Neuroprotective Effects of Vesatolimod in EAE: Modulating Immune Balance and Microglial Polarization
Abstract
1. Introduction
2. Results
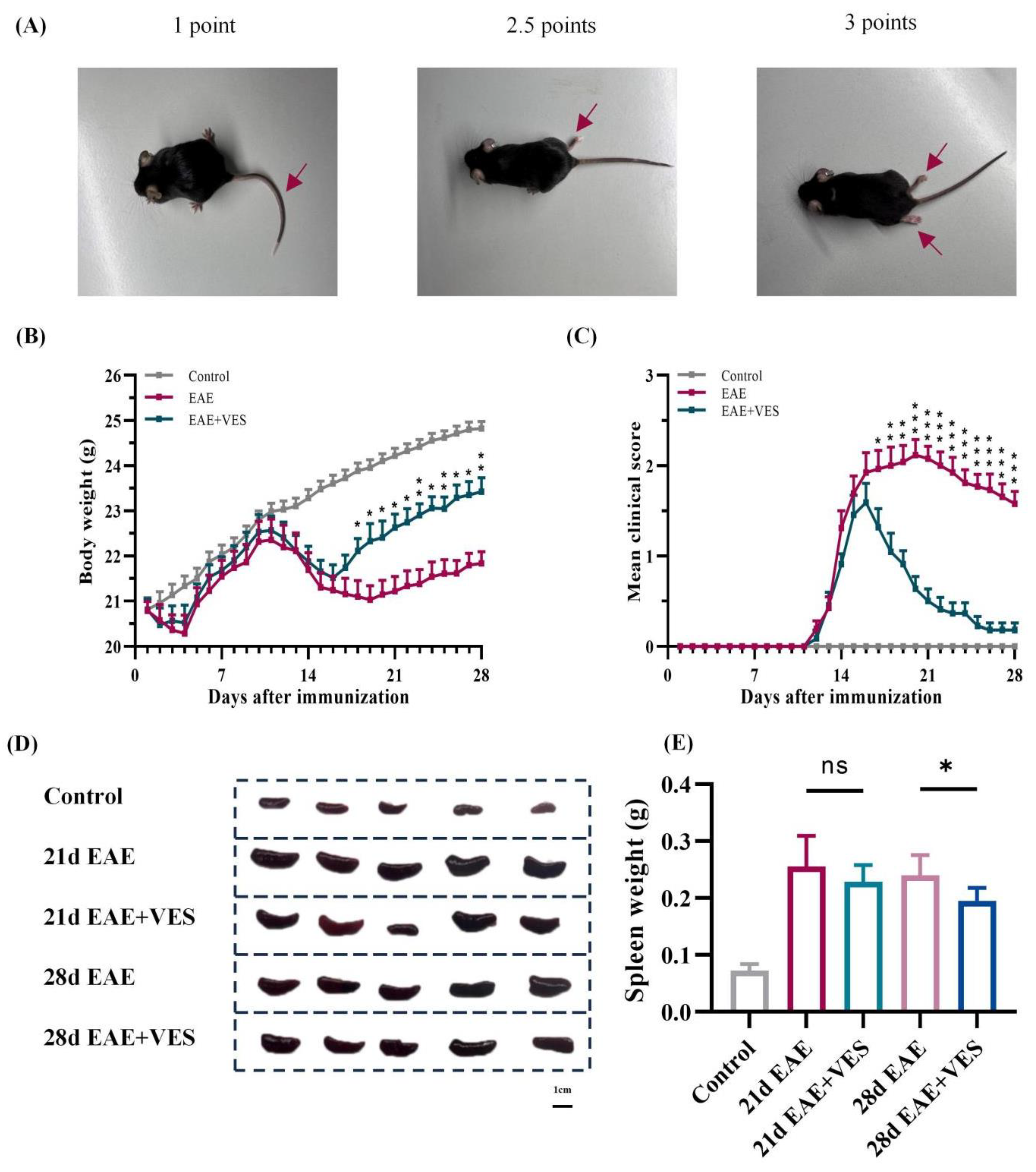
2.1. VES Alleviates EAE Symptoms and Enhances Neurological Recovery
2.2. VES Alleviates EAE-Induced Splenomegaly
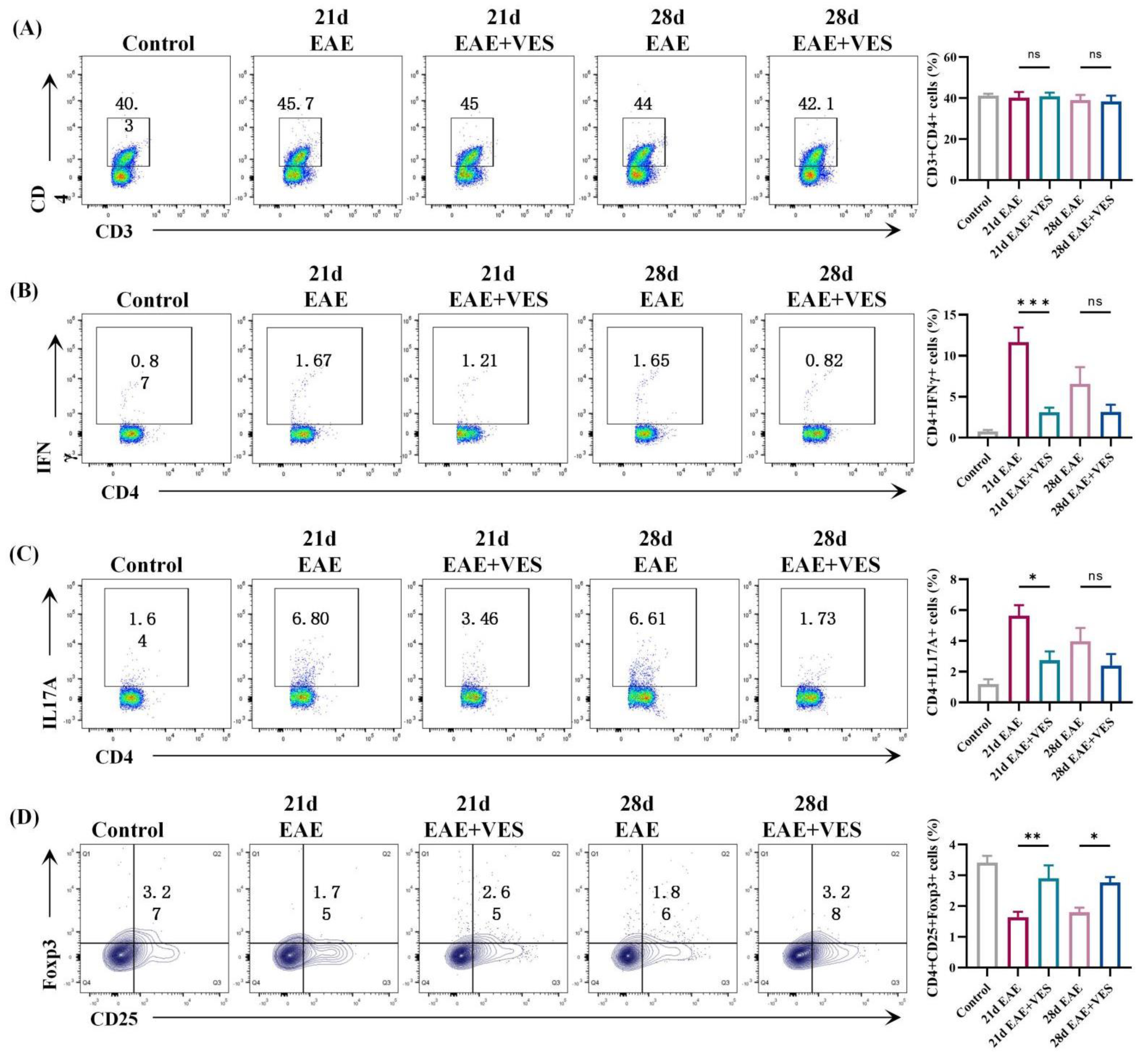
2.3. VES Regulates Peripheral CD4+ T Cell Subsets in EAE
2.4. VES Preserves BBB Integrity and Enhances Remyelination in EAE
2.5. VES Modulates Microglial Activation Status In Vivo and In Vitro
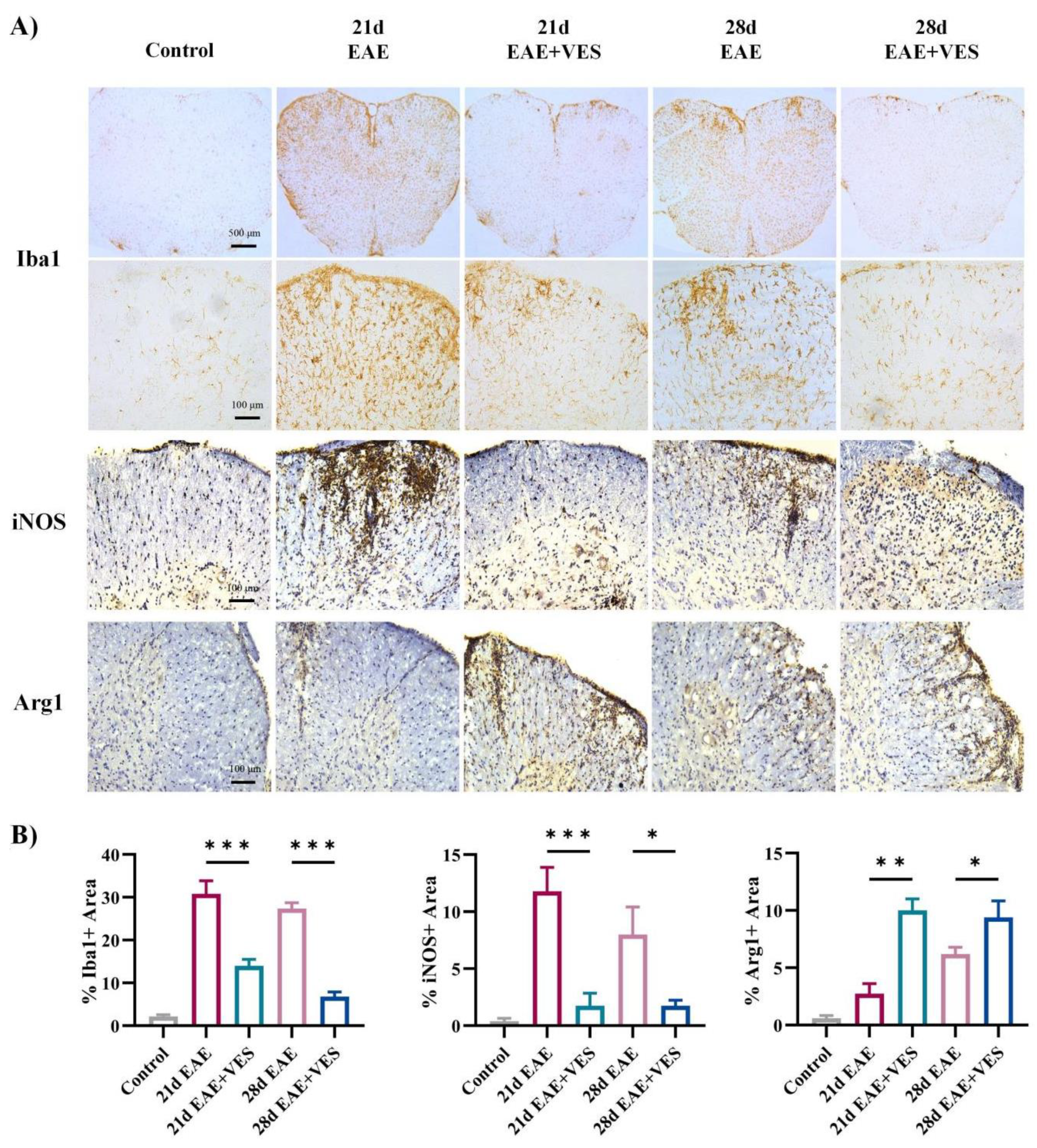
2.5.1. VES Attenuates Microglial Overactivation and Promotes a Shift in Activation State in the Spinal Cord of EAE Mice
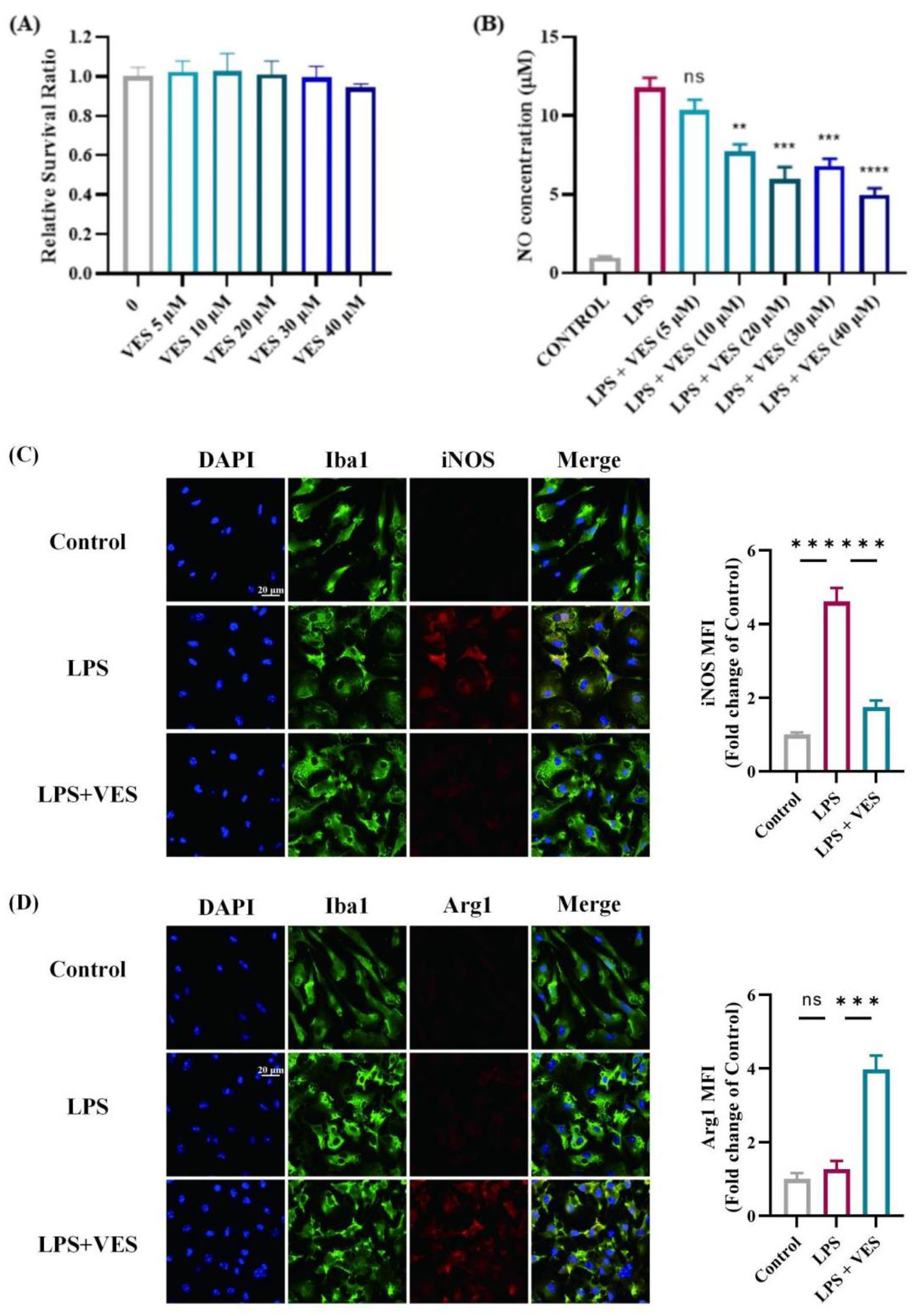
2.5.2. VES Directly Modulates Microglial Activation In Vitro
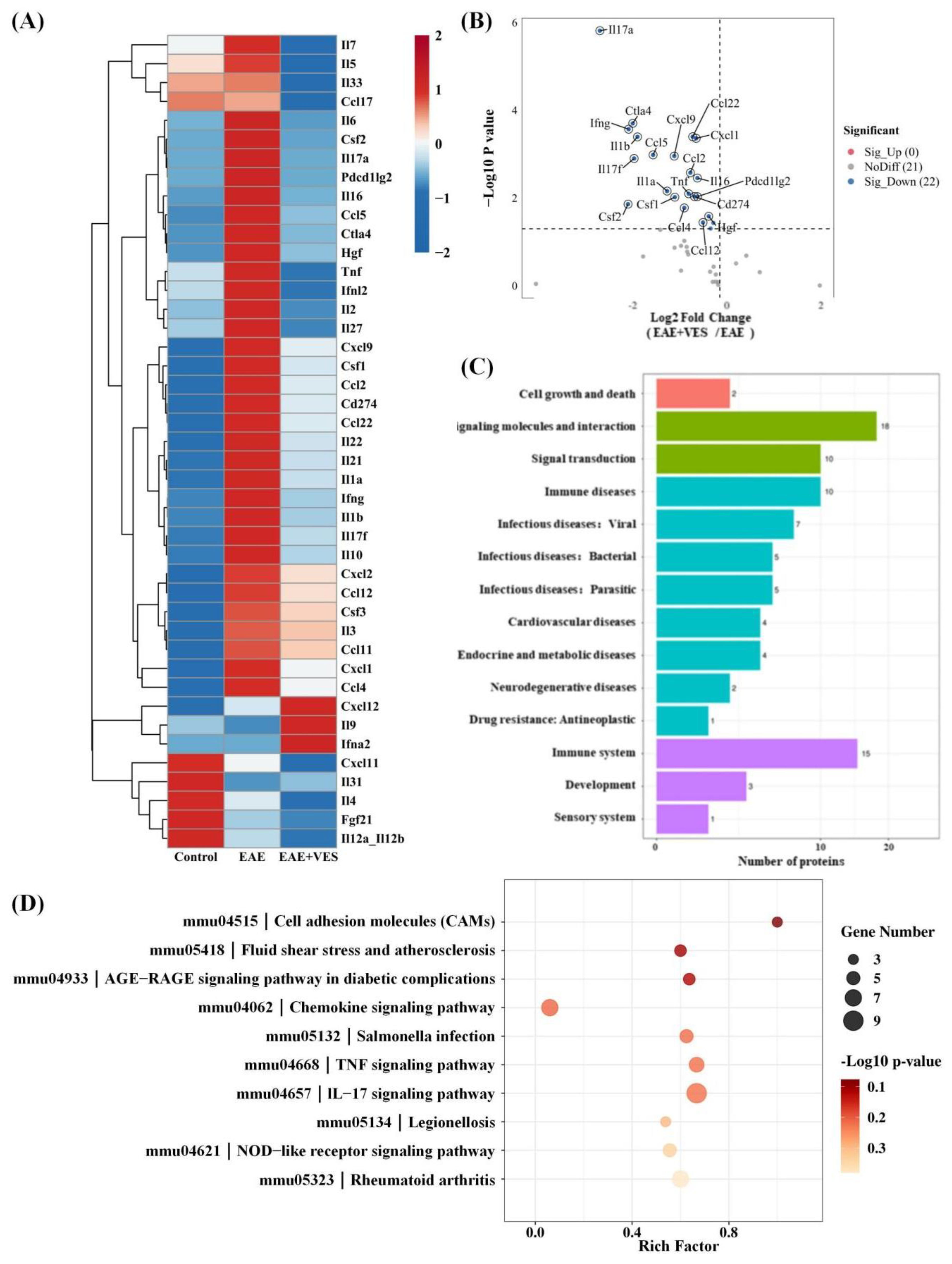
2.6. Proteomic Analysis of VES Modulation in the EAE Inflammatory Microenvironment
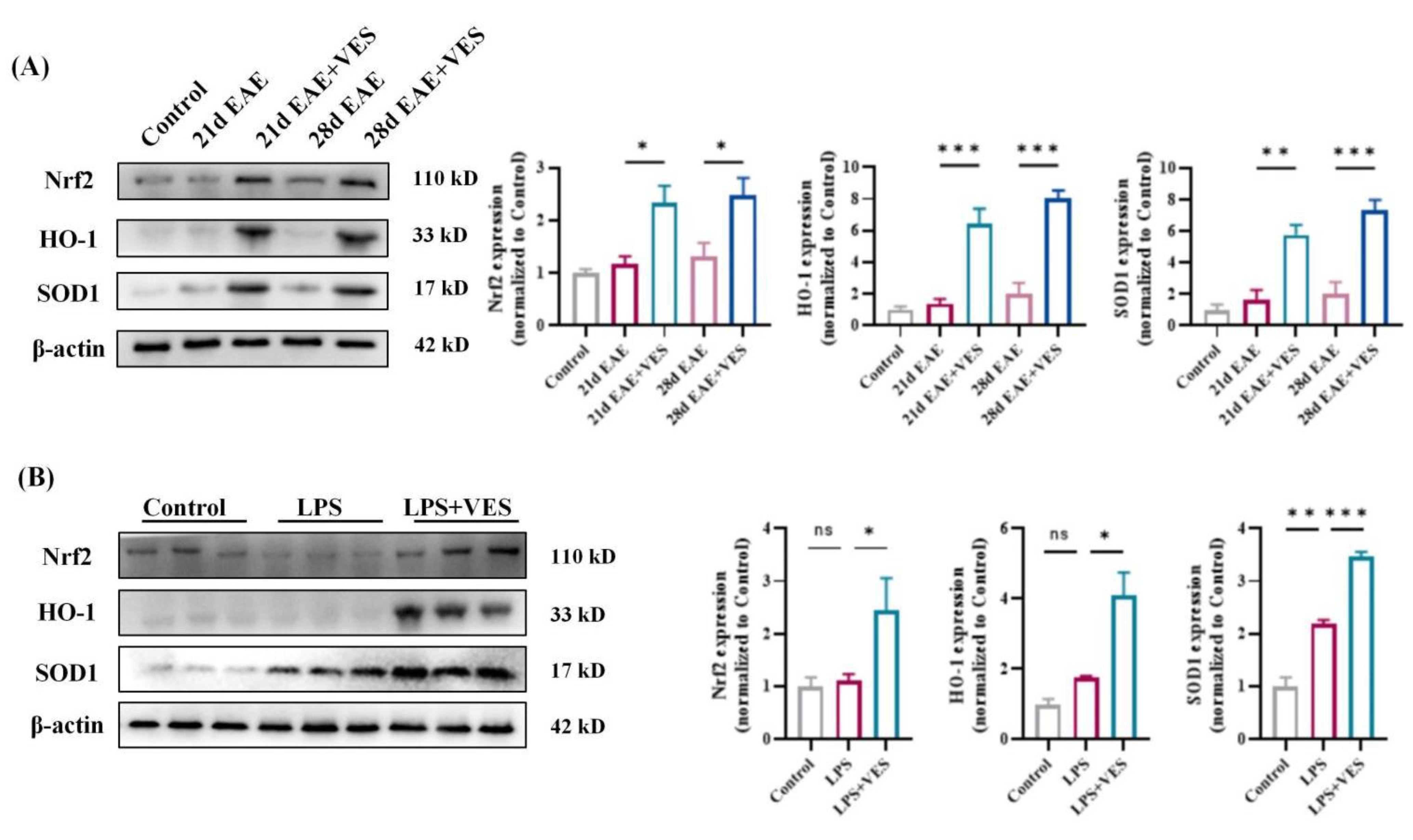
2.7. VES Confers Neuroprotection via Nrf2 Pathway Activation
2.7.1. In Vivo Activation of Nrf2 by VES
2.7.2. In Vitro Confirmation in Microglia
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Induction of EAE
4.3. Grouping and Treatment
4.4. Flow Cytometry
4.5. Evans Blue Assay for Blood–Brain Barrier (BBB) Permeability
4.6. Luxol Fast Blue (LFB) Staining and Immunohistochemistry
4.7. Olink Proteomics
4.8. Primary Microglial Cell Isolation and Culture
4.9. In Vitro Neuroinflammation Model Induction
4.10. Cell Viability Assay (CCK-8)
4.11. Nitric Oxide (NO) Production Assay
4.12. Immunofluorescence
4.13. Western Blotting
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Gaitán, M.I.; Ysrraelit, M.C.; Fiol, M.P. Progressive Multiple Sclerosis: From Pathogenic Mechanisms to Treatment. Brain 2017, 140, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Absinta, M.; Maric, D.; Gharagozloo, M.; Garton, T.; Smith, M.D.; Jin, J.; Fitzgerald, K.C.; Song, A.; Liu, P.; Lin, J.P.; et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 