Molecular Insights into the Biomedical Applications of Plagiomnium affine (Blandow ex Funck) T. Kop.: A Promising Source of Bioactive Metabolites
Abstract
1. Introduction
1.1. Methodology
1.2. The Taxonomy of P. affine
1.3. Morphology and Reproductive Biology of P. affine
1.4. Karyology of P. affine
2. Physiological and Chemical Properties of P. affine
2.1. P. affine as a Bioindicator
2.2. Role of P. affine in Regulation of Local Humidity
2.3. Cryoprotective Properties and Frozen Sensitivity of P. affine
2.4. P. affine Is a Source of Bioactive Metabolites
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 18S rDNA | Small subunit ribosomal RNA |
| 26S rDNA | Large subunit ribosomal RNA |
| C | DNA content |
| CHCl3 | Chloroform |
| cox1 | Cytochrome c oxidase subunit 1 |
| cpDNA | Chloroplast DNA |
| DMSO | Dimethylsulfoxide |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| ITS | Internal Transcribed Spacer |
| KN medium | Knop’s nutrient solution |
| matK | Maturase K gene |
| MS medium | Murashige and Skoog’s nutrient solution |
| mtDNA | Mitochondrial DNA |
| n | Numbers of chromosomes |
| nad5 | NADH dehydrogenase subunit 5 |
| PSII | Photosystem II |
| PHYP | Phytochrome genes |
| psbA-trnH | Spacer region between psbA and trnH genes |
| rDNA | Ribosomal DNA |
| rbcL | Ribulose-1,5-bisphosphate carboxylase large subunit |
| SL1344 | One of Salmonella enterica serotype Typhimurium |
| trnL-F | tRNA-Leu and its spacer region |
References
- Koponen, T. On the hypothesis of dioicous–monoicous species pairs in the Mniaceae (Bryophyta); morphology, sexual condition and distribution. Acta Musei Silesiae Sci. Nat. 2019, 68, 67–81. [Google Scholar] [CrossRef]
- Šoltés, R.; Gregušková, E. Accumulation characteristics of some elements in the moss Polytrichum commune (Bryophytes) based on XRF spectrometry. J. Environ. Protect. 2013, 4, 612–617. [Google Scholar] [CrossRef]
- Šoltés, R. Heavy metal concentrations in the mosses of the Tatra Mountains (Czecho-Slovakia). Multivariate analysis. Oecol. Mont. 1992, 1, 31–36. [Google Scholar]
- Vollár, M.; Gyovai, A.; Szűcs, P.; Zupkó, I.; Marschall, M.; Csupor-Löffler, B.; Bérdi, P.; Vecsernyés, A.; Csorba, A.; Liktor-Busa, E.; et al. Antiproliferative and antimicrobial activities of selected bryophytes. Molecules 2018, 23, 1520. [Google Scholar] [CrossRef]
- Mishra, R.; Pandey, V.K.; Chandra, R. Potential of bryophytes as therapeutics. Int. J. Pharm. Sci. Res. 2014, 5, 3584–3593. [Google Scholar] [CrossRef]
- Altuner, E.M.; Çeter, T.; Bayar, E.; Aydın, S.; Arıcı, F.; Süleymanoğlu, G.; Edis, A. Investigation on antimicrobial effects of some moss species collected from Kastamonu region. Commun. Fac. Sci. Univ. Ank. Ser. C Biol. 2011, 23, 33–43. [Google Scholar]
- Singh, R.; Sharma, P.; Kumar, V.; Mehta, A. Characterization of bioactive compounds from Plagiomnium cuspidatum moss collected from Uttarakhand, India. Appl. Sci. 2025, 12, 160. [Google Scholar] [CrossRef]
- Zhang, W.G.; Weng, J.; Shang, H.; Xie, Y.; Li, Y. Fabrication of temperature-regulating functional fabric based on n-octadecane/SWCNTs composite phase change material. Pigm. Resin Technol. 2022, 51, 564–573. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhu, L.; Wang, X.; Meng, F.; Xia, L.; Zhang, H. Advances in stigmasterol on its anti-tumor effect and mechanism of action. Front. Oncol. 2022, 12, 1101289. [Google Scholar] [CrossRef]
- Rütten, D.; Santarius, K.A. Age-related differences in frost sensitivity of the photosynthetic apparatus of two Plagiomnium species. Planta 1992, 187, 224–229. [Google Scholar] [CrossRef]
- Rütten, D.; Santarius, K.A. Cryoprotection of Plagiomnium affine induced by various natural and artificial substances. Can. J. Bot. 1993, 71, 715–723. [Google Scholar] [CrossRef]
- Sokal, I.; Kuta, E.; Przywara, L. Callus induction and gametophyte regeneration in moss cultures. Acta Biol. Cracov. Ser. Bot. 1997, 39, 35–42. [Google Scholar]
- Lamparter, T. Photomorphogenesis of Mosses; Springer: Dordrecht, The Netherlands, 2006; pp. 537–565. [Google Scholar] [CrossRef]
- Wyatt, R.; Stoneburner, A.; Odrzykoski, I.J. Phylogenetic relationships within Plagiomnium section Rosulata. J. Hattori. Bot. Lab. 1994, 76, 87–95. [Google Scholar] [CrossRef]
- Shaw, A.J.; Cox, C.J.; Goffinet, B. Global patterns of moss diversity: Taxonomic and molecular inferences. Taxon 2005, 54, 337–352. [Google Scholar] [CrossRef]
- Harris, E.S.J. Paraphyly and multiple causes of phylogenetic incongruence in the moss genus Plagiomnium (Mniaceae). Taxon 2008, 57, 417–433. [Google Scholar] [CrossRef]
- Goffinet, B.; Shaw, A.J. Bryophyte Biology; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- Crandall-Stotler, B.J.; Bartholomew-Began, S.E. Morphology of Mosses (Phylum Bryophyta). 2007. Available online: https://api.semanticscholar.org/CorpusID:6425938 (accessed on 1 May 2025).
