Evaluation of Inflammatory Markers in Perception Disorders in Major Psychiatric Pathology
Abstract
1. Introduction
- Leukocyte formula: increased levels of total leukocytes, granulocytes, and monocytes have been identified in patients experiencing their first psychotic episode, compared to healthy individuals [19]. According to another study, these markers decrease significantly after 4 weeks of antipsychotic treatment. This suggests a reduction in systemic inflammation as clinical status improves [30].
- Neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR): Research comparing acute psychotic disorder and substance-induced psychosis has indicated higher levels of NLL and MLL in both groups compared to control groups, suggesting that these ratios may be potential markers of inflammatory processes in acute psychosis [31].
- Platelet–lymphocyte ratio (PLR): Increased PLR has been associated with treatment resistance in schizophrenia, making it a potential marker for treatment response [32].
- Erythrocyte sedimentation rate (ESR): This was found to be elevated in patients experiencing their first psychotic episode. ESR levels remained unchanged in a significant proportion of patients after 4 weeks of treatment with antipsychotics. Non-responders had higher ESR levels compared to those who responded to treatment. Therefore, persistently elevated ESR may indicate a poor response to treatment [30].
- Systemic inflammation index (SII): This is a marker that reflects immune response and inflammation, defined as follows: SII = Platelets x Neutrophils/Lymphocytes. The increased SII value in patients with schizophrenia compared to healthy individuals suggests its potential as a diagnostic marker for identifying inflammatory changes during psychotic episodes [33].
2. Results
Demographic and Clinical Features
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howes, O.; McCutcheon, R.; Stone, J. Glutamate and dopamine in schizophrenia: An update for the 21st century. J. Psychopharmacol. 2015, 29, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Weidinger, E.; Leitner, B.; Schwarz, M.J. The role of inflammation in schizophrenia. Front. Neurosci. 2015, 9, 372. [Google Scholar] [CrossRef]
- Benros, M.E.; Nielsen, P.R.; Nordentoft, M.; Eaton, W.W.; Dalton, S.O.; Mortensen, P.B. Autoimmune diseases and severe infections as risk factors for schizophrenia: A 30-year population-based register study. Am. J. Psychiatry 2011, 168, 1303–1310. [Google Scholar] [CrossRef]
- Rømer, T.B.; Jeppesen, R.; Christensen, R.H.B.; Benros, M.E. Biomarkers in the cerebrospinal fluid of patients with psychotic disorders compared to healthy controls: A systematic review and meta-analysis. Mol. Psychiatry 2023, 28, 2277–2290. [Google Scholar] [CrossRef]
- Clausen, M.; Christensen, R.H.B.; da Re, M.; Benros, M.E. Immune cell alterations in psychotic disorders: A Comprehensive systematic review and meta-analysis. Biol. Psychiatry 2024, 96, 331–341. [Google Scholar] [CrossRef]
- Beatriz González-Castro, T.; Alfonso Tovilla-Zárate, C.; Esther Juárez-Rojop, I.; Hernández-Díaz, Y.; Lilia López-Narváez, M.; Felicita Ortiz-Ojeda, R. The association of cytokines genes (IL-6 and IL-10) with the susceptibility to schizophrenia: A systematic review and meta-analysis. Brain Res. 2024, 1822, 148667. [Google Scholar] [CrossRef]
- Suvisaari, J.; Mantere, O. Inflammation theories in psychotic disorders: A critical review. Infect. Disord.—Drug Targets 2013, 13, 59–70. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W.; Chen, F.; Hu, S.; Jiang, H. Maternal infection exposure and the risk of psychosis in the offspring: A systematic review and meta-analysis. J. Psychiatry Res. 2021, 135, 28–36. [Google Scholar] [CrossRef]
- Chaves, C.; Dursun, S.M.; Tusconi, M.; Hallak, J.E.C. Neuroinflammation and schizophrenia—Is there a link? Front. Psychiatry 2024, 15, 1356975. [Google Scholar] [CrossRef]
- Müller, N. Inflammation in schizophrenia: Pathogenetic aspects and therapeutic considerations. Schizophr. Bull. 2018, 44, 973–982. [Google Scholar] [CrossRef]
- Kose, M.; Pariante, C.M.; Dazzan, P.; Mondelli, V. The role of peripheral inflammation in clinical outcome and brain imaging abnormalities in psychosis: A systematic review. Front. Psychiatry 2021, 12, 612471. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Guo, M.; Guo, C.; He, K. The Interrelationships between Cytokines and Schizophrenia: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 8477. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A. Neuroinflammation in schizophrenia: The key role of the wnt/β-catenin pathway. Int. J. Mol. Sci. 2022, 23, 2810. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Mun, J.; Kimmel, H.L.; Nye, J.A.; Drake, D.F.; Hernandez, C.R.; Freeman, A.A.; Rye, D.B.; Goodman, M.M.; Howell, L.L.; et al. Chronic interferon-α decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology 2013, 38, 2179–2187. [Google Scholar] [CrossRef]
- Steiner, J. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an. JAMA Psychiatry 2013, 70, 271–278. [Google Scholar] [CrossRef]
- van Kesteren, C.F.M.G.; Gremmels, H.; de Witte, L.D.; Hol, E.M.; Van Gool, A.R.; Falkai, P.G.; Kahn, R.S.; Sommer, I.E.C. Immune involvement in the pathogenesis of schizophrenia: A meta-analysis on postmortem brain studies. Transl. Psychiatry 2017, 7, e1075. [Google Scholar] [CrossRef]
- Fond, G.; Lançon, C.; Korchia, T.; Auquier, P.; Boyer, L. The role of inflammation in the treatment of schizophrenia. Front. Psychiatry 2020, 11, 160. [Google Scholar] [CrossRef]
- Frydecka, D.; Krzystek-Korpacka, M.; Lubeiro, A.; Stramecki, F.; Stańczykiewicz, B.; Beszłej, J.A.; Piotrowski, P.; Kotowicz, K.; Szewczuk-Bogusławska, M.; Pawlak-Adamska, E.; et al. Profiling inflammatory signatures of schizophrenia: A cross-sectional and meta-analysis study. Brain Behav. Immun. 2018, 71, 28–36. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef]