2021, 597, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Bar-Or, A.; Cree, B.A.C.; Fox, R.J.; Giovannoni, G.; Gold, R.; Vermersch, P.; Arnold, D.L.; Arnould, S.; Scherz, T.; et al. Siponimod Versus Placebo in Secondary Progressive Multiple Sclerosis (EXPAND): A Double-Blind, Randomised, Phase 3 Study. Lancet 2018, 391, 1263–1273. [Google Scholar] [CrossRef]
- Giovannoni, G.; Soelberg Sorensen, P.; Cook, S.; Rammohan, K.; Rieckmann, P.; Comi, G.; Dangond, F.; Adeniji, A.K.; Vermersch, P. Safety and Efficacy of Cladribine Tablets in Patients with Relapsing-Remitting Multiple Sclerosis: Results from the Randomized Extension Trial of the CLARITY Study. Mult. Scler. 2018, 24, 1594–1604. [Google Scholar] [CrossRef]
- Olejnik, P.; Roszkowska, Z.; Adamus, S.; Kasarełło, K. Multiple Sclerosis: A Narrative Overview of Current Pharmacotherapies and Emerging Treatment Prospects. Pharmacol. Rep. 2024, 76, 926–943. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef]
- Luna, G.; Alping, P.; Burman, J.; Fink, K.; Fogdell-Hahn, A.; Gunnarsson, M.; Hillert, J.; Langer-Gould, A.; Lycke, J.; Nilsson, P.; et al. Infection Risks Among Patients With Multiple Sclerosis Treated with Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol. 2021, 77, 184–191, Erratum in JAMA Neurol. 2021, 78, 1413. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental Autoimmune Encephalomyelitis (EAE) as a Model for Multiple Sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Miyamura, S.; Matsuo, N.; Nagayasu, K.; Shirakawa, H.; Kaneko, S. Myelin Oligodendrocyte Glycoprotein 35–55 (MOG 35–55)-Induced Experimental Autoimmune Encephalomyelitis: A Model of Chronic Multiple Sclerosis. Bio Protoc. 2019, 9, e3453. [Google Scholar] [CrossRef]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Böttcher, C.; Amann, L.; Sagar; Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G.; et al. Spatial and Temporal Heterogeneity of Mouse and Human Microglia at Single-Cell Resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef]
