1. Introduction
Angiogenesis is a multifaceted process that plays a crucial role in the growth and progression of tumor cells. This occurs through the release of tumor-derived factors, extracellular matrix-associated cytokines, and the overexpression of pro-angiogenic mediators such as vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and angiopoietins [
1]. The radiological tumor response to anti-angiogenic therapies varies widely due to the heterogeneity of angiogenic mechanisms, the biological heterogeneity of tumors, and the intricate network of intracellular signaling pathways activated in response to hypoxia. Nevertheless, certain tumors eventually develop resistance to anti-angiogenic treatment [
2]. In the era of personalized medicine, the identification and application of prognostic and predictive biomarkers are essential for optimizing therapeutic strategies in oncology and across various medical conditions.
A topic of growing interest in assessing the benefits of antiangiogenic therapy is the analysis of panels of circulating biomarkers in relation to progression free survival (PFS), overall survival (OS) or radiological tumor response due to the benefits of accessibility, low cost and possibility of obtaining repeated samples for serial testing. Such studies have been conducted in ovarian cancers [
3], hepatocellular carcinomas [
4], angiosarcomas [
5], lung cancers [
6], gastric cancers [
7] and colorectal cancers (CRC), but the results regarding the prognostic or predictive role of circulating proangiogenic factors are contradictory [
8,
9,
10].
In the CALGB 80405 study, a panel of 24 plasma biomarkers was measured at baseline to evaluate their prognostic and predictive significance in 715 patients with metastatic colorectal cancer (CRC) exhibiting wild-type KRAS status. Patients were randomized to receive chemotherapy in combination with either bevacizumab or cetuximab. The analyzed markers were involved in angiogenesis, as well as in inflammation and generation of the immune response [
8]. Vascular endothelial growth factor A (VEGF-A) and PlGF proved to be prognostic factors for OS, but not for PFS. Elevated PlGF baseline levels were associated with a lack of PFS benefit from bevacizumab treatment, regardless of the chemotherapy regimen administered. Conversely, lower levels of VEGF-D were correlated with improved PFS outcomes, with the predictive effect being most pronounced in patients whose VEGF-D levels fell within the lowest quartile.
Interestingly, Zhang et al. investigated the expression levels of VEGF-A, along with the soluble forms of its receptors VEGFR1 (FLT-1), and VEGFR2, across two independent colon cancer datasets [
9]. Their analysis revealed that elevated expression of all three markers was associated with poor prognosis. The observation was also confirmed in another dataset, where high expression levels of the three factors were specifically linked to unfavorable outcomes in patients with RAS wild-type tumors. In contrast, a study by Delle Monache et al. found no significant link between progression-free survival (PFS) and baseline levels of VEGF-A or IL-8 in patients with metastatic colorectal cancer harboring RAS mutations who were treated with bevacizumab [
10].
In a recent review summarizing data from a phase II trial evaluating the efficacy of bevacizumab combined with irinotecan-based chemotherapy in metastatic CRC, the authors observed that, prior to radiological disease progression, certain subgroups of patients showed increased plasma levels of cytokines and proangiogenic factors. This increase may suggest a mechanism of resistance to antiangiogenic therapy. The authors observed an increase in PlGF values following bevacizumab administration, with the highest levels registered just before disease progression [
11].
Basic Fibroblast Growth Factor (bFGF/FGF-2), is involved in promoting cell growth, angiogenesis, and modulating the tumor microenvironment [
12]. However, little is known about its role in CRC. Jibiki et al. examined the clinical significance of bFGF in CRC and showed that bFGF levels differ significantly between early and advanced stage cancers, being influenced by tumor size and extent of lymphatic invasion. Patients with larger tumors and moderate lymphatic invasion show elevated bFGF levels. These data suggest a potential prognostic role of bFGF [
13].
The VEGF-C/VEGFR3 signaling pathway is crucial for promoting the proliferation, migration, and survival of lymphatic endothelial cells, as well as facilitating the metastatic process [
14]. In CRC, Tacconi et al. demonstrated that VEGF-C expression is upregulated, while VEGFR3 is present in both lymphatic vessels and tumor-associated macrophages. VEGF-C/VEGFR3 signaling synergically activates lymphatic endothelial cells and tumor-associated macrophages, resulting in the suppression of antitumor immunity and the enhancement of primary tumor growth [
15].
Tyrosine Kinase with Immunoglobulin-like and Epidermal Growth Factor-like Domains 2 (Tie2) is the receptor of angiopoietins and is involved in the modulation of angiogenesis and vascular permeability. Abnormal activation of the Tie2 signaling pathway has been linked to excessive angiogenesis in cancer and tumor progression, thus potentially becoming a target for antiangiogenic therapy [
16,
17]. In metastatic CRC, the results of a study showed that after bevacizumab administration, Tie2 levels correlated with an imaging marker of the tumor vasculature, suggesting that Tie2 is secreted by the tumor vasculature. Prior to antiangiogenic therapy, Tie2 levels independently correlated with the vascular characteristics of tumors. After treatment, Tie2 levels were associated with radiological tumor response and PFS [
18].
