Gut Microbiome as a Source of Probiotic Drugs for Parkinson’s Disease
Abstract
1. Introduction
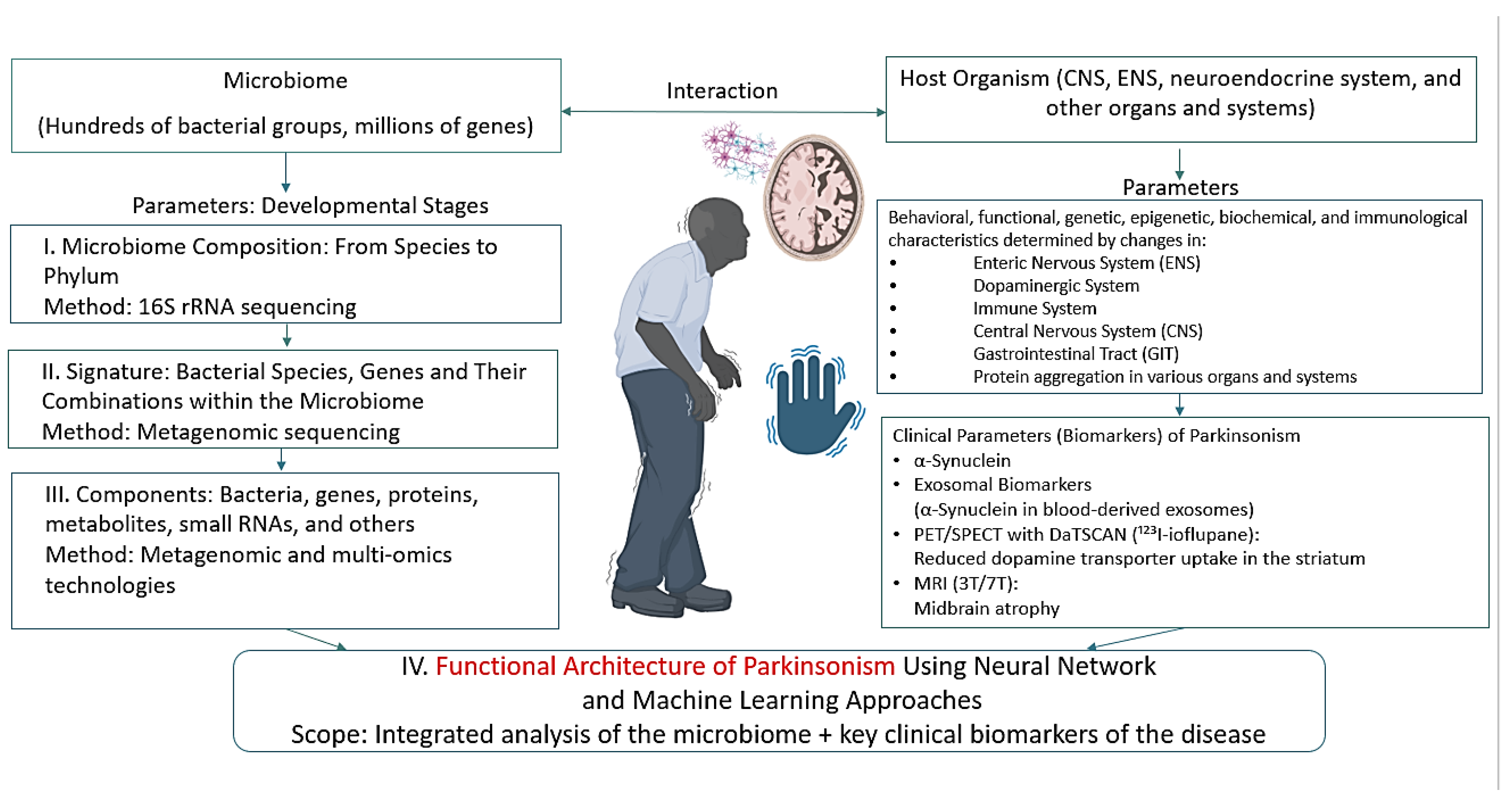
2. Current Understanding of the Development of Parkinson’s Disease
2.1. Etiology of Parkinson’s Disease
2.2. Motor and Non-Motor Symptoms in Parkinson’s Disease
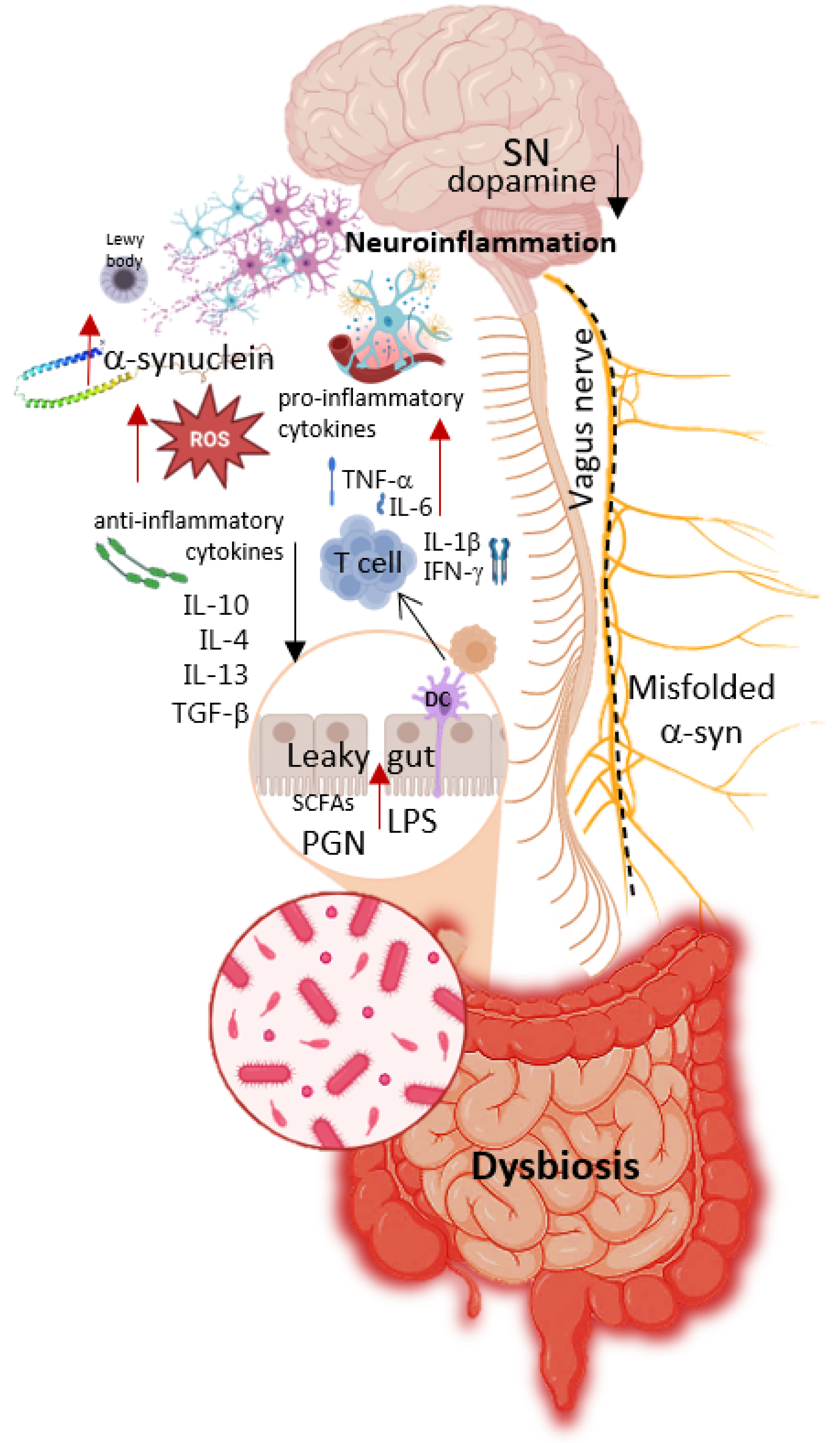
2.3. Pathogenesis of Parkinson’s Disease
2.4. Modeling Parkinson’s Disease in Rodents
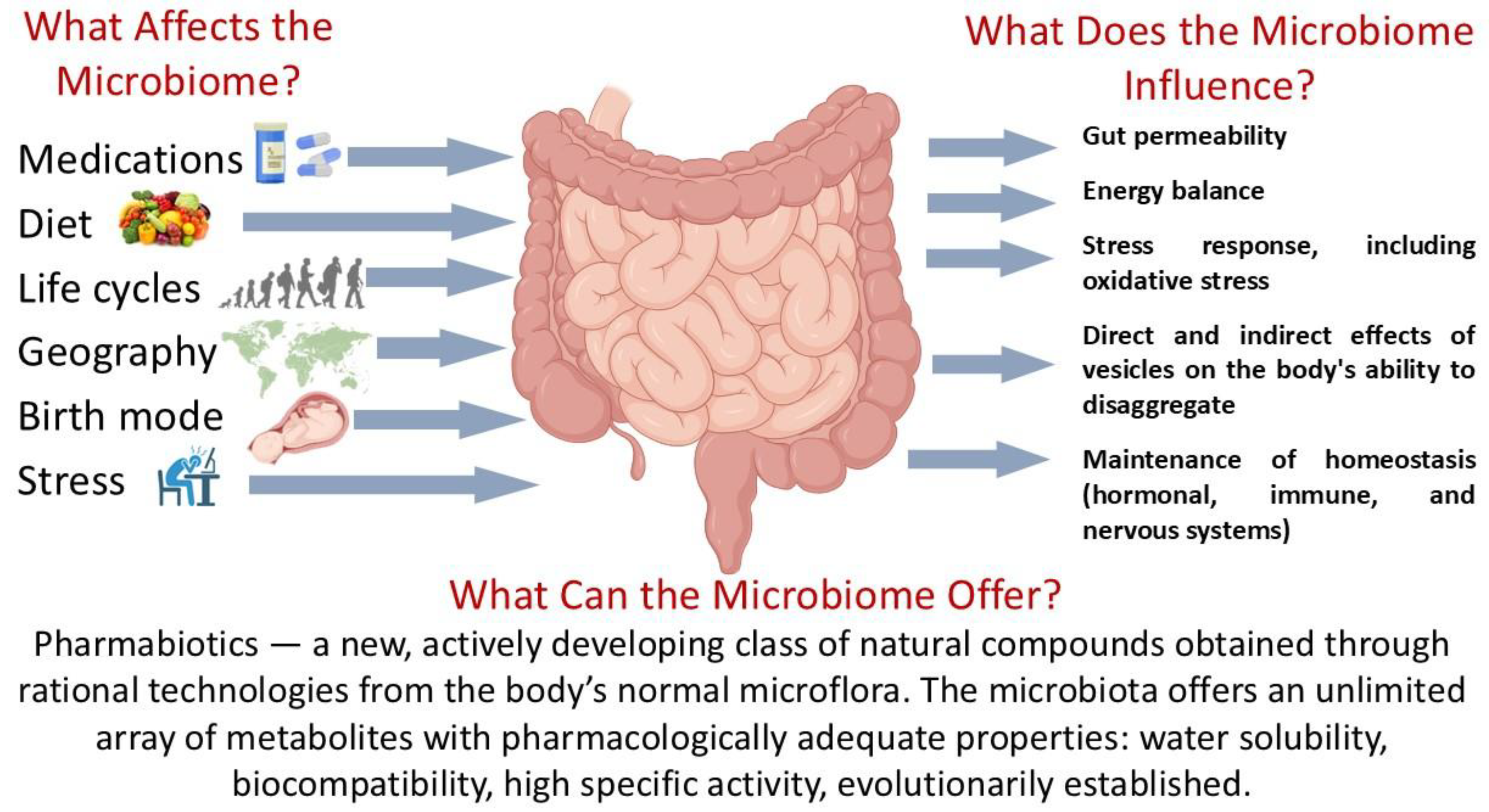
3. Gut Microbiome and Parkinson’s Disease
4. Probiotics and Metabiotics: Existing Potential for the Development of Live Biotherapeutic Products and Pharmabiotics
4.1. Probiotics in Parkinson’s Disease: Analysis of Neuroinflammatory, Immunomodulatory, and Neuromodulatory Activity in Animal Studies
4.2. Probiotics in Parkinson’s Disease: Human Studies
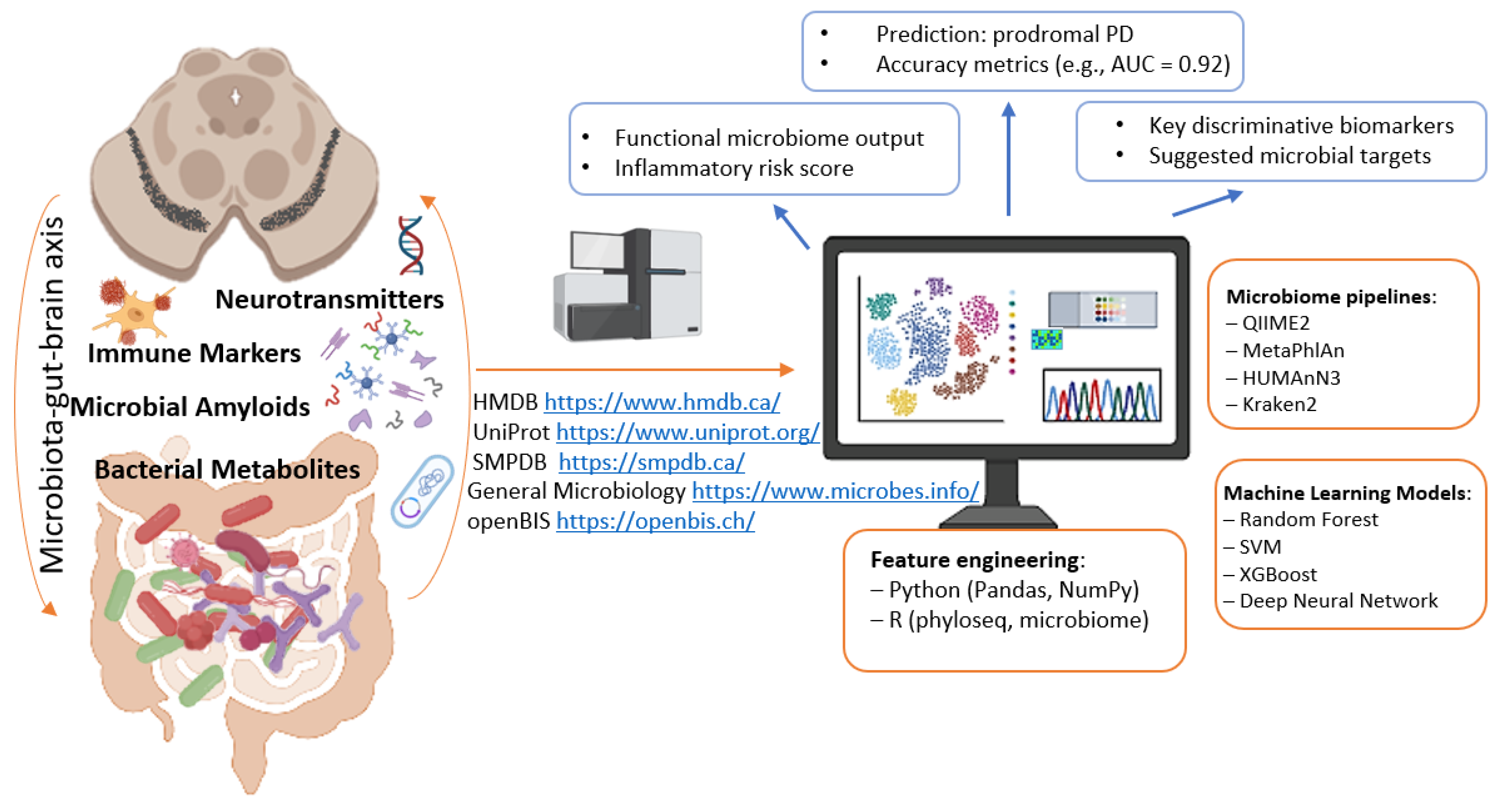
5. Neurodegenerative Diseases—Parkinson’s Disease—The Potential of Machine Learning for Diagnosis and Treatment
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| BBB | Blood-Brain Barrier |
| CFU | Colony Forming Units |
| CNS | Central Nervous System |
| COMT | Catechol-O-methyltransferase |
| ENS | Enteric Nervous System |
| EVs | Extracellular Vesicles |
| FMT | Fecal Microbiota Transplantation |
| GABA | Gamma-Aminobutyric Acid |
| GSH | Reduced Glutathione |
| IL-6 | Interleukin-6 |
| INF-γ | Interferon-gamma |
| LBPs | Live Biotherapeutic Products |
| LPS | Lipopolysaccharides |
| MPTP | 1-methyl-4-phenyl-1:2,3,6-tetrahydropyridine |
| NMS | Non-Motor Symptoms |
| PD | Parkinson’s Disease |
| QS | Quorum Sensing |
| ROS | Reactive Oxygen Species |
| SCFAs | Short-Chain Fatty Acids |
| SN | Substantia Nigra |
| SOD | Superoxide Dismutase |
| TH | Tyrosine Hydroxylase |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- Morais, L.H.; Schreiber, H.L., 4th; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Houser, M.C.; Tansey, M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? npj Park. Dis. 2017, 3, 3. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G.; Del Tredici, K.; Braak, H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013, 9, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubev, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; de Vos, R.A.; Bohl, J.; Del Tredici, K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 2006, 396, 67–72. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef]
- Huh, E.; Choi, J.G.; Choi, Y.; Ju, I.G.; Kim, B.; Shin, Y.J.; An, J.M.; Park, M.G.; Yim, S.V.; Chung, S.J.; et al. P. mirabilis-derived pore-forming haemolysin, HpmA drives intestinal alpha-synuclein aggregation in a mouse model of neurodegeneration. EBioMedicine 2023, 98, 104887. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, M.; Xue, J.; Xiang, L.; Li, Y.; Xiao, J.; Xiao, G.; Wang, H.L. EGCG ameliorates neuronal and behavioral defects by remodeling gut microbiota and TotM expression in Drosophila models of Parkinson’s disease. FASEB J. 2020, 34, 5931–5950. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Ma, Y.; Yan, X.; Duan, Y.; Zhang, X.; Dong, H.; Fang, R.; Zhang, Y.; Li, Q.; et al. Citrobacter rodentium infection impairs dopamine metabolism and exacerbates the pathology of Parkinson’s disease in mice. J. Neuroinflamm. 2024, 21, 153. [Google Scholar] [CrossRef]
- Hasegawa, M.; Nonaka, T.; Masuda-Suzukake, M. Prion-like mechanisms and potential therapeutic targets in neurodegenerative disorders. Pharmacol. Ther. 2017, 172, 22–33. [Google Scholar] [CrossRef]
- Alfaidi, M.; Barker, R.A.; Kuan, W.L. An update on immune-based alpha-synuclein trials in Parkinson’s disease. J. Neurol. 2024, 272, 21. [Google Scholar] [CrossRef]
- Haas-Neill, S.; Forsythe, P. A Budding Relationship: Bacterial Extracellular Vesicles in the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2020, 21, 8899. [Google Scholar] [CrossRef] [PubMed]
- Bleibel, L.; Dziomba, S.; Waleron, K.F.; Kowalczyk, E.; Karbownik, M.S. Deciphering psychobiotics’ mechanism of action: Bacterial extracellular vesicles in the spotlight. Front. Microbiol. 2023, 14, 1211447. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yin, Y.; Zhou, X. Insights into Microbiota-Host Crosstalk in the Intestinal Diseases Mediated by Extracellular Vesicles and Their Encapsulated MicroRNAs. Int. J. Mol. Sci. 2024, 25, 13001. [Google Scholar] [CrossRef] [PubMed]
- Illarioshkin, S.N. Modern concepts of Parkinson’s disease etiology. Nevrol. Zhurnal 2015, 20, 4–13. (In Russian) [Google Scholar] [CrossRef]
- Xiao, B.; Zhou, Z.; Chao, Y.; Tan, E.K. Pathogenesis of Parkinson’s Disease. Neurol. Clin. 2025, 43, 185–207. [Google Scholar] [CrossRef]
- Salmina, A.B.; Kapkaeva, M.R.; Vetchinova, A.S.; Illarioshkin, S.N. Novel Approaches Used to Examine and Control Neurogenesis in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 9608. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Huang, Z.; Fan, X.; Wang, X.; Chen, X.; Guo, H.; Liu, H.; Li, S.; Yu, S.; et al. Dopaminergic system and neurons: Role in multiple neurological diseases. Neuropharmacology 2024, 260, 110133. [Google Scholar] [CrossRef]
- Chen, Z.; Li, G.; Liu, J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 2020, 134, 104700. [Google Scholar] [CrossRef]
- Westenberger, A.; Brüggemann, N.; Klein, C. Genetics of Parkinson’s Disease: From Causes to Treatment. Cold Spring Harb. Perspect. Med. 2025, 15, a041774. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Marinus, J.; Zhu, K.; Marras, C.; Aarsland, D.; van Hilten, J.J. Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 2018, 17, 559–568. [Google Scholar] [CrossRef]
- Fullard, M.E.; Morley, J.F.; Duda, J.E. Olfactory Dysfunction as an Early Biomarker in Parkinson’s Disease. Neurosci. Bull. 2017, 33, 515–525. [Google Scholar] [CrossRef]
- Stefani, A.; Högl, B. Sleep in Parkinson’s disease. Neuropsychopharmacology 2020, 45, 121–128. [Google Scholar] [CrossRef]
- Weintraub, D.; Aarsland, D.; Chaudhuri, K.R.; Dobkin, R.D.; Leentjens, A.F.; Rodriguez-Violante, M.; Schrag, A. The neuropsychiatry of Parkinson’s disease: Advances and challenges. Lancet Neurol. 2022, 21, 89–102. [Google Scholar] [CrossRef]
- Kawahata, I.; Fukunaga, K. Degradation of Tyrosine Hydroxylase by the Ubiquitin-Proteasome System in the Pathogenesis of Parkinson’s Disease and Dopa-Responsive Dystonia. Int. J. Mol. Sci. 2020, 21, 3779. [Google Scholar] [CrossRef]
- Rausch, W.D.; Wang, F.; Radad, K. From the tyrosine hydroxylase hypothesis of Parkinson’s disease to modern strategies: A short historical overview. J. Neural. Transm. 2022, 129, 487–495. [Google Scholar] [CrossRef]
- Segura-Aguilar, J.; Paris, I.; Muñoz, P.; Ferrari, E.; Zecca, L.; Zucca, F.A. Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem. 2014, 129, 898–915. [Google Scholar] [CrossRef]
- Calabresi, P.; Di Lazzaro, G.; Marino, G.; Campanelli, F.; Ghiglieri, V. Advances in understanding the function of alpha-synuclein: Implications for Parkinson’s disease. Brain 2023, 146, 3587–3597. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Tanji, K.; Odagiri, S.; Miki, Y.; Mori, F.; Takahashi, H. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol. Neurobiol. 2013, 47, 495–508. [Google Scholar] [CrossRef]
- Atik, A.; Stewart, T.; Zhang, J. Alpha-Synuclein as a Biomarker for Parkinson’s Disease. Brain Pathol. 2016, 26, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, T.N.; Logvinenko, A.A.; Poleshchuk, V.V.; Illarioshkin, S.N. The state of systemic oxidative stress during Parkinson’s disease. Neurochem. J. 2017, 11, 340–345. [Google Scholar] [CrossRef]
- Caproni, S.; Di Fonzo, A.; Colosimo, C. Oxidative Stress: A New Pathophysiological Pathway in Parkinson’s Disease and a Potential Target of the Brain-Sport Crosstalk. Park. Dis. 2025, 2025, 6691390. [Google Scholar] [CrossRef] [PubMed]
