The Therapeutic Potential of Pulsed Electromagnetic Fields (PEMF) and Low-Intensity Pulsed Ultrasound (LIPUS) in Peripheral Nerve Regeneration: A Comprehensive Review
Abstract
1. Introduction
2. Therapeutic Modalities: PEMF and LIPUS
2.1. Pulsed Electromagnetic Field (PEMF)
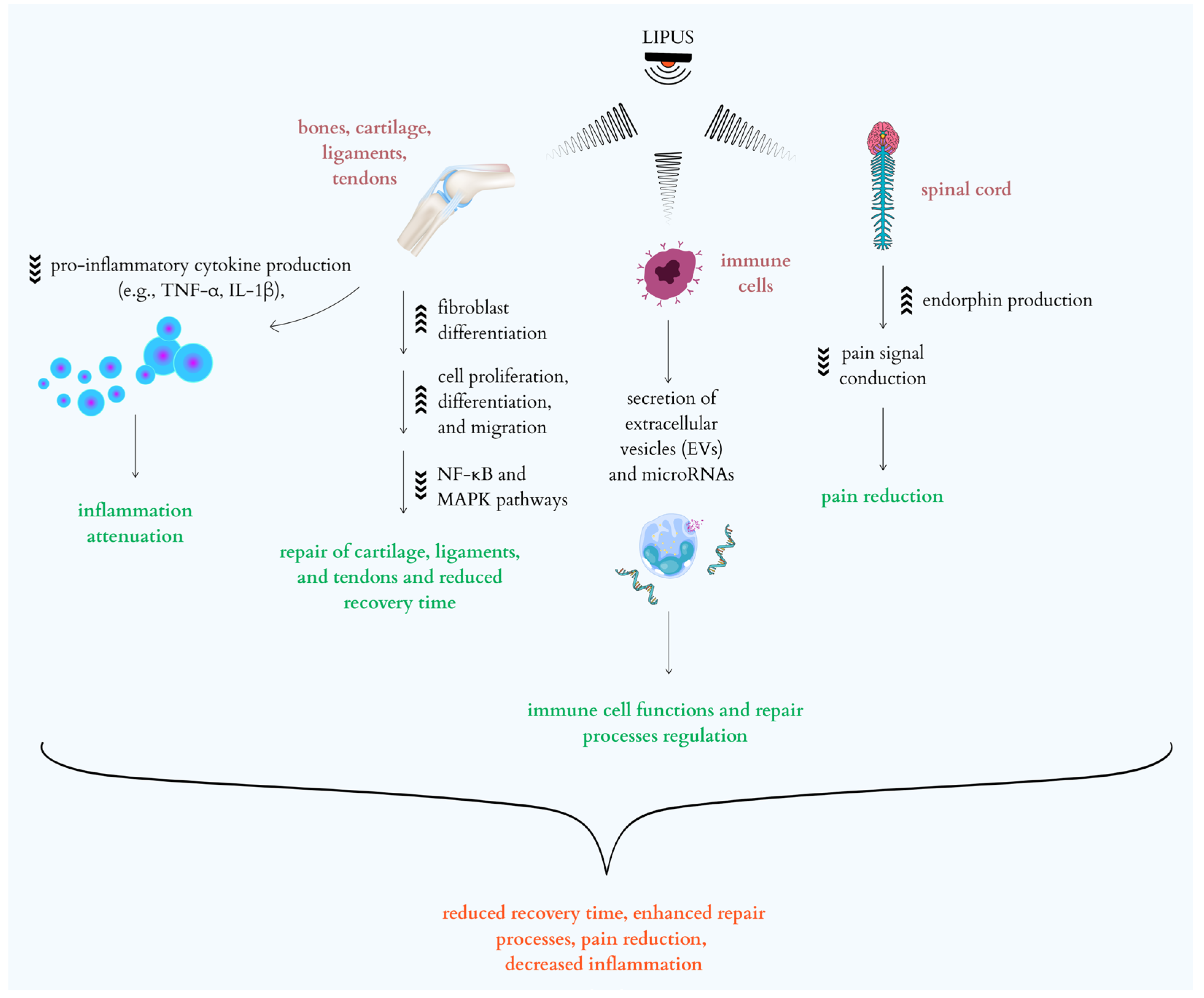
2.2. Low-Intensity Pulsed Ultrasound (LIPUS)
3. Mechanisms of Action at the Cellular and Molecular Level
3.1. PEMF Mechanisms
3.2. LIPUS Mechanisms
4. Effects on Peripheral Nerve Regeneration
4.1. PEMF Effects on Nerve Regeneration
4.2. LIPUS Effects on Nerve Regeneration
5. Limitations, Side Effects, and Contraindications of PEMF and LIPUS in Peripheral Nerve Regeneration
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, W. Objawy Kliniczne w Neurologii Wrocław; Urban&Partner: Wroclaw, Poland, 2019. [Google Scholar]
- Kozubski, W. Neurologia Wrocław; Wydawnictwo Urban & Partner: Krakow, Poland, 2016. [Google Scholar]
- Grand View Horizon Databooks. Available online: https://www.grandviewresearch.com/horizon/outlook/peripheral-nerve-injury-market/europe (accessed on 23 September 2025).
