mRNA Isoforms and Variants in Health and Disease
Abstract
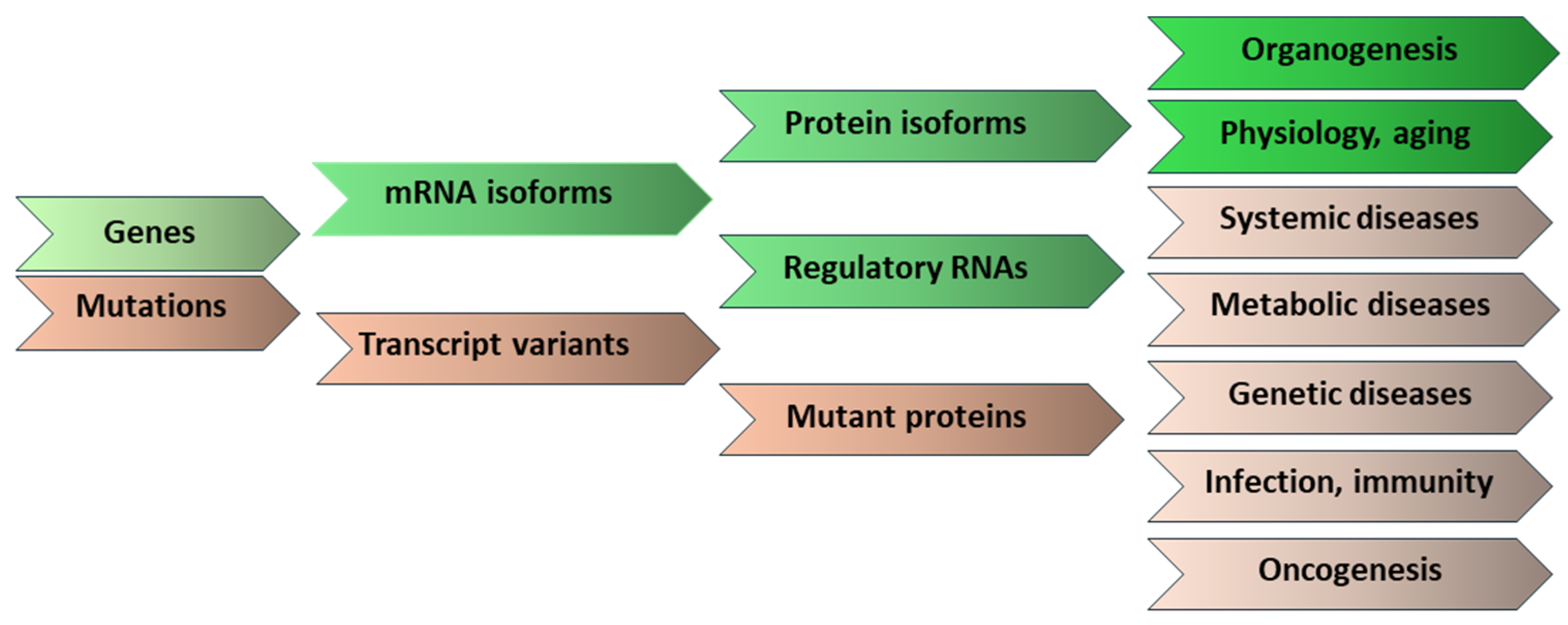
1. Introduction
2. Physiological Role of mRNA Isoforms
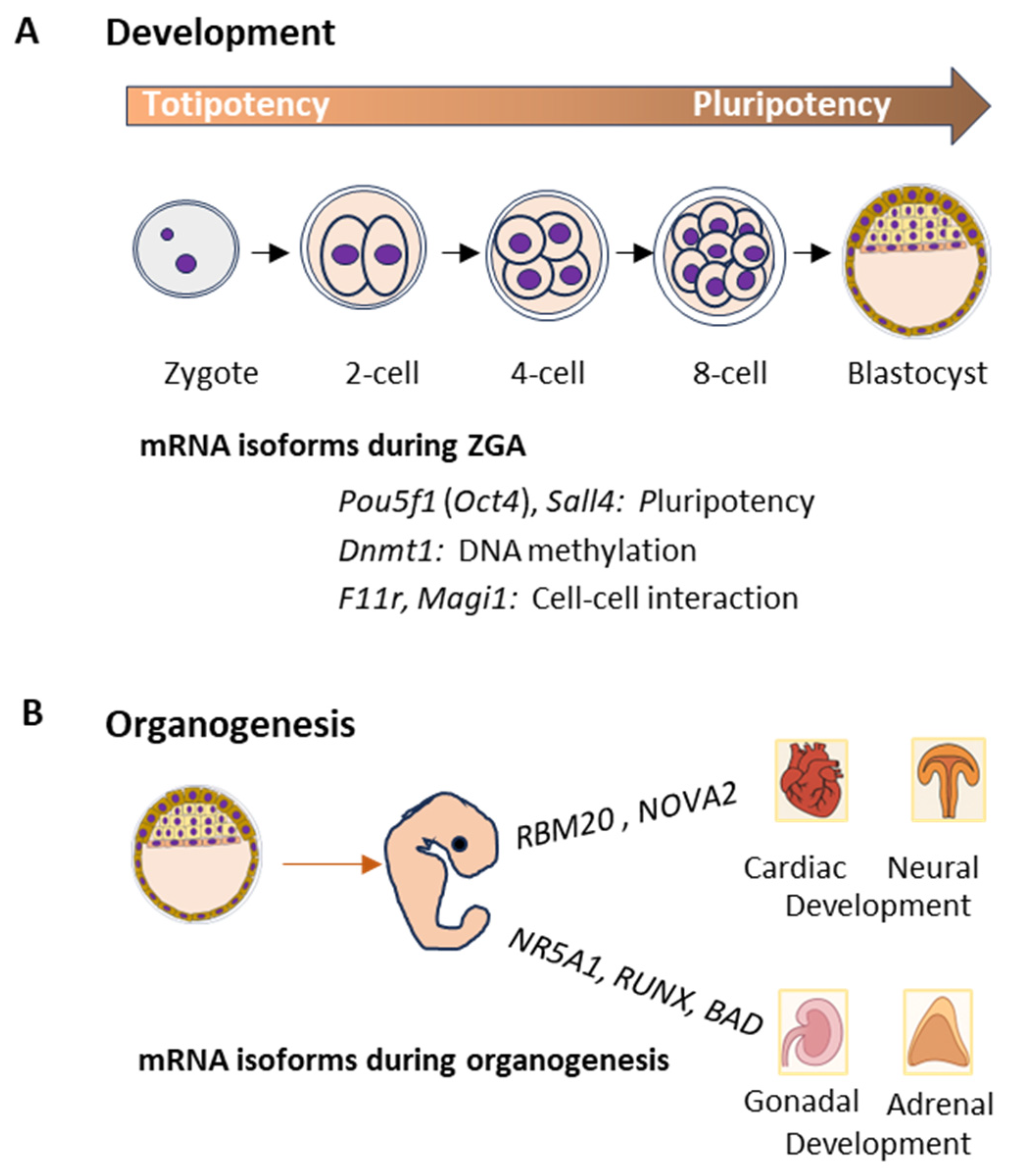
2.1. Development and Organogenesis
2.2. Physiological Functions
2.3. Aging
3. mRNA Transcript Variants in Disease Conditions
3.1. Systemic Diseases
3.1.1. Neurological Diseases
3.1.2. Cardiovascular Diseases
3.1.3. Respiratory Diseases
3.1.4. Gastrointestinal Diseases
3.1.5. Genitourinary Diseases
3.1.6. Musculoskeletal Diseases
3.2. Metabolic Diseases
3.3. Genetic Diseases
3.4. Autoimmune Diseases
3.5. Infectious Diseases
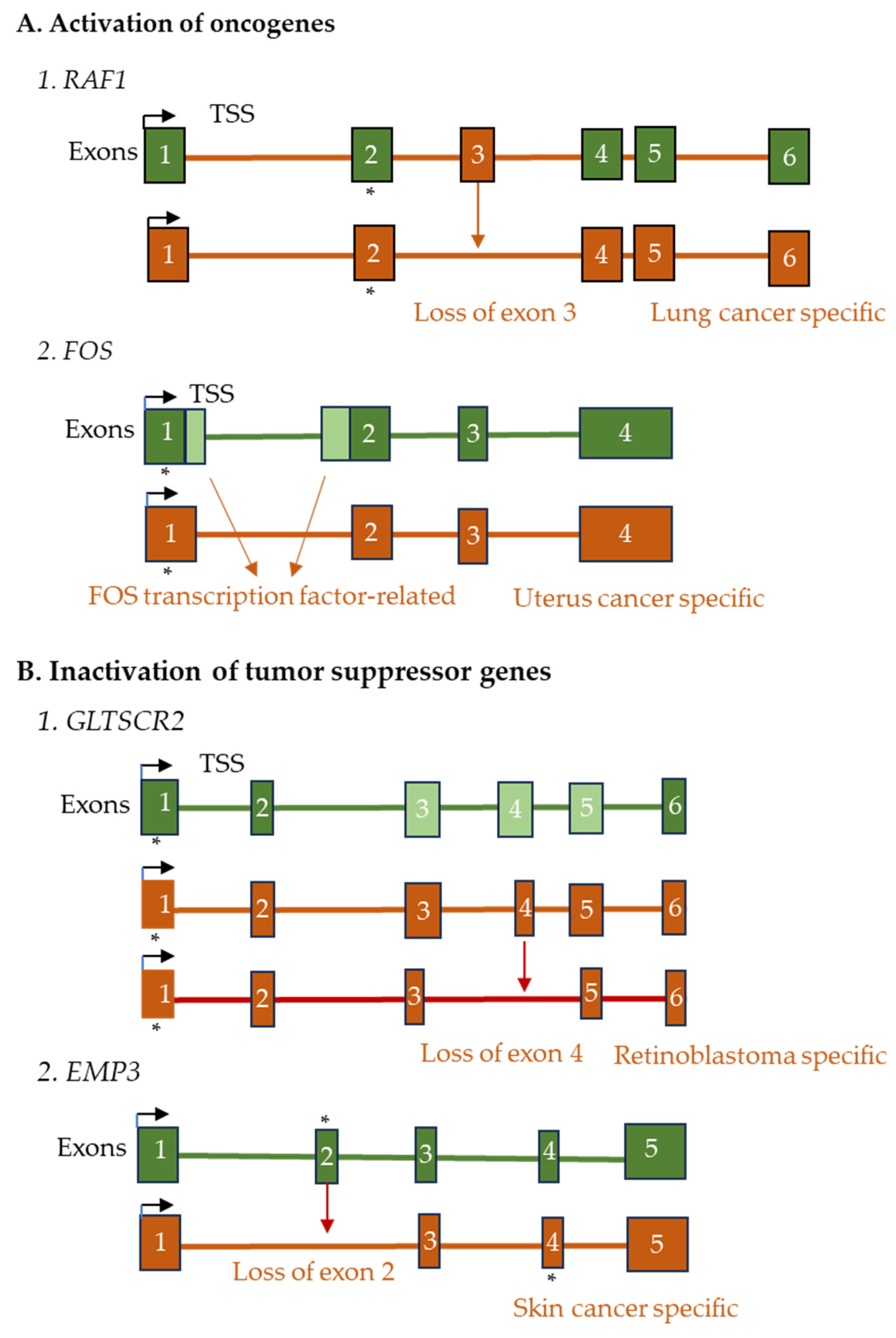
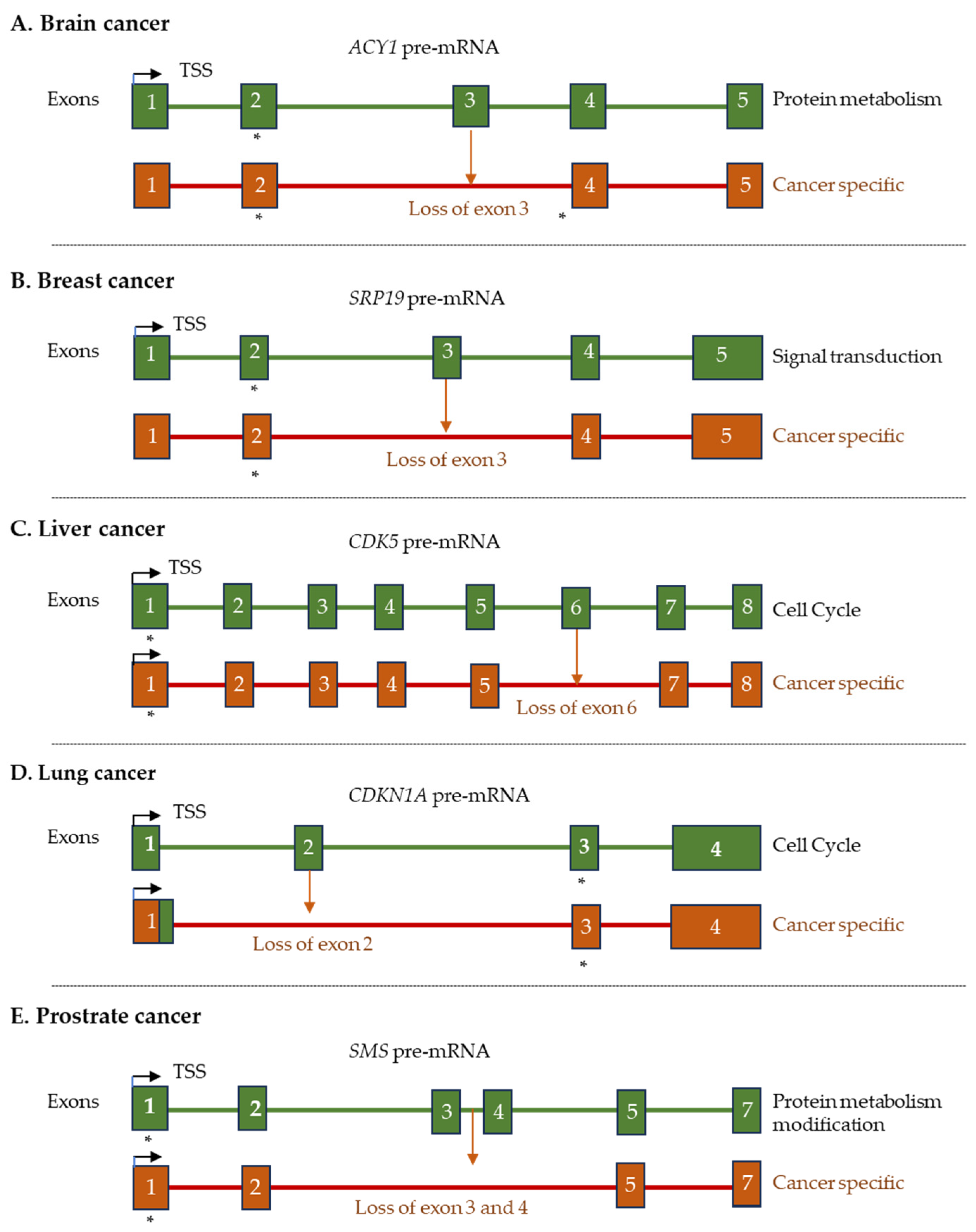
3.6. Benign and Malignant Tumors
4. Translational Impact of mRNA Isoforms and Variants
5. Detection of Full-Length mRNA Isoforms and Variants
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lisowiec, J.; Magner, D.; Kierzek, E.; Lenartowicz, E.; Kierzek, R. Structural determinants for alternative splicing regulation of the MAPT pre-mRNA. RNA Biol. 2015, 12, 330–342. [Google Scholar] [CrossRef][Green Version]
- Dhamija, S.; Menon, M.B. Non-coding transcript variants of protein-coding genes—What are they good for? RNA Biol. 2018, 15, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Vo, K.; Sharma, Y.; Paul, A.; Mohamadi, R.; Mohamadi, A.; Fields, P.E.; Rumi, M.A.K. Importance of Transcript Variants in Transcriptome Analyses. Cells 2024, 13, 1502. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.A.; Cochran, K.; Kozlowski, C.; Wang, J.; Alexander, G.; Cady, M.A.; Spencer, W.J.; Ruzycki, P.A.; Clark, B.S.; Laeremans, A.; et al. Comprehensive identification of mRNA isoforms reveals the diversity of neural cell-surface molecules with roles in retinal development and disease. Nat. Commun. 2020, 11, 3328. [Google Scholar] [CrossRef]
- Pai, A.A.; Luca, F. Environmental influences on RNA processing: Biochemical, molecular and genetic regulators of cellular response. Wiley Interdiscip. Rev. RNA 2019, 10, e1503. [Google Scholar] [CrossRef]
- Badr, E.; ElHefnawi, M.; Heath, L.S. Computational identification of tissue-specific splicing regulatory elements in human genes from RNA-Seq data. PLoS ONE 2016, 11, e0166978. [Google Scholar] [CrossRef]
- Kasprzak, A.; Szaflarski, W. Role of Alternatively Spliced Messenger RNA (mRNA) Isoforms of the Insulin-Like Growth Factor 1 (IGF1) in Selected Human Tumors. Int. J. Mol. Sci. 2020, 21, 6995. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, V.; Seluanov, A.; Mita, P.; McKerrow, W.; Fenyö, D.; Boeke, J.D.; Linker, S.B.; Gage, F.H.; Kreiling, J.A.; Petrashen, A.P.; et al. The role of retrotransposable elements in ageing and age-associated diseases. Nature 2021, 596, 43–53. [Google Scholar] [CrossRef]
- Patterson, M.N.; Scannapieco, A.E.; Au, P.H.; Dorsey, S.; Royer, C.A.; Maxwell, P.H. Preferential retrotransposition in aging yeast mother cells is correlated with increased genome instability. DNA Repair 2015, 34, 18–27. [Google Scholar] [CrossRef]
- You, N.; Liu, C.; Gu, Y.; Wang, R.; Jia, H.; Zhang, T.; Jiang, S.; Shi, J.; Chen, M.; Guan, M.-X.; et al. SpliceTransformer predicts tissue-specific splicing linked to human diseases. Nat. Commun. 2024, 15, 9129. [Google Scholar] [CrossRef]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Y. mRNA Metabolism in Cardiac Development and Disease: Life After Transcription. Physiol. Rev. 2020, 100, 673–694. [Google Scholar] [CrossRef]
- Baralle, M.; Romano, M. Age-Related Alternative Splicing: Driver or Passenger in the Aging Process? Cells 2023, 12, 2819. [Google Scholar] [CrossRef]
- Cooper, T.A.; Wan, L.; Dreyfuss, G. RNA and disease. Cell 2009, 136, 777–793. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, S.M.; Yoon, K.J. Epitranscriptomic regulation of transcriptome plasticity in development and diseases of the brain. BMB Rep. 2020, 53, 551–564. [Google Scholar] [CrossRef]
- Sharma, Y.; Vo, K.; Shila, S.; Paul, A.; Dahiya, V.; Fields, P.E.; Rumi, M.A.K. mRNA Transcript Variants Expressed in Mammalian Cells. Int. J. Mol. Sci. 2025, 26, 1052. [Google Scholar] [CrossRef]
- Le Quesne, J.P.; Spriggs, K.A.; Bushell, M.; Willis, A.E. Dysregulation of protein synthesis and disease. J. Pathol. 2010, 220, 140–151. [Google Scholar] [CrossRef]
- Liu, S.; Yu, Y.-P.; Ren, B.-G.; Ben-Yehezkel, T.; Obert, C.; Smith, M.; Wang, W.; Ostrowska, A.; Soto-Gutierrez, A.; Luo, J.-H. Long-read single-cell sequencing reveals expressions of hypermutation clusters of isoforms in human liver cancer cells. eLife 2023. [Google Scholar] [CrossRef]
- Schoch, K.; Tan, Q.K.; Stong, N.; Deak, K.L.; McConkie-Rosell, A.; McDonald, M.T.; Goldstein, D.B.; Jiang, Y.H.; Shashi, V. Alternative transcripts in variant interpretation: The potential for missed diagnoses and misdiagnoses. Genet. Med. 2020, 22, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Vo, K.; Shila, S.; Sharma, Y.; Pei, G.J.; Rosales, C.Y.; Dahiya, V.; Fields, P.E.; Rumi, M.A.K. Detection of mRNA Transcript Variants. Genes 2025, 16, 343. [Google Scholar] [CrossRef] [PubMed]
- Farley, B.M.; Ryder, S.P. Regulation of maternal mRNAs in early development. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 135–162. [Google Scholar] [CrossRef]
- Schulz, K.N.; Harrison, M.M. Mechanisms regulating zygotic genome activation. Nat. Rev. Genet. 2019, 20, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ahi, E.P. The importance of alternative splicing in adaptive evolution. Mol. Ecol. 2022, 31, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Onichtchouk, D. Pou5f1/oct4 in pluripotency control: Insights from zebrafish. Genesis 2012, 50, 75–85. [Google Scholar] [CrossRef]
- Aanes, H.; Østrup, O.; Andersen, I.S.; Moen, L.F.; Mathavan, S.; Collas, P.; Alestrom, P. Differential transcript isoform usage pre-and post-zygotic genome activation in zebrafish. BMC Genom. 2013, 14, 331. [Google Scholar] [CrossRef]
- Li, F.; Karimi, N.; Wang, S.; Pan, T.; Dong, J.; Wang, X.; Ma, S.; Shan, Q.; Liu, C.; Zhang, Y. mRNA isoform switches during mouse zygotic genome activation. Cell Prolif. 2024, 57, e13655. [Google Scholar] [CrossRef]
- Ju Lee, H.; Bartsch, D.; Xiao, C.; Guerrero, S.; Ahuja, G.; Schindler, C.; Moresco, J.J.; Yates, J.R.; Gebauer, F.; Bazzi, H.; et al. A post-transcriptional program coordinated by CSDE1 prevents intrinsic neural differentiation of human embryonic stem cells. Nat. Commun. 2017, 8, 1456. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.; Ranjouri, M.; Megino-Luque, C.; Newberg, J.Y.; Du, D.; Martin, K.; Miner, R.E.; Prater, M.S.; Wee, D.K.B.; Centeno, B.; et al. RBFOX2 deregulation promotes pancreatic cancer progression and metastasis through alternative splicing. Nat. Commun. 2023, 14, 8444. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Q.; Wang, H.; Yang, X.; Mu, H. Alternative splicing and related RNA binding proteins in human health and disease. Signal Transduct. Target. Ther. 2024, 9, 26. [Google Scholar] [CrossRef]
- Colino-Sanguino, Y.; Clark, S.J.; Valdes-Mora, F. The H2A. Z-nucleosome code in mammals: Emerging functions. Trends Genet. 2022, 38, 273–289. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Li, X.; Zhao, X.; Zhao, H.; Yang, W.; Zuo, Y.; Cai, L.; Xing, Y. Temporal dynamic analysis of alternative splicing during embryonic development in zebrafish. Front. Cell Dev. Biol. 2022, 10, 879795. [Google Scholar] [CrossRef]
- Tan, C.M.J.; Lewandowski, A.J. The transitional heart: From early embryonic and fetal development to neonatal life. Fetal Diagn. Ther. 2020, 47, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Fochi, S.; Lorenzi, P.; Galasso, M.; Stefani, C.; Trabetti, E.; Zipeto, D.; Romanelli, M.G. The emerging role of the RBM20 and PTBP1 ribonucleoproteins in heart development and cardiovascular diseases. Genes 2020, 11, 402. [Google Scholar] [CrossRef]