- Hammond, B.P.; Panda, S.P.; Kaushik, D.K.; Plemel, J.R. Microglia and Multiple Sclerosis. Adv. Neurobiol. 2024, 37, 445–456. [Google Scholar] [CrossRef]
- Chu, F.; Shi, M.; Zheng, C.; Shen, D.; Zhu, J.; Zheng, X.; Cui, L. The Roles of Macrophages and Microglia in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. J. Neuroimmunol. 2018, 318, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and Macrophages in Brain Homeostasis and Disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Afzali, A.M.; Korn, T. The role of the adaptive immune system in the initiation and persistence of multiple sclerosis. Semin. Immunol. 2025, 78, 101947. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Song, Y.; Pan, J.J.; Jiang, Y.; Shi, X.; Liu, C.; Ma, Y.; Luo, L.; Mamtilahun, M.; et al. M2 Microglia-Derived Extracellular Vesicles Promote White Matter Repair and Functional Recovery via miR-23a-5p After Cerebral Ischemia in Mice. Theranostics 2022, 12, 3553–3573. [Google Scholar] [CrossRef]
- Lanford, R.E.; Guerra, B.; Chavez, D.; Giavedoni, L.; Hodara, V.L.; Brasky, K.M.; Fosdick, A.; Frey, C.R.; Zheng, J.; Wolfgang, G.; et al. GS-9620, an Oral Agonist of Toll-like Receptor-7, Induces Prolonged Suppression of Hepatitis B Virus in Chronically Infected Chimpanzees. Gastroenterology 2013, 144, 1508–1517.e10. [Google Scholar] [CrossRef]
- Janssen, H.L.A.; Brunetto, M.R.; Kim, Y.J.; Ferrari, C.; Massetto, B.; Nguyen, A.H.; Joshi, A.; Woo, J.; Lau, A.H.; Gaggar, A.; et al. Safety, Efficacy and Pharmacodynamics of Vesatolimod (GS-9620) in Virally Suppressed Patients with Chronic Hepatitis B. J. Hepatol. 2018, 68, 431–440. [Google Scholar] [CrossRef]
- Kraemer, K.L.; McGinnis, K.A.; Fiellin, D.A.; Skanderson, M.; Gordon, A.J.; Robbins, J.; Zickmund, S.; Bryant, K.; Korthuis, P.T. Low Levels of Initiation, Engagement, and Retention in Substance Use Disorder Treatment Including Pharmacotherapy Among HIV-Infected and Uninfected Veterans. J. Subst. Abus. Treat. 2019, 103, 23–32. [Google Scholar] [CrossRef]