- Qiu, J.; Liao, X.; Deng, R. The prevalence and risk factors for peripheral nerve injury following arthroplasty: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2025, 20, 137. [Google Scholar] [CrossRef]
- Berry, D.; Ene, J.; Nathani, A.; Singh, M.; Li, Y.; Zeng, C. Effects of Physical Cues on Stem Cell-Derived Extracellular Vesicles toward Neuropathy Applications. Biomedicines 2024, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Gordon, K. Nerve Regeneration in the Peripheral Nervous System. J. Physiol. 2010, 43, 274–285. [Google Scholar]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, M.; Liu, N. Interactions between Schwann cell and extracellular matrix in peripheral nerve regeneration. Front. Neurol. 2024, 15, 1372168. [Google Scholar] [CrossRef]
- Chu, X.L.; Zhao, X.X.; Liu, S.Y.; Li, Y.J.; Ding, N.; Liu, M.Q.; Li, Q.W.; Li, Q. Research progress in different physical therapies for treating peripheral nerve injuries. Front. Neurol. 2025, 16, 1508604. [Google Scholar] [CrossRef]
- Raza, C.; Riaz, H.A.; Anjum, R.; Shakeel, N.U.A. Repair strategies for injured peripheral nerve: Review. Life Sci. 2020, 243, 117308. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Wan, H.; Li, J.H.; Li, J.M. Effect of low-intensity pulsed ultrasound on the expression of neurotrophin-3 and brain-derived neurotrophic factor in cultured Schwann cells. Microsurgery 2009, 29, 479–485. [Google Scholar] [CrossRef]
- Bassett, C.A. The development and application of pulsed electromagnetic fields (PEMFs) for ununited fractures and arthrodeses. Clin. Plast. Surg. 1985, 12, 259–277. [Google Scholar] [CrossRef]
- Markov, M. Magnetic Field Therapy. Electromagn. Biol. Med. 2007, 26, 1–23. [Google Scholar] [CrossRef]
- Funk, R.H.; Monsees, T.; Ozkucur, N. Electromagnetic effects-from cell biology to medicine. Prog. Histochem. Cytochem. 2009, 43, 177–264. [Google Scholar] [CrossRef]
- Liu, M.; Lee, C.; Laron, D.; Zhang, N.; Waldorff, E.I.; Ryaby, J.T.; Feeley, B.; Liu, X. Role of pulsed electromagnetic fields (PEMF) on tenocytes and myoblasts-potential application for treating rotator cuff tears. J. Orthop. Res. 2017, 35, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.A. Beneficial effects of electromagnetic fields. J. Cell. Biochem. 1993, 51, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Faldini, C.; Cadossi, M.; Deianiraa, L.; Betti, E.; Chiarello, E.; Giannini, S. Electromagnetic Bone Growth Stimulation in Patients with Femoral Neck Fractures Treated with Screws: Prospective Randomized Double-Blind Study. Orthop. Pract. 2010, 21, 282–287. [Google Scholar] [CrossRef]
- Zhou, S.; Wen, H.; He, X.; Han, X.; Li, H. Pulsed electromagnetic field ameliorates the progression of osteoarthritis via the Sirt1/NF-κB pathway. Arthritis Res. Ther. 2025, 27, 33. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, H.; Ye, W.; Yang, L.; He, C. Pulsed Electromagnetic Field Attenuates Osteoarthritis Progression in a Murine Destabilization-Induced Model through Inhibition of TNF-α and IL-6 Signaling. Cartilage 2021, 13, 1665S–1675S. [Google Scholar] [CrossRef]
- Yang, X.; He, H.; Ye, W.; Perry, T.A.; He, C. Effects of Pulsed Electromagnetic Field Therapy on Pain, Stiffness, Physical Function, and Quality of Life in Patients With Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Phys. Ther. 2020, 100, 1118–1131. [Google Scholar] [CrossRef]
- Cadossi, R.; Massari, L.; Racine-Avila, J.; Aaron, R.K. Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular Mechanisms of Skeletal Response. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e19. [Google Scholar] [CrossRef]