- Deshpande, A.; Shetty, P.M.V.; Frey, N.; Rangrez, A.Y. SRF: A seriously responsible factor in cardiac development and disease. J. Biomed. Sci. 2022, 29, 38. [Google Scholar] [CrossRef] [PubMed]
- Mengmeng, X.; Yuejuan, X.; Sun, C.; Yanan, L.; Fen, L.; Kun, S. Novel mutations of the SRF gene in Chinese sporadic conotruncal heart defect patients. BMC Med. Genet. 2020, 21, 95. [Google Scholar] [CrossRef]
- Mokalled, M.H.; Carroll, K.J.; Cenik, B.K.; Chen, B.; Liu, N.; Olson, E.N.; Bassel-Duby, R. Myocardin-related transcription factors are required for cardiac development and function. Dev. Biol. 2015, 406, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.L.; Titus, T.; Desvignes, T.; BreMiller, R.; Batzel, P.; Sydes, J.; Farnsworth, D.; Dillon, D.; Wegner, J.; Phillips, J.B.; et al. A fish with no sex: Gonadal and adrenal functions partition between zebrafish NR5A1 co-orthologs. Genetics 2021, 217, iyaa030. [Google Scholar] [CrossRef]
- Ruggiero, C.; Doghman, M.; Lalli, E. How genomic studies have improved our understanding of the mechanisms of transcriptional regulation by NR5A nuclear receptors. Mol. Cell. Endocrinol. 2015, 408, 138–144. [Google Scholar] [CrossRef]
- Tagami, A.; Ikeda, Y.; Ishizuka, K.; Maekawa, M. Conditional disruption of Nr5a1 directed by Sox9-Cre impairs adrenal development. Sci. Rep. 2024, 14, 12297. [Google Scholar] [CrossRef]
- Narita, T.; Higashijima, Y.; Kilic, S.; Liebner, T.; Walter, J.; Choudhary, C. Acetylation of histone H2B marks active enhancers and predicts CBP/p300 target genes. Nat. Genet. 2023, 55, 679–692. [Google Scholar] [CrossRef]
- Haberland, M.; Montgomery, R.L.; Olson, E.N. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat. Rev. Genet. 2009, 10, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Rui, X.; Bo, W.; Qing, G. The critical roles of histone deacetylase 3 in the pathogenesis of solid organ injury. Cell Death Dis. 2021, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Carim, S. Regulating enhancer activation. Nat. Cell Biol. 2023, 25, 372. [Google Scholar] [CrossRef]
- Jansz, N. DNA methylation dynamics at transposable elements in mammals. Essays Biochem. 2019, 63, 677–689. [Google Scholar] [CrossRef]
- Mittleman, B.E.; Pott, S.; Warland, S.; Zeng, T.; Mu, Z.; Kaur, M.; Gilad, Y.; Li, Y. Alternative polyadenylation mediates genetic regulation of gene expression. eLife 2020, 9, e57492. [Google Scholar] [CrossRef]
- Cesana, M.; Guo, M.H.; Cacchiarelli, D.; Wahlster, L.; Barragan, J.; Doulatov, S.; Vo, L.T.; Salvatori, B.; Trapnell, C.; Clement, K.; et al. A CLK3-HMGA2 Alternative Splicing Axis Impacts Human Hematopoietic Stem Cell Molecular Identity throughout Development. Cell Stem Cell 2018, 22, 575–588.e577. [Google Scholar] [CrossRef] [PubMed]
- Cleynen, I.; Van de Ven, W.J. The HMGA proteins: A myriad of functions. Int. J. Oncol. 2008, 32, 289–305. [Google Scholar] [CrossRef]
- Snijders Blok, L.; Vino, A.; den Hoed, J.; Underhill, H.R.; Monteil, D.; Li, H.; Reynoso Santos, F.J.; Chung, W.K.; Amaral, M.D.; Schnur, R.E.; et al. Heterozygous variants that disturb the transcriptional repressor activity of FOXP4 cause a developmental disorder with speech/language delays and multiple congenital abnormalities. Genet. Med. 2021, 23, 534–542. [Google Scholar] [CrossRef]
- Yamamoto, T. Genomic aberrations associated with the pathophysiological mechanisms of neurodevelopmental disorders. Cells 2021, 10, 2317. [Google Scholar] [CrossRef]
- Wang, R.; Helbig, I.; Edmondson, A.C.; Lin, L.; Xing, Y. Splicing defects in rare diseases: Transcriptomics and machine learning strategies towards genetic diagnosis. Brief. Bioinform. 2023, 24, bbad284. [Google Scholar] [CrossRef]
- Mensah, M.A.; Niskanen, H.; Magalhaes, A.P.; Basu, S.; Kircher, M.; Sczakiel, H.L.; Reiter, A.M.V.; Elsner, J.; Meinecke, P.; Biskup, S.; et al. Aberrant phase separation and nucleolar dysfunction in rare genetic diseases. Nature 2023, 614, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, S.; Chattopadhyay, K. A tale of (disordered) tail. Commun. Biol. 2023, 6, 411. [Google Scholar] [CrossRef]
- Imbriano, C.; Molinari, S. Alternative splicing of transcription factors genes in muscle physiology and pathology. Genes 2018, 9, 107. [Google Scholar] [CrossRef]
- Barrie, E.S.; Smith, R.M.; Sanford, J.C.; Sadee, W. mRNA transcript diversity creates new opportunities for pharmacological intervention. Mol. Pharmacol. 2012, 81, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.; Benovoy, D.; Dias, C.; Gurd, S.; Provencher, C.; Beaulieu, P.; Hudson, T.J.; Sladek, R.; Majewski, J. Genome-wide analysis of transcript isoform variation in humans. Nat. Genet. 2008, 40, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, R.E.; Andreadis, A.; Nadal-Ginard, B. Alternative splicing: A ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu. Rev. Biochem. 1987, 56, 467–495. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Pozo, F.; di Domenico, T.; Vazquez, J.; Tress, M.L. An analysis of tissue-specific alternative splicing at the protein level. PLoS Comput. Biol. 2020, 16, e1008287. [Google Scholar] [CrossRef]
- Beitelshees, A.L.; Navare, H.; Wang, D.; Gong, Y.; Wessel, J.; Moss, J.I.; Langaee, T.Y.; Cooper-DeHoff, R.M.; Sadee, W.; Pepine, C.J. CACNA1C gene polymorphisms, cardiovascular disease outcomes, and treatment response. Circ.—Cardiovasc. Genet. 2009, 2, 362–370. [Google Scholar] [CrossRef]
- Su, C.-H.; Tarn, W.-Y. Alternative splicing in neurogenesis and brain development. Front. Mol. Biosci. 2018, 5, 12. [Google Scholar] [CrossRef]
- Celotto, A.M.; Graveley, B.R. Alternative splicing of the Drosophila Dscam pre-mRNA is both temporally and spatially regulated. Genetics 2001, 159, 599–608. [Google Scholar] [CrossRef]
- Smith, P.H. Dscam Gene Expression in Invertebrate Immunity: Alternative Splicing in Response to Diverse Pathogens. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 2012. [Google Scholar]
- Chen, Y.; Xiao, D.; Zhang, L.; Cai, C.L.; Li, B.Y.; Liu, Y. The Role of Tbx20 in Cardiovascular Development and Function. Front. Cell Dev. Biol. 2021, 9, 638542. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, H.; Xu, L.; Xie, Z. N6-Methyladenosine Modification and Its Regulation of Respiratory Viruses. Front. Cell Dev. Biol. 2021, 9, 699997. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, B.; Nie, Z.; Duan, L.; Xiong, Q.; Jin, Z.; Yang, C.; Chen, Y. The role of m6A modification in the biological functions and diseases. Signal Transduct. Target. Ther. 2021, 6, 74. [Google Scholar] [CrossRef]
- Cai, T.; Atteh, L.L.; Zhang, X.; Huang, C.; Bai, M.; Ma, H.; Zhang, C.; Fu, W.; Gao, L.; Lin, Y.; et al. The N6-Methyladenosine Modification and Its Role in mRNA Metabolism and Gastrointestinal Tract Disease. Front. Surg. 2022, 9, 819335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wan, J.; Gao, X.; Zhang, X.; Jaffrey, S.R.; Qian, S.-B. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 2015, 526, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Di Giammartino, D.C.; Nishida, K.; Manley, J.L. Mechanisms and consequences of alternative polyadenylation. Mol. Cell 2011, 43, 853–866. [Google Scholar] [CrossRef]
- Mitschka, S.; Mayr, C. Context-specific regulation and function of mRNA alternative polyadenylation. Nat. Rev. Mol. Cell Biol. 2022, 23, 779–796. [Google Scholar] [CrossRef]
- Cao, J.; Kuyumcu-Martinez, M.N. Alternative polyadenylation regulation in cardiac development and cardiovascular disease. Cardiovasc. Res. 2023, 119, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Marasco, L.E.; Kornblihtt, A.R. The physiology of alternative splicing. Nat. Rev. Mol. Cell Biol. 2023, 24, 242–254. [Google Scholar] [CrossRef]