- Riddler, S.A.; Para, M.; Benson, C.A.; Mills, A.; Ramgopal, M.; DeJesus, E.; Brinson, C.; Cyktor, J.; Jacobs, J.; Koontz, D.; et al. Vesatolimod, a Toll-like Receptor 7 Agonist, Induces Immune Activation in Virally Suppressed Adults Living with Human Immunodeficiency Virus-1. Clin. Infect. Dis. 2021, 72, e815–e824. [Google Scholar] [CrossRef]
- Abdel-Mohsen, M.; Deeks, S.; Giron, L.; Hong, K.Y.; Goldman, A.; Zhang, L.; Huang, S.S.Y.; Verrill, D.; Guo, S.; Selzer, L.; et al. Circulating immune and plasma biomarkers of time to HIV rebound in HIV controllers treated with vesatolimod. Front. Immunol. 2024, 15, 1405348. [Google Scholar] [CrossRef]
- Gane, E.J.; Lim, Y.S.; Gordon, S.C.; Visvanathan, K.; Sicard, E.; Fedorak, R.N.; Roberts, S.; Massetto, B.; Ye, Z.; Pflanz, S.; et al. The Oral Toll-like Receptor-7 Agonist GS-9620 in Patients with Chronic Hepatitis B Virus Infection. J. Hepatol. 2015, 63, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, B.; Chen, X.; Song, N.; Wu, J.; Li, G.; Yu, P.; Han, Y.; Liu, J.; Qin, C. GS-9620 Inhibits Enterovirus 71 Replication Mainly through the NF-κB and PI3K-AKT Signaling Pathways. Antivir. Res. 2018, 153, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Song, Y.; Fang, J.; Yang, X.; Mu, S.; Zhang, J. Neuroprotective Effect of Vesatolimod in an Experimental Autoimmune Encephalomyelitis Mice Model. Int. Immunopharmacol. 2023, 116, 109717. [Google Scholar] [CrossRef]
- Farez, M.F.; Quintana, F.J.; Gandhi, R.; Izquierdo, G.; Lucas, M.; Weiner, H.L. Toll-like Receptor 2 and Poly(ADP-ribose) Polymerase 1 Promote Central Nervous System Neuroinflammation in Progressive EAE. Nat. Immunol. 2009, 10, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Kaskow, B.J.; Baecher-Allan, C. Effector T Cells in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a029025. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, W.; Su, G.; Hu, J.; Yang, P. Progranulin Suppressed Autoimmune Uveitis and Autoimmune Neuroinflammation by Inhibiting Th1/Th17 Cells and Promoting Treg Cells and M2 Macrophages. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1133. [Google Scholar] [CrossRef]
