Phlorizin Ameliorates Amyloid-β Toxicity and Enhances Fatty Acid β-Oxidation in Caenorhabditis elegans via NHR-49-Dependent Pathway
Abstract
1. Introduction
2. Result
2.1. PHZ Concentration-Dependently Increased C. elegans’ Resistance
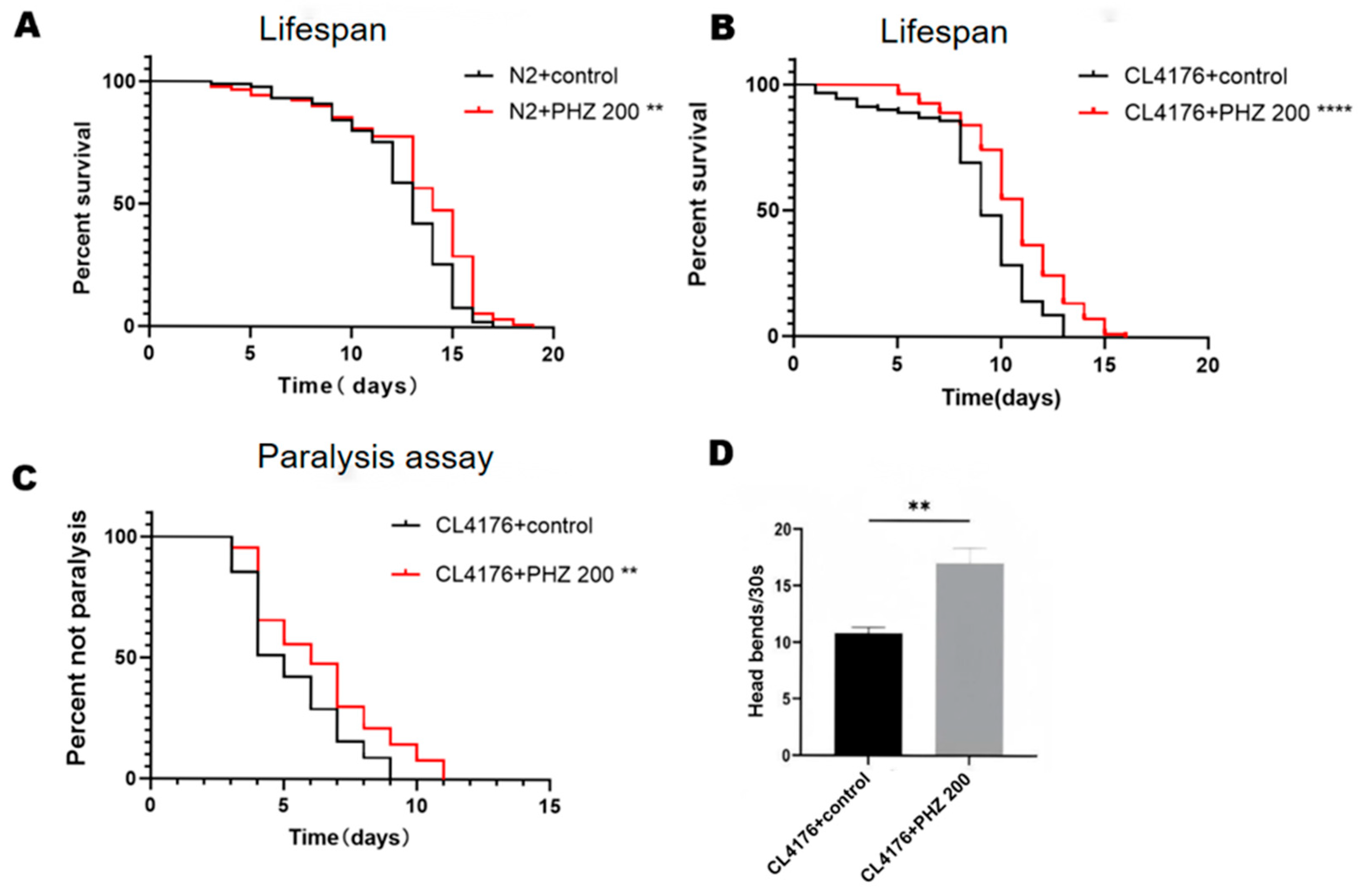
2.2. PHZ Extends the Lifespan of Wild-Type N2 and Transgenic C. elegans CL4176
2.3. PHZ Protects Against Aβ Toxicity
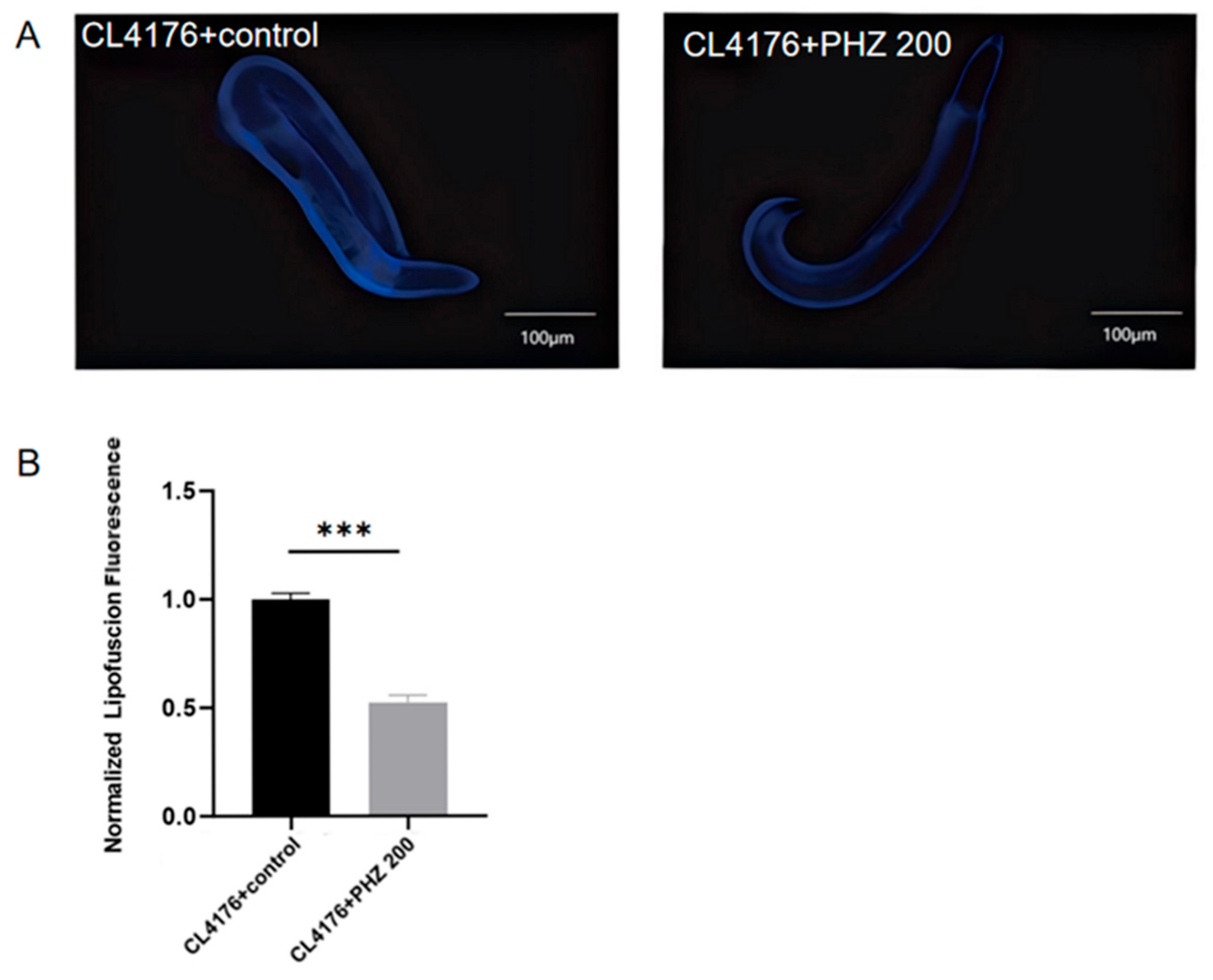
2.4. PHZ Reduced Lipofuscin Accumulation in CL4176
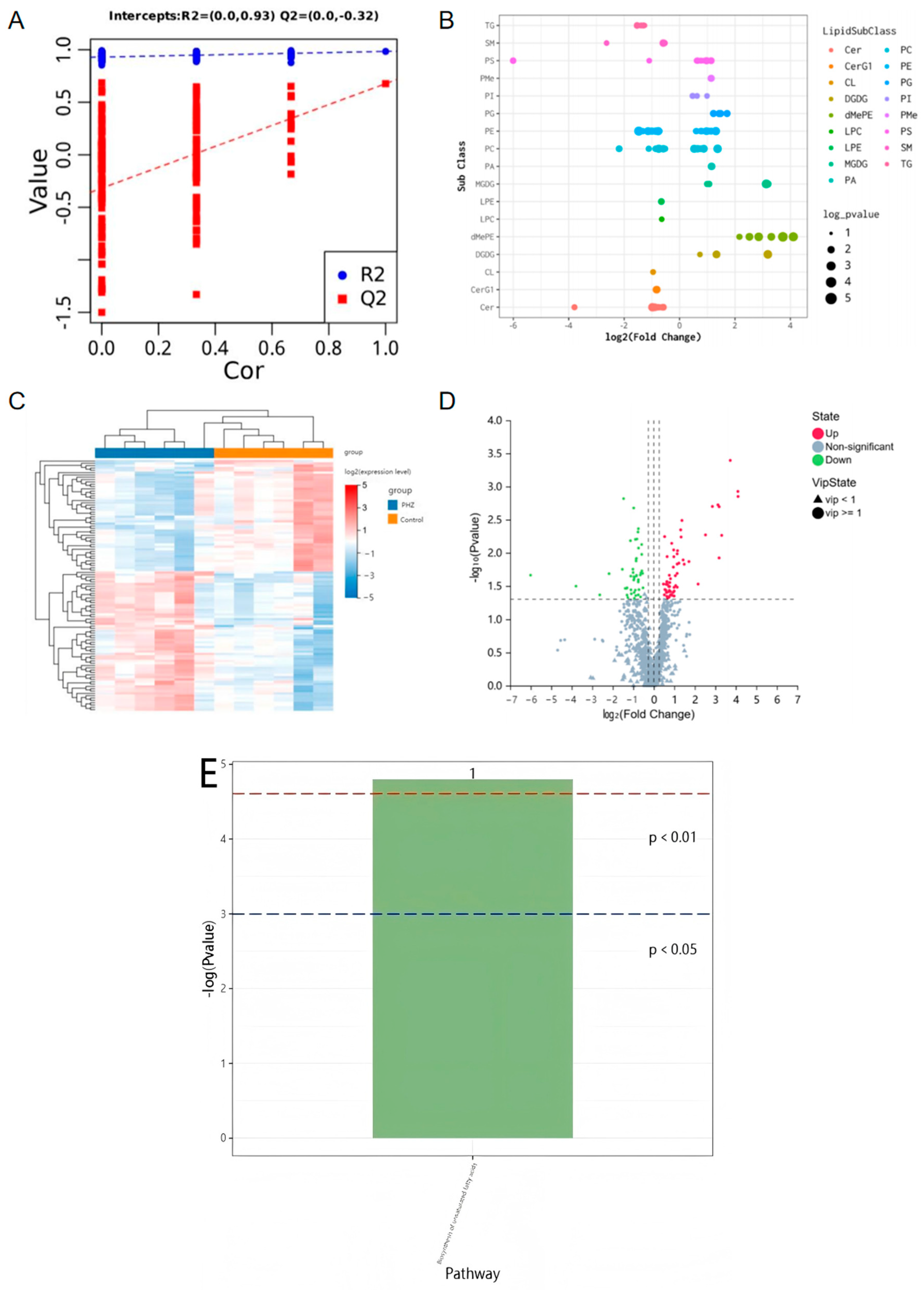
2.5. Lipidomics Reveals the Role of PHZ in Regulating Lipid Metabolism in CL4176
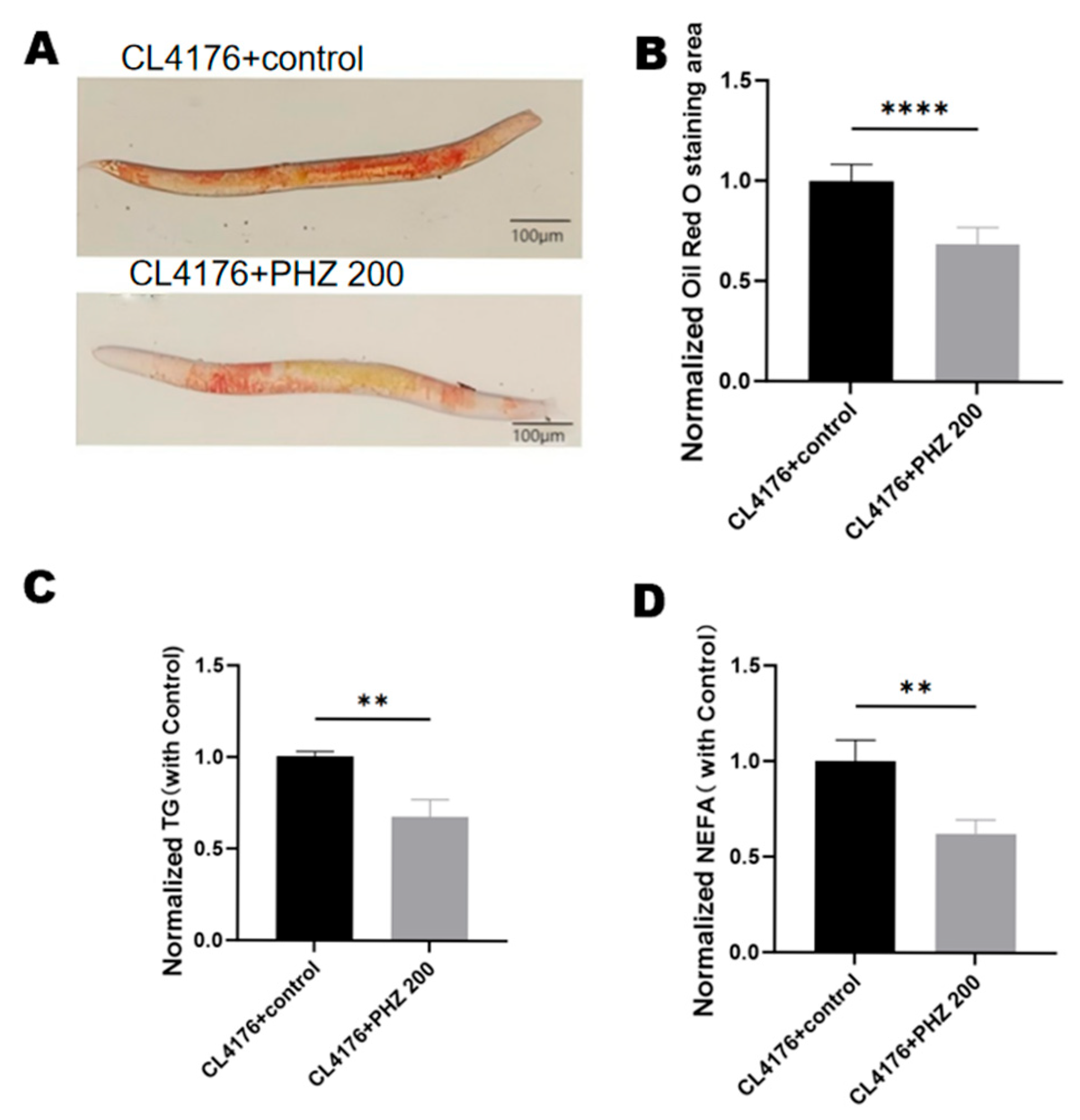
2.6. PHZ Reduces Lipid Accumulation in Transgenic C. elegans CL4176
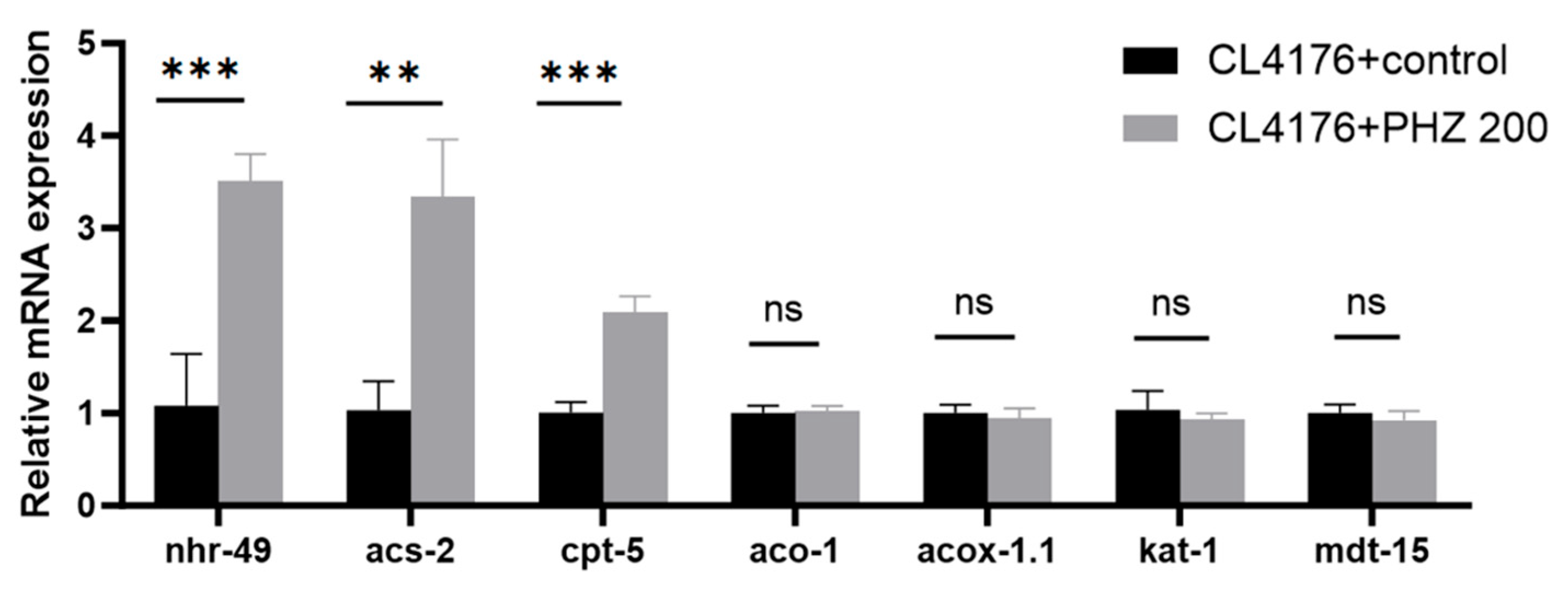
2.7. qRT-PCR Results Indicate That PHZ Protects AD Worms from Aβ Toxicity and Prolongs Lifespan by Enhencing Fatty Acids β-Oxidation
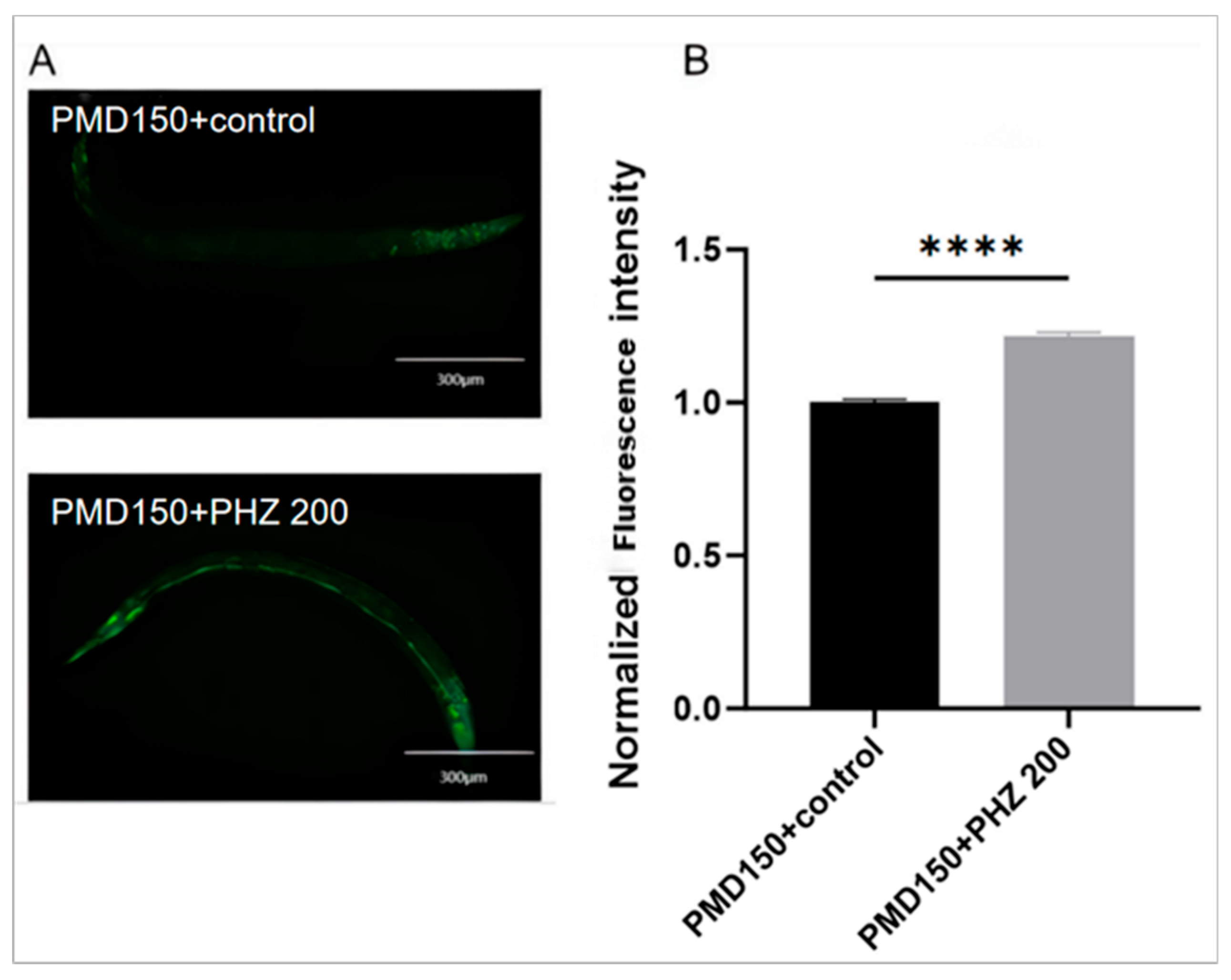
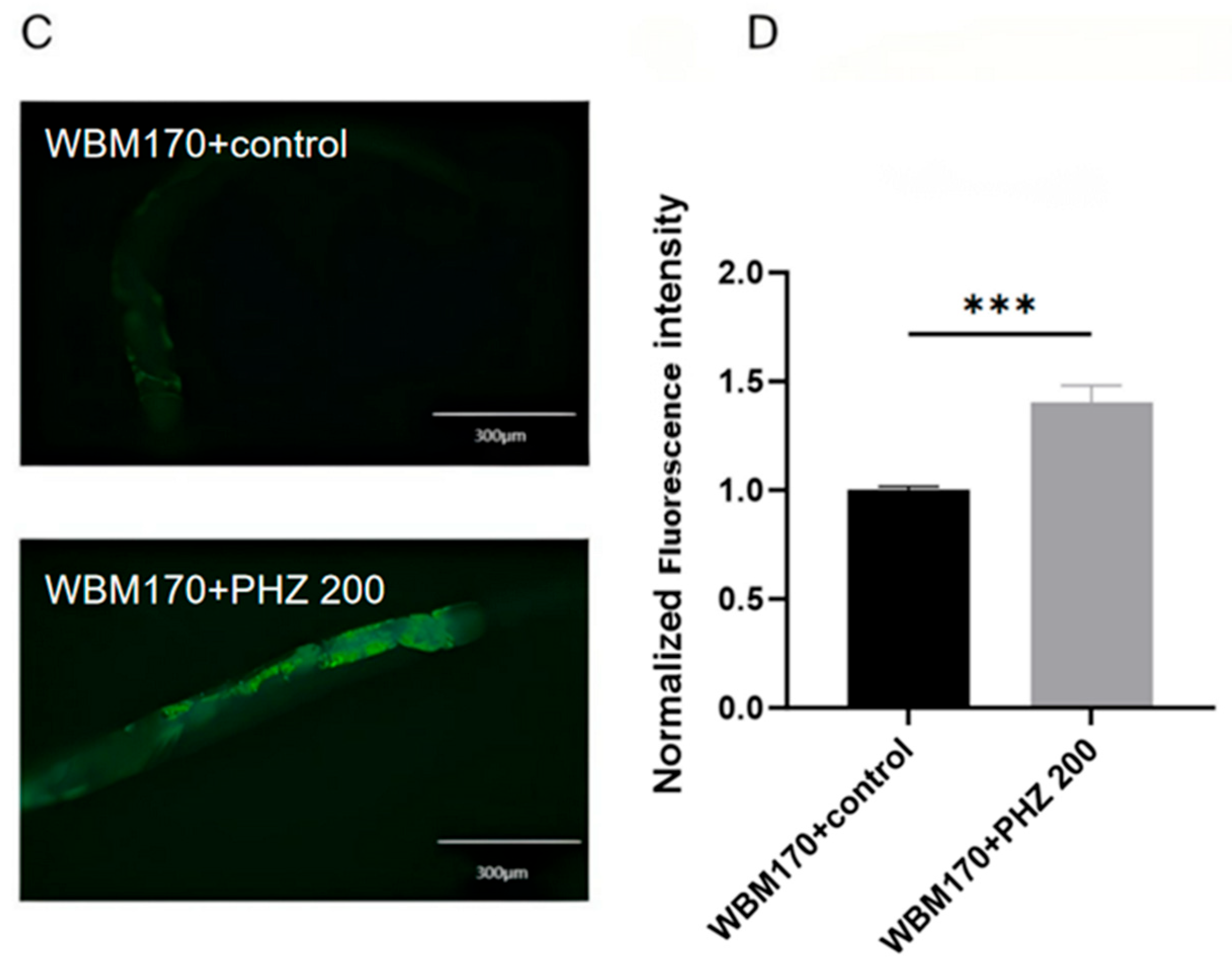
2.8. Molecular Docking Results Show That NHR-49 Binds to PHZ
2.9. NHR-49 Was Essential to PHZ Mediated Protection of Worms’ Lipid Metabolism
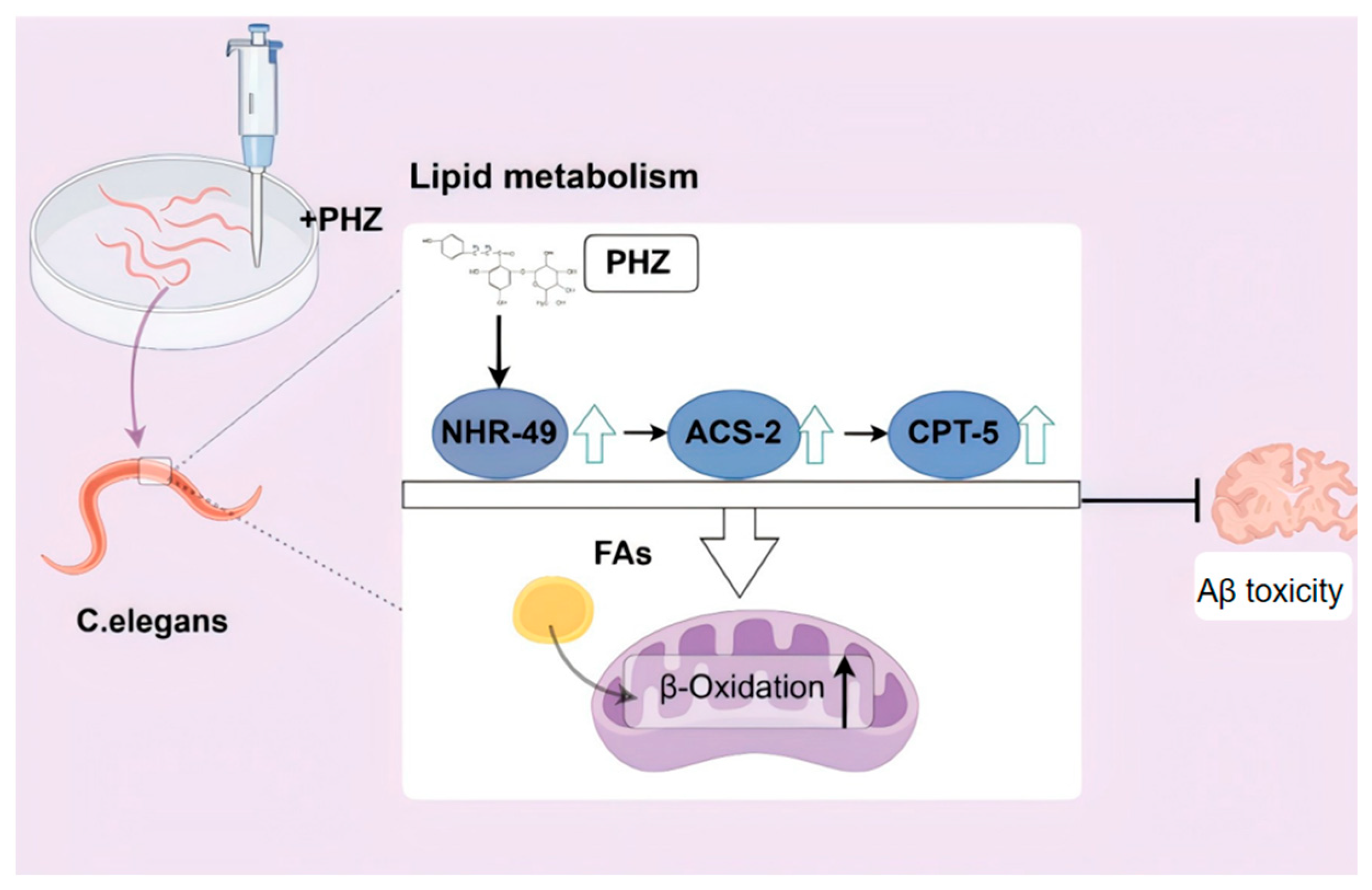
3. Discussion
4. Materials and Methods
4.1. Strains and Maintenance
4.2. Synchronization
4.3. Lifespan Assay
4.4. Paralysis Assay
4.5. Resistance Assay
4.6. Reactive Oxygen Species (ROS) Assay
4.7. Head-Bending Assay
4.8. Lipofuscin Assay
4.9. Triglyceride and NEFA Quantification
4.10. Oil Red O Assay
4.11. qRT-PCR Experiments
4.12. Fluorescence Detection Experiments
4.13. Lipidomics Assay
4.14. Molecular Docking
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wurtman, R. Biomarkers in the diagnosis and management of Alzheimer’s disease. Metab. Clin. Exp. 2015, 64 (Suppl. S1), S47–S50. [Google Scholar] [CrossRef]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef]
- Ooi, K.M.; Vacy, K.; Boon, W.C. Fatty acids and beyond: Age and Alzheimer’s disease related changes in lipids reveal the neuro-nutraceutical potential of lipids in cognition. Neurochem. Int. 2021, 149, 105143. [Google Scholar] [CrossRef]