The link between cyclophilin A (CypA) and angiogenesis was investigated recent studies, which demonstrated that under hypoxic conditions, CypA is upregulated and acetylated, leading to enhanced autophagy in endothelial cells. This acetylated form of CypA was shown to promote proliferation, migration, and the formation of tubular networks in pulmonary arterial endothelial cells. These effects were accompanied by increased endothelial cell motility and angiogenic activity, highlighting a role for CypA in modulating vascular remodeling and angiogenesis in response to cellular stress [
19]. Additionally, Peng et al. demonstrated that CypA plays a key role in regulating oxidative stress and the production of reactive oxygen species, mechanisms through which it contributes to the development of chemoresistance in CRC. Furthermore, increased levels of CypA were correlated with lack of radiologic response to chemotherapy, suggesting its implications in the mechanisms of treatment resistance and its potential predictive role [
20]. Additionally, in a recent study, we demonstrated that CypA correlates with improved OS, being a favorable prognostic factor [
21].
In our study, we propose a novel perspective on the dual prognostic and predictive roles of bevacizumab therapy initiation in CRC by simultaneously evaluating, in the same cohort of patients, a comprehensive panel of angiogenesis-related biomarkers, namely bFGF, PlGF, VEGF-A, VEGF-C, VEGF-D, FLT-1, Tie2, and CypA, alongside baseline patient characteristics, including age, primary tumor location, RAS mutational status, metastatic sites, and the associated chemotherapy regimen. While the majority of these circulating angiogenic biomarkers were assessed only at baseline, CypA measurements were available both at baseline and after six months of treatment, as previously reported in our recent study [
21]. We subsequently developed mathematical prediction models integrating clinical parameters with these biomarkers to identify those with relevance for both OS and PFS. This approach enables a more personalized treatment strategy by identifying patients most likely to benefit from bevacizumab, potentially minimizing unnecessary exposure to adverse effects and reducing healthcare costs.
3. Discussion
In colorectal cancer, angiogenesis facilitates tumor progression by sustaining growth and enabling dissemination to distant sites. Targeting this process with antiangiogenic therapies has significantly improved patient outcomes. However, lack of imagistic response to treatment remains a major challenge, limiting long-term efficacy. Thus, elucidating the mechanisms underlying angiogenesis and therapeutic resistance is key to refining treatment strategies. Furthermore, identifying reliable predictive and prognostic biomarkers could facilitate optimal patient selection for antiangiogenic therapy initiation, allowing for a more personalized and effective approach.
