Pharmacological and Non-Pharmacological Interventions in Diabetes Mellitus: Effects on Epicardial Adipose Tissue
Abstract
1. Introduction
2. EAT Characteristics
2.1. Anatomy
2.2. White or Brown?
2.3. Role of EAT
2.4. EAT Assessment
3. How Does EAT Change in Diabetes?
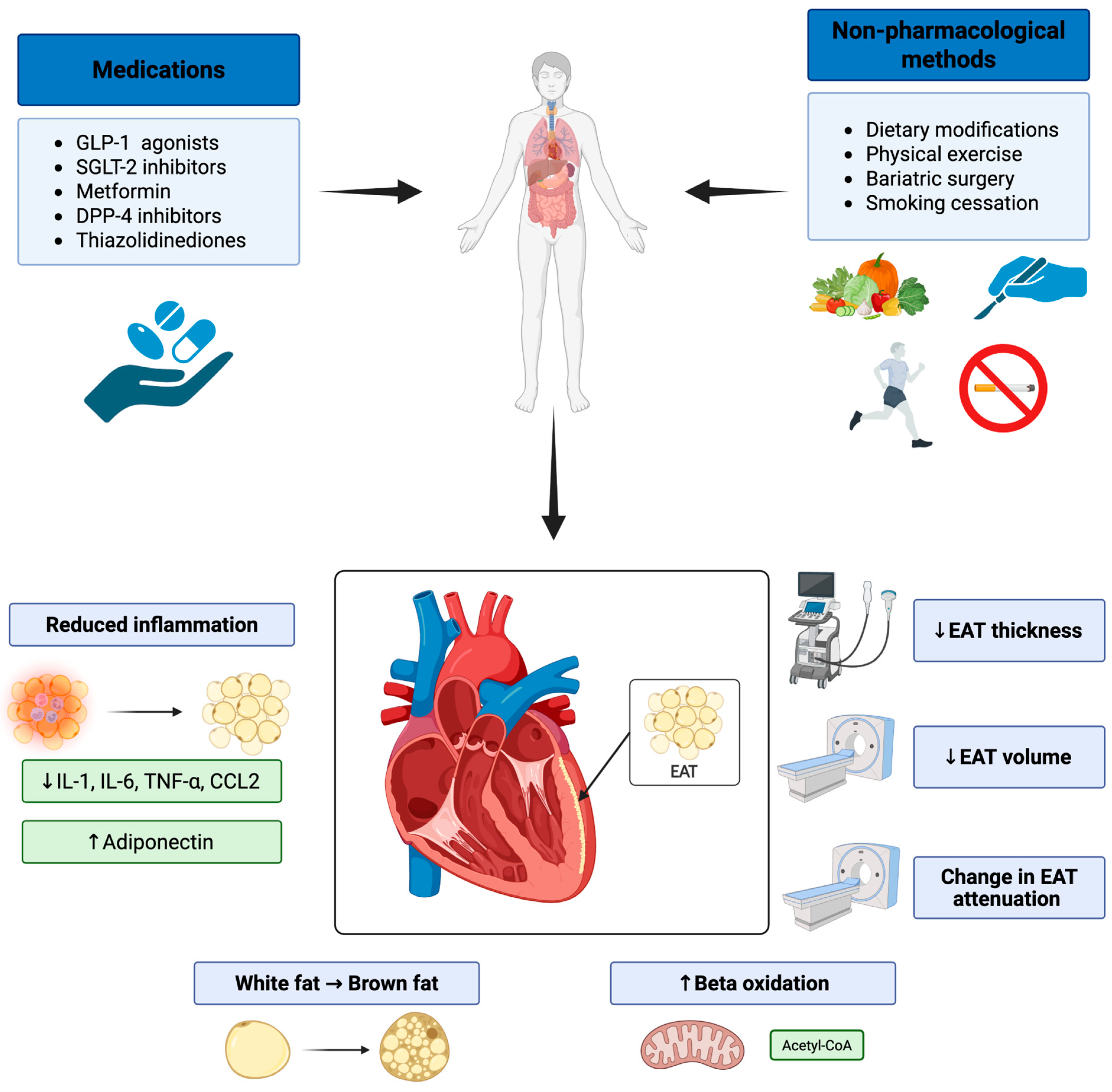
4. Pharmacological Interventions
4.1. Glucagon-like Peptide-1 Receptor Agonists
4.2. Sodium-Glucose Cotransporter-2 Inhibitors
4.3. Metformin
4.4. Thiazolidinediones
4.5. Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
4.6. Insulin
4.7. Sulfonylureas
5. Non-Pharmacological Interventions
5.1. Physical Exercise
5.2. Diet
5.3. Bariatric Surgery
5.4. Combination or Comparison of Weight Reduction Methods in Terms of EAT Changes
5.5. Smoking Cessation
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacks, D.B.; Arnold, M.; Bakris, G.L.; Bruns, D.E.; Horvath, A.R.; Lernmark, A.; Metzger, B.E.; Nathan, D.M.; Kirkman, M.S. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Diabetes Care 2023, 46, e151–e199. [Google Scholar] [CrossRef]
- Mlynarska, E.; Czarnik, W.; Dzieza, N.; Jedraszak, W.; Majchrowicz, G.; Prusinowski, F.; Stabrawa, M.; Rysz, J.; Franczyk, B. Type 2 Diabetes Mellitus: New Pathogenetic Mechanisms, Treatment and the Most Important Complications. Int. J. Mol. Sci. 2025, 26, 1094. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Xue, A.; Wu, Y.; Zhu, Z.; Zhang, F.; Kemper, K.E.; Zheng, Z.; Yengo, L.; Lloyd-Jones, L.R.; Sidorenko, J.; Wu, Y.; et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat. Commun. 2018, 9, 2941. [Google Scholar] [CrossRef]
- Desai, S.; Deshmukh, A. Mapping of Type 1 Diabetes Mellitus. Curr. Diabetes Rev. 2020, 16, 438–441. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Strain, W.D.; Paldanius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef]
- Zhang, S.J.; Wang, S.W.; Liu, S.Y.; Li, P.; Huang, D.L.; Zeng, X.X.; Lan, T.; Ruan, Y.P.; Shi, H.J.; Zhang, X. Epicardial adipose tissue: A new link between type 2 diabetes and heart failure-a comprehensive review. Heart Fail. Rev. 2025, 30, 477–491. [Google Scholar] [CrossRef]
- Bornachea, O.; Vea, A.; Llorente-Cortes, V. Interplay between epicardial adipose tissue, metabolic and cardiovascular diseases. Clin. Investig. Arter. 2018, 30, 230–239. [Google Scholar] [CrossRef]
- Naryzhnaya, N.V.; Koshelskaya, O.A.; Kologrivova, I.V.; Kharitonova, O.A.; Evtushenko, V.V.; Boshchenko, A.A. Hypertrophy and Insulin Resistance of Epicardial Adipose Tissue Adipocytes: Association with the Coronary Artery Disease Severity. Biomedicines 2021, 9, 64. [Google Scholar] [CrossRef]
- Nerlekar, N.; Brown, A.J.; Muthalaly, R.G.; Talman, A.; Hettige, T.; Cameron, J.D.; Wong, D.T.L. Association of Epicardial Adipose Tissue and High-Risk Plaque Characteristics: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e006379. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Hirata, Y.; Tabata, M.; Dagvasumberel, M.; Sato, H.; Kurobe, H.; Fukuda, D.; Soeki, T.; Kitagawa, T.; Takanashi, S.; et al. Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arter. Thromb. Vasc. Biol. 2013, 33, 1077–1084. [Google Scholar] [CrossRef]
- Wong, C.X.; Sun, M.T.; Odutayo, A.; Emdin, C.A.; Mahajan, R.; Lau, D.H.; Pathak, R.K.; Wong, D.T.; Selvanayagam, J.B.; Sanders, P.; et al. Associations of Epicardial, Abdominal, and Overall Adiposity With Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2016, 9, e004378. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, H.; Guo, L.; Hong, K. Relationship between epicardial adipose tissue volume and atrial fibrillation: A systematic review and meta-analysis. Herz 2016, 41, 421–427. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef]
- Conte, M.; Petraglia, L.; Cabaro, S.; Valerio, V.; Poggio, P.; Pilato, E.; Attena, E.; Russo, V.; Ferro, A.; Formisano, P.; et al. Epicardial Adipose Tissue and Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front. Cardiovasc. Med. 2022, 9, 932262. [Google Scholar] [CrossRef]
- Anagnostopoulos, I.; Kousta, M.; Kossyvakis, C.; Paraskevaidis, N.T.; Vrachatis, D.; Deftereos, S.; Giannopoulos, G. Epicardial Adipose Tissue and Atrial Fibrillation Recurrence following Catheter Ablation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 6369. [Google Scholar] [CrossRef]
- Momot, K.; Krauz, K.; Pruc, M.; Szarpak, L.; Rodkiewicz, D.; Mamcarz, A. Association Between Left Atrial Epicardial Adipose Tissue Attenuation Assessed by Cardiac Computed Tomography and Atrial Fibrillation Recurrence Following Catheter Ablation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4771. [Google Scholar] [CrossRef]
- Tan, S.Y.; Mei Wong, J.L.; Sim, Y.J.; Wong, S.S.; Mohamed Elhassan, S.A.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. 2019, 13, 364–372, Erratum in Diabetes Metab. Syndr. 2021, 15, 469. [Google Scholar] [CrossRef]
- Lazaros, G.; Antonopoulos, A.; Antoniades, C.; Tousoulis, D. The Role of Epicardial Fat in Pericardial Diseases. Curr. Cardiol. Rep. 2018, 20, 40. [Google Scholar] [CrossRef]
- Corradi, D.; Maestri, R.; Callegari, S.; Pastori, P.; Goldoni, M.; Luong, T.V.; Bordi, C. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc. Pathol. 2004, 13, 313–316. [Google Scholar] [CrossRef]
- Jermendy, A.L.; Kolossvary, M.; Drobni, Z.D.; Tarnoki, A.D.; Tarnoki, D.L.; Karady, J.; Voros, S.; Lamb, H.J.; Merkely, B.; Jermendy, G.; et al. Assessing genetic and environmental influences on epicardial and abdominal adipose tissue quantities: A classical twin study. Int. J. Obes. 2018, 42, 163–168. [Google Scholar] [CrossRef]
- Ernault, A.C.; Meijborg, V.M.F.; Coronel, R. Modulation of Cardiac Arrhythmogenesis by Epicardial Adipose Tissue: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1730–1745. [Google Scholar] [CrossRef]
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 536–543. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial and pericardial fat: Close, but very different. Obesity 2009, 17, 625. [Google Scholar] [CrossRef]
- Bambace, C.; Telesca, M.; Zoico, E.; Sepe, A.; Olioso, D.; Rossi, A.; Corzato, F.; Di Francesco, V.; Mazzucco, A.; Santini, F.; et al. Adiponectin gene expression and adipocyte diameter: A comparison between epicardial and subcutaneous adipose tissue in men. Cardiovasc. Pathol. 2011, 20, e153–e156. [Google Scholar] [CrossRef]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N.; Bahouth, S.W.; Ojha, S.; Frontini, A.; Budge, H.; Cinti, S.; Symonds, M.E. Adult epicardial fat exhibits beige features. J. Clin. Endocrinol. Metab. 2013, 98, E1448–E1455. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N.; Holman, B.; Cheema, P.; Chary, A.; Parks, F.; Karas, J.; Optican, R.; Bahouth, S.W.; Garrett, E.; et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: Epicardial fat functioning as brown fat. J. Clin. Endocrinol. Metab. 2009, 94, 3611–3615. [Google Scholar] [CrossRef]