- Tong, B.; Ba, Y.; Li, Z.; Yang, C.; Su, K.; Qi, H.; Zhang, D.; Liu, X.; Wu, Y.; Chen, Y.; et al. Targeting dysregulated lipid metabolism for the treatment of Alzheimer’s disease and Parkinson’s disease: Current advancements and future prospects. Neurobiol. Dis. 2024, 196, 106505. [Google Scholar] [CrossRef]
- Bogie, J.F.J.; Haidar, M.; Kooij, G.; Hendriks, J.J.A. Fatty acid metabolism in the progression and resolution of CNS disorders. Adv. Drug Deliv. Rev. 2020, 159, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, K.J.; Kajy, M.; Ward, K.M.; Burghardt, P.R. Metabolomics, Lipidomics, and Antipsychotics: A Systematic Review. Biomedicines 2023, 11, 3295. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Strachan, M.W.; Visseren, F.L.; Kappelle, L.J.; Whitmer, R.A. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: Towards targeted interventions. Lancet Diabetes Endocrinol. 2014, 2, 246–255. [Google Scholar] [CrossRef]
- Nilsson, M.I.; Tarnopolsky, M.A. Mitochondria and Aging-The Role of Exercise as a Countermeasure. Biology 2019, 8, 40. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, S.J.; Jung, U.J.; Ryu, R.; Choi, M.S. Phlorizin Supplementation Attenuates Obesity, Inflammation, and Hyperglycemia in Diet-Induced Obese Mice Fed a High-Fat Diet. Nutrients 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Akhila, P.V.; Suchiang, K. Hesperidin ameliorates Amyloid-β toxicity and enhances oxidative stress resistance and lifespan of Caenorhabditis elegans through acr-16 mediated activation of the autophagy pathway. Free Radic. Biol. Med. 2023, 209 Pt 2, 366–380. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A review. Diabetes/Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, J.; Yi, K.; Peng, L.; Xie, J.; Gou, X.; Peng, T.; Tang, L. Phlorizin ameliorates obesity-associated endotoxemia and insulin resistance in high-fat diet-fed mice by targeting the gut microbiota and intestinal barrier integrity. Gut Microbes 2020, 12, 1–18. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Liu, S.; Hu, C.; Meng, Y. Phlorizin ameliorates cognitive and behavioral impairments via the microbiota-gut-brain axis in high-fat and high-fructose diet-induced obese male mice. Brain Behav. Immun. 2025, 123, 193–210. [Google Scholar] [CrossRef]
