Pluripotent Cells Expressing APOE4 Exhibit a Pronounced Pro-Apoptotic Phenotype Accompanied by Markers of Hyperinflammation and a Blunted NF-κB Response
Abstract
1. Alzheimer’s Disease
2. NF-κB
3. Apolipoprotein E
4. Results
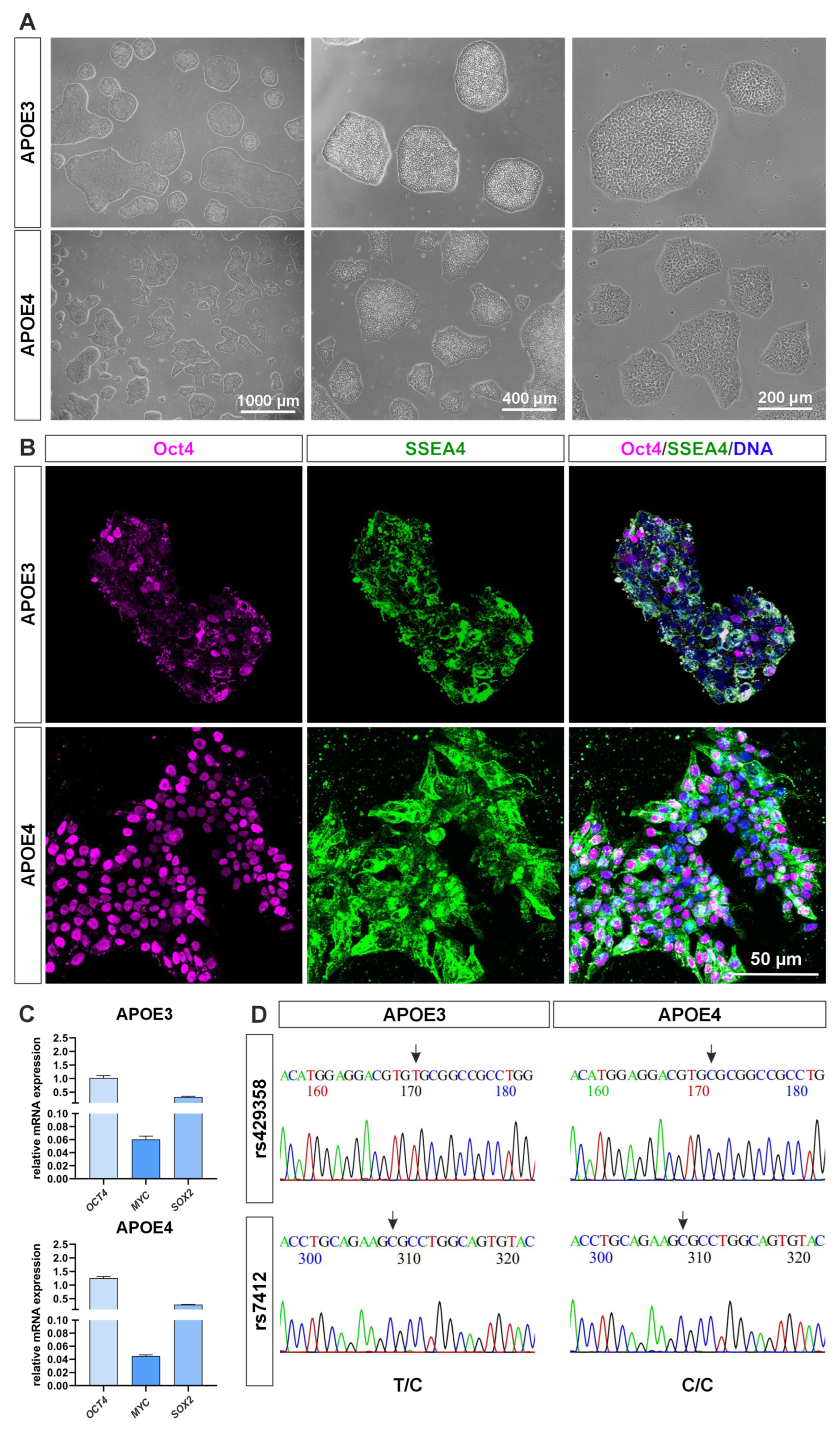
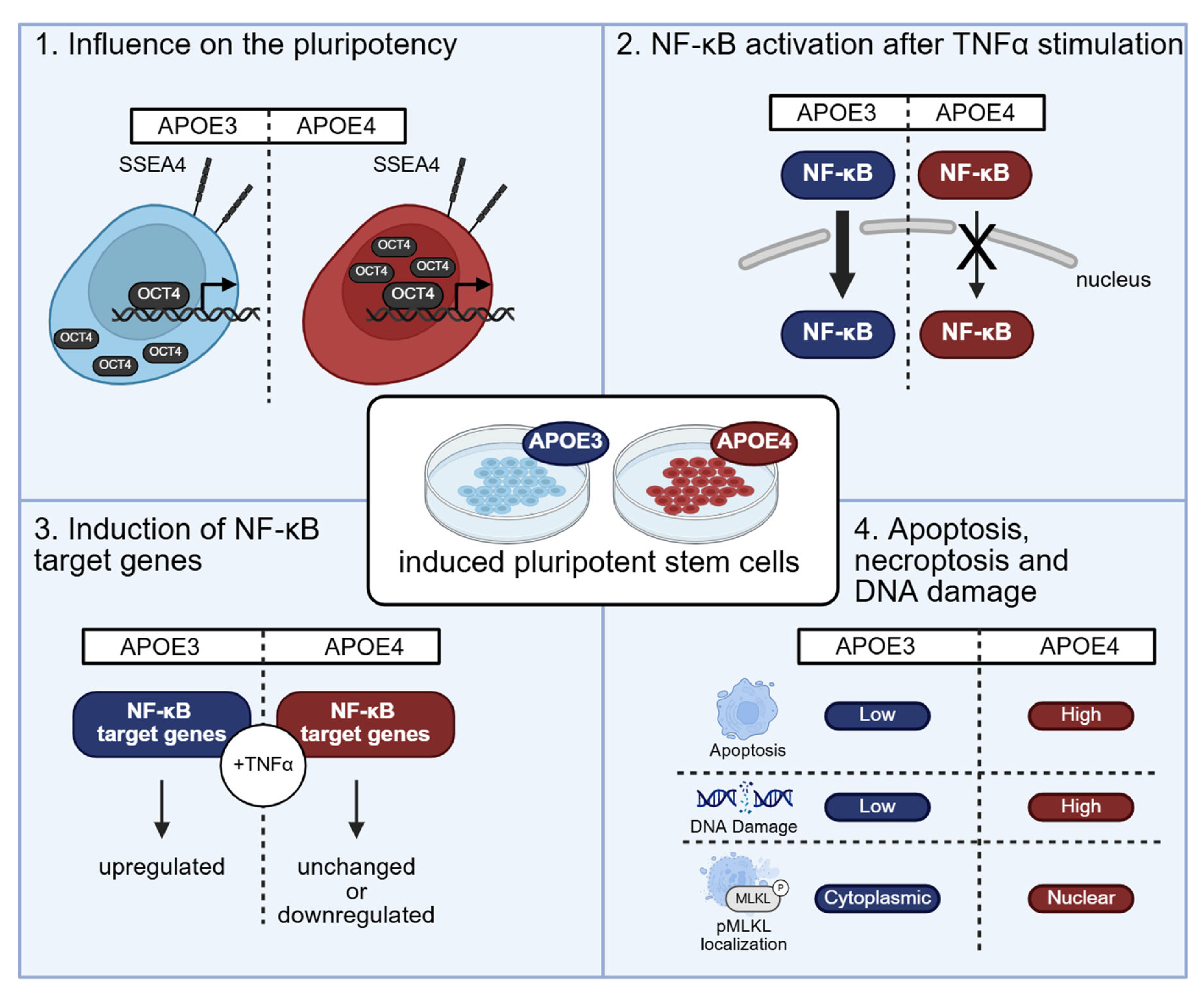
4.1. The APOE4 Allele Variant Shows Molecular Signs of Higher Pluripotency
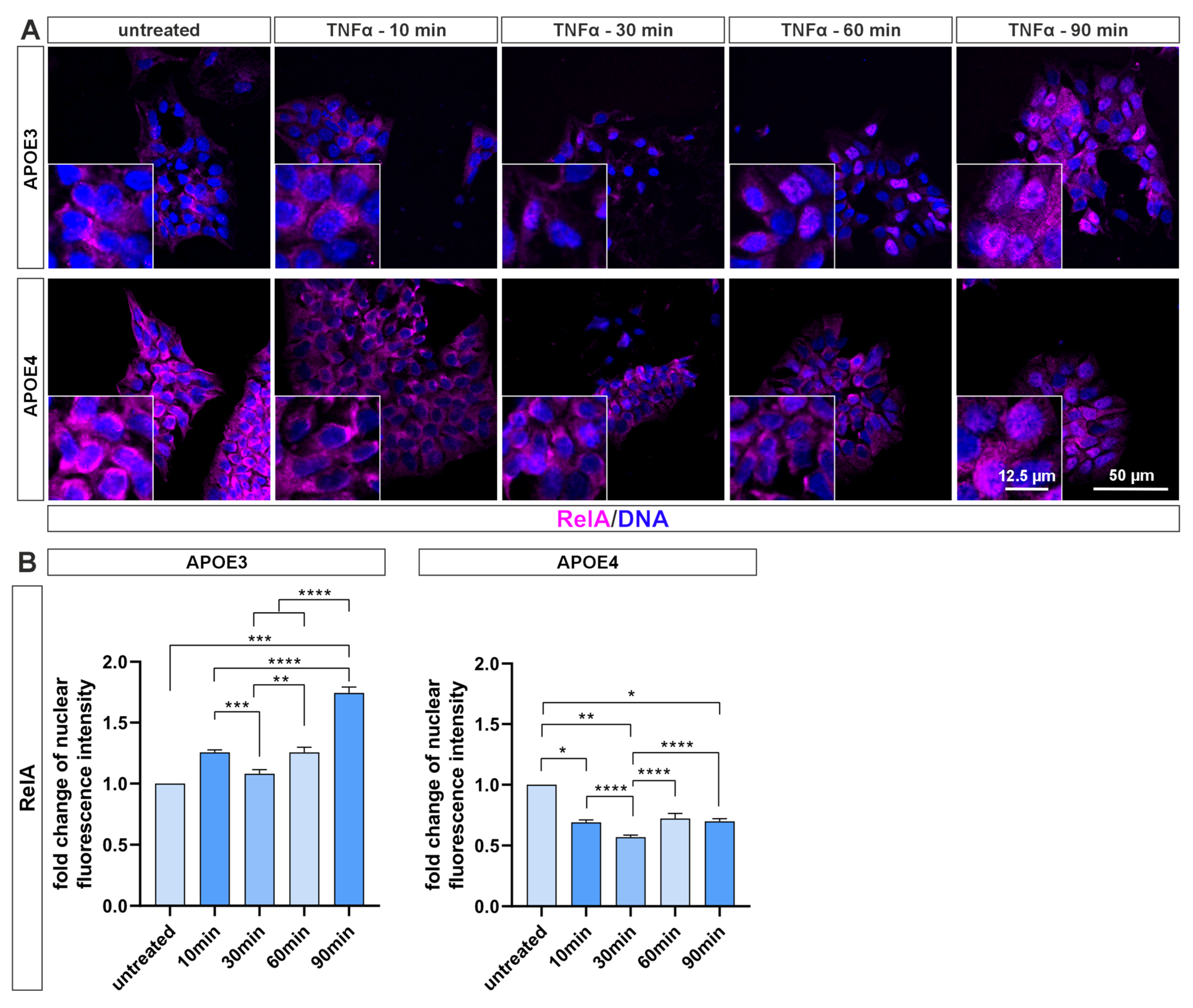
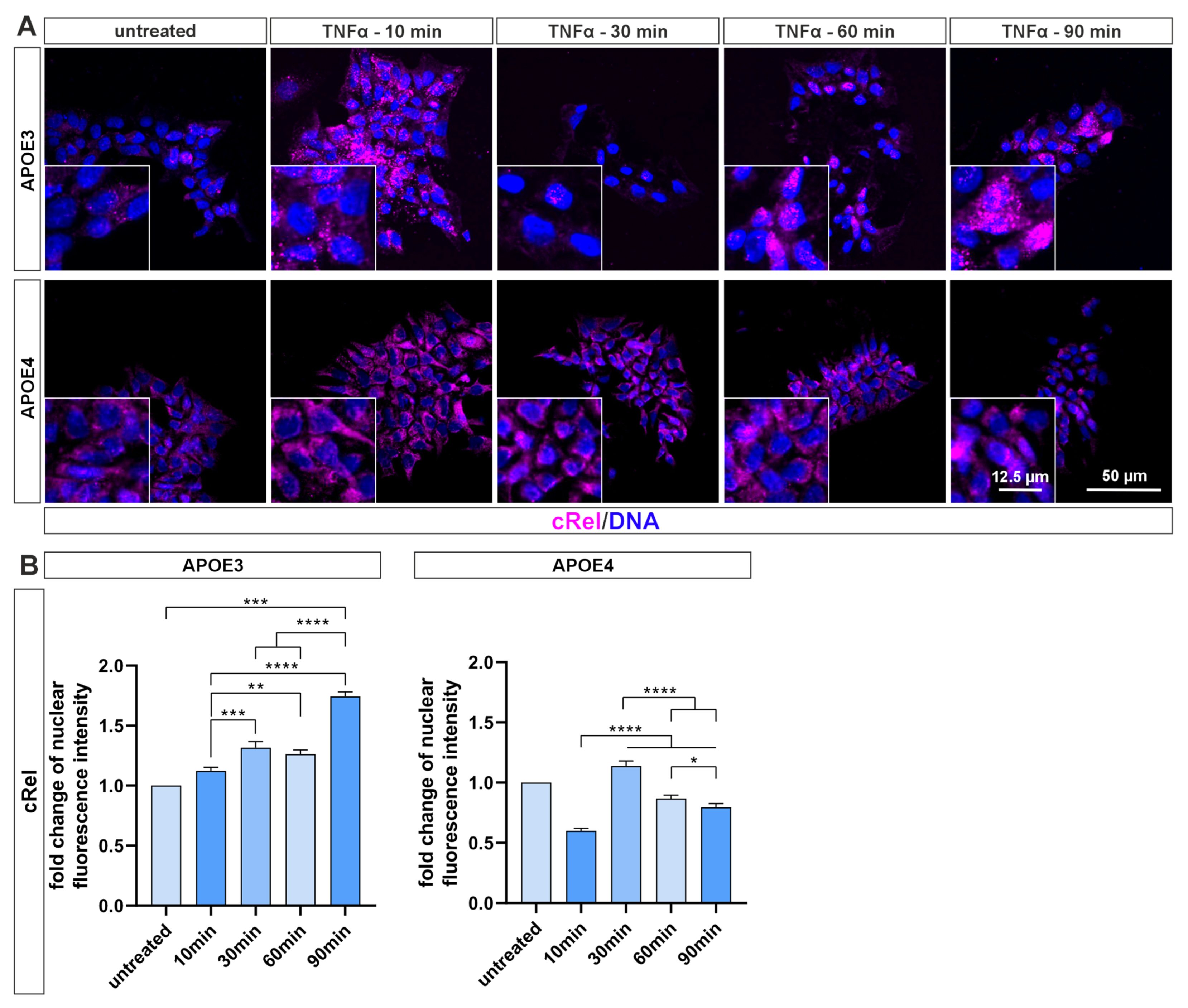
4.2. NF-κB Activation Is Blunted in Pluripotent Cells with APOE4 Alleles
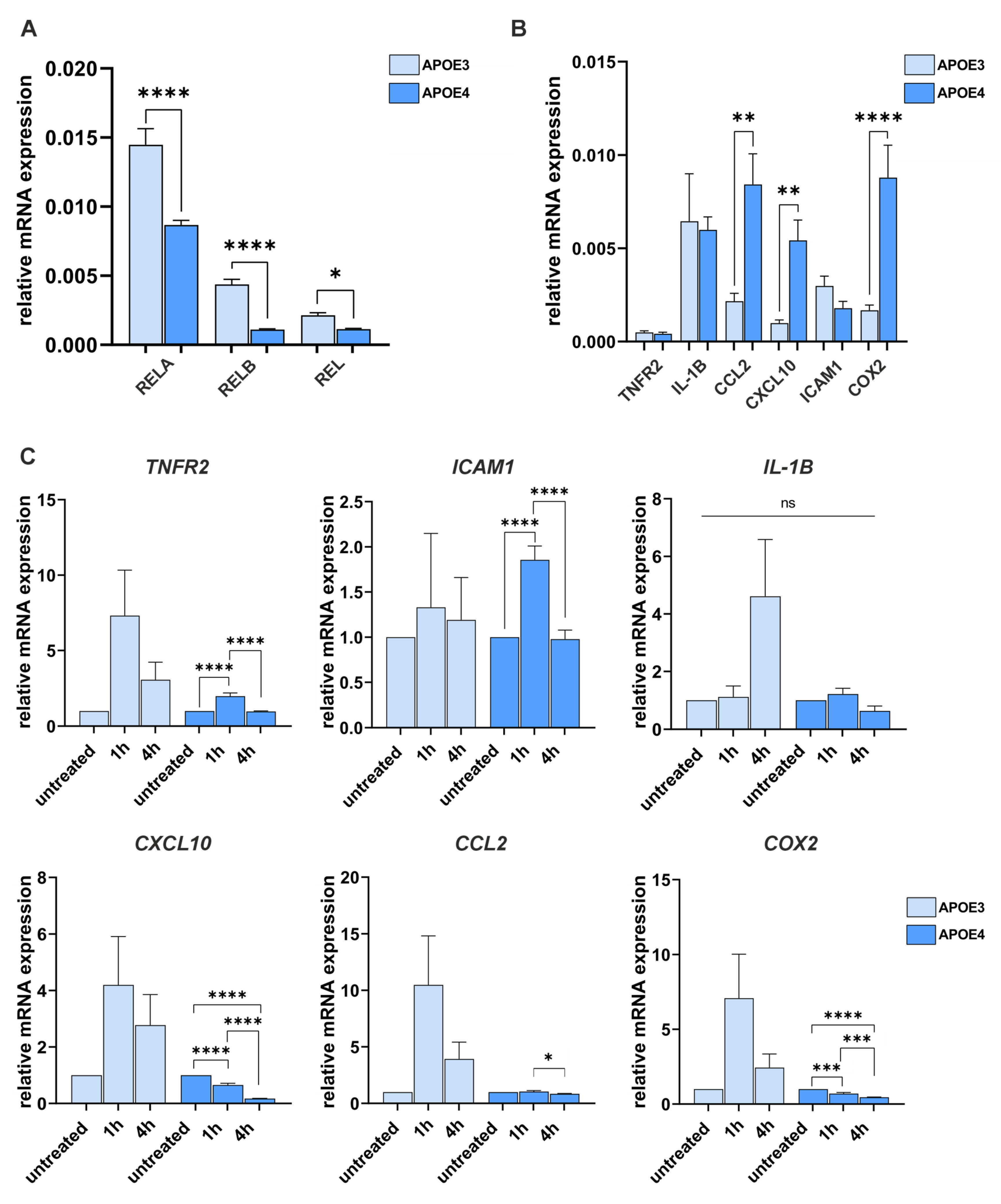
4.3. The APOE4 Allele Has a Gene-Specific Influence on the Expression and Induction of NF-κB Target Genes
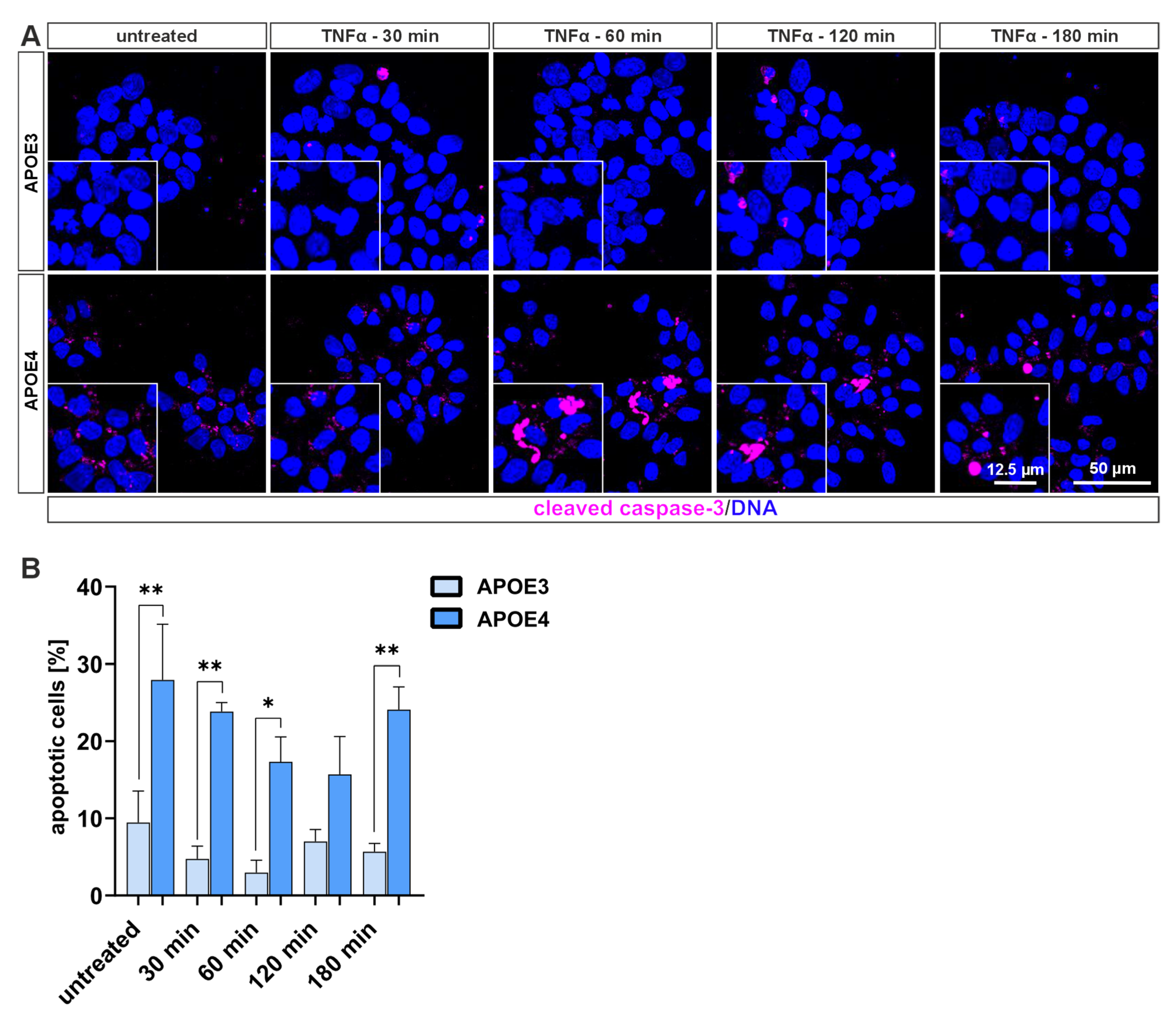
4.4. Cells Harboring the Homozygous APOE4 Genotype Undergo Apoptosis More Frequently than Those with APOE3
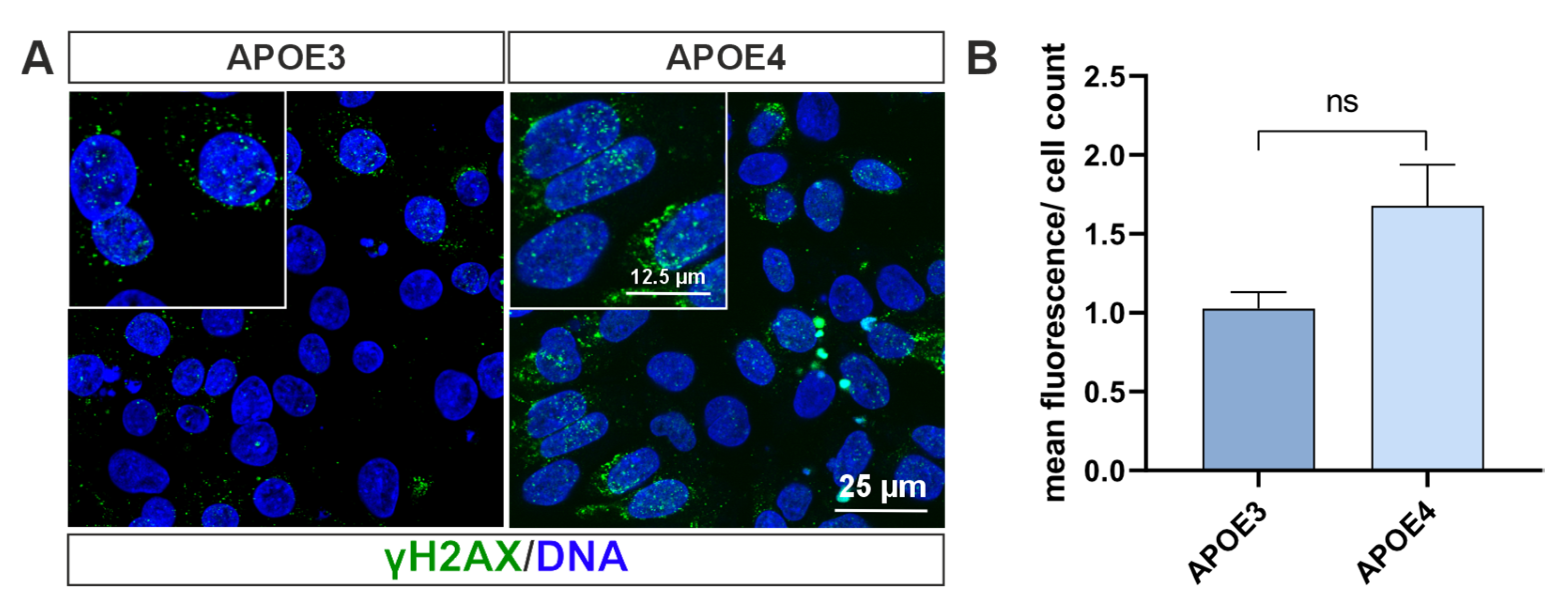
4.5. Double Strand Break-Bearing Cells Are Mainly Present in APOE4 Genotype
5. Discussion
6. Materials and Methods
6.1. Cell Culture
6.2. Genotyping of iPS Cell Lines
6.3. TNF-α Treatments
6.4. Immunocytochemistry
6.5. qPCR
6.6. Nomenclature
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-beta |
| AD | Alzheimer’s Disease |
| APOE | Apolipoprotein E |
| APP | Amyloid Precursor Protein |
| CCL2 | C-C Motif Chemokine Ligand 2 (also known as MCP-1) |
| cIAP | Cellular Inhibitor of Apoptosis Protein |
| CTCF | Corrected Total Cell Fluorescence |
| MYC | Cellular Myelocytomatosis Oncogene |
| COX2 | Cyclooxygenase-2 |
| CXCL10 | C-X-C Motif Chemokine Ligand 10 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DNA | Desoxyribonucleic Acid |
| DSB | Double-Strand Break |
| ES | Embryonic Stem (cells) |
| h | Hour |
| IκBα | Inhibitor of kappa B alpha |
| ICC | Immunocytochemistry |
| ICAM1 | Intercellular Adhesion Molecule 1 |
| IKK | IκB Kinase |
| IL-1B | Interleukin-1 beta |
| iPS | Induced Pluripotent Stem (cells) |
| Min | Minute |
| ml | Milliliter |