- Brondani, L.A.; Assmann, T.S.; de Souza, B.M.; Boucas, A.P.; Canani, L.H.; Crispim, D. Meta-analysis reveals the association of common variants in the uncoupling protein (UCP) 1–3 genes with body mass index variability. PLoS ONE 2014, 9, e96411. [Google Scholar] [CrossRef]
- Gharanei, S.; Shabir, K.; Brown, J.E.; Weickert, M.O.; Barber, T.M.; Kyrou, I.; Randeva, H.S. Regulatory microRNAs in Brown, Brite and White Adipose Tissue. Cells 2020, 9, 2489. [Google Scholar] [CrossRef]
- Sharp, L.Z.; Shinoda, K.; Ohno, H.; Scheel, D.W.; Tomoda, E.; Ruiz, L.; Hu, H.; Wang, L.; Pavlova, Z.; Gilsanz, V.; et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE 2012, 7, e49452. [Google Scholar] [CrossRef]
- Evans, B.A.; Merlin, J.; Bengtsson, T.; Hutchinson, D.S. Adrenoceptors in white, brown, and brite adipocytes. Br. J. Pharmacol. 2019, 176, 2416–2432. [Google Scholar] [CrossRef]
- Lapa, C.; Arias-Loza, P.; Hayakawa, N.; Wakabayashi, H.; Werner, R.A.; Chen, X.; Shinaji, T.; Herrmann, K.; Pelzer, T.; Higuchi, T. Whitening and Impaired Glucose Utilization of Brown Adipose Tissue in a Rat Model of Type 2 Diabetes Mellitus. Sci. Rep. 2017, 7, 16795. [Google Scholar] [CrossRef]
- Iacobellis, G. Aging Effects on Epicardial Adipose Tissue. Front. Aging 2021, 2, 666260. [Google Scholar] [CrossRef]
- Fainberg, H.P.; Birtwistle, M.; Alagal, R.; Alhaddad, A.; Pope, M.; Davies, G.; Woods, R.; Castellanos, M.; May, S.T.; Ortori, C.A.; et al. Transcriptional analysis of adipose tissue during development reveals depot-specific responsiveness to maternal dietary supplementation. Sci. Rep. 2018, 8, 9628. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Antoniades, C. The role of epicardial adipose tissue in cardiac biology: Classic concepts and emerging roles. J. Physiol. 2017, 595, 3907–3917. [Google Scholar] [CrossRef]
- Marchington, J.M.; Pond, C.M. Site-specific properties of pericardial and epicardial adipose tissue: The effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int. J. Obes. 1990, 14, 1013–1022. [Google Scholar]
- Iacobellis, G.; Pistilli, D.; Gucciardo, M.; Leonetti, F.; Miraldi, F.; Brancaccio, G.; Gallo, P.; di Gioia, C.R. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005, 29, 251–255. [Google Scholar] [CrossRef]
- Iacobellis, G.; di Gioia, C.R.; Di Vito, M.; Petramala, L.; Cotesta, D.; De Santis, V.; Vitale, D.; Tritapepe, L.; Letizia, C. Epicardial adipose tissue and intracoronary adrenomedullin levels in coronary artery disease. Horm. Metab. Res. 2009, 41, 855–860. [Google Scholar] [CrossRef]
- Iacobellis, G.; Mahabadi, A.A. Is epicardial fat attenuation a novel marker of coronary inflammation? Atherosclerosis 2019, 284, 212–213. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Wang, Y.; Zhou, N.; Shu, J.; Stamm, C.; Jiang, M.; Luo, F. Association of epicardial adipose tissue attenuation with coronary atherosclerosis in patients with a high risk of coronary artery disease. Atherosclerosis 2019, 284, 230–236. [Google Scholar] [CrossRef]
- Wang, C.P.; Hsu, H.L.; Hung, W.C.; Yu, T.H.; Chen, Y.H.; Chiu, C.A.; Lu, L.F.; Chung, F.M.; Shin, S.J.; Lee, Y.J. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin. Endocrinol. 2009, 70, 876–882. [Google Scholar] [CrossRef]
- Philouze, C.; Obert, P.; Nottin, S.; Benamor, A.; Barthez, O.; Aboukhoudir, F. Dobutamine Stress Echocardiography Unmasks Early Left Ventricular Dysfunction in Asymptomatic Patients with Uncomplicated Type 2 Diabetes: A Comprehensive Two-Dimensional Speckle-Tracking Imaging Study. J. Am. Soc. Echocardiogr. 2018, 31, 587–597. [Google Scholar] [CrossRef]
- Gunes, H.; Gunes, H.; Temiz, F. The Relationship Between Epicardial Adipose Tissue and Insulin Resistance in Obese Children. Arq. Bras. Cardiol. 2020, 114, 675–682. [Google Scholar] [CrossRef]
- Ayton, S.L.; Yeo, J.L.; Gulsin, G.S.; Dattani, A.; Bilak, J.; Deshpande, A.; Arnold, J.R.; Singh, A.; Graham-Brown, M.P.M.; Ng, L.; et al. Association of epicardial adipose tissue with early structural and functional cardiac changes in Type 2 diabetes. Eur. J. Radiol. 2024, 174, 111400. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.; Blythe, H.; Wood, E.G.; Sandhar, B.; Sarker, S.J.; Balmforth, D.; Ambekar, S.G.; Yap, J.; Edmondson, S.J.; Di Salvo, C.; et al. Obesity and diabetes are major risk factors for epicardial adipose tissue inflammation. JCI Insight. 2021, 6. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Santos, I.; Perez-Belmonte, L.M.; Macias-Gonzalez, M.; Mataro, M.J.; Castellano, D.; Lopez-Garrido, M.; Porras-Martin, C.; Sanchez-Fernandez, P.L.; Gomez-Doblas, J.J.; Cardona, F.; et al. Type 2 diabetes is associated with decreased PGC1alpha expression in epicardial adipose tissue of patients with coronary artery disease. J. Transl. Med. 2016, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Camarena, V.; Sant, D.; Mohseni, M.; Salerno, T.; Zaleski, M.L.; Wang, G.; Iacobellis, G. Novel atherogenic pathways from the differential transcriptome analysis of diabetic epicardial adipose tissue. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 739–750. [Google Scholar] [CrossRef]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef]
- Xie, W.; Wang, Y.; Xiao, S.; Qiu, L.; Yu, Y.; Zhang, Z. Association of gestational diabetes mellitus with overall and type specific cardiovascular and cerebrovascular diseases: Systematic review and meta-analysis. BMJ 2022, 378, e070244. [Google Scholar] [CrossRef] [PubMed]
- Henningsson, M.; Brundin, M.; Scheffel, T.; Edin, C.; Viola, F.; Carlhall, C.J. Quantification of epicardial fat using 3D cine Dixon MRI. BMC Med. Imaging 2020, 20, 80. [Google Scholar] [CrossRef]
- Morano, S.; Romagnoli, E.; Filardi, T.; Nieddu, L.; Mandosi, E.; Fallarino, M.; Turinese, I.; Dagostino, M.P.; Lenzi, A.; Carnevale, V. Short-term effects of glucagon-like peptide 1 (GLP-1) receptor agonists on fat distribution in patients with type 2 diabetes mellitus: An ultrasonography study. Acta Diabetol. 2015, 52, 727–732. [Google Scholar] [CrossRef]
- Iacobellis, G.; Mohseni, M.; Bianco, S.D.; Banga, P.K. Liraglutide causes large and rapid epicardial fat reduction. Obesity 2017, 25, 311–316. [Google Scholar] [CrossRef]
- Iacobellis, G.; Villasante Fricke, A.C. Effects of Semaglutide Versus Dulaglutide on Epicardial Fat Thickness in Subjects with Type 2 Diabetes and Obesity. J. Endocr. Soc. 2020, 4, bvz042. [Google Scholar] [CrossRef]
- Kramer, C.M.; Borlaug, B.A.; Zile, M.R.; Ruff, D.; DiMaria, J.M.; Menon, V.; Ou, Y.; Zarante, A.M.; Hurt, K.C.; Murakami, M.; et al. Tirzepatide Reduces LV Mass and Paracardiac Adipose Tissue in Obesity-Related Heart Failure: SUMMIT CMR Substudy. J. Am. Coll. Cardiol. 2025, 85, 699–706. [Google Scholar] [CrossRef]
- Cinti, F.; Leccisotti, L.; Sorice, G.P.; Capece, U.; D’Amario, D.; Lorusso, M.; Gugliandolo, S.; Morciano, C.; Guarneri, A.; Guzzardi, M.A.; et al. Dapagliflozin treatment is associated with a reduction of epicardial adipose tissue thickness and epicardial glucose uptake in human type 2 diabetes. Cardiovasc. Diabetol. 2023, 22, 349. [Google Scholar] [CrossRef] [PubMed]