- Mahoney-Sánchez, L.; Bouchaoui, H.; Ayton, S.; Devos, D.; Duce, J.A.; Devedjian, J.C. Ferroptosis and its potential role in the physiopathology of Parkinson’s Disease. Prog. Neurobiol. 2021, 196, 101890. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, X.; Yin, Y.; Thomas, E.R.; Liu, K.; Wang, W.; Li, X. The interplay of iron, oxidative stress, and α-synuclein in Parkinson’s disease progression. Mol. Med. 2025, 31, 154. [Google Scholar] [CrossRef]
- Pajares, M.; Rojo, A.I.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Wang, C.; Yang, T.; Liang, M.; Xie, J.; Song, N. Astrocyte dysfunction in Parkinson’s disease: From the perspectives of transmitted α-synuclein and genetic modulation. Transl. Neurodegener. 2021, 10, 39. [Google Scholar] [CrossRef]
- Isik, S.; Yeman Kiyak, B.; Akbayir, R.; Seyhali, R.; Arpaci, T. Microglia Mediated Neuroinflammation in Parkinson’s Disease. Cells 2023, 127, 1012. [Google Scholar] [CrossRef]
- Khan, E.; Hasan, I.; Haque, M.E. Parkinson’s Disease: Exploring Different Animal Model Systems. Int. J. Mol. Sci. 2023, 24, 9088. [Google Scholar] [CrossRef]
- Okyere, S.K.; Zeng, C.; Yue, D.; Hu, Y. Neurotoxic Mechanism and Shortcomings of MPTP, 6-OHDA, Rotenone and Paraquat-induced Parkinson’s Disease Animal Models. Venoms Toxins 2021, 1, 27–40. [Google Scholar] [CrossRef]
- Ungerstedt, U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 1968, 5, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Simola, N.; Morelli, M.; Carta, A.R. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox. Res. 2007, 11, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Masini, D.; Plewnia, C.; Bertho, M.; Scalbert, N.; Caggiano, V.; Fisone, G. A Guide to the Generation of a 6-Hydroxydopamine Mouse Model of Parkinson’s Disease for the Study of Non-Motor Symptoms. Biomedicines 2021, 9, 598. [Google Scholar] [CrossRef]
- Mondal, S.; Firdous, S.M. Unrevealing the molecular mechanisms of MPTP-induced Parkinson’s in experimental animals. Med. Chem. Res. 2025. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J.; Remião, F.; Carmo, H.; Duarte, J.A.; Navarro, A.S.; Bastos, M.L.; Carvalho, F. Paraquat exposure as an etiological factor of Parkinson’s disease. Neurotoxicology 2006, 27, 1110–1122. [Google Scholar] [CrossRef]
- Johnson, M.E.; Bobrovskaya, L. An update on the rotenone models of Parkinson’s disease: Their ability to reproduce the features of clinical disease and model gene-environment interactions. Neurotoxicology 2015, 46, 101–116. [Google Scholar] [CrossRef]
- Sharma, P.; Mittal, P. Paraquat (herbicide) as a cause of Parkinson’s Disease. Park. Relat. Disord. 2024, 119, 105932. [Google Scholar] [CrossRef]
- Kovtun, A.S.; Averina, O.V.; Zakharevich, N.V.; Kasianov, A.S.; Danilenko, V.N. In silico identification of metagenomic signature describing neurometabolic potential of normal human gut microbiota. Russ. J. Genet. 2018, 54, 1101–1110. [Google Scholar] [CrossRef]
- Averina, O.V.; Poluektova, E.U.; Marsova, M.V.; Danilenko, V.N. Biomarkers and Utility of the Antioxidant Potential of Probiotic Lactobacilli and Bifidobacteria as Representatives of the Human Gut Microbiota. Biomedicines 2021, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Joos, R.; Boucher, K.; Lavelle, A.; Arumugam, M.; Blaser, M.J.; Claesson, M.J.; Clarke, G.; Cotter, P.D.; De Sordi, L.; Dominguez-Bello, M.G.; et al. Examining the healthy human microbiome concept. Nat. Rev. Microbiol. 2025, 23, 192–205, Erratum in Nat. Rev. Microbiol. 2025, 23, 206. https://doi.org/10.1038/s41579-024-01145-8. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, K.; Su, N.; Yuan, C.; Zhang, N.; Hu, X.; Fu, Y.; Zhao, F. Microbiota-gut-brain axis in health and neurological disease: Interactions between gut microbiota and the nervous system. J. Cell. Mol. Med. 2024, 28, e70099. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shao, Y.; Hu, Y.; Qian, J.; Wang, P.; Tian, L.; Ni, Y.; Li, S.; Al-Nusaif, M.; Liu, C.; et al. Fecal microbiota from patients with Parkinson’s disease intensifies inflammation and neurodegeneration in A53T mice. CNS Neurosci. Ther. 2024, 30, e70003. [Google Scholar] [CrossRef]
- Borghammer, P.; Van Den Berge, N. Brain-first versus gut-first Parkinson’s disease: A hypothesis. J. Park. Dis. 2019, 9, S281–S295. [Google Scholar] [CrossRef]
- Horsager, J.; Borghammer, P. Brain-first vs. body-first Parkinson’s disease: An update on recent evidence. Park. Relat. Disord. 2024, 122, 106101. [Google Scholar] [CrossRef]
- Metcalfe-Roach, A.; Cirstea, M.S.; Yu, A.C.; Ramay, H.R.; Coker, O.; Boroomand, S.; Kharazyan, F.; Martino, D.; Sycuro, L.K.; Appel-Cresswell, S.; et al. Metagenomic analysis reveals large-scale disruptions of the gut microbiome in Parkinson’s disease. Mov. Disord. 2024, 39, 1740–1751. [Google Scholar] [CrossRef]
- Suresh, S.B.; Malireddi, A.; Abera, M.; Noor, K.; Ansar, M.; Boddeti, S.; Nath, T.S. Gut Microbiome and Its Role in Parkinson’s Disease. Cureus 2024, 16, e73150. [Google Scholar] [CrossRef]
- Gallop, A.; Weagley, J.; Paracha, S.U.; Grossberg, G. The Role of The Gut Microbiome in Parkinson’s Disease. J. Geriatr. Psychiatry Neurol. 2021, 34, 253–262. [Google Scholar] [CrossRef]
- Li, Z.; Liang, H.; Hu, Y.; Lu, L.; Zheng, C.; Fan, Y.; Wu, B.; Zou, T.; Luo, X.; Zhang, X.; et al. Gut bacterial profiles in Parkinson’s disease: A systematic review. CNS Neurosci. Ther. 2023, 29, 140–157. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ueyama, J.; Ito, M.; Hamaguchi, T.; Takimoto, K.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Mori, H.; Kurokawa, K.; et al. Meta-analysis of shotgun sequencing of gut microbiota in Parkinson’s disease. npj Park. Dis. 2024, 10, 106. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, J.; Zhao, D.; Chen, B.; Wang, Q.; Li, Y.; Chen, J.; Bai, C.; Guo, X.; Hu, N.; et al. Microbial biomarker discovery in Parkinson’s disease through a network-based approach. npj Park. Dis. 2024, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Yang, J.Y.; Yan, Z.H.; Hu, S.; Li, J.Q.; Xu, Z.X.; Jian, Y.P. Recent findings in Akkermansia muciniphila-regulated metabolism and its role in intestinal diseases. Clin. Nutr. 2022, 41, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Shkoporov, A.N.; Chaplin, A.V.; Shcherbakova, V.A.; Suzina, N.E.; Kafarskaia, L.I.; Bozhenko, V.K.; Efimov, B.A. Ruthenibacterium lactatiformans gen. nov., sp. nov., an anaerobic, lactate-producing member of the family Ruminococcaceae isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2016, 66, 3041–3049. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe-Roach, A.; Finlay, B.B. The role of the gut microbiome in Parkinson’s disease. Future Neurol. 2025, 20, 2494981. [Google Scholar] [CrossRef]
- VanOtterloo, L.M.; Trent, M.S. Microbial Primer: Lipopolysaccharide—A remarkable component of the Gram-negative bacterial surface. Microbiology 2024, 170, 1439. [Google Scholar] [CrossRef]
- Brown, G.C.; Camacho, M.; Williams-Gray, C.H. The Endotoxin Hypothesis of Parkinson’s Disease. Mov. Disord. 2023, 38, 1143–1155. [Google Scholar] [CrossRef]
- Chen, S.J.; Chen, C.C.; Liao, H.Y.; Lin, Y.T.; Wu, Y.W.; Liou, J.M.; Wu, M.S.; Kuo, C.H.; Lin, C.H. Association of Fecal and Plasma Levels of Short-Chain Fatty Acids with Gut Microbiota and Clinical Severity in Patients with Parkinson Disease. Neurology 2022, 98, e848–e858. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Q.; Su, R. Interplay of human gastrointestinal microbiota metabolites: Short-chain fatty acids and their correlation with Parkinson’s disease. Medicine 2024, 103, e37960. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Rund, L.; Hutchinson, N.T.; Woods, J.A.; Steelman, A.J.; Johnson, R.W. Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Sci. Rep. 2023, 13, 2819. [Google Scholar] [CrossRef] [PubMed]