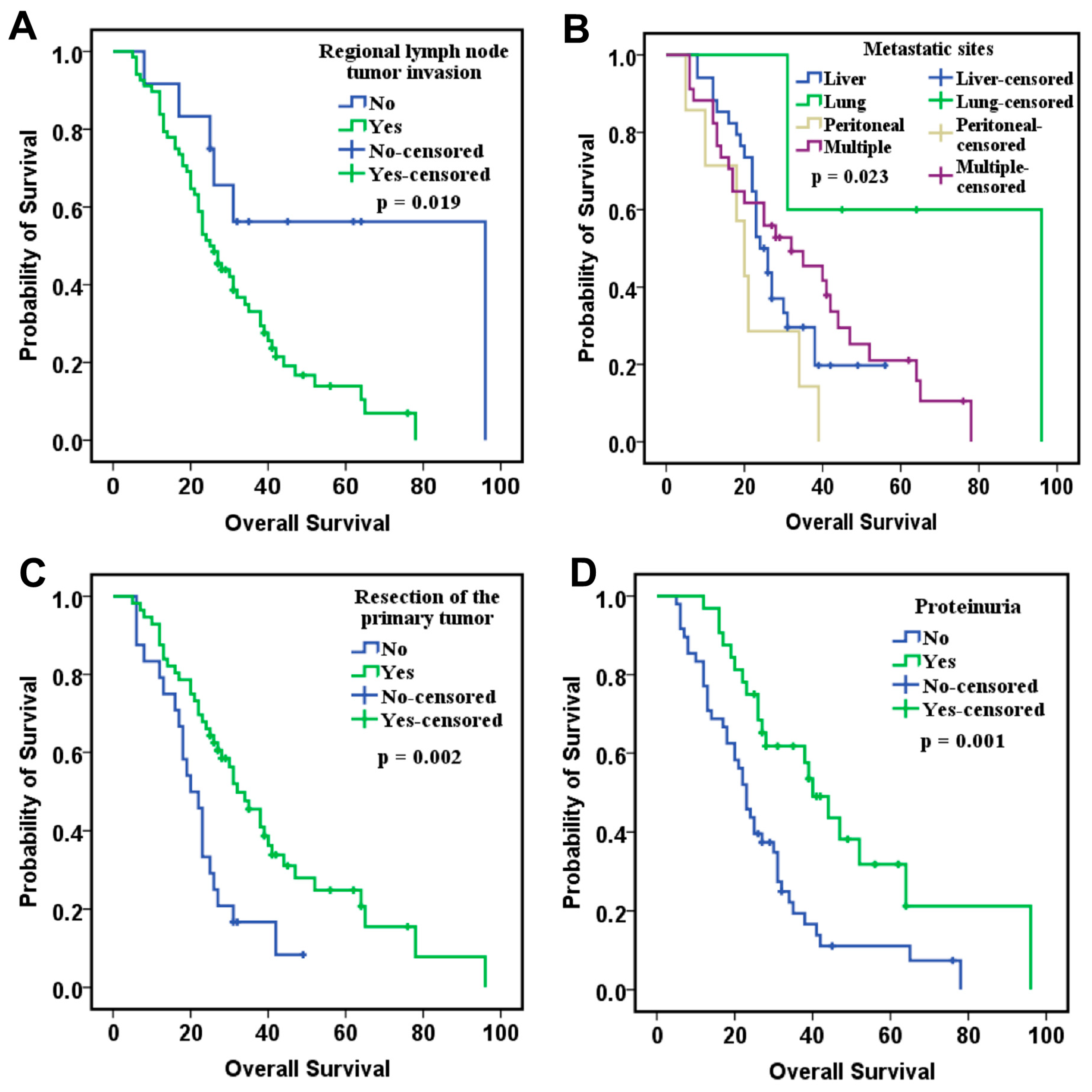
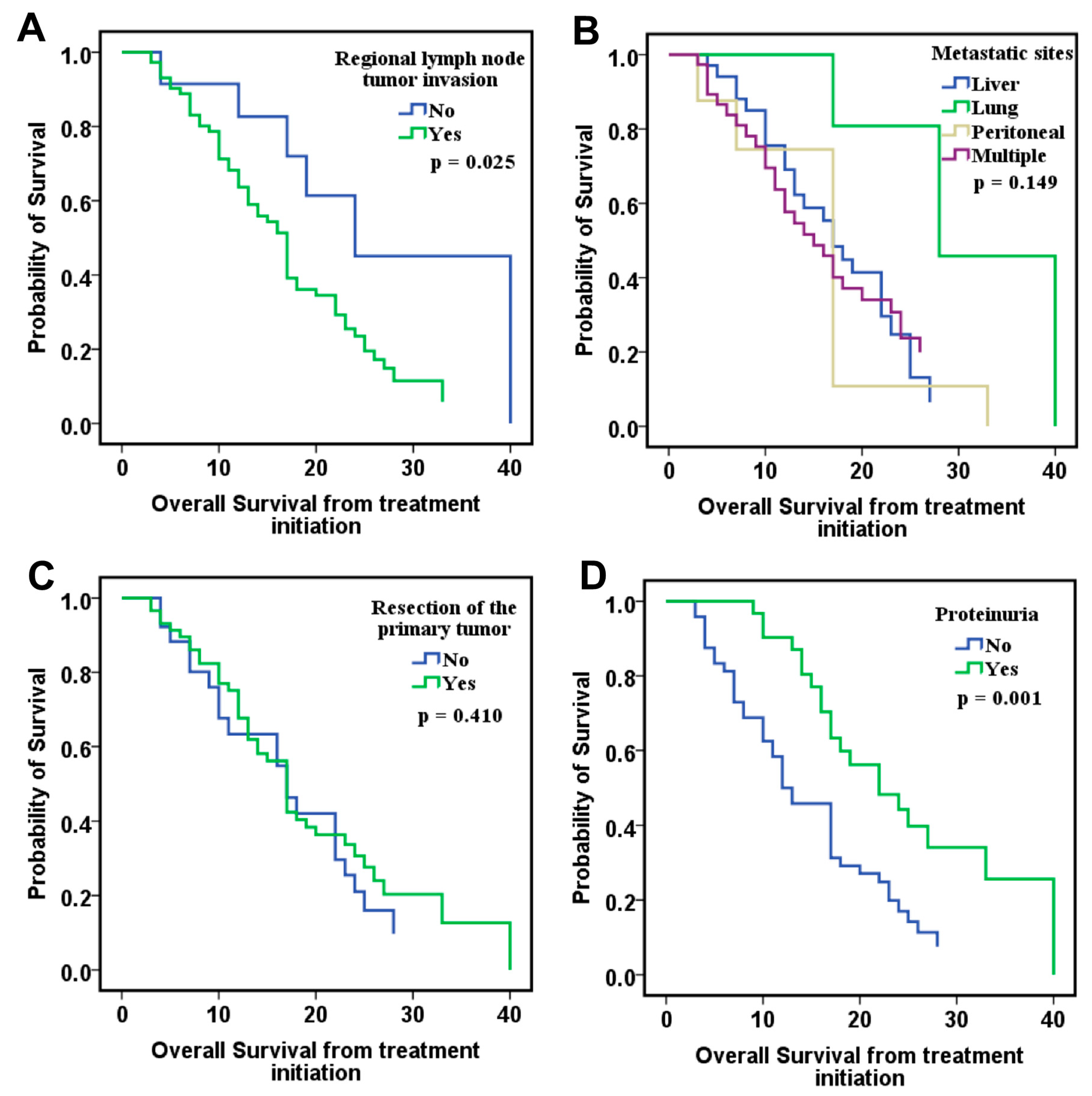
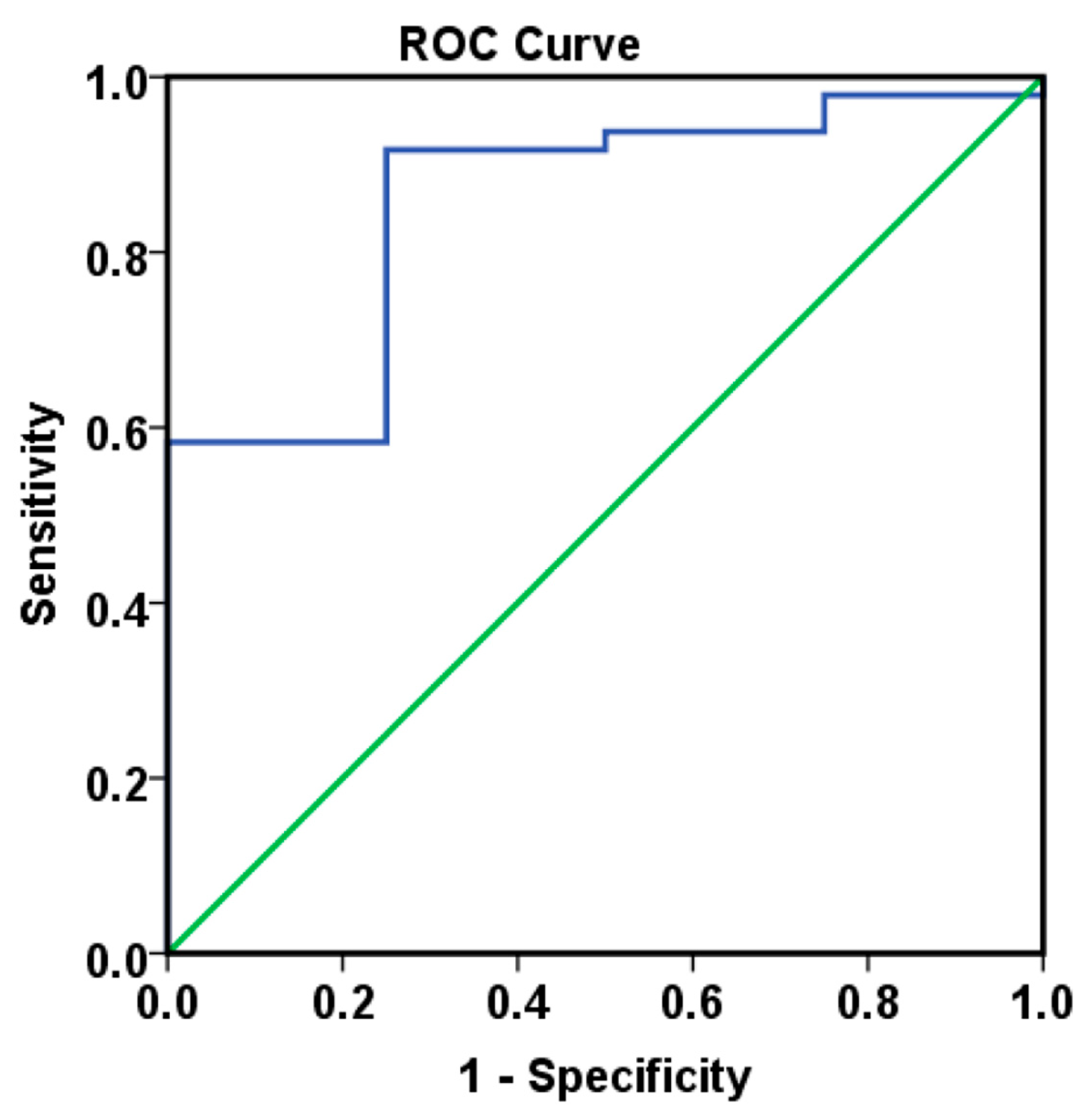
Our study investigates the involvement of eight angiogenic factors in the angiogenesis process in CRC and explores the role of circulating biomarkers in optimizing therapeutic decision-making. The study cohort included 80 patients, but CypA values were available for only 52 of them. The results showed that VEGF-A, VEGF-D, and bFGF levels correlated with OS in the ITT group. Patients with low levels of VEGF-A and VEGF-D and high levels of bFGF had the best survival, suggesting that these markers may serve as favorable prognostic factors. Moreover, when CypA was added to the analysis model in the PDS group, along with clinical prognostic factors, low levels of VEGF-A, VEGF-D, and CypA, combined with high levels of VEGF-C and PlGF, were significantly associated with improved OS.
Our results regarding the prognostic role of circulating VEGF-A are consistent with other studies in the literature [
8,
9,
22], as summarized in
Table 12. Additionally, existing data on tumor-tissue angiogenesis biomarker expression have shown concordant results with circulating biomarkers, further reinforcing their prognostic value, as also demonstrated in our study (
Table 13).