- Requena-Ibanez, J.A.; Santos-Gallego, C.G.; Rodriguez-Cordero, A.; Vargas-Delgado, A.P.; Mancini, D.; Sartori, S.; Atallah-Lajam, F.; Giannarelli, C.; Macaluso, F.; Lala, A.; et al. Mechanistic Insights of Empagliflozin in Nondiabetic Patients With HFrEF: From the EMPA-TROPISM Study. JACC Heart Fail. 2021, 9, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Bouchi, R.; Terashima, M.; Sasahara, Y.; Asakawa, M.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; et al. Ipragliflozin Reduces Epicardial Fat Accumulation in Non-Obese Type 2 Diabetic Patients with Visceral Obesity: A Pilot Study. Diabetes Ther. 2017, 8, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Bouchi, R.; Terashima, M.; Sasahara, Y.; Asakawa, M.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: A pilot study. Cardiovasc. Diabetol. 2017, 16, 32. [Google Scholar] [CrossRef]
- Sato, T.; Aizawa, Y.; Yuasa, S.; Kishi, S.; Fuse, K.; Fujita, S.; Ikeda, Y.; Kitazawa, H.; Takahashi, M.; Sato, M.; et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc. Diabetol. 2018, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Ziyrek, M.; Kahraman, S.; Ozdemir, E.; Dogan, A. Metformin monotherapy significantly decreases epicardial adipose tissue thickness in newly diagnosed type 2 diabetes patients. Rev. Port. Cardiol. (Engl. Ed.) 2019, 38, 419–423. [Google Scholar] [CrossRef]
- Gunes, H.; Gunes, H.; Ozmen, S.; Celik, E.; Temiz, F. Effects of metformin on epicardial adipose tissue and atrial electromechanical delay of obese children with insulin resistance. Cardiol. Young 2020, 30, 1429–1432. [Google Scholar] [CrossRef]
- Moody, A.J.; Molina-Wilkins, M.; Clarke, G.D.; Merovci, A.; Solis-Herrera, C.; Cersosimo, E.; Chilton, R.J.; Iozzo, P.; Gastaldelli, A.; Abdul-Ghani, M.; et al. Pioglitazone reduces epicardial fat and improves diastolic function in patients with type 2 diabetes. Diabetes Obes. Metab. 2023, 25, 426–434. [Google Scholar] [CrossRef]
- Lima-Martinez, M.M.; Paoli, M.; Rodney, M.; Balladares, N.; Contreras, M.; D’Marco, L.; Iacobellis, G. Effect of sitagliptin on epicardial fat thickness in subjects with type 2 diabetes and obesity: A pilot study. Endocrine 2016, 51, 448–455. [Google Scholar] [CrossRef]
- Hiruma, S.; Shigiyama, F.; Hisatake, S.; Mizumura, S.; Shiraga, N.; Hori, M.; Ikeda, T.; Hirose, T.; Kumashiro, N. A prospective randomized study comparing effects of empagliflozin to sitagliptin on cardiac fat accumulation, cardiac function, and cardiac metabolism in patients with early-stage type 2 diabetes: The ASSET study. Cardiovasc. Diabetol. 2021, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Elisha, B.; Azar, M.; Taleb, N.; Bernard, S.; Iacobellis, G.; Rabasa-Lhoret, R. Body Composition and Epicardial Fat in Type 2 Diabetes Patients Following Insulin Detemir Versus Insulin Glargine Initiation. Horm. Metab. Res. 2016, 48, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Malavazos, A.E.; Iacobellis, G.; Dozio, E.; Basilico, S.; Di Vincenzo, A.; Dubini, C.; Menicanti, L.; Vianello, E.; Meregalli, C.; Ruocco, C.; et al. Human epicardial adipose tissue expresses glucose-dependent insulinotropic polypeptide, glucagon, and glucagon-like peptide-1 receptors as potential targets of pleiotropic therapies. Eur. J. Prev. Cardiol. 2023, 30, 680–693. [Google Scholar] [CrossRef]
- Iacobellis, G.; Camarena, V.; Sant, D.W.; Wang, G. Human Epicardial Fat Expresses Glucagon-Like Peptide 1 and 2 Receptors Genes. Horm. Metab. Res. 2017, 49, 625–630. [Google Scholar] [CrossRef]
- Baggio, L.L.; Yusta, B.; Mulvihill, E.E.; Cao, X.; Streutker, C.J.; Butany, J.; Cappola, T.P.; Margulies, K.B.; Drucker, D.J. GLP-1 Receptor Expression Within the Human Heart. Endocrinology 2018, 159, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Dozio, E.; Vianello, E.; Malavazos, A.E.; Tacchini, L.; Schmitz, G.; Iacobellis, G.; Corsi Romanelli, M.M. Epicardial adipose tissue GLP-1 receptor is associated with genes involved in fatty acid oxidation and white-to-brown fat differentiation: A target to modulate cardiovascular risk? Int. J. Cardiol. 2019, 292, 218–224. [Google Scholar] [CrossRef]
- Chondronikola, M.; Volpi, E.; Borsheim, E.; Porter, C.; Annamalai, P.; Enerback, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M.; et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014, 63, 4089–4099. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013, 123, 3404–3408. [Google Scholar] [CrossRef]
- Berg, G.; Barchuk, M.; Lobo, M.; Nogueira, J.P. Effect of glucagon-like peptide-1 (GLP-1) analogues on epicardial adipose tissue: A meta-analysis. Diabetes Metab. Syndr. 2022, 16, 102562. [Google Scholar] [CrossRef]
- Hong, J.Y.; Park, K.Y.; Kim, B.J.; Hwang, W.M.; Kim, D.H.; Lim, D.M. Effects of Short-Term Exenatide Treatment on Regional Fat Distribution, Glycated Hemoglobin Levels, and Aortic Pulse Wave Velocity of Obese Type 2 Diabetes Mellitus Patients. Endocrinol. Metab. 2016, 31, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Shingu, Y.; Amorim, P.A.; Schenkl, C.; Schwarzer, M.; Doenst, T. GLP-1 Improves Diastolic Function and Survival in Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Transl. Res. 2018, 11, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Goldberger, J.J.; Lamelas, J.; Martinez, C.A.; Sterling, C.M.; Bodenstab, M.; Frasca, D. Liraglutide effects on epicardial adipose tissue micro-RNAs and intra-operative glucose control. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103726. [Google Scholar] [CrossRef]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef]
- MacIsaac, R.J.; Deed, G.; D’Emden, M.; Ekinci, E.I.; Hocking, S.; Sumithran, P.; Rasalam, R. Challenging Clinical Perspectives in Type 2 Diabetes with Tirzepatide, a First-in-Class Twincretin. Diabetes Ther. 2023, 14, 1997–2014. [Google Scholar] [CrossRef] [PubMed]
- Krauss, Z.; Hintz, A.; Fisk, R. Tirzepatide: Clinical review of the “twincretin” injectable. Am. J. Health Syst. Pharm. 2023, 80, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, J.; Kong, B.; Shuai, W.; Huang, H. Tirzepatide attenuates lipopolysaccharide-induced left ventricular remodeling and dysfunction by inhibiting the TLR4/NF-kB/NLRP3 pathway. Int. Immunopharmacol. 2023, 120, 110311. [Google Scholar] [CrossRef]
- Taktaz, F.; Scisciola, L.; Fontanella, R.A.; Pesapane, A.; Ghosh, P.; Franzese, M.; Tortorella, G.; Puocci, A.; Sommella, E.; Signoriello, G.; et al. Evidence that tirzepatide protects against diabetes-related cardiac damages. Cardiovasc. Diabetol. 2024, 23, 112. [Google Scholar] [CrossRef]
- Sharif, S.; Van der Graaf, Y.; Cramer, M.J.; Kapelle, L.J.; de Borst, G.J.; Visseren, F.L.J.; Westerink, J.; SMART study group. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 220. [Google Scholar] [CrossRef]