- Yang, C.; Ma, Y.; Lu, Q.; Qu, Y.; Li, Y.; Cheng, S.; Xiao, C.; Chen, J.; Wang, C.; Wang, F.; et al. 2-Bromo-1,4-Naphthalenedione Promotes CD8+ T Cell Expansion and Limits Th1/Th17 to Mitigate Experimental Autoimmune Encephalomyelitis. J. Neuroinflammation 2024, 21, 181. [Google Scholar] [CrossRef]
- Goverman, J. Autoimmune T Cell Responses in the Central Nervous System. Nat. Rev. Immunol. 2009, 9, 393–407. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-Brain Barrier Breakdown in Alzheimer Disease and Other Neurodegenerative Disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Bennett, J.; Basivireddy, J.; Kollar, A.; Biron, K.E.; Reickmann, P.; Jefferies, W.A.; McQuaid, S. Blood-brain barrier disruption and enhanced vascular permeability in the multiple sclerosis model EAE. J. Neuroimmunol. 2010, 229, 180–191. [Google Scholar] [CrossRef]
- Wei, S.S.; Chen, L.; Yang, F.Y.; Wang, S.Q.; Wang, P. The Role of Fibronectin in Multiple Sclerosis and the Effect of Drug Delivery Across the Blood-Brain Barrier. Neural Regen. Res. 2023, 18, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Macías-Islas, M.Á.; Flores-Alvarado, L.J.; Mireles-Ramírez, M.A.; González-Renovato, E.D.; Hernández-Navarro, V.E.; Sánchez-López, A.L.; Alatorre-Jiménez, M.A. Role of the Blood-Brain Barrier in Multiple Sclerosis. Arch. Med. Res. 2014, 45, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; Ffrench-Constant, C. Regenerating CNS Myelin—From Mechanisms to Experimental Medicines. Nat. Rev. Neurosci. 2017, 18, 753–769. [Google Scholar] [CrossRef]
- Fancy, S.P.; Chan, J.R.; Baranzini, S.E.; Franklin, R.J.; Rowitch, D.H. Myelin Regeneration: A Recapitulation of Development? Annu. Rev. Neurosci. 2011, 34, 21–43. [Google Scholar] [CrossRef]