- Massari, L.; Benazzo, F.; Moretti, B.; Dallari, D.; Perugia, D.; Meani, E.; Cadossi, R. Electrical Stimulation of Osteogenesis: Efficacy and Technologies Compared. GIOT 2011, 6, 1–8. [Google Scholar]
- Tang, X.; Coughlin, D.; Ballatori, A.; Berg-Johansen, B.; Waldorff, E.I.; Zhang, N.; Ryaby, J.T.; Aliston, T.; Lotz, J.C. Pulsed Electromagnetic Fields Reduce Interleukin-6 Expression in Intervertebral Disc Cells Via Nuclear Factor-κβ and Mitogen-Activated Protein Kinase p38 Pathways. Spine 2019, 44, E1290–E1297. [Google Scholar] [CrossRef]
- Jin, K.; Zhao, D.; Zhou, J.; Zhang, X.; Wang, Y.; Wu, Z. Pulsed electromagnetic fields inhibit IL-37 to alleviate CD8+ T cell dysfunction and suppress cervical cancer progression. Apoptosis 2024, 29, 2108–2127. [Google Scholar] [CrossRef]
- Lee, C.G.; Park, C.; Hwang, S.; Hong, J.E.; Jo, M.; Eom, M.; Lee, Y.; Rhee, K.J. Pulsed Electromagnetic Field (PEMF) Treatment Reduces Lipopolysaccharide-Induced Septic Shock in Mice. Int. J. Mol. Sci. 2022, 23, 5661. [Google Scholar] [CrossRef]
- Tepper, O.M.; Callaghan, M.J.; Chang, E.I.; Galiano, R.D.; Bhatt, K.A.; Baharestani, S.; Gan, J.; Simon, B.; Hopper, R.A.; Levine, J.P.; et al. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 2004, 18, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Battistello, E.; Giacomelli, L.; Varani, K.; Vincenzi, F.; Borea, P.A.; Gessi, S. Targeting A3 and A2A adenosine receptors in the fight against cancer. Expert Opin. Ther. Targets 2019, 23, 669–678. [Google Scholar] [CrossRef]
- Cook, S.D.; Salkeld, S.L.; Patron, L.P.; Doughty, E.S.; Jones, D.G. The effect of lowintensity pulsed ultrasound on autologous osteochondral plugs in a canine model. Am. J. Sports Med. 2008, 36, 1733–1741. [Google Scholar] [CrossRef]
- Hu, J.; Qu, J.; Xu, D.; Zhang, T.; Qin, L.; Lu, H. Combined application of low-intensity pulsed ultrasound and functional electrical stimulation accelerates bone-tendon junction healing in a rabbit model. J. Orthop. Res. 2014, 32, 204–209. [Google Scholar] [CrossRef]
- Ikai, H.; Tamura, T.; Watanabe, T.; Itou, M.; Sugaya, A.; Iwabuchi, S.; Mikuni-Takagaki, Y.; Deguchi, S. Low-intensity pulsed ultrasound accelerates periodontal wound healing after flap surgery. J. Periodontal Res. 2008, 43, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.; Mollon, B.; Bance, S.; Busse, J.W.; Bhandari, M. Low-intensity pulsed ultrasonography versus electrical stimulation for fracture healing: A systematic review and network meta-analysis. Can. J. Surg. 2014, 57, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, Y.; Tang, J.; Peng, J.; Wang, Y.; Guo, Q.; Guo, Z.; Li, P.; Xiao, B.; Zhang, J. Low-intensity pulsed ultrasound treatment improved the rate of autograft peripheral nerve regeneration in rat. Sci. Rep. 2016, 6, 22773. [Google Scholar] [CrossRef]
- Jiang, X.; Savchenko, O.; Li, Y.; Qi, S.; Yang, T.; Zhang, W.; Chen, J. A Review of Low-Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans. Biomed. Eng. 2019, 66, 2704–2718. [Google Scholar] [CrossRef]
- Li, X.; Zhong, Y.; Zhang, L.; Xie, M. Recent advances in the molecular mechanisms of low-intensity pulsed ultrasound against inflammation. J. Mol. Med. 2023, 101, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Li, Y.; Li, Y.; Ji, H.; Mang, G.; Fu, S.; Jiang, S.; Choi, S.; Wang, X.; Tong, Z.; et al. Low-intensity pulsed ultrasound protects from inflammatory dilated cardiomyopathy through inciting extracellular vesicles. Cardiovasc. Res. 2024, 120, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Cao, S.; Yang, L.; Liu, J.; Gui, B.; Wang, H.; Jiang, N.; Zhou, Q.; Deng, Q. Low-intensity pulsed ultrasound accelerates diabetic wound healing by ADSC-derived exosomes via promoting the uptake of exosomes and enhancing angiogenesis. Int. J. Mol. Med. 2024, 53, 23. [Google Scholar] [CrossRef]