- Mirza, S.; Hossain, M.; Mathews, C.; Martinez, P.; Pino, P.; Gay, J.L.; Rentfro, A.; McCormick, J.B.; Fisher-Hoch, S.P. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: A cross-sectional study. Cytokine 2012, 57, 136–142. [Google Scholar] [CrossRef]
- Gallou, G.; Ruelland, A.; Legras, B.; Maugendre, D.; Allannic, H.; Cloarec, L. Plasma malondialdehyde in type 1 and type 2 diabetic patients. Clin. Chim. Acta 1993, 214, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.J.H.; Foster-Davies, H.; Salem, A.; Hoole, A.L.; Obaid, D.R.; Halcox, J.P.J.; Stephens, J.W. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomised controlled trials. Diabetes Obes. Metab. 2021, 23, 1806–1822. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schutt, K.; Muller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Lo, K.B.; Gul, F.; Ram, P.; Kluger, A.Y.; Tecson, K.M.; McCullough, P.A.; Rangaswami, J. The Effects of SGLT2 Inhibitors on Cardiovascular and Renal Outcomes in Diabetic Patients: A Systematic Review and Meta-Analysis. Cardiorenal. Med. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; DeMets, D.L.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; Bengtsson, O.; Ponikowski, P.; Sabatine, M.S.; et al. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur. J. Heart Fail. 2019, 21, 665–675. [Google Scholar] [CrossRef]
- Palmer, S.C.; Tendal, B.; Mustafa, R.A.; Vandvik, P.O.; Li, S.; Hao, Q.; Tunnicliffe, D.; Ruospo, M.; Natale, P.; Saglimbene, V.; et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2021, 372, m4573, Erratum in BMJ 2022, 376, o109. [Google Scholar] [CrossRef]
- Baartscheer, A.; Schumacher, C.A.; Wust, R.C.; Fiolet, J.W.; Stienen, G.J.; Coronel, R.; Zuurbier, C.J. Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia 2017, 60, 568–573. [Google Scholar] [CrossRef]
- Clancy, C.E.; Chen-Izu, Y.; Bers, D.M.; Belardinelli, L.; Boyden, P.A.; Csernoch, L.; Despa, S.; Fermini, B.; Hool, L.C.; Izu, L.; et al. Deranged sodium to sudden death. J. Physiol. 2015, 593, 1331–1345. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, E.; Agra, R.M.; Fernandez, A.L.; Adrio, B.; Garcia-Caballero, T.; Gonzalez-Juanatey, J.R.; Eiras, S. Effects of dapagliflozin on human epicardial adipose tissue: Modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovasc. Res. 2018, 114, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial Tissue Characterization and Fibrosis by Imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Van Gaal, L.; Leiter, L.A.; Vijapurkar, U.; List, J.; Cuddihy, R.; Ren, J.; Davies, M.J. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism 2018, 85, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, V.A.; Parisi, V.; Moschetta, D.; Valerio, V.; Conte, M.; Massaiu, I.; Bozzi, M.; Celeste, F.; Leosco, D.; Iaccarino, G.; et al. Efficacy of cardiometabolic drugs in reduction of epicardial adipose tissue: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2023, 22, 23. [Google Scholar] [CrossRef]
- Bao, Y.; Hu, Y.; Shi, M.; Zhao, Z. SGLT2 inhibitors reduce epicardial adipose tissue more than GLP-1 agonists or exercise interventions in patients with type 2 diabetes mellitus and/or obesity: A systematic review and network meta-analysis. Diabetes Obes. Metab. 2025, 27, 1096–1112. [Google Scholar] [CrossRef]
- Shah, A.S.; Zeitler, P.S.; Wong, J.; Pena, A.S.; Wicklow, B.; Arslanian, S.; Chang, N.; Fu, J.; Dabadghao, P.; Pinhas-Hamiel, O.; et al. ISPAD Clinical Practice Consensus Guidelines 2022: Type 2 diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 872–902. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycaemia in type 2 diabetes: A patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012, 55, 1577–1596. [Google Scholar] [CrossRef]
- Wrobel, M.P.; Marek, B.; Kajdaniuk, D.; Rokicka, D.; Szymborska-Kajanek, A.; Strojek, K. Metformin—A new old drug. Endokrynol. Pol. 2017, 68, 482–496. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Chen, X.; Peng, S.; Chen, S.; Zhou, G.; Wei, Y.; Lu, X.; Zhou, C.; Ye, Y.; et al. Selective blockade of interleukin 6 trans-signaling depresses atrial fibrillation. Heart Rhythm 2023, 20, 1759–1770. [Google Scholar] [CrossRef]
- Wu, N.; Xu, B.; Xiang, Y.; Wu, L.; Zhang, Y.; Ma, X.; Tong, S.; Shu, M.; Song, Z.; Li, Y.; et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: A meta-analysis. Int. J. Cardiol. 2013, 169, 62–72. [Google Scholar] [CrossRef]
- Temiz, F.; Gunes, H.; Gunes, H. Evaluation of Atrial Electromechanical Delay in Children with Obesity. Medicina 2019, 55, 228. [Google Scholar] [CrossRef]
- Gunes, H.; Sokmen, A.; Kaya, H.; Gungor, O.; Kerkutluoglu, M.; Guzel, F.B.; Sokmen, G. Evaluation of Atrial Electromechanical Delay to Predict Atrial Fibrillation in Hemodialysis Patients. Medicina 2018, 54, 58. [Google Scholar] [CrossRef] [PubMed]
- Tascanov, M.B. The Relationship Between Prolidase Activity and Atrial Electromechanical Changes in Patients with Paroxysmal Atrial Fibrillation. Comb. Chem. High Throughput Screen. 2019, 22, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Po, S.S.; Zhang, B.; Bai, F.; Li, J.; Qin, F.; Liu, N.; Sun, C.; Xiao, Y.; Tu, T.; et al. Metformin regulates adiponectin signalling in epicardial adipose tissue and reduces atrial fibrillation vulnerability. J. Cell. Mol. Med. 2020, 24, 7751–7766. [Google Scholar] [CrossRef] [PubMed]
- Elia, E.M.; Pustovrh, C.; Amalfi, S.; Devoto, L.; Motta, A.B. Link between metformin and the peroxisome proliferator-activated receptor gamma pathway in the uterine tissue of hyperandrogenized prepubertal mice. Fertil. Steril. 2011, 95, 2534–2537.e1. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, Y.W.; Han, J.; Liang, H.; Wang, N.; Cheng, Y. 12/15-Lipoxygenase metabolites of arachidonic acid activate PPARgamma: A possible neuroprotective effect in ischemic brain. J. Lipid Res. 2015, 56, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.H.; El Kiki, S.M.; Galal, S.M. Metformin and low dose radiation modulates cisplatin-induced oxidative injury in rat via PPAR-gamma and MAPK pathways. Arch. Biochem. Biophys. 2017, 616, 13–19. [Google Scholar] [CrossRef]
- Su, J.R.; Lu, Z.H.; Su, Y.; Zhao, N.; Dong, C.L.; Sun, L.; Zhao, S.F.; Li, Y. Relationship of Serum Adiponectin Levels and Metformin Therapy in Patients with Type 2 Diabetes. Horm. Metab. Res. 2016, 48, 92–98. [Google Scholar] [CrossRef]
- Evia-Viscarra, M.L.; Rodea-Montero, E.R.; Apolinar-Jimenez, E.; Munoz-Noriega, N.; Garcia-Morales, L.M.; Leanos-Perez, C.; Figueroa-Barron, M.; Sanchez-Fierros, D.; Reyes-Garcia, J.G. The effects of metformin on inflammatory mediators in obese adolescents with insulin resistance: Controlled randomized clinical trial. J. Pediatr. Endocrinol. Metab. 2012, 25, 41–49. [Google Scholar] [CrossRef]