- Voet, S.; Prinz, M.; van Loo, G. Microglia in Central Nervous System Inflammation and Multiple Sclerosis Pathology. Trends Mol. Med. 2019, 25, 112–123. [Google Scholar] [CrossRef]
- Charabati, M.; Wheeler, M.A.; Weiner, H.L.; Quintana, F.J. Multiple sclerosis: Neuroimmune crosstalk and therapeutic targeting. Cell 2023, 186, 1309–1327. [Google Scholar] [CrossRef]
- Guerrero, B.L.; Sicotte, N.L. Microglia in Multiple Sclerosis: Friend or Foe? Front. Immunol. 2020, 11, 374. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 Microglia: The Good, the Bad, and the Inflamed. J. Neuroinflammation 2014, 11, 98. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 Polarization and Metabolic States. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- La Torre, M.E.; Cianciulli, A.; Monda, V.; Monda, M.; Filannino, F.M.; Antonucci, L.; Valenzano, A.; Cibelli, G.; Porro, C.; Messina, G.; et al. α-Tocopherol Protects Lipopolysaccharide-Activated BV2 Microglia. Molecules 2023, 28, 3340. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Barger, S.W.; Mrak, R.E.; Griffin, W.S. Vitamin E Suppression of Microglial Activation Is Neuroprotective. J. Neurosci. Res. 2001, 66, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; Innamorato, N.G.; Martín-Moreno, A.M.; De Ceballos, M.L.; Yamamoto, M.; Cuadrado, A. Nrf2 Regulates Microglial Dynamics and Neuroinflammation in Experimental Parkinson’s Disease. Glia 2010, 58, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic Targeting of the NRF2 and KEAP1 Partnership in Chronic Diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef]
- Khan, A.; Shal, B.; Khan, A.U.; Bibi, T.; Islam, S.U.; Baig, M.W.; Haq, I.U.; Ali, H.; Ahmad, S.; Khan, S. Withametelin, a Novel Phytosterol, Alleviates Neurological Symptoms in EAE Mouse Model of Multiple Sclerosis via Modulation of Nrf2/HO-1 and TLR4/NF-κB Signaling. Neurochem. Int. 2021, 151, 105211. [Google Scholar] [CrossRef]