- Crum, H.A.; Anderson, L.E. Mosses of Eastern North America; Columbia University Press: New York, NY, USA, 1981; ISBN 9780231045162. [Google Scholar]
- Leitch, I.J.; Leitch, A.R. Genome size diversity and evolution in land plants. Biol. J. Linn. Soc. 2013, 111, 88. [Google Scholar] [CrossRef]
- Koponen, T.; Sun, Y. Preliminary study on phylogenetic position and delimitation of the ciliate arthrodontous genera of the moss family Mniaceae. J. Bryol. 2017, 39, 23–38. [Google Scholar] [CrossRef]
- Koponen, T. A monograph of Plagiomnium sect. Rosulata (Mniaceae). Ann. Bot. Fenn. 1971, 8, 305–367. [Google Scholar]
- Wyatt, R.; Odrzykoski, I.J. Plagiomnium floridanum sp. nov. (Mniaceae), a new moss from the southeastern United States. Bryologist 2012, 115, 527–535. [Google Scholar] [CrossRef]
- Cronberg, N.; Andersson, K.; Wyatt, R.; Odrzykoski, I.J. Clonal distribution, fertility and sex ratios of the moss Plagiomnium affine (Bland.) T.Kop. in forests of contrasting age. J. Bryol. 2003, 25, 155–162. [Google Scholar] [CrossRef]
- Lindberg, S.O. Observationes Mniaceis europaeis. Not. Sällsk. Fauna Flora Fenn. 1868, 9, 39–88. [Google Scholar]
- Mamczarz, H. Taxonomic relations of Plagiomnium affine (Funck.) Kop. and P. elatum (BSG) Kop. Acta. Soc. Bot. Pol. 1974, 43, 3–13. [Google Scholar] [CrossRef][Green Version]
- Cronberg, N.; Wyatt, R.; Odrzykoski, I.J.; Andersson, K. Genetic diversity of the moss Plagiomnium affine in forests of contrasting age. Lindbergia 2005, 30, 49–58. [Google Scholar][Green Version]
- Spence, J.R. A major new treatment to the Bryaceae of Europe. Bryologist 2021, 124, 638–640. [Google Scholar] [CrossRef]
- Smith, A.J.E. The Moss Flora of Britain and Ireland; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Yi, Y.-J.; He, S. Plagiomnium guizhouense Y.-J.Yi and S.He sp. nov. (Mniaceae) found in southwestern China. J. Bryol. 2014, 36, 291–294. [Google Scholar] [CrossRef]
- Hofmann, H. Plagiomnium Affine (Funck) T.J.Kop. Moosflora der Schweiz, Swissbryophytes Working Group, 2016. Available online: www.swissbryophytes.ch (accessed on 28 April 2025).
- Pasiche-Lisboa, C.J.; Jesús, I.S. Moss protonemata are dispersed by water, wind, and snails. Am. J. Bot. 2018, 105, 788–795. [Google Scholar] [CrossRef]
- Lisboa, C.J.P. Protonematal Dispersal by Water, Wind, and Animal. Master’s Thesis, University of Puerto Rico, Mayagüez Campus, Mayagüez, Puerto Rico, 2013. [Google Scholar]
- Andersson, K. Dispersal of Spermatozoids from Splash-Cups of the Moss Plagiomnium affine. Lindbergia 2002, 27, 90–96. [Google Scholar]
- Kłos, J.; Kuta, E.; Przywara, L. Karyology of Plagiomnium I. Plagiomnium affine (Schrad.) T. Kop. J. Bryol. 2001, 23, 9–16. [Google Scholar] [CrossRef]
- Stoneburner, A.; Wyatt, R. New and confirmed chromosome counts for the Mniaceae. Bryologist 2023, 126, 385–397. [Google Scholar] [CrossRef]
- Suire, C.; Bourgeois, G.; Koponen, T. Some chemical constituents of thirteen mosses from the traditional Mniaceae family. J. Hattori Bot. Lab. 2000, 89, 233–246. [Google Scholar] [CrossRef]
- Harmens, H.; Norris, D.A.; Steinnes, E. Mosses as biomonitors of atmospheric heavy metal deposition: Spatial patterns and temporal trends in Europe. Environ. Poll. 2010, 158, 3144–3156. [Google Scholar] [CrossRef] [PubMed]
- Van de Koot, W.Q.M.; Msonda, J.; Olver, O.P.; Doonan, J.H.; Nibau, C. Variation in water-holding capacity in Sphagnum species depends on both plant and colony structure. Plants 2024, 13, 1061. [Google Scholar] [CrossRef] [PubMed]
- Petschinger, K.; Adlassnig, W.; Sabovljevic, M.S.; Lang, I. Lamina cell shape and cell wall thickness are useful indicators for metal tolerance—An example in bryophytes. Plants 2021, 10, 274. [Google Scholar] [CrossRef]
- Carter, J.G.; Altaba, C.R.; Anderson, L.C.; Campbell, D.; Fang, Z.; Harries, P.J.; Skelton, P.W. The paracladistic approach to phylogenetic taxonomy. Paleontol. Contrib. 2015, 12, 1–9. [Google Scholar] [CrossRef]
- Lobachevska, O.V.; Karpinets, L. Water exchange of the forest ecosystems epigeic bryophytes depending on changes of the structural and functional organization of their turfs and the influence of local growth environmental conditions. Bìologìčnì Studìï 2024, 18, 139–156. [Google Scholar] [CrossRef]
- Aryal, B.; Raut, B.K.; Bhattarai, S.; Bhandari, S.; Tandan, P.; Gyawali, K.; Parajuli, N. Potential therapeutic applications of plant-derived alkaloids against inflammatory and neurodegenerative diseases. Evid.-Based Complement. Altern. Med. 2022, 2022, 7299778. [Google Scholar] [CrossRef]