- Krueger, M.E.; Boles, J.S.; Simon, Z.D.; Alvarez, S.D.; McFarland, N.R.; Okun, M.S.; Zimmermann, E.M.; Forsmark, C.E.; Tansey, M.G. Comparative analysis of Parkinson’s and inflammatory bowel disease gut microbiomes reveals shared butyrate-producing bacteria depletion. npj Park. Dis. 2025, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; An, K.; Wang, D.; Yu, H.; Li, J.; Min, Z.; Xiong, Y.; Xue, Z.; Mao, Z. Short-Chain Fatty Acid Aggregates Alpha-Synuclein Accumulation and Neuroinflammation via GPR43-NLRP3 Signaling Pathway in a Model Parkinson’s Disease. Mol. Neurobiol. 2025, 62, 6612–6625. [Google Scholar] [CrossRef] [PubMed]
- Renaldi, R.; Wiguna, T.; Persico, A.M.; Tanra, A.J. p-Cresol and p-Cresyl Sulphate Boost Oxidative Stress: A Systematic Review of Recent Evidence. Basic Clin. Pharmacol. Toxicol. 2025, 137, e70065. [Google Scholar] [CrossRef]
- Paul, K.C.; Zhang, K.; Walker, D.I.; Sinsheimer, J.; Yu, Y.; Kusters, C.; Del Rosario, I.; Folle, A.D.; Keener, A.M.; Bronstein, J.; et al. Untargeted serum metabolomics reveals novel metabolite associations and disruptions in amino acid and lipid metabolism in Parkinson’s disease. Mol. Neurodegener. 2023, 18, 100. [Google Scholar] [CrossRef]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Boertien, J.M.; Murtomäki, K.; Pereira, P.A.B.; van der Zee, S.; Mertsalmi, T.H.; Levo, R.; Nojonen, T.; Mäkinen, E.; Jaakkola, E.; Laine, P.; et al. Fecal microbiome alterations in treatment-naive de novo Parkinson’s disease. npj Park. Dis. 2022, 8, 129. [Google Scholar] [CrossRef]
- Pereira, F.C.; Ge, X.; Kristensen, J.M.; Kirkegaard, R.H.; Maritsch, K.; Szamosvári, D.; Imminger, S.; Seki, D.; Shazzad, J.B.; Zhu, Y.; et al. The Parkinson’s disease drug entacapone disrupts gut microbiome homoeostasis via iron sequestration. Nat. Microbiol. 2024, 9, 3165–3183. [Google Scholar] [CrossRef]
- Cirstea, M.S.; Creus-Cuadros, A.; Lo, C.; Yu, A.C.; Serapio-Palacios, A.; Neilson, S.; Appel-Cresswell, S.; Finlay, B.B. A novel pathway of levodopa metabolism by commensal Bifidobacteria. Sci. Rep. 2023, 13, 19155. [Google Scholar] [CrossRef]
- Xu, K.; Sheng, S.; Zhang, F. Relationship Between Gut Bacteria and Levodopa Metabolism. Curr. Neuropharmacol. 2023, 21, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Auffret, M.; Meuric, V.; Boyer, E.; Bonnaure-Mallet, M.; Vérin, M. Oral Health Disorders in Parkinson’s Disease: More than Meets the Eye. J. Park. Dis. 2021, 11, 1507–1535. [Google Scholar] [CrossRef] [PubMed]
- Murcia-Flores, L.; Sánchez-García, A.; Pecci-Lloret, M.P.; Rodríguez-Lozano, F.J. Association between oral dysbiosis and Parkinson’s disease: A systematic review. Front. Cell. Infect. Microbiol. 2025, 15, 1564362. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Psychotropics and the Microbiome: A Chamber of Secrets. Psychopharmacology 2019, 236, 1411–1432. [Google Scholar] [CrossRef]
- Alam, M.; Abbas, K.; Mustafa, M.; Usmani, N.; Habib, S. Microbiome-based therapies for Parkinson’s disease. Front Nutr. 2024, 11, 1496616. [Google Scholar] [CrossRef]
- Ghosh, D.; Ghosh, S.; Sharma, M.; Datta, S. A review of gut microbiota as a therapeutic approach for Parkinson’s disease. MGM J. Med. Sci. 2024, 11, 763–771. [Google Scholar] [CrossRef]
- da Silva, T.F.; Glória, R.A.; Americo, M.F.; Freitas, A.D.S.; de Jesus, L.C.L.; Barroso, F.A.L.; Laguna, J.G.; Coelho-Rocha, N.D.; Tavares, L.M.; le Loir, Y.; et al. Unlocking the Potential of Probiotics: A Comprehensive Review on Research, Production, and Regulation of Probiotics. Probiotics Antimicrob. Proteins 2024, 16, 1687–1723. [Google Scholar] [CrossRef]
- Tan, A.H.; Hor, J.W.; Chong, C.W.; Lim, S.Y. Probiotics for Parkinson’s disease: Current evidence and future directions. JGH Open 2020, 5, 414–419. [Google Scholar] [CrossRef]
- Mirzaei, H.; Sedighi, S.; Kouchaki, E.; Barati, E.; Dadgostar, E.; Aschner, M.; Tamtaji, O.R. Probiotics and the Treatment of Parkinson’s Disease: An Update. Cell. Mol. Neurobiol. 2022, 42, 2449–2457. [Google Scholar] [CrossRef]
- Zhu, F.; Yin, S.; Wang, Y.; Zhong, Y.; Ji, Q.; Wu, J. Effects of Probiotics on Neurodegenerative Disease-Related Symptoms and Systemic Inflammation: A Systematic Review. Int. J. Gen. Med. 2024, 17, 5941–5958. [Google Scholar] [CrossRef]
- Rout, M.; Prusty, S.K.; Kar, D.M. Probiotic: A Gut Microbiota-Based Therapeutic Approaches for the Treatment of Parkinson’s Disease. Curr. Rev. Clin. Exp. Pharmacol. 2024, 20, 322–333. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, Y.; Lee, S.; Oh, M.S. Probiotics as Potential Treatments for Neurodegenerative Diseases: A Review of the Evidence from in vivo to Clinical Trial. Biomol. Ther. 2025, 33, 54–74. [Google Scholar] [CrossRef]
- Kumar, D.; Bishnoi, M.; Kondepudi, K.K.; Sharma, S.S. Gut Microbiota-Based Interventions for Parkinson’s Disease: Neuroprotective Mechanisms and Current Perspective. Probiotics Antimicrob. Proteins 2025, 17, 2438–2460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wong, K.Y.; Xie, H. Modulation of Intestinal Inflammation and Protection of Dopaminergic Neurons in Parkinson’s Disease Mice through a Probiotic Formulation Targeting NLRP3 Inflammasome. J. Neuroimmune Pharmacol. 2025, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Patel, A.; Acharya, S.; Patel, A.; Shah, U.; Patel, S.; Solanki, N.; Patel, M. Novel approaches to improve systemic bioavailability of curcumin using probiotics for rotenone-induced Parkinson’s disease in rodents. Am. J. Transl. Res. 2025, 17, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Abou-Hany, H.O.; Shata, A.; Hellal, D.; El-Baz, A.M.; ElSaid, Z.H.; Haleem, A.A.; Morsy, N.E.; Abozied, R.M.; Elbrolosy, B.M.; et al. Vinpocetine and Lactobacillus Attenuated Rotenone-Induced Parkinson’s Disease and Restored Dopamine Synthesis in Rats through Modulation of Oxidative Stress, Neuroinflammation, and Lewy Bodies Inclusion. J. Neuroimmune Pharmacol. 2025, 20, 22. [Google Scholar] [CrossRef]
- Wang, W.; Shi, S.; Su, M.; Li, Y.; Yao, X.; Jiang, J.; Yao, W.; Qin, X.; Wang, Z.; Tang, C. Akkermansia muciniphila Akk11 Supplementation Attenuates MPTP-Induced Neurodegeneration by Inhibiting Microglial NLRP3 Inflammasome. Probiotics Antimicrob. Proteins 2025, 17, 2348–2361. [Google Scholar] [CrossRef]
- Francisco, M.; Grau, R. Biofilm proficient Bacillus subtilis prevents neurodegeneration in Caenorhabditis elegans Parkinson’s disease models via PMK-1/p38 MAPK and SKN-1/Nrf2 signaling. Sci. Rep. 2025, 15, 9864. [Google Scholar] [CrossRef]
- Sheikh, K.; Arasteh, J.; Tajabadi Ebrahimi, M.; Hesampour, A. Membrane vesicles from Lactobacillus acidophilus reduce intestinal inflammation and increase 5-HT in the substantia nigra of rats with Parkinson’s disease. Arch. Med. Res. 2025, 56, 103143. [Google Scholar] [CrossRef]
- Qi, Y.; Dong, Y.; Chen, J.; Xie, S.; Ma, X.; Yu, X.; Yu, Y.; Wang, Y. Lactiplantibacillus plantarum SG5 inhibits neuroinflammation in MPTP-induced PD mice through GLP-1/PGC-1α pathway. Exp. Neurol. 2025, 383, 115001. [Google Scholar] [CrossRef]
- Aktas, B.; Aslim, B.; Ozdemir, D.A. A Neurotherapeutic Approach with Lacticaseibacillus rhamnosus E9 on Gut Microbiota and Intestinal Barrier in MPTP-Induced Mouse Model of Parkinson’s Disease. Sci. Rep. 2024, 14, 15460. [Google Scholar] [CrossRef]
- Pérez Visñuk, D.; LeBlanc, J.G.; de Moreno de LeBlanc, A. Neuroprotective Effects Exerted by a Combination of Selected Lactic Acid Bacteria in a Mouse Parkinsonism Model under Levodopa-Benserazide Treatment. Neurochem. Res. 2024, 49, 2940–2956. [Google Scholar] [CrossRef]