- Upadhayay, S.; Mehan, S.; Prajapati, A.; Sethi, P.; Suri, M.; Zawawi, A.; Almashjary, M.N.; Tabrez, S. Nrf2/HO-1 Signaling Stimulation Through Acetyl-11-Keto-Beta-Boswellic Acid (AKBA) Provides Neuroprotection in Ethidium Bromide-Induced Experimental Model of Multiple Sclerosis. Genes 2022, 13, 1324. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Y.; Ban, T.; Chen, K.; Zhen, X.; Dai, Q.; Zhang, G. Serum Amyloid A Drive Microglia Shift to a Resolving Phenotype through Nrf2. Neuropharmacology 2025, 270, 110374. [Google Scholar] [CrossRef]
- Farjam, M.; Zhang, G.X.; Ciric, B.; Rostami, A. Emerging Immunopharmacological Targets in Multiple Sclerosis. J. Neurol. Sci. 2015, 358, 22–30. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of Multiple Sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Robinson, A.P.; Harp, C.T.; Noronha, A.; Miller, S.D. The Experimental Autoimmune Encephalomyelitis (EAE) Model of MS: Utility for Understanding Disease Pathophysiology and Treatment. Handb. Clin. Neurol. 2014, 122, 173–189. [Google Scholar] [CrossRef]
- Ghorbani, M.M.; Farazmandfar, T.; Abediankenari, S.; Hassannia, H.; Maleki, Z.; Shahbazi, M. Treatment of EAE Mice with Treg, G-MDSC and IL-2: A New Insight into Cell Therapy for Multiple Sclerosis. Immunotherapy 2022, 14, 789–798. [Google Scholar] [CrossRef]
- Yu, H.Z.; Zhu, B.Q.; Zhu, L.; Li, S.; Wang, L.M. NR4A1 Agonist Cytosporone B Attenuates Neuroinflammation in a Mouse Model of Multiple Sclerosis. Neural Regen. Res. 2022, 17, 2765–2770. [Google Scholar] [CrossRef]
- Lu, K.; Liu, L.; Xu, X.; Zhao, F.; Deng, J.; Tang, X.; Wang, X.; Zhao, B.Q.; Zhang, X.; Zhao, Y. ADAMTS13 Ameliorates Inflammatory Responses in Experimental Autoimmune Encephalomyelitis. J. Neuroinflammation 2020, 17, 67. [Google Scholar] [CrossRef]
- Kuo, P.C.; Brown, D.A.; Scofield, B.A.; Yu, I.C.; Chang, F.L.; Wang, P.Y.; Yen, J.H. 3H-1,2-Dithiole-3-thione as a Novel Therapeutic Agent for the Treatment of Experimental Autoimmune Encephalomyelitis. Brain Behav. Immun. 