| µg | Microgram |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NFT | Neurofibrillary Tangles |
| NIK | NF-κB-inducing Kinase |
| NMDA | N-Methyl-D-Aspartate |
| OCT4 | Octamer-binding Transcription Factor 4 |
| pMLKL | Phosphorylated Mixed Lineage Kinase Domain-Like Protein |
| PBS | Phosphate-Buffered Saline |
| PCR | Polymerase Chain Reaction |
| PKA | Protein Kinase A |
| RIPK1 | Receptor-Interacting Serine/Threonine-Protein Kinase 1 |
| RNA | Ribonucleic Acid |
| RT-qPCR | Reverse Transcription quantitative PCR |
| SEM | Standard Error of the Mean |
| SNPs | Single Nucleotide Polymorphisms |
| SOX2 | SRY-box Transcription Factor 2 |
| SSEA4 | Stage-Specific Embryonic Antigen-4 |
| TNFα | Tumor Necrosis Factor alpha |
| TNFR | Tumor Necrosis Factor Receptor |
| TRAF2 | TNF Receptor-Associated Factor 2 |
| TRADD | TNFR1-Associated Death Domain Protein |
References
- Alzheimer, A. Über eigenartige Krankheitsfälle des späteren Alters. Z. Gesamte Neurol. Psychiatr. 1911, 4, 356–385. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Datta, D.; Tredici, K.D.; Braak, H. Hypothesis: Tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, 115–124. [Google Scholar] [CrossRef]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Czaniera, N.J.; Schulten, W.; Kaltschmidt, C. NF-κB in Alzheimer’s Disease: Friend or Foe? Opposite Functions in Neurons and Glial Cells. Int. J. Mol. Sci. 2024, 25, 11353. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Helweg, L.P.; Greiner, J.F.W.; Kaltschmidt, C. NF-κB in neurodegenerative diseases: Recent evidence from human genetics. Front. Mol. Neurosci. 2022, 15, 954541. [Google Scholar] [CrossRef]
- Piras, S.; Furfaro, A.L.; Domenicotti, C.; Traverso, N.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. RAGE Expression and ROS Generation in Neurons: Differentiation versus Damage. Oxidative Med. Cell. Longev. 2016, 2016, 9348651. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Uherek, M.; Wellmann, H.; Volk, B.; Kaltschmidt, C. Inhibition of NF-κB potentiates amyloid β-mediated neuronal apoptosis. Proc. Natl. Acad. Sci. USA 1999, 96, 9409–9414. [Google Scholar] [CrossRef]