- Freitag, P.; Mues, R.; Brill-Fess, C.; Stoll, M.; Zinsmeister, H.D.; Markham, K.R. Isoorientin 3′-O-sophoroside and 3′-O-neohesperidoside from the moss Plagiomnium affine. Phytochemistry 1986, 25, 669–671. [Google Scholar] [CrossRef]
- Ziqubu, K.; Dludla, P.V.; Joubert, E.; Muller, C.J.F.; Louw, J.; Tiano, L.; Nkambule, B.B.; Kappo, A.P.; Mazibuko-Mbej, S.E. Isoorientin: A dietary flavone with the potential to ameliorate diverse metabolic complications. Pharmacol. Re. 2020, 158, 104867. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Du, L.Y.; Zhao, M.; Tao, J.H.; Qian, D.W.; Jiang, S.; Shang, E.X.; Guo, J.M.; Liu, P.; Su, S.L.; Duan, J.A. The metabolic profiling of isorhamnetin-3-O-neohesperidoside produced by human intestinal flora employing UPLC-Q-TOF/MS. J. Chromatogr. Sci. 2017, 55, 243–250. [Google Scholar] [CrossRef]
- Hoffmann, H.R.; Matusch, R.; Baniahmad, A. Isolation of Atraric Acid, Synthesis of Atraric Acid Derivatives, and Use of Atraric Acid and the Derivatives Thereof for the Treatment of Benign Prostatic Hyperplasia, Prostate Carcinoma, and Spinobulbar Muscular Atrophy. U.S. Patent US 2009/0143466A1, 4 June 2009. [Google Scholar]
- Zviely, M. Methyl 2,4-dihydroxy-3,6-dimethylbenzoate. Perf. Flavor. 2011, 36, 54–57. [Google Scholar]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and tocotrienols—Bioactive dietary compounds; what is certain, what is doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Carvalho, A.M.S.; Heimfarth, L.; Pereira, E.W.M.; Oliveira, F.S.; Menezes, I.R.A.; Coutinho, H.D.M.; Picot, L.; Antonioll, A.R.; Quintans, J.S.S.; Quintans-Júnior, L.J. Phytol, a chlorophyll component, produces antihyperalgesic, antiinflammatory, and antiarthritic effects: Possible NFκB pathway involvement and reduced levels of the proinflammatory cytokines TNF-α and IL-6. J. Nat. Prod. 2020, 83, 1107–1117. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Mohamed, I.N. Interdependence of anti-inflammatory and antioxidant properties of squalene–implication for cardiovascular health. Life 2021, 11, 103. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H. The health beneficial properties of Rhodomyrtus tomentosa as potential functional food. Biomolecules 2019, 9, 76. [Google Scholar] [CrossRef]
- Agostoni, C.; Moreno, L.; Shamir, R. Palmitic acid and health: Introduction. Crit. Rev. Food Sci. Nutr. 2016, 56, 1941–1942. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Chemical Constituents of Bryophyta. Prog. Chem. Org. Nat. Prod. 2013, 95, 563–605. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A. Chemical constituents of bryophytes: Structures and biological activity. J. Nat. Prod. 2017, 81, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, G.; Wan, X.; Wang, F.; Han, P.; Bao, S.; Wang, K.; Song, T.; Jiang, H. Highly efficient biosynthesis of γ-bisabolene with a new sesquiterpene synthase AcTPS5 by dual cytoplasmic-peroxisomal engineering in Saccharomyces cerevisiae. Fermentation 2023, 9, 779. [Google Scholar] [CrossRef]
- Matsuo, A.; Nakayama, M.; Hayashi, S. δ-Cuparenol, a new sesquiterpene phenol from the liverwort, Bazzania pompeana. Chem. Lett. 1972, 1, 341–342. [Google Scholar] [CrossRef]
- Jeong, H.U.; Kwon, S.S.; Kong, T.Y.; Kim, J.Y.; Lee, H.S. Inhibitory effects of cedrol, β-cedrene, and thujopsene on cytochrome P450 enzyme activities in human liver microsomes. J. Toxicol. Environ. Health A 2014, 77, 1522–1532. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Y.G.; Wang, Z.; Ding, H. Recent development on the [5+2] cycloadditions and their application in natural product synthesis. Chem. Commun. 2019, 55, 1859. [Google Scholar] [CrossRef]
- Tashiro, T.; Kurosawa, S.; Mori, K. Revision of the structure of the major aggregation pheromone of the broad-horned flour beetle (Gnatocerus cornutus) to (1S,4R,5R)-α-acoradiene by its synthesis. Biosci. Biotechnol. Biochem. 2004, 68, 663–670. [Google Scholar] [CrossRef][Green Version]
- Li, M.; Wang, L.; Li, S.; Hua, C.; Gao, H.; Ning, D.; Li, C.; Zhang, C.; Jiang, F. Chemical composition, antitumor properties, and mechanism of the essential oil from Plagiomnium acutum T. Kop. Int. J. Mol. Sci. 2022, 23, 14790. [Google Scholar] [CrossRef] [PubMed]