- Wang, K.; Wu, D.; Zhang, H.; Das, A.; Basu, M.; Malin, J.; Cao, K.; Hannenhalli, S. Comprehensive map of age-associated splicing changes across human tissues and their contributions to age-associated diseases. Sci. Rep. 2018, 8, 10929. [Google Scholar] [CrossRef]
- Bhadra, M.; Howell, P.; Dutta, S.; Heintz, C.; Mair, W.B. Alternative splicing in aging and longevity. Hum. Genet. 2020, 139, 357–369. [Google Scholar] [CrossRef]
- Pal, S.; Tyler, J.K. Epigenetics and aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Dang, W.; Donahue, G.; Dai, J.; Dorsey, J.; Cao, X.; Liu, W.; Cao, K.; Perry, R.; Lee, J.Y.; et al. H3K36 methylation promotes longevity by enhancing transcriptional fidelity. Genes. Dev. 2015, 29, 1362–1376. [Google Scholar] [CrossRef]
- Masuda, K.; Marasa, B.; Martindale, J.L.; Halushka, M.K.; Gorospe, M. Tissue- and age-dependent expression of RNA-binding proteins that influence mRNA turnover and translation. Aging 2009, 1, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gómez, A.; Izquierdo, J.M. The multifunctional faces of T-cell intracellular antigen 1 in health and disease. Int. J. Mol. Sci. 2022, 23, 1400. [Google Scholar] [CrossRef] [PubMed]
- López-Domínguez, J.A.; Rodríguez-López, S.; Ahumada-Castro, U.; Desprez, P.Y.; Konovalenko, M.; Laberge, R.M.; Cárdenas, C.; Villalba, J.M.; Campisi, J. Cdkn1a transcript variant 2 is a marker of aging and cellular senescence. Aging 2021, 13, 13380–13392. [Google Scholar] [CrossRef]
- Hudgins, A.D.; Tazearslan, C.; Tare, A.; Zhu, Y.; Huffman, D.; Suh, Y. Age-and tissue-specific expression of senescence biomarkers in mice. Front. Genet. 2018, 9, 59. [Google Scholar] [CrossRef]
- Papadakis, A. Aging Associated Changes of Transcriptional Elongation Speed and Transcriptional Error Rate. Ph.D. Thesis, Universität zu Köln, Cologne, Germany, 2024. [Google Scholar]
- Debès, C.; Papadakis, A.; Grönke, S.; Karalay, Ö.; Tain, L.S.; Mizi, A.; Nakamura, S.; Hahn, O.; Weigelt, C.; Josipovic, N.; et al. Ageing-associated changes in transcriptional elongation influence longevity. Nature 2023, 616, 814–821. [Google Scholar] [CrossRef]
- Gruner, H.; Cortés-López, M.; Cooper, D.A.; Bauer, M.; Miura, P. CircRNA accumulation in the aging mouse brain. Sci. Rep. 2016, 6, 38907. [Google Scholar] [CrossRef]
- Tumasian, R.A., 3rd; Harish, A.; Kundu, G.; Yang, J.H.; Ubaida-Mohien, C.; Gonzalez-Freire, M.; Kaileh, M.; Zukley, L.M.; Chia, C.W.; Lyashkov, A.; et al. Skeletal muscle transcriptome in healthy aging. Nat. Commun. 2021, 12, 2014. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, N.; Matai, L.; Jain, V.; Garg, A.; Mukhopadhyay, A. A chromatin modifier integrates insulin/IGF-1 signalling and dietary restriction to regulate longevity. Aging Cell 2016, 15, 694–705. [Google Scholar] [CrossRef]
- Testa, G.; Biasi, F.; Poli, G.; Chiarpotto, E. Calorie restriction and dietary restriction mimetics: A strategy for improving healthy aging and longevity. Curr. Pharm. Des. 2014, 20, 2950–2977. [Google Scholar] [CrossRef]
- Green, C.L.; Lamming, D.W.; Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 2022, 23, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.K.; Sun, S.; Lee, M.; Li, W.; Skvir, N.; Neretti, N.; Vijg, J.; Secombe, J. Expression of retrotransposons contributes to aging in Drosophila. Genetics 2023, 224, iyad073. [Google Scholar] [CrossRef]
- Kwon, H.C.; Bae, Y.; Lee, S.V. The Role of mRNA Quality Control in the Aging of Caenorhabditis elegans. Mol. Cells 2023, 46, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Smith, C.W.; Jiggins, C.D. Alternative splicing as a source of phenotypic diversity. Nat. Rev. Genet. 2022, 23, 697–710. [Google Scholar] [CrossRef]
- Kim, H.K.; Pham, M.H.C.; Ko, K.S.; Rhee, B.D.; Han, J. Alternative splicing isoforms in health and disease. Pflügers Arch.—Eur. J. Physiol. 2018, 470, 995–1016. [Google Scholar] [CrossRef] [PubMed]
- Raj, B.; Blencowe, B.J. Alternative Splicing in the Mammalian Nervous System: Recent Insights into Mechanisms and Functional Roles. Neuron 2015, 87, 14–27. [Google Scholar] [CrossRef]
- Bian, S.; Sun, T. Functions of Noncoding RNAs in Neural Development and Neurological Diseases. Mol. Neurobiol. 2011, 44, 359–373. [Google Scholar] [CrossRef]
- Lopez Soto, E.J.; Gandal, M.J.; Gonatopoulos-Pournatzis, T.; Heller, E.A.; Luo, D.; Zheng, S. Mechanisms of Neuronal Alternative Splicing and Strategies for Therapeutic Interventions. J. Neurosci. 2019, 39, 8193–8199. [Google Scholar] [CrossRef]
- Furlanis, E.; Scheiffele, P. Regulation of Neuronal Differentiation, Function, and Plasticity by Alternative Splicing. Annu. Rev. Cell Dev. Biol. 2018, 34, 451–469. [Google Scholar] [CrossRef]
- Lyons, M.R.; West, A.E. Mechanisms of specificity in neuronal activity-regulated gene transcription. Prog. Neurobiol. 2011, 94, 259–295. [Google Scholar] [CrossRef]
- Yap, E.-L.; Greenberg, M.E. Activity-Regulated Transcription: Bridging the Gap between Neural Activity and Behavior. Neuron 2018, 100, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Lin, S. mRNA alternative polyadenylation (APA) in regulation of gene expression and diseases. Genes Dis. 2023, 10, 165–174. [Google Scholar] [CrossRef]
- Liu, C.S.; Park, C.; Ngo, T.; Saikumar, J.; Palmer, C.R.; Shahnaee, A.; Romanow, W.J.; Chun, J. RNA isoform diversity in human neurodegenerative diseases. eNeuro 2024, 11, ENEURO.0296-24.2024. [Google Scholar] [CrossRef] [PubMed]
- Sandbrink, R.; Masters, C.L.; Beyreuther, K. APP gene family. Alternative splicing generates functionally related isoforms. Ann. N. Y. Acad. Sci. 1996, 777, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Duan, J.; Cai, Z.; Arai, C.; Di, C.; Venters, C.C.; Xu, J.; Jones, M.; So, B.-R.; Dreyfuss, G. TOMM40-APOE chimera linking Alzheimer’s highest risk genes: A new pathway for mitochondria regulation and APOE4 pathogenesis. bioRxiv 2024. [Google Scholar] [CrossRef]
- De Paoli-Iseppi, R.; Joshi, S.; Gleeson, J.; Prawer, Y.D.J.; Yu, Y.; Agarwal, R.; Li, A.; Hull, A.; Whitehead, E.M.; Seo, Y. Long-read sequencing reveals the RNA isoform repertoire of neuropsychiatric risk genes in human brain. medRxiv 2024. [Google Scholar] [CrossRef]
- Han, X.; Li, W.; Chen, C.; Liu, J.; Sun, J.; Wang, F.; Wang, C.; Mu, J.; Gu, X.; Liu, F.; et al. Genetic variants and mRNA expression levels of KLF4 and KLF5 with hypertension: A combination of case-control study and cohort study. J. Biomed. Res. 2024, 39, 103–113. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Han, Z.; Dong, J.; Pang, D.; Fu, Y.; Li, L. KLF4 alleviates cerebral vascular injury by ameliorating vascular endothelial inflammation and regulating tight junction protein expression following ischemic stroke. J. Neuroinflamm. 2020, 17, 107. [Google Scholar] [CrossRef]
- Ni, H.; Haemmig, S.; Deng, Y.; Chen, J.; Simion, V.; Yang, D.; Sukhova, G.; Shvartz, E.; Wara, A.K.M.K.; Cheng, H.S.; et al. A Smooth Muscle Cell–Enriched Long Noncoding RNA Regulates Cell Plasticity and Atherosclerosis by Interacting With Serum Response Factor. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2399–2416. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, H.-Y. Transcription factors: Key regulatory targets of vascular smooth muscle cell in atherosclerosis. Mol. Med. 2023, 29, 2. [Google Scholar] [CrossRef]
- Cuthbertson, I.; Morrell, N.W.; Caruso, P. BMPR2 mutation and metabolic reprogramming in pulmonary arterial hypertension. Circ. Res. 2023, 132, 109–126. [Google Scholar] [CrossRef]