- Furukawa, K.; Mattson, M.P. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J. Neurochem. 1998, 70, 1876–1886. [Google Scholar] [CrossRef]
- Guerrini, L.; Blasi, F.; Denis-Donini, S. Synaptic activation of NF-kappa B by glutamate in cerebellar granule neurons in vitro. Proc. Natl. Acad. Sci. USA 1995, 92, 9077–9081. [Google Scholar] [CrossRef]
- Kaltschmidt, C.; Kaltschmidt, B.; Baeuerle, P.A. Stimulation of ionotropic glutamate receptors activates transcription factor NF-kappa B in primary neurons. Proc. Natl. Acad. Sci. USA 1995, 92, 9618–9622. [Google Scholar] [CrossRef]
- Meffert, M.K.; Chang, J.M.; Wiltgen, B.J.; Fanselow, M.S.; Baltimore, D. NF-kappa B functions in synaptic signaling and behavior. Nat. Neurosci. 2003, 6, 1072–1078. [Google Scholar] [CrossRef]
- Ghosh, G.; van Duyne, G.; Ghosh, S.; Sigler, P.B. Structure of NF-kappa B p50 homodimer bound to a kappa B site. Nature 1995, 373, 303–310. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Greiner, J.F.W.; Kadhim, H.M.; Kaltschmidt, C. Subunit-Specific Role of NF-κB in Cancer. Biomedicines 2018, 6, 44. [Google Scholar] [CrossRef]
- Zhao, M.; Chauhan, P.; Sherman, C.A.; Singh, A.; Kaileh, M.; Mazan-Mamczarz, K.; Ji, H.; Joy, J.; Nandi, S.; De, S.; et al. NF-κB subunits direct kinetically distinct transcriptional cascades in antigen receptor-activated B cells. Nat. Immunol. 2023, 24, 1552–1564. [Google Scholar] [CrossRef]
- Fricke, F.; Malkusch, S.; Wangorsch, G.; Greiner, J.F.; Kaltschmidt, B.; Kaltschmidt, C.; Widera, D.; Dandekar, T.; Heilemann, M. Quantitative single-molecule localization microscopy combined with rule-based modeling reveals ligand-induced TNF-R1 reorganization toward higher-order oligomers. Histochem. Cell Biol. 2014, 142, 91–101. [Google Scholar] [CrossRef]