- Jacomb, I.; Stanton, C.; Vasudevan, R.; Powell, H.; O’Donnell, M.; Lenroot, R.; Bruggemann, J.; Balzan, R.; Galletly, C.; Liu, D.; et al. C-Reactive protein: Higher during acute psychotic episodes and related to cortical thickness in schizophrenia and healthy controls. Front. Immunol. 2018, 9, 2230. [Google Scholar] [CrossRef]
- Maes, M.; Delange, J.; Ranjan, R.; Meltzer, H.Y.; Desnyder, R.; Cooremans, W.; Scharpé, S. Acute phase proteins in schizophrenia, mania and major depression: Modulation by psychotropic drugs. Psychiatry Res. 1997, 66, 1–11. [Google Scholar] [CrossRef]
- Juchnowicz, D.; Dzikowski, M.; Rog, J.; Waszkiewicz, N.; Karakuła, K.H.; Zalewska, A.; Maciejczyk, M.; Karakula-Juchnowicz, H. The usefulness of a complete blood count in the prediction of the first episode of schizophrenia diagnosis and its relationship with oxidative stress. PLoS ONE 2023, 18, e0292756. [Google Scholar] [CrossRef]
- Cavaleri, D.; De Pietra, A.; Gazzola, M.; Crocamo, C.; Bartoli, F.; Carrà, G. Complete blood count-based inflammation indexes and symptom severity in people with schizophrenia spectrum disorders: An analysis based on structural equation modeling. Psychoneuroendocrinology 2024, 168, 107134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhou, X.; Chen, K.; Fu, Z.; Zhang, P.; Zhu, Q. Neutrophil/lymphocyte ratio is increased in the acute phase of schizophrenia and regardless of the use and types of antipsychotic drugs. BMC Psychiatry 2024, 24, 876. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Tomioka, H.; Mera, K.; Tazaki, T.; Nishiyama, H.; Yamada, H.; Sanada, K.; Inamoto, A.; Iwanami, A. Neutrophil-Lymphocyte Ratio in Patients With Acute Schizophrenia. Cureus 2024, 16, e52181. [Google Scholar] [CrossRef] [PubMed]
- Bioque, M.; Llorca-Bofí, V.; Salmerón, S.; García-Bueno, B.; MacDowell, K.S.; Moreno, C.; Sáiz, P.A.; González-Pinto, A.; Hidalgo-Figueroa, M.; Barcones, M.F.; et al. Association between neutrophil to lymphocyte ratio and inflammatory biomarkers in patients with a first episode of psychosis. J. Psychiatry Res. 2024, 172, 334–339. [Google Scholar] [CrossRef]
- Özdin, S.; Böke, Ö. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in different stages of schizophrenia. Psychiatry Res. 2019, 271, 131–135. [Google Scholar] [CrossRef]
- Inaltekin, A.; Yağci, İ. Evaluation of Simple Markers of Inflammation and Systemic Immune Inflammation Index in Schizophrenia, Bipolar Disorder Patients and Healthy Controls. Şizofreni Hastalarında, Bipolar ve Sağlıklı Kontrol Grubunda Basit İnflamasyon Belirteçlerinin ve Sistemik İmmün İnflamasyon İndeksinin Değerlendirilmesi. Turk. Psikiyatr. Derg. Turk. J. Psychiatry 2023, 34, 11–15. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Gong, M.; Yang, Y.; Ling, Y.; Fang, X.; Zhu, T.; Wang, Z.; Zhang, X.; Zhang, C. Systemic inflammatory biomarkers in Schizophrenia are changed by ECT administration and related to the treatment efficacy. BMC Psychiatry 2024, 24, 53. [Google Scholar] [CrossRef]
- Stefanovic, V.; Mihajlovic, G.; Nenadovic, M.; Djukic-Dejanovic, S.; Borovcanin, M.; Trajkovic, G. The effect of antipsychotic drugs on nonspecific inflammation markers in the first episode of schizophrenia. Vojnosanit. Pregl. 2015, 72, 1085–1092. [Google Scholar] [CrossRef]
- Onur, D.; Neslihan, A.K.; Samet, K. A comparative study of complete blood count inflammatory markers in substance-free acute psychotic disorder and substance-induced psychosis. Early Interv. Psychiatry 2021, 15, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Labonté, C.; Zhand, N.; Park, A.; Harvey, P.D. Complete blood count inflammatory markers in treatment-resistant schizophrenia: Evidence of association between treatment responsiveness and levels of inflammation. Psychiatry Res. 2022, 308, 114382. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, T.; Li, G.; Feng, J.; Deng, L.; Xu, H.; Yin, L.; Ma, J.; Chen, D.; Chen, J. Investigation of systemic immune-inflammation index, neutrophil/high-density lipoprotein ratio, lymphocyte/high-density lipoprotein ratio, and monocyte/high-density lipoprotein ratio as indicators of inflammation in patients with schizophrenia and bipolar disorder. Front. Psychiatry 2022, 13, 941728. [Google Scholar] [CrossRef]
- Cohen, J. Set Correlation and Contingency Tables. Appl. Psychol. Meas. 1988, 12, 425–434. [Google Scholar] [CrossRef]
- Trifu, S.; Marica, S.; Braileanu, D.; Carp, E.G.; Gutt, A.M. Teaching Psychiatric Concepts of Neurosis, Psychosis and Borderline Pathology. Conceptual Boundaries. Procedia—Soc. Behav. Sci. 2015, 203, 125–129. [Google Scholar] [CrossRef][Green Version]
- Semiz, M.; Yldirim, O.; Canan, F.; Demir, S.; Hasbek, E.; Tuman, T.; Kayka, N.; Tosun, M. Elevated neutrophil/lymphocyte ratio in patients with schizophrenia. Psychiatr. Danub. 2014, 26, 220–225. [Google Scholar][Green Version]
- Lee, J.S.; Kim, N.Y.; Na, S.H.; Youn, G.H.Y.; Shin, C.S. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine 2018, 97, e11138. [Google Scholar] [CrossRef]
- Figueiredo, I.C.; Cargaleiro, I.; Mueller, S.; Aguiar, P.; Batuca, J.R.; Pereira, S.A.; Diogo, L.; Ureche-Angelescu, I.; Monteiro, E.C. F13. Platelet-lymphocyte ratio as a short-term treatment-response predictor in schizophrenia’s relapse. Schizophr. Bull. 2019, 45 (Suppl. 2), S258–S259. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, C.; Wu, L.; Li, Z.; Li, H.; Zhang, J. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: A 20-year follow-up cohort study of 42,875 US adults. J. Clin. Med. 2023, 12, 1128. [Google Scholar] [CrossRef]
- Ferguson, C.J. An effect size primer: A guide for clinicians and researchers. Prof. Psychol. Res. Pract. 2009, 40, 532–538. [Google Scholar] [CrossRef]
- Vito, D. Multiple comparisons: Bonferroni Corrections and False Discovery Rates. Cornell University Statistical Consulting Unit. 2016. Available online: https://physiology.med.cornell.edu/people/banfelder/qbio/resources_2008/1.5_Bonferroni_FDR.pdf (accessed on 20 September 2025).