- Ichijo, S.; Shindo, T.; Eguchi, K.; Monma, Y.; Nakata, T.; Morisue, Y.; Kanai, H.; Osumi, N.; Yasuda, S.; Shimokawa, H. Low-intensity pulsed ultrasound therapy promotes recovery from stroke by enhancing angio-neurogenesis in mice in vivo. Sci. Rep. 2021, 11, 4958. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, E.; Lu, L.; Zhang, H.; Yang, X.; Hao, Y. Effectiveness of low-intensity pulsed ultrasound on osteoarthritis: Molecular mechanism and tissue engineering. Front. Med. 2024, 11, 1292473. [Google Scholar] [CrossRef]
- Lin, Z.; Gao, L.; Hou, N.; Zhi, X.; Zhang, Y.; Che, Z.; Deng, A. Application of low-intensity pulsed ultrasound on tissue resident stem cells: Potential for ophthalmic diseases. Front. Endocrinol. 2023, 14, 1153793. [Google Scholar] [CrossRef]
- Zuo, K.J.; Gordon, T.; Chan, K.M.; Borschel, G.H. Electrical stimulation to enhance peripheral nerve regeneration: Update in molecular investigations and clinical translation. Exp. Neurol. 2020, 332, 113397. [Google Scholar] [CrossRef]
- Xie, Z.; Askari, A. Na+/K+-ATPase as a signal transducer. Eur. J. Biochem. 2002, 269, 2434–2439. [Google Scholar] [CrossRef]
- Thiel, G.; Schmidt, T.; Rössler, O.G. Ca2+ Microdomains, Calcineurin and the Regulation of Gene Transcription. Cells 2021, 10, 875. [Google Scholar] [CrossRef]
- Martini, F.; Pellati, A.; Mazzoni, E.; Salati, S.; Caruso, G.; Contartese, D.; De Mattei, M. Bone Morphogenetic Protein-2 Signaling in the Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells Induced by Pulsed Electromagnetic Fields. Int. J. Mol. Sci. 2020, 21, 2104. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, L.; Pellati, A.; Rizzo, P.; Aquila, G.; Massari, L.; De Mattei, M.; Ongaro, A. Notch pathway is active during osteogenic differentiation of human bone marrow mesenchymal stem cells induced by pulsed electromagnetic fields. J. Tissue Eng. Regen. Med. 2018, 12, 304–315. [Google Scholar] [CrossRef]
- Robertson, J.A.; Thomas, A.W.; Bureau, Y.; Prato, F.S. The influence of extremely low frequency magnetic fields on cytoprotection and repair. Bioelectromagnetics 2007, 28, 16–30. [Google Scholar] [CrossRef]
- Gaynor, J.S.; Hagberg, S.; Gurfein, B.T. Veterinary applications of pulsed electromagnetic field therapy. Res. Vet. Sci. 2018, 119, 1–8. [Google Scholar] [CrossRef]
- Li, J.K.; Lin, J.C.; Liu, H.C.; Chang, W.H. Release from Osteoblasts in Response to Different Intensities of Pulsed Electromagnetic Field Stimulation. Electromagn. Biol. Med. 2007, 26, 153–165. [Google Scholar] [CrossRef]
- Varani, K.; Vincenzi, F.; Ravani, A.; Pasquini, S.; Merighi, S.; Gessi, S.; Setti, S.; Cadossi, M.; Borea, P.A.; Cadossi, R. Adenosine Receptors as a Biological Pathway for the Anti-Inflammatory and Beneficial Effects of Low Frequency Low Energy Pulsed Electromagnetic Fields. Mediat. Stanu Zapalnego 2017, 2017, 2740963. [Google Scholar] [CrossRef]
- Kaadan, A.; Salati, S.; Setti, S.; Aaron, R. Augmentation of Deficient Bone Healing by Pulsed Electromagnetic Fields-From Mechanisms to Clinical Outcomes. Bioengineering 2024, 11, 1223. [Google Scholar] [CrossRef] [PubMed]
- Petecchia, L.; Sbrana, F.; Utzeri, R.; Vercellino, M.; Usai, C.; Visai, L.; Vassalli, M.; Gavazzo, P. Electro-magnetic field promotes osteogenic differentiation of BM-hMSCs through a selective action on Ca2+-related mechanisms. Sci. Rep. 2015, 5, 13856. [Google Scholar] [CrossRef] [PubMed]
