Genome Editing by Grafting
Abstract
1. Introduction
2. The Delivery of CRISPR/Cas Components in Plant Cells
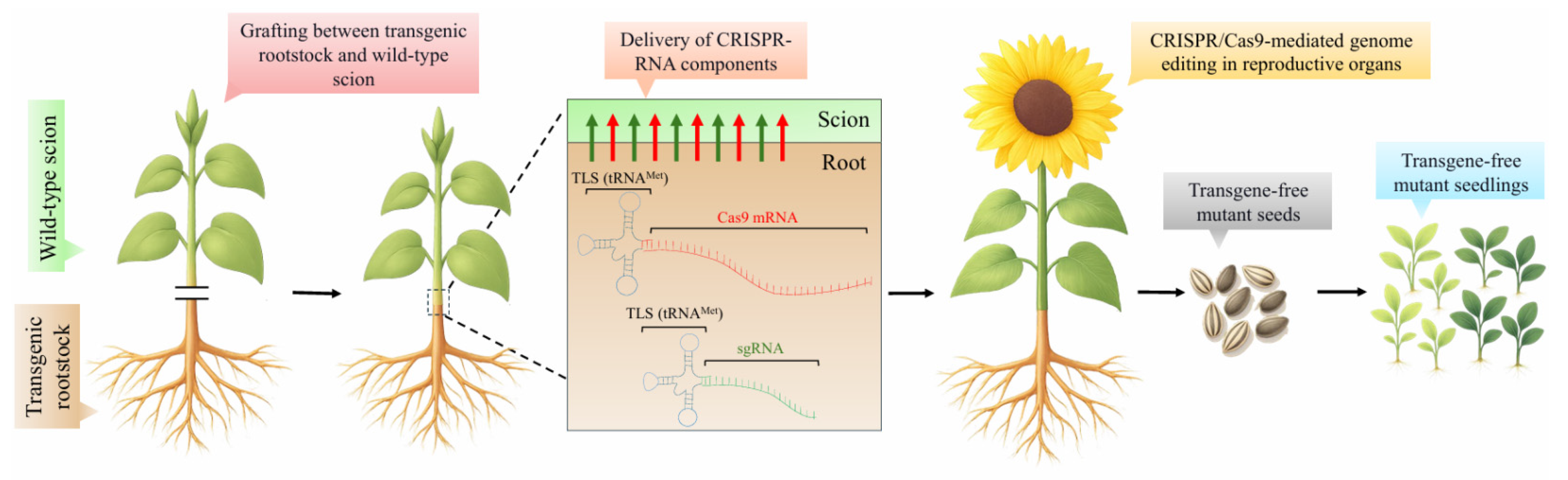
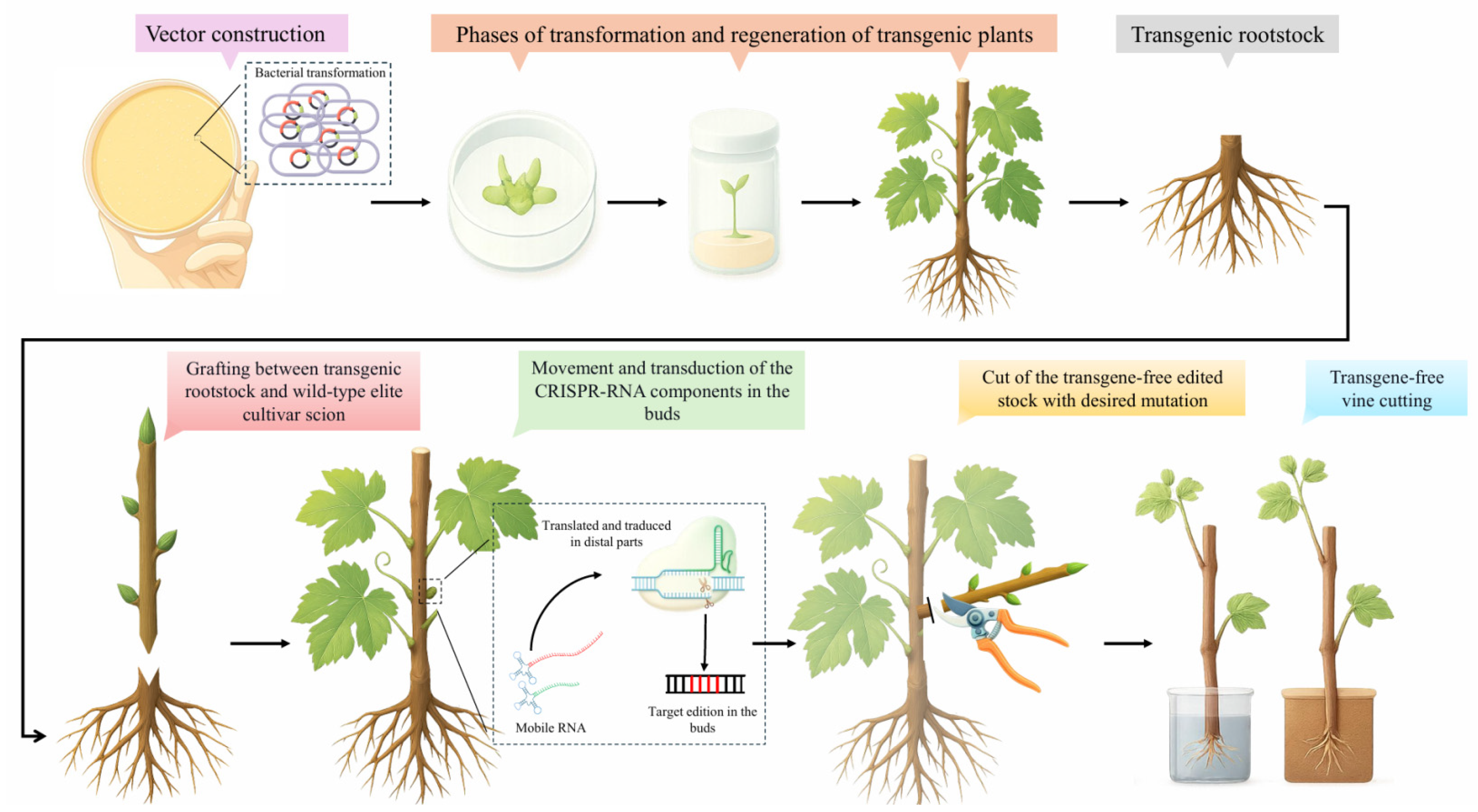
3. Genome Editing by Grafting (GEG)
4. Existing Gaps in the Implementation of the GEG
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Richter, C.; Chang, J.T.; Fineran, P.C. Function and regulation of clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated (Cas) systems. Viruses 2012, 4, 2291–2311. [Google Scholar] [CrossRef]
- Li, B.; Sun, C.; Li, J.; Gao, C. Targeted genome-modification tools and their advanced applications in crop breeding. Nat. Rev. Genet. 2024, 25, 603–622. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Viviani, A.; Spada, M.; Giordani, T.; Fambrini, M.; Pugliesi, C. Origin of the genome editing systems: Application for crop improvement. Biologia 2022, 77, 3353–3383. [Google Scholar] [CrossRef]
- Liu, M.; Rehman, S.; Tang, X.; Gu, K.; Fan, Q.; Chen, D.; Ma, W. Methodologies for improving HDR efficiency. Front. Genet. 2019, 9, 691. [Google Scholar] [CrossRef]
- Rogo, U.; Simoni, S.; Fambrini, M.; Giordani, T.; Pugliesi, C.; Mascagni, F. Future-proofing agriculture: De novo domestication for sustainable and resilient crops. Int. J. Mol. Sci. 2024, 25, 2374. [Google Scholar] [CrossRef]
- Wang, L.; Han, H. Strategies for improving the genome-editing efficiency of class 2 CRISPR/Cas system. Heliyon 2024, 10, e38588. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023, 11, 1143157. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Nerkar, G.; Devarumath, S.; Purankar, M.; Kumar, A.; Valarmathi, R.; Devarumath, R.; Appunu, C. Advances in crop breeding through precision genome editing. Front. Genet. 2022, 13, 880195. [Google Scholar] [CrossRef]
- Pandey, S.; Divakar, S.; Singh, A. Genome editing prospects for heat stress tolerance in cereal crops. Plant Physiol. Biochem. 2024, 215, 108989. [Google Scholar] [CrossRef]
- Gilbertson, L.; Puchta, H.; Slotkin, R.K. The future of genome editing in plants. Nat. Plants 2025, 11, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Rogo, U.; Viviani, A.; Pugliesi, C.; Fambrini, M.; Usai, G.; Castellacci, M.; Simoni, S. Improving crop tolerance to abiotic stress for sustainable agriculture: Progress in manipulating ascorbic acid metabolism via genome editing. Sustainability 2025, 17, 719. [Google Scholar] [CrossRef]
- Yuan, P.; Usman, M.; Liu, W.; Adhikari, A.; Zhang, C.; Njiti, V.; Xia, Y. Advancements in plant gene editing technology: From construct design to enhanced transformation efficiency. Biotechnol. J. 2024, 19, e202400457. [Google Scholar] [CrossRef]
- Kim, H.S.; Kweon, J.; Kim, Y. Recent advances in CRISPR-based functional genomics for the study of disease-associated genetic variants. Exp. Mol. Med. 2024, 56, 861–869. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, D.; Xiong, X.; Yan, B.; Xie, W.; Sheen, J.; Li, J.-F. A potent Cas9-derived gene activator for plant and mammalian cells. Nat. Plants 2017, 3, 930–936. [Google Scholar] [CrossRef]
- Pan, C.; Wu, X.; Markel, K.; Malzahn, A.A.; Kundagrami, N.; Sretenovic, S.; Zhang, Y.; Cheng, Y.; Shih, P.M.; Qi, Y. CRISPR-Act3.0 for highly efficient multiplexed gene activation in plants. Nat. Plants 2021, 7, 942–953. [Google Scholar] [CrossRef]
- Hilton, I.B.; D’Ippolito, A.M.; Vockley, C.M.; Thakore, P.I.; Crawford, G.E.; Reddy, T.E.; Gersbach, C.A. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 2015, 33, 510–517. [Google Scholar] [CrossRef]
- Wang, Z.; Bart, R.S. Using targeted genome methylation for crop improvement. J. Exp. Bot. 2025, 76, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Tanenbaum, M.E.; Gilbert, L.A.; Qi, L.S.; Weissman, J.S.; Vale, R.D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 2014, 159, 635–646. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, W.; Tan, L.; Chen, T.; He, Y.; Irving, P.S.; Weeks, K.M.; Zhang, Q.C.; Dong, X. Pervasive downstream RNA hairpins dynamically dictate start-codon selection. Nature 2023, 621, 423–430. [Google Scholar] [CrossRef]
- Hendelman, A.; Zebell, S.; Rodriguez-Leal, D.; Dukler, N.; Robitaille, G.; Wu, X.; Kostyun, J.; Tal, L.; Wang, P.; Bartlett, M.E.; et al. Conserved pleiotropy of an ancient plant homeobox gene uncovered by cis-regulatory dissection. Cell 2021, 184, 1724–1739.e16. [Google Scholar] [CrossRef] [PubMed]