- Wright, E.M. SGLT2 Inhibitors: Physiology and Pharmacology. Kidney360 2021, 2, 2027–2037. [Google Scholar] [CrossRef]
- Mei, X.; Li, Y.; Zhang, X.; Zhai, X.; Yang, Y.; Li, Z.; Li, L. Maternal Phlorizin Intake Protects Offspring from Maternal Obesity-Induced Metabolic Disorders in Mice via Targeting Gut Microbiota to Activate the SCFA-GPR43 Pathway. J. Agric. Food Chem. 2024, 72, 4703–4725. [Google Scholar] [CrossRef] [PubMed]
- Kamdi, S.P.; Raval, A.; Nakhate, K.T. Phloridzin attenuates lipopolysaccharide-induced cognitive impairment via antioxidant, anti-inflammatory and neuromodulatory activities. Cytokine 2021, 139, 155408. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Wang, C.; Huang, H.; Wei, D.; Fu, L.; Liu, G.; Zhou, Q.; Yang, J.; Fu, Y. Phlorizin mitigates high glucose-induced metabolic disorders through the IIS pathway in Caenorhabditis elegans. Food Funct. 2025, 16, 3004–3017. [Google Scholar] [CrossRef]
- Zhao, H.; Zhai, B.W.; Zhang, M.Y.; Huang, H.; Zhu, H.L.; Yang, H.; Ni, H.Y.; Fu, Y.J. Phlorizin from Lithocarpus litseifolius [Hance] Chun ameliorates FFA-induced insulin resistance by regulating AMPK/PI3K/AKT signaling pathway. Phytomed. Int. J. Phytother. Phytopharm. 2024, 130, 155743. [Google Scholar] [CrossRef]
- Kumsta, C.; Chang, J.T.; Schmalz, J.; Hansen, M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 2017, 8, 14337. [Google Scholar] [CrossRef]
- Wan, Q.L.; Meng, X.; Dai, W.; Luo, Z.; Wang, C.; Fu, X.; Yang, J.; Ye, Q.; Zhou, Q. N(6)-methyldeoxyadenine and histone methylation mediate transgenerational survival advantages induced by hormetic heat stress. Sci. Adv. 2021, 7, eabc3026. [Google Scholar] [CrossRef] [PubMed]
- Viña, J. The free radical theory of frailty: Mechanisms and opportunities for interventions to promote successful aging. Free Radic. Biol. Med. 2019, 134, 690–694. [Google Scholar] [CrossRef]