A retrospective analysis of four clinical trials evaluating the prognostic and predictive values of circulating VEGF-A levels in 1816 patients with CRC, lung cancer, and renal cell carcinoma treated with bevacizumab and chemotherapy showed that circulating VEGF-A has a prognostic role, but it was not predictive for the benefit of bevacizumab-based treatment [
22]. Results from another more recent study, including 715 patients with metastatic CRC who received chemotherapy with either bevacizumab or cetuximab, showed that VEGF-A and PlGF were prognostic markers for OS, but not for PFS. Additionally, higher levels of VEGF-A were indicative for a higher risk of death, regardless of the associated treatment [
8].
In fact, studies in the literature have shown that VEGF-A is an extremely important factor in vascular development, and angiogenesis is indispensable for tumor transformation and progression [
22,
33,
34]. As a result of high levels of VEGF-A expression, malignant cells can maintain their regenerative capacity, leading to a dedifferentiated phenotype and promoting metastasis. The pathophysiological mechanisms [
22,
33,
34,
35] highlight VEGF-A as a key target for antiangiogenic therapy, such as bevacizumab, a monoclonal antibody that inhibits VEGF-A activity and tumor angiogenesis.
Although bevacizumab has provided substantial benefits in metastatic CRC treatment [
36,
37], tumor response to antiangiogenic therapy remains variable among patients. Resistance to antiangiogenic therapy is a common challenge in cancer treatment and most commonly occurs due to alternative angiogenic escape mechanisms that activate signaling pathways independent of VEGF. A potential resistance mechanism involves the overexpression of VEGF-D, which can activate compensatory signaling within the VEGF pathway through VEGFR2, counteracting the effects of VEGF-A inhibition by bevacizumab. Consequently, patients with elevated VEGF-D levels may derive limited therapeutic benefit from bevacizumab due to the presence of an alternative proangiogenic signaling pathway [
8].
Interestingly, VEGF-A is overexpressed in CRC, particularly in tumors that have developed distant metastases. Its mRNA expression is increased in both tumor tissue and plasma of CRC patients compared to healthy individuals [
9]. An analysis of the expression levels of VEGF-A and its two soluble receptors in two independent CRC cohorts revealed that high VEGF-A expression correlated with a shorter time to disease progression, suggestive for its role as a negative prognostic factor. Moreover, combined overexpression of VEGF-A, VEGFR1, and VEGFR2 conferred a very poor prognosis [
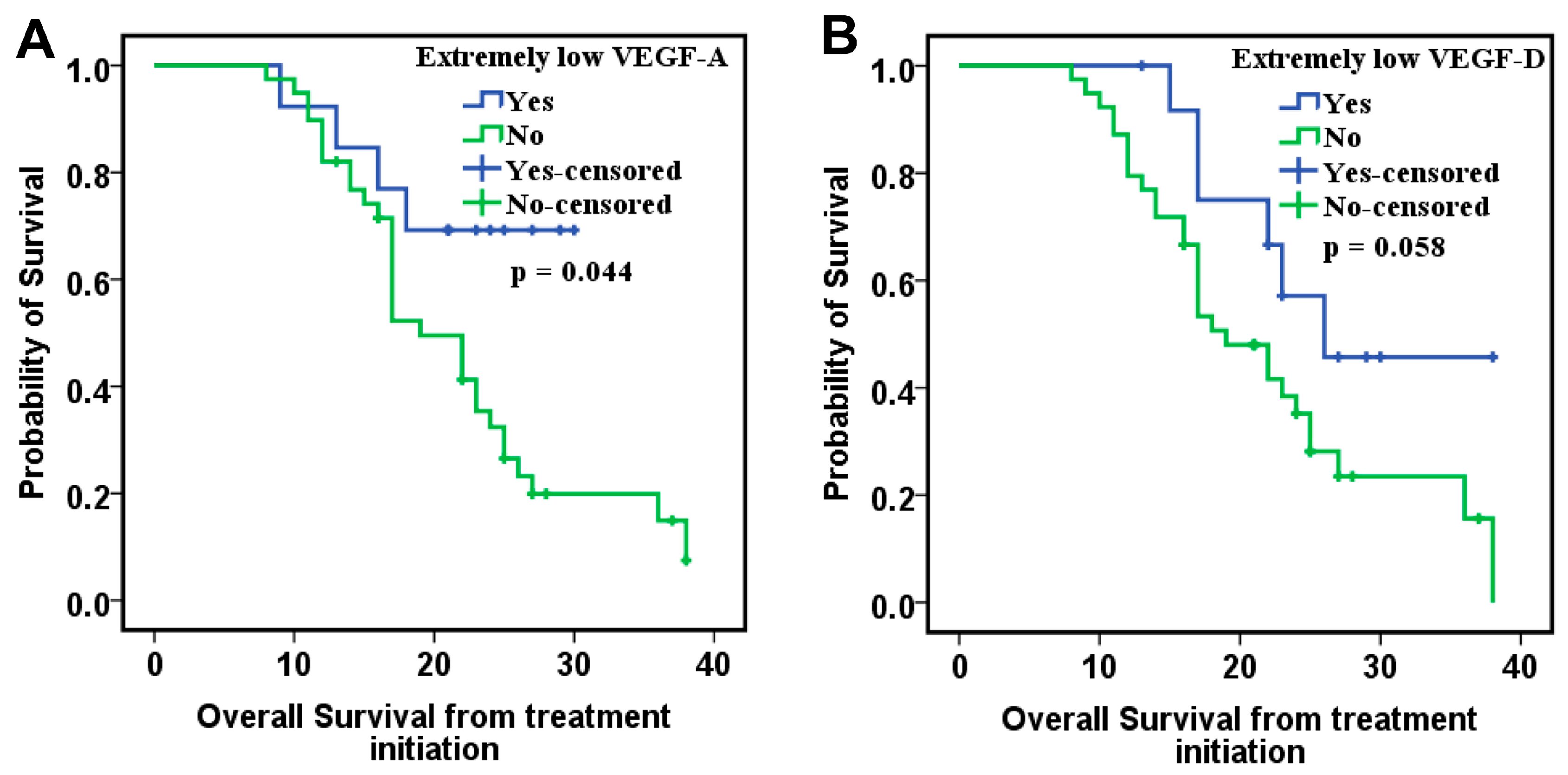
9]. In our study, CRC patients with VEGF-A levels above the 25th percentile had significantly lower OS in the PDS cohort. In addition, patients with elevated VEGF-A levels exhibited a higher risk of death in both the ITT and PDS cohorts.