- Gage, J.L.; Mali, S.; McLoughlin, F.; Khaipho-Burch, M.; Monier, B.; Bailey-Serres, J.; Vierstra, R.D.; Buckler, E.S. Variation in upstream open reading frames contributes to allelic diversity in maize protein abundance. Proc. Natl. Acad. Sci. USA 2022, 119, e2112516119. [Google Scholar] [CrossRef]
- Xiong, X.; Li, Z.; Liang, J.; Liu, K.; Li, C.; Li, J.-F. A cytosine base editor toolkit with varying activity windows and target scopes for versatile gene manipulation in plants. Nucleic Acids Res. 2022, 50, 3565–3580. [Google Scholar] [CrossRef]
- Xue, C.; Qiu, F.; Wang, Y.; Li, B.; Zhao, K.T.; Chen, K.; Gao, C. Tuning plant phenotypes by precise, graded downregulation of gene expression. Nat. Biotechnol. 2023, 41, 1758–1764. [Google Scholar] [CrossRef]
- Shen, R.; Yao, Q.; Tan, X.; Ren, W.; Zhong, D.; Zhang, X.; Li, X.; Dong, C.; Cao, X.; Tian, Y.; et al. In-locus gene silencing in plants using genome editing. New Phytol. 2024, 243, 2501–2511. [Google Scholar] [CrossRef]
- Wu, M.; Chen, A.; Li, X.; Li, X.; Hou, X.; Liu, X. Advancements in delivery strategies and non-tissue culture regeneration systems for plant genetic transformation. Adv. Biotechnol. 2024, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Xu, M.; Radani, Y.; Yang, L. Technological development and application of plant genetic transformation. Int. J. Mol. Sci. 2023, 24, 10646. [Google Scholar] [CrossRef]
- Bélanger, J.G.; Copley, T.R.; Hoyos-Villegas, V.; Charron, J.B.; O’Donoughue, L. A comprehensive review of in planta stable transformation strategies. Plant Methods 2024, 20, 79, Erratum in Plant Methods 2024, 20, 158. https://doi.org/10.1186/s13007-024-01282-4. [Google Scholar] [CrossRef]
- Altpeter, F.; Springer, N.M.; Bartley, L.E.; Blechl, A.E.; Brutnell, T.P.; Citovsky, V.; Conrad, L.J.; Gelvin, S.B.; Jackson, D.P.; Kausch, A.P.; et al. Advancing crop transformation in the era of genome editing. Plant Cell 2016, 28, 1510–1520. [Google Scholar] [CrossRef]
- Sukegawa, S.; Saika, H.; Toki, S. Plant genome editing: Ever more precise and wide reaching. Plant J. 2021, 106, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Venezia, M.; Creasy Krainer, K.M. Current advancements and limitation of gene editing in orphan crops. Front. Plant Sci. 2021, 12, 742932. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, W.; Katin-Grazzini, L.; Ding, J.; Gu, X.; Li, Y.; Gu, T.; Wang, R.; Lin, X.; Deng, Z.; et al. A method for the production and expedient screening of CRISPR/Cas9-mediated non-transgenic mutant plants. Hortic. Res. 2018, 5, 13. [Google Scholar] [CrossRef]
- He, Y.; Mudgett, M.; Zhao, Y. Advances in gene editing without residual transgenes in plants. Plant Physiol. 2022, 188, 1757–1768. [Google Scholar] [CrossRef]
- Laforest, L.C.; Nadakuduti, S.S. Advances in delivery mechanisms of CRISPR gene-editing reagents in plants. Front. Genome Ed. 2022, 4, 830178. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, D.; Jogam, P.; Allini, V.R.; Abbagani, S.; Alok, A. The present and potential future methods for delivering CRISPR/Cas9 components in plants. J. Genet. Eng. Biotechnol. 2020, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, J.; Ghoshal, B.; Wang, M.; Jacobsen, S.E. CRISPR-Cas-mediated transcriptional control and epi-mutagenesis. Plant Physiol. 2022, 188, 1811–1824. [Google Scholar] [CrossRef]
- Ahmad, M. Plant breeding advancements with “CRISPR-Cas” genome editing technologies will assist future food security. Front. Plant Sci. 2023, 14, 1133036. [Google Scholar] [CrossRef]
- Prestwich, B.D.; Cardi, T.; Bakhsh, A.; Nicolia, A.; Bhati, K.K. Novel delivery methods for CRISPR-based plant genome editing. In A Roadmap for Plant Genome; Ricroch, A., Eriksson, D., Miladinović, D., Sweet, J., Van Laere, K., Woźniak-Gientka, E., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 41–67. [Google Scholar] [CrossRef]
- Maher, M.F.; Nasti, R.A.; Vollbrecht, M.; Starker, C.G.; Clark, M.D.; Voytas, D.F. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 2020, 38, 84–89. [Google Scholar] [CrossRef]
- Long, Y.; Wang, C.; Liu, C.; Li, H.; Pu, A.; Dong, Z.; Wei, X.; Wan, X. Molecular mechanisms controlling grain size and weight and their biotechnological breeding applications in maize and other cereal crops. J. Adv. Res. 2024, 62, 27–46. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Unver, T.; Zhang, B. CRISPR/Cas: A powerful tool for gene function study and crop improvement. J. Adv. Res. 2021, 29, 207–221. [Google Scholar] [CrossRef]