- Valvaikar, S.; Vaidya, B.; Sharma, S.; Bishnoi, M.; Kondepudi, K.K.; Sharma, S.S. Supplementation of probiotic Bifidobacterium breve Bif11 reverses neurobehavioural deficits, inflammatory changes and oxidative stress in Parkinson’s disease model. Neurochem. Int. 2024, 174, 05691. [Google Scholar] [CrossRef]
- Dong, Y.; Qi, Y.; Chen, J.; Han, S.; Su, W.; Ma, X.; Yu, Y.; Wang, Y. Neuroprotective Effects of Bifidobacterium animalis subsp. lactis NJ241 in a Mouse Model of Parkinson’s Disease: Implications for Gut Microbiota and PGC-1α. Mol. Neurobiol. 2024, 61, 7534–7548. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.P.; Yeh, T.H.; Lin, Y.T.; Pan, C.H.; Lee, Y.K.; Wu, C.H.; Huang, H.Y. Supplementation with Bifidobacterium animalis subsp. lactis MH-022 for remission of motor impairments in a 6-OHDA-induced Parkinson’s disease rat model by reducing inflammation, reshaping the gut microbiome, and fostering specific microbial taxa. Food Funct. 2024, 15, 9368–9389. [Google Scholar] [CrossRef]
- Li, T.; Zhang, C.; Qiannan Wang, Q.; Wang, S.; Yu, L.; Qixiao Zhai, Q.; Tian, F. Exploring the therapeutic potential of Bifidobacterium longum subsp. longum CCFM1029 in Parkinson’s disease: Insights from behavioral, neurophysiological, gut microbiota, and microbial metabolites analysis. Foods 2024, 5, 156. [Google Scholar] [CrossRef]
- Stavrovskaya, A.V.; Voronkov, D.N.; Marsova, M.V.; Olshansky, A.S.; Gushchina, A.S.; Danilenko, V.N.; Illarioshkin, S.N. Effects of the pharmabiotic U-21 in a combined neuroinflammatory model of Parkinson’s disease in rats. Bull. Exp. Biol. Med. 2024, 177, 193–199. (In Russian) [Google Scholar] [CrossRef]
- Li, T.; Chu, C.; Yu, L.; Zhai, Q.; Wang, S.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. Neuroprotective effects of Bifidobacterium breve CCFM1067 in MPTP-induced mouse models of Parkinson’s disease. Nutrients 2022, 14, 4678. [Google Scholar] [CrossRef]
- Wu, H.; Wei, J.; Zhao, X.; Liu, Y.; Chen, Z.; Wei, K.; Lu, J.; Chen, W.; Jiang, M.; Li, S.; et al. Neuroprotective effects of an engineered Escherichia coli Nissle 1917 on Parkinson’s disease in mice by delivering GLP-1 and modulating gut microbiota. Bioeng. Transl. Med. 2022, 8, e10351. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.J.; Han, Y.Y.; Xu, X.; Yang, A.X.; Wei, J.; Hong, D.J.; Fang, X.; Chen, T.T. Neuroprotective effect of engineered Clostridium butyricum-pMTL007-GLP-1 on Parkinson’s disease mice models via promoting mitophagy. Bioeng. Transl. Med. 2024, 8, e10505. [Google Scholar] [CrossRef]
- Poluektova, E.; Yunes, R.; Danilenko, V. The Putative Antidepressant Mechanisms of Probiotic Bacteria: Relevant Genes and Proteins. Nutrients 2021, 13, 1591. [Google Scholar] [CrossRef]
- Kaur, H.; Nookala, S.; Singh, S.; Mukundan, S.; Nagamoto-Combs, K.; Combs, C.K. Sex-Dependent Effects of Intestinal Microbiome Manipulation in a Mouse Model of Alzheimer’s Disease. Cells 2021, 10, 2370. [Google Scholar] [CrossRef]
- Al Ebrahim, R.N.; Alekseeva, M.G.; Bazhenov, S.V.; Fomin, V.V.; Mavletova, D.A.; Nesterov, A.A.; Poluektova, E.U.; Danilenko, V.N.; Manukhov, I.V. ClpL Chaperone as a Possible Component of the Disaggregase Activity of Limosilactobacillus fermentum U-21. Biology 2024, 3, 592. [Google Scholar] [CrossRef]
- Khan, A.N.; Khan, R.H. Protein Misfolding and Related Human Diseases: A Comprehensive Review of Toxicity, Proteins Involved, and Current Therapeutic Strategies. Int. J. Biol. Macromol. 2022, 223, 143–160. [Google Scholar] [CrossRef]
- Meredith, M.E.; Salameh, T.S.; Banks, W.A. Intranasal Delivery of Proteins and Peptides in the Treatment of Neurodegenerative Diseases. AAPS J. 2015, 17, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Braschi, C.; Capsoni, S.; Narducci, R.; Poli, A.; Sansevero, G.; Brandi, R.; Maffei, L.; Cattaneo, A.; Berardi, N. Intranasal delivery of BDNF rescues memory deficits in AD11 mice and reduces brain microgliosis. Aging Clin. Exp. Res. 2021, 33, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Aggarwal, N.; Cui, B.; Foo, G.W.; He, Y.; Srivastava, S.K.; Li, S.; Seah, M.Z.X.; Wun, K.S.; Ling, H.; et al. Engineered commensals for targeted nose-to-brain drug delivery. Cell 2025, 188, 1545–1562.e16. [Google Scholar] [CrossRef]
- Poupet, C.; Chassard, C.; Nivoliez, A.; Bornes, S. Caenorhabditis elegans, a Host to Investigate the Probiotic Properties of Beneficial Microorganisms. Front. Nutr. 2020, 7, 135. [Google Scholar] [CrossRef]
- Goya, M.E.; Xue, F.; Sampedro-Torres-Quevedo, C.; Arnaouteli, S.; Riquelme-Dominguez, L.; Romanowski, A.; Brydon, J.; Ball, K.L.; Stanley-Wall, N.R.; Doitsidou, M. Probiotic Bacillus subtilis Protects against α-Synuclein Aggregation in C. elegans. Cell Rep. 2020, 30, 367–380.e7. [Google Scholar] [CrossRef]
- Gusarov, I.; Gautier, L.; Smolentseva, O.; Shamovsky, I.; Eremina, S.; Mironov, A.; Nudler, E. Bacterial nitric oxide extends the lifespan of C. elegans. Cell 2013, 152, 818–830. [Google Scholar] [CrossRef]
- Marsova, M.; Poluektova, E.; Odorskaya, M.; Ambaryan, A.; Revishchin, A.; Pavlova, G.; Danilenko, V. Protective effects of Lactobacillus fermentum U-21 against paraquat-induced oxidative stress in Caenorhabditis elegans and mouse models. World J. Microbiol. Biotechnol. 2020, 36, 104. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Rehman, A.; Yu, S.; Andino, N.M. Brain fogginess, gas and bloating: A link between SIBO, probiotics and metabolic acidosis. Clin. Transl. Gastroenterol. 2018, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Leung, B.P.Y.; Tsui, B.S.Y.; Kan, I. D-lactic acidosis in short bowel syndrome: Are probiotics friend or foe? A case report. Hong Kong Med. J. 2025, 31, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell. Fact. 2020, 19, 168. [Google Scholar] [CrossRef]
- Yunes, R.A.; Poluektova, E.U.; Belkina, T.V.; Danilenko, V.N. Lactobacilli: Legal regulation and prospects for new generation drugs. Appl. Biochem. Microbiol. 2022, 58, 652–664. [Google Scholar] [CrossRef]
- Fan, H.X.; Sheng, S.; Li, D.D.; Li, J.J.; Wang, G.Q.; Zhang, F. Heat-killed Lactobacillus murinus confers neuroprotection against dopamine neuronal loss by targeting NLRP3 inflammasome. Bioeng. Transl. Med. 2022, 8, e10455. [Google Scholar] [CrossRef]
- Głowacka, P.; Oszajca, K.; Pudlarz, A.; Szemraj, J.; Witusik-Perkowska, M. Postbiotics as Molecules Targeting Cellular Events of Aging Brain-The Role in Pathogenesis, Prophylaxis and Treatment of Neurodegenerative Diseases. Nutrients 2024, 16, 2244. [Google Scholar] [CrossRef]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef]
- Koukoulis, T.F.; Beauchamp, L.C.; Kaparakis-Liaskos, M.; McQuade, R.M.; Purnianto, A.; Finkelstein, D.I.; Barnham, K.J.; Vella, L.J. Do Bacterial Outer Membrane Vesicles Contribute to Chronic Inflammation in Parkinson’s Disease? J. Park. Dis. 2024, 14, 227–244. [Google Scholar] [CrossRef]
- Krzyżek, P.; Marinacci, B.; Vitale, I.; Grande, R. Extracellular Vesicles of Probiotics: Shedding Light on the Biological Activity and Future Applications. Pharmaceutics 2023, 15, 522. [Google Scholar] [CrossRef]
- Xie, J.; Haesebrouck, F.; Van Hoecke, L.; Vandenbroucke, R.E. Bacterial extracellular vesicles: An emerging avenue to tackle diseases. Trends Microbiol. 2023, 31, 1206–1224. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.E.Q.; Naveed, M.; Wang, X.; Liu, Y.; Li, M. The biological activity and potential of probiotics-derived extracellular vesicles as postbiotics in modulating microbiota-host communication. J. Nanobiotechnol. 2025, 23, 349. [Google Scholar] [CrossRef] [PubMed]