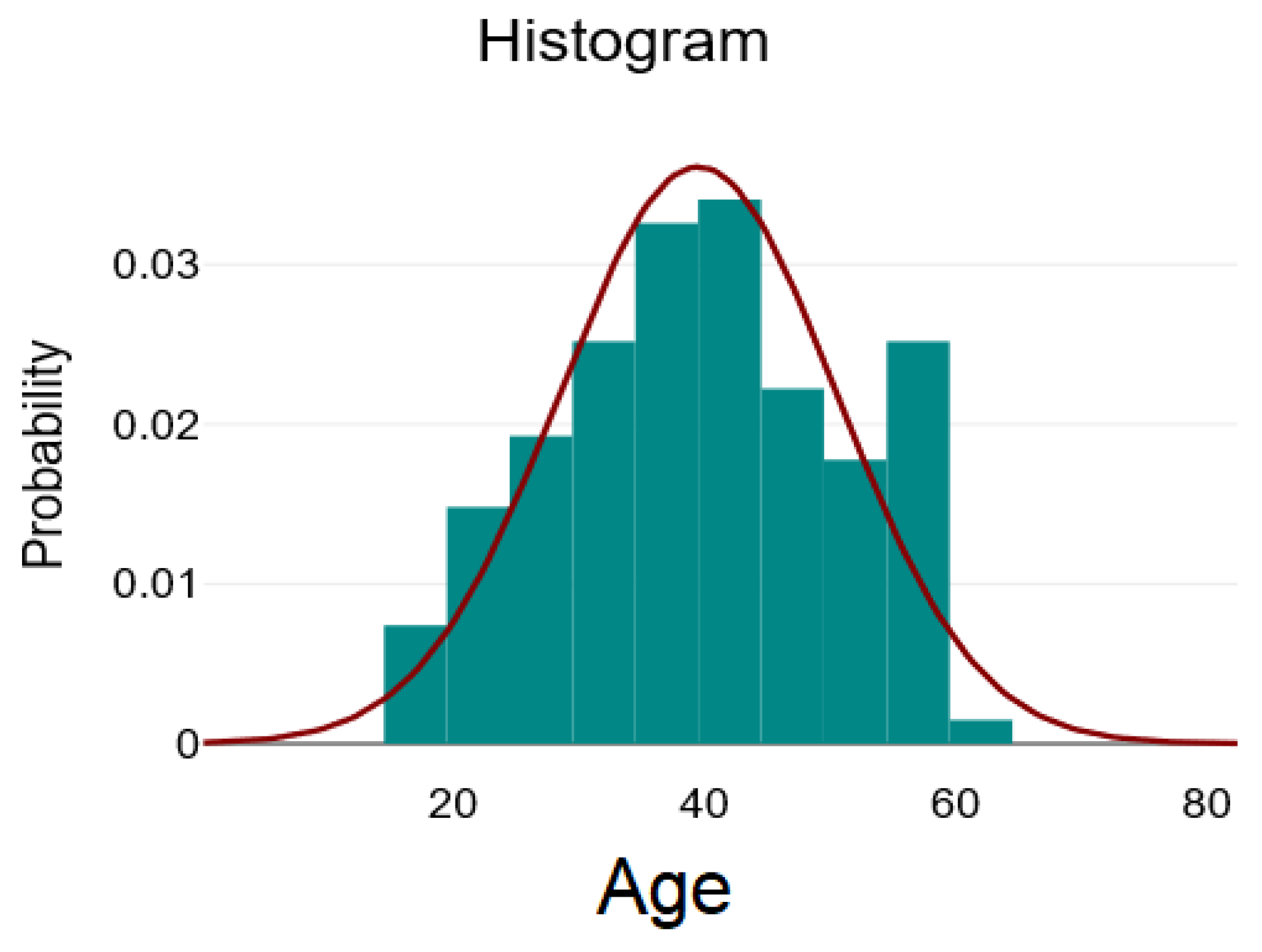
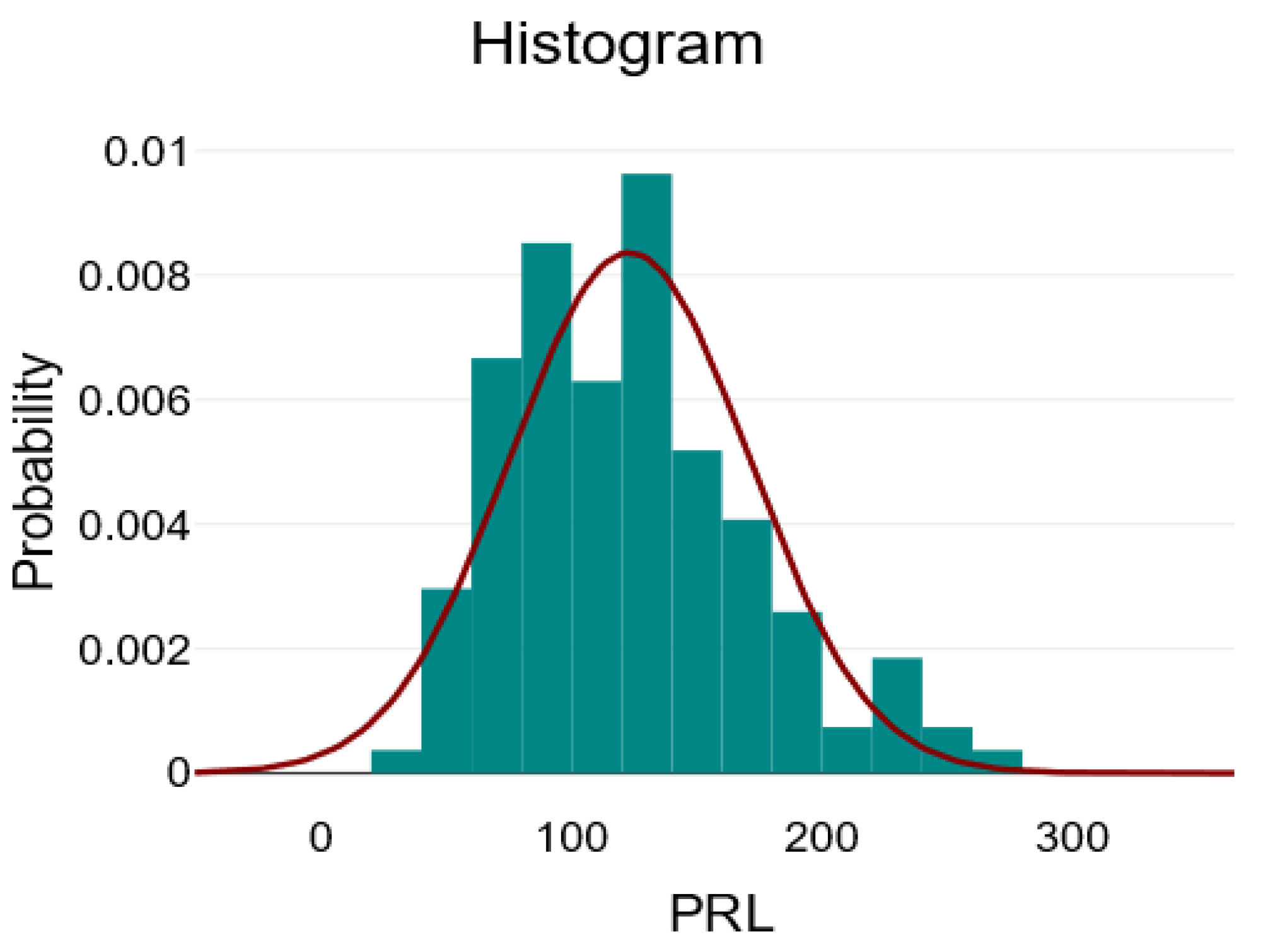

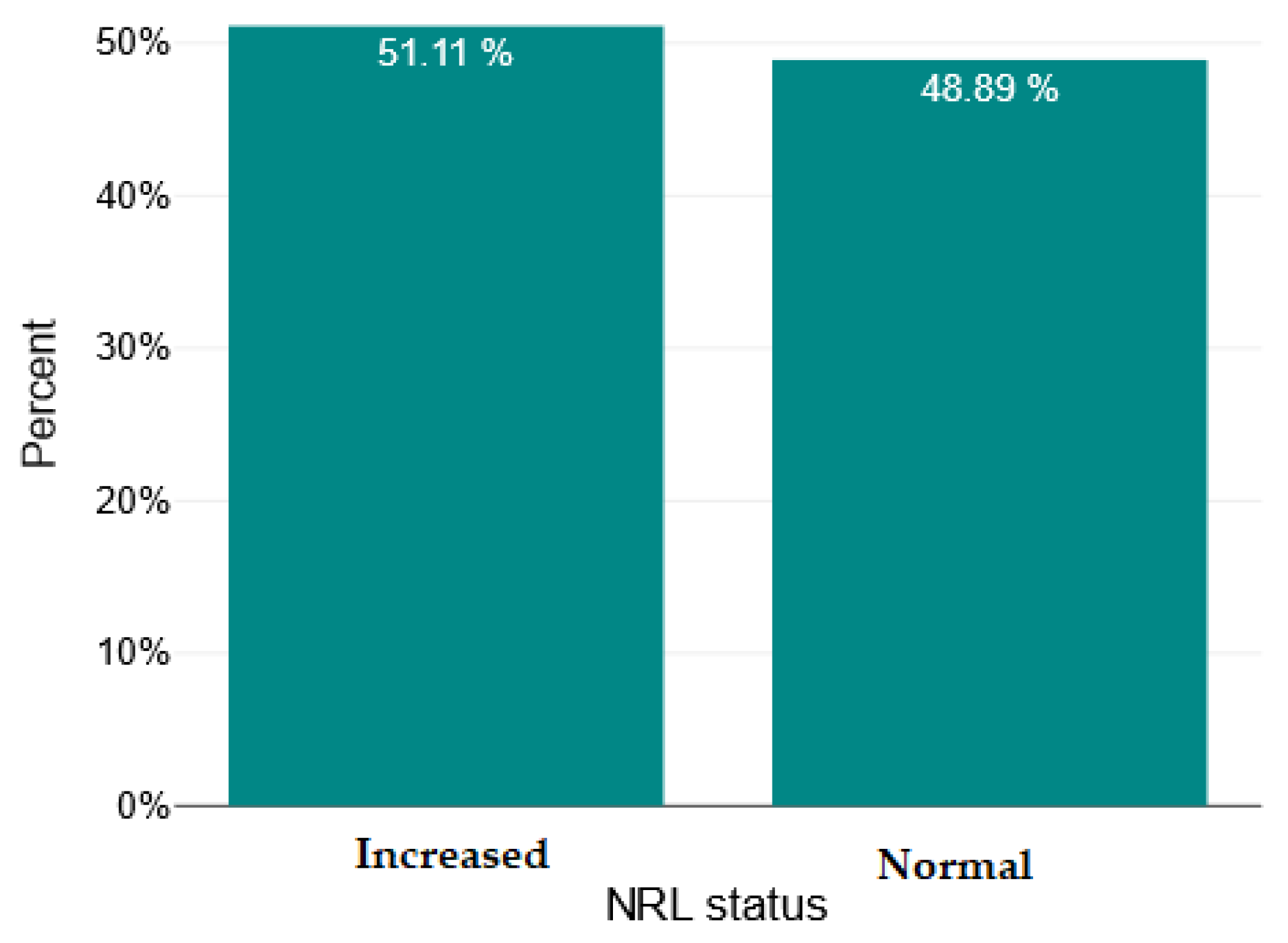
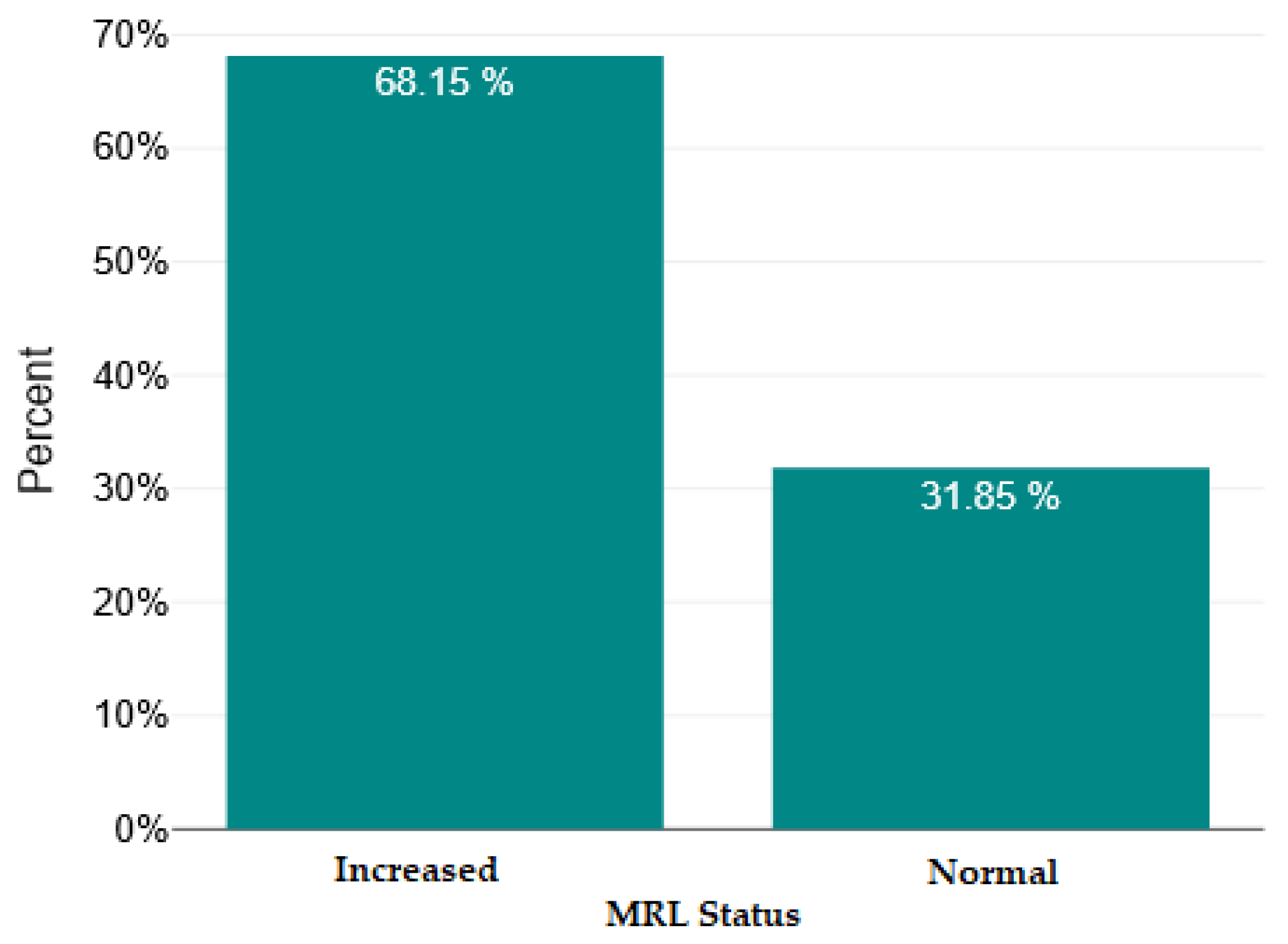
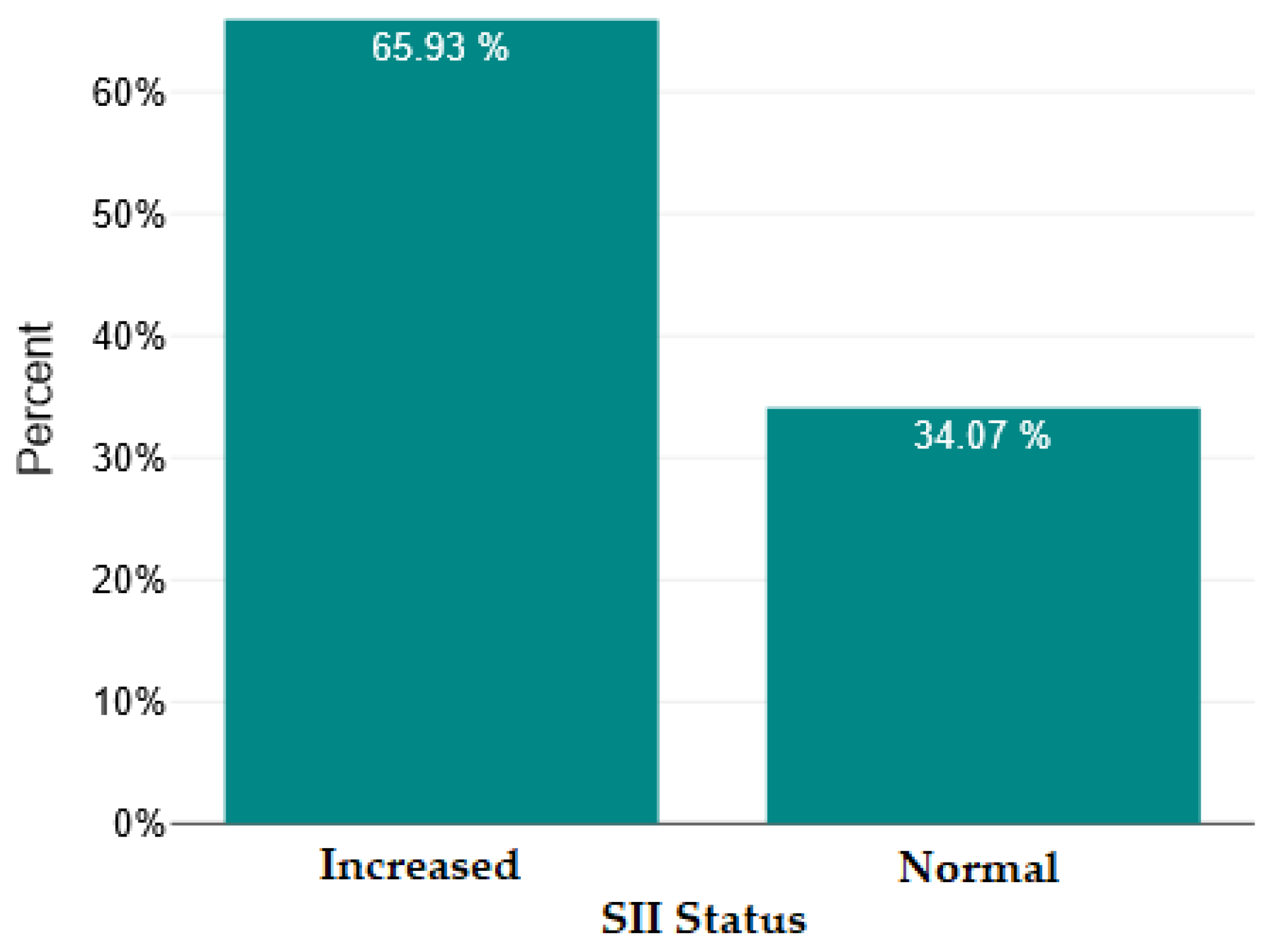
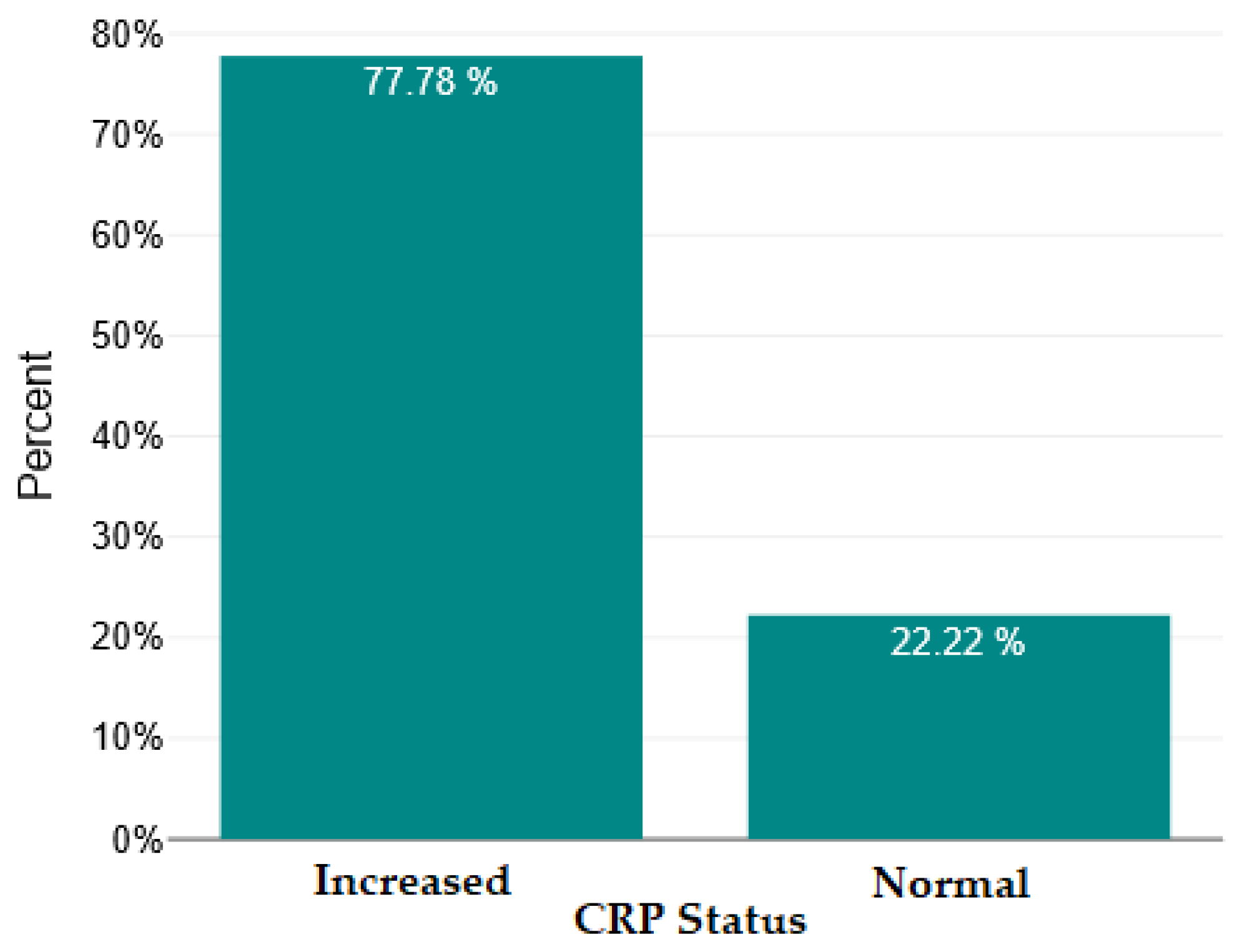
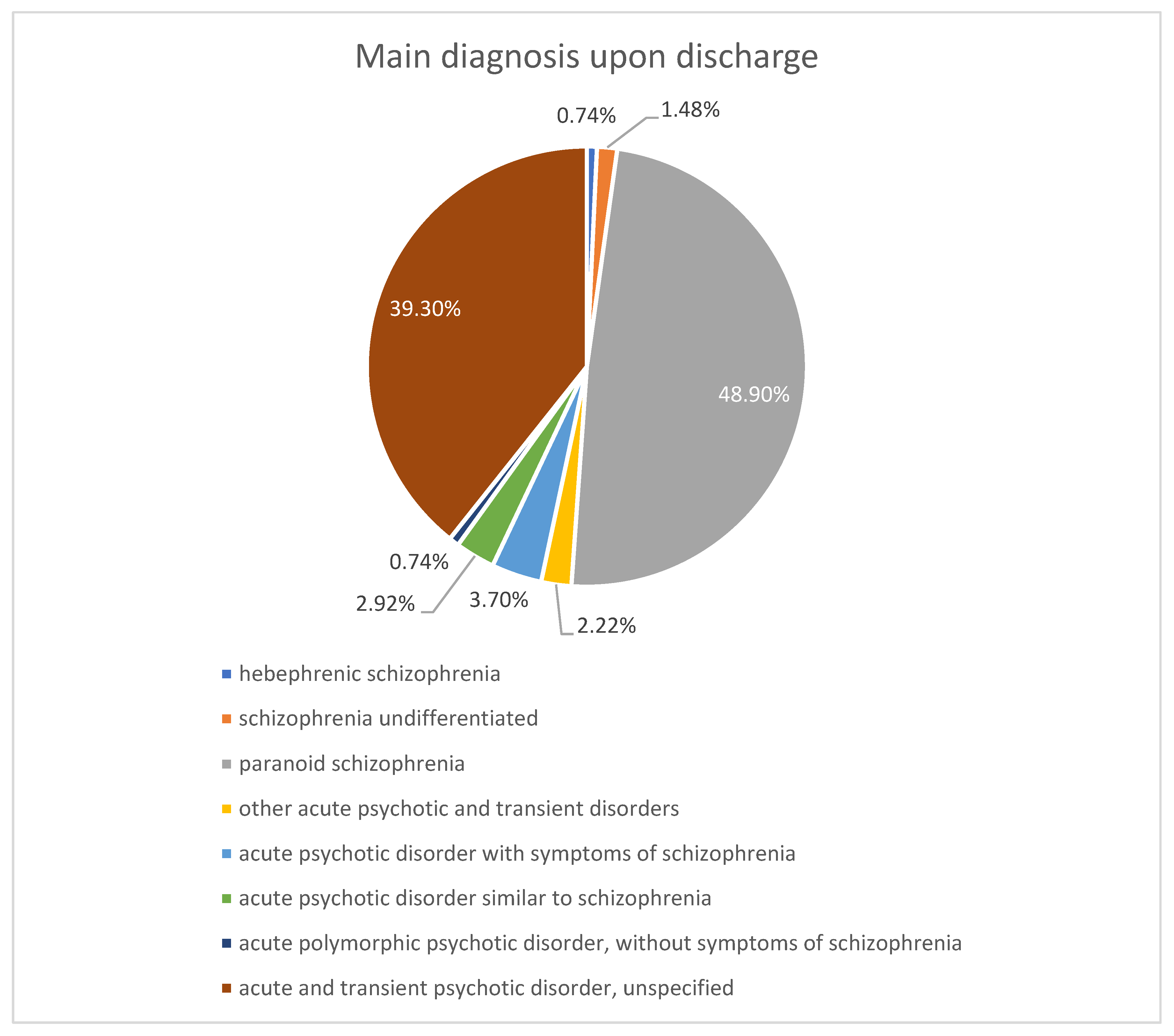

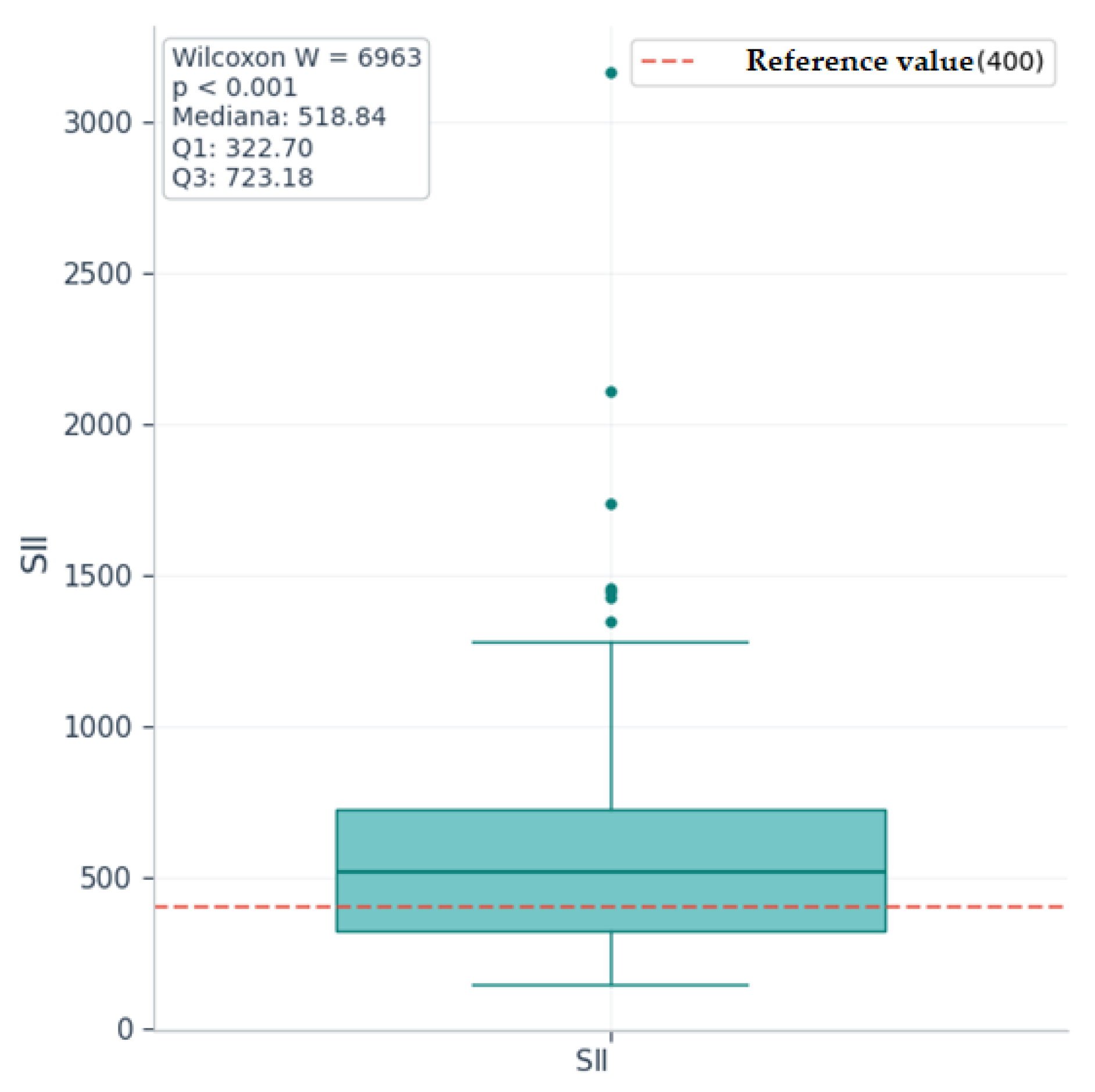

| Mean ± SD/ Median [Q1–Q3] | Minimum | Maximum | Skew | |
|---|---|---|---|---|
| Age | 39.5 ± 11.09 | 18 | 64 | 0 |
| Leukocytes | 7.26 [6.22–9.02] | 3.62 | 16.99 | 1.14 |
| Neutrophils | 4.22 [3.28–5.75] | 1.62 | 11.65 | 1.13 |
| Lymphocytes | 2.24 ± 0.76 | 1 | 5.33 | 0.96 |
| Monocyte | 0.62 [0.5–0.76] | 0.24 | 3.12 | 3.86 |
| NRL | 2.11 [1.41–2.79] | 0.6 | 7.66 | 1.43 |
| PRL | 123.04 ± 47.89 | 39.69 | 271.71 | 0.73 |
| MRL | 0.29 [0.23–0.38] | 0.1 | 1.15 | 2.34 |