- He, W.F.; Qin, R.; Gao, Y.H.; Zhou, J.; Wei, J.J.; Liu, J.; Hou, X.F.; Ma, H.P.; Xian, C.J.; Li, X.Y.; et al. The interdependent relationship between the nitric oxide signaling pathway and primary cilia in pulse electromagnetic field-stimulated osteoblastic differentiation. FASEB J. 2022, 36, 22376. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Zhang, W.; He, J.; Xu, B.; Lei, B.; Wang, Z.H.; Cates, C.; Rousselle, T.; Li, J. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metab. Clin. Exp. 2018, 83, 52–63. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, Z.; He, H.; Li, M.; Lan, Y.; Hui, J.; Bie, P.; Chen, Y.; Liu, H.; Fan, H.; et al. Lycium barbarum glycopeptide alleviates neuroinflammation in spinal cord injury via modulating docosahexaenoic acid to inhibiting MAPKs/NF-kB and pyroptosis pathways. J. Transl. Med. 2023, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yao, X.; Sun, B.; Jiang, W.; Liao, C.; Dai, X.; Chen, Y.; Chen, J.; Ding, R.; Liu, Z.; et al. Pretreatment with Kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Free. Radic. Biol. Med. 2021, 168, 142–154. [Google Scholar] [CrossRef]
- Hou, X.L.; Tong, Q.; Wang, W.Q.; Shi, C.Y.; Xiong, W.; Chen, J.; Liu, X.; Fang, J.G. Suppression of Inflammatory Responses by Dihydromyricetin, a Flavonoid from Ampelopsis grossedentata, via Inhibiting the Activation of NF-κB and MAPK Signaling Pathway. J. Nat. Prod. 2015, 78, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Maosheng, X.; Liang, W. Review on experimental study and clinical application of low-intensity pulsed ultrasound in inflammation. Quant. Imaging Med. Surg. 2021, 11, 443–462. [Google Scholar]
- Li, X.; Zhong, Y.; Zhou, W.; Song, Y.; Li, W.; Jin, Q.; Gao, T.; Zhang, L.; Xie, M. Low-intensity pulsed ultrasound (LIPUS) enhances the anti-inflammatory effects of bone marrow mesenchymal stem cells (BMSCs)-derived extracellular vesicles. Cell. Mol. Biol. Lett. 2023, 28, 9, Erratum in Cell. Mol. Biol. Lett. 2024, 29, 96. [Google Scholar] [CrossRef]
- Deng, Q.; Yao, X.; Fang, S.; Sun, Y.; Liu, L.; Li, C.; Li, G.; Guo, Y.; Liu, J. Mast cell-mediated microRNA functioning in immune regulation and disease pathophysi-ology. Clin. Exp. Med. 2025, 25, 38. [Google Scholar] [CrossRef]
- Di Silvestre, D.; Garavelli, S.; Procaccini, C.; Prattichizzo, F.; Passignani, G.; De Rosa, V.; Mauri, P.; Matarese, G.; De Candia, P. CD4+ T-Cell Activation Prompts Suppressive Function by Extracellular Vesicle-Associated MicroRNAs. Front. Cell Dev. Biol. 2021, 9, 753884. [Google Scholar] [CrossRef]
- Liao, B.; Guan, M.; Tan, Q.; Wang, G.; Zhang, R.; Huang, J.; Liu, M.; Chen, H.; Li, K.; Bai, D.; et al. Low-intensity pulsed ultrasound inhibits fibroblast-like synoviocyte proliferation and reduces synovial fibrosis by regulating Wnt/β-catenin signaling. J. Orthop. Transl. 2021, 30, 41–50. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Wang, Z.; Zhu, R.; Cheng, L.; Cheng, Q. Low-intensity pulsed ultrasound promotes mesenchymal stem cell transplantation-based articular cartilage regeneration via inhibiting the TNF signaling pathway. Stem Cell Res. Ther. 2023, 14, 93. [Google Scholar] [CrossRef]
