/CD in the jejunum after dietary inclusion with C271 and C377. Dietary supplementation with different
Bacillus strains increased the relative abundance of anaerobic microbes, including Bacteroides, Alistipes, and Lactobacillus in the cecal microbiome of 42-day-old broilers. Lactobacillus strains are generally considered beneficial for gut and host health due to their immunomodulatory capacity, pathogen inhibition, and bacteriocin synthesis [
53]. The increased abundance of Bacteroides and Alistipes suggests enhanced fermentation capacity and improved gut health. The LEfSe analysis, following rigorous multiple-testing correction, revealed distinct strain-specific modulation of cecal microbiota. Although the C271 group shared overall structural similarity with CON, its unique enrichment at the kingdom level, Bacteria (LDA = 4.23, q = 0.014), suggests that
B. amyloliquefaciens C271 may induce a broad, nonspecific stimulation of bacterial biomass. The specific enrichment of Alistipes in the C377 group (LDA = 4.10, q = 0.010), combined with its clustering tendency with ANT in β-diversity analysis, strongly indicates that
B. siamensis C377 may shape a microbial environment partially resembling that of antibiotic treatment, likely through its antimicrobial activity. These findings underscore the critical importance of strain-specific selection for achieving targeted modulation of the gut microbiota.