| SII | 518.84 [322.7–723.18] | 146.43 | 3165.43 | 2.88 |
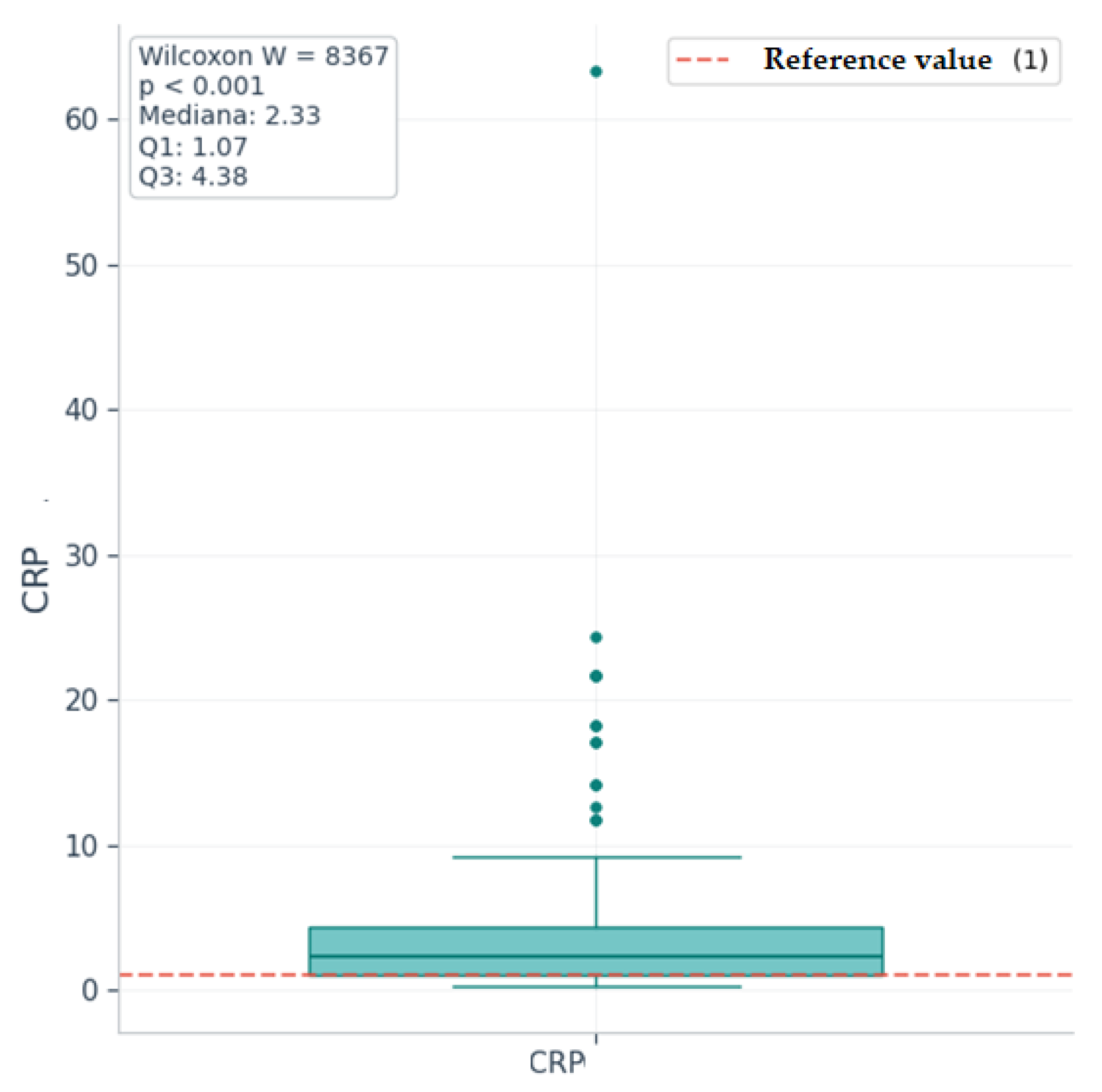
| CRP | 2.33 [1.07–4.38] | 0.3 | 63.4 | 4.76 |
| ESR | 9 [4–13.25] | 2 | 73 | 2.9 |
| Schizophrenia (n = 69) | Acute Psychotic Disorder (n = 66) | |
|---|---|---|
| Age (Average ± SD) | 43.58 ± 9.59 | 35.23 ± 11 |
| Sex, n (%) | ||
| - Male | 63 (91.3%) | 64 (96.97%) |
| - Female | 6 (8.7%) | 2 (3.03%) |
| Environment of origin, n (%) | ||
| - Urban | 61 (88.41%) | 52 (78.79%) |
| - Countryside | 8 (11.59%) | 14 (21.21%) |
| Level of education, n (%) | ||
| - <9 years | 16 (23.19%) | 11 (16.67%) |
| - 9–12 years | 39 (56.52%) | 40 (60.61%) |
| - >12 years | 14 (20.29%) | 15 (22.73%) |
| Relationship status, n (%) | ||
| - Stable partner | 20 (28.99%) | 28 (42.42%) |
| - No steady partner | 49 (71.01%) | 38 (57.58%) |