- Kelly, G.; Plesser, E.; Bdolach, E.; Arroyave, M.; Belausov, E.; Doron-Faigenboim, A.; Rozen, A.; Zemach, H.; Zach, Y.Y.; Goldenberg, L.; et al. In planta genome editing in citrus facilitated by co-expression of CRISPR/Cas and developmental regulators. Plant J. 2025, 122, e70155. [Google Scholar] [CrossRef]
- Kelliher, T.; Starr, D.; Su, X.; Tang, G.; Chen, Z.; Carter, J.; Wittich, P.E.; Dong, S.; Green, J.; Burch, E.; et al. One-step genome editing of elite crop germplasm during haploid induction. Nat. Biotechnol. 2019, 37, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zeng, X.; Yang, Y.; Li, R.; Zhao, Z. Applications and prospects of CRISPR/Cas9 technology in the breeding of major tropical crops. Plants 2024, 13, 3388. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, L.; Zhao, B.; Zhao, Y.; Xie, Y.; Zheng, Z.; Li, Y.; Sun, J.; Wang, H. Development of a haploid-inducer mediated genome editing system for accelerating maize breeding. Mol. Plant 2019, 12, 597–602. [Google Scholar] [CrossRef]
- Sheng, H.; Gao, P.; Yang, C.; Quilichini, T.D.; Kochian, L.V.; Datla, R.; Xiang, D. Advances in genome editing through haploid induction systems. Int. J. Mol. Sci. 2025, 26, 4779. [Google Scholar] [CrossRef] [PubMed]
- Bykonya, A.G.; Lavrov, A.V.; Smirnikhina, S.A. Methods for CRISPR-Cas as ribonucleoprotein complex delivery in vivo. Mol. Biotechnol. 2023, 65, 181–195. [Google Scholar] [CrossRef]
- Karp, H.; Zoltek, M.; Wasko, K.; Vazquez, A.L.; Brim, J.; Ngo, W.; Schepartz, A.; Doudna, J.A. Packaged delivery of CRISPR-Cas9 ribonucleoproteins accelerates genome editing. Nucleic Acids Res. 2025, 53, gkaf105. [Google Scholar] [CrossRef]
- DeWitt, M.A.; Corn, J.E.; Carroll, D. Genome editing via delivery of Cas9 ribonucleoprotein. Methods 2017, 121–122, 9–15. [Google Scholar] [CrossRef]
- Han, A.R.; Shin, H.R.; Kweon, J.; Lee, S.B.; Lee, S.E.; Kim, E.-Y.; Kweon, J.; Chang, E.-J.; Kim, Y.; Kim, S.W. Highly efficient genome editing via CRISPR-Cas9 ribonucleoprotein (RNP) delivery in mesenchymal stem cells. BMB Rep. 2024, 57, 60–65. [Google Scholar] [CrossRef]
- Molaei, Z.; Jabbarpour, Z.; Omidkhoda, A.; Ahmadbeigi, N. Exploring non-viral methods for the delivery of CRISPR-Cas ribonucleoprotein to hematopoietic stem cells. Stem Cell Res. Ther. 2024, 15, 233. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J.; Won, K.-H. A stable DNA-free screening system for CRISPR/RNPs-mediated gene editing in hot and sweet cultivars of Capsicum annuum. BMC Plant Biol. 2020, 20, 449. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Fang, H.; Roberts, N.; Zhang, L.; Vakulskas, C.A.; Niedz, R.P.; Culver, J.N.; Qi, Y. Highly efficient genome editing in plant protoplasts by ribonucleoprotein delivery of CRISPR-Cas12a nucleases. Front. Genome Ed. 2022, 4, 780238. [Google Scholar] [CrossRef] [PubMed]
- Sahab, S.; Runa, F.; Ponnampalam, M.; Kay, P.T.; Jaya, E.; Viduka, K.; Panter, S.; Tibbits, J.; Hayden, M.J. Efficient multi-allelic genome editing via CRISPR-Cas9 ribonucleoprotein-based delivery to Brassica napus mesophyll protoplasts. Front. Plant Sci. 2024, 15, 1397632. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, E.V.; Khusnutdinov, E.A.; Chemeris, A.V.; Kuluev, B.R. Toolkits for CRISPR/CAS genome editing in plants. Russ. J. Plant Physiol. 2022, 69, 3. [Google Scholar] [CrossRef]
- Sioson, V.A.; Kim, M.; Joo, J. Challenges in delivery systems for CRISPR-based genome editing and opportunities of nanomedicine. Biomed. Eng. Lett. 2021, 11, 217–233. [Google Scholar] [CrossRef]
- Demirci, S.; Essawi, K.; Germino-Watnick, P.; Liu, X.; Hakami, W.; Tisdale, J.F. Advances in CRISPR delivery methods: Perspectives and challenges. CRISPR J. 2022, 5, 660–676. [Google Scholar] [CrossRef]
- Bloomer, H.; Khirallah, J.; Li, Y.; Xu, Q. CRISPR/Cas9 ribonucleoprotein-mediated genome and epigenome editing in mammalian cells. Adv. Drug Deliv. Rev. 2022, 181, 114087. [Google Scholar] [CrossRef]
- Khromov, A.V.; Makhotenko, A.V.; Makarova, S.S.; Suprunova, T.P.; Kalinina, N.O.; Taliansky, M.E. Delivery of CRISPR/Cas9 ribonucleoprotein complex into plant apical meristem cells leads to large deletions in an editing gene. Russ. J. Bioorg. Chem. 2020, 46, 1242–1249. [Google Scholar] [CrossRef]
- Zhang, Y.; Iaffaldano, B.; Qi, Y. CRISPR ribonucleoprotein-mediated genetic engineering in plants. Plant Commun. 2021, 2, 100168. [Google Scholar] [CrossRef]