- Haasbroek, K.; Otaka, S.; Sakiyama, C.; Iwase, S.; Yagi, M.; Yonei, Y. Extracellular vesicles from probiotic microorganisms enhance microglia amyloid β phagocytosis. Glycative Stress Res. 2025, 12, 25–38. [Google Scholar] [CrossRef]
- Yang, Y.; Li, N.; Gao, Y.; Xu, F.; Chen, H.; Zhang, C.; Ni, X. The activation impact of lactobacillus-derived extracellular vesicles on lipopolysaccharide-induced microglial cell. BMC Microbiol. 2024, 24, 70. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Debnath, N.; Pradhan, D.; Mehta, P.K.; Kumar, A.; Yadav, M.L.; Yadav, A.K. Probiotic Lactobacillus-Derived Extracellular Vesicles: Insights Into Disease Prevention and Management. Mol. Nutr. Food Res. 2025, 69, e70013. [Google Scholar] [CrossRef]
- Grishina, Y.V.; Vatlin, A.A.; Mavletova, D.A.; Odorskaya, M.V.; Senkovenko, A.M.; Ilyasov, R.A.; Danilenko, V.N. Metabolites Potentially Determine the High Antioxidant Properties of Limosilactobacillus fermentum U-21. BioTech 2023, 12, 39. [Google Scholar] [CrossRef]
- Odorskaya, M.V.; Mavletova, D.A.; Nesterov, A.A.; Tikhonova, O.V.; Soloveva, N.A.; Reznikova, D.A.; Galanova, O.O.; Vatlin, A.A.; Slynko, N.M.; Vasilieva, A.R.; et al. The use of omics technologies in creating LBP and postbiotics based on the Limosilactobacillus fermentum U-21. Front. Microbiol. 2024, 15, 1416688. [Google Scholar] [CrossRef]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol. Dietol. 2011, 57, 117–121. [Google Scholar] [PubMed]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A.; et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef]
- Georgescu, D.; Ancusa, O.E.; Georgescu, L.A.; Ionita, I.; Reisz, D. Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: Is there hope? Clin. Interv. Aging 2016, 11, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Borzabadi, S.; Oryan, S.; Eidi, A.; Aghadavod, E.; Daneshvar Kakhaki, R.; Tamtaji, O.R.; Taghizadeh, M.; Asemi, Z. The Effects of Probiotic Supplementation on Gene Expression Related to Inflammation, Insulin and Lipid in Patients with Parkinson’s Disease: A Randomized, Double-blind, PlaceboControlled Trial. Arch. Iran. Med. 2018, 21, 289–295. [Google Scholar] [PubMed]
- Ibrahim, A.; Ali, R.A.R.; Manaf, M.R.A.; Ahmad, N.; Tajurruddin, F.W.; Qin, W.Z.; Desa, S.H.M.; Ibrahim, N.M. Multistrain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomized controlled trial. PLoS ONE 2020, 15, e0244680. [Google Scholar] [CrossRef] [PubMed]
- Ghyselinck, J.; Verstrepen, L.; Moens, F.; Abbeele, P.V.D.; Bruggeman, A.; Said, J.; Smith, B.; Barker, L.A.; Jordan, C.; Leta, V.; et al. Influence of probiotic bacteria on gut -microbiota composition and gut wall function in an in-vitro model in patients with Parkinson’s disease. Int. J. Pharm. X 2021, 3, 100087. [Google Scholar] [CrossRef]
- Lu, C.-S.; Chang, H.-C.; Weng, Y.-H.; Chen, C.-C.; Kuo, Y.-S.; Tsai, Y.-C. The Add-On Effect of Lactobacillus plantarum PS128 in Patients With Parkinson’s Disease: A Pilot Study. Front. Nutr. 2021, 8, 650053. [Google Scholar] [CrossRef]
- Tan, A.H.; Lim, S.Y.; Chong, K.K.; Manap, M.A.A.A.; Hor, J.W.; Lim, J.L.; Low, S.C.; Chong, C.W.; Mahadeva, S.; Lang, A.E. Probiotics for Constipation in Parkinson Disease: A Randomized Placebo-Controlled Study. Neurology 2021, 96, e772–e782. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, F.; Liu, Y.; Ma, T.; Jin, H.; Quan, K.; Leng, B.; Zhao, J.; Yuan, X.; Li, Z.; et al. Probiotics synergized with conventional regimen in managing Parkinson’s disease. npj Park. Dis. 2022, 8, 62. [Google Scholar] [CrossRef]
- Yang, X.; He, X.; Xu, S.; Zhang, Y.; Mo, C.; Lai, Y.; Song, Y.; Yan, Z.; Ai, P.; Qian, Y.; et al. Effect of Lacticaseibacillus paracasei strain Shirota supplementation on clinical responses and gut microbiome in Parkinson’s disease. Food Funct. 2023, 14, 6828–6839. [Google Scholar] [CrossRef]
- Mehrabani, S.; Khorvash, F.; Heidari, Z.; Tajabadi-Ebrahimi, M.; Amani, R. The effects of synbiotic supplementation on oxidative stress markers, mental status, and quality of life in patients with Parkinson’s disease: A double-blind, placebo-controlled, randomized controlled trial. J. Funct. Foods 2023, 100, 105397. [Google Scholar] [CrossRef]
- Ghalandari, N.; Assarzadegan, F.; Habibi, S.A.H.; Esmaily, H.; Malekpour, H. Efficacy of probiotics in improving motor functionand alleviating constipation in Parkinson’s disease: A randomized controlled trial. Iran. J. Pharm. Res. 2023, 22, e137840. [Google Scholar] [CrossRef]
- Andreozzi, V.; Cuoco, S.; Balestrieri, M.; Fierro, F.; Ferrara, N.; Erro, R.; Di Filippo, M.; Barbella, G.; Memoli, M.C.; Silvestri, A.; et al. Synbiotic supplementation may globally improve non-motor symptoms in patients with stable Parkinson’s disease: Results from an open label single-arm study. Sci. Rep. 2024, 14, 23095. [Google Scholar] [CrossRef]
- Magistrelli, L.; Contaldi, E.; Visciglia, A.; Deusebio, G.; Pane, M.; Amoruso, A. The Impact of Probiotics on Clinical Symptoms and Peripheral Cytokines Levels in Parkinson’s Disease: Preliminary In Vivo Data. Brain Sci. 2024, 14, 1147. [Google Scholar] [CrossRef]
- Zali, A.; Hajyani, S.; Salari, M.; Tajabadi-Ebrahimi, M.; Mortazavian, A.M.; Pakpour, B. Co-administration of probiotics and vitamin D reduced disease severity and complications in patients with Parkinson’s disease: A randomized controlled clinical trial. Psychopharmacology 2024, 241, 1905–1914. [Google Scholar] [CrossRef]
- Ramadan, M.E.; Mostafa, T.M.; Ghali, A.A.; El-Afify, D.R. Randomized controlled trial evaluating synbiotic supplementation as an adjuvant therapy in the treatment of Parkinson’s disease. Inflammopharmacology 2025, 33, 3897–3908. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Z.; Cheng, S.H.; Chang, M.Y.; Lin, Y.F.; Wu, C.C.; Tsai, Y.C. Neuroprotective Effects of Lactobacillus plantarum PS128 in a Mouse Model of Parkinson’s Disease: The Role of Gut Microbiota and MicroRNAs. Int. J. Mol. Sci. 2023, 24, 6794. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, M.; Zhao, W.; Kwok, L.-Y.; Zhang, W. Effects of probiotics and its fermented milk on constipation: A systematic review. Food Sci. Hum. Well. 2023, 12, 2124–2134. [Google Scholar] [CrossRef]
- Neiworth-Petshow, E.M.; Baldwin-Sayre, C. Naturopathic Treatment of Gastrointestinal Dysfunction in the Setting of Parkinson’s Disease. Integr. Med. 2018, 17, 44–50. [Google Scholar] [PubMed]
- Averina, O.V.; Poluektova, E.U.; Zorkina, Y.A.; Kovtun, A.S.; Danilenko, V.N. Human Gut Microbiota for Diagnosis and Treatment of Depression. Int. J. Mol. Sci. 2024, 25, 5782. [Google Scholar] [CrossRef]
- Ferrari, S.; Mulè, S.; Parini, F.; Galla, R.; Ruga, S.; Rosso, G.; Brovero, A.; Molinari, C.; Uberti, F. The influence of the gut-brain axis on anxiety and depression: A review of the literature on the use of probiotics. J. Tradit. Complement. Med. 2024, 14, 237–255. [Google Scholar] [CrossRef]
- Çınar, E.; Tel, B.C.; Şahin, G. Neuroinflammation in Parkinson’s Disease and its Treatment Opportunities. Balk. Med. J. 2022, 39, 318–333. [Google Scholar] [CrossRef]
- Chen, X.; Yan, L.; Yang, J.; Xu, C.; Yang, L. The impact of probiotics on oxidative stress and inflammatory markers in patients with diabetes: A meta-research of meta-analysis studies. Front. Nutr. 2025, 12, 1552358. [Google Scholar] [CrossRef] [PubMed]
- Salles, B.I.M.; Cioffi, D.; Ferreira, S.R.G. Probiotics supplementation and insulin resistance: A systematic review. Diabetol. Metab. Syndr. 2020, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.C.; Garbim Junior, E.E.; Jahnke, V.S.; Lisbôa Moura, J.G.; Brasil, C.S.; Schimitt da Cunha, P.H.; Lora, P.S.; Gemelli, T. Impact of probiotic supplementation in a patient with type 2 diabetes on glycemic and lipid profile. Clin. Nutr. ESPEN 2022, 49, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Mal, S.; Dwivedi, A.R.; Kumar, V.; Kumar, N.; Kumar, B.; Kumar, V. Role of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) in Different Disease States: Recent Updates. Curr. Med. Chem. 2021, 28, 3193–3215. [Google Scholar] [CrossRef]