| Schizophrenia | Acute Psychosis | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD/Median [Q1–Q3] | Min | Max | Skew | Mean ± SD/Median [Q1–Q3] | Min | Max | Skew | |
| WBC | 7.58 [6.57–9.4] | 3.65 | 15.8 | 1.05 | 6.94 [6.06–8.6] | 3.62 | 16.99 | 1.3 |
| NEU | 4.41 [3.65–6.34] | 1.97 | 10.14 | 0.87 | 3.86 [3.04–5.59] | 1.62 | 11.65 | 1.42 |
| LYM | 2.26 [1.72–2.6] | 1 | 5.33 | 1.13 | 2.2 [1.57–2.62] | 1.02 | 4.42 | 0.76 |
| MON | 0.63 [0.5–0.79] | 0.24 | 2.42 | 3.02 | 0.62 [0.49–0.71] | 0.3 | 3.12 | 4.2 |
| NRL | 2.25 [1.48–2.97] | 0.6 | 5.5 | 0.9 | 1.85 [1.35–2.65] | 0.82 | 7.66 | 1.81 |
| PRL | 124.89 ± 48.16 | 48.78 | 241.35 | 0.78 | 121.12 ± 47.9 | 39.69 | 271.71 | 0.7 |
| MRL | 0.29 [0.23–0.38] | 0.12 | 0.91 | 1.62 | 0.28 [0.23–0.38] | 0.1 | 1.15 | 2.56 |
| SII | 528.84 [355.6–724.56] | 162.45 | 2107.91 | 1.8 | 465.01 [289.77–712.73] | 146.43 | 3165.43 | 3.68 |