2016, 57, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Weng, W.T.; Scofield, B.A.; Paraiso, H.C.; Brown, D.A.; Wang, P.Y.; Yu, I.C.; Yen, J.H. Dimethyl Itaconate, an Itaconate Derivative, Exhibits Immunomodulatory Effects on Neuroinflammation in Experimental Autoimmune Encephalomyelitis. J. Neuroinflammation 2020, 17, 138. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.T.; Kuo, P.C.; Brown, D.A.; Scofield, B.A.; Furnas, D.; Paraiso, H.C.; Wang, P.Y.; Yu, I.C.; Yen, J.H. 4-Ethylguaiacol Modulates Neuroinflammation and Th1/Th17 Differentiation to Ameliorate Disease Severity in Experimental Autoimmune Encephalomyelitis. J. Neuroinflammation 2021, 18, 110. [Google Scholar] [CrossRef] [PubMed]


| Cytokines | Cytokines | Cytokines | Cytokines |
|---|---|---|---|
| IL1a | IL12 | Cxcl11 | Ccl17 |
| ILIb | IL16 | Cxcl12 | Fg121 |
| IL2 | ILI7a | Ccl22 | Cila4 |
| IL3 | ILI7f | Csl1 | Cd274 |
| IL4 | IL21 | Csl2 | Tnf |
| IL5 | IL27 | Csl3 | IFNa2 |
| IL6 | IL31 | Ccl2 | IFNg |
| IL7 | IL33 | Ccl4 | IFNl2 |
| IL9 | Cxcl1 | Ccl5 | Hgf |
| II10 | Cxcl2 | Ccl11 | Pdcd1lg2 |
| ILI2a, ILI2b | Cxcl9 | Ccl12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, J.; Mu, S. Neuroprotective Effects of Vesatolimod in EAE: Modulating Immune Balance and Microglial Polarization. Int. J. Mol. Sci. 2025, 26, 9297. https://doi.org/10.3390/ijms26199297
Chen X, Zhang J, Mu S. Neuroprotective Effects of Vesatolimod in EAE: Modulating Immune Balance and Microglial Polarization. International Journal of Molecular Sciences. 2025; 26(19):9297. https://doi.org/10.3390/ijms26199297
Chicago/Turabian StyleChen, Xueyu, Jian Zhang, and Shuhua Mu. 2025. "Neuroprotective Effects of Vesatolimod in EAE: Modulating Immune Balance and Microglial Polarization" International Journal of Molecular Sciences 26, no. 19: 9297. https://doi.org/10.3390/ijms26199297
APA StyleChen, X., Zhang, J., & Mu, S. (2025). Neuroprotective Effects of Vesatolimod in EAE: Modulating Immune Balance and Microglial Polarization. International Journal of Molecular Sciences, 26(19), 9297. https://doi.org/10.3390/ijms26199297