- Yajima, A.; Yamaguchi, A.; Nukada, T.; Yabuta, G. Asymmetric total synthesis of ent-sandaracopimaradiene, a biosynthetic intermediate of oryzalexins. Biosci. Biotechnol. Biochem. 2007, 71, 2822–2829. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.L. An Update on vitamin e, tocopherol and tocotrienol—Perspectives. Molecules 2010, 15, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Calpe-Berdiel, L.; Escolà-Gil, J.C.; Blanco-Vaca, F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis 2009, 203, 18–31. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef]
- Miszczuk, E.; Bajguz, A.; Kiraga, Ł.; Crowley, K.; Chłopecka, M. Phytosterols and the digestive system: A review study from insights into their potential health benefits and safety. Pharmaceuticals 2024, 17, 557. [Google Scholar] [CrossRef]
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef]
- Hamed, A.B.; Mantawy, E.M.; El-Bakly, W.M.; Abdel-Mottaleb, Y.; Azab, S.S. Methyl palmitate: The naturally occurring cardioprotective agent. Arch. Pharm. Sci. Ain. Shams. Univ. 2020, 4, 47–62. [Google Scholar] [CrossRef]
- Hunter, J.E.; Zhang, J.; Kris-Etherton, P.M. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: A systematic review. Am. J. Clin. Nutr. 2009, 91, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Gül, Ü.; Canturk, Z.; Ilhan, S.; Birgi, F. The bioactive properties of a bryophyte collected from Bilecik (Turkey) Province. S. Afr. J. Bot. 2023, 156, 91–98. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Hassan, H.M.; Elmaidomyf, A.H.; Abdelmohsen, U.R. Pharmacological and natural products diversity of the brown algae genus Sargassum. RSC Adv. 2020, 10, 24951. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Paudel, Y.N.; Yang, X.; Ren, Q.; Zhang, S.; Ji, S.; Liu, K.; Jin, M. Schaftoside suppresses pentylenetetrazol-induced seizures in zebrafish via suppressing apoptosis, modulating inflammation, and oxidative stress. ACS Chem. Neurosci. 2021, 12, 2542–2552. [Google Scholar] [CrossRef]
- Guan, S.; Sun, L.; Wang, X.; Huang, X.; Luo, T. Isoschaftoside inhibits lipopolysaccharide-induced inflammation in microglia through regulation of HIF-1α-mediated metabolic reprogramming. Evid-Based Complement Altern. Med. 2022, 2022, 5227335. [Google Scholar] [CrossRef]
- Besson, E.; Chopin, J.; Markham, K.R.; Mues, R.; Wong, H.; Bouillant, M.L. Identification of neoschaftoside as 6-C-β-d-glucopyranosyl-8-C-β-l-arabinopyranosylapigenin. Phytochemistry 1984, 23, 159–161. [Google Scholar] [CrossRef]
- Marrassini, C.; Davicino, R.; Acevedo, C.; Anesini, C.; Gorzalczany, S.; Ferraro, G. Vicenin-2, a potential anti-inflammatory constituent of Urtica circularis. J. Nat. Prod. 2011, 74, 1503–1507. [Google Scholar] [CrossRef]
- Nignpense, B.E.; Latif, S.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Bioaccessibility and antioxidant activity of polyphenols from pigmented barley and wheat. Foods 2022, 11, 3697. [Google Scholar] [CrossRef]
- Min, S.Y.; Park, C.H.; Yu, H.W.; Park, Y.J. Anti-inflammatory and anti-allergic effects of saponarin and its impact on signaling pathways of RAW 264.7, RBL-2H3, and HaCaT cells. Int. J. Mol. Sci. 2021, 22, 8431. [Google Scholar] [CrossRef]
- Harris, E.S.J. Phylogenetic and environmental lability of flavonoids in a medicinal moss. Biochem. Syst. Ecol. 2009, 37, 541–550. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Dahija, S.; Hassan, S.T.S. Biflavonoids: Important contributions to the health benefits of Ginkgo (Ginkgo biloba L.). Plants 2022, 11, 1381. [Google Scholar] [CrossRef]
- Ihlenfeldt, W.D.; Bolton, E.E.; Bryant, S.H. The PubChem chemical structure sketcher. J. Cheminform. 2009, 1, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keyl, A.; Nakielski, J.; Reski, R. Bryophytes as metabolic engineering platforms: Progress and prospects. New Phytol. 2024, 243, 396–410. [Google Scholar] [CrossRef]
- Sabovljević, M.; Grubisic, D.; Vujicic, M.; Djalovic, I.; Jockovic, N. On a search of biologically active compounds in bryophytes. Acta Biol. Hung. 2013, 64, 450–458. [Google Scholar] [CrossRef]
- Schneider, T.; Graef, V.; Lemke, N.; Becker, S.; Reski, R.; Menger-Krug, E. Valuable fatty acids in bryophytes—Production, biosynthesis, analysis and applications. Int. J. Mol. Sci. 2019, 20, 5620. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Bryophytes as a source of bioactive volatile terpenoids: New results and future prospects. Food Chem. Toxicol. 2019, 132, 110649. [Google Scholar] [CrossRef]
- Koid, C.W.; Shaipulah, N.F.M.; Lee, G.E.; Gradstein, S.R.; Asakawa, Y.; Gradstein, R.S.; Asakawa, Y.; Andriani, Y.; Mohammed, A.; Norhazrina, N.; et al. Volatile organic compounds of bryophytes from Peninsular Malaysia and their roles in bryophytes. Plants 2022, 11, 2575. [Google Scholar] [CrossRef]