- Nanjan, M.J.; Mohammed, M.; Prashantha Kumar, B.R.; Chandrasekar, M.J.N. Thiazolidinediones as antidiabetic agents: A critical review. Bioorg. Chem. 2018, 77, 548–567. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S158–S178, Erratum in Diabetes Care 2024, 47, 1238. [Google Scholar] [CrossRef]
- Lee, K.A.; Jin, H.Y.; Kim, Y.J.; Im, Y.J.; Kim, E.Y.; Park, T.S. Treatment Patterns of Type 2 Diabetes Assessed Using a Common Data Model Based on Electronic Health Records of 2000–2019. J. Korean Med. Sci. 2021, 36, e230. [Google Scholar] [CrossRef]
- Huang, L.Y.; Yeh, H.L.; Yang, M.C.; Shau, W.Y.; Su, S.; Lai, M.S. Therapeutic inertia and intensified treatment in diabetes mellitus prescription patterns: A nationwide population-based study in Taiwan. J. Int. Med. Res. 2016, 44, 1263–1271. [Google Scholar] [CrossRef]
- Filipova, E.; Uzunova, K.; Kalinov, K.; Vekov, T. Effects of pioglitazone therapy on blood parameters, weight and BMI: A meta-analysis. Diabetol. Metab. Syndr. 2017, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Pan, X.; Zhang, X.; Tong, N. The effect of thiazolidinediones on body fat redistribution in adults: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2024, 25, e13675. [Google Scholar] [CrossRef]
- Jonker, J.T.; Lamb, H.J.; van der Meer, R.W.; Rijzewijk, L.J.; Menting, L.J.; Diamant, M.; Bax, J.J.; de Roos, A.; Romijn, J.A.; Smit, J.W. Pioglitazone compared with metformin increases pericardial fat volume in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2010, 95, 456–460. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N.; Cheema, P.; Bahouth, S.W.; Garrett, E.; Wolf, R.Y.; Wolford, D.; Samaha, J. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: Changes associated with pioglitazone. Diabetes Care 2011, 34, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Distel, E.; Penot, G.; Cadoudal, T.; Balguy, I.; Durant, S.; Benelli, C. Early induction of a brown-like phenotype by rosiglitazone in the epicardial adipose tissue of fatty Zucker rats. Biochimie 2012, 94, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, X.; Zheng, Y.; Li, J.; Wang, Y.; Lin, Y.; He, Z.; Zhao, W.; Chen, C.; Qiu, K.; et al. Association of glucose-lowering medications with cardiovascular outcomes: An umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020, 8, 192–205. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Lee, C.; Kohler, S. Safety and Tolerability of Combinations of Empagliflozin and Linagliptin in Patients with Type 2 Diabetes: Pooled Data from Two Randomized Controlled Trials. Adv. Ther. 2018, 35, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Takano, H.; Hasegawa, H.; Tadokoro, H.; Hashimoto, N.; Takemura, G.; Kobayashi, Y. The effects of dipeptidyl peptidase-4 on cardiac fibrosis in pressure overload-induced heart failure. J. Pharmacol. Sci. 2017, 135, 164–173. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Varin, E.M.; Ussher, J.R.; Campbell, J.E.; Bang, K.W.; Abdullah, T.; Baggio, L.L.; Drucker, D.J. Inhibition of Dipeptidyl Peptidase-4 Impairs Ventricular Function and Promotes Cardiac Fibrosis in High Fat-Fed Diabetic Mice. Diabetes 2016, 65, 742–754. [Google Scholar] [CrossRef]
- Dimitriadis, G.; Mitrou, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. 1), S52–S59. [Google Scholar] [CrossRef]
- Mittendorfer, B.; Johnson, J.D.; Solinas, G.; Jansson, P.A. Insulin Hypersecretion as Promoter of Body Fat Gain and Hyperglycemia. Diabetes 2024, 73, 837–843. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Mei, B.; Fang, X.; Cai, G.; Cai, N.; Wu, Q.; Huang, Z.; Ge, C.; Liang, H.; et al. A small nucleolar RNA, SNORD126, promotes adipogenesis in cells and rats by activating the PI3K-AKT pathway. J. Cell. Physiol. 2021, 236, 3001–3014. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Y.; Chen, C.; Zhang, L.; Wang, J.; Yang, C.; Wu, T.; Yang, S.; Tao, C.; Wang, Y. Bone Morphogenetic Protein 2 Enhances Porcine Beige Adipogenesis via AKT/mTOR and MAPK Signaling Pathways. Int. J. Mol. Sci. 2024, 25, 3915. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Trabzon, G.; Gungor, S.; Gullu, S.D.; Caliskan, O.F.; Gullu, U.U. Evaluation of epicardial adipose tissue in children with type 1 diabetes. Pediatr. Res. 2025, 97, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V.; Chacko, E.C.; Pappachan, J.M. Non-pharmacological Treatment Options in the Management of Diabetes Mellitus. Eur. Endocrinol. 2018, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, R.; Rajani, R.; Cheng, V.Y.; Shmilovich, H.; Nakanishi, R.; Otaki, Y.; Gransar, H.; Slomka, P.J.; Hayes, S.W.; Thomson, L.E.; et al. Weight change modulates epicardial fat burden: A 4-year serial study with non-contrast computed tomography. Atherosclerosis 2012, 220, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Burchfiel, C.M.; Sharp, D.S.; Curb, J.D.; Rodriguez, B.L.; Hwang, L.J.; Marcus, E.B.; Yano, K. Physical activity and incidence of diabetes: The Honolulu Heart Program. Am. J. Epidemiol. 1995, 141, 360–368. [Google Scholar] [CrossRef]
- Honkala, S.M.; Motiani, K.K.; Eskelinen, J.J.; Savolainen, A.; Saunavaara, V.; Virtanen, K.A.; Loyttyniemi, E.; Kapanen, J.; Knuuti, J.; Kalliokoski, K.K.; et al. Exercise Training Reduces Intrathoracic Fat Regardless of Defective Glucose Tolerance. Med. Sci. Sports Exerc. 2017, 49, 1313–1322. [Google Scholar] [CrossRef]
- Jonker, J.T.; de Mol, P.; de Vries, S.T.; Widya, R.L.; Hammer, S.; van Schinkel, L.D.; van der Meer, R.W.; Gans, R.O.; Webb, A.G.; Kan, H.E.; et al. Exercise and type 2 diabetes mellitus: Changes in tissue-specific fat distribution and cardiac function. Radiology 2013, 269, 434–442. [Google Scholar] [CrossRef]
- Fernandez-del-Valle, M.; Gonzales, J.U.; Kloiber, S.; Mitra, S.; Klingensmith, J.; Larumbe-Zabala, E. Effects of resistance training on MRI-derived epicardial fat volume and arterial stiffness in women with obesity: A randomized pilot study. Eur. J. Appl. Physiol. 2018, 118, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.H.; Wedell-Neergaard, A.S.; Lehrskov, L.L.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Effect of Aerobic and Resistance Exercise on Cardiac Adipose Tissues: Secondary Analyses from a Randomized Clinical Trial. JAMA Cardiol. 2019, 4, 778–787. [Google Scholar] [CrossRef]
- Wilund, K.R.; Tomayko, E.J.; Wu, P.T.; Ryong Chung, H.; Vallurupalli, S.; Lakshminarayanan, B.; Fernhall, B. Intradialytic exercise training reduces oxidative stress and epicardial fat: A pilot study. Nephrol. Dial. Transplant. 2010, 25, 2695–2701. [Google Scholar] [CrossRef]
- Rosety, M.A.; Pery, M.T.; Rodriguez-Pareja, M.A.; Diaz, A.; Rosety, J.; Garcia, N.; Brenes-Martin, F.; Rosety-Rodriguez, M.; Toro, R.; Ordonez, F.J.; et al. A Short-Term Circuit Resistance Programme Reduced Epicardial Fat in Obese Aged Women. Nutr. Hosp. 2015, 32, 2193–2197. [Google Scholar] [CrossRef]