- Haas, T.L.; Emmerich, C.H.; Gerlach, B.; Schmukle, A.C.; Cordier, S.M.; Rieser, E.; Feltham, R.; Vince, J.; Warnken, U.; Wenger, T.; et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 2009, 36, 831–844. [Google Scholar] [CrossRef]
- Gatz, M.; Reynolds, C.A.; Fratiglioni, L.; Johansson, B.; Mortimer, J.A.; Berg, S.; Fiske, A.; Pedersen, N.L. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 2006, 63, 168–174. [Google Scholar] [CrossRef]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Shvetcov, A.; Johnson, E.C.B.; Winchester, L.M.; Walker, K.A.; Wilkins, H.M.; Thompson, T.G.; Rothstein, J.D.; Krish, V.; Imam, F.B.; Burns, J.M.; et al. APOE ε4 carriers share immune-related proteomic changes across neurodegenerative diseases. Nat. Med. 2025, 31, 2590–2601. [Google Scholar] [CrossRef]
- Belloy, M.E.; Napolioni, V.; Greicius, M.D. A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward. Neuron 2019, 101, 820–838. [Google Scholar] [CrossRef]
- Yang, L.G.; March, Z.M.; Stephenson, R.A.; Narayan, P.S. Apolipoprotein E in lipid metabolism and neurodegenerative disease. Trends Endocrinol. Metab. 2023, 34, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Martens, S.; Bridelance, J.; Roelandt, R.; Vandenabeele, P.; Takahashi, N. MLKL in cancer: More than a necroptosis regulator. Cell Death Differ. 2021, 28, 1757–1772. [Google Scholar] [CrossRef]
- Madabhushi, R.; Pan, L.; Tsai, L.-H. DNA damage and its links to neurodegeneration. Neuron 2014, 83, 266–282. [Google Scholar] [CrossRef]
- Welch, G.; Tsai, L. Mechanisms of DNA damage-mediated neurotoxicity in neurodegenerative disease. EMBO Rep. 2022, 23, e54217. [Google Scholar] [CrossRef]
- Rahmanian, N.; Shokrzadeh, M.; Eskandani, M. Recent advances in γH2AX biomarker-based genotoxicity assays: A marker of DNA damage and repair. DNA Repair 2021, 108, 103243. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.-L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron 2018, 98, 1141–1154.e7, Erratum in Neuron 2018, 98, 1294. [Google Scholar] [CrossRef]