- Cogan, J.; Austin, E.; Hedges, L.; Womack, B.; West, J.; Loyd, J.; Hamid, R. Role of BMPR2 alternative splicing in heritable pulmonary arterial hypertension penetrance. Circulation 2012, 126, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.W.; Hu, Y.W.; Ho, J.W.K.; Ikeda, S.; Polster, S.; John, R.; Hall, J.L.; Bisping, E.; Pieske, B.; dos Remedios, C.G.; et al. Heart Failure–Associated Changes in RNA Splicing of Sarcomere Genes. Circ.—Cardiovasc. Genet. 2010, 3, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wei, Z.; Nie, Y.; Chen, H.Z. Therapeutic potential of alternative splicing in cardiovascular diseases. eBioMedicine 2024, 101, 104995. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhou, D.; Poulsen, O.; Hartley, I.; Imamura, T.; Xie, E.X.; Haddad, G.G. Exploring miRNA-mRNA regulatory network in cardiac pathology in Na+/H+ exchanger isoform 1 transgenic mice. Physiol. Genom. 2018, 50, 846–861. [Google Scholar] [CrossRef]
- Park, J.Y.; Li, W.; Zheng, D.; Zhai, P.; Zhao, Y.; Matsuda, T.; Vatner, S.F.; Sadoshima, J.; Tian, B. Comparative analysis of mRNA isoform expression in cardiac hypertrophy and development reveals multiple post-transcriptional regulatory modules. PLoS ONE 2011, 6, e22391. [Google Scholar] [CrossRef]
- Fang, L.; Sun, Q.; Roth, M. Immunologic and non-immunologic mechanisms leading to airway remodeling in asthma. Int. J. Mol. Sci. 2020, 21, 757. [Google Scholar] [CrossRef]
- Grzela, K.; Litwiniuk, M.; Zagorska, W.; Grzela, T. Airway remodeling in chronic obstructive pulmonary disease and asthma: The role of matrix metalloproteinase-9. Arch. Immunol. Ther. Exp. 2016, 64, 47–55. [Google Scholar] [CrossRef]
- Panek, M.G.; Karbownik, M.S.; Górski, K.M.; Koćwin, M.; Kardas, G.; Marynowski, M.; Kuna, P. New insights into the regulation of TGF-β/Smad and MPK signaling pathway gene expressions by nasal allergen and methacholine challenge test in asthma. Clin. Transl. Allergy 2022, 12, e12172. [Google Scholar] [CrossRef]
- Khalenkow, D.; Brandsma, C.-A.; Timens, W.; Choy, D.F.; Grimbaldeston, M.A.; Rosenberger, C.M.; Slebos, D.-J.; Kerstjens, H.A.; Faiz, A.; Koppelman, G.H. Alternative Splicing Is a Major Factor Shaping Transcriptome Diversity in Mild and Severe Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2024, 70, 414–423. [Google Scholar] [CrossRef]
- Lackey, L.; Coria, A.; Ghosh, A.J.; Grayeski, P.; Hatfield, A.; Shankar, V.; Platig, J.; Xu, Z.; Ramos, S.B.; Silverman, E.K. Alternative poly-adenylation modulates α1-antitrypsin expression in chronic obstructive pulmonary disease. PLoS Genet. 2021, 17, e1009912. [Google Scholar] [CrossRef]
- Corley, M.; Solem, A.; Phillips, G.; Lackey, L.; Ziehr, B.; Vincent, H.A.; Mustoe, A.M.; Ramos, S.B.V.; Weeks, K.M.; Moorman, N.J.; et al. An RNA structure-mediated, posttranscriptional model of human α-1-antitrypsin expression. Proc. Natl. Acad. Sci. USA 2017, 114, E10244–E10253. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, D.; Cichy, W.; Przysławski, J.; Drzymała-Czyż, S. The role of microbiota and enteroendocrine cells in maintaining homeostasis in the human digestive tract. Adv. Med. Sci. 2021, 66, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Shen, C.-K.J.; Majumder, P. RNA Modifications and RNA Metabolism in Neurological Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 11870. [Google Scholar] [CrossRef] [PubMed]
- LaForce, G.R.; Philippidou, P.; Schaffer, A.E. mRNA isoform balance in neuronal development and disease. WIREs RNA 2023, 14, e1762. [Google Scholar] [CrossRef]
- Miura, K.; Fujibuchi, W.; Sasaki, I. Alternative pre-mRNA splicing in digestive tract malignancy. Cancer Sci. 2011, 102, 309–316. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vanuytsel, T.; Farré, R.; Verstockt, S.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.; Schuit, F.; Vermeire, S.; Arijs, I.; et al. Genetic and Transcriptomic Bases of Intestinal Epithelial Barrier Dysfunction in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1718–1729. [Google Scholar] [CrossRef]
- McCole, D.F. IBD Candidate Genes and Intestinal Barrier Regulation. Inflamm. Bowel Dis. 2014, 20, 1829–1849. [Google Scholar] [CrossRef]
- Carrion, S.A.; Michal, J.J.; Jiang, Z. Alternative Transcripts Diversify Genome Function for Phenome Relevance to Health and Diseases. Genes 2023, 14, 2051. [Google Scholar] [CrossRef]
- Nilsen, T.W.; Graveley, B.R. Expansion of the eukaryotic proteome by alternative splicing. Nature 2010, 463, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.X.; Rao, X.; Liu, Y. Modulator-dependent RBPs changes alternative splicing outcomes in kidney cancer. Front. Genet. 2020, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Torban, E.; Goodyer, P. Wilms’ tumor gene 1: Lessons from the interface between kidney development and cancer. Am. J. Physiol.—Ren. Physiol. 2024, 326, F3–F19. [Google Scholar] [CrossRef]
- Trink, Y.; Urbach, A.; Dekel, B.; Hohenstein, P.; Goldberger, J.; Kalisky, T. Characterization of alternative splicing in high-risk Wilms’ tumors. Int. J. Mol. Sci. 2024, 25, 4520. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, L.; Chen, D.; Li, F. The function of pre-mRNA alternative splicing in mammal spermatogenesis. Int. J. Biol. Sci. 2020, 16, 38. [Google Scholar] [CrossRef]
- Wu, D.; Khan, F.A.; Huo, L.; Sun, F.; Huang, C. Alternative splicing and MicroRNA: Epigenetic mystique in male reproduction. RNA Biol. 2022, 19, 162–175. [Google Scholar] [CrossRef]
- Zalewski, A.; Ma, N.S.; Legeza, B.; Renthal, N.; Flück, C.E.; Pandey, A.V. Vitamin D-dependent rickets type 1 caused by mutations in CYP27B1 affecting protein interactions with adrenodoxin. J. Clin. Endocrinol. Metab. 2016, 101, 3409–3418. [Google Scholar] [CrossRef]
- Rohdin, C.; Wang, C.; Brander, G.; Rondahl, V.; Karlsson, Å.; Friling, L.; Fischetti, A.; Meadows, J.; Häggström, J.; Jäderlund, K.H. Mutations in the CYP27B1 gene cause vitamin D dependent rickets in pugs. J. Vet. Intern. Med. 2023, 37, 1507–1513. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, G.S. Vitamin D Regulation of Osteoblast Growth and Differentiation. In Nutrition and Gene Expression; Crc Press: Boca Raton, FL, USA, 2018; pp. 391–429. [Google Scholar]
- Zhou, R.; Chun, R.F.; Lisse, T.S.; Garcia, A.J.; Xu, J.; Adams, J.S.; Hewison, M. Vitamin D and alternative splicing of RNA. J. Steroid Biochem. Mol. Biol. 2015, 148, 310–317. [Google Scholar] [CrossRef]
- Estañ, M.C.; Fernández-Núñez, E.; Zaki, M.S.; Esteban, M.I.; Donkervoort, S.; Hawkins, C.; Caparros-Martin, J.A.; Saade, D.; Hu, Y.; Bolduc, V. Recessive mutations in muscle-specific isoforms of FXR1 cause congenital multi-minicore myopathy. Nat. Commun. 2019, 10, 797. [Google Scholar] [CrossRef]
- Mroczek, M.; Longman, C.; Farrugia, M.E.; Garcia, S.K.; Ardicli, D.; Topaloglu, H.; Hernández-Laín, A.; Orhan, D.; Alikasifoglu, M.; Duff, J. FXR1-related congenital myopathy: Expansion of the clinical and genetic spectrum. J. Med. Genet. 2022, 59, 1069–1074. [Google Scholar] [CrossRef]
- Pistoni, M.; Ghigna, C.; Gabellini, D. Alternative splicing and muscular dystrophy. RNA Biol. 2010, 7, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Culligan, K.G.; Mackey, A.J.; Finn, D.M.; Maguire, P.B.; Ohlendieck, K. Role of dystrophin isoforms and associated proteins in muscular dystrophy. Int. J. Mol. Med. 1998, 2, 639–687. [Google Scholar] [CrossRef] [PubMed]
- Verdile, V.; Guizzo, G.; Ferrante, G.; Paronetto, M.P. RNA targeting in inherited neuromuscular disorders: Novel therapeutic strategies to counteract mis-splicing. Cells 2021, 10, 2850. [Google Scholar] [CrossRef]
- Coovert, D.D.; Le, T.T.; McAndrew, P.E.; Strasswimmer, J.; Crawford, T.O.; Mendell, J.R.; Coulson, S.E.; Androphy, E.J.; Prior, T.W.; Burghes, A.H. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 1997, 6, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Bebee, T.W.; Gladman, J.T.; Chandler, D.S. Splicing regulation of the survival motor neuron genes and implications for treatment of spinal muscular atrophy. Front. Biosci. 2010, 15, 1191. [Google Scholar] [CrossRef]