Overall, VEGF-A reflects the angiogenic activity of colorectal cancer, and high levels are consistently associated with adverse outcomes. As a circulating factor, it has prognostic value and may aid in patient risk stratification. When considered together with other angiogenic markers, it could further strengthen clinical decision-making.
VEGF-D is another factor identified in our study with prognostic significance. Patients with low VEGF-D levels had significantly better OS in the PDS cohort and presented a lower risk of death in both the ITT and PDS cohorts, along with other markers and clinicopathological factors included in the analyzed model.
There are studies in the literature that have shown that increased VEGF-D expression is correlated with a poor prognosis and a more aggressive tumor evolution [
23,
27,
28,
29,
38]. In ovarian cancer, the expression of VEGF-A, VEGF-D and VEGFR1 proteins was higher in metastases compared to primary tumors [
27]. Another study analyzed the changes in the expression profile of VEGF-C, VEGF-D and VEGFR3, involved in lymphangiogenesis in endometrial cancer and the results showed that VEGF-D and VEGFR-3 were highly elevated compared to the control group. This change may indicate that, as the endometrium undergoes dedifferentiation, VEGF-D-dependent processes are intensified in malignant cells [
28]. In gastric cancer, VEGF-A and VEGF-D were significantly overexpressed in tumor tissue compared to the stroma. Patients with high VEGF-A levels had significantly worse overall survival (OS) than those with low levels. A similar trend was seen for VEGF-D, with higher concentrations associated with a tendency toward poorer OS [
38].
In CRC, Ose et al. found that doubling VEGF-D levels was linked to a threefold increased risk of death from rectal cancer, with no significant association in colon cancer [
23]. Additionally, VEGF-D has been demonstrated to correlate with PFS in metastatic CRC patients treated with chemotherapy and bevacizumab as first-line therapy [
29]. Patients with low VEGF-D expression in the tumor tissue showed the greatest benefit from treatment, both for OS and PFS. In another more recent study, Nixon et al. analyzed a panel of 24 soluble proteins with potential prognostic role in the plasma of patients with metastatic CRC who received chemotherapy in combination with either bevacizumab or cetuximab. The results of this study showed that high levels of VEGF-D were predictive of a lack of benefit on PFS following bevacizumab therapy, in addition to PlGF [
8]. Furthermore, patients with extremely low levels of VEGF-D, values below the 25th quartile, had better OS and PFS. These results correlate with the results of our study. Patients with extremely low levels of VEGF-D had the most favorable OS.
An important aspect is the relationship between VEGF-D and disease progression in CRC patients treated with bevacizumab. Lieu et al. demonstrated that circulating VEGF-D and PlGF levels increased at the time of imaging-confirmed progression [
24]. This increase suggests a compensatory mechanism through which tumors reactivate angiogenesis to counteract the effects of VEGF-A inhibition, thereby avoiding therapeutic control and promoting malignant progression. The data highlight the role of VEGF-D as a potential prognostic biomarker and its involvement in the mechanisms of resistance to antiangiogenic treatment [
8,
24].
On the other hand, Taniguchi et al. obtained surprising results regarding the predictive role of VEGF-D for second-line antiangiogenic therapy in metastatic CRC [
25]. Patients with high VEGF-D levels had a longer PFS and OS following ramucirumab and chemotherapy; however, statistical significance was achieved exclusively for PFS. The results are contradictory to those obtained in first-line bevacizumab treatment, in which patients with low VEGF-D levels had a better prognosis. Unlike bevacizumab, which only inhibits VEGF-A, ramucirumab (a monoclonal antibody that targets VEGFR2) prevents signaling at the receptor level, regardless of the activating ligand, such as VEGF-A, VEGF-C or VEGF-D [
39]. This strategy is important in the context of resistance to bevacizumab therapy, where tumors can compensate by overexpressing VEGF-D or other ligands that continue to activate VEGFR2 [
33,
40].