- Salazar, N.; González, S.; Nogacka, A.M.; Rios-Covián, D.; Arboleya, S.; Gueimonde, M.; Reyes-Gavilán, C.G.L. Microbiome: Effects of Ageing and Diet. Curr. Issues Mol. Biol. 2020, 36, 33–62. [Google Scholar] [CrossRef] [PubMed]
- Link, P.; Roth, K.; Sporer, F.; Wink, M. Carlina acaulis Exhibits Antioxidant Activity and Counteracts Aβ Toxicity in Caenorhabditis elegans. Molecules 2016, 21, 871. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, Y.; Zhang, Z.; Yi, M.; Zhu, L.; Peng, W. The interaction between ageing and Alzheimer’s disease: Insights from the hallmarks of ageing. Transl. Neurodegener. 2024, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.D.; Chen, X.; Yu, L.; Hu, M.L.; Pan, Y.R.; Qin, D.L.; Wu, J.M.; Li, L.; Law, B.Y.; Wong, V.K.; et al. Targeting autophagy to discover the Piper wallichii petroleum ether fraction exhibiting antiaging and anti-Alzheimer’s disease effects in Caenorhabditis elegans. Phytomed. Int. J. Phytother. Phytopharm. 2023, 117, 154916. [Google Scholar] [CrossRef]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef] [PubMed]
- Watterson, A.; Tatge, L.; Wajahat, N.; Arneaud, S.L.B.; Solano Fonseca, R.; Beheshti, S.T.; Metang, P.; Mihelakis, M.; Zuurbier, K.R.; Corley, C.D.; et al. Intracellular lipid surveillance by small G protein geranylgeranylation. Nature 2022, 605, 736–740. [Google Scholar] [CrossRef]
- Luo, R.; Su, L.Y.; Li, G.; Yang, J.; Liu, Q.; Yang, L.X.; Zhang, D.F.; Zhou, H.; Xu, M.; Fan, Y.; et al. Activation of PPARA-mediated autophagy reduces Alzheimer disease-like pathology and cognitive decline in a murine model. Autophagy 2020, 16, 52–69. [Google Scholar] [CrossRef]
- Wang, Y.X. PPARs: Diverse regulators in energy metabolism and metabolic diseases. Cell Res. 2010, 20, 124–137. [Google Scholar] [CrossRef]
- Paul, D.; Chipurupalli, S.; Justin, A.; Raja, K.; Mohankumar, S.K. Caenorhabditis elegans as a possible model to screen anti-Alzheimer’s therapeutics. J. Pharmacol. Toxicol. Methods 2020, 106, 106932. [Google Scholar] [CrossRef]