- Fernandes, S.; Revanna, J.; Pratt, J.; Hayes, N.; Marchetto, M.C.; Gage, F.H. Modeling Alzheimer’s disease using human cell derived brain organoids and 3D models. Front. Neurosci. 2024, 18, 1434945. [Google Scholar] [CrossRef]
- Götz, J.; Bodea, L.-G.; Goedert, M. Rodent models for Alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 583–598. [Google Scholar] [CrossRef]
- Miyashita, A.; Kikuchi, M.; Hara, N.; Ikeuchi, T. Genetics of Alzheimer’s disease: An East Asian perspective. J. Hum. Genet. 2023, 68, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Marchetto, M.C.N.; Yeo, G.W.; Kainohana, O.; Marsala, M.; Gage, F.H.; Muotri, A.R. Transcriptional Signature and Memory Retention of Human-Induced Pluripotent Stem Cells. PLoS ONE 2009, 4, e7076. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.-Y.; Weick, J.P.; Yu, J.; Ma, L.-X.; Zhang, X.-Q.; Thomson, J.A.; Zhang, S.-C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 2010, 107, 4335–4340. [Google Scholar] [CrossRef]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.; Ji, H.; Ehrlich, L.; et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef]
- Shutova, M.V.; Surdina, A.V.; Ischenko, D.S.; Naumov, V.A.; Bogomazova, A.N.; Vassina, E.M.; Alekseev, D.G.; Lagarkova, M.A.; Kiselev, S.L. An integrative analysis of reprogramming in human isogenic system identified a clone selection criterion. Cell Cycle 2016, 15, 986–997. [Google Scholar] [CrossRef]
- Whyte, W.A.; Orlando, D.A.; Hnisz, D.; Abraham, B.J.; Lin, C.Y.; Kagey, M.H.; Rahl, P.B.; Lee, T.I.; Young, R.A. Master Transcription Factors and Mediator Establish Super-Enhancers at Key Cell Identity Genes. Cell 2013, 153, 307–319. [Google Scholar] [CrossRef]
- Theendakara, V.; Peters-Libeu, C.A.; Bredesen, D.E.; Rao, R.V. Transcriptional Effects of ApoE4: Relevance to Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 5243–5254. [Google Scholar] [CrossRef]