- Lou, H.; Xiang, H.; Zeng, W.; Jiang, J.; Zhang, J.; Xu, L.; Zhao, C.; Gao, Q.; Li, Z. Protocol for transformation-free genome editing in plants using RNA virus vectors for CRISPR-Cas delivery. STAR Protoc. 2024, 5, 103437. [Google Scholar] [CrossRef]
- Mikhaylova, E. Virus-Induced Genome Editing (VIGE): One step away from an agricultural revolution. Int. J. Mol. Sci. 2025, 26, 4599. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Janssen, J.M.; Liu, J.; Tasca, F.; Hoeben, R.C.; Gonçalves, M.A.F.V. Precision genome editing using combinatorial viral vector delivery of CRISPR-Cas9 nucleases and donor DNA constructs. Nucleic Acids Res. 2025, 53, gkae1213. [Google Scholar] [CrossRef]
- Uranga, M.; Daròs, J.-A. Tools and targets: The dual role of plant viruses in CRISPR-Cas genome editing. Plant Genome 2023, 16, e20220. [Google Scholar] [CrossRef] [PubMed]
- Uranga, M. Virus-induced genome editing: Methods and applications in plant breeding. In CRISPR and Plant Functional Genomics; Chen, J.-T., Ed.; CRC Press: Boca Raton, FL, USA, 2024; pp. 81–106. [Google Scholar] [CrossRef]
- Lee, H.; Baik, J.E.; Kim, K.N. Development of an efficient and heritable virus-induced genome editing system in Solanum lycopersicum. Hortic. Res. 2024, 12, uhae364. [Google Scholar] [CrossRef] [PubMed]
- Uranga, M.; Martín-Hernández, A.M.; De Storme, N.; Pasin, F. CRISPR-Cas systems and applications for crop bioengineering. Front. Bioeng. Biotechnol. 2024, 12, 1483857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, S.; Li, X.; Zhang, R.; Li, J. Virus-induced gene editing and its applications in plants. Int. J. Mol. Sci. 2022, 23, 10202. [Google Scholar] [CrossRef]
- Liu, D.; Ellison, E.E.; Myers, E.A.; Donahue, L.I.; Xuan, S.; Swanson, R.; Qi, S.; Prichard, L.E.; Starker, C.G.; Voytas, D.F. Heritable gene editing in tomato through viral delivery of isopentenyl transferase and single-guide RNAs to latent axillary meristematic cells. Proc. Natl. Acad. Sci. USA 2024, 121, e2406486121. [Google Scholar] [CrossRef]
- Shen, Y.; Ye, T.; Li, Z.; Kimutai, T.H.; Song, H.; Dong, X.; Wan, J. Exploiting viral vectors to deliver genome editing reagents in plants. aBIOTECH 2024, 5, 247–261. [Google Scholar] [CrossRef]
- Zhao, C.; Lou, H.; Liu, Q.; Pei, S.; Liao, Q.; Li, Z. Efficient and transformation-free genome editing in pepper enabled by RNA virus-mediated delivery of CRISPR/Cas9. J. Integr. Plant Biol. 2024, 66, 2079–2082. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kang, B.; Venkatesh, J.; Lee, J.-H.; Lee, S.; Kim, J.-M.; Back, S.; Kwon, J.-K.; Kang, B.-C. Development of virus-induced genome editing methods in Solanaceous crops. Hortic. Res. 2023, 11, uhad233. [Google Scholar] [CrossRef]
- Gong, Z.; Previtera, D.A.; Wang, Y.; Botella, J.R. Geminiviral-induced genome editing using miniature CRISPR/Cas12j (CasΦ) and Cas12f variants in plants. Plant Cell Rep. 2024, 43, 71. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.; Kamalu, M.; Shi, H.; Li, Z.; Amerasekera, J.; Zhong, Z.; Adler, B.A.; Song, M.M.; Vohra, K.; Wirnowski, G.; et al. Viral delivery of an RNA-guided genome editor for transgene-free germline editing in Arabidopsis. Nat. Plants 2025, 11, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Baysal, C.; Kausch, A.P.; Cody, J.P.; Altpeter, F.; Voytas, D.F. Rapid and efficient in planta genome editing in sorghum using foxtail mosaic virus-mediated sgRNA delivery. Plant J. 2025, 121, e17196. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, C.; Sun, K.; Deng, Y.; Li, Z. Engineered biocontainable RNA virus vectors for non-transgenic genome editing across crop species and genotypes. Mol. Plant 2023, 16, 616–631. [Google Scholar] [CrossRef]
- Yoshida, T.; Ishikawa, M.; Toki, S.; Ishibashi, K. Heritable tissue-culture-free gene editing in Nicotiana benthamiana through viral delivery of SpCas9 and sgRNA. Plant Cell Physiol. 2024, 65, 1743–1750. [Google Scholar] [CrossRef]
- Qiao, J.-H.; Zang, Y.; Gao, Q.; Liu, S.; Zhang, X.-W.; Hu, W.; Wang, Y.; Han, C.; Li, D.; Wang, X.-B. Transgene- and tissue culture-free heritable genome editing using RNA virus-based delivery in wheat. Nat. Plants 2025, 11, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Ayanoğlu, F.B.; Elçin, A.E.; Elçin, Y.M. Bioethical issues in genome editing by CRISPR-Cas9 technology. Turk. J. Biol. 2020, 44, 110–120. [Google Scholar] [CrossRef]
- Gentzel, I.N.; Ohlson, E.W.; Redinbaugh, M.G.; Wang, G.-L. VIGE: Virus-induced genome editing for improving abiotic and biotic stress traits in plants. Stress. Biol. 2022, 2, 2. [Google Scholar] [CrossRef]