- Ryde, I.; Bourtsoukidis, E.; Foken, T.; Hellén, H.; Rinnan, R. Volatile organic compound emissions from subarctic mosses and lichens. Atmos. Environ. 2022, 290, 119357. [Google Scholar] [CrossRef]
- Kollar, A.; Boucher, T.; Walker, L.; Yost, J.M.; Jenks, M.A.; Moffett, M. Measuring volatile emissions from moss gametophytes: A review of methodologies and new applications. Appl. Plant Sci. 2022, 10, e11412. [Google Scholar] [CrossRef]
- Blanco, A.; Blanco, G. Antioxidants. In Medical Biochemistry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 221–231. [Google Scholar] [CrossRef]
- Tyszkowski, R.; Mehrzad, R. Inflammation: A multifaceted and omnipresent phenomenon. In Inflammation and Obesity; Elsevier: Amsterdam, The Netherlands, 2023; pp. 19–30. [Google Scholar] [CrossRef]
- Özdemir, S. Inflammation: Complexity and significance of cellular and molecular responses. J. Acute Dis. 2024, 13, 3–7. [Google Scholar] [CrossRef]
- Becker, R.; Trpin, B.; Brenner, M.; Hao, L.; Duncan, S.; Bégout, P. Anti-diabetic agents. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Dowarah, J.; Singh, V.P. Anti-diabetic drugs: Recent approaches and advancements. Bioorg. Med. Chem. 2020, 28, 115263. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P. An overview on anti-diabetic drugs and development. Sci. Tech. J. 2016, 4, 113–123. [Google Scholar] [CrossRef]
- He, P.; Zang, L.; Zhu, S. New obesity treatment agent: β-Caryophyllene. Curr. Drug Ther. 2024, 19, 379–392. [Google Scholar] [CrossRef]
- Mikhail, N.; Wali, S. Semaglutide: The first anti-obesity agent shown to decrease cardiovascular events. Ann. Cardiovasc. Dis. 2024, 8, 1036. [Google Scholar] [CrossRef]
- Scheen, A.; Flines, J.; Paquot, N. Anti-obesity drugs: From previous disappointments to new hopes. Rev. Med. Liège 2023, 78, 147–152. [Google Scholar]
- Ando, Y.; Nei, D.; Kono, S.; Nabetani, H. Current state and future issues of technology development concerned with freezing and thawing of foods. J. Jpn. Soc. Food Sci. Technol. 2017, 64, 391–428. [Google Scholar] [CrossRef][Green Version]
- Nishishita, N.; Muramatsu, M.; Kawamata, S. An effective freezing/thawing method for human pluripotent stem cells cultured in chemically-defined and feeder-free conditions. Am. J. Stem Cells 2025, 4, 38. [Google Scholar][Green Version]
- Pegg, D.E. Principles of cryopreservation. Methods Cell Biol. 2007, 82, 39–57. [Google Scholar][Green Version]
- Hong, W.; Xiao, T.; Lin, G.; Liu, C.; Li, H.; Li, Y.; Hu, H.; Wu, S.; Wang, S.; Liang, Z.; et al. Structure-based design and synthesis of anti-fibrotic compounds derived from para-positioned 3,4,5-trisubstituted benzene. Bioorg. Chem. 2024, 144, 107113. [Google Scholar] [CrossRef]
- Ramadoss, R.; Sathish, S.; Sohn, H.; Madhavan, T. Potency of anti-fibrotic herbs on fibrogenesis: A theoretical evaluation. Phytomed. Plus 2023, 3, 100496. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.; Cai, J.; Gao, J.; Hu, Y. Research progress on the antifibrotic activity of traditional chinese medicine polysaccharides. Chem. Biodivers. 2024, 22, e202402012. [Google Scholar] [CrossRef] [PubMed]
- Lepori, M.; Sartori, C.; Duplain, H.; Nicod, P.; Scherrer, U. Interaction between cholinergic and nitrergic vasodilation: A novel mechanism of blood pressure control. Cardiovasc. Res. 2001, 51, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Perera, K.C.; Ekanayaka, S.K.; Chandrasiri, N.; Jayatilleke, K.; Kottahachchi, J. In-vitro evaluation of bactericidal activity of antiseptics and disinfectants commonly used in healthcare settings. Galle Med. J. 2021, 26, 23. [Google Scholar] [CrossRef]
- Bäumler, W.; Eckl, D.B.; Holzmann, T.; Schneider-Brachert, W. Antimicrobial coatings for environmental surfaces in hospitals: A potential new pillar for prevention strategies in hygiene. Crit. Rev. Microbiol. 2022, 48, 531–564. [Google Scholar] [CrossRef]
- Hayek, S.N. Wound Cleansing, Topical Antiseptics and Wound. 2009. Available online: https://shadyhayek.com/wp-content/uploads/2012/02/Wound-cleansing-topical-antiseptics-and-wound-healing.pdf (accessed on 5 May 2025).
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Vaux, D.L.; Korsmeyer, S.J. Cell death in development. Cell 1999, 96, 245–254. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef]
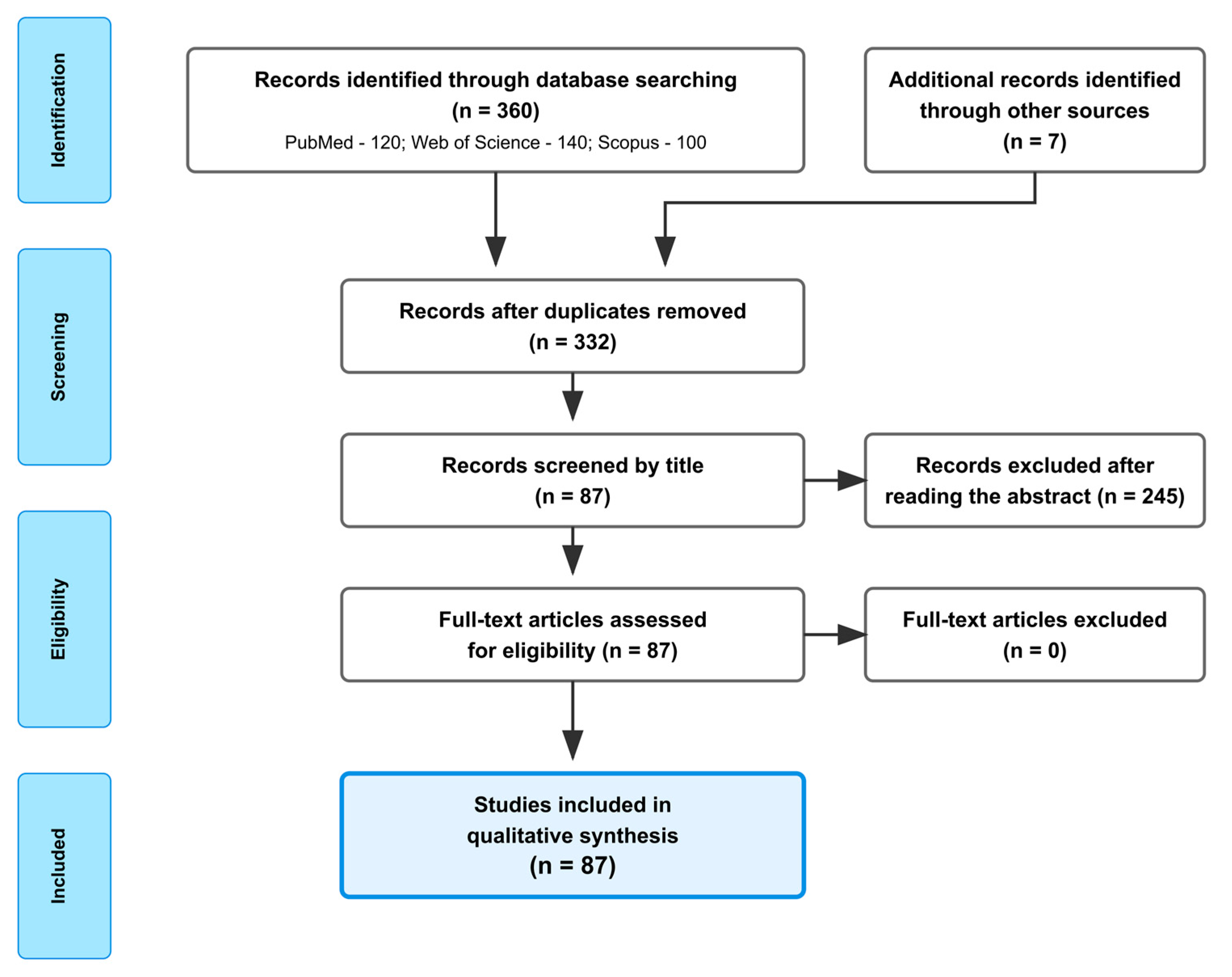
| Criteria | Description |
|---|---|