- Bose, S.; Cho, J. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch. Pharm. Res. 2013, 36, 1039–1050. [Google Scholar] [CrossRef]
- Bufi, A.A.; Di Stefano, J.; Papait, A.; Silini, A.R.; Parolini, O.; Ponsaerts, P. The central role of CXCL10-CXCR3 signaling in neuroinflammation and neuropathology. Cytokine Growth Factor Rev. 2025, 84, 20–34. [Google Scholar] [CrossRef]
- Moussa, N.; Dayoub, N. Exploring the role of COX-2 in Alzheimer’s disease: Potential therapeutic implications of COX-2 inhibitors. Saudi Pharm. J. 2023, 31, 101729. [Google Scholar] [CrossRef] [PubMed]
- Ortí-Casañ, N.; Wu, Y.; Naudé, P.J.W.; De Deyn, P.P.; Zuhorn, I.S.; Eisel, U.L.M. Targeting TNFR2 as a Novel Therapeutic Strategy for Alzheimer’s Disease. Front. Neurosci. 2019, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Wijdan, S.A.; Bokhari, S.M.N.A.; Alvares, J.; Latif, V. The role of interleukin-1 beta in inflammation and the potential of immune-targeted therapies. Pharmacol. Res.-Rep. 2025, 3, 100027. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta—A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef]
- Lee, S.J.; Hou, J.; Benveniste, E.N. Transcriptional regulation of intercellular adhesion molecule-1 in astrocytes involves NF–κB and C/EBP isoforms. J. Neuroimmunol. 1998, 92, 196–207. [Google Scholar] [CrossRef]
- Arnaud, L.; Benech, P.; Greetham, L.; Stephan, D.; Jimenez, A.; Jullien, N.; García-González, L.; Tsvetkov, P.O.; Devred, F.; Sancho-Martinez, I.; et al. APOE4 drives inflammation in human astrocytes via TAGLN3 repression and NF-κB activation. Cell Rep. 2022, 40, 111200. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 2001, 107, 135–142. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Steele, O.G.; Stuart, A.C.; Minkley, L.; Shaw, K.; Bonnar, O.; Anderle, S.; Penn, A.C.; Rusted, J.; Serpell, L.; Hall, C.; et al. A multi-hit hypothesis for an APOE4-dependent pathophysiological state. Eur. J. Neurosci. 2022, 56, 5476–5515. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Mattson, M.P. Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer’s disease. Neurobiol. Dis. 2020, 138, 104795. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Alexander, G.E. Exercise, APOE genotype, and the evolution of the human lifespan. Trends Neurosci. 2014, 37, 247–255. [Google Scholar] [CrossRef]