VEGF-D exhibits a consistent pattern in colorectal cancer, where very low circulating or tissue levels are associated with favorable prognosis. This suggests that VEGF-D could serve as a negative prognostic biomarker and may help identify patients more likely to benefit from bevacizumab therapy.
bFGF belongs to the FGF family and is involved in cell proliferation, angiogenesis, and metastasis through interaction with its four tyrosine kinase receptors, acting as a proangiogenic factor. In CRC, data from studies show that elevated levels of bFGF are associated with more aggressive tumors and an increased risk of recurrence [
12,
13,
30]. One study analyzed bFGF expression levels in patients with various forms of cancer, including colon cancer, to assess their association with clinicopathological characteristics of the neoplasms [
30]. The results showed that elevated levels of bFGF protein expression were associated with more aggressive tumors. Another study showed significant differences in bFGF levels between colorectal cancers in stages I-IIIb compared with those in stage IV, suggesting an association between elevated levels of this factor and tumor progression. Significant differences were also observed between cases with minimal and moderate lymphatic invasion, indicating a possible link between elevated bFGF expression and the ability of the tumor to invade the lymphatic system [
13]. However, there is contradictory data regarding the negative prognostic role of elevated bFGF levels in CRC [
26]. Kasurinen et al. classified CRC patients into four phenotypic subtypes, immune, canonical, metabolic, and mesenchymal, based on the immunohistochemically determined CD3-CD8 index in tumor and stroma, proliferation index, and stroma-tumor ratio. They evaluated serum levels and tissue expression of angiogenic factors VEGF, bFGF, and PDGF in each subtype. Surprisingly, in the metabolic subgroup, higher serum concentrations of all three markers were associated with significantly improved survival. Additionally, high initial serum bFGF levels proved to be favorable prognostic factors in the canonical subtype [
26]. This observation contrasts with other studies that associate high levels of bFGF with a poor prognosis, highlighting the complexity and heterogeneity of CRC. Importantly, our findings are in agreement with the results reported in the aforementioned study, supporting the association of elevated bFGF levels with a favorable prognosis. A possible explanation may be related to the fact that a bFGF-stimulated angiogenesis process may favor a more efficient immune tumor microenvironment, which may inhibit tumor growth. There are data suggesting that FGF can modulate the tumor microenvironment being directly associated with infiltration of M2 macrophages and dendritic cells, which would explain a possible association with a favorable prognosis in CRC [
12,
41,
42].
Another surprising finding in our study was that increased VEGF-C levels correlated with better prognosis and higher disease control rates. In other CRC studies, high levels of VEGF-C were associated with an unfavorable prognosis, being linked to an increased rate of lymph node metastases and lower OS [
31,
32,
43]. A possible explanation for our results may be related to the association of increased VEGF-C expression with different molecular subtypes of CRC, such as those with microsatellite instability or with well-differentiated histological grades, but this is more related to the specific biology of the tumor, not to the direct role of VEGF-C.
CypA is a multifunctional protein involved in protein folding by catalyzing proline bond isomerization, cellular signaling, and modulating inflammatory responses [
44]. In cancer, CypA stimulates tumor cell proliferation, angiogenesis, and metastasis by activating pathways such as NF-κB and ERK1/2 [
45]. In our previous study, we also showed that lower levels of CypA, both before and after one month of bevacizumab plus chemotherapy, independently predicted better OS and were associated with improved prognosis in metastatic CRC patients [
21].