| Exclusion | Editorials, letters, books, encyclopedias, non-English publications, duplicate publications, conference abstracts without full data |
| Inclusion | No restriction on study location; studies on bryophytes (mosses) addressing one or more of the following: bioactive metabolites, biomedical applications, occurrence of flavonoids or other secondary metabolites, cryoprotective properties, DNA content, chromosome number, life cycle, taxonomy, rhizoids, polyploidy, genetics, karyology, ecological functions |
| Metabolite | Molecular Monoisotopic Masses in Daltons (Da) ** | Properties/Functions Important to Humans | Bibliography |
|---|---|---|---|
| Acyclic hydrogenated diterpene alcohol | |||
Phytol ***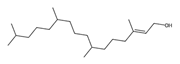 | ≈296.3079 | It is suggested to have antinociceptive, antioxidant 1, anti-inflammatory 2, and anticarcinogenic 9 properties, as well as an anticonvulsant and anxiolytic-like agent. | [37,51] |
| Aliphatic hydrocarbons | |||
n-Octadecane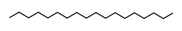 | ≈254.2974 | A component of cuticular waxes in plants and secretions of some microorganisms; used in industrial applications as a compound of lubricants, waxes, and phase change materials for heat storage. | [37] |
n-Heptacosane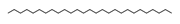 | ≈380.4382 | Natural saturated hydrocarbon, lipid derivative, anti-inflammatory 2 properties, and antibacterial 8 activities; in the cuticle of leaves, fruits, and seeds of plants, protects against water loss and acts as a barrier to pathogens; they are also present in animals, especially insects. Applied in skincare and haircare cosmetics. | [37,72] |
| flavonoids | |||
Isoorientin ***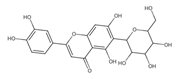 | ≈448.1006 | C-glucosyl flavone, antioxidant 1, anti-inflammatory 2, antidiabetic 3, and anti-obesity 4 agent. | [44,45] |
Isoorientin 3′-O-sophoroside *** | ≈790.2166 | Antioxidant 1 and anti-inflammatory 2 can be applied in nutraceutical formulations and cosmetic products. For the mechanism of action and biomedical applications, further research is required. | [44,46] |
Isoorientin 3′-O-neohesperidoside ***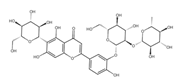 | ≈756.2113 | Antioxidant 1 modulator, anti-inflammatory 2, and cytoprotective agent; may be considered as a dietary supplement or an ingredient in food and cosmetics. | [44,47] |
| phenolic compounds | |||
Methyl 2,4-dihydroxy-3,6-dimethylbenzoate (atraric acid) * | 196.1999 | Anti-androgenic agent, components in perfumery, cosmetics, detergents, fabric softeners, candles, and incense; concentrated may cause mild skin irritation. | [37,48,49] |
| sesquiterpenes/sesquiterpenoids | |||
β-Bisabolene (tentatively identified)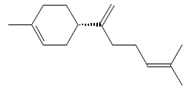 | ≈204.3511 | Naturally present in plants, it can be applied as an anti-inflammatory 2, antibacterial 8, and anticancer 9 (for breast cancer) factor, especially against Staphylococcus aureus, in the pharmaceutical and cosmetic industries. | [37,57] |
δ-Cuparenol | ≈219.1747 | Primarily isolated from the liverwort Bazzania pompeana, an agent with biomedical applications, especially as an anti-inflammatory 2 and antibacterial 8 activities, can be used for skin protection. | [37,58] |
ent-β-cedrene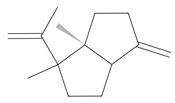 | ≈204.1878 | Anti-inflammatory 2, antiseptic 8, and antispasmodic essential oil evoking sedative, diuretic, insecticidal, tonic, and astringent effects. | [37,59] |
α-Cedrene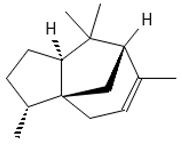 | ≈204.1878 | Natural essential oils (e.g., cedar, juniper, cypress) with anti-inflammatory 2 and analgesic properties with arthritis properties are also applied as antiseptic 8 agent, suggested as anticancer 9 metabolites (e.g., anti-mouth, liver, and lung cancer), and as fragrances, and they are used in cosmetics and as the dainty sweet taste in the food industry. | [37,60] |
α-Acoradiene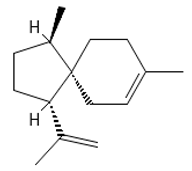 | ≈204.1878 | Natural essential oil with aromatic properties and antioxidant 1 activities; it plays a vital role in protective and regenerative mechanisms as part of inflammation defense, immune processes, and antibacterial 8 and antiviral properties; it is applied in cosmetics (for skin and hair) and pharmaceutical industries. | [37,61] |
| diterpenoids | |||
Dolabella-7,8-dien-18-ol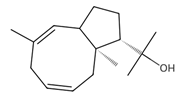 | ≈290.2609 | Essential oil of, e.g., Pseudocorythion acutum may present antioxidative 1 as well as anti-inflammatory 2 effects; it can be applied as an anti-UV skin protector in the cosmetic industry. | [37,61,62] |