- Xue, Y.C.; Ng, C.S.; Xiang, P.; Liu, H.; Zhang, K.; Mohamud, Y.; Luo, H. Dysregulation of RNA-binding proteins in amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2020, 13, 78. [Google Scholar] [CrossRef]
- Perrone, B.; La Cognata, V.; Sprovieri, T.; Ungaro, C.; Conforti, F.L.; Andò, S.; Cavallaro, S. Alternative splicing of ALS genes: Misregulation and potential therapies. Cell. Mol. Neurobiol. 2020, 40, 1–14. [Google Scholar] [CrossRef]
- Dlamini, Z.; Mokoena, F.; Hull, R. Abnormalities in alternative splicing in diabetes: Therapeutic targets. J. Mol. Endocrinol. 2017, 59, R93–R107. [Google Scholar] [CrossRef]
- Singha, D.; Mondal, M.; Ghosh, D.; Choudhury, D.; Chakravarti, B.; Kar, R.K.; Malakar, P. The role of alternative splicing and splicing factors in diabetes: Current status and future perspectives. Wiley Interdiscip. Rev.—RNA 2024, 15, e1831. [Google Scholar] [CrossRef]
- Nutter, C.A.; Kuyumcu-Martinez, M.N. Emerging roles of RNA-binding proteins in diabetes and their therapeutic potential in diabetic complications. Wiley Interdiscip. Rev.—RNA 2018, 9, e1459. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Li, Z.; Han, J.; Xia, P.; Shen, Y.; Ma, J.; Liu, X.; Zhang, J.; Yu, P. Advances in the study of RNA-binding proteins in diabetic complications. Mol. Metab. 2022, 62, 101515. [Google Scholar] [CrossRef]
- Ghiasi, S.M.; Rutter, G.A. Consequences for pancreatic β-cell identity and function of unregulated transcript processing. Front. Endocrinol. 2021, 12, 625235. [Google Scholar] [CrossRef] [PubMed]
- Juan-Mateu, J.; Villate, O.; Eizirik, D.L. Mechanisms in endocrinology: Alternative splicing: The new frontier in diabetes research. Eur. J. Endocrinol. 2016, 174, R225–R238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Jiang, M.; Ma, L.; Hu, J.; Zhang, H.-H. Post-transcriptional control by RNA-binding proteins in diabetes and its related complications. Front. Physiol. 2022, 13, 953880. [Google Scholar] [CrossRef]
- Wong, C.-M.; Xu, L.; Yau, M.Y.-C. Alternative mRNA splicing in the pathogenesis of obesity. Int. J. Mol. Sci. 2018, 19, 632. [Google Scholar] [CrossRef]
- Shao, X.; Wang, M.; Wei, X.; Deng, S.; Fu, N.; Peng, Q.; Jiang, Y.; Ye, L.; Xie, J.; Lin, Y. Peroxisome proliferator-activated receptor-γ: Master regulator of adipogenesis and obesity. Curr. Stem Cell Res. Ther. 2016, 11, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.; Villena, A.J. Targeting mitochondrial biogenesis to treat insulin resistance. Curr. Pharm. Des. 2014, 20, 5527–5557. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Alsharhan, H.; Ficicioglu, C. Disorders of phenylalanine and tyrosine metabolism. Transl. Sci. Rare Dis. 2020, 5, 3–58. [Google Scholar] [CrossRef]
- Williams, R.A.; Mamotte, C.D.; Burnett, J.R. Phenylketonuria: An inborn error of phenylalanine metabolism. Clin. Biochem. Rev. 2008, 29, 31. [Google Scholar]
- Yahya-Graison, E.A.; Aubert, J.; Dauphinot, L.; Rivals, I.; Prieur, M.; Golfier, G.; Rossier, J.; Personnaz, L.; Creau, N.; Blehaut, H. Classification of human chromosome 21 gene-expression variations in Down syndrome: Impact on disease phenotypes. Am. J. Hum. Genet. 2007, 81, 475–491. [Google Scholar] [CrossRef]
- Palmer, C.R.; Liu, C.S.; Romanow, W.J.; Lee, M.H.; Chun, J. Altered cell and RNA isoform diversity in aging Down syndrome brains. Proc. Natl. Acad. Sci. USA 2021, 118, e2114326118, Erratum in Proc. Natl. Acad. Sci. USA 2022, 119, e2212181119. https://doi.org/10.1073/pnas.2212181119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Yang, G.; Cai, L.; Yang, F.; Zhang, Y.; Zeng, Y.; Ma, Q.; Zeng, F. The Study of Alternative Splicing Events in Human Induced Pluripotent Stem Cells From a Down’s Syndrome Patient. Front. Cell Dev. Biol. 2021, 9, 661381. [Google Scholar] [CrossRef]
- Toiber, D.; Azkona, G.; Ben-Ari, S.; Torán, N.; Soreq, H.; Dierssen, M. Engineering DYRK1A overdosage yields Down syndrome-characteristic cortical splicing aberrations. Neurobiol. Dis. 2010, 40, 348–359. [Google Scholar] [CrossRef] [PubMed]
- de C Gomes, F.; Mattos, M.F.; Goloni-Bertollo, E.M.; Pavarino, É.C. Alzheimer’s disease in the Down syndrome: An overview of genetics and molecular aspects. Neurol. India 2021, 69, 32–41. [Google Scholar] [CrossRef]
- Paw, B.H.; Neufeld, E.F. Normal transcription of the beta-hexosaminidase alpha-chain gene in the Ashkenazi Tay-Sachs mutation. J. Biol. Chem. 1988, 263, 3012–3015. [Google Scholar] [CrossRef] [PubMed]
- Solovyeva, V.V.; Shaimardanova, A.A.; Chulpanova, D.S.; Kitaeva, K.V.; Chakrabarti, L.; Rizvanov, A.A. New Approaches to Tay-Sachs Disease Therapy. Front. Physiol. 2018, 9, 1663. [Google Scholar] [CrossRef]
- Deletang, K.; Taulan-Cadars, M. Splicing mutations in the CFTR gene as therapeutic targets. Gene Ther. 2022, 29, 399–406. [Google Scholar] [CrossRef]
- Ramananda, Y.; Naren, A.P.; Arora, K. Functional consequences of CFTR interactions in cystic fibrosis. Int. J. Mol. Sci. 2024, 25, 3384. [Google Scholar] [CrossRef]
- Ho, P.J. The regulation of beta globin gene expression and beta thalassemia. Pathology 1999, 31, 315–324. [Google Scholar] [PubMed]
- Peixeiro, I.; Silva, A.L.; Romão, L. Control of human β-globin mRNA stability and its impact on beta-thalassemia phenotype. Haematologica 2011, 96, 905. [Google Scholar] [CrossRef]
- Kantor, J.A.; Turner, P.H.; Nienhuis, A.W. Beta thalassemia: Mutations which affect processing of the β-globin mRNA precursor. Cell 1980, 21, 149–157. [Google Scholar] [CrossRef]
- Benz, E.J.; Forget, B.G.; Hillman, D.G.; Cohen-Solal, M.; Pritchard, J.; Cavallesco, C.; Prensky, W.; Housman, D. Variability in the amount of beta-globin mRNA in beta0 thalassemia. Cell 1978, 14, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Goodnow, C.C. Pathways for self-tolerance and the treatment of autoimmune diseases. Lancet 2001, 357, 2115–2121. [Google Scholar] [CrossRef]
- Lazzari, E.; Jefferies, C.A. IRF5-mediated signaling and implications for SLE. Clin. Immunol. 2014, 153, 343–352. [Google Scholar] [CrossRef]
- Stone, R.C.; Du, P.; Feng, D.; Dhawan, K.; Rönnblom, L.; Eloranta, M.-L.; Donnelly, R.; Barnes, B.J. RNA-Seq for enrichment and analysis of IRF5 transcript expression in SLE. PLoS ONE 2013, 8, e54487. [Google Scholar] [CrossRef]
- Ibáñez-Costa, A.; Perez-Sanchez, C.; Patiño-Trives, A.M.; Luque-Tevar, M.; Font, P.; Arias de la Rosa, I.; Roman-Rodriguez, C.; Abalos-Aguilera, M.C.; Conde, C.; Gonzalez, A.; et al. Splicing machinery is impaired in rheumatoid arthritis, associated with disease activity and modulated by anti-TNF therapy. Ann. Rheum. Dis. 2022, 81, 56–67. [Google Scholar] [CrossRef]
- Li, H.; Reksten, T.R.; Ice, J.A.; Kelly, J.A.; Adrianto, I.; Rasmussen, A.; Wang, S.; He, B.; Grundahl, K.M.; Glenn, S.B.; et al. Identification of a Sjögren’s syndrome susceptibility locus at OAS1 that influences isoform switching, protein expression, and responsiveness to type I interferons. PLoS Genet. 2017, 13, e1006820. [Google Scholar] [CrossRef]
- Del Papa, N.; Minniti, A.; Lorini, M.; Carbonelli, V.; Maglione, W.; Pignataro, F.; Montano, N.; Caporali, R.; Vitali, C. The role of interferons in the pathogenesis of Sjögren’s syndrome and future therapeutic perspectives. Biomolecules 2021, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Voulgarelis, M.; Tzioufas, A.G. Pathogenetic mechanisms in the initiation and perpetuation of Sjögren’s syndrome. Nat. Rev. Rheumatol. 2010, 6, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, J.; Tian, J.; Wang, S. CD8+ T Lymphocytes: Crucial Players in Sjögren’s Syndrome. Front. Immunol. 2021, 11, 602823. [Google Scholar] [CrossRef]