- Ferré-González, L.; Lloret, A.; Cháfer-Pericás, C. Systematic review of brain and blood lipidomics in Alzheimer’s disease mouse models. Prog. Lipid Res. 2023, 90, 101223. [Google Scholar] [CrossRef]
- Javed, H.; Vaibhav, K.; Ahmed, M.E.; Khan, A.; Tabassum, R.; Islam, F.; Safhi, M.M.; Islam, F. Effect of hesperidin on neurobehavioral, neuroinflammation, oxidative stress and lipid alteration in intracerebroventricular streptozotocin induced cognitive impairment in mice. J. Neurol. Sci. 2015, 348, 51–59. [Google Scholar] [CrossRef]
- Shen, T.; Wang, H.; Tang, B.; Zhu, G.; Wang, X. The impact of RNA binding proteins and the associated long non-coding RNAs in the TCA cycle on cancer pathogenesis. RNA Biol. 2023, 20, 223–234. [Google Scholar] [CrossRef]
- Berry, T.; Abohamza, E.; Moustafa, A.A. A disease-modifying treatment for Alzheimer’s disease: Focus on the trans-sulfuration pathway. Rev. Neurosci. 2020, 31, 319–334. [Google Scholar] [CrossRef]
- Xue, Q.; Kang, R.; Klionsky, D.J.; Tang, D.; Liu, J.; Chen, X. Copper metabolism in cell death and autophagy. Autophagy 2023, 19, 2175–2195. [Google Scholar] [CrossRef]
- Jiang, Y.; Huo, Z.; Qi, X.; Zuo, T.; Wu, Z. Copper-induced tumor cell death mechanisms and antitumor theragnostic applications of copper complexes. Nanomedicine 2022, 17, 303–324. [Google Scholar] [CrossRef]
- Lee, D.; Son, H.G.; Jung, Y.; Lee, S.V. The role of dietary carbohydrates in organismal aging. Cell. Mol. Life Sci. CMLS 2017, 74, 1793–1803. [Google Scholar] [CrossRef]
- Iglesias, J.; Morales, L.; Barreto, G.E. Metabolic and Inflammatory Adaptation of Reactive Astrocytes: Role of PPARs. Mol. Neurobiol. 2017, 54, 2518–2538. [Google Scholar] [CrossRef] [PubMed]
- Morant-Ferrando, B.; Jimenez-Blasco, D.; Alonso-Batan, P.; Agulla, J.; Lapresa, R.; Garcia-Rodriguez, D.; Yunta-Sanchez, S.; Lopez-Fabuel, I.; Fernandez, E.; Carmeliet, P.; et al. Fatty acid oxidation organizes mitochondrial supercomplexes to sustain astrocytic ROS and cognition. Nat. Metab. 