- Chauhan, H.; Alok, A.; Singh, K. Tissue-culture free gene editing in plants using virus-induced gene editing: A brief overview. Nucleus, 2025; in press. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Jiang, X.; Ma, T.; Guo, Y.; Wu, X.; Guo, Y.; Cheng, X. Considerations in engineering viral vectors for genome editing in plants. Virology 2024, 589, 109922. [Google Scholar] [CrossRef]
- Qiu, F.; Xue, C.; Liu, J.; Li, B.; Gao, Q.; Liang, R.; Chen, K.; Gao, C. An efficient mRNA delivery system for genome editing in plants. Plant Biotechnol. J. 2025, 23, 1348–1358. [Google Scholar] [CrossRef]
- Kitagawa, M.; Tran, T.M.; Jackson, D. Traveling with purpose: Cell-to-cell transport of plant mRNAs. Trends Cell Biol. 2024, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Maizel, A.; Markmann, K.; Timmermans, M.; Wachter, A. To move or not to move: Roles and specificity of plant RNA mobility. Curr. Opin. Plant Biol. 2020, 57, 52–60. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, H.; Li, D.; Li, L.; Wang, Z.; Yuan, W.; Xing, Y.; Li, C.; Liang, D. Root-to-shoot long-distance mobile miRNAs identified from Nicotiana rootstocks. Int. J. Mol. Sci. 2021, 22, 12821. [Google Scholar] [CrossRef] [PubMed]
- Thieme, C.J.; Rojas-Triana, M.; Stecyk, E.; Schudoma, C.; Zhang, W.; Yang, L.; Miñambres, M.; Walther, D.; Schulze, W.X.; Paz-Ares, J.; et al. Endogenous Arabidopsis messenger RNAs transported to distant tissues. Nat. Plants 2015, 1, 15025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Thieme, C.J.; Kollwig, G.; Apelt, F.; Yang, L.; Winter, N.; Andresen, N.; Walther, D.; Kragler, F. tRNA-related sequences trigger systemic mRNA transport in plants. Plant Cell 2016, 28, 1237–1249. [Google Scholar] [CrossRef]
- Calderwood, A.; Kopriva, S.; Morris, R.J. Transcript abundance explains mRNA mobility data in Arabidopsis thaliana. Plant Cell 2016, 28, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zheng, Y.; Huang, J.; Zhou, X.; Li, R.; Zha, M.; Wang, S.; Huang, Z.; Lan, H.; Turgeon, R.; et al. Elucidation of the mechanisms of long-distance mRNA movement in a Nicotiana benthamiana/tomato heterograft system. Plant Physiol. 2018, 177, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Notaguchi, M. The use of grafting to study systemic signaling in plants. Plant Cell Physiol. 2017, 58, 1291–1301. [Google Scholar] [CrossRef]
- Melnyk, C.W. Plant grafting: Insights into tissue regeneration. Regeneration 2016, 4, 3–14. [Google Scholar] [CrossRef]
- Feng, M.; Augstein, F.; Kareem, A.; Melnyk, C.W. Plant grafting: Molecular mechanisms and applications. Mol. Plant 2024, 17, 75–91. [Google Scholar] [CrossRef]
- Goldschmidt, E.E. Plant grafting: New mechanisms, evolutionary implications. Front. Plant Sci. 2014, 5, 727. [Google Scholar] [CrossRef]
- Ning, K.; Zhou, W.; Cai, X.; Yan, L.; Ma, Y.; Xie, A.; Wang, Y.; Xu, P. Rootstock-scion exchanging mRNAs participate in watermelon fruit quality improvement. Int. J. Mol. Sci. 2025, 26, 5121. [Google Scholar] [CrossRef]
- Kim, G.; LeBlanc, M.L.; Wafula, E.K.; de Pamphilis, C.W.; Westwood, J.H. Plant science. Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 2014, 345, 808–811. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, L.; Jittayasothorn, Y.; Kang, Y.; Jiao, C.; Fei, Z.; Zhong, G.-Y. Messenger RNA exchange between scions and rootstocks in grafted grapevines. BMC Plant Biol. 2015, 15, 251. [Google Scholar] [CrossRef]
- Notaguchi, M.; Higashiyama, T.; Suzuki, T. Identification of mRNAs that move over long distances using an RNA-Seq analysis of Arabidopsis/Nicotiana benthamiana heterografts. Plant Cell Physiol. 2015, 56, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, Y.; Ham, B.-K.; Chen, J.; Yoshida, A.; Kochian, L.V.; Fei, Z.; Lucas, W.J. Vascular-mediated signalling involved in early phosphate stress response in plants. Nat. Plants 2016, 2, 16033. [Google Scholar] [CrossRef] [PubMed]
- Ham, B.-K.; Lucas, W.J. Phloem-mobile RNAs as systemic signaling agents. Annu. Rev. Plant Biol. 2017, 68, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Canio, W.; Kessler, S.; Sinha, N. Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science 2001, 293, 287–289. [Google Scholar] [CrossRef]
- Kitagawa, M.; Wu, P.; Balkunde, R.; Cunniff, P.; Jackson, D. An RNA exosome subunit mediates cell-to-cell trafficking of a homeobox mRNA via plasmodesmata. Science 2022, 375, 177–182. [Google Scholar] [CrossRef]