- Evsyukova, I.; Somarelli, J.A.; Gregory, S.G.; Garcia-Blanco, M.A. Alternative splicing in multiple sclerosis and other autoimmune diseases. RNA Biol. 2010, 7, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.M.; Trifilieff, E.; Pender, M.P. Correlation Between Anti-Myelin Proteolipid Protein (PLP) Antibodies and Disease Severity in Multiple Sclerosis Patients With PLP Response-Permissive HLA Types. Front. Immunol. 2020, 11, 1891. [Google Scholar] [CrossRef]
- Ohno, K.; Ohkawara, B.; Shen, X.-M.; Selcen, D.; Engel, A.G. Clinical and pathologic features of congenital myasthenic syndromes caused by 35 genes—A comprehensive review. Int. J. Mol. Sci. 2023, 24, 3730. [Google Scholar] [CrossRef]
- Marín-Sánchez, A.; Álvarez-Sierra, D.; González, O.; Lucas-Martin, A.; Sellés-Sánchez, A.; Rudilla, F.; Enrich, E.; Colobran, R.; Pujol-Borrell, R. Regulation of TSHR expression in the thyroid and thymus may contribute to TSHR tolerance failure in graves’ disease patients via two distinct mechanisms. Front. Immunol. 2019, 10, 1695. [Google Scholar] [CrossRef]
- Brand, O.J.; Barrett, J.C.; Simmonds, M.J.; Newby, P.R.; McCabe, C.J.; Bruce, C.K.; Kysela, B.; Carr-Smith, J.D.; Brix, T.; Hunt, P.J. Association of the thyroid stimulating hormone receptor gene (TSHR) with Graves’ disease. Hum. Mol. Genet. 2009, 18, 1704–1713. [Google Scholar] [CrossRef]
- Latif, R.; Mezei, M.; Morshed, S.A.; Ma, R.; Ehrlich, R.; Davies, T.F. A Modifying Autoantigen in Graves’ Disease. Endocrinology 2019, 160, 1008–1020. [Google Scholar] [CrossRef]
- Li, J.; He, L.; Zhang, Y.; Xue, C.; Cao, Y. A novel method for genome-wide profiling of dynamic host-pathogen interactions using 3′ end enriched RNA-seq. Sci. Rep. 2017, 7, 8681. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.M.; Lynch, K.W. Control of alternative splicing in immune responses: Many regulators, many predictions, much still to learn. Immunol. Rev. 2013, 253, 216–236. [Google Scholar] [CrossRef]
- Su, Z.; Huang, D. Alternative splicing of pre-mRNA in the control of immune activity. Genes 2021, 12, 574. [Google Scholar] [CrossRef]
- Dubois, J.; Terrier, O.; Rosa-Calatrava, M. Influenza viruses and mRNA splicing: Doing more with less. mBio 2014, 5. [Google Scholar] [CrossRef]
- Te Velthuis, A.J.; Fodor, E. Influenza virus RNA polymerase: Insights into the mechanisms of viral RNA synthesis. Nat. Rev. Microbiol. 2016, 14, 479–493. [Google Scholar] [CrossRef]
- Picton, A.C.P.; Paximadis, M.; Koor, G.W.; Bharuthram, A.; Shalekoff, S.; Lassauniere, R.; Ive, P.; Tiemessen, C.T. Reduced CCR5 Expression and Immune Quiescence in Black South African HIV-1 Controllers. Front. Immunol. 2021, 12, 781263. [Google Scholar] [CrossRef]
- Judge, M.; Parker, E.; Naniche, D.; Souëf, P.L. Gene Expression: The Key to Understanding HIV-1 Infection? Microbiol. Mol. Biol. Rev. 2020, 84, 10.1128/mmbr.00080-00019. [Google Scholar] [CrossRef] [PubMed]
- Erkelenz, S. Regulation of Human and HIV-1 Splice Sites. Ph.D. Thesis, Heinrich-Heine-Universität, Düsseldorf, Germany, 2012. [Google Scholar]
- Mummidi, S.; Adams, L.M.; VanCompernolle, S.E.; Kalkonde, M.; Camargo, J.F.; Kulkarni, H.; Bellinger, A.S.; Bonello, G.; Tagoh, H.; Ahuja, S.S.; et al. Production of specific mRNA transcripts, usage of an alternate promoter, and octamer-binding transcription factors influence the surface expression levels of the HIV coreceptor CCR5 on primary T cells. J. Immunol. 2007, 178, 5668–5681. [Google Scholar] [CrossRef] [PubMed]
- Purcell, D.F.; Martin, M.A. Alternative splicing of human immunodeficiency virus type 1 mRNA modulates viral protein expression, replication, and infectivity. J. Virol. 1993, 67, 6365–6378. [Google Scholar] [CrossRef]
- Brelot, A.; Chakrabarti, L.A. CCR5 Revisited: How Mechanisms of HIV Entry Govern AIDS Pathogenesis. J. Mol. Biol. 2018, 430, 2557–2589. [Google Scholar] [CrossRef]
- Gornalusse, G.G.; Mummidi, S.; Gaitan, A.A.; Jimenez, F.; Ramsuran, V.; Picton, A.; Rogers, K.; Manoharan, M.S.; Avadhanam, N.; Murthy, K.K.; et al. Epigenetic mechanisms, T-cell activation, and CCR5 genetics interact to regulate T-cell expression of CCR5, the major HIV-1 coreceptor. Proc. Natl. Acad. Sci. USA 2015, 112, E4762–E4771. [Google Scholar] [CrossRef]
- Chinen, J.; Shearer, W.T. Molecular virology and immunology of HIV infection. J. Allergy Clin. Immunol. 2002, 110, 189–198. [Google Scholar] [CrossRef]
- Sehrawat, S.; Garcia-Blanco, M.A. RNA virus infections and their effect on host alternative splicing. Antivir. Res. 2023, 210, 105503. [Google Scholar] [CrossRef]
- Evans, E.L., III. Investigating Species-Specific Blocks to HIV-1 Replication and Vif-Induced Metaphase Arrest. Ph.D. Thesis, The University of Wisconsin-Madison, Madison, WI, USA, 2019. [Google Scholar]
- Liu, Y.-H.; Xu, H.-Q.; Zhu, S.-S.; Hong, Y.-F.; Li, X.-W.; Li, H.-X.; Xiong, J.-P.; Xiao, H.; Bu, J.-H.; Zhu, F.; et al. ASVirus: A Comprehensive Knowledgebase for the Viral Alternative Splicing. J. Chem. Inf. Model. 2025, 65, 2722–2729. [Google Scholar] [CrossRef]
- Amir, N.; Taube, R. Role of long noncoding RNA in regulating HIV infection—A comprehensive review. mBio 2024, 15, e01925-23. [Google Scholar] [CrossRef]
- Zhao, H.-J.; Hu, Y.-F.; Han, Q.-J.; Zhang, J. Innate and adaptive immune escape mechanisms of hepatitis B virus. World J. Gastroenterol. 2022, 28, 881. [Google Scholar] [CrossRef] [PubMed]
- Kuipery, A.; Gehring, A.J.; Isogawa, M. Mechanisms of HBV immune evasion. Antivir. Res. 2020, 179, 104816. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-C.; Garcia-Blanco, M.A. Role of alternative splicing in regulating host response to viral infection. Cells 2021, 10, 1720. [Google Scholar] [CrossRef] [PubMed]
- Plotch, S.J.; Bouloy, M.; Ulmanen, I.; Krug, R.M. A unique cap (m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell 1981, 23, 847–858. [Google Scholar] [CrossRef]
- Lyu, M.; Lai, H.; Wang, Y.; Zhou, Y.; Chen, Y.; Wu, D.; Chen, J.; Ying, B. Roles of alternative splicing in infectious diseases: From hosts, pathogens to their interactions. Chin. Med. J. 2023, 136, 767–779. [Google Scholar] [CrossRef]
- Carpenter, S.; Ricci, E.P.; Mercier, B.C.; Moore, M.J.; Fitzgerald, K.A. Post-transcriptional regulation of gene expression in innate immunity. Nat. Rev. Immunol. 2014, 14, 361–376. [Google Scholar] [CrossRef]
- Kalam, H.; Fontana, M.F.; Kumar, D. Alternate splicing of transcripts shape macrophage response to Mycobacterium tuberculosis infection. PLoS Pathog. 2017, 13, e1006236. [Google Scholar] [CrossRef]
- Hong, W.; Yang, H.; Wang, X.; Shi, J.; Zhang, J.; Xie, J. The Role of mRNA Alternative Splicing in Macrophages Infected with Mycobacterium tuberculosis: A Field Needing to Be Discovered. Molecules 2024, 29, 1798. [Google Scholar] [CrossRef]
- Baena, A.; Porcelli, S.A. Evasion and subversion of antigen presentation by Mycobacterium tuberculosis. Tissue Antigens 2009, 74, 189–204. [Google Scholar] [CrossRef]
- Corre, M.; Lebreton, A. Regulation of cold-inducible RNA-binding protein (CIRBP) in response to cellular stresses. Biochimie 2024, 217, 3–9. [Google Scholar] [CrossRef]
- Corre, M.; Boehm, V.; Besic, V.; Kurowska, A.; Viry, A.; Mohammad, A.; Sénamaud-Beaufort, C.; Thomas-Chollier, M.; Lebreton, A. Alternative splicing induced by bacterial pore-forming toxins sharpens CIRBP-mediated cell response to Listeria infection. Nucleic Acids Res. 2023, 51, 12459–12475. [Google Scholar] [CrossRef]
- Neves-da-Rocha, J.; Bitencourt, T.A.; de Oliveira, V.M.; Sanches, P.R.; Rossi, A.; Martinez-Rossi, N.M. Alternative Splicing in Heat Shock Protein Transcripts as a Mechanism of Cell Adaptation in Trichophyton rubrum. Cells 2019, 8, 1206. [Google Scholar] [CrossRef] [PubMed]
- Muzafar, S.; Sharma, R.D.; Chauhan, N.; Prasad, R. Intron distribution and emerging role of alternative splicing in fungi. FEMS Microbiol. Lett. 2021, 368, fnab135. [Google Scholar] [CrossRef] [PubMed]