2023, 5, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Comerota, M.M.; Gedam, M.; Xiong, W.; Jin, F.; Deng, L.; Wang, M.C.; Wang, J.; Zheng, H. Oleoylethanolamide facilitates PPARα and TFEB signaling and attenuates Aβ pathology in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, S.; Strosznajder, A.K.; Jeżyna, M.; Strosznajder, J.B. The Novel Role of PPAR Alpha in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochem. Res. 2020, 45, 972–988. [Google Scholar] [CrossRef]
- Kummer, M.P.; Schwarzenberger, R.; Sayah-Jeanne, S.; Dubernet, M.; Walczak, R.; Hum, D.W.; Schwartz, S.; Axt, D.; Heneka, M.T. Pan-PPAR modulation effectively protects APP/PS1 mice from amyloid deposition and cognitive deficits. Mol. Neurobiol. 2015, 51, 661–671. [Google Scholar] [CrossRef]
- Corbett, G.T.; Gonzalez, F.J.; Pahan, K. Activation of peroxisome proliferator-activated receptor α stimulates ADAM10-mediated proteolysis of APP. Proc. Natl. Acad. Sci. USA 2015, 112, 8445–8450. [Google Scholar] [CrossRef]
- Chandra, S.; Roy, A.; Jana, M.; Pahan, K. Cinnamic acid activates PPARα to stimulate Lysosomal biogenesis and lower Amyloid plaque pathology in an Alzheimer’s disease mouse model. Neurobiol. Dis. 2019, 124, 379–395. [Google Scholar] [CrossRef]
- Roy, A.; Kundu, M.; Jana, M.; Mishra, R.K.; Yung, Y.; Luan, C.H.; Gonzalez, F.J.; Pahan, K. Identification and characterization of PPARα ligands in the hippocampus. Nat. Chem. Biol. 2016, 12, 1075–1083. [Google Scholar] [CrossRef]
- Raha, S.; Ghosh, A.; Dutta, D.; Patel, D.R.; Pahan, K. Activation of PPARα enhances astroglial uptake and degradation of β-amyloid. Sci. Signal 2021, 14, eabg4747. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Peng, Q.; Su, L.; Yu, X.; Ma, C.W.; Liang, M.; Yin, X.; Zou, Y.; Huang, Z. Novel Bioactive Peptides from Meretrix meretrix Protect Caenorhabditis elegans against Free Radical-Induced Oxidative Stress through the Stress Response Factor DAF-16/FOXO. Mar. Drugs 2018, 16, 444. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Lin, D.; Guo, W.; Xu, Z.; Wang, L.; Guan, S. Ginsenoside Prolongs the Lifespan of C. elegans via Lipid Metabolism and Activating the Stress Response Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 9668. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, X.; Zhang, C.; Li, J.; Zhao, Y.; Zhu, Y.; Zhang, J.; Zhou, X. Toxicity and multigenerational effects of bisphenol S exposure to Caenorhabditis elegans on developmental, biochemical, reproductive and oxidative stress. Toxicol. Res. 2019, 8, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Schulz, T.J.; Zarse, K.; Voigt, A.; Urban, N.; Birringer, M.; Ristow, M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007, 6, 280–293. [Google Scholar] [CrossRef] [PubMed]
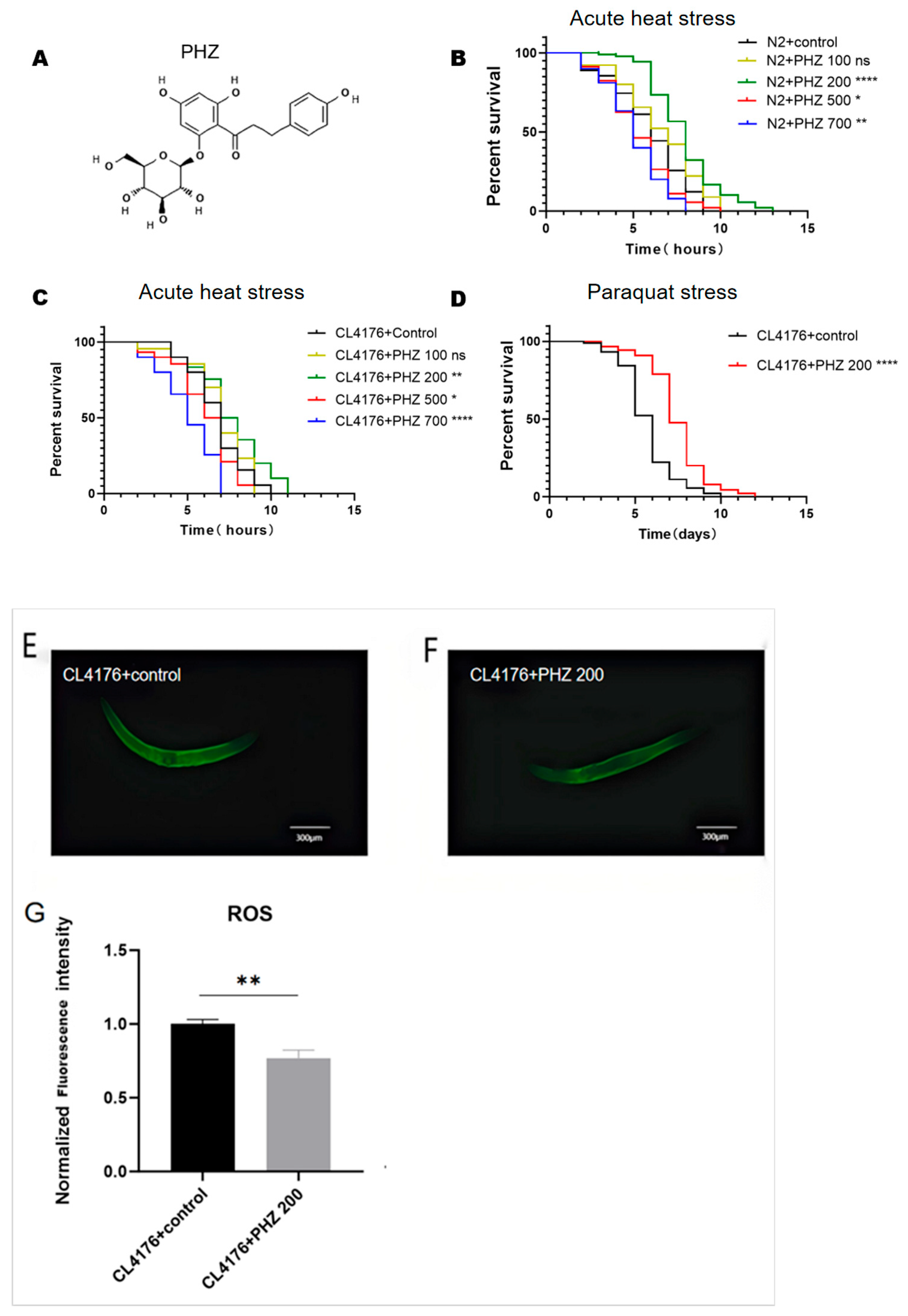

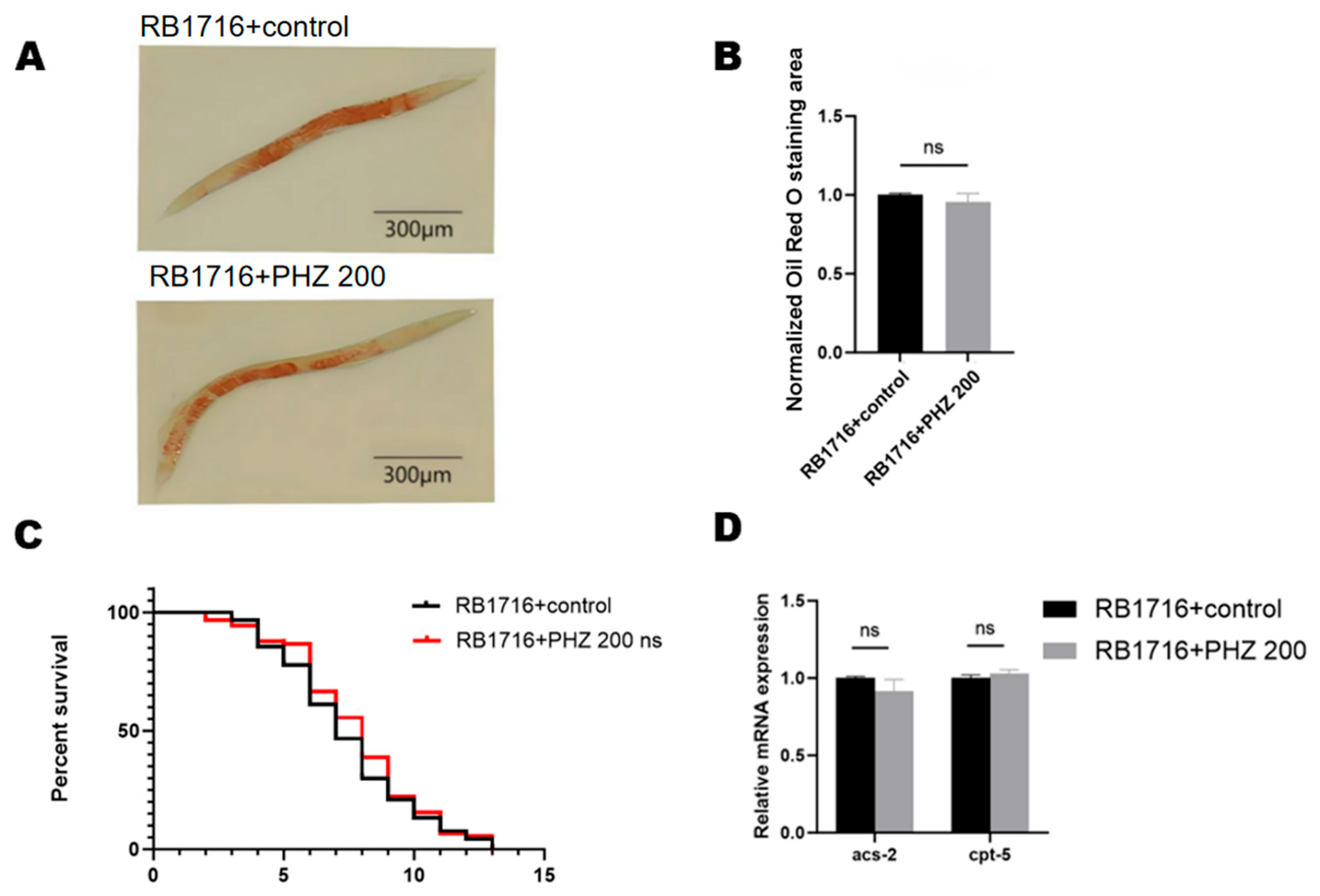
| C. elegans Model | Treatment | Mean | ±SD | % | p Value (Log-Rank Significance) |
|---|---|---|---|---|---|
| N2 | 0 µM | 5.922 | 0.81 | ||
| 100 µM | 6.633 | 0.24 | 12.01 | ns | |
| 200 µM | 7.878 | 0.93 | 33.03 | p < 0.0001 | |
| 500 µM | 5.333 | 0.38 | −9.95 | p < 0.05 | |
| 700 µM | 5.022 | 0.56 | −15.20 | p < 0.01 | |
| CL4176 | 0 µM | 7.8 | 0.82 | ||
| 100 µM | 8.056 | 0.57 | 3.28 | ns | |
| 200 µM | 8.756 | 1.32 | 12.26 | p < 0.01 | |
| 500 µM | 7.122 | 0.52 | −8.69 | p < 0.05 | |
| 700 µM | 6.122 | 1.04 | −21.51 | p < 0.0001 |
| C. elegans Model | Treatment | Mean | ±SD | % | p Value (Log-Rank Significance) |
|---|---|---|---|---|---|
| N2 (lifespan) | 0 µM | 9.722 | 0.79 | ||
| 200 µM | 11.522 | 1.32 | 18.51 | p < 0.01 | |
| CL4176 (lifespan) | 0 µM | 7.288 | 1.08 | ||
| 200 µM | 10.122 | 0.71 | 38.89 | p < 0.0001 | |
| CL4176 (paralysis) | 0 µM | 4.322 | 0.28 | ||
| 200 µM | 5.377 | 0.91 | 24.41 | p < 0.01 | |
| CL4176 (paraquat) | 0 µM | 5.7 | 1.03 | ||
| 200 µM | 6.433 | 0.89 | 12.86 | p < 0.0001 |
| C. elegans | Relevant Genetic Background | Characteristics |
|---|---|---|
| N2 | Wild type | Applied in aging, basic biology with high fecundity |
| CL4176 | dvIs27 [myo-3p::A-Beta (1-42)::let-8513’UTR) + rol-6 (su1006)] | Applied in Alzheimer’s disease mechanisms and drug screening, temperature-induced paralysis |
| PMD150 | utsIs4 [nhr-49p::nhr-49::GFP + myo-2p::mCherry] | NHR-49::GFP; green fluorescent protein-tagged NHR-49 |