- Hong, C.T.; Chen, J.H.; Huang, T.W. Probiotics treatment for Parkinson disease: A systematic review and meta-analysis of clinical trials. Aging 2022, 14, 7014–7025. [Google Scholar] [CrossRef]
- Xie, L.; Chen, D.; Zhu, X.; Cheng, C. Efficacy and safety of probiotics in Parkinson’s constipation: A systematic review and meta-analysis. Front. Pharmacol. 2023, 13, 1007654. [Google Scholar] [CrossRef]
- Ghalandari, N.; Assarzadegan, F.; Mahdavi, H.; Jamshidi, E.; Esmaily, H. Evaluating the effectiveness of probiotics in relieving constipation in Parkinson’s disease: A systematic review and meta-analysis. Heliyon 2023, 9, e14312. [Google Scholar] [CrossRef]
- Jin, X.; Dong, W.; Chang, K. Efficacy of probiotic supplements on Parkinson’s disease and meta-analysis. Complement. Ther. Med. 2024, 82, 103045. [Google Scholar] [CrossRef]
- Park, J.M.; Lee, S.C.; Ham, C.; Kim, Y.W. Effect of probiotic supplementation on gastrointestinal motility, inflammation, motor, non-motor symptoms and mental health in Parkinson’s disease: A meta-analysis of randomized controlled trials. Gut Pathog. 2023, 15, 9. [Google Scholar] [CrossRef]
- Ugrumov, M. Development of early diagnosis of Parkinson’s disease: Illusion or reality? CNS Neurosci. Ther. 2020, 26, 997–1009. [Google Scholar] [CrossRef]
- Katunina, E.A.; Blokhin, V.; Nodel, M.R.; Pavlova, E.N.; Kalinkin, A.L.; Kucheryanu, V.G.; Alekperova, L.; Selikhova, M.V.; Martynov, M.Y.; Ugrumov, M.V. Searching for Biomarkers in the Blood of Patients at Risk of Developing Parkinson’s Disease at the Prodromal Stage. Int. J. Mol. Sci. 2023, 24, 1842. [Google Scholar] [CrossRef]
- Blokhin, V.; Pavlova, E.N.; Katunina, E.A.; Nodel, M.R.; Kataeva, G.V.; Moskalets, E.R.; Pronina, T.S.; Ugrumov, M.V. Dopamine Synthesis in the Nigrostriatal Dopaminergic System in Patients at Risk of Developing Parkinson’s Disease at the Prodromal Stage. J. Clin. Med. 2024, 13, 875. [Google Scholar] [CrossRef] [PubMed]
- Axial Therapeutics|United States, Biotechnology Company|HOME. Available online: https://www.axialbiotherapeutics.com (accessed on 1 August 2025).
- Research|Bened Biomedical Co., Ltd. Available online: https://www.benedbiomed.com/research (accessed on 1 August 2025).
- 4D Pharma Plc—Developing Science, Delivering Therapies. Available online: https://www.4dpharmaplc.com/ (accessed on 1 August 2025).
- ADS024. Available online: https://www.ads024.com/ads024 (accessed on 1 August 2025).
- Ianiro, G.; Bibbò, S.; Porcari, S.; Settanni, C.R.; Giambò, F.; Curta, A.R.; Quaranta, G.; Scaldaferri, F.; Masucci, L.; Sanguinetti, M.; et al. Fecal microbiota transplantation for recurrent infection in patients with inflammatory bowel disease: Experience of a large-volume European FMT center. Gut Microbes 2021, 13, 1994834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ning, J.; Bao, X.Q.; Shang, M.; Ma, J.; Li, G.; Zhang, D. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 2021, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Tong, Q.; Xu, M.; Gu, J.; Ye, H. Gut Microbiota-Induced Modulation of the Central Nervous System Function in Parkinson’s Disease Through the Gut-Brain Axis and Short-Chain Fatty Acids. Mol. Neurobiol. 2025, 62, 2480–2492. [Google Scholar] [CrossRef]
- Cheng, Y.; Tan, G.; Zhu, Q.; Wang, C.; Ruan, G.; Ying, S.; Qie, J.; Hu, X.; Xiao, Z.; Xu, F.; et al. Efficacy of fecal microbiota transplantation in patients with Parkinson’s disease: Clinical trial results from a randomized, placebo-controlled design. Gut Microbes 2023, 15, 2284247. [Google Scholar] [CrossRef]
- DuPont, H.L.; Suescun, J.; Jiang, Z.D.; Brown, E.L.; Essigmann, H.T.; Alexander, A.S.; DuPont, A.W.; Iqbal, T.; Utay, N.S.; Newmark, M.; et al. Fecal microbiota transplantation in Parkinson’s disease-A randomized repeat-dose, placebo-controlled clinical pilot study. Front. Neurol. 2023, 14, 1104759. [Google Scholar] [CrossRef]
- Bruggeman, A.; Vandendriessche, C.; Hamerlinck, H.; De Looze, D.; Tate, D.J.; Vuylsteke, M.; De Commer, L.; Devolder, L.; Raes, J.; Verhasselt, B.; et al. Safety and efficacy of faecal microbiota transplantation in patients with mild to moderate Parkinson’s disease (GUT-PARFECT): A double-blind, placebo-controlled, randomised, phase 2 trial. EClinicalMedicine 2024, 71, 102563. [Google Scholar] [CrossRef]
- Scheperjans, F.; Levo, R.; Bosch, B.; Lääperi, M.; Pereira, P.A.B.; Smolander, O.P.; Aho, V.T.E.; Vetkas, N.; Toivio, L.; Kainulainen, V.; et al. Fecal Microbiota Transplantation for Treatment of Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2024, 81, 925–938. [Google Scholar] [CrossRef]
- Sampson, T.R. Fecal Microbiome Transplants For Parkinson Disease. JAMA Neurol. 2024, 81, 911–913. [Google Scholar] [CrossRef]
- Rojas-Velazquez, D.; Kidwai, S.; Liu, T.C.; El-Yacoubi, M.A.; Garssen, J.; Tonda, A.; Lopez-Rincon, A. Understanding Parkinson’s: The microbiome and machine learning approach. Maturitas 2025, 193, 108185. [Google Scholar] [CrossRef] [PubMed]
- Rabie, H.; Akhloufi, M.A. A review of machine learning and deep learning for Parkinson’s disease detection. Discov. Artif. Intell. 2025, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Pietrucci, D.; Teofani, A.; Unida, V.; Cerroni, R.; Biocca, S.; Stefani, A.; Desideri, A. Can Gut Microbiota Be a Good Predictor for Parkinson’s Disease? A Machine Learning Approach. Brain Sci. 2020, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Wirbel, J.; Ansorge, R.; Schudoma, C.; Ducarmon, Q.R.; Narbad, A.; Zeller, G. Machine learning-based meta-analysis reveals gut microbiome alterations associated with Parkinson’s disease. Nat. Commun. 2025, 16, 4227. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, H.; Zhang, M. Deep learning-based differential gut flora for prediction of Parkinson’s. PLoS ONE 2025, 20, e0310005. [Google Scholar] [CrossRef]
- Nie, S.; Wang, J.; Deng, Y.; Ye, Z.; Ge, Y. Inflammatory microbes and genes as potential biomarkers of Parkinson’s disease. Npj Biofilms Microbiomes 2022, 8, 101. [Google Scholar] [CrossRef]
- Morton, J.T.; Jin, D.M.; Mills, R.H.; Shao, Y.; Rahman, G.; McDonald, D.; Zhu, Q.; Balaban, M.; Jiang, Y.; Cantrell, K.; et al. Multi-level analysis of the gut-brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat. Neurosci. 2023, 26, 1208–1217. [Google Scholar] [CrossRef]
- Kovtun, A.S.; Averina, O.V.; Angelova, I.Y.; Yunes, R.A.; Zorkina, Y.A.; Morozova, A.Y.; Pavlichenko, A.V.; Syunyakov, T.S.; Karpenko, O.A.; Kostyuk, G.P.; et al. Alterations of the Composition and Neurometabolic Profile of Human Gut Microbiota in Major Depressive Disorder. Biomedicines 2022, 10, 2162. [Google Scholar] [CrossRef]
- Angelova, I.Y.; Kovtun, A.S.; Averina, O.V.; Koshenko, T.A.; Danilenko, V.N. Unveiling the Connection between Microbiota and Depressive Disorder through Machine Learning. Int. J. Mol. Sci. 2023, 24, 16459. [Google Scholar] [CrossRef]
- Danilenko, V.; Devyatkin, A.; Marsova, M.; Shibilova, M.; Ilyasov, R.; Shmyrev, V. Common Inflammatory Mechanisms in COVID-19 and Parkinson’s Diseases: The Role of Microbiome, Pharmabiotics and Postbiotics in Their Prevention. J. Inflamm. Res. 2021, 14, 6349–6381. [Google Scholar] [CrossRef]
- Zhang, J.; Bishir, M.; Barbhuiya, S.; Chang, S.L. Meta-Analysis of the Mechanisms Underlying COVID-19 Modulation of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 13554. [Google Scholar] [CrossRef]
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live biotherapeutic products: The importance of a defined regulatory framework. Exp. Mol. Med. 2020, 52, 1397–1406. [Google Scholar] [CrossRef]