- Agosto, L.M.; Mallory, M.J.; Ferretti, M.B.; Blake, D.; Krick, K.S.; Gazzara, M.R.; Garcia, B.A.; Lynch, K.W. Alternative splicing of HDAC7 regulates its interaction with 14-3-3 proteins to alter histone marks and target gene expression. Cell Rep. 2023, 42, 112273. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Bhattacharjee, R.; Das, S.; Mukherjee, S.; Ali, N. The paradigm of intracellular parasite survival and drug resistance in leishmanial parasite through genome plasticity and epigenetics: Perception and future perspective. Front. Cell. Infect. Microbiol. 2023, 13, 1001973. [Google Scholar] [CrossRef]
- Piazzi, M.; Bavelloni, A.; Salucci, S.; Faenza, I.; Blalock, W.L. Alternative Splicing, RNA Editing, and the Current Limits of Next Generation Sequencing. Genes 2023, 14, 1386. [Google Scholar] [CrossRef] [PubMed]
- Stamm, S.; Ben-Ari, S.; Rafalska, I.; Tang, Y.; Zhang, Z.; Toiber, D.; Thanaraj, T.; Soreq, H. Function of alternative splicing. Gene 2005, 344, 1–20. [Google Scholar] [CrossRef]
- Duggan, M.C. The Role of Novel NRAS Isoforms in Melanoma Disease Progression and BRAF Inhibitor Resistance. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2017. [Google Scholar]
- Chen, W.; Geng, D.; Chen, J.; Han, X.; Xie, Q.; Guo, G.; Chen, X.; Zhang, W.; Tang, S.; Zhong, X. Roles and mechanisms of aberrant alternative splicing in melanoma—Implications for targeted therapy and immunotherapy resistance. Cancer Cell Int. 2024, 24, 101. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, J.; Wu, Z.; Yin, Y.; Wu, Q.; Wu, Y.; Zheng, J.; Wang, C.; Chen, H.; Qazi, T.J. Insight of novel biomarkers for papillary thyroid carcinoma through multiomics. Front. Oncol. 2023, 13, 1269751. [Google Scholar] [CrossRef]
- Wojtyś, W.; Oroń, M. How Driver Oncogenes Shape and Are Shaped by Alternative Splicing Mechanisms in Tumors. Cancers 2023, 15, 2918. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Braga, L.; Volpe, M.C.; Maiocchi, S.; Generali, D. The predictive and prognostic role of RAS–RAF–MEK–ERK pathway alterations in breast cancer: Revision of the literature and comparison with the analysis of cancer genomic datasets. Cancers 2022, 14, 5306. [Google Scholar] [CrossRef]
- Milde-Langosch, K. The Fos family of transcription factors and their role in tumourigenesis. Eur. J. Cancer 2005, 41, 2449–2461. [Google Scholar] [CrossRef]
- Dou, Z.; Zhao, D.; Chen, X.; Xu, C.; Jin, X.; Zhang, X.; Wang, Y.; Xie, X.; Li, Q.; Di, C.; et al. Aberrant Bcl-x splicing in cancer: From molecular mechanism to therapeutic modulation. J. Exp. Clin. Cancer Res. 2021, 40, 194. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, J.; Gu, C.; Yang, Y. Alternative splicing and cancer: A systematic review. Signal Transduct. Target. Ther. 2021, 6, 78. [Google Scholar] [CrossRef]
- Fu, T.; Amoah, K.; Chan, T.W.; Bahn, J.H.; Lee, J.-H.; Terrazas, S.; Chong, R.; Kosuri, S.; Xiao, X. Massively parallel screen uncovers many rare 3′ UTR variants regulating mRNA abundance of cancer driver genes. Nat. Commun. 2024, 15, 3335. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Cho, Y.-E.; Park, J.-H. The nucleolar protein GLTSCR2 is an upstream negative regulator of the oncogenic nucleophosmin-MYC axis. Am. J. Pathol. 2015, 185, 2061–2068. [Google Scholar] [CrossRef]
- Takhar, M.K. The Role of the Retinoblastoma Protein on Hypoxia-Inducible Factor Dependent Tumor Cell Transformation: Microarray Validation. Master’s Thesis, Simon Fraser University, Burnaby, BC, Canada, 2015. [Google Scholar]
- Montano, N.; Cenci, T.; Martini, M.; D’Alessandris, Q.G.; Pelacchi, F.; Ricci-Vitiani, L.; Maira, G.; De Maria, R.; Larocca, L.M.; Pallini, R. Expression of EGFRvIII in glioblastoma: Prognostic significance revisited. Neoplasia 2011, 13, 1113–1121. [Google Scholar] [CrossRef]
- Batool, S.M.; Muralidharan, K.; Hsia, T.; Falotico, S.; Gamblin, A.S.; Rosenfeld, Y.B.; Khanna, S.K.; Balaj, L.; Carter, B.S. Highly sensitive EGFRvIII detection in circulating extracellular vesicle RNA of glioma patients. Clin. Cancer Res. 2022, 28, 4070–4082. [Google Scholar] [CrossRef]
- Yang, J.; Yan, J.; Liu, B. Targeting EGFRvIII for glioblastoma multiforme. Cancer Lett. 2017, 403, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Bielli, P.; Pagliarini, V.; Pieraccioli, M.; Caggiano, C.; Sette, C. Splicing Dysregulation as Oncogenic Driver and Passenger Factor in Brain Tumors. Cells 2019, 9, 10. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, P.; Yang, H.; He, Y.; Dang, Y.W.; Feng, Z.B.; Chen, G. Clinical role and biological function of CDK5 in hepatocellular carcinoma: A study based on immunohistochemistry, RNA-seq and in vitro investigation. Oncotarget 2017, 8, 108333–108354. [Google Scholar] [CrossRef][Green Version]
- Nikhil, K.; Shah, K. CDK5: An oncogene or an anti-oncogene: Location location location. Mol. Cancer 2023, 22, 186. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, A.; Pasini, A.; Pirini, F.; Ravaioli, S.; Giordano, E.; Tesei, A.; Calistri, D.; Ulivi, P.; Fabbri, F.; Foca, F.; et al. CDKN1A upregulation and cisplatin-pemetrexed resistance in non-small cell lung cancer cells. Int. J. Oncol. 2020, 56, 1574–1584. [Google Scholar] [CrossRef]
- He, C.; Zhou, F.; Zuo, Z.; Cheng, H.; Zhou, R. A global view of cancer-specific transcript variants by subtractive transcriptome-wide analysis. PLoS ONE 2009, 4, e4732. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Bhat, T.A.; Walsh, E.M.; Chaudhary, A.K.; O’Malley, J.; Rhim, J.S.; Wang, J.; Morrison, C.D.; Attwood, K.; Bshara, W. Cytochrome c deficiency confers apoptosome and mitochondrial dysfunction in African-American men with prostate cancer. Cancer Res. 2019, 79, 1353–1368. [Google Scholar] [CrossRef]
- Moradi, A.; Sharma, H.; Sharma, R.D.; Fernando, A.; Barrero, R.A.; Batra, J. Identification of candidate mRNA isoforms for prostate Cancer-risk SNPs utilizing Iso-eQTL and sQTL methods. Int. J. Mol. Sci. 2022, 23, 12406. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.D.; Nam, S.W. Pathogenic diversity of RNA variants and RNA variation-associated factors in cancer development. Exp. Mol. Med. 2020, 52, 582–593. [Google Scholar] [CrossRef]
- Sreenath, T.L.; Dobi, A.; Petrovics, G.; Srivastava, S. Oncogenic activation of ERG: A predominant mechanism in prostate cancer. J. Carcinog. 2011, 10, 37. [Google Scholar] [CrossRef]
- Adamo, P.; Ladomery, M.R. The oncogene ERG: A key factor in prostate cancer. Oncogene 2016, 35, 403–414. [Google Scholar] [CrossRef]
- Tran, M.N.; Choi, W.; Wszolek, M.F.; Navai, N.; Lee, I.L.; Nitti, G.; Wen, S.; Flores, E.R.; Siefker-Radtke, A.; Czerniak, B.; et al. The p63 protein isoform ΔNp63α inhibits epithelial-mesenchymal transition in human bladder cancer cells: Role of MIR-205. J. Biol. Chem. 2013, 288, 3275–3288. [Google Scholar] [CrossRef]
- Andreotti, V.; Bisio, A.; Bressac-de Paillerets, B.; Harland, M.; Cabaret, O.; Newton-Bishop, J.; Pastorino, L.; Bruno, W.; Bertorelli, R.; De Sanctis, V.; et al. The 2A/p16 5′UTR sequence and translational regulation: Impact of novel variants predisposing to melanoma. Pigment. Cell Melanoma Res. 2016, 29, 210–221. [Google Scholar] [CrossRef]
- Tovar-Parra, D.; Gil-Quiñones, S.R.; Nova, J.; Gutierrez-Castaneda, L.D. 3′UTR-CDKN2A and CDK4 germline variants are associated with susceptibility to cutaneous melanoma. In Vivo 2021, 35, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Liu, T.; Wang, X.; Cheng, S.; Zhu, X.; Huang, C.; Duan, L.; Zhao, X.; Guo, F.; Wang, X. PD-1IR2 promotes tumor evasion via deregulating CD8+ T cell function. J. Immunother. Cancer 2025, 13, e010529. [Google Scholar] [CrossRef]
- Tzaban, S.; Stern, O.; Zisman, E.; Eisenberg, G.; Klein, S.; Frankenburg, S.; Lotem, M. Alternative splicing of modulatory immune receptors in T lymphocytes: A newly identified and targetable mechanism for anticancer immunotherapy. Front. Immunol. 2025, 15, 1490035. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, J.; Xiang, J. Alternative Splicing in Tumorigenesis and Cancer Therapy. Biomolecules 2025, 15, 789. [Google Scholar] [CrossRef]