- Trumble, B.C.; Charifson, M.; Kraft, T.; Garcia, A.R.; Cummings, D.K.; Hooper, P.; Lea, A.J.; Eid Rodriguez, D.; Koebele, S.V.; Buetow, K.; et al. Apolipoprotein-ε4 is associated with higher fecundity in a natural fertility population. Sci. Adv. 2023, 9, eade9797. [Google Scholar] [CrossRef]
- Trumble, B.C.; Stieglitz, J.; Blackwell, A.D.; Allayee, H.; Beheim, B.; Finch, C.E.; Gurven, M.; Kaplan, H. Apolipoprotein E4 is associated with improved cognitive function in Amazonian forager-horticulturalists with a high parasite burden. FASEB J. 2017, 31, 1508–1515. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; van den Hoff, M.J.B.; Moorman, A.F.M. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F.M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
| Primary Antibody | Host | Dilution | Manufacturer |
|---|---|---|---|
| SSEA4 | Mouse | 1:100 | Thermo Fisher Scientific, Waltham, MA, USA |
| OCT4 | Rabbit | 1:200 | Thermo Fisher Scientific, Waltham, MA, USA |
| RelA | Rabbit | 1:400 | Cell Signaling Technology, Danvers & Boston, MA, USA |
| RelB | Rabbit | 1:100 | Cell Signaling Technology, Danvers & Boston, MA, USA |
| cRel | Rabbit | 1:100 | Cell Signaling Technology, Danvers & Boston, MA, USA |
| pMLKL | Rabbit | 1:200 | Cell Signaling Technology, Danvers & Boston, MA, USA |
| cleaved caspase-3 | Rabbit | 1:400 | Cell Signaling Technology, Danvers & Boston, MA, USA |
| γH2AX | Mouse | 1:500 | Thermo Fisher Scientific, Waltham, MA, USA |
| Primer | Sequence (5′ to 3′) |
|---|---|
| GAPDH fwd | CATGAGAAGTATGACAACAGCCT |
| GAPDH rev | AGTCCTTCCACGATACCAAAGT |
| RPLP0 fwd | TGGTCATCCAGCAGGTGTTCGA |
| RPLP0 rev | ACAGACACTGGCAACATTGCGG |