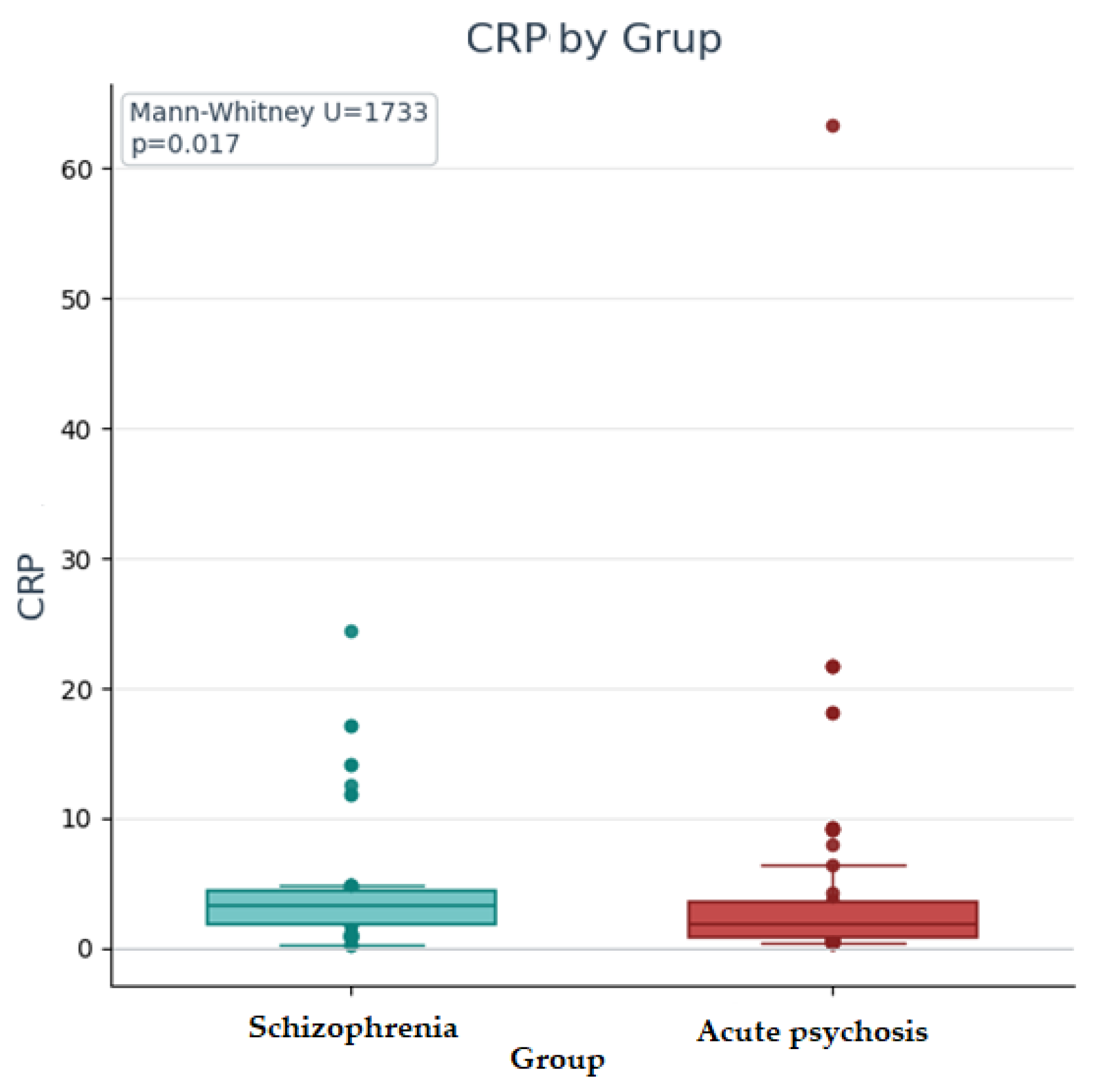
| CRP | 3.3 [1.82–4.38] | 0.3 | 24.4 | 2.53 | 1.88 [0.82–3.59] | 0.35 | 63.4 | 4.28 |
| ESR | 10 [6–15] | 2 | 39 | 1.19 | 7 [4–12] | 2 | 73 | 3.56 |
| Marker | Mediana | Normal Upper Limit | W | Z | N | p-Value | Interpretation |
|---|---|---|---|---|---|---|---|
| NRL | 2.11 | 2 | 5366 | 1.7 | 135 | 0.089 | Insignificant |
| SII | 518.84 | 400 | 6963 | 5.21 | 135 | <0.001 | Highly significant |
| CRP | 2.33 | 1 | 8367 | 8.3 | 135 | <0.001 | Highly significant |
| MRL | 0.29 | 0.25 | 6881.5 | 5.24 | 134 | <0.001 | Highly significant |
| U | z | p (Asymptotic) | r | Interpretation | |
|---|---|---|---|---|---|
| Leukocytes | 1928 | −1.54 | 0.124 | 0.13 | Insignificant |
| Neutrophils | 1945 | −1.46 | 0.144 | 0.13 | Insignificant |