- Tan, Y.; Guo, Y.; Reed-Maldonado, A.; Li, Z.; Lin, G.; Xia, S.; Lue, T. Low-intensity pulsed ultrasound stimulates proliferation of stem/progenitor cells: What we need to know to translate basic science research into clinical applications. Asian J. Androl. 2021, 23, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lv, M.; Lin, J.; Guo, J.; Lin, J.; Li, S.; Sun, Y.; Zhang, X. Latest Progress of LIPUS in Fracture Healing. J. Ultrasound Med. 2024, 43, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Maung, W.; Nakata, H.; Miura, M.; Miyasaka, M.; Kim, Y.; Kasugai, S.; Kuroda, S. Low intensity pulsed ultrasound stimulates osteogenic differentiation of periosteal cells in vitro. Tissue Eng. 2021, 27, 63–73. [Google Scholar] [CrossRef]
- Liang, W.; Liang, B.; Yan, K.; Zhang, G.; Zhuo, J.; Cai, Y. Low-Intensity Pulsed Ultrasound: A Physical Stimulus with Immunomodulatory and Anti-inflammatory Potential. Ann. Biomed. Eng. 2024, 52, 1955–1981. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Rodrigues, A.A., Jr.; Glover, L.E.; Voltarelli, J.; Borlongan, C.V. Peripheral Nerve Repair with Cultured Schwann Cells: Getting Closer to the Clinics. Sci. World J. 2012, 2012, 413091. [Google Scholar] [CrossRef]
- Mert, T.; Gunay, I.; Gocmen, C.; Kaya, M.; Polat, S. Regenerative effects of pulsed magnetic field on injured peripheral nerves. Altern. Ther. Health Med. 2006, 12, 42–49. [Google Scholar] [PubMed]
- Al-Majed, A.A.; Neumann, C.M.; Brushart, T.M.; Gordon, T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J. Neurosci. 2000, 20, 2602–2608. [Google Scholar] [CrossRef]
- Haastert-Talini, K.; Schmitte, R.; Korte, N.; Klode, D.; Ratzka, A.; Grothe, C. Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps. J. Neurotrauma 2011, 28, 661–674. [Google Scholar] [CrossRef]
- Huang, T.; Liu, L.; Qian, Z.; Tu, K.; Li, Y.; Xie, L. Using GeneReg to construct time delay gene regulatory networks. BMC Res. Notes 2010, 3, 142. [Google Scholar] [CrossRef]
- McLean, N.A.; Popescu, B.F.; Gordon, T.; Zochodne, D.W.; Verge, V.M. Delayed nerve stimulation promotes axon-protective neurofilament phosphorylation, accelerates immune cell clearance and enhances remyelination in vivo in focally demyelinated nerves. PLoS ONE 2014, 9, 110174. [Google Scholar] [CrossRef]
- Sharma, H.S. Selected combination of neurotrophins potentiate neuroprotection and functional recovery following spinal cord injury in the rat. Acta Neurochir. Suppl. 2010, 106, 295–300. [Google Scholar]
- Hei, W.H.; Byun, S.H.; Kim, J.S.; Kim, S.; Seo, Y.K.; Park, J.C.; Kim, S.M.; Jahng, J.W.; Lee, J.H. Effects of electromagnetic field (PEMF) exposure at different frequency and duration on the peripheral nerve regeneration: In vitro and in vivo study. Int. J. Neurosci. 2016, 126, 739–748. [Google Scholar]
- Hei, W.H.; Kim, S.; Park, J.C.; Seo, Y.K.; Kim, S.M.; Jahng, J.W.; Lee, J.H. Schwann-like cells differentiated from human dental pulp stem cells combined with a pulsed electromagnetic field can improve peripheral nerve regeneration. Bioelectromagnetics 2016, 37, 163–174. [Google Scholar] [CrossRef]
- Hu, H.; Yang, W.; Zeng, Q.; Chen, W.; Zhu, Y.; Liu, W.; Wang, S.; Wang, B.; Shao, Z.; Zhang, Y. Promising application of Pulsed Electromagnetic Fields (PEMFs) in musculoskeletal disorders. Biomed. Pharmacother. 2020, 131, 110767. [Google Scholar] [CrossRef]
- Flatscher, J.; Loriè, P.; Mittermayr, R.; Meznik, P.; Slezak, P.; Redl, H.; Slezak, C. Pulsed Electromagnetic Fields (PEMF)—Physiological Response and Its Potential in Trauma Treatment. Int. J. Mol. Sci. 2023, 24, 11239. [Google Scholar] [CrossRef]
- Sandberg, M.; Whitman, W.; Bissette, R.; Ross, C.; Tsivian, M.; Walker, S. Pulsed Electromagnetic Field Therapy Alters the Genomic Profile of Bladder Cancer Cell Line HT-1197. J. Pers. Med. 2025, 15, 143. [Google Scholar] [CrossRef]