| No. | Microbial Intervention | Animal | BP Model Inductor | Duration of the Experiment; Dose of Bacteria per Animal per Day | Conducted Research | Result | Target in the Animal’s Body | Active Components of Bacteria | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | VSL#3 | C57BL/6 mice; 6 mice/group | MPTP I.P. 30 mg/kg 5 days | 6 weeks; 4 × 109 CFU | 1. In striatum | 1. DA↑,DOPAK↑, NVA↑, NE no changes | Inhibition of the intestinal NLRP3 inflammasome | [95] | |
| 2. In SN | 2. TH+ neurons↑ | ||||||||
| 3. In striatum | 3. GDNF↑, BDNF↑, TNF-α↓, IL-β↓ | ||||||||
| 4. In serum | 4. TNF-α↓, IL-1β↓, IL-6↓, IL-17↓, IFN-γ↓, GM-CFS↓ | ||||||||
| 5. In liver | 5. TNF-α↓, IL-1β↓ | ||||||||
| 6. In intestine | 6. TNF-α↓, IL-1β↓, IL-6↓, IL-17↓, caspase-1↓, NLRP3↓ | ||||||||
| 2 | L. rhamnosus (Unique Biotech Ltd., Telangana, India) + curcumin 500 mg/kg | Sprague Dawley rats; 6 rats/group | Rotenone S.C. 2.5 mg/kg 3 weeks | 3 weeks; 0.1 × 109– 1 × 109 CFU | 1. Behavioral tests | 1. Hanging wire↑, akinesia↓, catalepsy↓ | [96] | ||
| 2. Brain homogenate | 2. DA↑, AchE↓, SOD↑, catalase↑, GSH↑, MDA↓ | ||||||||
| 3. Histopathology of brain sections | 3. Improvement in the histology of the brain. | ||||||||
| 3 | L. fermentum, L. delbrueckii, +vinpocetine 20 mg/kg | Sprague Dawley rats; 10 rats/group | Rotenone I.P. 2.5 mg/kg | 8.5 weeks | 1. Behavioral tests | 1. Motor symptoms improved in movement coordination and strength. | [97] | ||
| 2. In striatum | 2. DA↑, GSH↑, nitrite↓, MDA↓, IL-1↓, TNF-α↓, α-synuclein↓ tau↓ | ||||||||
| 3. In SN | 3. TH+↑ | ||||||||
| 4 | A. municiphila Akk11 | C57BL/6 mice; 8 mice/group | MPTP 30 mg/kg +probenecid 250 mg/kg I.P. 6 days. | 4 weeks, 109 CFU | 1. Behavioral tests | 1. open field↑, pole↓, rotarod↑ tests | Inhibition of microglial TLR4/NF-κB/NLRP3 inflammasome activation | [98] | |
| 2. SN | 2. TH+ ↑ TH protein expression↑ | ||||||||
| 3.Expression of cytolines in SN | 3. IL-1β↓, TNF-α↓, IL-6↓, TGF-β↑, IL-10↑, Arg-1↑ | ||||||||
| 4. Microglia and the NLRP3 inflammasome | 4. Activation of microglia ↓ | ||||||||
| 5. Colon | 5. Colonic integrity↑, IL-1β↓, TNF-α↓, IL-6↓. | ||||||||
| 5 | B. subtilis NCIB3610 | Caenorhabditis elegans | 6-OHDA 75 mM | 1. Dopaminergic neuron . | 1. Dopaminergic neuron injury↓ | PMK-1 (p38 MAPK)/SKN-1 (Nrf2) signaling | Bacterial biofilm (hydrophobic BskA protein) and QS peptide CSF | [99] | |
| 2. Human alpha-synuclein in transgenic worms | 2. Aggregation of alpha-synuclein↓ | ||||||||
| 6 | L. acidophilus PTCC 1643, membrane vesicles | Wistar rats; 5 rats/group | 6-OHDA bilateral injection in SN; The rats rested for one month after surgery and entered the study after PD was confirmed | 4 weeks, 3 d/wk; equivalent 1 × 107 CFU | 1. Behavioral test. | 1. Beam-walking test ↑ | [100] | ||
| 2. SN | 2. Protein and genes (receptor) of 5-HT ↑, protein and gene (receptor) of GABA ↓, TH ↑ | ||||||||
| 3. The intestine | 3. The expression of TLR-4 and α-synuclein gene ↓ | ||||||||
| 7 | L. plantarum SG5 | C57BL/6 mice; 8 mice/group | MPTP I.P. 30 mg/kg 5 days | 5 weeks; 1 × 109 CFU | 1. Behavioral tests | 1. Rotarod↑, hanging test↑ | The GLP-1/PGC-1α signaling pathway | [101] | |
| 2. SN | 2. Dopaminergic neuron count↑, TH↑ α-syn↓, activation of glial cells↓ NF-κB↓ IL-1β↓, ZO-1↑, GLP-1R↑ PGC-1α+↑. | ||||||||
| 3. BBB | 3. BBB integrity↑ | ||||||||
| 4. Gut and colon | 4. Gut microbiota richness↑ diversity↑, intestinal transit↑, intestinal barrier integrity (ZO-1↑, occludin↑)↑, NF-κB↓, IL-6↓, IL-1β↓, GLP-1↑. | ||||||||
| 5. Serum | 5. GLP-1↑ | ||||||||
| 8 | L. rhamnosus E9 | C57BL/6 mice; 15 mice/group | MPTP I.P. 30 mg/kg 5 days | 2 weeks; 108 CFU | 1. Behavioral tests. | 1. Open field↑, catalepsy↓, wire hanging test↑ | EPS | [102] | |
| 2. Striatum | 2. TH gene and protein↑, DA↑, DR1↓, DAT↑, ROS↓ | ||||||||
| 3. Gut | 3. Occludin gene and protein↑, remodulation of the cecal microbiota at the phylum and genus level, Firmicutes/Bacteroidota↑ | ||||||||
| 9 | L. plantarum CRL2130, S. thermophilus CRL808, S. thermophilus CRL807 | C57BL/6 aged (1-year-old) mice; 7 mice/group | MPTP I.P. 20 mg/kg +Probenecid I.C. 250 mg/kg | 6 weeks; 1.8 ± 2 × 107 CFU of every strain | 1. Behavioral tests. | 1. Pole test↓, transversal beam test↓,, nasal bridge adhesive removal test↓, foot sliding test↓ | Vitamins: riboflavin, folic acid | [103] | |
| 2. SN | 2. TH + cells↑ | ||||||||
| 3. Brain | 3. IL-6↓, TNF-α↓, IL-10↑ | ||||||||
| 4. Serum | 4. IL-6↓, TNF-α↓, IL-10↑ | ||||||||
| 5. Gut and small intestine | 5. Villi length/crypts depth↑, dysbiosis↓ (Lactobacillceae↑ Prevotellacea↑Alistipes↑) | ||||||||
| 10 | B. breve Bif11 | Sprague Dawley rats; 3–9 rats/group | MPTP 100 μg in SN | 3 weeks; 1–2 × 1010 CFU | 1. Behavioral tests | 1. Y-maze spontaneous alteration↑, novel object recognition↑, rotarod↑, passive avoidance↑. | [104] | ||
| 2. Midbrain | 2. TH ↑, MDA↓, GSH↑, nitrite↓ iNOS↓, IL-6↓, IL-1β↓, NF-κB↓, CRP↓ | ||||||||
| 3. Feces | 3. SCFA↑ | ||||||||
| 4. Intestine | 4. Intestinal epithelial permeability↑. | ||||||||
| 11 | B. animalis subsp. lactis NJ241 | C57BL/6 mice | MPTP I.P. 30 mg/kg | 4 weeks; 1 × 109 CFU | 1. Behavioral Tests | 1. Open field↑, wire hanging ability↑ | GLP-1R/PGC-1α signaling | [105] | |
| 2. SN | 2. TH+ cells↑, TH protein↑, glial activation↓, IL-1β, NF-κB, GLP-1R↑, PGC-1α↑ | ||||||||
| 3. BBB | 3. ZO-1↑ occludin ↑ in SN | ||||||||
| 4. Gut and colon | 4. Gastrointestinal motility↑, gut dysbiosis↓, GLP↑ | ||||||||
| 5. Feces | 5. SCFA↑ | ||||||||
| 6. Serum | 6. GLP↑ | ||||||||
| 12 | B. animalis subsp. lactis MH-022 | Rat | 6-OHDA | 1. Behavioral Tests | 1. Motor deficits↓ | Mitochondria | SCFAs | [106] | |
| 2. SN | 2. Dopaminergic neurons↑, antioxidant capacity↑, inflammation↓ | ||||||||
| 3. Gut | 3. Normalization of the gut microbiota composition, SCFA↑. | ||||||||
| 13 | B. longum subsp. longum C7 CCFM1029 | C57BL/6 J mice; 10 mice/group | MPTP I.P. 30 mg/kg | 6 weeks; 5 × 108 CFU | 1. Behavioral Tests | 1. Pole↓, beam walking↓, rotarod↑, open field↑ | The modification of the gut microbiota and microbially produced metabolites. | [107] | |
| 2. Striatum | 2. LDOPA↑, DA↑, DOPAC↑, 5-HT↑, 5-HIAA↑, HVA↑, BDNF↑, GDNF↑, GFAP↓, Iba1↓, TNF-α↓, IL-1β↓, IL-6↓, ZO-1↑, occludin↑, claudin-1↑ | ||||||||
| 3. SN | 3. TH+↑ | ||||||||
| 4. Gut and colon | 4. TNF-α↓, IL-1β↓, IL-6↓, ZO-1↑, occludin↑, claudin-1↑, indole-3-acetic acid↓, spermidine↓, N-acetylhistamine↓, 3-acrylic acid↑, docosatrienoic acid↑, indole-3-butyric acid↑, SCFA↑, gut dysbiosis↓ | ||||||||
| 14 | L. fermentum U-21 | Wistar rats; 6/8 rats/group | Paraquat I.P. 8 mg/kg; LPS I.N. 4 mg/kg | 2.5 weeks; 1 × 108 CFU | 1. Behavioral tests | 1. Beam walking↓. | [108] | ||
| 2. SN | 2. Phosphorylated α-synuclein↓, inflammatory glial response↓, complement component C3↓. |
| N | Type of Research | Composition and Form of Probiotic | Number of Patients; Average Age (Experiment/Placebo) | Duration of the Experiment | Conducted Research | Result | References |
|---|---|---|---|---|---|---|---|
| 1. | L. casei Shirota 6.5 × 109 CFU; fermented milk | 40 | 5 weeks | Stools consistency, bloating, abdominal pain | Stools consistency↑, bloating↓, abdominal pain↓ | [139] | |
| 2. | S. salivarius subsp thermophilus, E. faecium, L. rhamnosus GG, L. acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp bulgaricus, B. breve, B. animalis subsp lactis, 2.5 × 1011 CFU + prebotic fiber; fermented milk | 80/40; 72/69 years | 4 weeks | Rome III–confirmed constipation | Number of complete bowel movements per week (CBM)↑ | [140] | |