- Wang, X.; Liu, T.; Li, Y.; Ding, A.; Zhang, C.; Gu, Y.; Zhao, X.; Cheng, S.; Cheng, T.; Wu, S.; et al. A splicing isoform of PD-1 promotes tumor progression as a potential immune checkpoint. Nat. Commun. 2024, 15, 9114. [Google Scholar] [CrossRef]
- Kushlinskii, N.E.; Kovaleva, O.V.; Gratchev, A.N.; Alferov, A.A.; Kuzmin, Y.B.; Sokolov, N.Y.; Tsekatunov, D.A.; Ryzhavskaya, I.B.; Kuznetsov, I.N.; Kushlinskii, D.N.; et al. Assessing the Clinical Relevance of Soluble PD-1 and PD-L1: A Multi-Cohort Study Across Diverse Tumor Types and Prognostic Implications. Biomedicines 2025, 13, 500. [Google Scholar] [CrossRef]
- Cho, N.; Kim, S.-Y.; Lee, S.-G.; Park, C.; Choi, S.; Kim, E.-M.; Kim, K.K. Alternative splicing of PBRM1 mediates resistance to PD-1 blockade therapy in renal cancer. EMBO J. 2024, 43, 5421–5444. [Google Scholar] [CrossRef]
- Huang, P.; Wen, F.; Tuerhong, N.; Yang, Y.; Li, Q. Neoantigens in cancer immunotherapy: Focusing on alternative splicing. Front. Immunol. 2024, 15, 1437774. [Google Scholar] [CrossRef]
- Dondi, A.; Lischetti, U.; Jacob, F.; Singer, F.; Borgsmüller, N.; Coelho, R.; Aebersold, R.; Ak, M.; Al-Quaddoomi, F.S.; Albert, S.I.; et al. Detection of isoforms and genomic alterations by high-throughput full-length single-cell RNA sequencing in ovarian cancer. Nat. Commun. 2023, 14, 7780. [Google Scholar] [CrossRef] [PubMed]
- Marco-Puche, G.; Lois, S.; Benítez, J.; Trivino, J.C. RNA-Seq Perspectives to Improve Clinical Diagnosis. Front. Genet. 2019, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Wang, F.; Wu, Y.; Hu, H.; Xing, Z.; Zhu, J.; Xu, S.; Han, T.; Liu, G.; Wu, Z.; et al. Large-scale transcript variants dictate neoepitopes for cancer immunotherapy. Sci. Adv. 2025, 11, eado5600. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, T.; Sammeth, M.; Friedländer, M.R.; ‘t Hoen, P.A.; Monlong, J.; Rivas, M.A.; Gonzalez-Porta, M.; Kurbatova, N.; Griebel, T.; Ferreira, P.G. Transcriptome and genome sequencing uncovers functional variation in humans. Nature 2013, 501, 506–511. [Google Scholar] [CrossRef]
- Fuzio, P.; Napoli, A.; Ciampolillo, A.; Lattarulo, S.; Pezzolla, A.; Nuzziello, N.; Liuni, S.; Giorgino, F.; Maiorano, E.; Perlino, E. Clusterin transcript variants expression in thyroid tumor: A potential marker of malignancy? BMC Cancer 2015, 15, 349. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Bresnahan, S.T.; Taylor Head, S.; Harrison, T.A.; Yu, Y.; Huff, C.D.; Pasaniuc, B.; Lindström, S.; Bhattacharya, A. Isoform-level analyses of 6 cancers uncover extensive genetic risk mechanisms undetected at the gene-level. Br. J. Cancer 2025. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer: Current status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Supplitt, S.; Karpinski, P.; Sasiadek, M.; Laczmanska, I. Current Achievements and Applications of Transcriptomics in Personalized Cancer Medicine. Int. J. Mol. Sci. 2021, 22, 1422. [Google Scholar] [CrossRef]
- Chen, E.Y. Gaining Insights into Vertebrate Vascular Development: Characterization of Zebrafish Morphants Identified from a Morpholino-Based Vascular Screen. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2004. [Google Scholar]
- Anglada-Girotto, M.; Ciampi, L.; Bonnal, S.; Head, S.A.; Miravet-Verde, S.; Serrano, L. In silico RNA isoform screening to identify potential cancer driver exons with therapeutic applications. Nat. Commun. 2024, 15, 7039. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, H.; Wiesmüller, L.; Chen, M. Canonical and non-canonical functions of p53 isoforms: Potentiating the complexity of tumor development and therapy resistance. Cell Death Dis. 2024, 15, 412. [Google Scholar] [CrossRef]
- Alsafadi, S.; Houy, A.; Battistella, A.; Popova, T.; Wassef, M.; Henry, E.; Tirode, F.; Constantinou, A.; Piperno-Neumann, S.; Roman-Roman, S.; et al. Cancer-associated SF3B1 mutations affect alternative splicing by promoting alternative branchpoint usage. Nat. Commun. 2016, 7, 10615. [Google Scholar] [CrossRef]
- Liu, Z.; Yoshimi, A.; Wang, J.; Cho, H.; Chun-Wei Lee, S.; Ki, M.; Bitner, L.; Chu, T.; Shah, H.; Liu, B. Mutations in the RNA splicing factor SF3B1 promote tumorigenesis through MYC stabilization. Cancer Discov. 2020, 10, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Brooks, A.N.; Fan, J.; Wan, Y.; Gambe, R.; Li, S.; Hergert, S.; Yin, S.; Freeman, S.S.; Levin, J.Z.; et al. Transcriptomic Characterization of SF3B1 Mutation Reveals Its Pleiotropic Effects in Chronic Lymphocytic Leukemia. Cancer Cell 2016, 30, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Türeci, Ö.; Vormehr, M.; Diken, M.; Kreiter, S.; Huber, C.; Sahin, U. Targeting the Heterogeneity of Cancer with Individualized Neoepitope Vaccines. Clin. Cancer Res. 2016, 22, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef]
- Rukov, J.L.; Shomron, N. MicroRNA pharmacogenomics: Post-transcriptional regulation of drug response. Trends Mol. Med. 2011, 17, 412–423. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M.; Pirmohamed, M. Precision medicine in cardiovascular therapeutics: Evaluating the role of pharmacogenetic analysis prior to drug treatment. J. Intern. Med. 2024, 295, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Hagemann-Jensen, M.; Ziegenhain, C.; Sandberg, R. Scalable single-cell RNA sequencing from full transcripts with Smart-seq3xpress. Nat. Biotechnol. 2022, 40, 1452–1457. [Google Scholar] [CrossRef]
- Hahaut, V.; Pavlinic, D.; Carbone, W.; Schuierer, S.; Balmer, P.; Quinodoz, M.; Renner, M.; Roma, G.; Cowan, C.S.; Picelli, S. Fast and highly sensitive full-length single-cell RNA sequencing using FLASH-seq. Nat. Biotechnol. 2022, 40, 1447–1451. [Google Scholar] [CrossRef]
- Shi, Z.-X.; Chen, Z.-C.; Zhong, J.-Y.; Hu, K.-H.; Zheng, Y.-F.; Chen, Y.; Xie, S.-Q.; Bo, X.-C.; Luo, F.; Tang, C.; et al. High-throughput and high-accuracy single-cell RNA isoform analysis using PacBio circular consensus sequencing. Nat. Commun. 2023, 14, 2631. [Google Scholar] [CrossRef]
- Joglekar, A.; Hu, W.; Zhang, B.; Narykov, O.; Diekhans, M.; Marrocco, J.; Balacco, J.; Ndhlovu, L.C.; Milner, T.A.; Fedrigo, O.; et al. Single-cell long-read sequencing-based mapping reveals specialized splicing patterns in developing and adult mouse and human brain. Nat. Neurosci. 2024, 27, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.-H.; Mao, A.; Salzberg, S.L.; Pertea, M. Splam: A deep-learning-based splice site predictor that improves spliced alignments. Genome Biol. 2024, 25, 243. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shila, S.; Dahiya, V.; Hisle, C.; Bahadursingh, E.; Thiyagarajan, R.; Fields, P.E.; Rumi, M.A.K. mRNA Isoforms and Variants in Health and Disease. Int. J. Mol. Sci. 2025, 26, 9356. https://doi.org/10.3390/ijms26199356
Shila S, Dahiya V, Hisle C, Bahadursingh E, Thiyagarajan R, Fields PE, Rumi MAK. mRNA Isoforms and Variants in Health and Disease. International Journal of Molecular Sciences. 2025; 26(19):9356. https://doi.org/10.3390/ijms26199356
Chicago/Turabian StyleShila, Sharmin, Vinesh Dahiya, Charles Hisle, Elizabeth Bahadursingh, Ramkumar Thiyagarajan, Patrick E. Fields, and M. A. Karim Rumi. 2025. "mRNA Isoforms and Variants in Health and Disease" International Journal of Molecular Sciences 26, no. 19: 9356. https://doi.org/10.3390/ijms26199356
APA StyleShila, S., Dahiya, V., Hisle, C., Bahadursingh, E., Thiyagarajan, R., Fields, P. E., & Rumi, M. A. K. (2025). mRNA Isoforms and Variants in Health and Disease. International Journal of Molecular Sciences, 26(19), 9356. https://doi.org/10.3390/ijms26199356