- Bademoğlu, G.; Erdal, N.; Uzun, C.; Taşdelen, B. The effects of pulsed electromagnetic field on experimentally induced sciatic nerve injury in rats. Electromagn. Biol. Med. 2021, 40, 408–419. [Google Scholar] [CrossRef]
- Baptista, A.F.; Goes, B.T.; Menezes, D.; Gomes, F.C.; Zugaib, J.; Stipursky, J.; Gomes, J.R.; Oliveira, J.T.; Vannier-Santos, M.A.; Martinez, A.M. PEMF fails to enhance nerve regeneration after sciatic nerve crush lesion. J. Peripher. Nerv. Syst. 2009, 14, 285–293. [Google Scholar] [CrossRef]
- Ito, A.; Wang, T.; Nakahara, R.; Kawai, H.; Nishitani, K.; Aoyama, T.; Kuroki, H. Ultrasound therapy with optimal intensity facilitates peripheral nerve regeneration in rats through suppression of pro-inflammatory and nerve growth inhibitor gene expression. PLoS ONE 2020, 15, e0234691. [Google Scholar] [CrossRef]
- Wang, T.; Ito, A.; Xu, S.; Kawai, H.; Kuroki, H.; Aoyama, T. Low-Intensity Pulsed Ultrasound Prompts Both Functional and Histologic Improvements While Upregulating the Brain-Derived Neurotrophic Factor Expression after Sciatic Crush Injury in Rats. Ultrasound Med. Biol. 2021, 47, 1136–1140. [Google Scholar] [CrossRef]
- Peng, D.Y.; Reed-Maldonado, A.B.; Lin, G.T.; Xia, S.J.; Lue, T.F. Low-intensity pulsed ultrasound for regenerating peripheral nerves: Potential for penile nerve. Asian J. Androl. 2020, 22, 335–341. [Google Scholar] [CrossRef]
- Hirota, H.; Kiyama, H.; Kishimoto, T.; Taga, T. Accelerated nerve regeneration in mice by upregulated expression of interleukin (IL) 6 and IL-6 receptor after trauma. J. Exp. Med. 1996, 183, 2627–2634. [Google Scholar] [CrossRef]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef]
- Ni, X.J.; Wang, X.D.; Zhao, Y.H.; Sun, H.L.; Hu, Y.M.; Yao, J.; Wang, Y. The effect of low-intensity ultrasound on brain-derived neurotropic factor expression in a rat sciatic nerve crushed injury model. Ultrasound Med. Biol. 2017, 43, 461–468. [Google Scholar] [CrossRef]
- Yue, Y.; Yang, X.; Zhang, L.; Xiao, X.; Nabar, N.R.; Lin, Y.; Hao, L.; Zhang, D.; Huo, J.; Li, J.; et al. Low-intensity pulsed ultrasound upregulates pro-myelination indicators of Schwann cells enhanced by co-culture with adipose-derived stem cells. Cell Prolif. 2016, 49, 720–728. [Google Scholar] [CrossRef]
- Wen, J.; Deng, X.; Huang, C.; An, Z.; Liu, M. Low-Intensity Pulsed Ultrasound Enhanced Neurite Guidance Growth through Netrin-1/DCC Signal Pathway in Primary Cultured Cortical Neurons of Rats. ACS Chem. Neurosci. 2021, 12, 1931–1939. [Google Scholar] [CrossRef]
- Daeschler, S.C.; Harhaus, L.; Schoenle, P.; Boecker, A.; Kneser, U.; Bergmeister, K.D. Ultrasound and shock-wave stimulation to promote axonal regeneration following nerve surgery: A systematic review and meta-analysis of preclinical studies. Sci. Rep. 2018, 8, 3168. [Google Scholar] [CrossRef] [PubMed]
- Haffey, P.R.; Bansal, N.; Kaye, E.; Ottestad, E.; Aiyer, R.; Noori, S.; Gulati, A. The regenerative potential of therapeutic ultrasound on neural tissue: A pragmatic review. Pain Med. 2020, 21, 1494–1506. [Google Scholar] [CrossRef]
- Siwak, M.; Piotrzkowska, D.; Skrzypek, M.; Majsterek, I. Effects of PEMF and LIPUS Therapy on the Expression of Genes Related to Peripheral Nerve Regeneration in Schwann Cells. Int. J. Mol. Sci. 2024, 25, 12791. [Google Scholar] [CrossRef]
- Keyan, Z.; Zhang, L.; Xu, X.; Jing, J.; Xu, C. Pulsed Electromagnetic Fields Improved Peripheral Nerve Regeneration After Delayed Repair of One Month. Bioelectromagnetics 2023, 44, 133–143. [Google Scholar] [CrossRef]