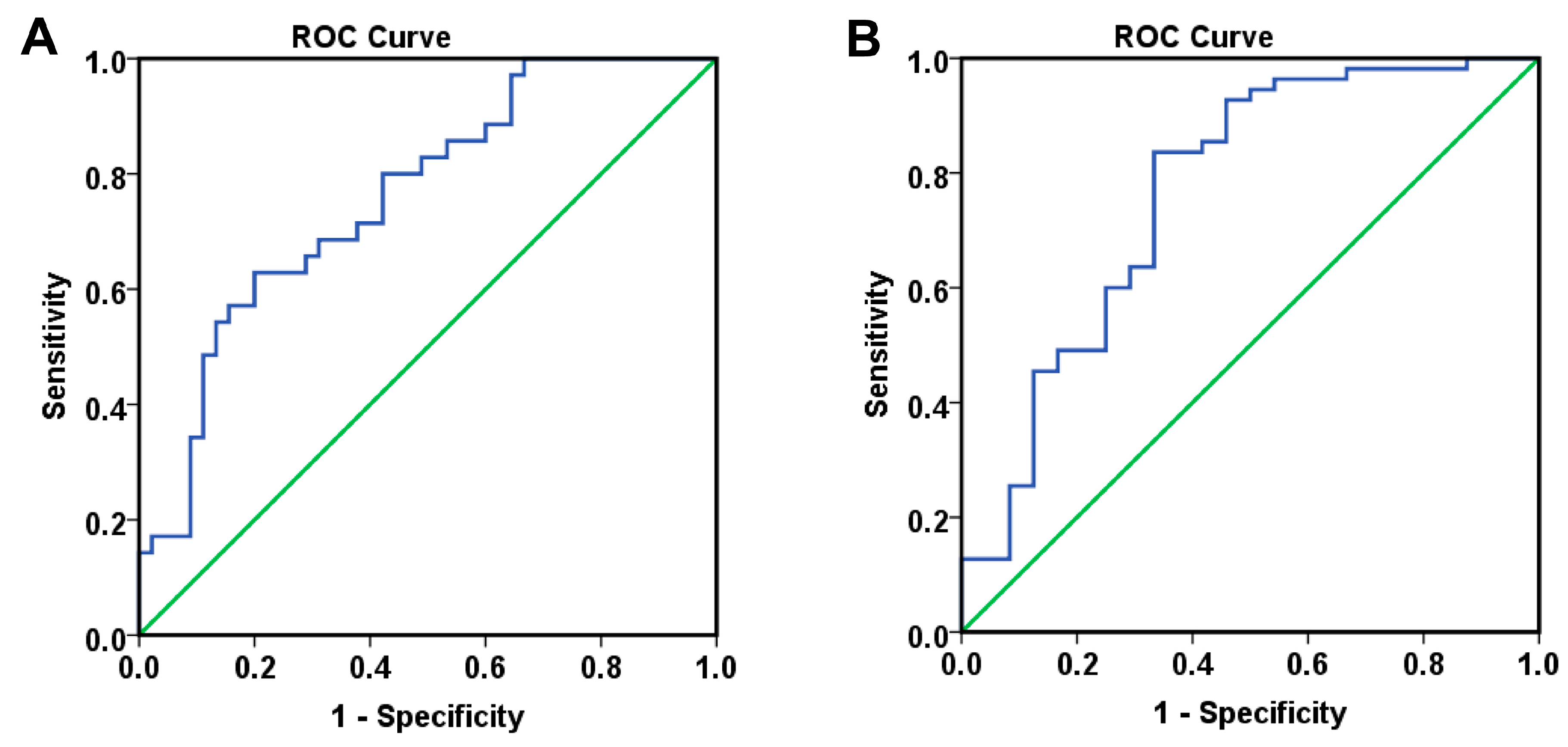
Finally, combining these angiogenic biomarkers with clinical parameters, such as lymph node invasion, and time-dependent variables, including proteinuria, hypertension, and CypA levels at 6 months, allowed the development of logistic regression models that demonstrated good to very good predictive capacity for overall survival, measured from either diagnosis or treatment initiation, as well as for progression-free survival (PFS).
One notable limitation of our study is that tumor angiogenesis was assessed indirectly through circulating biomarkers, rather than by direct tissue-based methods such as immunohistochemistry. Circulating angiogenic factors may not fully reflect the complexity and heterogeneity of the tumor vasculature, which is largely determined by local microenvironmental signals, including hypoxia, stromal interactions, and extracellular matrix remodeling. Nevertheless, systemic angiogenic markers may capture not only local angiogenic activity but also systemic processes, such as the mobilization of endothelial progenitor cells and paracrine signaling influencing vascular remodeling at distant sites. While our approach does not provide a direct histological assessment of angiogenesis, it offers a clinically feasible and biologically relevant perspective that complements tissue-based analyses. Furthermore, future studies could benefit from stratifying patients according to histotype and tumor grading, as these factors may influence angiogenic patterns and clinical outcomes. Together, incorporating both circulating and tissue-level markers, along with stratification by histological features, will be essential to achieve a more comprehensive characterization of tumor angiogenesis. Another limitation of our study is the relatively small cohort size, which may restrict the generalizability of our findings, as well as the lack of external validation. Nevertheless, the results provide valuable insights into the potential role of circulating angiogenic biomarkers, in combination with clinical variables, for predicting survival in bevacizumab-treated colorectal cancer. Importantly, these findings establish a foundation for future research with larger, independent cohorts, which will be essential to validate, refine, and potentially translate these prognostic models into clinical decision-making.
Overall, identifying predictive and prognostic biomarkers for antiangiogenic therapy in colorectal cancer is crucial for optimizing treatment and improving patient outcomes. A deeper understanding of the molecular factors influencing therapeutic response can guide personalized interventions and enhance efficacy. Our study offers new insights into the role of circulating biomarkers, such as VEGF-A, VEGF-D, VEGF-C, bFGF, and CypA, highlighting both novel findings and controversial aspects regarding their significance in this context.
Importantly, by combining multiple parameters identified as independent prognostic factors in multivariate analyses, including both clinical characteristics and paraclinical data, we were able to construct robust mathematical models with high predictive performance for both overall survival (OS) and progression-free survival (PFS). These models demonstrated excellent discriminatory power in stratifying patients according to risk, and allowed for the estimation of individual survival probabilities. This integrative approach supports a more personalized management of patients with colorectal cancer treated with antiangiogenic agents such as bevacizumab, potentially guiding clinical decisions and improving the selection of patients most likely to benefit from therapy.