| TNFR2 fwd | CGTTCTCCAACACGACTTCATCC |
| TNFR2 rev | ACGTGCAGACTGCATCCATGCT |
| IL-1B fwd | ATGATGGCTTATTACAGTGGCAA |
| IL-1B rev | GTCGGAGATTCGTAGCTGGA |
| CCL2 fwd | CAGCCAGATGCAATCAATGCC |
| CCL2 rev | TGGAATCCTGAACCCACTTCT |
| CXCL10 fwd | GGTGAGAAGAGATGTCTGAATCC |
| CXCL10 rev | GTCCATCCTTGGAAGCACTGCA |
| ICAM1 fwd | ATGGCAACGACTCCTTCTCG |
| ICAM1 rev | GCCGGAAAGCTGTAGATGGT |
| COX2 fwd | CGGTGAAACTCTGGCTAGACAG |
| COX2 rev | GCAAACCGTAGATGCTCAGGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulten, W.; Czaniera, N.J.; Buschheuer, A.L.; Liermann, A.; Wiegand, A.; Kaltschmidt, B.; Kaltschmidt, C. Pluripotent Cells Expressing APOE4 Exhibit a Pronounced Pro-Apoptotic Phenotype Accompanied by Markers of Hyperinflammation and a Blunted NF-κB Response. Int. J. Mol. Sci. 2025, 26, 9283. https://doi.org/10.3390/ijms26199283
Schulten W, Czaniera NJ, Buschheuer AL, Liermann A, Wiegand A, Kaltschmidt B, Kaltschmidt C. Pluripotent Cells Expressing APOE4 Exhibit a Pronounced Pro-Apoptotic Phenotype Accompanied by Markers of Hyperinflammation and a Blunted NF-κB Response. International Journal of Molecular Sciences. 2025; 26(19):9283. https://doi.org/10.3390/ijms26199283
Chicago/Turabian StyleSchulten, Wiebke, Nele Johanne Czaniera, Anna Lena Buschheuer, Antonia Liermann, Axel Wiegand, Barbara Kaltschmidt, and Christian Kaltschmidt. 2025. "Pluripotent Cells Expressing APOE4 Exhibit a Pronounced Pro-Apoptotic Phenotype Accompanied by Markers of Hyperinflammation and a Blunted NF-κB Response" International Journal of Molecular Sciences 26, no. 19: 9283. https://doi.org/10.3390/ijms26199283
APA StyleSchulten, W., Czaniera, N. J., Buschheuer, A. L., Liermann, A., Wiegand, A., Kaltschmidt, B., & Kaltschmidt, C. (2025). Pluripotent Cells Expressing APOE4 Exhibit a Pronounced Pro-Apoptotic Phenotype Accompanied by Markers of Hyperinflammation and a Blunted NF-κB Response. International Journal of Molecular Sciences, 26(19), 9283. https://doi.org/10.3390/ijms26199283