Sandaracopimaradiene (tentatively identified)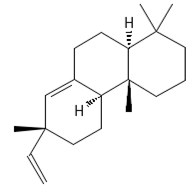 | 272.4681 | Diterpene hydrocarbon with anti-inflammatory 2 and antiparasitic properties applied in traditional medicine, e.g., for respiratory and digestive illness treatment; as the antispasmodic agent, relief of smooth muscle spasms can be a therapeutic agent for, e.g., abdominal pain or intestinal cramps; inhibits the growth of Staphylococcus aureus, Candida albicans, and Mycobacterium smegmatis and acts as an antimalarial agent, inhibiting the growth of Plasmodium falciparum, the parasite responsible for malaria 8. | [37,63] |
| triterpens | |||
Squalene ***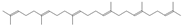 | ≈410.3912 | The precursor of secondary metabolites; anticancer 9, anti-inflammatory 2, as well as cardioprotective and antioxidant agent 1. | [37,52] |
| tocopherols | |||
β-Tocopherol ***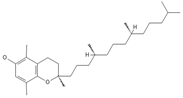 | ≈416.3654 | Vitamin E has antioxidant 1, anticancer 9, or anti-heart disease properties by protecting cell membranes, their integrity, and their functionality; supports the immune system; improves skin health; and has anti-inflammatory 2 effects; it can be used as a dietary supplement 3. | [37,50,64] |
| phytosterols | |||
Campesterol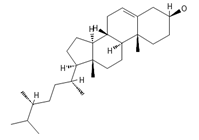 | 400.3705 | Present in plants with antioxidant properties, it lowers low-density lipoprotein (LDL) cholesterol levels, reducing the risk of cardiovascular diseases and helping to prevent obesity 4, diabetes 3, and cancer 9. It also decreases the levels of metabolites such as β-carotene, lycopene, and vitamin E. | [37,65,66] |
β-Sitosterol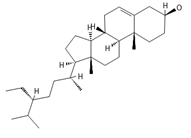 | ≈414.7180 | A plant-derived sterol with antioxidant properties reduces LDL cholesterol levels, contributes to the improvement of heart health, and reduces the risk of cardiovascular diseases; it may reduce cancer 9 and digestive risks, e.g., of liver damage, by its anti-inflammatory and gastrointestinal protective properties. | [37,66,67] |
Stigmasterol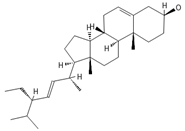 | ≈412.3705 | A phytosterol with antioxidant 1 properties and cholesterol-lowering effects, it is suggested to have anticancer 9 properties by apoptosis induction, inflammatory bowel disease, stomach ulcers, and protecting the liver from toxin-induced damage. | [9,37,66] |
| fatty acids | |||
Hop-22(29)-ene *** (C30)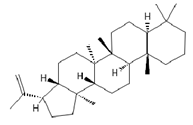 | ≈410.3913 | Hop-22(29)-ene and its derivatives, such as 21αH-hop-22(29)-en-3-ol, are the agents with antioxidant 1 and anti-inflammatory 2 properties; biomedical applications in cancer 9 treatment and the production of dietary supplements; further clinical research is required. | [37,53] |
Palmitic acid (C16) ***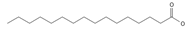 | ≈256.2402 | Plays a key role in the palmitoylation of proteins and palmitoylated signal molecules. | [37,54] |
| fatty acid esters | |||
Methyl palmitate (C17)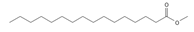 | ≈270.2558 | Antioxidant 1, anti-inflammatory 2, anti-apoptotic, anti-fibrotic 6, and vasodilator 7 properties, as well as a cardioprotective agent. | [37,68,69] |
Ethyl palmitate (C18) | ≈284.2715 | Anti-inflammatory 2 and histoprotective effects. | [37,68] |
Methyl stearate (C18)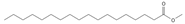 | ≈298.2871 | Stearic acid ester is present in vegetable oils with skincare properties and is used in cosmetics and pharmaceuticals as well as a food stabilizer. It may cause allergic reactions. | [37,70] |
Methyl behenate (C22)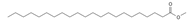 | ≈354.3497 | Synthetic compound, substrate of behenyl behenate; applied in the cosmetic industry as a stabilizer and emulsifier. | [37] |
| Lactones | |||
Dihydroambrettolide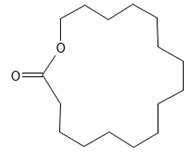 | ≈254.2290 | High fragrance durability on the skin; pleasant-smelling compound; widely used in the perfume industry. | [37] |
| Flavones | |||
Schaftoside | ≈564.1479 | Di-C-glycoside with antioxidative 1, anti-inflammatory 2, and anti-melanogenic activities, as well as antiepileptic properties. | [44,73] |
Isoschaftoside, Apigenin 6-C-α-L-arabinoside 8-C-β-D-glucoside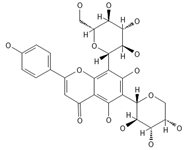 | ≈564.1479 | Di-C-glycoside with neuroprotective and anti-inflammatory 2 properties. | [44,74] |