- Ye, K.; Li, Z.; Yin, Y.; Zhou, J.; Li, D.; Gan, Y.; Peng, D.; Xiao, M.; Zhao, L.; Dai, Y.; et al. LIPUS-SCs-Exo promotes peripheral nerve regeneration in cavernous nerve crush injury-induced ED rats via PI3K/Akt/FoxO signaling pathway. CNS Neurosci. Ther. 2023, 29, 3239–3258. [Google Scholar] [CrossRef]
- Juckett, L.; Saffari, T.; Ormseth, B.; Senger, J.; Moore, A. The Effect of Electrical Stimulation on Nerve Regeneration Following Peripheral Nerve Injury. Biomolecules 2022, 12, 1856. [Google Scholar] [CrossRef]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef]

| Pulsed Electromagnetic Field (PEMF) | Low-Intensity Pulsed Ultrasound (LIPUS) | |
|---|---|---|
| Feature/Parameter | Pulsed Electromagnetic Field (PEMF) | Low-Intensity Pulsed Ultrasound (LIPUS) |
| Mechanism of action | Ion mobilization (Na+, Ca2+), biochemical cascades (cGMP, cAMP), gene activation, growth factor increase, inflammation modulation (adenosine A2A/A3A, JNK MAPK), osteogenesis | Mechanical phenomena (cavitation, microbubbles, microjets, acoustic streaming), activation of signaling pathways (proliferation, differentiation, migration), modulation of inflammation (TNF-α, IL-1β, IL-6), secretion of EVs and miRNAs |
| Main applications | Fractures, pain, inflammation, edema, osteoarthritis, intracerebral hemorrhage, intervertebral disk degeneration, cancer therapy, sepsis, angiogenesis | Fractures, bone diseases, joints, tendons, cartilage regeneration, ligaments, diabetic wounds, kidney function, heart, adipose tissue, neurological diseases, cell therapy |
| Recommended parameters | Trapezoidal/sawtooth signal, intensity 1.2–2 mT, frequency 15–75 Hz | Intensity 200–500 mW/cm2, daily/every other day application (1–10 min) |
| FDA approval | Approved for bone non-unions (1979) | Approved for fresh fractures and delayed unions (1994, 2000) |
| Therapy | Limitations | Side Effects | Contraindications |
|---|---|---|---|
| PEMF | Lack of long-term data, need for parameter optimization | No cytotoxicity/genotoxicity in in vitro studies | Electronic implants, pregnancy, cancers |
| LIPUS | Limited effectiveness in extensive damage, incomplete clinical translation | Potential DNA damage with excessive exposure (unconfirmed in vitro) | Pregnancy, bone growth plates, cancers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrzkowska, D.; Siwak, M.; Adamkiewicz, J.; Dziki, L.; Majsterek, I. The Therapeutic Potential of Pulsed Electromagnetic Fields (PEMF) and Low-Intensity Pulsed Ultrasound (LIPUS) in Peripheral Nerve Regeneration: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 9311. https://doi.org/10.3390/ijms26199311
Piotrzkowska D, Siwak M, Adamkiewicz J, Dziki L, Majsterek I. The Therapeutic Potential of Pulsed Electromagnetic Fields (PEMF) and Low-Intensity Pulsed Ultrasound (LIPUS) in Peripheral Nerve Regeneration: A Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(19):9311. https://doi.org/10.3390/ijms26199311
Chicago/Turabian StylePiotrzkowska, Danuta, Mateusz Siwak, Julia Adamkiewicz, Lukasz Dziki, and Ireneusz Majsterek. 2025. "The Therapeutic Potential of Pulsed Electromagnetic Fields (PEMF) and Low-Intensity Pulsed Ultrasound (LIPUS) in Peripheral Nerve Regeneration: A Comprehensive Review" International Journal of Molecular Sciences 26, no. 19: 9311. https://doi.org/10.3390/ijms26199311
APA StylePiotrzkowska, D., Siwak, M., Adamkiewicz, J., Dziki, L., & Majsterek, I. (2025). The Therapeutic Potential of Pulsed Electromagnetic Fields (PEMF) and Low-Intensity Pulsed Ultrasound (LIPUS) in Peripheral Nerve Regeneration: A Comprehensive Review. International Journal of Molecular Sciences, 26(19), 9311. https://doi.org/10.3390/ijms26199311