- Kim, M.K.; Tomita, T.; Kim, M.J.; Sasai, H.; Maeda, S.; Tanaka, K. Aerobic exercise training reduces epicardial fat in obese men. J. Appl. Physiol. (1985) 2009, 106, 5–11. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, X.; Zhang, D.; Jiang, C.; Yu, W. Effects of breaking up prolonged sitting via exercise snacks intervention on the body composition and plasma metabolomics of sedentary obese adults: A randomized controlled trial. Endocr. J. 2025, 72, 183–192. [Google Scholar] [CrossRef]
- Islam, H.; Gibala, M.J.; Little, J.P. Exercise Snacks: A Novel Strategy to Improve Cardiometabolic Health. Exerc. Sport. Sci. Rev. 2022, 50, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Delany, J.P.; Otto, A.D.; Kuller, L.; Vockley, J.; South-Paul, J.E.; Thomas, S.B.; Brown, J.; McTigue, K.; Hames, K.C.; et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: A randomized trial. JAMA 2010, 304, 1795–1802. [Google Scholar] [CrossRef]
- Iacobellis, G.; Singh, N.; Wharton, S.; Sharma, A.M. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity 2008, 16, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Tanaka, K.; Kim, M.J.; Matuso, T.; Endo, T.; Tomita, T.; Maeda, S.; Ajisaka, R. Comparison of epicardial, abdominal and regional fat compartments in response to weight loss. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 760–766. [Google Scholar] [CrossRef]
- Pacifico, L.; Bonci, E.; Di Martino, M.; Versacci, P.; Andreoli, G.; Silvestri, L.M.; Chiesa, C. A double-blind, placebo-controlled randomized trial to evaluate the efficacy of docosahexaenoic acid supplementation on hepatic fat and associated cardiovascular risk factors in overweight children with nonalcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Barrio-Lopez, M.T.; Ruiz-Canela, M.; Goni, L.; Valiente, A.M.; Garcia, S.R.; de la, O.V.; Anton, B.D.; Fernandez-Friera, L.; Castellanos, E.; Martinez-Gonzalez, M.A.; et al. Mediterranean diet and epicardial adipose tissue in patients with atrial fibrillation treated with ablation: A substudy of the ‘PREDIMAR’ trial. Eur. J. Prev. Cardiol. 2024, 31, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, B.; Rovio, S.P.; Ruohonen, S.; Hutri-Kahonen, N.; Kahonen, M.; Viikari, J.S.A.; Pahkala, K.; Raitakari, O.T. Determinants of echocardiographic epicardial adipose tissue in a general middle-aged population—The Cardiovascular Risk in Young Finns Study. Sci. Rep. 2024, 14, 11982. [Google Scholar] [CrossRef]
- Kondo, H.; Abe, I.; Gotoh, K.; Fukui, A.; Takanari, H.; Ishii, Y.; Ikebe, Y.; Kira, S.; Oniki, T.; Saito, S.; et al. Interleukin 10 Treatment Ameliorates High-Fat Diet-Induced Inflammatory Atrial Remodeling and Fibrillation. Circ. Arrhythm. Electrophysiol. 2018, 11, e006040. [Google Scholar] [CrossRef]
- Walker, M.E.; Matthan, N.R.; Goldbaum, A.; Meng, H.; Lamon-Fava, S.; Lakshman, S.; Jang, S.; Molokin, A.; Solano-Aguilar, G.; Urban, J.F., Jr.; et al. Dietary patterns influence epicardial adipose tissue fatty acid composition and inflammatory gene expression in the Ossabaw pig. J. Nutr. Biochem. 2019, 70, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.E.; Matthan, N.R.; Solano-Aguilar, G.; Jang, S.; Lakshman, S.; Molokin, A.; Faits, T.; Urban, J.F., Jr.; Johnson, W.E.; Lamon-Fava, S.; et al. A Western-type dietary pattern and atorvastatin induce epicardial adipose tissue interferon signaling in the Ossabaw pig. J. Nutr. Biochem. 2019, 67, 212–218. [Google Scholar] [CrossRef]
- Pezeshkian, M.; Rashidi, M.R.; Varmazyar, M.; Hanaee, J.; Darbin, A.; Nouri, M. Influence of a high cholesterol regime on epicardial and subcutaneous adipose tissue fatty acids profile in rabbits. Metab. Syndr. Relat. Disord. 2011, 9, 403–409. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Okawa, C.; Yamada, H.; Yanagi, S.; Uematsu, E.; Sugasawa, N.; Kurobe, H.; Hirata, Y.; Kim-Kaneyama, J.R.; Lei, X.F.; et al. The pathophysiological role of oxidized cholesterols in epicardial fat accumulation and cardiac dysfunction: A study in swine fed a high caloric diet with an inhibitor of intestinal cholesterol absorption, ezetimibe. J. Nutr. Biochem. 2016, 35, 66–73. [Google Scholar] [CrossRef]
- Hsu, J.L.; Farrell, T.M. Updates in Bariatric Surgery. Am. Surg. 2024, 90, 925–933. [Google Scholar] [CrossRef]
- Sandoval, D.A.; Patti, M.E. Glucose metabolism after bariatric surgery: Implications for T2DM remission and hypoglycaemia. Nat. Rev. Endocrinol. 2023, 19, 164–176. [Google Scholar] [CrossRef]
- van Schinkel, L.D.; Sleddering, M.A.; Lips, M.A.; Jonker, J.T.; de Roos, A.; Lamb, H.J.; Jazet, I.M.; Pijl, H.; Smit, J.W. Effects of bariatric surgery on pericardial ectopic fat depositions and cardiovascular function. Clin. Endocrinol. 2014, 81, 689–695. [Google Scholar] [CrossRef]
- Gaborit, B.; Jacquier, A.; Kober, F.; Abdesselam, I.; Cuisset, T.; Boullu-Ciocca, S.; Emungania, O.; Alessi, M.C.; Clement, K.; Bernard, M.; et al. Effects of bariatric surgery on cardiac ectopic fat: Lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J. Am. Coll. Cardiol. 2012, 60, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Meulendijks, E.R.; Janssen-Telders, C.; Hulsman, E.L.; Lobe, N.; Zappala, P.; Terpstra, M.M.; Wesselink, R.; de Vries, T.A.C.; Al-Shama, R.F.; van Veen, R.N.; et al. The change of epicardial adipose tissue characteristics and vulnerability for atrial fibrillation upon drastic weight loss. Adipocyte 2024, 13, 2395565. [Google Scholar] [CrossRef] [PubMed]
- Willens, H.J.; Byers, P.; Chirinos, J.A.; Labrador, E.; Hare, J.M.; de Marchena, E. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am. J. Cardiol. 2007, 99, 1242–1245. [Google Scholar] [CrossRef]
- Graziani, F.; Leone, A.M.; Cialdella, P.; Basile, E.; Pennestri, F.; Della Bona, R.; Iaconelli, A.; Liuzzo, G.; Biasucci, L.M.; Cardillo, M.T.; et al. Effects of bariatric surgery on cardiac remodeling: Clinical and pathophysiologic implications. Int. J. Cardiol. 2013, 168, 4277–4279. [Google Scholar] [CrossRef]
- Altin, C.; Erol, V.; Aydin, E.; Yilmaz, M.; Tekindal, M.A.; Sade, L.E.; Gulay, H.; Muderrisoglu, H. Impact of weight loss on epicardial fat and carotid intima media thickness after laparoscopic sleeve gastrectomy: A prospective study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 501–509. [Google Scholar] [CrossRef]
- Kaya, B.C.; Elkan, H. The impact of weight loss after laparoscopic sleeve gastrectomy on early markers of atherosclerotic vascular disease: A prospective study. Kardiol. Pol. 2020, 78, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, A.; Alexiadou, K.; Liaskos, C.; Argyrakopoulou, G.; Balla, I.; Tentolouris, N.; Moyssakis, I.; Katsilambros, N.; Vafiadis, I.; Alexandrou, A.; et al. Improvement in cardiovascular indices after Roux-en-Y gastric bypass or sleeve gastrectomy for morbid obesity. Obes. Surg. 2013, 23, 31–38. [Google Scholar] [CrossRef]
- Henry, J.A.; Abdesselam, I.; Deal, O.; Lewis, A.J.; Rayner, J.; Bernard, M.; Dutour, A.; Gaborit, B.; Kober, F.; Soghomonian, A.; et al. The effect of bariatric surgery type on cardiac reverse remodelling. Int. J. Obes. 2024, 48, 808–814. [Google Scholar] [CrossRef]