| 3. | L. acidophilus, B. infantis; tablets | 20 probiotic/ 20 trimebutine; 70/76 years | 12 weeks | NMS-GI | Probiotics improve abdominal pain and bloating as much as trimebutine, but slightly less for constipation with incomplete evacuation. | [141] | |
| 4. | RCT | L. acidophilus, B. bifidum, L. reuteri, L. fermentum, 8 × 109 CFU | 25/25; 67/67 years | 12 weeks | In peripheral blood mononuclear cells of PD patients: IL-1, IL-8, TNF-α, TGF-β, PPAR-γ, LDLR, VEGF; Plasma NO and GSH | IL-1↓, IL-8↓, TNF-α↓, TGF-β↑, PPAR-γ↑ | [142] |
| 5. | RCT | Hexbio® L. acidophilus BCMC1 12130, L. casei BCMC1 12313, L. lactis BCMC1 12451, L. lactis BCMC1 02290, B. infantis BCMC1 02129, B. longum BCMC1 02120, 3 × 1010 CFU; +FOS; sachets (B-Crobes Laboratory, Perak, Malaysia) | 22/26; 69/70 years | 8 weeks | Garrigues Questionnaire (GQ) bowel opening frequency (BOF) | BOF↑ | [143] |
| gut transit time (GTT) | GTT↓ | ||||||
| PDQ39-SI | no changes | ||||||
| UPDRS-II, UPDRS-III | no changes | ||||||
| NMSS | NMSS↓ | ||||||
| 6. | Symprove L. acidophilus NCIMB 30175, L. plantarum NCIMB 30173, .L. rhamnosus NCIMB 30174, E. faecium NCIMB 30176 (Symprove Ltd., Surrey, UK) | 3 patients; stool samples in an in vitro gut model | 48 h | 1. Colonic media from in an in vitro gut model in cell culture models— effect on epithelial tight-junction integrity, wound healing, production of inflammatory markers. | 1. Tight junction integrity improved, wound healing was seen to occur faster, MCP-1↓ IL-8↓ IL-6↑ IL-10↑ | [144] | |
| 2. Bacterial composition and metabolic activity in the microbiotas of an in vitro gut model | 2. Change in bacterial composition in the microbiotas; promotion of SCFA and lactate production | ||||||
| 7. | Single-arm, baseline-controlled trial | L. plantarum PS128 (PS128) 6 × 1010 CFU; capsules (Bened Biomedical Co., Ltd., Taipei, Taiwan) | 25; 61 years | 12 weeks | ON and OFF period duration | ON↑ OFF↓ | [145] |
| UPDRS | UPDRS total,↓ UPDRS-III↓ | ||||||
| PDQ-39 | PDQ-39↓ | ||||||
| PGI-C | PGI-C (17 patients)↑ | ||||||
| NMSS | no changes | ||||||
| PAC-SYM | no changes | ||||||
| BDI-II | no changes | ||||||
| Metabolic parameters | plasma myeloperoxidase↓, urine creatinine↓ | ||||||
| 8. | RCT | Multistrain probiotic, 10 × 109 CFU; capsules | 34/38; 71/69 years | 4 weeks | Number of spontaneous bowel movements (SBM) | SBM↑ | [146] |
| stool consistency | stool consistency↑ | ||||||
| quality of life | quality of life↑ | ||||||
| fecal calprotectin | no changes | ||||||
| 9. | RCT | Probio-M8 B. animalis subsp. lactis 3 × 1010 CFU; sachets | 50/50; 69 years | 12 weeks | PAC-QOL, UPDRS-III, MMSE, PDQ-39, HAMA, PDSS, HAMD-17, GI-related symptoms | PAC-QOL ↓, UPDRS-III ↓, MMSE↑, PDQ-39↓, HAMA↓, PDSS↑, HAMD-17↓, GI-related symptoms↓ | [147] |
| gut microbiome | Change in gut microbiome composition | ||||||
| 7 kinds of SCFAs in serum | acetic acid ↑ | ||||||
| 12 types of neurotransmitters in serum | dopamin↑, glutamine↓, tryptophan↓ | ||||||
| 10. | RCT | L. paracasei strain Shirota 1010 CFU; fermented milk. | 65/63 67/70 years | 12 weeks | Constipation | constipation-related symptoms↓ | [148] |
| NMSS | NMSS↓ | ||||||
| HAMD-17 | HAMD-17↓ | ||||||
| HAMA | HAMA↓ | ||||||
| PDQ-39 | PDQ-39↓ | ||||||
| fecal microbiota composition | Lacticaseibacillus↑ | ||||||
| faecal metabolites | L-tyrosine↓ | ||||||
| serum metabolites | L-tyrosine↑ | ||||||
| 11. | RCT | L. acidophilus LAA-5, L. rhamnosus LAR-7, L. plantarum LAP-10, B. longum BIA-8, S. thermophilus, 5 × 109 CFU +inulin; sachets. | 40/40; 68/69 years | 12 weeks | Serum biomarkers of oxidative stress | TAC↑ OSI↓ MDA↓ GSH no changes | [149] |
| PDQ-39 | PDQ-39↓ | ||||||
| mental status | BDI-II↓ HADS no changes, FSS no changes | ||||||
| 12. | RCT | Comflor® L. plantarum, L. casei, L. acidophilus, Lactobacillus bulgaricus, B. infantis, B. longum, B. breve, S. thermophilus, total of 4.5 × 1011 CFU; capsules (Fara Daroo Fanavar Mehr Co., Tehran, Iran). | 14/13; 68/68 years | 8 weeks | Defecation | Frequency of defecation↑ | [150] |
| Sense of complete evacuation | no changes | ||||||
| Bristol stool consistency | Bristol stool consistency↑ | ||||||
| UPDRS | no changes | ||||||
| 13. | A single-arm, open label study | Enterolactis Duo L. paracasei DG (DSM 34154) 8 × 109 CFU + inulin, vitamins B1, B2, B6; 4 times/day; sachets | 30 65 years | 12 weeks | MDS-UPDRS | MDS-UPDRS I-1, I-6, I-11↓ | [151] |
| SCOPA-AUT | SCOPA-AUT↓ | ||||||
| TAS-20 | TAS-20↓ | ||||||
| HAM-D | HAM-D↓ | ||||||
| DIFt | DIFt↓ | ||||||
| PAS-A | PAS-A↓ | ||||||
| PAC-SYM | PAC-SYM↓ | ||||||
| STAI-Y | no changes | ||||||
| MoCA | no changes | ||||||
| RMET | no changes | ||||||
| BDI-II | no changes | ||||||
| MDS-UPDRS II, III, IV | no changes | ||||||
| fecal microbiota composition | Changes in the abundance of 8 taxa, (the genus Faecalibacterium↑) | ||||||
| fecal microbiota metabolites | butyrate/acetate↑ | ||||||
| 14. | Preliminary in vivo data | B. animalis subsp. lactis BS01, B. longum 03, B. adolescentis BA02 ≥ 1 × 109 CFU each + FOS | 20/20; 68 years | 12 weeks | UPDRS | UPDRS-III ↓ | [152] |
| NMSS | NMSS↓ (particularly in gastrointestinal symptoms) | ||||||
| plasma level of cytokines | IL-6↓, TNF-α no changes, IFN-γ no changes, TGF-β no changes | ||||||
| 15. | RCT | BioZen D L. acidophilus, L. rhamnosus, L. reuteri, L. paracasei, B. longum, B. coagulans 2 × 109 CFU + vitamin D; capsules | 23/23; 56/56 | 12 weeks | BAI | BAI↓ | [153] |
| GSRS | GSRS↓ | ||||||
| UPDRS | UPDRS I, III, IV, total↓ | ||||||
| serum level of cytokines and antioxidants | IFN-γ↓, IL-6↓, IL-1β↓, MDA↓, IL-10↑, TAC↑ | ||||||
| 16. | RCT (open-label design) | Livia® L. acidophilus 109 CFU + inulin 3 g; twice a day; sachets (Pharma Zad, Cairo, Egypt) | 33/33 45–65 years | 12 weeks | MDS-UPDRS | MDS-URDS-I↓ | [154] |
| serum levels of cytokines and BDNF | TNF-α↓, MDA↓, BDNF↑ |
| Purpose | Drug/Active Agent | Research Stage | Developer/Manufacturer | |
|---|---|---|---|---|
| 1 | Parkinson’s disease | AB-202 (Microbiome-directed product) | Preclinical studies | Axial Biotherapeutics (USA) [173] |
| 2 | Parkinsonism and other neurodegenerative/neuroinflammatory conditions | PS128 (L. plantarum PS128) | Preclinical studies | Bened Biomedical (Taiwan) [174] |
| 3 | Neurodegeneration | MRx0029, (LPB) MRx0005 (LPB) | Preclinical studies | 4D Pharma (United Kingdom) [175] |
| 4 | Neurodegenerative/neuroinflammatory diseases | ADS024 (Bacillus velezensis ADS024) | Preclinical studies | Adiso therapeutic (USA) [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poluektova, E.U.; Stavrovskaya, A.; Pavlova, A.; Yunes, R.; Marsova, M.; Koshenko, T.; Illarioshkin, S.; Danilenko, V. Gut Microbiome as a Source of Probiotic Drugs for Parkinson’s Disease. Int. J. Mol. Sci. 2025, 26, 9290. https://doi.org/10.3390/ijms26199290
Poluektova EU, Stavrovskaya A, Pavlova A, Yunes R, Marsova M, Koshenko T, Illarioshkin S, Danilenko V. Gut Microbiome as a Source of Probiotic Drugs for Parkinson’s Disease. International Journal of Molecular Sciences. 2025; 26(19):9290. https://doi.org/10.3390/ijms26199290
Chicago/Turabian StylePoluektova, Elena U, Alla Stavrovskaya, Anastasia Pavlova, Roman Yunes, Maria Marsova, Tatiana Koshenko, Sergey Illarioshkin, and Valery Danilenko. 2025. "Gut Microbiome as a Source of Probiotic Drugs for Parkinson’s Disease" International Journal of Molecular Sciences 26, no. 19: 9290. https://doi.org/10.3390/ijms26199290
APA StylePoluektova, E. U., Stavrovskaya, A., Pavlova, A., Yunes, R., Marsova, M., Koshenko, T., Illarioshkin, S., & Danilenko, V. (2025). Gut Microbiome as a Source of Probiotic Drugs for Parkinson’s Disease. International Journal of Molecular Sciences, 26(19), 9290. https://doi.org/10.3390/ijms26199290