| Lymphocytes | 2194 | −0.37 | 0.715 | 0.03 | Insignificant |
| Monocytes | 2094 | −0.81 | 0.42 | 0.07 | Insignificant |
| NRL | 2022 | −1.12 | 0.262 | 0.1 | Insignificant |
| MRL | 2223 | −0.24 | 0.812 | 0.02 | Insignificant |
| SII | 2007 | −1.19 | 0.235 | 0.1 | Insignificant |
| CRP | 1733 | −2.4 | 0.017 | 0.21 | Insignificant according to the corrected threshold (0.005) |
| ESR | 1691 | −1.71 | 0.088 | 0.15 | Insignificant |
| t | df | p | Cohen’s d | Interpretation | |
|---|---|---|---|---|---|
| PRL | 0.46 | 133 | 0.649 | 0.08 | Insignificant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segarceanu, L.-M.; Zanfir, A.-G.; Minca, D.G.; Trifu, S. Evaluation of Inflammatory Markers in Perception Disorders in Major Psychiatric Pathology. Int. J. Mol. Sci. 2025, 26, 9299. https://doi.org/10.3390/ijms26199299
Segarceanu L-M, Zanfir A-G, Minca DG, Trifu S. Evaluation of Inflammatory Markers in Perception Disorders in Major Psychiatric Pathology. International Journal of Molecular Sciences. 2025; 26(19):9299. https://doi.org/10.3390/ijms26199299
Chicago/Turabian StyleSegarceanu, Laura-Maria, Andrei-Gabriel Zanfir, Dana Galieta Minca, and Simona Trifu. 2025. "Evaluation of Inflammatory Markers in Perception Disorders in Major Psychiatric Pathology" International Journal of Molecular Sciences 26, no. 19: 9299. https://doi.org/10.3390/ijms26199299
APA StyleSegarceanu, L.-M., Zanfir, A.-G., Minca, D. G., & Trifu, S. (2025). Evaluation of Inflammatory Markers in Perception Disorders in Major Psychiatric Pathology. International Journal of Molecular Sciences, 26(19), 9299. https://doi.org/10.3390/ijms26199299