- Henry, J.A.; Abdesselam, I.; Deal, O.; Lewis, A.J.; Rayner, J.; Bernard, M.; Dutour, A.; Gaborit, B.; Kober, F.; Soghomonian, A.; et al. Changes in epicardial and visceral adipose tissue depots following bariatric surgery and their effect on cardiac geometry. Front. Endocrinol. 2023, 14, 1092777. [Google Scholar] [CrossRef]
- Asteria, C.; Secchi, F.; Morricone, L.; Malavazos, A.E.; Francesconi, S.; Milani, V.; Giovanelli, A. Open-bore MRI Scanner Assessment of Epicardial Adipose Tissue after Bariatric Surgery: A Pilot Study. Endocr. Metab. Immune Disord. Drug Targets 2025, 25, 173–188. [Google Scholar] [CrossRef]
- Hunt, S.C.; Davidson, L.E.; Adams, T.D.; Ranson, L.; McKinlay, R.D.; Simper, S.C.; Litwin, S.E. Associations of Visceral, Subcutaneous, Epicardial, and Liver Fat with Metabolic Disorders up to 14 Years After Weight Loss Surgery. Metab. Syndr. Relat. Disord. 2021, 19, 83–92. [Google Scholar] [CrossRef]
- Leroux-Stewart, J.; Elisha, B.; Tagougui, S.; Suppere, C.; Bernard, S.; Mircescu, H.; Desjardin, K.; Messier, V.; Iacobellis, G.; Rabasa-Lhoret, R. Effect of caloric restriction with or without physical activity on body composition and epicardial fat in type 2 diabetic patients: A pilot randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 921–929. [Google Scholar] [CrossRef]
- Wu, F.Z.; Huang, Y.L.; Wu, C.C.; Wang, Y.C.; Pan, H.J.; Huang, C.K.; Yeh, L.R.; Wu, M.T. Differential Effects of Bariatric Surgery Versus Exercise on Excessive Visceral Fat Deposits. Medicine 2016, 95, e2616. [Google Scholar] [CrossRef]
- Serrano-Ferrer, J.; Crendal, E.; Walther, G.; Vinet, A.; Dutheil, F.; Naughton, G.; Lesourd, B.; Chapier, R.; Courteix, D.; Obert, P. Effects of lifestyle intervention on left ventricular regional myocardial function in metabolic syndrome patients from the RESOLVE randomized trial. Metabolism 2016, 65, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Fenk, S.; Fischer, M.; Strack, C.; Schmitz, G.; Loew, T.; Lahmann, C.; Baessler, A. Successful weight reduction improves left ventricular diastolic function and physical performance in severe obesity. Int. Heart J. 2015, 56, 196–202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ersan Demirci, D.; Demirci, D.; Eke, R.N. Reversal of metabolic syndrome with weight loss decreases epicardial fat more than weight loss alone in women with obesity. Turk. Kardiyol. Dern. Ars. 2022, 50, 45–56. [Google Scholar] [CrossRef]
- Bairapareddy, K.C.; Maiya, A.G.; Kumar, P.; Nayak, K.; Guddattu, V.; Nayak, V. Effect of aerobic exercise on echocardiographic epicardial adipose tissue thickness in overweight individuals. Diabetes Metab. Syndr. Obes. 2018, 11, 303–312. [Google Scholar] [CrossRef]
- Liang, K.W.; Tsai, I.C.; Lee, W.J.; Lin, S.Y.; Lee, W.L.; Lee, I.T.; Fu, C.P.; Wang, J.S.; Sheu, W.H. Correlation between reduction of superior interventricular groove epicardial fat thickness and improvement of insulin resistance after weight loss in obese men. Diabetol. Metab. Syndr. 2014, 6, 115. [Google Scholar] [CrossRef]
- Fu, C.P.; Sheu, W.H.; Lee, I.T.; Tsai, I.C.; Lee, W.J.; Liang, K.W.; Lee, W.L.; Lin, S.Y. Effects of weight loss on epicardial adipose tissue thickness and its relationship between serum soluble CD40 ligand levels in obese men. Clin. Chim. Acta 2013, 421, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Mikamo, H.; Jiang, M.; Noro, M.; Suzuki, Y.; Hiruta, N.; Unoki-Kubota, H.; Schneider, W.J.; Bujo, H. Susceptibilities of epicardial and subcutaneous fat tissue for browning-gene expression and diet-induced volume reduction are different. Mol. Med. Rep. 2018, 17, 6542–6550. [Google Scholar] [CrossRef]
- Durlach, V.; Verges, B.; Al-Salameh, A.; Bahougne, T.; Benzerouk, F.; Berlin, I.; Clair, C.; Mansourati, J.; Rouland, A.; Thomas, D.; et al. Smoking and diabetes interplay: A comprehensive review and joint statement. Diabetes Metab. 2022, 48, 101370. [Google Scholar] [CrossRef]
- Qu, D.; Liu, J.; Lau, C.W.; Huang, Y. IL-6 in diabetes and cardiovascular complications. Br. J. Pharmacol. 2014, 171, 3595–3603. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell. Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef]
- Mach, L.; Bedanova, H.; Soucek, M.; Karpisek, M.; Nemec, P.; Orban, M. Tobacco smoking and cytokine levels in human epicardial adipose tissue: Impact of smoking cessation. Atherosclerosis 2016, 255, 37–42. [Google Scholar] [CrossRef]
- Milanese, G.; Silva, M.; Bruno, L.; Goldoni, M.; Benedetti, G.; Rossi, E.; Ferrari, C.; Grutta, L.; Maffei, E.; Toia, P.; et al. Quantification of epicardial fat with cardiac CT angiography and association with cardiovascular risk factors in symptomatic patients: From the ALTER-BIO (Alternative Cardiovascular Bio-Imaging markers) registry. Diagn. Interv. Radiol. 2019, 25, 35–41. [Google Scholar] [CrossRef]
- Hartiala, O.; Magnussen, C.G.; Bucci, M.; Kajander, S.; Knuuti, J.; Ukkonen, H.; Saraste, A.; Rinta-Kiikka, I.; Kainulainen, S.; Kahonen, M.; et al. Coronary heart disease risk factors, coronary artery calcification and epicardial fat volume in the Young Finns Study. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Foldyna, B.; Hadzic, I.; Zeleznik, R.; Langenbach, M.C.; Raghu, V.K.; Mayrhofer, T.; Lu, M.T.; Aerts, H. Deep learning analysis of epicardial adipose tissue to predict cardiovascular risk in heavy smokers. Commun. Med. 2024, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Monti, A.; Murdolo, G.; Di Renzi, P.; Pirro, M.R.; Borgognoni, F.; Vincentelli, G.M. Correlation between epicardial fat and cigarette smoking: CT imaging in patients with metabolic syndrome. Scand. Cardiovasc. J. 2014, 48, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Gac, P.; Czerwinska, K.; Poreba, M.; Macek, P.; Mazur, G.; Poreba, R. Environmental Tobacco Smoke Exposure Estimated Using the SHSES Scale and Epicardial Adipose Tissue Thickness in Hypertensive Patients. Cardiovasc. Toxicol. 2021, 21, 79–87. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Angelini, F.; Belli, L.; Cicero, A.F.G.; Da Ros, R.; De Pergola, G.; Gaudio, G.V.; Lupi, A.; Sartore, G.; et al. Nutraceuticals and Supplements in Management of Prediabetes and Diabetes. Nutrients 2024, 17, 14. [Google Scholar] [CrossRef] [PubMed]
| Author, Reference | Pharmacological Intervention | Study Population | Method of EAT Assessment | Observation Time | Change in EAT Characteristics |
|---|---|---|---|---|---|
| GLP-1 receptor agonists | |||||
| Morano et al. [54] | Exenatide or liraglutide | 25 patients with T2DM
| Echo | 3 months | ↓ EAT thickness in both exenatide and liraglutide groups No significant difference between groups |
| Iacobellis et al. [55] | Metformin or metformin + liraglutide | 95 patients with T2DM
| Echo | 3, 6 months | ↓ EAT thickness in metformin + liraglutide group No significant difference in metformin group |