- Kehr, J.; Kragler, F. Long distance RNA movement. New Phytol. 2018, 218, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y. Insights into mobile small-RNAs mediated signaling in plants. Plants 2022, 11, 3155. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Bhide, A.J.; Banerjee, A.K. Mobile RNAs and proteins: Impact on plant growth and productivity. J. Exp. Bot. 2025, 76, 3927–3942. [Google Scholar] [CrossRef]
- Sharma, P.; Lin, T.; Grandellis, C.; Yu, M.; Hannapel, D.J. The BEL1-like family of transcription factors in potato. J. Exp. Bot. 2014, 65, 709–723. [Google Scholar] [CrossRef]
- Cho, S.K.; Sharma, P.; Butler, N.M.; Kang, I.-H.; Shah, S.; Rao, A.G.; Hannapel, D.J. Polypyrimidine tract-binding proteins of potato mediate tuberization through an interaction with StBEL5 RNA. J. Exp. Bot. 2015, 66, 6835–6847. [Google Scholar] [CrossRef]
- Du, K.; Zhang, D.; Dan, Z.; Bao, L.; Mu, W.; Zhang, J. Identification of long-distance mobile mRNAs responding to drought stress in heterografted tomato plants. Int. J. Mol. Sci. 2025, 26, 3168. [Google Scholar] [CrossRef]
- Fu, M.; Xu, Z.; Ma, H.; Hao, Y.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X.; Han, Z.; Wu, T. Characteristics of long-distance mobile mRNAs from shoot to root in grafted plant species. Hortic. Plant J. 2024, 10, 25–37. [Google Scholar] [CrossRef]
- Huang, N.-C.; Yu, T.-S. The sequences of Arabidopsis GA-INSENSITIVE RNA constitute the motifs that are necessary and sufficient for RNA long-distance trafficking. Plant J. 2009, 59, 921–929. [Google Scholar] [CrossRef]
- Li, C.; Zhang, K.; Zeng, X.; Jackson, S.; Zhou, Y.; Hong, Y. A cis element within Flowering Locus T mRNA determines its mobility and facilitates trafficking of heterologous viral RNA. J. Virol. 2009, 83, 3540–3548. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Chen, W.; Wang, Y.; Zhang, P.; Shi, N.; Hong, Y. Mobile Flowering Locus T RNA—Biological relevance and biotechnological potential. Front. Plant Sci. 2022, 12, 792192. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Medrano, R.; Xoconostle-Cázares, B.; Lucas, W.J. Phloem long-distance transport of CmNACP mRNA: Implications for supracellular regulation in plants. Development 1999, 126, 4405–4419. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Perrera, V.; Saplaoura, E.; Apelt, F.; Bahin, M.; Kramdi, A.; Olas, J.; Mueller-Roeber, B.; Sokolowska, E.; Zhang, W.; et al. m5C methylation guides systemic transport of messenger RNA over graft junctions in plants. Curr. Biol. 2019, 29, 2465–2476.e5. [Google Scholar] [CrossRef]
- Yang, L.; Machin, F.; Wang, S.; Saplaoura, E.; Kragler, F. Heritable transgene-free genome editing in plants by grafting of wild-type shoots to transgenic donor rootstocks. Nat. Biotechnol. 2023, 41, 958–967. [Google Scholar] [CrossRef]
- Darqui, F.S.; Radonic, L.M.; Beracochea, V.C.; Hopp, H.E.; López Bilbao, M. Peculiarities of the transformation of Asteraceae family species: The cases of sunflower and lettuce. Front. Plant Sci. 2021, 12, 767459. [Google Scholar] [CrossRef]
- Yot, P.; Pinck, M.; Haenni, A.L.; Duranton, H.M.; Chapeville, F. Valine-specific tRNA-like structure in turnip yellow mosaic virus RNA. Proc. Natl. Acad. Sci. USA 1970, 67, 1345–1352. [Google Scholar] [CrossRef]
- Cheng, C.L.; Dewdney, J.; Nam, H.G.; den Boer, B.G.; Goodman, H.M. A new locus (NIA 1) in Arabidopsis thaliana encoding nitrate reductase. EMBO J. 1988, 7, 3309–3314. [Google Scholar] [CrossRef]
- Wang, R.; Tischner, R.; Gutiérrez, R.A.; Hoffman, M.; Xing, X.; Chen, M.; Coruzzi, G.; Crawford, N.M. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol. 2004, 136, 2512–2522. [Google Scholar] [CrossRef]
- Nagai, T.; Ibata, K.; Park, E.S.; Kubota, M.; Mikoshiba, K.; Miyawaki, A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002, 20, 87–90. [Google Scholar] [CrossRef]
- Awan, M.J.A.; Aslam, M.Q.; Naqvi, R.Z.; Amin, I.; Mansoor, S. A graft that crafts nontransgenic and genome-edited plants. Trends Plant Sci. 2023, 28, 614–616. [Google Scholar] [CrossRef]
- Hu, J.; Gao, C. CRISPR-edited plants by grafting. Nat. Biotechnol. 2023, 41, 909–910. [Google Scholar] [CrossRef] [PubMed]
- Zaman, Q.U.; Raza, A.; Gill, R.A.; Hussain, M.A.; Wang, H.F.; Varshney, R.K. New possibilities for trait improvement via mobile CRISPR-RNA. Trends Biotechnol. 2023, 41, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; Liu, T.; Folta, K.; Sarkhosh, A. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hortic. Res. 2022, 9, uhac032. [Google Scholar] [CrossRef] [PubMed]