Neoschaftoside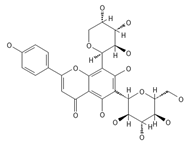 | ≈564.1479 | Di-C-glycoside, a flavone derivative of apigenin with antimicrobial 8, anti-inflammatory, and anticancer 9 properties, can reduce the risk of cardiovascular disease. | [44,75] |
Vicenin-2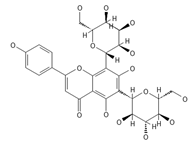 | ≈594.1584 | Di-C-glycoside with anti-inflammatory 2 activities. | [44,76] |
Chrysoeriol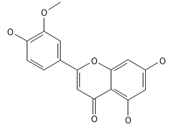 | ≈300.0634 | In di-C-glycoside form, like neosisoschaftoside, present in, e.g., grains of purple barley, with high bioavailability after digestion, it is important for gastrointestinal health support. | [44,77] |
Saponarin, luteolin 6-C-glycoside-7-O-glikozyd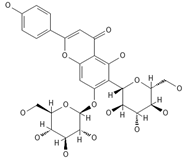 | ≈594.1580 | Flavone-O-diglucoside, with antioxidant 1, anti-inflammatory 2, anti-allergic, and skin-protective properties, used in dietary supplements and cosmetics, may enhance mitochondrial metabolism. | [37,78,79] |
| Biflavones | |||
Amentoflavone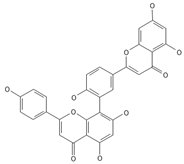 | ≈538.0900 | Together with its derivatives like 7′-O-β-D-glucosylamentoflavone, exhibits antioxidant 1, anti-inflammatory 2, antiviral, antiseptic 8, and anticancer 9 properties. | [37,80] |
Sequoiaflavone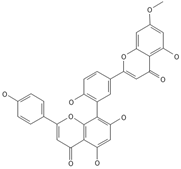 | ≈552.1056 | Antioxidant 1, anti-inflammatory 2 antiviral, antiseptic 8, and anticancer 9 properties. | [37,80] |
Bilobetin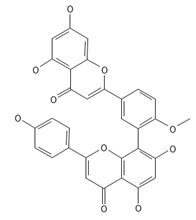 | ≈552.1056 | Antioxidant 1, anti-inflammatory 2 antiviral, antiseptic 8, and anticancer 9 properties. | [37,80] |
Podocarpusflavone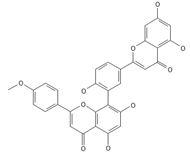 | ≈552.1056 | Antioxidant 1, anti-inflammatory 2 antiviral, antiseptic 8, and anticancer 9 properties. | [37,80] |
Ginkgetin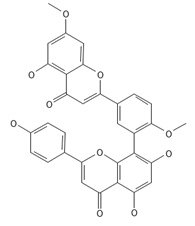 | ≈566.1213 | The situation is similar for N-octadecane, although it is also not a unique metabolite for P. affine. It is an interesting cryoprotectant 5 and may be used in the cosmetics and pharmaceutical industries. | [37,80] |
Isoginkgetin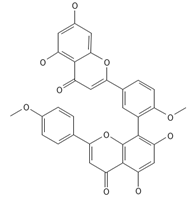 | ≈566.1213 | Together with its glycoside derivative like 7′-O-β-D-glucosyl-isoginkgetin, exhibits antioxidant 1, anti-inflammatory 2 antiviral, antiseptic 8, and anticancer 9 properties. | [37,80] |
5′-methoxybilobetine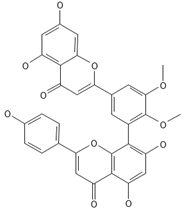 | ≈582.1162 | Antioxidant 1, anti-inflammatory 2 antiviral, antiseptic 8, and anticancer 9 properties. | [37,80] |
Sciadopitysins | ≈566.1110 | Antioxidant 1, anti-inflammatory 2 antiviral, antiseptic 8, and anticancer 9 properties. | [37,80] |
2,3-Dihydroisoginkgetin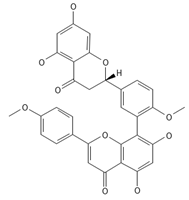 | ≈568.1370 | Antioxidant 1, anti-inflammatory 2 antiviral, antiseptic 8, and anticancer 9 properties. | [37,80] |
2,3-Dihydrosciadopitysin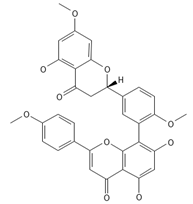 | ≈582.1553 | Antioxidant 1, anti-inflammatory 2 antiviral, antiseptic 8, and anticancer 9 properties. | [37,80] |
| Extracts | HeLa 10 μg mL−1 | HeLa 30 μg mL−1 | A2780 10 μg mL−1 | A2780 30 μg mL−1 | T47D 10 μg mL−1 | T47D 30 μg mL−1 |
|---|---|---|---|---|---|---|
| A | <25 | 41.79 | <25 | <25 | <25 | 42.41 |
| B | 55.53 | <25 | 42.11 | 42.49 | 56.05 | <25 |
| C | 42.04 | 50.67 | <25 | 26.86 | 53.3 | 57.53 |
| D | <25 | <25 | <25 | <25 | <25 | <25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupa, J.; Kaźmierczak, A.; Kołodziejczyk, I. Molecular Insights into the Biomedical Applications of Plagiomnium affine (Blandow ex Funck) T. Kop.: A Promising Source of Bioactive Metabolites. Int. J. Mol. Sci. 2025, 26, 9341. https://doi.org/10.3390/ijms26199341
Krupa J, Kaźmierczak A, Kołodziejczyk I. Molecular Insights into the Biomedical Applications of Plagiomnium affine (Blandow ex Funck) T. Kop.: A Promising Source of Bioactive Metabolites. International Journal of Molecular Sciences. 2025; 26(19):9341. https://doi.org/10.3390/ijms26199341
Chicago/Turabian StyleKrupa, Julia, Andrzej Kaźmierczak, and Izabela Kołodziejczyk. 2025. "Molecular Insights into the Biomedical Applications of Plagiomnium affine (Blandow ex Funck) T. Kop.: A Promising Source of Bioactive Metabolites" International Journal of Molecular Sciences 26, no. 19: 9341. https://doi.org/10.3390/ijms26199341
APA StyleKrupa, J., Kaźmierczak, A., & Kołodziejczyk, I. (2025). Molecular Insights into the Biomedical Applications of Plagiomnium affine (Blandow ex Funck) T. Kop.: A Promising Source of Bioactive Metabolites. International Journal of Molecular Sciences, 26(19), 9341. https://doi.org/10.3390/ijms26199341