| Iacobellis et al. [56] | Semaglutide or dulaglutide or metformin | 80 patients with T2DM and obesity
| Echo | 12 weeks | ↓ EAT thickness in semaglutide and dulaglutide groups, no significant difference between them No significant EAT thickness reduction in metformin group |
| Kramer et al. [57] | Tirzepatide | 175 patients with obesity-related HFpEF | MRI | 52 weeks | No significant changes in EAT volume |
| SGLT-2 inhibitors | |||||
| Cinti et al. [58] | Dapagliflozin | 14 patients with T2DM
| PET/CT during Euglycemic hyperinsulinemic clamp | 4 weeks | ↓ EAT thickness ↓ glucose uptake by EAT |
| Requena-Ibáñez et al. [59] | Empagliflozin | 84 nondiabetic HFrEF patients
| MRI | 6 months | ↓ EAT volume |
| Fukuda et al. [60] | Ipragliflozin | 9 non-obese patients with T2DM | MRI | 12 weeks | ↓ EAT volume, |
| Bouchi et al. [61] | Luseogliflozin | 19 patients with T2DM | MRI | 12 weeks | ↓ EAT volume |
| Sato et al. [62] | Dapagliflozin | 40 patients with T2DM and CAD
| CT | 6 months | ↓ EAT volume |
| Metformin | |||||
| Ziyrek et al. [63] | Metformin | 40 patients newly diagnosed with T2DM | Echo | 3 months | ↓ EAT thickness |
| Gunes et al. [64] | Metformin | 30 obese children with insulin resistance | Echo | 3 months | ↓ EAT thickness |
| Thiazolidinediones | |||||
| Moody et al. [65] | Pioglitazone | 24 individuals
| MRI | 24 weeks | ↓ EAT volume |
| DPP-4 inhibitors | |||||
| Lima-Martinez et al. [66] | Sitagliptin | 26 patients with T2DM inadequately controlled with metformin | Echo | 24 weeks | ↓ EAT thickness |
| Hiruma et al. [67] | Sitagliptin or empagliflozin | 44 patients with T2DM
| MRI | 12 weeks | ↓ EAT area No significant difference in EAT reduction between groups |
| Insulin | |||||
| Elisha et al. [68] | Insulin Detemir or Insulin Glargine | 36 patients inadequetly controlled T2DM
| Echo | 6 months | ↓ EAT thickness No significant difference between groups |
| Author, Reference | Intervention | Study Population | Method of EAT Assessment | Observation Time | Change in EAT Characteristics |
| Honkala et al. [139] | HIIT or MICT | 44 men
| CT | 2 weeks | ↓ EAT volume |
| Jonker et al. [140] | moderate-intensity exercise followed by a high-altitude trekking expedition with exercise of long duration | 12 patients with T2DM | MRI | 6 months | No significant changes in EAT volume |
| Fernandez-del-Valle et al. [141] | high-intensity, moderate-volume muscular endurance resistance training | 11 young females with obesity *
| MRI | 3 weeks | ↓ EAT volume in intervention group |
| Christensen et al. [142] | endurance or resistance training | 39 physically inactive participants with abdominal obesity a
| MRI | 12 weeks | Decrease in EAT mass significantly greater in endurance and resistance groups compared to the control group. |
| Wilund et al. [143] | intradialytic exercise training (cycling) | 17 hemodialysis patients
| Echo | 4 months | ↓ EAT thickness in the exercising group |
| Rosety et al. [144] | circuit training program, 3 days per week | 48 obese women over 65 years old a
| Echo | 12 weeks | ↓ EAT thickness in the exercising group |
| Kim et al. [145] | supervised exercise training program | 24 obese middle-aged men (57.7% with metabolic syndrome) | Echo | 12 weeks | ↓ EAT thickness |
| Zhou et al. [146] | 4 days of sprint exercises by stair-climbing per week (“exercise snacks”) | 27 participants *
| CT | 12 weeks | ↓ EAT volume |
| Author, Reference | Intervention | Study Population | Method of EAT Assessment | Observation Time | Change in EAT Characteristics |
|---|---|---|---|---|---|
| Iacobellis et al. [149] | very low-calorie diet weight loss program | 20 severely obese individuals a | Echo | 6 months | ↓ EAT thickness |
| Kim et al. [150] | low-calorie diet | 27 moderately obese men * | Echo | 12 weeks | ↓ EAT thickness |
| Pacifico et al. [151] | DHA supplementation | 51 overweight children a
| Echo | 6 months | ↓ EAT thickness |
|
Author, Reference | Intervention | Study Population | Method of EAT Assessment | Observation Time | Change in EAT Characteristics |
|---|---|---|---|---|---|
| van Schinkel et al. [161] | RYGB | 10 obese patients with T2DM | MRI | 16 weeks | ↓ EAT volume |
| Gaborit et al. [162] | RYGB | 23 morbidly obese patients (26% DM) | MRI | 6 months | ↓ EAT volume |
| Meulendijks et al. [163] | RYGB or SG | 36 patients over 40 years old (5% DM) | CT | 1 year | ↓ EAT volume ↑ EAT attenuation |
| Willens et al. [164] |
| 23 patients with severe obesity * | Echo | 8.3 ± 3.7 months | ↓ EAT thickness |
| Graziani et al. [165] | bariatric surgery (not specified) | 80 obese patients *
| Echo | 2 years | ↓ EAT thickness |
| Altin et al. [166] | laparoscopic SG | 105 patients (26.7% DM) | Echo | 6 months | ↓ EAT thickness |
| Kaya and Elkan [167] | laparoscopic SG | 71 patients (36.6% DM) | Echo | 6 months | ↓ EAT thickness |
| Kokkinos et al. [168] | RYGB or SG | 37 patients
| Echo | 6 months | ↓ EAT thickness in both groups; significantly greater decrease in RYGB group |
| Henry et al. [169] | RYGB or laparoscopic SG or LAGB | 58 patients
| MRI | short-term (median 251–273 days) and longer-term (median 983–1027 days) | ↓ EAT volume; significantly greater decrease in RYGB group |
| Henry et al. [170] | gastric bypass or SG or adjustable gastric band | 62 patients (15% diabetes)
| MRI | short-term (median 212 days), medium-term (median 428 days), long-term (median 1030 days) | ↓ EAT volume |
| Asteria et al. [171] | laparoscopic SG or RYGB | 15 patients *
| MRI | 12 months | ↓ EAT volume; significantly greater decrease in RYGB group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuleta, K.; Krauz, K.; Żmuda, J.; Momot, K.; Zarębiński, M.; Poprawa, I.; Wojciechowska, M. Pharmacological and Non-Pharmacological Interventions in Diabetes Mellitus: Effects on Epicardial Adipose Tissue. Int. J. Mol. Sci. 2025, 26, 9271. https://doi.org/10.3390/ijms26199271
Kuleta K, Krauz K, Żmuda J, Momot K, Zarębiński M, Poprawa I, Wojciechowska M. Pharmacological and Non-Pharmacological Interventions in Diabetes Mellitus: Effects on Epicardial Adipose Tissue. International Journal of Molecular Sciences. 2025; 26(19):9271. https://doi.org/10.3390/ijms26199271
Chicago/Turabian StyleKuleta, Krzysztof, Kamil Krauz, Jakub Żmuda, Karol Momot, Maciej Zarębiński, Izabela Poprawa, and Małgorzata Wojciechowska. 2025. "Pharmacological and Non-Pharmacological Interventions in Diabetes Mellitus: Effects on Epicardial Adipose Tissue" International Journal of Molecular Sciences 26, no. 19: 9271. https://doi.org/10.3390/ijms26199271
APA StyleKuleta, K., Krauz, K., Żmuda, J., Momot, K., Zarębiński, M., Poprawa, I., & Wojciechowska, M. (2025). Pharmacological and Non-Pharmacological Interventions in Diabetes Mellitus: Effects on Epicardial Adipose Tissue. International Journal of Molecular Sciences, 26(19), 9271. https://doi.org/10.3390/ijms26199271