- Walubengo, D.; Orina, I.; Kubo, Y.; Owino, W. Physico-chemical and postharvest quality characteristics of intra and interspecific grafted tomato fruits. J. Agric. Food Res. 2022, 7, 100261. [Google Scholar] [CrossRef]
- Karaca, M.; Ince, A.G.; Reddy, U.K. Interspecific grafting between Gossypium hirsutum, G. barbadense and G. herbaceum lines. Sci. Rep. 2020, 10, 18649. [Google Scholar] [CrossRef]
- Loupit, G.; Brocard, L.; Ollat, N.; Cookson, S.J. Grafting in plants: Recent discoveries and new applications. J. Exp. Bot. 2023, 74, 2433–2447. [Google Scholar] [CrossRef]
- Lokya, V.; Singh, S.; Chaudhary, R.; Jangra, A.; Tiwari, S. Emerging trends in transgene-free crop development: Insights into genome editing and its regulatory overview. Plant Mol. Biol. 2025, 115, 84. [Google Scholar] [CrossRef]
- Augstein, F.; Melnyk, C.W. Modern and historical uses of plant grafting to engineer development, stress tolerance, chimeras, and hybrids. Plant J. 2025, 121, e70057. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.; Tripathi, A.; Singh, P.; Jones, M.R.W.; Nanda, A.K.; Musseau, C.; Craze, M.; Bowden, S.; Walker, J.F.; Bentley, A.R.; et al. Monocotyledonous plants graft at the embryonic root-shoot interface. Nature 2022, 602, 280–286. [Google Scholar] [CrossRef]
- Jones, T.R.; Patel, S. Gene editing for graft compatibility in apple trees: A future direction. Plant Sci. Biotechnol. 2021, 37, 158–170. [Google Scholar]
- Heeney, M.; Frank, M.H. The mRNA mobileome: Challenges and opportunities for deciphering signals from the noise. Plant Cell 2023, 35, 1817–1833. [Google Scholar] [CrossRef]
- Guan, D.; Xia, Y.; Zhang, S. Analyzing and predicting phloem mobility of macromolecules with an online database. Methods Mol. Biol. 2019, 2014, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Urzì, O.; Gasparro, R.; Ganji, N.R.; Alessandro, R.; Raimondo, S. Plant-RNA in extracellular vesicles: The secret of cross-kingdom communication. Membranes 2022, 12, 352. [Google Scholar] [CrossRef]
- Chukhchin, D.G.; Vashukova, K.; Novozhilov, E. Bordered pit formation in cell walls of spruce tracheids. Plants 2021, 10, 1968. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, C.; Gao, B.; Zhang, Y.; Stewart, E.; Jez, J.; Nakajima, K.; Chen, X. Microtubules promote the non–cell autonomous action of microRNAs by inhibiting their cytoplasmic loading onto ARGONAUTE1 in Arabidopsis. Dev. Cell 2022, 57, 995–1008.e5. [Google Scholar] [CrossRef]
- Xoconostle-Cázares, B.; Xiang, Y.; Ruiz-Medrano, R.; Wang, H.-L.; Monzer, J.; Yoo, B.-C.; McFarland, K.C.; Franceschi, V.R.; Lucas, W.J. Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 1999, 283, 94–98. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, B.C.; Rojas, M.R.; Gomez-Ospina, N.; Staehelin, L.A.; Lucas, W.J. Selective trafficking of non-cell-autonomous proteins mediated by NtNCAPP1. Science 2003, 299, 392–396. [Google Scholar] [CrossRef]
- Haywood, V.; Yu, T.-S.; Huang, N.C.; Lucas, W.J. Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J. 2005, 42, 49–68. [Google Scholar] [CrossRef]
- Ham, B.-K.; Brandom, J.L.; Xoconostle-Cázares, B.; Ringgold, V.; Lough, T.J.; Lucas, W.J. A polypyrimidine tract binding protein, pumpkin RBP50, forms the basis of a phloem-mobile ribonucleoprotein complex. Plant Cell 2009, 21, 197–215. [Google Scholar] [CrossRef]
- Paajanen, P.; Tomkins, M.; Hoerbst, F.; Veevers, R.; Heeney, M.; Thomas, H.R.; Apelt, F.; Saplaoura, E.; Gupta, S.; Frank, M.; et al. Re-analysis of mobile mRNA datasets raises questions about the extent of long-distance mRNA communication. Nat. Plants 2025, 11, 977–984. [Google Scholar] [CrossRef]
- Gao, L.; Kantar, M.B.; Moxley, D.; Ortiz-Barrientos, D.; Rieseberg, L.H. Crop adaptation to climate change: An evolutionary perspective. Mol. Plant 2023, 16, 1518–1546. [Google Scholar] [CrossRef]
- Telem, R.S.; Wani, S.H.; Singh, N.B.; Nandini, R.; Sadhukhan, R.; Bhattacharya, S.; Mandal, N. Cisgenics—A sustainable approach for crop improvement. Curr. Genom. 2013, 14, 468–476. [Google Scholar] [CrossRef]
- Ren, C.; Mohamed, M.S.M.; Aini, N.; Kuang, Y.; Liang, Z. CRISPR/Cas in grapevine genome editing: The best is yet to come. Horticulturae 2024, 10, 965. [Google Scholar] [CrossRef]