| WBM170 | wbmEx57 [acs-2p::GFP + rol-6 (su1006)]. Pick Rollers to maintain. | ACS-2::GFP; green fluorescent protein-tagged ACS-2 |
| RB1716 | nhr-49 (ok2165) loss-of-function mutation | Increased lipid deposition, β-oxidation defects |
| Gene | Seqence Forward (5′–3′) | Seqence Reverse (5′–3′) |
|---|---|---|
| nhr-49 | CAGATGACGCACCCACAAGATATG | GAATGAACTCGGAGAGCAGAGAATC |
| acs-2 | GCAGCCTCGCTCTACACTCT | GACTCCTGCAAATGCACATGC |
| cpt-5 | GCTCGGCGTGCTCCATATCATC | TCAAGATTCGGCAACGGAAGACG |
| aco-1 | GATGGAAGTGGTGTTCTTGGATGG | AGTCGGTACTGGTAACGGTATCAC |
| acox-1.1 | TTCAACAACTACCGTATCCCAAGAAC | AATCGCCTGTCCAGTAAGCATATAAC |
| kat-1 | CCGCCACGCACCCACTC | CATTAACTTCCCATTGAGCAACATCTG |
| mdt-15 | CTCCAGATCCACAACCAACATCAG | GCGGCAACAGCAGCAGTG |
| β-actin | GCCGGAGACGACGCTCCACGCG | GCCTCGTCTCCGACGTACGAGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Fu, Y.; Li, X.; Zhang, Y.; Li, L.; Yi, T.; Jiang, H.; Lu, Y. Phlorizin Ameliorates Amyloid-β Toxicity and Enhances Fatty Acid β-Oxidation in Caenorhabditis elegans via NHR-49-Dependent Pathway. Int. J. Mol. Sci. 2025, 26, 9303. https://doi.org/10.3390/ijms26199303
Zhang X, Fu Y, Li X, Zhang Y, Li L, Yi T, Jiang H, Lu Y. Phlorizin Ameliorates Amyloid-β Toxicity and Enhances Fatty Acid β-Oxidation in Caenorhabditis elegans via NHR-49-Dependent Pathway. International Journal of Molecular Sciences. 2025; 26(19):9303. https://doi.org/10.3390/ijms26199303
Chicago/Turabian StyleZhang, Xuya, Yan Fu, Xue Li, Yali Zhang, Lingling Li, Tianxing Yi, Hong Jiang, and Yi Lu. 2025. "Phlorizin Ameliorates Amyloid-β Toxicity and Enhances Fatty Acid β-Oxidation in Caenorhabditis elegans via NHR-49-Dependent Pathway" International Journal of Molecular Sciences 26, no. 19: 9303. https://doi.org/10.3390/ijms26199303
APA StyleZhang, X., Fu, Y., Li, X., Zhang, Y., Li, L., Yi, T., Jiang, H., & Lu, Y. (2025). Phlorizin Ameliorates Amyloid-β Toxicity and Enhances Fatty Acid β-Oxidation in Caenorhabditis elegans via NHR-49-Dependent Pathway. International Journal of Molecular Sciences, 26(19), 9303. https://doi.org/10.3390/ijms26199303