- Campos, G.; Chialva, C.; Miras, S.; Lijavetzky, D. New technologies and strategies for grapevine breeding through genetic transformation. Front. Plant Sci. 2021, 12, 767522. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, L.; Malnoy, M.; Gribaudo, I. Breeding next generation tree fruits: Technical and legal challenges. Hortic. Res. 2017, 4, 17067. [Google Scholar] [CrossRef]
- Nurtaza, A.; Dyussembekova, D.; Islamova, S.; Samatova, I.; Zhanybekova, Z.; Umirzakova, A.; Magzumova, G.; Muranets, A.; Kakimzhanova, A. In vitro conservation and genetic diversity analysis of rare species Ribes janczewskii. Sci. Rep. 2024, 14, 31117. [Google Scholar] [CrossRef] [PubMed]
- Yassitepe, J.E.C.T.; da Silva, V.C.H.; Hernandes-Lopes, J.; Dante, R.A.; Gerhardt, I.R.; Fernandes, F.R.; da Silva, P.A.; Vieira, L.R.; Bonatti, V.; Arruda, P. Maize transformation: From plant material to the release of genetically modified and edited varieties. Front. Plant Sci. 2021, 12, 766702. [Google Scholar] [CrossRef]
- Long, Y.; Yang, Y.; Pan, G.; Shen, Y. New insights into tissue culture plant-regeneration mechanisms. Front. Plant. Sci. 2022, 13, 926752. [Google Scholar] [CrossRef]
- Carra, A.; Wijerathna-Yapa, A.; Pathirana, R.; Carimi, F. Development and applications of somatic embryogenesis in grapevine (Vitis spp.). Plants 2024, 13, 3131. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, F.; Wiersma, J.; Scofield, S.; Zhang, C.; Alizadeh, H.; Mohammadi, M. Facts, uncertainties, and opportunities in wheat molecular improvement. Heredity 2024, 133, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Salgotra, R.K.; Chauhan, B.S. Genetic diversity, conservation, and utilization of plant genetic resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef]
- Kaur, A.; Sangha Kaur, M. Crop Wild Relatives (CWRs) a genetic pool for crop improvement: A review. Agric. Rev. 2025, 46, 109–115. [Google Scholar] [CrossRef]
- Czembor, P.C.; Piechota, U.; Song, J.; Mańkowski, D.; Radecka-Janusik, M.; Piaskowska, D.; Słowacki, P.; Kilian, A. Genome-wide association study of seedling leaf rust resistance in European winter wheat cultivars. J. Appl. Genet. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Sun, Y.; Wang, Y.; Luo, L.; Song, Y. Advances in miniature CRISPR-Cas proteins and their applications in gene editing. Arch. Microbiol. 2024, 206, 231. [Google Scholar] [CrossRef]
- Saito, M.; Xu, P.; Faure, G.; Maguire, S.; Kannan, S.; Altae-Tran, H.; Vo, S.; Desimone, A.; Macrae, R.K.; Zhang, F. Fanzor is a eukaryotic programmable RNA-guided endonuclease. Nature 2023, 620, 660–668. [Google Scholar] [CrossRef]
- Nandy, S.; Pathak, B.; Zhao, S.; Srivastava, V. Heat-shock-inducible CRISPR/Cas9 system generates heritable mutations in rice. Plant Direct 2019, 3, e00145. [Google Scholar] [CrossRef]
- Liang, Z.; Wei, S.; Wu, Y.; Guo, Y.; Zhang, B.; Yang, H. Temporally gene knockout using heat shock–inducible genome-editing system in plants. Plant Genome 2023, 16, e20376. [Google Scholar] [CrossRef]
- Sen, M.K.; Sellamuthu, G.; Mondal, S.K.; Varshney, R.K.; Roy, A. Epigenome editing for herbicide resistance crops. Trends Plant Sci. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
| Method | DNA Integration | Use of Viral Vectors/Bacteria | Main Advantages | Main Disadvantages |
|---|---|---|---|---|
| Genome editing by grafting (GEG) | No | Yes (only in the rootstock) |
|
|
| Virus-Induced Genome Editing (VIGE) | No | Yes |
|
|
| Agrobacterium-mediated transformation | Yes | Yes |
|
|
| Biolistic bombardment | Yes/No | No |
|
|
| Direct physical delivery (PEG-mediated uptake, microinjection, electroporation) | No (unless DNA is delivered) | No |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simoni, S.; Fambrini, M.; Pugliesi, C.; Rogo, U. Genome Editing by Grafting. Int. J. Mol. Sci. 2025, 26, 9294. https://doi.org/10.3390/ijms26199294
Simoni S, Fambrini M, Pugliesi C, Rogo U. Genome Editing by Grafting. International Journal of Molecular Sciences. 2025; 26(19):9294. https://doi.org/10.3390/ijms26199294
Chicago/Turabian StyleSimoni, Samuel, Marco Fambrini, Claudio Pugliesi, and Ugo Rogo. 2025. "Genome Editing by Grafting" International Journal of Molecular Sciences 26, no. 19: 9294. https://doi.org/10.3390/ijms26199294
APA StyleSimoni, S., Fambrini, M., Pugliesi, C., & Rogo, U. (2025). Genome Editing by Grafting. International Journal of Molecular Sciences, 26(19), 9294. https://doi.org/10.3390/ijms26199294







